Kinetic Study of Manganese Oxidative Precipitation Reaction by Using SO2/Air Gas Mixture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Apparatus
2.3. Methods
2.4. Analysis and Characterization
3. Results and Discussion
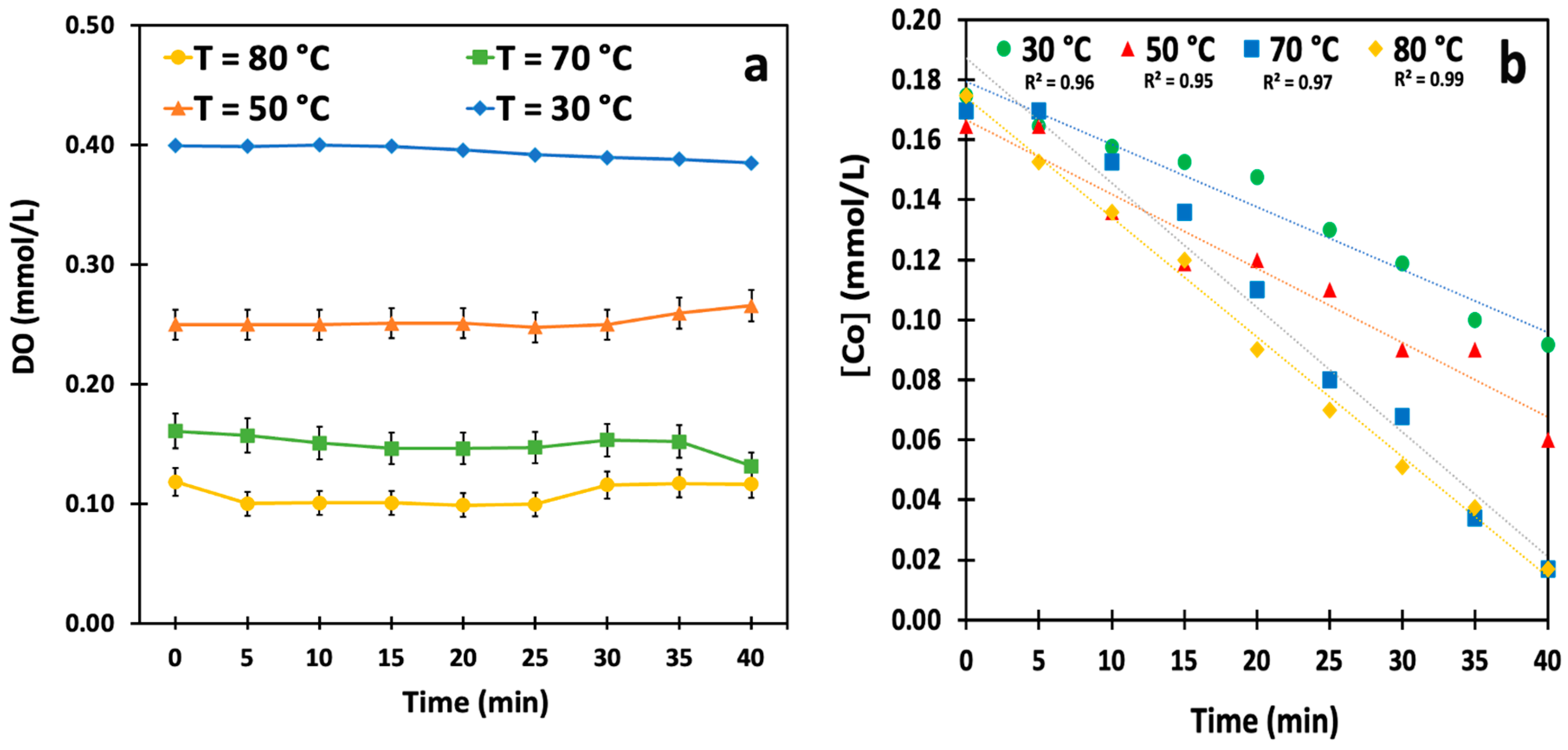
3.1. Effect of Temperature on the Kinetics of the Reaction
3.2. Solution Potential in Mn-Co-SO2-Air System
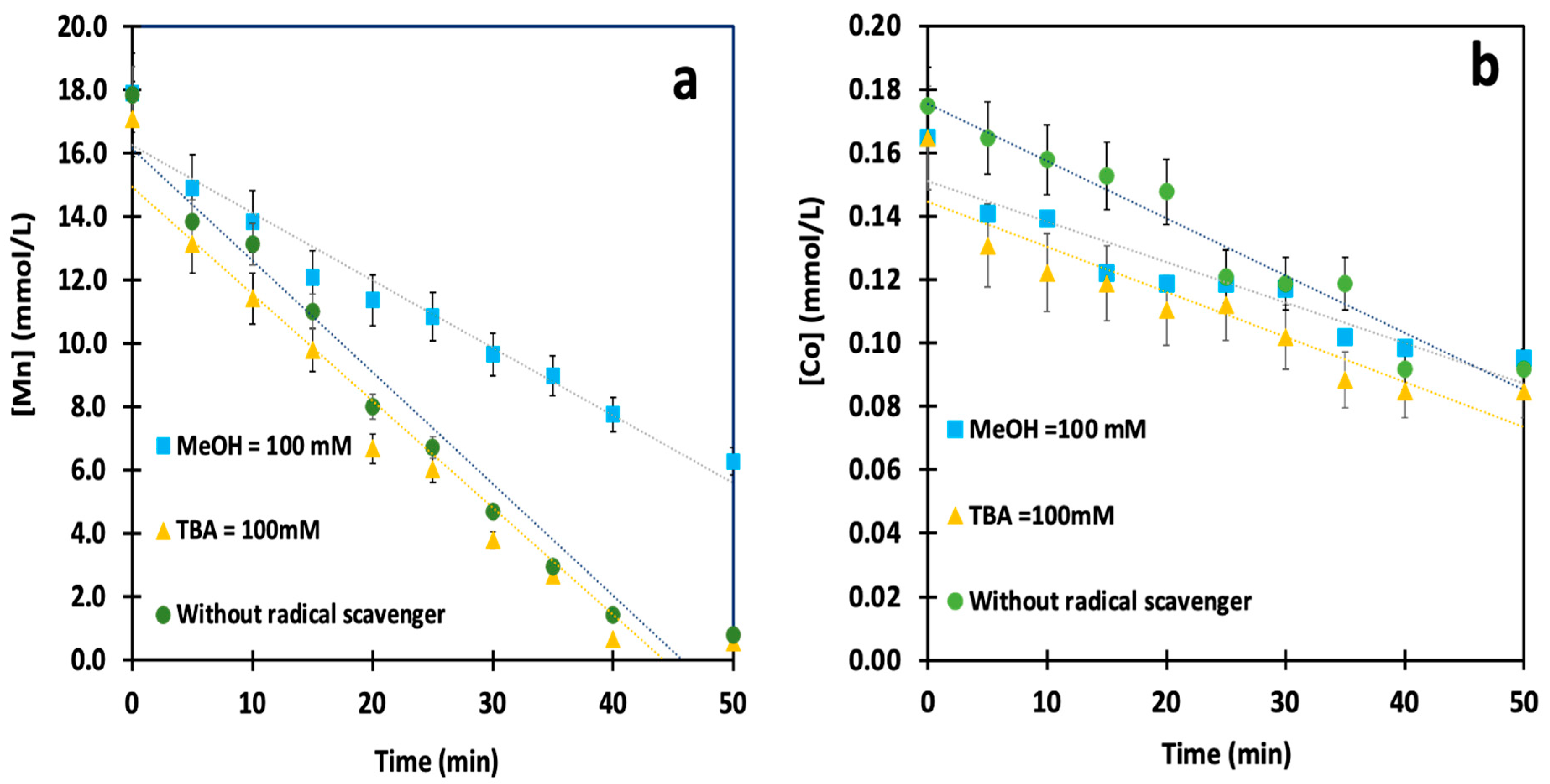
3.3. Determination of Principal Reactive Radicals
3.4. Characterization of the Precipitates
3.4.1. Scanning Electron Microscopy Analysis
3.4.2. X-ray Diffraction Analysis
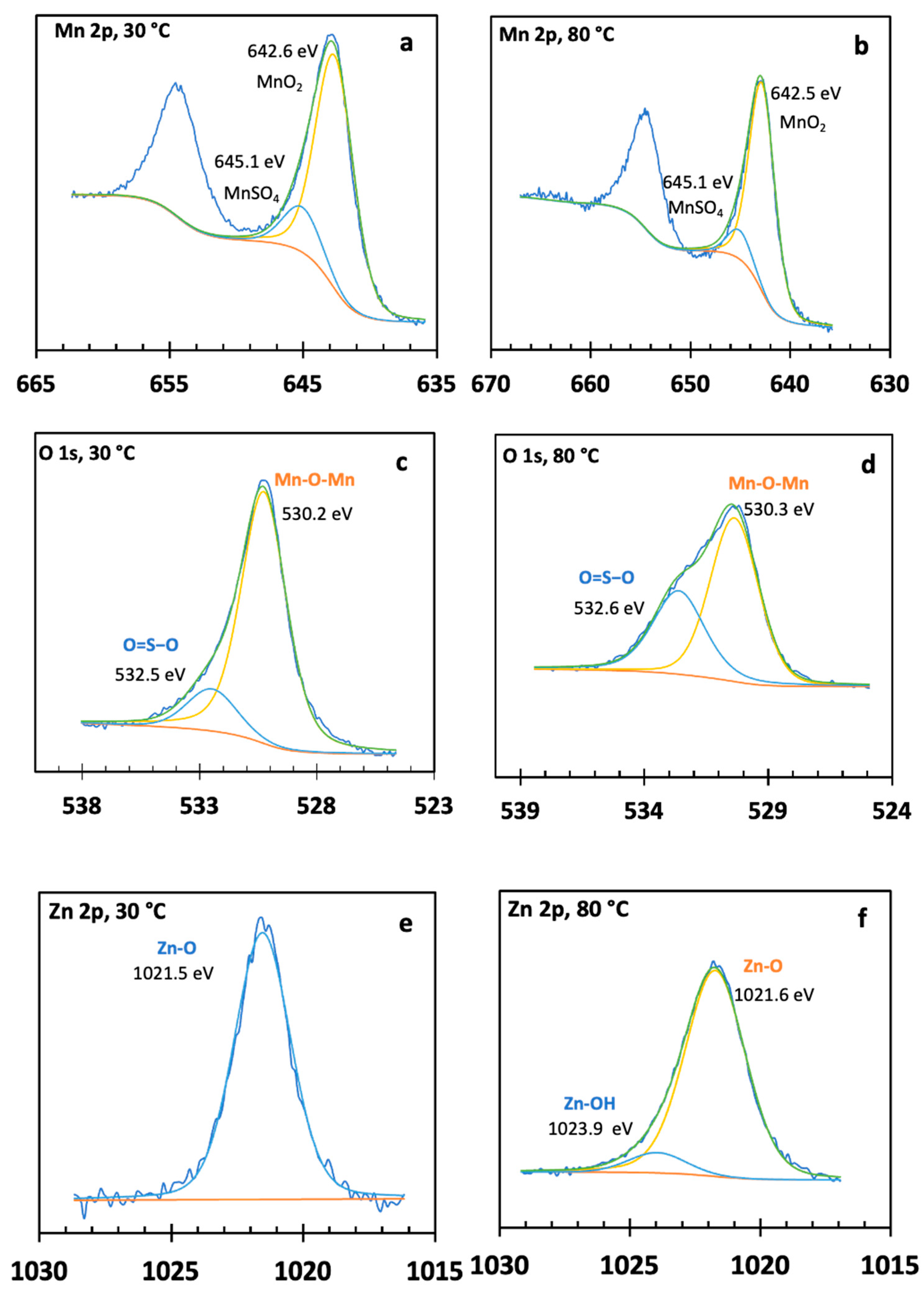
3.4.3. X-ray Photoelectron Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taghvaie Nakhjiri, A.; Sanaeepur, H.; Ebadi Amooghin, A.; Shirazi, M.M.A. Recovery of precious metals from industrial wastewater towards resource recovery and environmental sustainability: A critical review. Desalination 2022, 527, 115510. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, Y. Sustainable Electrochemical Extraction of Metal Resources from Waste Streams: From Removal to Recovery. ACS Sustain. Chem. Eng. 2020, 8, 4693–4707. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, F.; Jiang, L.; Zuo, J.; Jin, W.; Ye, W.; Zhuang, S.; Duan, N. Dynamic evolution of structure–activity of anode on lead release and overpotential change in zinc electrowinning. Chem. Eng. J. 2023, 451, 138944. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Feng, L.; He, Z.; Kang, X.; Yu, L.; He, Y.; Qin, Z.; Zhao, Q.; Qiu, Y.; et al. Synthesis and study of TiMn2 intermetallic compound anode materials with different structures for zinc electrowinning. Intermetallics 2023, 161, 107989. [Google Scholar] [CrossRef]
- Ye, W.; Xu, F.; Jiang, L.; Duan, N.; Li, J.; Zhang, F.; Zhang, G.; Chen, L. A novel functional lead-based anode for efficient lead dissolution inhibition and slime generation reduction in zinc electrowinning. J. Clean. Prod. 2021, 284, 124767. [Google Scholar] [CrossRef]
- Zhang, F.; Zuo, J.; Jin, W.; Xu, F.; Jiang, L.; Xi, D.; Wen, Y.; Li, J.; Yu, Z.; Li, Z.; et al. Size effect of γ-MnO2 precoated anode on lead-containing pollutant reduction and its controllable fabrication in industrial-scale for zinc electrowinning. Chemosphere 2022, 287, 132457. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Xu, F.; Jiang, L.; Duan, N.; Li, J.; Ma, Z.; Zhang, F.; Chen, L. Lead release kinetics and film transformation of Pb-MnO2 pre-coated anode in long-term zinc electrowinning. J. Hazard. Mater. 2021, 408, 124931. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Jiang, W.; Chen, C.; Yu, B.; Xu, R. Facile synthesis MnCo2O4 modifying PbO2 composite electrode with enhanced OER electrocatalytic activity for zinc electrowinning. Sep. Purif. Technol. 2021, 272, 118916. [Google Scholar] [CrossRef]
- He, S.; Xu, R.; Sun, L.; Fan, Y.; Zhao, Z.; Liu, H.; Lv, H. Electrochemical characteristics of Co3O4-doped β-PbO2 composite anodes used in long-period zinc electrowinning. Hydrometallurgy 2020, 194, 105357. [Google Scholar] [CrossRef]
- Admiraal, A.K.; Hof, A.F.; den Elzen, M.G.J.; van Vuuren, D.P. Costs and benefits of differences in the timing of greenhouse gas emission reductions. Mitig. Adapt. Strateg. Glob. Chang. 2016, 21, 1165–1179. [Google Scholar] [CrossRef]
- Exner, K.S.; Lim, T.; Joo, S.H. Circumventing the OCl versus OOH scaling relation in the chlorine evolution reaction: Beyond dimensionally stable anodes. Curr. Opin. Electrochem. 2022, 34, 100979. [Google Scholar] [CrossRef]
- Duby, P. The history of progress in dimensionally stable anodes. JoM 1993, 45, 41–43. [Google Scholar] [CrossRef]
- Rossetto de Menezes, D. Performance Evaluation of Mixed Metal Oxide Anodes for Zinc Electrowinning. Master’s Thesis, Laval University, Quebec, QC, Canada, 2021. [Google Scholar]
- Patil, D.S.; Chavan, S.M.; Oubagaranadin, J.U.K. A review of technologies for manganese removal from wastewaters. J. Environ. Chem. Eng. 2016, 4, 468–487. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, C.Y. Manganese metallurgy review. Part III: Manganese control in zinc and copper electrolytes. Hydrometallurgy 2007, 89, 178–188. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, C.Y.; Pranolo, Y. Investigation of methods for removal and recovery of manganese in hydrometallurgical processes. Hydrometallurgy 2010, 101, 58–63. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, H.; Hu, C.; Yan, Y. Efficient and continuous removal of phenol by activating PMS via nitrogen doped carbon nanotube membrane in the structured fixed bed. J. Water Process Eng. 2023, 54, 104029. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, H.; Yao, B.; Gao, X.; Yang, X.; Zhou, Y. Activation of peroxymonosulfate (PMS) by spinel ferrite and their composites in degradation of organic pollutants: A Review. Chem. Eng. J. 2021, 414, 128800. [Google Scholar] [CrossRef]
- Zhang, W.; Muir, D.M.; Singh, P. Iron(II) oxidation by SO2/O2 in acidic media: Part II. Effect of copper. Hydrometallurgy 2000, 58, 117–125. [Google Scholar] [CrossRef]
- Zhang, W.; Singh, P.; Muir, D.M. SO2/O2 as an oxidant in hydrometallurgy. Miner. Eng. 2000, 13, 1319–1328. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, P.; Wang, Z.; Shang, R.; Wang, S. UV/persulfate preoxidation to improve coagulation efficiency of Microcystis aeruginosa. J. Hazard. Mater. 2017, 322, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Ushani, U.; Lu, X.; Wang, J.; Zhang, Z.; Dai, J.; Tan, Y.; Wang, S.; Li, W.; Niu, C.; Cai, T.; et al. Sulfate radicals-based advanced oxidation technology in various environmental remediation: A state-of-the–art review. Chem. Eng. J. 2020, 402, 126232. [Google Scholar] [CrossRef]
- Honarmandrad, Z.; Sun, X.; Wang, Z.; Naushad, M.; Boczkaj, G. Activated persulfate and peroxymonosulfate based advanced oxidation processes (AOPs) for antibiotics degradation—A review. Water Resour. Ind. 2023, 29, 100194. [Google Scholar] [CrossRef]
- Fang, G.; Wu, W.; Deng, Y.; Zhou, D. Homogenous activation of persulfate by different species of vanadium ions for PCBs degradation. Chem. Eng. J. 2017, 323, 84–95. [Google Scholar] [CrossRef]
- Hou, J.; He, X.; Zhang, S.; Yu, J.; Feng, M.; Li, X. Recent advances in cobalt-activated sulfate radical-based advanced oxidation processes for water remediation: A review. Sci. Total Environ. 2021, 770, 145311. [Google Scholar] [CrossRef] [PubMed]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Brienza, M.; Katsoyiannis, I.A. Sulfate Radical Technologies as Tertiary Treatment for the Removal of Emerging Contaminants from Wastewater. Sustainability 2017, 9, 1604. [Google Scholar] [CrossRef]
- Tang, D.; Zhang, G.; Guo, S. Efficient activation of peroxymonosulfate by manganese oxide for the degradation of azo dye at ambient condition. J. Colloid Interface Sci. 2015, 454, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tong, T.; Wang, N.; Ma, W.; Sun, B.; Chu, J.; Lin, K.A.; Du, Y. Facile Synthesis of Yolk–Shell Mn3O4 Microspheres as a High-Performance Peroxymonosulfate Activator for Bisphenol A Degradation. Ind. Eng. Chem. Res. 2019, 58, 21304–21311. [Google Scholar] [CrossRef]
- Huang, Z.; Bao, H.; Yao, Y.; Lu, W.; Chen, W. Novel green activation processes and mechanism of peroxymonosulfate based on supported cobalt phthalocyanine catalyst. Appl. Catal. B Environ. 2014, 154–155, 36–43. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Ma, J.; Lin, C.; Li, X.; Zhang, H. Activation of peroxymonosulfate by base: Implications for the degradation of organic pollutants. Chemosphere 2016, 151, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Singh, P.; Muir, D. Oxidative precipitation of manganese with SO2/O2 and separation from cobalt and nickel. Hydrometallurgy 2002, 63, 127–135. [Google Scholar] [CrossRef]
- Ménard, V. Controlled Oxidative Precipitation of Manganese from an Industrial Zinc Sulphate Solution Using a Sulphur Dioxide and Oxygen Gas Mixture. Master’s Thesis, McGill University, Montreal, QC, Canada, 2004. [Google Scholar]
- Askarian, M.; Mousavi, F.; Mollaabbasi, R.; Benguerel, E.; Brown, C.; Houlachi, G.; Alamdari, H. Towards Using MMO Anodes in Zinc Electrorefining: Mn Removal by Simulated Plant Off-Gas. Metals 2023, 13, 1675. [Google Scholar] [CrossRef]
- Shi, M.; Li, Q.; Wang, Q.; Yan, X.; Li, B.; Feng, L.; Wu, C.; Qiu, R.; Zhang, H.; Yang, Z.; et al. A review on the transformation of birnessite in the environment: Implication for the stabilization of heavy metals. J. Environ. Sci. 2024, 139, 496–515. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Roepke, E.W.; Flynn, E.D.; Rosenfeld, C.E.; Balgooyen, S.; Ginder-Vogel, M.; Schuler, C.J.; Santelli, C.M. Aqueous Co removal by mycogenic Mn oxides from simulated mining wastewaters. Chemosphere 2023, 327, 138467. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Garibay, R.; Bello-Teodoro, S.; Rojas-Montes, J.C. Thermodynamic simulation of the reaction mechanism of Mn2+ oxidation with an SO2/O2 mixture. Hydrometallurgy 2018, 176, 260–265. [Google Scholar] [CrossRef]
- Mulaudzi, N.; Mahlangu, T. Oxidative precipitation of Mn (II) from cobalt leach solutions using dilute SO2/air gas mixture. J. S. Afr. Inst. Min. Metall. 2009, 109, 375–382. [Google Scholar]
- Chen, X.; Li, W.; Zeng, Z.; Reed, D.; Li, X.; Liu, X. Engineering stable Zn-MnO2 batteries by synergistic stabilization between the carbon nanofiber core and birnessite-MnO2 nanosheets shell. Chem. Eng. J. 2021, 405, 126969. [Google Scholar] [CrossRef]
- Abedi, Z.; Leistenschneider, D.; Chen, W.; Ivey, D.G. Improved Capacitive Behavior of Birnessite Type Mn Oxide Coated on Activated Carbon Fibers. J. Electrochem. Soc. 2022, 169, 010507. [Google Scholar] [CrossRef]
- Ma, Z.; Shao, G.; Fan, Y.; Wang, G.; Song, J.; Shen, D. Construction of Hierarchical α-MnO2 Nanowires@Ultrathin δ-MnO2 Nanosheets Core–Shell Nanostructure with Excellent Cycling Stability for High-Power Asymmetric Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 9050–9058. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Li, Z.; Han, Y.; Xie, L.; Zhao, X.; Zhu, Z. Fabrication and Characterization of a MnO2/Ti3C2Tx Based Gas Sensor for Highly Sensitive and Selective Detection of Lung Cancer Marker Hexanal. Chem. Eng. J. 2023, 451, 139029. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division, Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Ilyas, U.; Lee, P.; Tan, T.L.; Ramanujan, R.V.; Zhang, S.; Chen, R.; Sun, H.D.; Rawat, R.S. High temperature ferromagnetic ordering in c-axis oriented ZnO:Mn nanoparticle thin films by tailoring substrate temperature. Int. J. Mod. Phys. Conf. Ser. 2014, 32, 1460341. [Google Scholar] [CrossRef]
- González-Garnica, M.; Galdámez-Martínez, A.; Malagón, F.; Ramos, C.D.; Santana, G.; Abolhassani, R.; Kumar Panda, P.; Kaushik, A.; Mishra, Y.K.; Karthik, T.V.K.; et al. One dimensional Au-ZnO hybrid nanostructures based CO2 detection: Growth mechanism and role of the seed layer on sensing performance. Sens. Actuators B Chem. 2021, 337, 129765. [Google Scholar] [CrossRef]




| Precipitate | Mn | O | Zn | S | K | Co |
|---|---|---|---|---|---|---|
| 30 °C | 53.16 | 24.28 | 17.22 | 4.08 | 0.94 | 0.32 |
| 50 °C | 44.84 | 29.45 | 18.94 | 5.07 | 1.49 | 0.21 |
| 80 °C | 44.30 | 21.40 | 29.54 | 3.88 | 0.58 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Askarian, M.; Mousavi, F.; Dufault-Bedard, V.; Houlachi, G.; Alamdari, H. Kinetic Study of Manganese Oxidative Precipitation Reaction by Using SO2/Air Gas Mixture. Metals 2024, 14, 412. https://doi.org/10.3390/met14040412
Askarian M, Mousavi F, Dufault-Bedard V, Houlachi G, Alamdari H. Kinetic Study of Manganese Oxidative Precipitation Reaction by Using SO2/Air Gas Mixture. Metals. 2024; 14(4):412. https://doi.org/10.3390/met14040412
Chicago/Turabian StyleAskarian, Masoomeh, Fariba Mousavi, Vincent Dufault-Bedard, Georges Houlachi, and Houshang Alamdari. 2024. "Kinetic Study of Manganese Oxidative Precipitation Reaction by Using SO2/Air Gas Mixture" Metals 14, no. 4: 412. https://doi.org/10.3390/met14040412
APA StyleAskarian, M., Mousavi, F., Dufault-Bedard, V., Houlachi, G., & Alamdari, H. (2024). Kinetic Study of Manganese Oxidative Precipitation Reaction by Using SO2/Air Gas Mixture. Metals, 14(4), 412. https://doi.org/10.3390/met14040412






