Comprehensive Unveiling of the Oxidation Resistance and Corrosion Protection of an Oxide Layer Formed on the Gd-Alloyed AZ80 Alloy Surface
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Isothermal Oxidation Evaluation
2.3. Corrosion Tests
2.4. Microstructure and Surface Morphology Identification
3. Results and Discussion
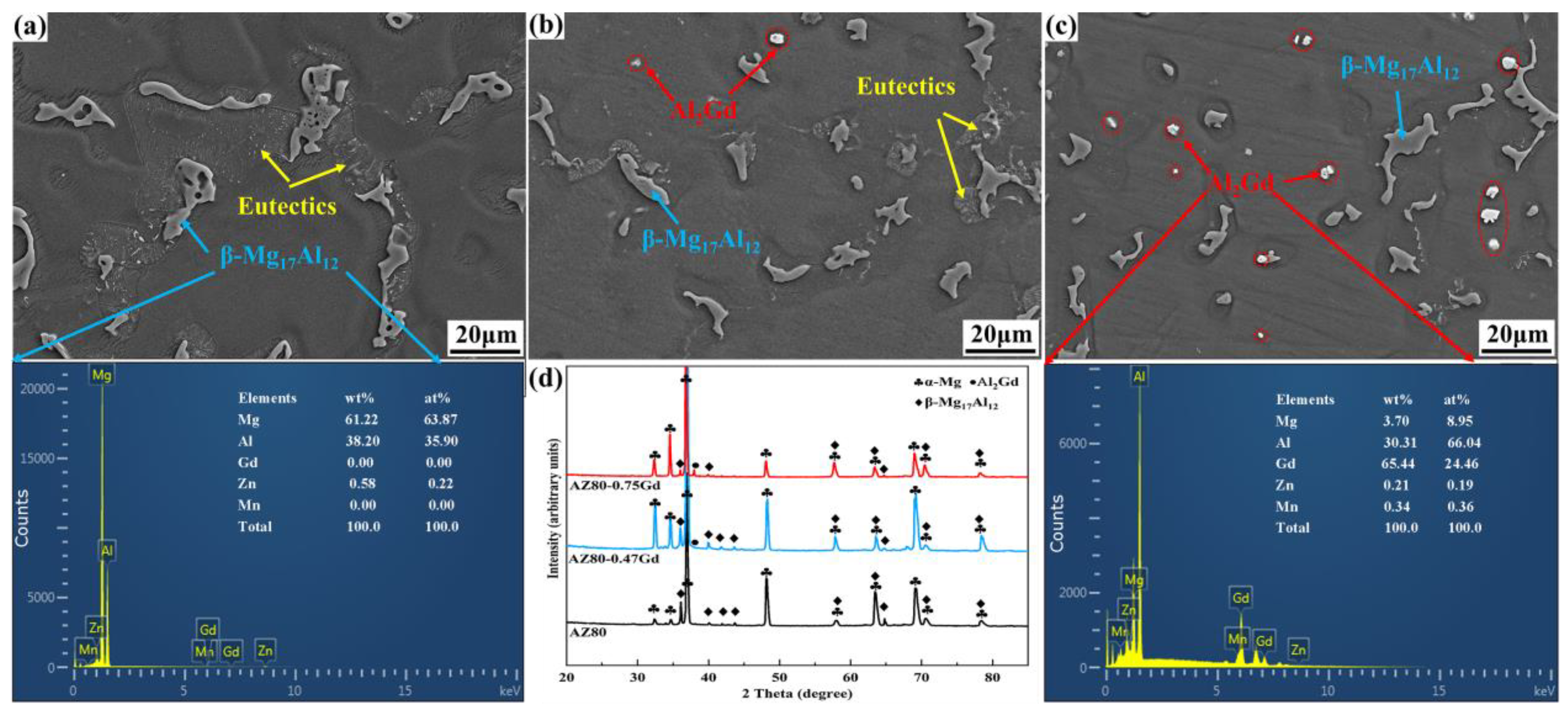
3.1. Microstructure and Phase Composition
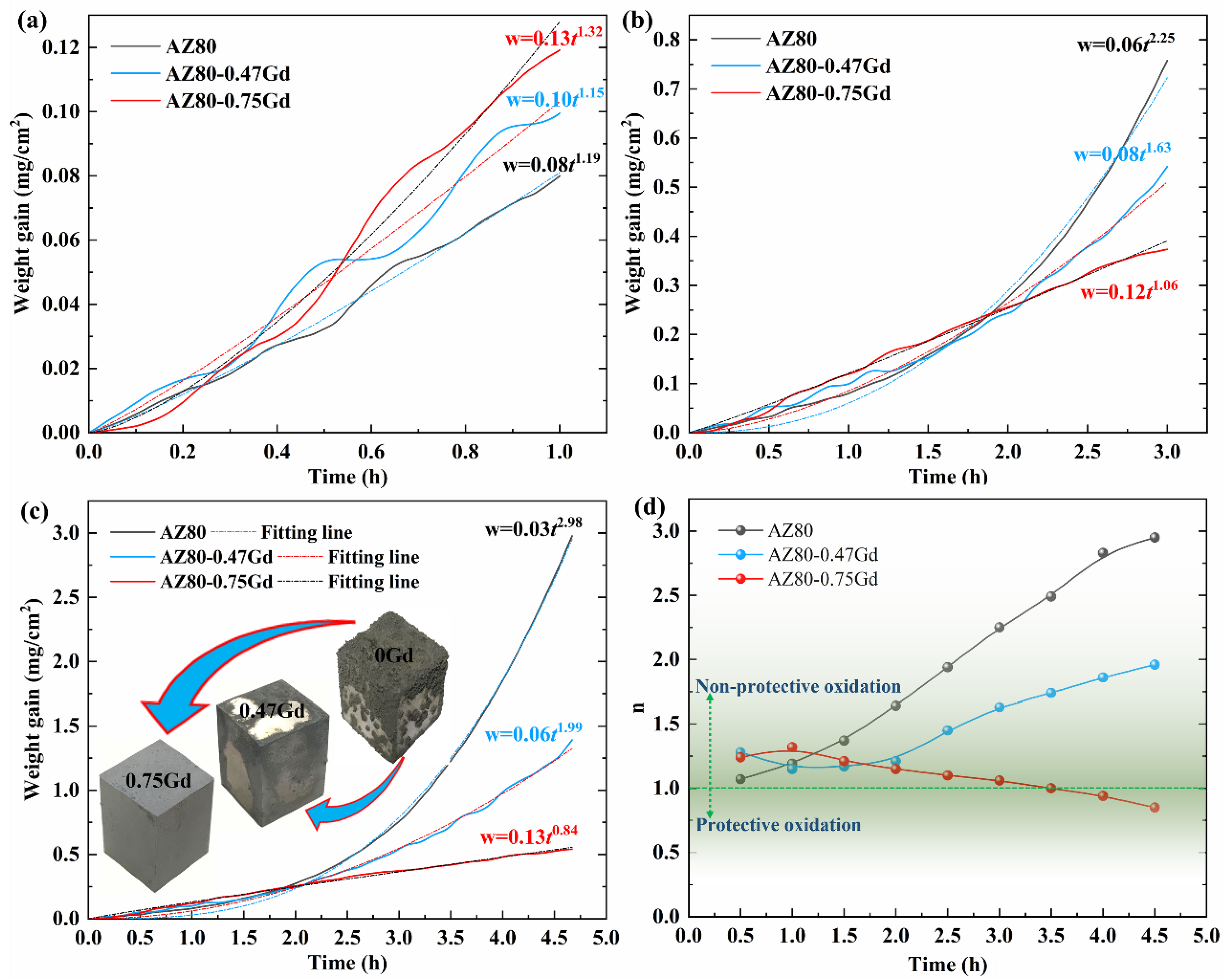
3.2. Oxidation Behavior and Kinetics
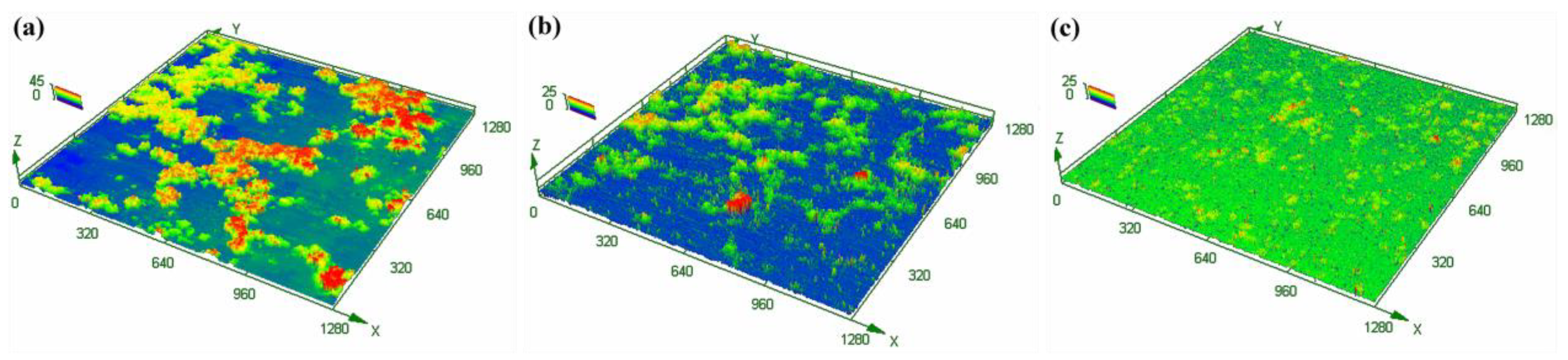
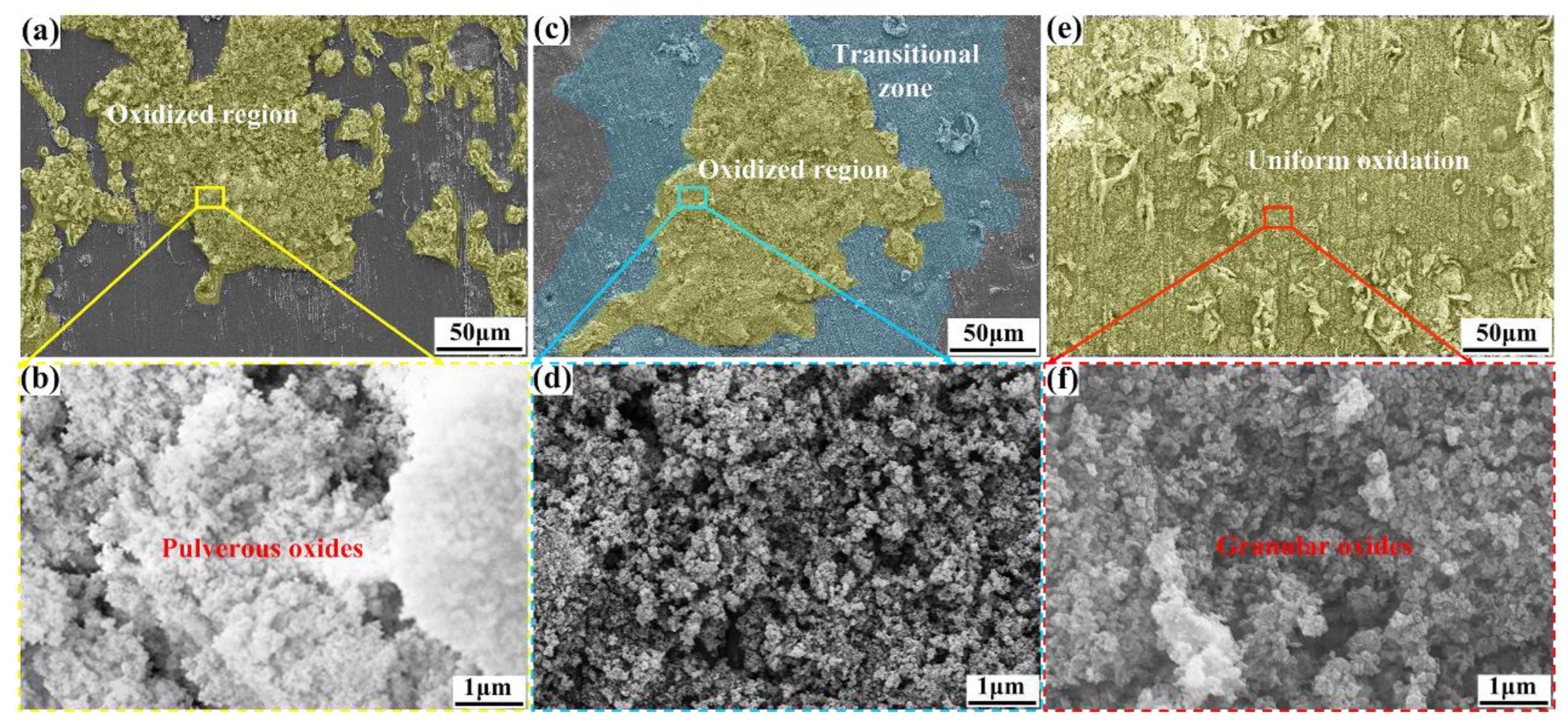
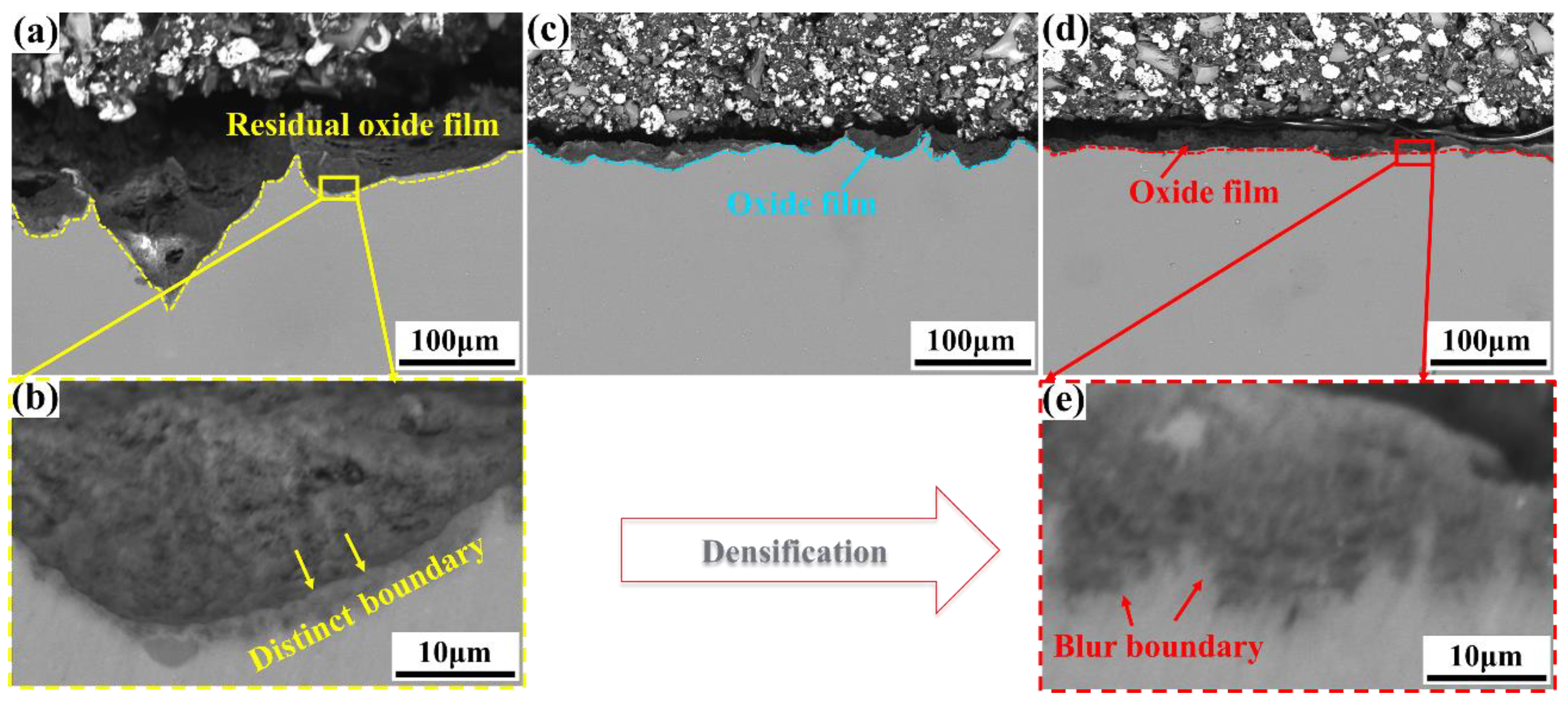
3.3. Oxide Film Characteristic
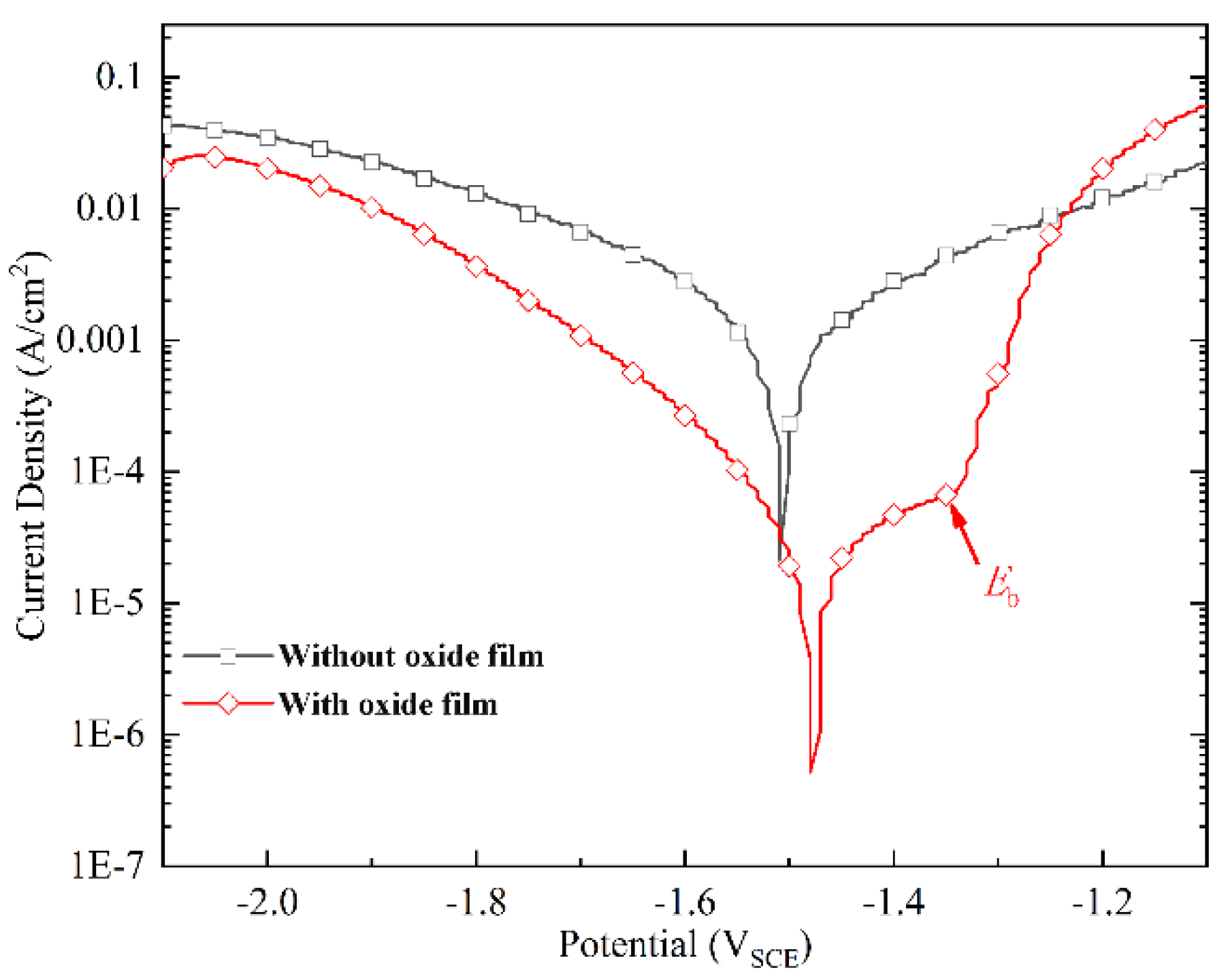
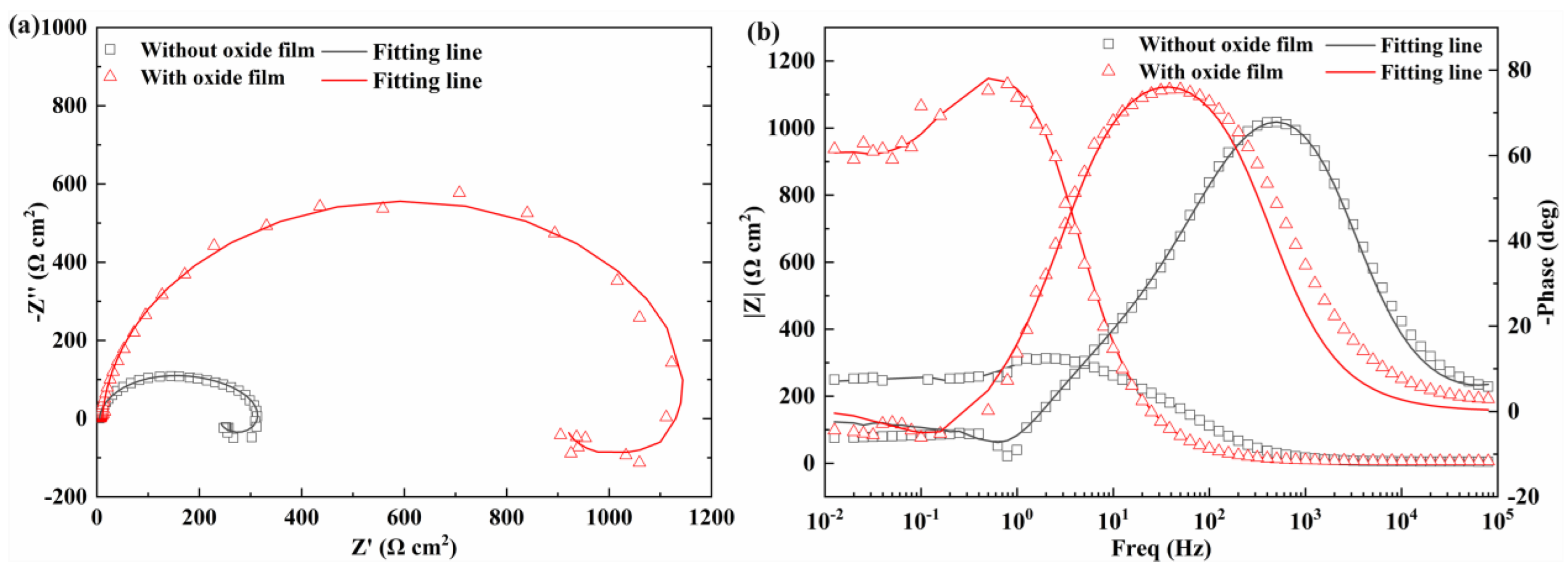
3.4. Corrosion Protection Performance
4. Conclusions
- (1)
- The addition of Gd into the AZ80 alloy modulated the phase compositions. Gd alloying eliminated the content of the low melting point phase of β-Mg17Al12 and promoted the generation of the high melting point phase of Al2Gd, resulting in the thermostability of the alloy.
- (2)
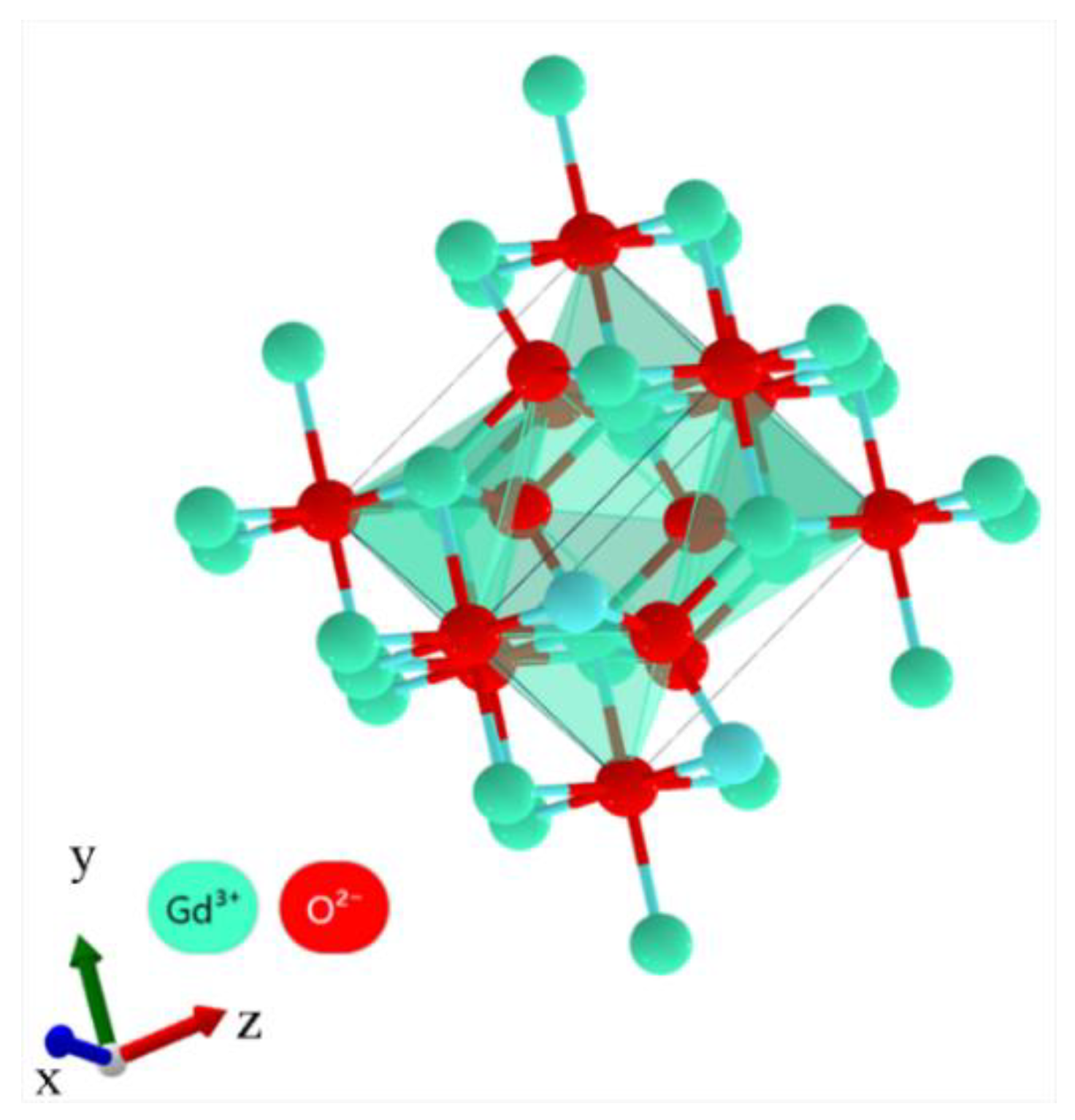
- When Gd was added, the enhancement in the thermal stability of the alloy and the formation of Gd2O3 in the oxide product promoted the uniform oxidation, layer formation, and densification of the oxides, thus improving the resistance to oxidation of the alloy and transforming the oxidation kinetics from a superlinear rate law to an approximate parabolic rate law.
- (3)
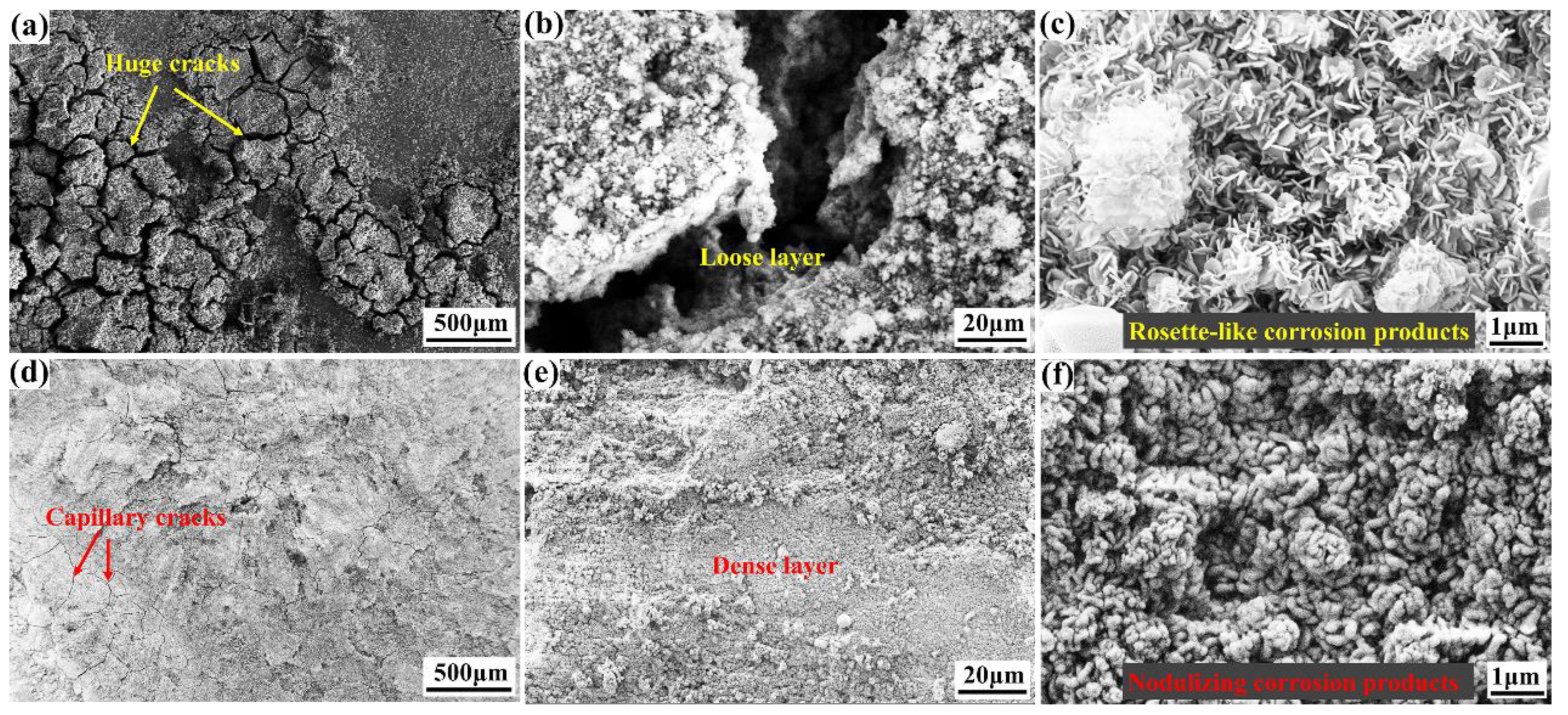
- The compact oxide layer demonstrates a blurred boundary with the magnesium matrix, indicating excellent adhesion with the substrate. After the erosion of the compact oxide layer containing Gd2O3, the products were roundly nodulated, resulting in corrosion passivation and showing corrosion protection performance.
- (4)
- The densification of the magnesium alloy oxide film improved its corrosion protection performance, which provides a new method for preparing magnesium alloy protective layers via high-temperature oxidation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, L.D.; Ding, S.J.; Cai, X.C.; Wu, Y.; Li, Z.J.; Wen, K.K.; Xin, S.W.; Sun, B.R.; Huang, M.X.; Shen, T.D. Unveiling initial oxidation behavior of Mg-Y-Zn long-period stacking ordered (LPSO) phase. Corros. Sci. 2022, 208, 110624. [Google Scholar] [CrossRef]
- Pan, H.; Ren, Y.; Fu, H.; Zhao, H.; Wang, L.; Meng, X.; Qin, G. Recent developments in rare-earth free wrought magnesium alloys having high strength: A review. J. Alloys Compd. 2016, 663, 321–331. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloy 2021, 9, 705–747. [Google Scholar] [CrossRef]
- Ming, Y.; You, G.; Li, Q.; Yao, F.; Zhang, J.; Jiang, B. Flame resistance and oxidation behaviour of Mg–xCa (x = 0.3, 1.0, 2.0 and 3.6 wt%) alloys. Corros. Sci. 2023, 215, 111059. [Google Scholar] [CrossRef]
- Villegas-Armenta, L.A.; Drew, R.A.L.; Pekguleryuz, M.O. The ignition behavior of Mg–Ca binary alloys: The role of heating rate. Oxid. Met. 2020, 93, 545–558. [Google Scholar] [CrossRef]
- Patel, V.; Li, W.; Andersson, J.; Li, N. Enhancing grain refinement and corrosion behavior in AZ31B magnesium alloy via stationary shoulder friction stir processing. J. Mater. Res. Technol. 2022, 17, 3150–3156. [Google Scholar] [CrossRef]
- Fan, Y.; Cao, J.; Zhang, J.; Zhao, Y.; Fang, R.; Wang, Y.; Chen, Z. Stable production of dissimilar steel joints in construction machinery by narrow gap oscillating laser welding. J. Mater. Res. Technol. 2024, 30, 1403–1413. [Google Scholar] [CrossRef]
- Kim, Y.M.; Yim, C.D.; Kim, H.S.; You, B.S. Key factor influencing the ignition resistance of magnesium alloys at elevated temperatures. Scr. Mater. 2011, 65, 958–961. [Google Scholar] [CrossRef]
- Fan, M.; Cui, Y.; Zhang, Z.; Liu, L.; Li, Q.; Luo, Q. Enhanced oxidation resistance in Mg-Y-Zn-Ho alloys via introducing dense low-oxygen-diffusion Ho2O3 oxide film. Corros. Sci. 2023, 213, 110976. [Google Scholar] [CrossRef]
- Cai, Y.; Yan, H.; Zhu, M.; Zhang, K.; Yi, X.; Chen, R. High-temperature oxidation behavior and corrosion behavior of high strength Mg-xGd alloys with high Gd content. Corros. Sci. 2021, 193, 109872. [Google Scholar] [CrossRef]
- Tian, J.; Deng, J.; Zhou, Y.; Chang, Y.; Liang, W.; Ma, J. Slip behavior during tension of rare earth magnesium alloys processed by different rolling methods. J. Mater. Res. Technol. 2023, 22, 473–488. [Google Scholar] [CrossRef]
- He, M.; Chen, L.; Yin, M.; Xu, S.; Liang, Z. Review on magnesium and magnesium-based alloys as biomaterials for bone immobilization. J. Mater. Res. Technol. 2023, 23, 4396–4419. [Google Scholar] [CrossRef]
- ASTM G44; Standard Practice for Exposure of Metals and Alloys by Alternate Immersion in Neutral 3.5% Sodium Chloride Solution. ASTM International: West Conshohocken, PA, USA, 2013.
- Czerwinski, F. The reactive element effect on high-temperature oxidation of magnesium. Int. Mater. Rev. 2015, 60, 264–296. [Google Scholar] [CrossRef]
- Raghavan, V. Al–Gd–Mg (aluminum–gadolinium–magnesium). J. Phase Eqilib. Diffus. 2007, 28, 464–468. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, K.; Yao, C.; Dong, J.; Li, Z.; Emmelmann, C. Effect of Al2Gd on microstructure and properties of laser clad Mg–Al–Gd coatings. Appl. Surf. Sci. 2015, 330, 393–404. [Google Scholar] [CrossRef]
- Wagner, C. Reaktionstypen bei der oxydation von legierungen. Z. Elektrochem. 1959, 63, 772–782. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.; Pettit, F. Introduction to the High-Temperature Oxidation of Metals; Cambridge University Press: Cambridge, UK, 2006; pp. 39–65. [Google Scholar]
- Wu, J.; Yuan, Y.; Yang, L.; Chen, T.; Li, D.; Wu, L.; Jiang, B.; Steinbrück, M.; Pan, F. The oxidation behavior of Mg-Er binary alloys at 500 °C. Corros. Sci. 2022, 195, 109961. [Google Scholar] [CrossRef]
- Cheng, C.; Le, Q.; Li, D.; Hu, W.; Wang, T.; Guo, R.; Hu, C. Effect of Y on high-temperature oxidation behavior and products of AZ80 alloy. Mater. Chem. Phys. 2021, 269, 124732. [Google Scholar] [CrossRef]
- Cheng, C.; Le, Q.; Hu, C.; Wang, T.; Liao, Q.; Li, X. Preparation of a novel robustness mineralized layer on surface of AZ80-0.38Nd (wt.%) alloy and investigation of its properties. Appl. Surf. Sci. 2022, 600, 153970. [Google Scholar] [CrossRef]
- Wang, L.; Snihirova, D.; Deng, M.; Wang, C.; Vaghefinazari, B.; Wiese, G.; Langridge, M.; Höche, D.; Lamaka, S.V. Insight into physical interpretation of high frequency time constant in electrochemical impedance spectra of Mg. Corros. Sci. 2021, 187, 109501. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Q.; Liu, W.; Yan, G.; Li, C.; Yang, L.; Zhou, Y. Biaxial stress in La2Ce2O7/YSZ thermal barrier coatings for oxidation evolution. Surf. Coat. Technol. 2022, 447, 128809. [Google Scholar] [CrossRef]
- Yan, G.; Sun, Y.; Zhao, X.; Liu, W.; Wang, Q.; Li, C.; Yang, L.; Zhou, Y. The enhanced thermal shock resistance performance induced by interface effect in blade-level La2Ce2O7/YSZ thermal barrier coating. Appl. Surf. Sci. 2023, 619, 156723. [Google Scholar] [CrossRef]
- Jia, G.; Liu, K.; Zheng, Y.; Song, Y.; Yang, M.; You, H. Highly uniform Gd(OH)3 and Gd2O3: Eu3+ nanotubes: Facile synthesis and luminescence properties. J. Phys. Chem. C 2009, 113, 6050–6055. [Google Scholar] [CrossRef]
- Available online: https://materialsproject.org/materials/mp-504886?formula=Gd2O3 (accessed on 20 February 2024).
- Wang, R.; Ji, Y.; Cheng, T.L.; Xue, F.; Chen, L.Q.; Wen, Y.H. Phase-field modeling of alloy oxidation at high temperatures. Acta Mater. 2023, 248, 118776. [Google Scholar] [CrossRef]
- Song, L.; Zhang, T.C.; Zhang, Y.; Chen, B.C.; Wu, M.; Zhou, S.Q.; Mei, Z. Simulations on the oxidation of Al-Mg alloy nanoparticles using the ReaxFF reactive force field. Mater. Today Commun. 2023, 35, 106180. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, Z.; Yue, K.; Chen, C.; Liu, L.; Le, Q. Revealing the effect of Gd alloying on oxidation resistance and oxide film protection performance of AZ80 alloy. Mater. Chem. Phys. 2024, 315, 129082. [Google Scholar] [CrossRef]
- Cubides, Y.; Zhao, D.; Nash, L.; Yadav, D.; Xie, K.; Karaman, I.; Castaneda, H. Effects of dynamic recrystallization and strain-induced dynamic precipitation on the corrosion behavior of partially recrystallized Mg–9Al–1Zn alloys. J. Magnes. Alloy 2020, 8, 1016–1037. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Q.; Chen, Y.; Wan, G. Anodic dissolution dictates the negative difference effect (NDE) of magnesium corrosion more in chemical pathway. Mater. Lett. 2018, 232, 54–57. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Atrens, A.D.; Gentle, I.; Atrens, A. Possible dissolution pathways participating in the Mg corrosion reaction. Corros. Sci. 2015, 92, 173–181. [Google Scholar] [CrossRef]
- Ma, Y.; Xiong, H.; Chen, B. Effect of heat treatment on microstructure and corrosion behavior of Mg-5Al-1Zn-1Sn magnesium alloy. Corros. Sci. 2021, 191, 109759. [Google Scholar] [CrossRef]
- Xu, R.Z.; Yang, X.B.; Li, P.H.; Suen, K.W.; Wu, S.; Chu, P.K. Eelectrochemical properties and corrosion resistance of carbon-ion-implanted magnesium. Corros. Sci. 2014, 82, 173–179. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, G.Q.; Zeng, S.B.; Zhang, S.; Long, F.; Shi, Q.Y. The corrosion behavior of Mg-9Al-xRE magnesium alloys modified by friction stir processing. J. Alloys Compd. 2021, 851, 156835. [Google Scholar] [CrossRef]
- Liu, S.; Qi, Y.; Peng, Z.; Liang, J. A chemical-free sealing method for micro-arc oxidation coatings on AZ31 mg alloy. Surf. Coat. Technol. 2021, 406, 126655. [Google Scholar] [CrossRef]
- Reyes-Riverol, R.; Lieblich, M.; Fajardo, S. Corrosion resistance and anomalous hydrogen evolution in chloride containing solutions of extruded cast and powder metallurgical Mg-1Zn alloy. Corros. Sci. 2022, 208, 110635. [Google Scholar] [CrossRef]
- Feliu, S., Jr.; Samaniego, A.; Barranco, V.; El-Hadad, A.A.; Llorente, I.; Serra, C.; Galván, J.C. A study on the relationships between corrosion properties and chemistry of thermally oxidised surface films formed on polished commercial magnesium alloys AZ31 and AZ61. Appl. Surf. Sci. 2014, 295, 219–230. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Guo, Z.; Yang, Z.; Qian, W.; Chen, Y.; Li, H.; Zhao, Q.; Xing, Y.; Zhao, Y. Improved corrosion resistance of ZrO2/MgO coating for magnesium alloys by manipulating the pore structure. J. Mater. Res. Technol. 2023, 24, 2403–2415. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Liu, M.; Shi, Z.; Cao, F.; Dargusch, M.S. Review of recent developments in the field of magnesium corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Ren, J.; Kang, H.; Guo, E.; Li, J.; Wang, T. Corrosion behavior of as-cast Mg–5Sn based alloys with in additions in 3.5 wt% NaCl solution. Corros. Sci. 2020, 164, 108318. [Google Scholar] [CrossRef]



| Alloy | Al | Zn | Mn | Gd | Others | Mg | Named |
|---|---|---|---|---|---|---|---|
| AZ80 | 8.12 | 0.57 | 0.33 | - | <0.1 | Bal. | AZ80 |
| AZ80-0.5Gd | 8.21 | 0.52 | 0.35 | 0.47 | <0.1 | Bal. | AZ80-0.47Gd |
| AZ80-0.8Gd | 8.18 | 0.56 | 0.37 | 0.75 | <0.1 | Bal. | AZ80-0.75Gd |
| Samples | Ecorr (VSCE) | icorr (A/cm2) |
|---|---|---|
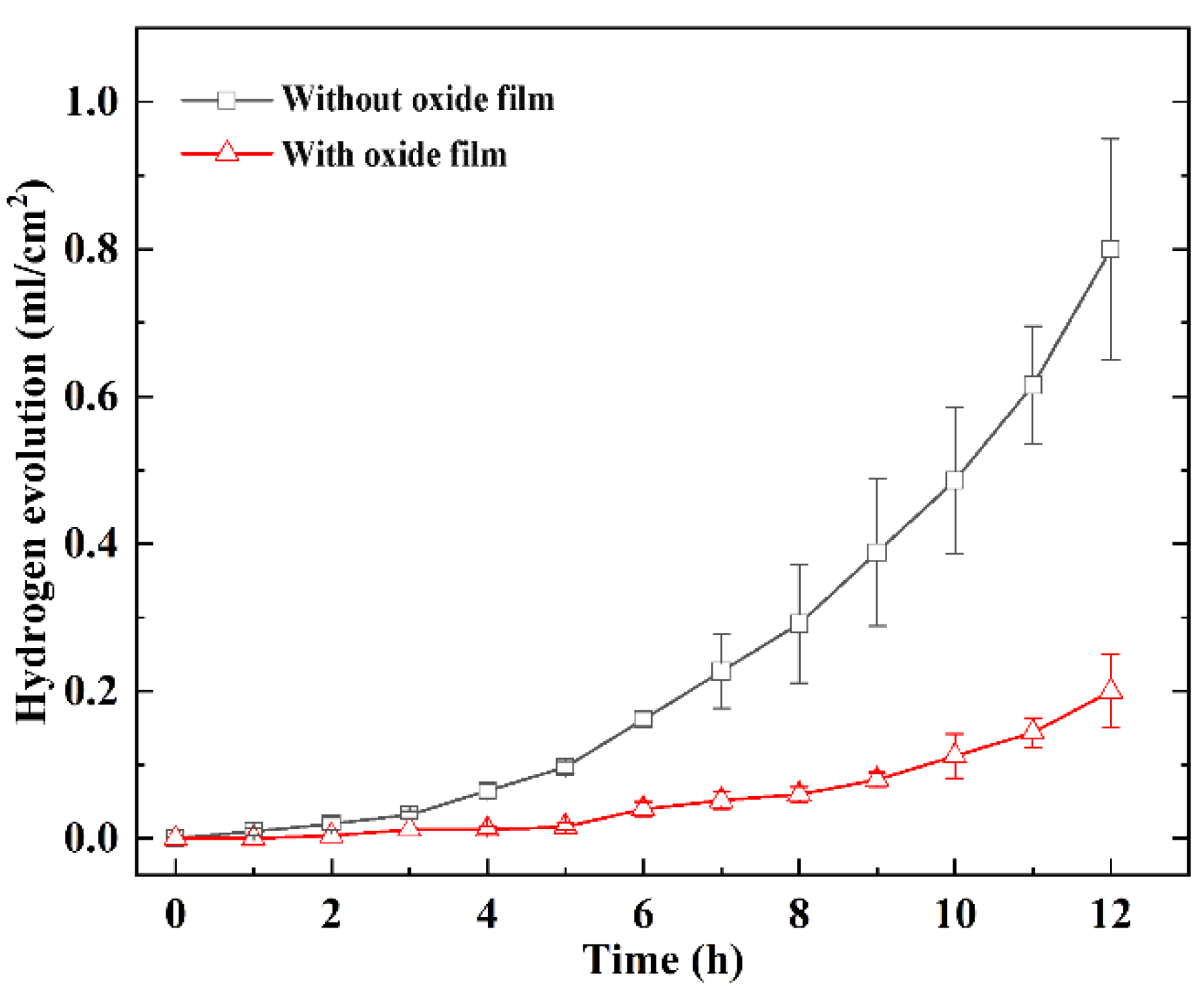
| Without oxide film | −1.51 | 1.6 × 10−3 |
| With oxide film | −1.48 | 6.7 × 10−5 |
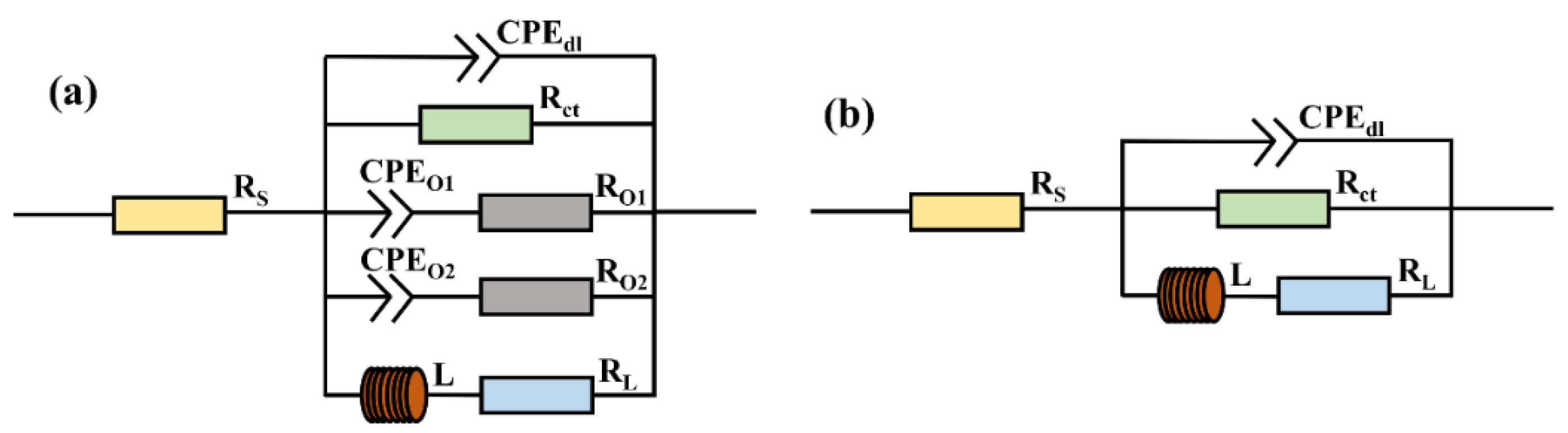
| Sample | RS Ω·cm2 | CPEdl Ω−1·cm−2·sn | ndl | Rct Ω·cm2 | CPEO1 Ω−1·cm−2·sn | nO1 | RO1 Ω·cm2 | CPEO2 Ω−1·cm−2·sn | nO2 | RO2 Ω·cm2 | L H·cm2 | RL Ω·cm2 | χ2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Without | 15.74 | 2.56 × 10−5 | 1 | 370.7 | 1.23 × 10−5 | 0.97 | 5.47 | 1.28 × 10−4 | 0.56 | 245.5 | 170.8 | 711.5 | 1.5 × 10−3 |
| With | 10.27 | 4.89 × 10−5 | 0.96 | 1182 | -- | -- | -- | -- | -- | -- | 4177 | 3842 | 2.8 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Zhou, G.; Qu, B.; Wang, L.; Malik, A.; Chen, Z. Comprehensive Unveiling of the Oxidation Resistance and Corrosion Protection of an Oxide Layer Formed on the Gd-Alloyed AZ80 Alloy Surface. Metals 2024, 14, 474. https://doi.org/10.3390/met14040474
Cheng C, Zhou G, Qu B, Wang L, Malik A, Chen Z. Comprehensive Unveiling of the Oxidation Resistance and Corrosion Protection of an Oxide Layer Formed on the Gd-Alloyed AZ80 Alloy Surface. Metals. 2024; 14(4):474. https://doi.org/10.3390/met14040474
Chicago/Turabian StyleCheng, Chunlong, Gaolin Zhou, Bo Qu, Liang Wang, Abdul Malik, and Zheng Chen. 2024. "Comprehensive Unveiling of the Oxidation Resistance and Corrosion Protection of an Oxide Layer Formed on the Gd-Alloyed AZ80 Alloy Surface" Metals 14, no. 4: 474. https://doi.org/10.3390/met14040474
APA StyleCheng, C., Zhou, G., Qu, B., Wang, L., Malik, A., & Chen, Z. (2024). Comprehensive Unveiling of the Oxidation Resistance and Corrosion Protection of an Oxide Layer Formed on the Gd-Alloyed AZ80 Alloy Surface. Metals, 14(4), 474. https://doi.org/10.3390/met14040474





