The Separation Behavior of TiB2 during Cl2-Free Degassing Treatment of 5083 Aluminum Melt
Abstract
:1. Introduction
2. Experimental Methodology
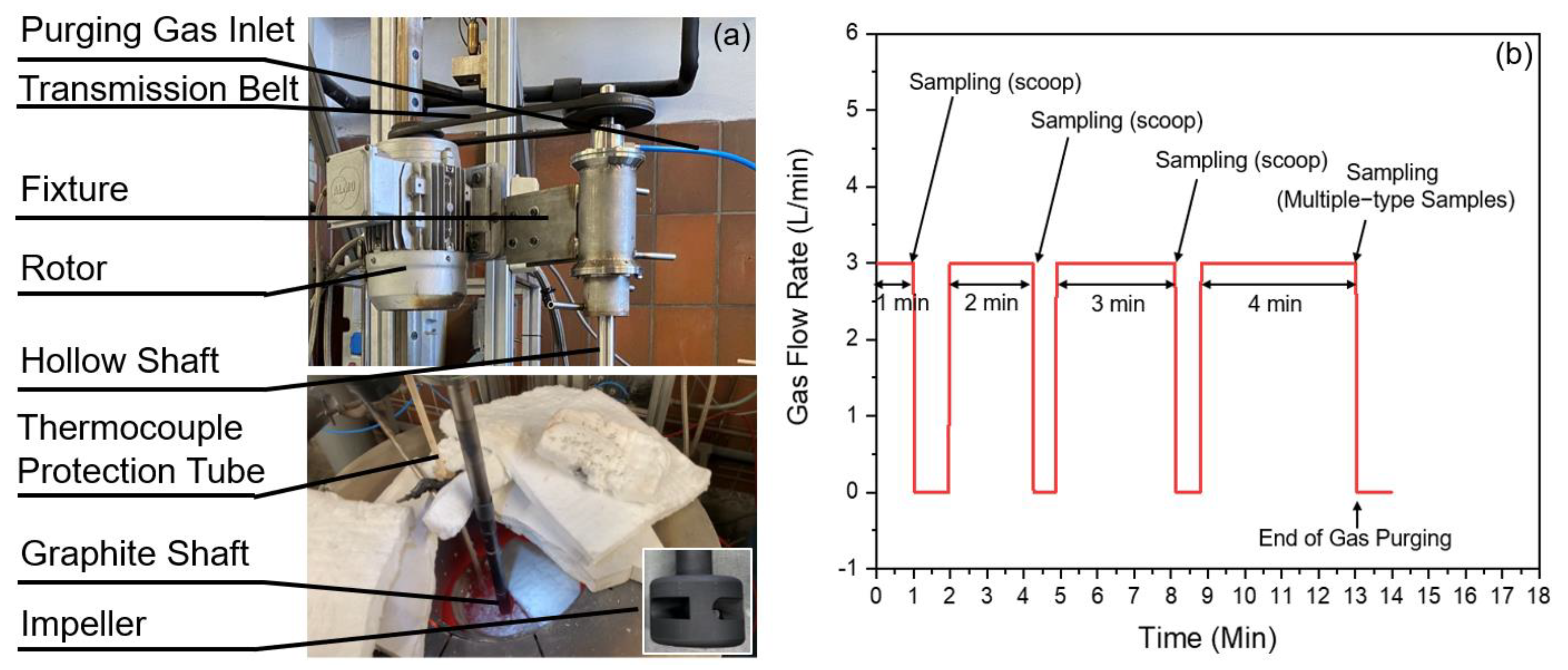
2.1. Set-Up and Materials
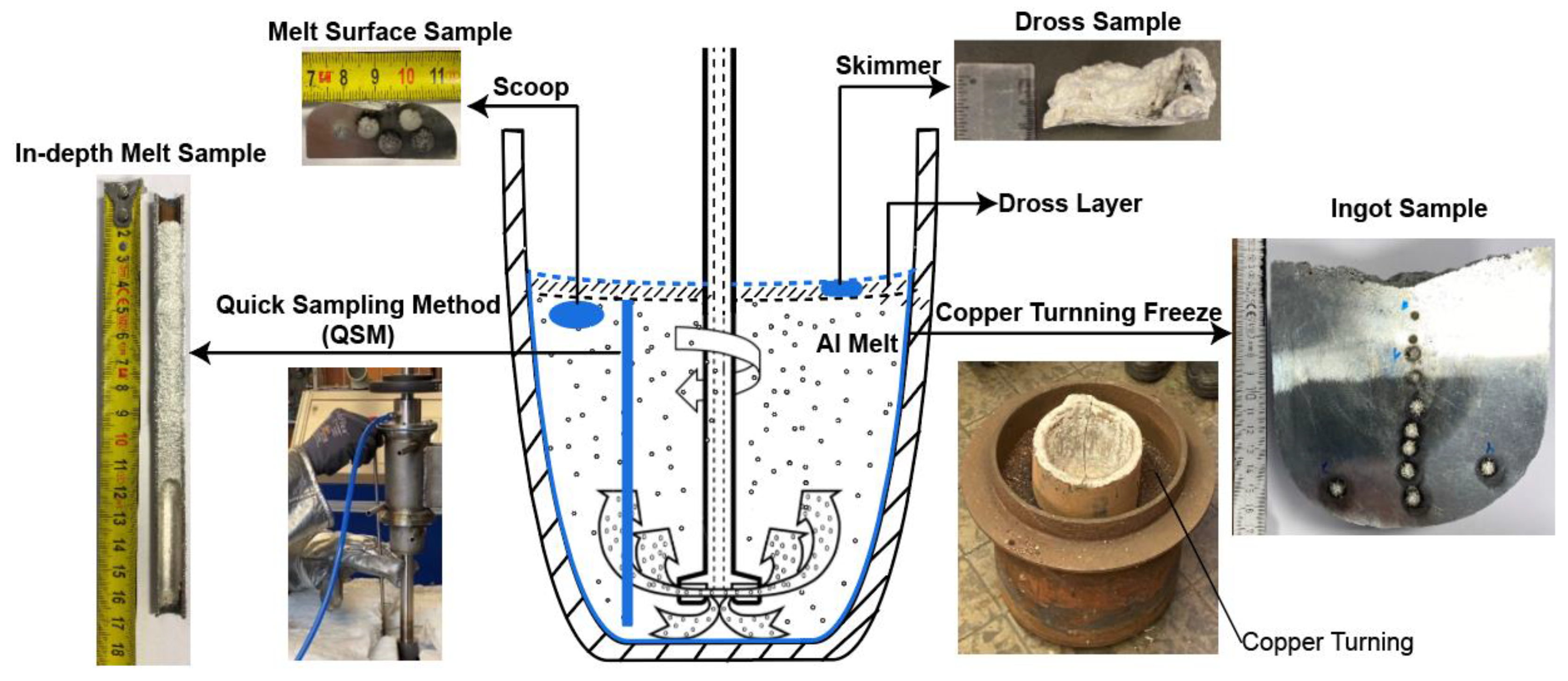
2.2. Trial Procedures
2.3. TiB2 Evaluation
3. Results
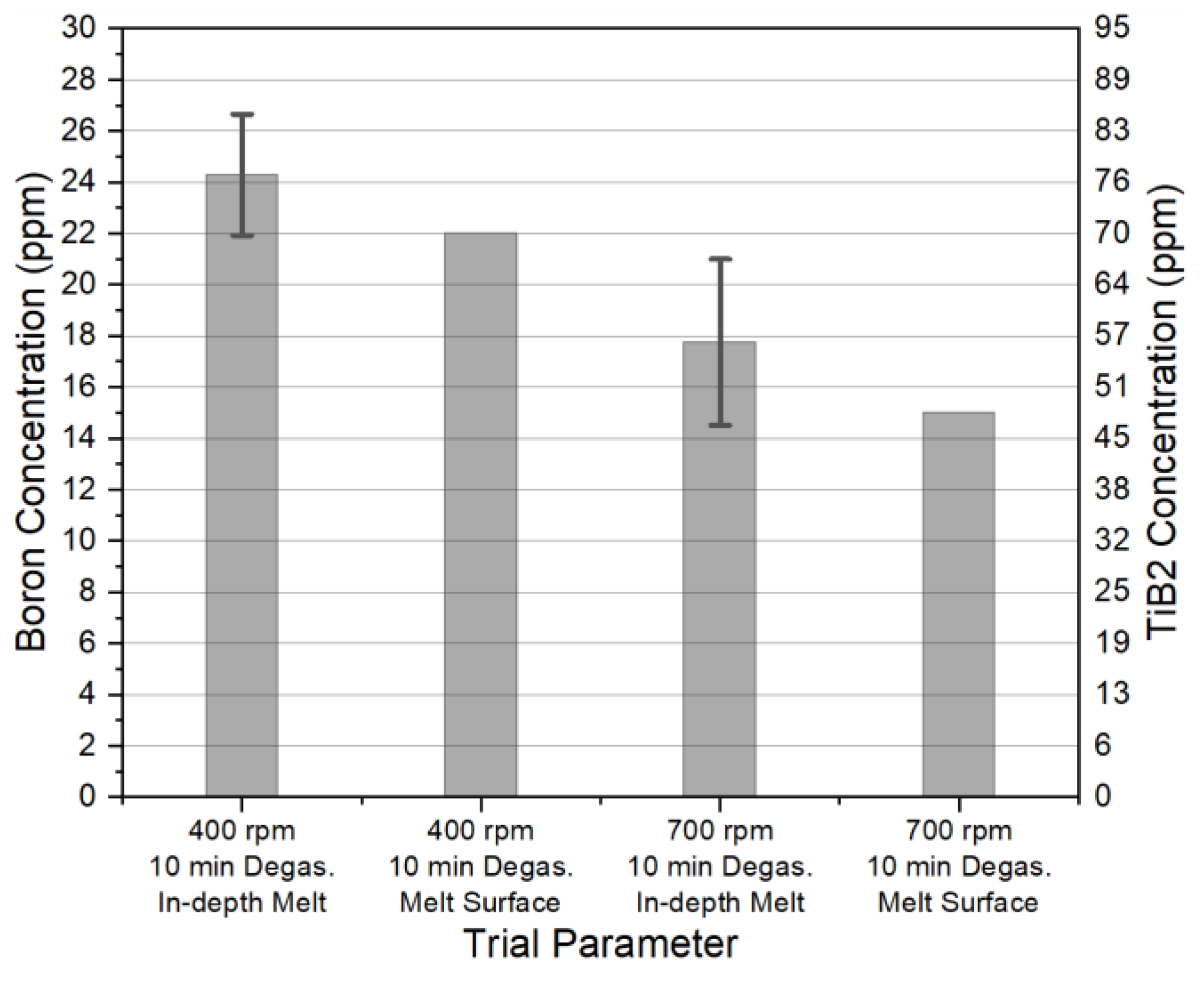
3.1. TiB2 Distribution
3.2. Influence of Impeller Rotation Speed on TiB2 Removal Kinetics
4. Conclusions
- Particle mapping results suggest that during degassing, TiB2 particles are separated to the dross layer, while their concentration in main part of the melt is relatively homogeneous.
- A significant removal of TiB2 particles during the Cl2-free degassing process was confirmed. The removal rate of TiB2 particles increased with the impeller rotary speed. At 700 rpm, the removal rate constant of TiB2 particles was 0.08 min−1.
- Conventional deterministic flotation model estimates of the inclusion removal rate constant is ca. 10 times lower compared with the experimental results. The EE mechanism is believed to be responsible for TiB2 removal during the Cl2-free degassing process. Instead of bubbles, the EE mechanism considers mainly the contribution of melt turbulence and the hetero-agglomeration of TiB2 and OF to the removal effect.
- From the point of view of preventing the fade of TiB2, it is suggested to add an Al-Ti-B grain refiner at the end phase of batch degassing treatments or after in-liner degassing. Reducing the impeller rotary speed or shortening the degassing treatment time (for in-line degassers, the time refers to the residence time) can also help; nevertheless, one needs to consider if the adjusted process is able to remove other harmful dissolved impurities and inclusions sufficiently.
- To validate the EE mechanism proposed to be accountable for TiB2 removal during Cl2-free degassing treatments, more theoretical and experimental work is required. The establishment of an analytical model deserves fundamental attention for predicting TiB2 removal during Cl2-free degassing treatments. From an experimental perspective, it is necessary to assess the influence of the dross layer’s properties on TiB2 removal efficiency. Be it wet or dry, or containing oxide films or other types of inclusions, it will be interesting to know how the dross layer affects the TiB2 separation behavior in the corresponding melt.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitehead, A.J.; Copper, P.S.; McCarthy, R.W. An Evaluation of Metal Cleanliness and Grain Refinement of 5182 Aluminum Alloy DC Cast Ingot Using Al-3% Ti-0.15%C and Al-3%Ti-1%B Grain Refiners. In Proceedings of the Light Metals; The Minerals, Metals & Materials Society, San Diego, CA, USA, 28 February–4 March 1999; pp. 763–772. [Google Scholar]
- EL Haj, B.A.; Hamadellah, A.; Bouayad, A.; El Akili, C. Review of Grain Refinement Performance of Aluminium Cast Alloys. Metall. Mater. Eng. 2023, 29, 1–15. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, T.; Fu, J.; Zu, Q. Development of Inoculants for Aluminum Alloy: A Review. Materials 2023, 16, 5500. [Google Scholar] [CrossRef] [PubMed]
- Milani, V.; Timelli, G. Solid Salt Fluxes for Molten Aluminum Processing—A Review. Metals 2023, 13, 832. [Google Scholar] [CrossRef]
- Dion-Martin, O.; Desmeules, J.-F.; Dumont, R. Molten Aluminium Transfer: Review and Comparison of Different Technologies. In Proceedings of the Light Metals; The Minerals, Metals & Materials Society, Virtual, 15–18 March 2021; pp. 769–777. [Google Scholar]
- Rathinasuriyan, C.; Karthik, K.; Sridhar, K. Investigation of Degassing on Aluminum Alloy by Rotatory Impeller Degasser. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Samaha, S.; Robichaud, P.; Gauthier, P.; Colbert, J.; Wang, T.; Hammersmith, G.; Event, P.; Maltais, B. Chlorine Free Degas System for Aluminium Slab Casting and Its Effect on Sheet Products. In Proceedings of the Light Metals; The Minerals, Metals & Materials Society, Anaheim, CA, USA, 27 February–3 March 2022; pp. 655–665. [Google Scholar]
- Liu, G.; Ren, Y.; Ma, W.; Morita, K.; Lei, Y.; Zhan, S.; Lv, G.; Li, S.; Wang, Z.; Li, R. Recent Advances and Future Trend of Aluminum Alloy Melt Purification: A Review. J. Mater. Res. Technol. 2024, 28, 4647–4662. [Google Scholar] [CrossRef]
- Wu, J.; Djavanroodi, F.; Gode, C.; Attarilar, S.; Ebrahimi, M. Melt Refining and Purification Processes in Al Alloys: A Comprehensive Study. Mater. Res. Express 2022, 9, 032001. [Google Scholar] [CrossRef]
- Raabe, D.; Ponge, D.; Uggowitzer, P.J.; Roscher, M.; Paolantonio, M.; Liu, C.; Antrekowitsch, H.; Kozeschnik, E.; Seidmann, D.; Gault, B.; et al. Making Sustainable Aluminum by Recycling Scrap: The Science of “Dirty” Alloys. Prog. Mater. Sci. 2022, 128, 100947. [Google Scholar] [CrossRef]
- Snow, G.; Pattle, D.; Walker, G.R.D.U. An Efficient Degassing System for the Aluminium Cast House. In Proceedings of the Light Metals; The Minerals, Metals & Materials Society, Denver, CO, USA, 24–26 February 1987; pp. 717–727. [Google Scholar]
- Socha, L.; Prášil, T.; Gryc, K.; Svizelova, J.; Saternus, M.; Merder, T.; Pieprzyca, J.; Nuska, P. Assessment of Refining Efficiency during the Refining Cycle in a Foundry Degassing Unit in Industrial Conditions. Sci. Rep. 2024, 14, 1415. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.S.; Kang, H.J.; Lee, G.H.; Yoon, P.H.; Park, J.Y.; Choi, Y.S.; Shin, S. Effect of Gas Bubbling Filtration Treatment Conditions on Melt Quality of Alsimgcu Alloy. Metals 2021, 11, 841. [Google Scholar] [CrossRef]
- Sigworth, G.K.; Williams, E.M.; Chesonis, D.C. Gas Fluxing of Molten Aluminum: An Overview. In Essential Readings in Light Metals: Cast Shop for Aluminum Production; Springer: Cham, Switherland, 2013; ISBN 978-3-319-48574-4. [Google Scholar]
- Simensen, C.J. The Effect of Melt Refining upon Inclusions in Aluminum. Metall. Trans. B 1982, 13, 31–34. [Google Scholar] [CrossRef]
- Schaffer, P.L.; Dahle, A.K. The Effect of Degassing on Grain Refinement in Commercial Purity Aluminum. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2009, 40, 481–485. [Google Scholar] [CrossRef]
- Khorasani, A.N. Effect of Degassing on Aluminium Silicon Alloys. AFS Trans. 1995, 103, 515–519. [Google Scholar]
- Gu, H.; Zou, Y.Z.; Bin Xu, Z.; Zeng, J.M. Investigation on Purification and Degassing of TiB2/Al Composite Fabricated by LSM Method. Key Eng. Mater. 2007, 353–358, 3051–3054. [Google Scholar] [CrossRef]
- Gudmundsson, T.; Saevarsdottir, G.; Sigfusson, T.I.; Mccartney, D.G. Chlorination of TiB2 Grain Refined Aluminium Melts. In Proceedings of the Light Metals; The Minerals, Metals & Materials Society, Orlando, FL, USA, 9–13 February 1997; pp. 851–855. [Google Scholar]
- Li, C.; Zhang, X.; Gökelma, M.; Stets, W.; Friedrich, B. A Quick Sampling Method for Mapping Particle Distribution in Al-Melt. In Proceedings of the European Metallurgical Conference, Düsseldorf, Germany, 23–26 June 2019; pp. 35–50. [Google Scholar]
- Quested, T.E. Understanding Mechanisms of Grain Refinement of Aluminium Alloys by Inoculation. Mater. Sci. Technol. 2004, 20, 1357–1369. [Google Scholar] [CrossRef]
- Engh, T.A.; Sigworth, G.K.; Kvithyld, A. Principles of Metal Refining and Recycling; Oxford University Press: Oxford, UK, 2021; ISBN 978-0-19-881192-3. [Google Scholar]
- Wang, D.; Liu, Q. Hydrodynamics of Froth Flotation and Its Effects on Fine and Ultrafine Mineral Particle Flotation: A Literature Review. Miner. Eng. 2021, 173, 107220. [Google Scholar] [CrossRef]
- Dai, Z.; Fornasiero, D.; Ralston, J. Particle-Bubble Collision Models—A Review. Adv. Colloid Interface Sci. 2000, 85, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ochoa, F.; Gomez, E. Theoretical Prediction of Gas-Liquid Mass Transfer Coefficient, Specific Area and Hold-up in Sparged Stirred Tanks. Chem. Eng. Sci. 2004, 59, 2489–2501. [Google Scholar] [CrossRef]
- Johansen, S.T.; Gradahl, S.; Grontvedt, P.O.; Tetlie, P.; Gammelsæter, R.; Venas, K.; Skaret, P.; Myrboastad, E.; Rasch, B. The Bubble Size and Mass Transfer Mechanisms in Rotor Stirred Reactors. In Proceedings of the Light Metals; The Minerals, Metals & Materials Society, Orlando, FL, USA, 9–13 February 1997; pp. 663–666. [Google Scholar]
- Johansen, S.T.; Graadahl, S.; Tetlie, P.; Rasch, B.; Myrboastad, E. Can Rotor-Based Refining Units Be Developed and Optimised on Water Model Experiment? In Proceedings of the Light Metals; The Minerals, Metals & Materials Society, San Antonio, TX, USA, 15–19 February 1998; pp. 805–810. [Google Scholar]
- Li, C.; Gökelma, M.; Dang, T.; Huang, J.; Huang, C.; Li, J.; Friedrich, B. Assessment of Melt Cleanliness of Secondary 5000 Aluminum Alloy via Non-Metallic Inclusions Characterization. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2023, 54, 578–592. [Google Scholar] [CrossRef]
- George, P.; Nguyen, A.V.; Jameson, G.J. Assessment of True Flotation and Entrainment in the Flotation of Submicron Particles by Fine Bubbles. Miner. Eng. 2004, 17, 847–853. [Google Scholar] [CrossRef]



| Reference | Method of Refinement | Alloy | Gas | Scale | Removal | Proposed Removal Principle |
|---|---|---|---|---|---|---|
| Schaffer et al. [16] | Lance | CPAl 1 | Ar | 5 kg | Limited | Sedimentation |
| Khorasani [17] | Impeller | A356-Sr | N2/Ar | 450 kg | Significant | Floatation |
| Gu et al. [18] | N.A. | CPAl-5% TiB2 | C2Cl6 | N.A. | Significant | Floatation |
| Gudmundsson et al. [19] | Impeller | CPAl (with Na,K) | Ar-5%Cl2 | 130 kg | Limited | / |
| Simensen [15] | Impeller (SNIF) | CPAl | N.A. | Plant-scale | Negligible | / |
| 5083 | Mg (wt. %) | Si (wt. %) | Ti (wt. %) | Mn (wt. %) | Al (wt. %) |
|---|---|---|---|---|---|
| As-received | 4.72 | 0.06 | 0.04 | 0.52 | Balance |
| / | Process Window | Sampling Operations | ||||||
|---|---|---|---|---|---|---|---|---|
| Trial Nr. | Inclusion | Rotor Speed (rpm) | Gas Flow Rate (L/min) | Duration (10 min) | Scoop | Dross | QSM 1 | Ingot |
| F-5kg-TB-400 | TiB2 | 400 | 3 | 10 | Yes | Yes | Yes | Yes |
| F-5kg-TB-550 | TiB2 | 550 | 3 | 10 | Yes | No | No | No |
| F-5kg-TB-700 | TiB2 | 700 | 3 | 10 | Yes | Yes | Yes | Yes |
| Parameter | Gas Flow Rate | Cross-Sectional Area | Bubble Diameter 1 | Collision Efficiency 2 | Flotation Rate Constant |
|---|---|---|---|---|---|
| Symbol | G | At | db | Ec | kf |
| Unit | (L/min) | (m2) | (mm) | / | (min−1) |
| Value | 3 | 0.011 | 10 | 0.00016 | 0.0063 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Gökelma, M.; Stets, W.; Friedrich, B. The Separation Behavior of TiB2 during Cl2-Free Degassing Treatment of 5083 Aluminum Melt. Metals 2024, 14, 402. https://doi.org/10.3390/met14040402
Li C, Gökelma M, Stets W, Friedrich B. The Separation Behavior of TiB2 during Cl2-Free Degassing Treatment of 5083 Aluminum Melt. Metals. 2024; 14(4):402. https://doi.org/10.3390/met14040402
Chicago/Turabian StyleLi, Cong, Mertol Gökelma, Wolfram Stets, and Bernd Friedrich. 2024. "The Separation Behavior of TiB2 during Cl2-Free Degassing Treatment of 5083 Aluminum Melt" Metals 14, no. 4: 402. https://doi.org/10.3390/met14040402






