Laser Powder Bed Fusion Processing of Low Cost CoCrFeNiMoxNby High Entropy Alloys with Promising High-Temperature Properties via In Situ Alloying Commercial Powders
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Thermodynamic Predictions
3.2. Porosity Analysis
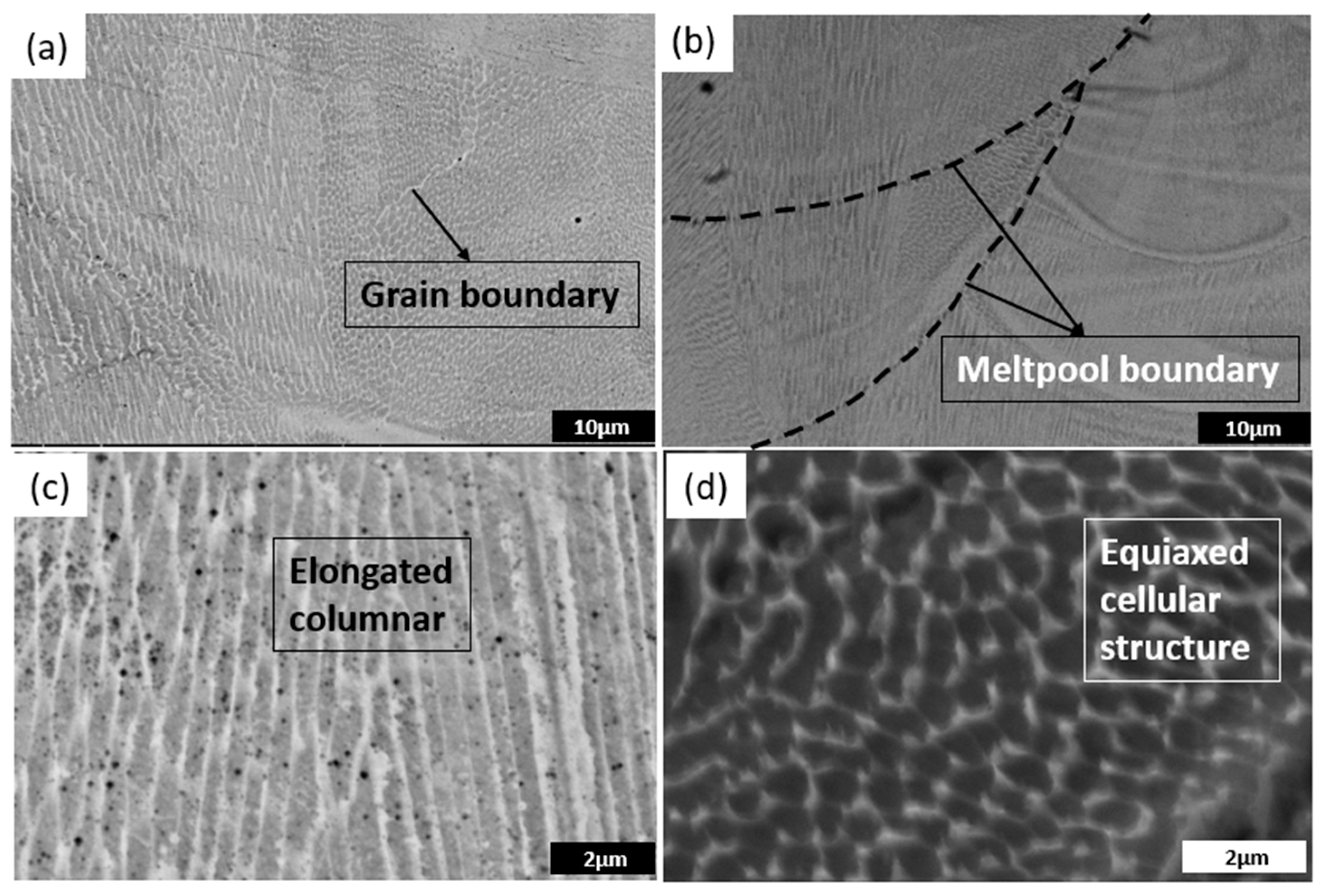
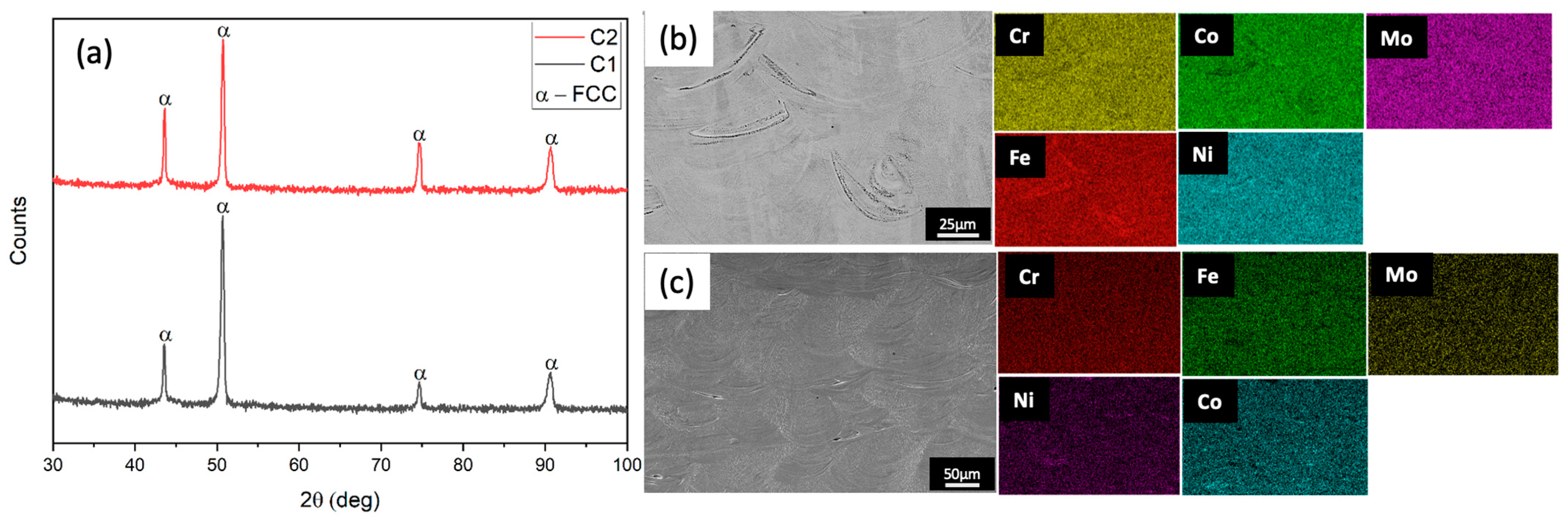
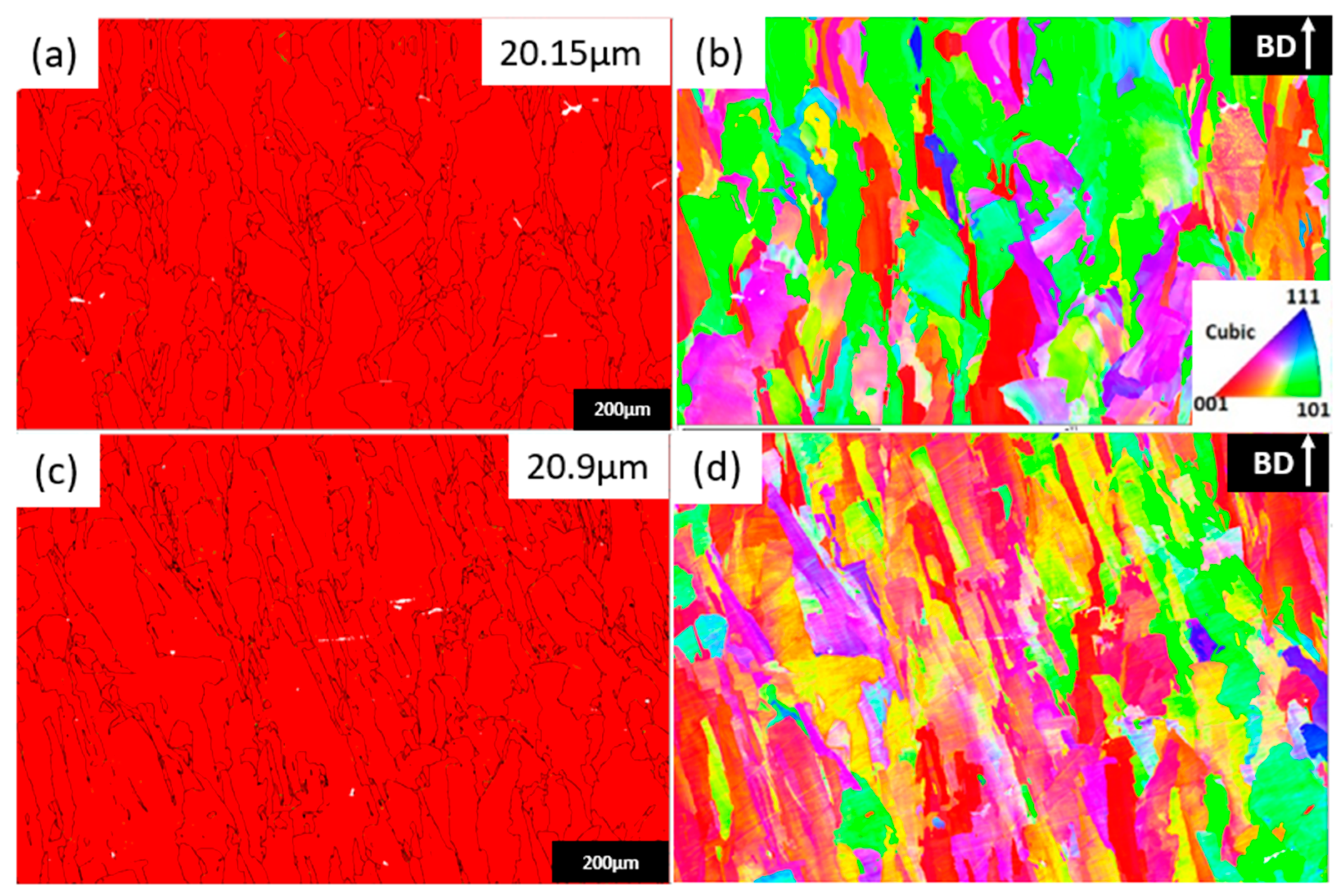
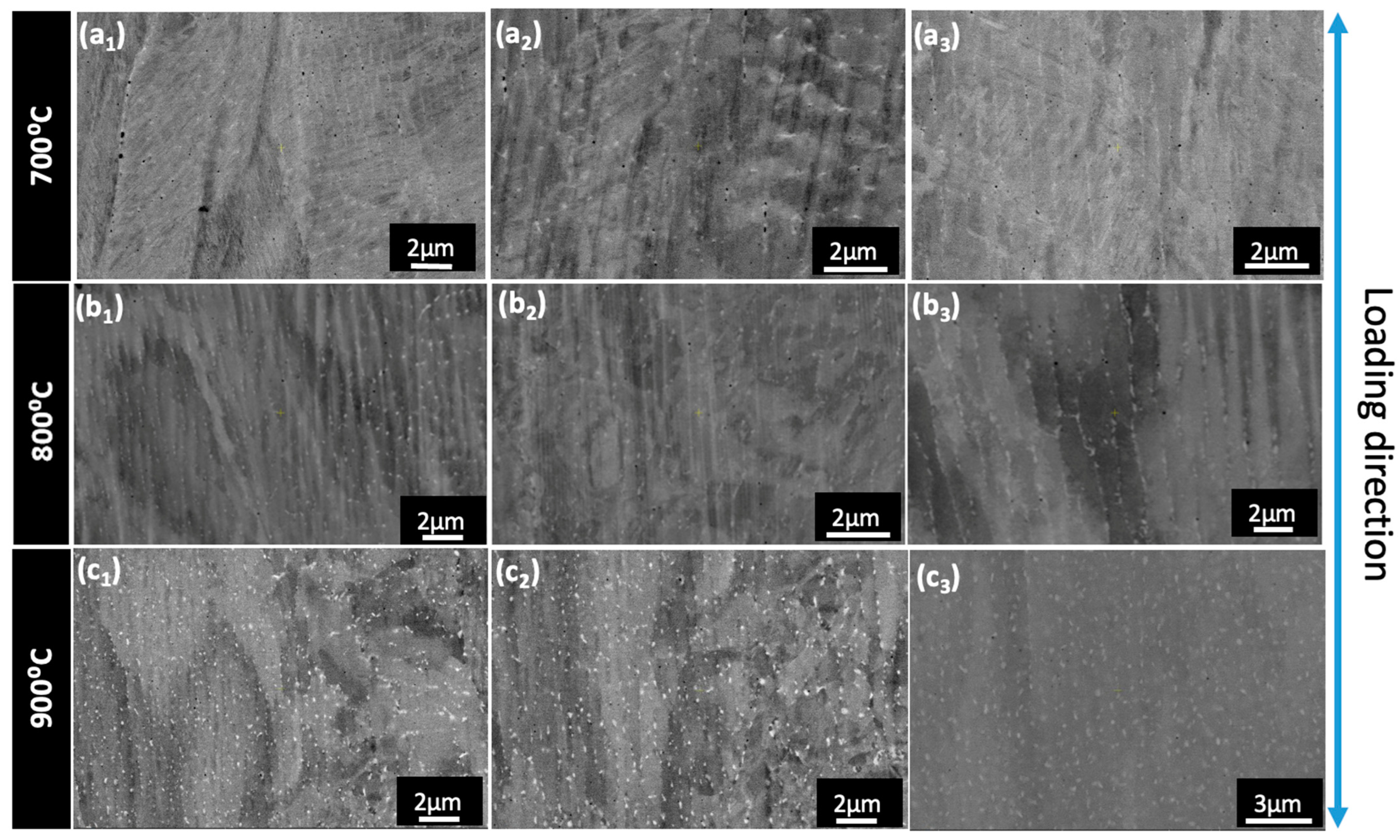
3.3. Microstructural Analysis
3.4. Mechanical Properties
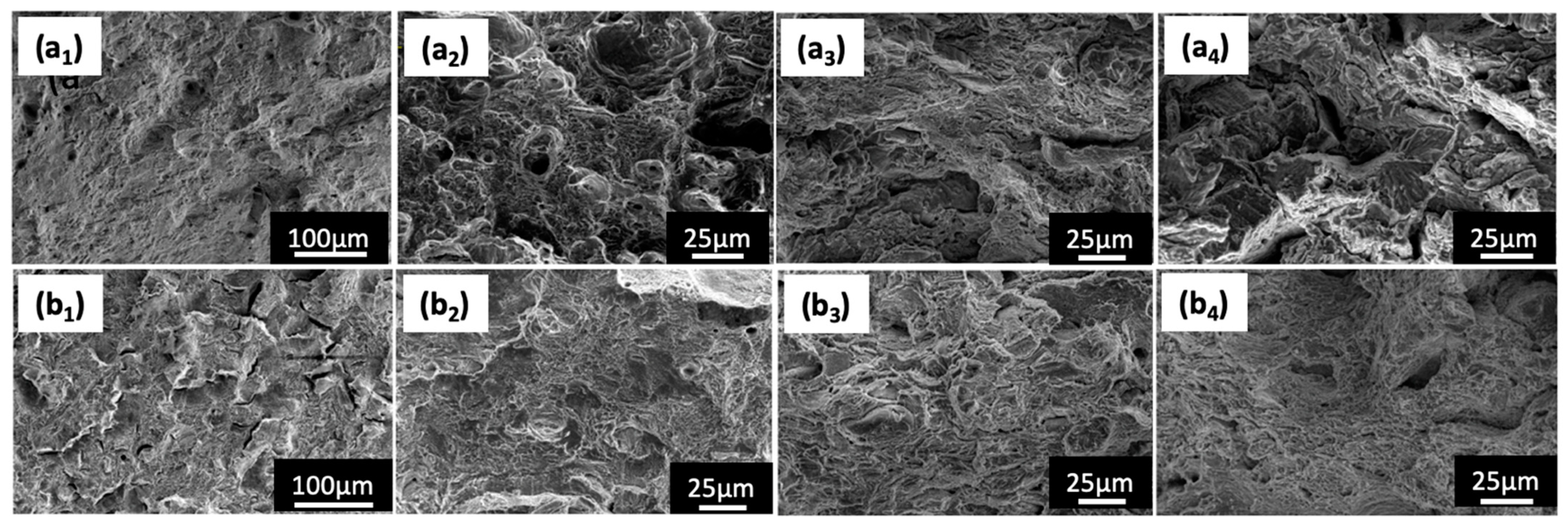
3.5. Fracture Behaviour
4. Conclusions
- The designed alloy compositions C1 and C2 achieved excellent printability after optimization, reaching densities close to 99.9% with no defects. In the as-built state, the alloys had a complete FCC matrix with almost a homogenous distribution of elements with no deleterious secondary phases;
- Both C1 and C2 alloys achieved competitive mechanical properties, with C2 alloys exhibiting better strength from room temperature to 900 °C than C1 due to the former´s higher Mo and Nb content, enabling higher lattice distortion. Considering that no microstructure homogenization treatment has been carried out yet to improve the properties further, this approach to manufacturing HEAs through the hypothesis of pre-alloyed powder mixing is indeed promising;
- Both the alloys exhibited good ductility at all temperatures except for C1 at 900 °C which suffered embrittlement due to the presence of Nb and Mo-rich laves precipitates along the grain boundaries, as opposed to C2, where the precipitates were dispersed throughout the matrix;
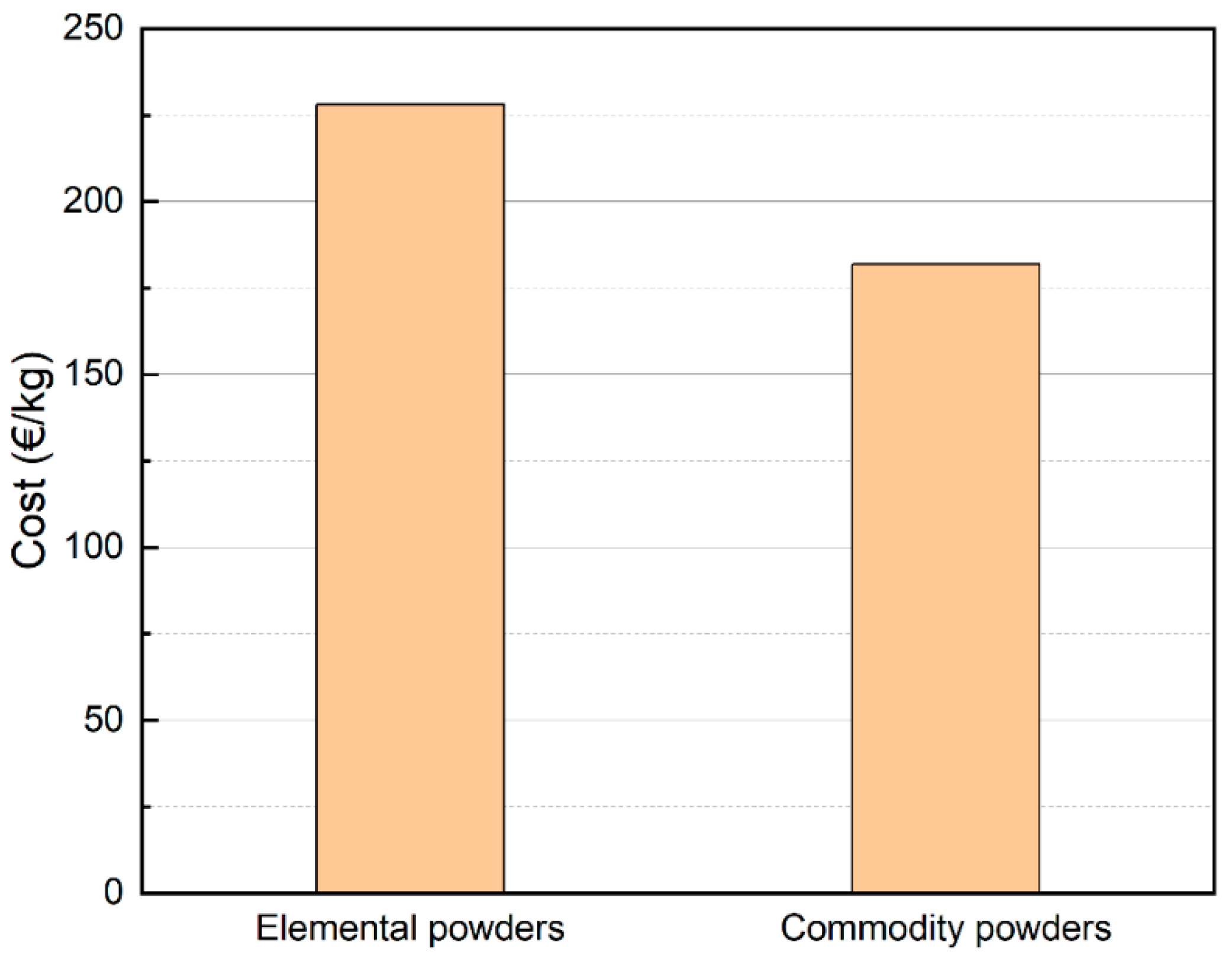
- Utilizing this approach resulted in a significant reduction in the cost of raw materials by 20%, not factoring in savings from ingot casting and gas atomization. Consequently, this method facilitates the commercialization of HEAs by providing an efficient and cost-effective avenue. Furthermore, it opens the possibility of employing scrap-based compositions to achieve a HEA microstructure.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Wang, W.; Liu, B.; Lv, Y.; Yang, W.; Xu, D.; Liu, Y. A review on fundamental of high entropy alloys with promising high–temperature properties. J. Alloys Compd. 2018, 760, 15–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Yeh, J.W. Alloy design strategies and future trends in high-entropy alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Torralba, J.M.; Campos, M. High Entropy Alloys Manufactured by Additive Manufacturing. Metals 2020, 10, 639. [Google Scholar] [CrossRef]
- Torralba, J.M.; Alvaredo, P.; García-Junceda, A. High-entropy alloys fabricated via powder metallurgy. A critical review. Powder Metall. 2019, 62, 84–114. [Google Scholar] [CrossRef]
- Cordova, L.; Campos, M.; Tinga, T. Revealing the Effects of Powder Reuse for Selective Laser Melting by Powder Characterization. JOM 2019, 71, 1062–1072. [Google Scholar] [CrossRef]
- Cobalt Infocard, European Chemicals Agency. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.028.325?_disssubsinfo_WAR_disssubsinfoportlet_backURL=https%3A%2F%2Fecha.europa.eu%2Finformation-on-chemicals%3Fp_p_id%3Ddisssimplesearchhomepage_WAR_disssearchportlet%26p_p_lifecycle%3D0%26 (accessed on 25 August 2021).
- Grohol, D.M. Constanze Veeh, Study on the Critical Raw Materials for the EU. 2023. Available online: https://op.europa.eu/en/publication-detail/-/publication/57318397-fdd4-11ed-a05c-01aa75ed71a1 (accessed on 1 March 2024).
- Proposal for a Regulation of the European Parliament and of the Council Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) 168/2013, (EU) 2018/858, 2018/1724 and (EU) 2019/1020; European Commission: Brussels, Belgiei, 2023; pp. 1–23.
- Annexes to the Proposal for a Regulation of the European Parliament and of the Council, for Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) 168/2013, (EU) 2018/858, 2018/1724 and (EU) 2019/1020, 0079; European Commission: Brussels, Belgiei, 2023; pp. 1–23. [CrossRef]
- Simonelli, M.; Aboulkhair, N.T.; Cohen, P.; Murray, J.W.; Clare, A.T.; Tuck, C.; Hague, R.J.M. A comparison of Ti-6Al-4V in-situ alloying in Selective Laser Melting using simply-mixed and satellited powder blend feedstocks. Mater. Charact. 2018, 143, 118–126. [Google Scholar] [CrossRef]
- Ewald, S.; Kies, F.; Hermsen, S.; Voshage, M.; Haase, C.; Schleifenbaum, J.H. Rapid Alloy Development of Extremely High-Alloyed Metals Using Powder Blends in Laser Powder Bed Fusion. Materials 2019, 12, 1706. [Google Scholar] [CrossRef]
- Chen, P.; Li, S.; Zhou, Y.; Yan, M.; Attallah, M.M. Fabricating CoCrFeMnNi high entropy alloy via selective laser melting in-situ alloying. J. Mater. Sci. Technol. 2020, 43, 40–43. [Google Scholar] [CrossRef]
- Sun, M.; Wang, B.; Zhang, J.; Lu, B. Intermetallics In-situ synthesis of CoCrFeMnNi high-entropy alloy by selective laser melting. Intermetallics 2023, 156, 107866. [Google Scholar] [CrossRef]
- Hou, Y.; Su, H.; Zhang, H.; Wang, X.; Wang, C. Fabricating Homogeneous FeCoCrNi High-Entropy Alloys via SLM In Situ Alloying. Metals 2021, 11, 942. [Google Scholar] [CrossRef]
- Farquhar, L.; Maddison, G.; Hardwick, L.; Livera, F.; Todd, I.; Goodall, R. In-Situ Alloying of CoCrFeNiX High Entropy Alloys by Selective Laser Melting. Metals 2022, 12, 456. [Google Scholar] [CrossRef]
- Nickel Infocard, European Chemicals Agency. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.028.283 (accessed on 25 August 2021).
- Torralba, J.M.; Kumarán, S.V. Development of competitive high-entropy alloys using commodity powders. Mater. Lett. 2021, 301, 130202. [Google Scholar] [CrossRef]
- Kumaran, S.V.; Garbiec, D.; Torralba, J.M. A novel and sustainable method to develop non-equiatomic CoCrFeNiMox high entropy alloys via spark plasma sintering using commercial commodity powders and evaluation of its mechanical behaviour. Mater. Sci. Eng. A 2023, 878, 145207. [Google Scholar] [CrossRef]
- Knieps, M.S.; Reynolds, W.J.; Dejaune, J.; Clare, A.T.; Evirgen, A. In-situ alloying in powder bed fusion: The role of powder morphology. Mater. Sci. Eng. A 2021, 807, 140849. [Google Scholar] [CrossRef]
- Hariharan, K.; Sivaprasad, K. Sustainable Low-Cost Method for Production of High-Entropy Alloys from Alloy Scraps. J. Sustain. Metall. 2022, 2, 625–631. [Google Scholar] [CrossRef]
- Navazani, M.; Kada, S.R.; Fabijanic, D.; Barnett, M. Increasing ductility via Cu addition in AlxCrFeMnNi: Towards a scrap-based high entropy alloy. Intermetallics 2024, 164, 108100. [Google Scholar] [CrossRef]
- Chao, Q.; Joseph, J.; Annasamy, M.; Hodgson, P.; Barnett, M.R.; Fabijanic, D. AlxCoCrFeNi high entropy alloys from metal scrap: Microstructure and mechanical properties. J. Alloys Compd. 2024, 976, 173002. [Google Scholar] [CrossRef]
- Liu, W.H.; Yang, T.; Liu, C.T. Precipitation hardening in CoCrFeNi-based high entropy alloys. Mater. Chem. Phys. 2018, 210, 2–11. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Juan, C.C.; Chen, S.T.; Sheu, T.S.; Yeh, J.W.; Chen, S.K. Phase diagrams of high-entropy alloy system Al-Co-Cr-Fe-Mo-Ni. JOM 2013, 65, 1829–1839. [Google Scholar] [CrossRef]
- Jiang, H.; Qiao, D.; Lu, Y.; Ren, Z.; Cao, Z.; Wang, T.; Li, T. Direct solidification of bulk ultrafine-microstructure eutectic high-entropy alloys with outstanding thermal stability. Scr. Mater. 2019, 165, 145–149. [Google Scholar] [CrossRef]
- Gao, X.; Liu, T.; Zhang, X.; Fang, H.; Qin, G.; Chen, R. Precipitation phase and twins strengthening behaviors of as-cast non-equiatomic CoCrFeNiMo high entropy alloys. J. Alloys Compd. 2022, 918, 165584. [Google Scholar] [CrossRef]
- ASTM E21-20 Standard; Standard Test Methods for Elevated Temperature Tension Tests of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; ISBN 0-471-41526-X. [Google Scholar]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Guo, S.; Hu, Q.; Ng, C.; Liu, C.T. More than entropy in high-entropy alloys: Forming solid solutions or amorphous phase. Intermetallics 2013, 41, 96–103. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, L.; Wikman, S.; Cui, D.; Shen, Z. Intragranular cellular segregation network structure strengthening 316L stainless steel prepared by selective laser melting. J. Nucl. Mater. 2016, 470, 170–178. [Google Scholar] [CrossRef]
- Birnbaum, A.J.; Steuben, J.C.; Barrick, E.J.; Iliopoulos, A.P.; Michopoulos, J.G. Intrinsic strain aging, Σ3 boundaries, and origins of cellular substructure in additively manufactured 316L. Addit. Manuf. 2019, 29, 100784. [Google Scholar] [CrossRef]
- Lin, D.; Xu, L.; Li, X.; Jing, H.; Qin, G.; Pang, H.; Minami, F. A Si-containing FeCoCrNi high-entropy alloy with high strength and ductility synthesized in situ via selective laser melting. Addit. Manuf. 2020, 35, 101340. [Google Scholar] [CrossRef]
- Moravcik, I.; Cizek, J.; Zapletal, J.; Kovacova, Z.; Vesely, J.; Minarik, P.; Kitzmantel, M.; Neubauer, E.; Dlouhy, I. Microstructure and mechanical properties of Ni1,5Co1,5CrFeTi0,5 high entropy alloy fabricated by mechanical alloying and spark plasma sintering. Mater. Des. 2017, 119, 141–150. [Google Scholar] [CrossRef]
- Liu, W.H.; Lu, Z.P.; He, J.Y.; Luan, J.H.; Wang, Z.J.; Liu, B.; Liu, Y.; Chen, M.W.; Liu, C.T. Ductile CoCrFeNiMox high entropy alloys strengthened by hard intermetallic phases. Acta Mater. 2016, 116, 332–342. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, W.; Yan, P.; Li, S.; Dai, Z.; Jiao, L.; Qiu, T.; Wang, X. High temperature tensile properties of as-cast and forged CrMnFeCoNi high entropy alloy. Mater. Sci. Eng. A 2022, 850, 143570. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, J.; Huang, Y.; Lu, Y.; Sun, X. Deformation mechanism during high-temperature tensile test in an eutectic high-entropy alloy AlCoCrFeNi2.1. Mater. Sci. Eng. A 2018, 724, 148–155. [Google Scholar] [CrossRef]
- Jo, M.G.; Suh, J.Y.; Kim, M.Y.; Kim, H.J.; Jung, W.S.; Kim, D.I.; Han, H.N. High temperature tensile and creep properties of CrMnFeCoNi and CrFeCoNi high-entropy alloys. Mater. Sci. Eng. A 2022, 838, 142748. [Google Scholar] [CrossRef]
- Palguna, Y.; Kotla, S.; Korla, R. High temperature deformation behavior of Al0.2CoCrFeNiMo0.5 high entropy alloy: Dynamic strain ageing. J. Alloys Compd. 2023, 930, 167422. [Google Scholar] [CrossRef]
- Wang, Q.; Amar, A.; Jiang, C.; Luan, H.; Zhao, S.; Zhang, H.; Le, G.; Liu, X.; Wang, X.; Yang, X.; et al. CoCrFeNiMo0.2 high entropy alloy by laser melting deposition: Prospective material for low temperature and corrosion resistant applications. Intermetallics 2020, 119, 106727. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, Y.; Geng, C.; Xu, J.; Wang, Y. Microstructural evolution, mechanical and corrosion behaviors of as-annealed CoCrFeNiMox (x = 0, 0.2, 0.5, 0.8, 1) high entropy alloys. J. Alloys Compd. 2020, 820, 153273. [Google Scholar] [CrossRef]
- Fan, R.; Wang, L.; Zhao, L.; Wang, L.; Zhao, S.; Zhang, Y.; Cui, B. Synergistic effect of Nb and Mo alloying on the microstructure and mechanical properties of CoCrFeNi high entropy alloy. Mater. Sci. Eng. A 2022, 829, 142153. [Google Scholar] [CrossRef]
- Wei, D.; Gong, W.; Tsuru, T.; Lobzenko, I.; Li, X.; Harjo, S.; Kawasaki, T.; Do, H.S.; Bae, J.W.; Wagner, C.; et al. Kato, Si-addition contributes to overcoming the strength-ductility trade-off in high-entropy alloys. Int. J. Plast. 2022, 159, 103443. [Google Scholar] [CrossRef]
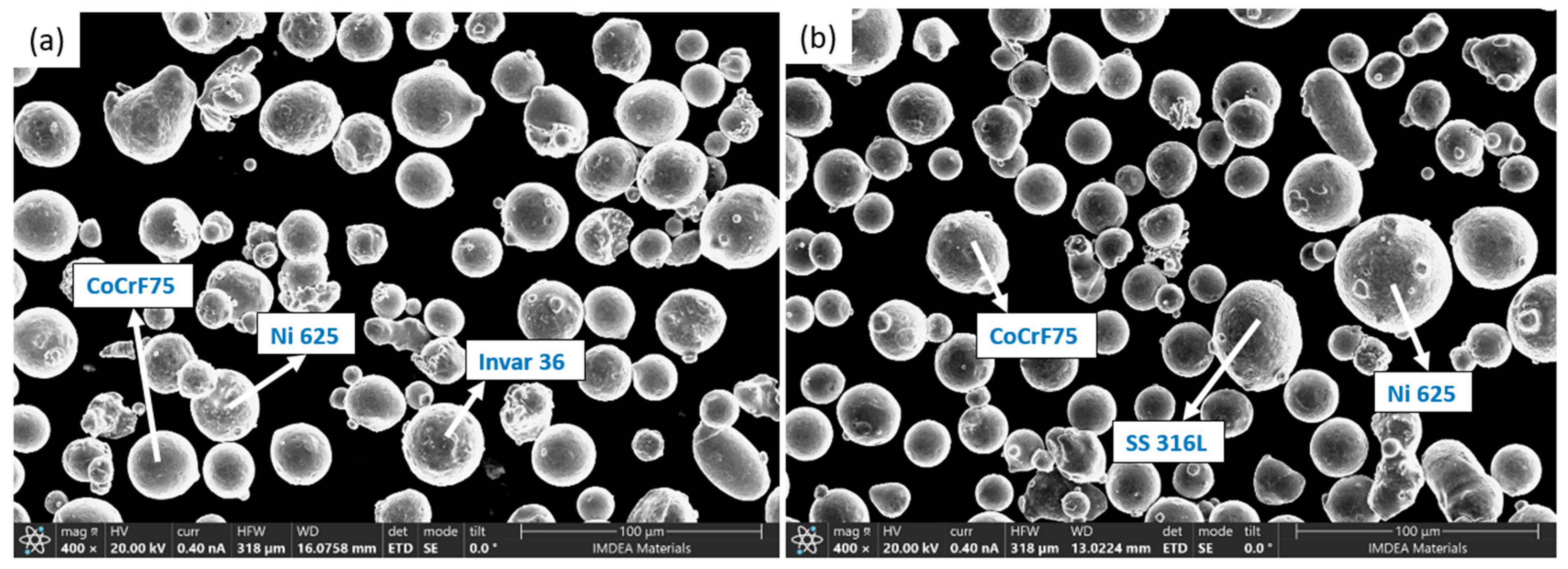



| Alloy | Size (d50) (µm) | Role | wt. (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Ni | Fe | Cr | Mo | Co | Nb | |||
| Ni625 | 32 | Source of Ni, Cr, Fe, Mo | 56.87 | 5 | 22 | 10 | 1 | 3.8 |
| INVAR 36 | 29.9 | Source of Fe and Ni | 36 | 63.28 | - | - | - | - |
| CoCrF75 | 30 | Source of Co, Cr, and Mo | 0.41 | 0.75 | 30 | 7 | 60.41 | - |
| 316L | 31.6 | Source of Fe, Cr, Ni, Mo | 12.55 | 65.85 | 17.68 | 2.33 | - | - |
| Alloy | wt. % | at. % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ni625 | INVAR 36 | CoCrF75 | 316L | Ni | Fe | Cr | Mo | Co | Nb | |
| C1 | 20 | 38 | 42 | - | 25.2 | 26.6 | 19.2 | 3 | 25.45 | 1.65 |
| C2 | 30 | - | 37 | 33 | 21.4 | 24.8 | 26.62 | 3.9 | 22.6 | 2.5 |
| Alloy | Laser Power (W) | Hatch Distance (µm) | Scan Speed (mm/s) | Layer Thickness (µm) | Porosity (%) |
|---|---|---|---|---|---|
| C1 | 150 | 70 | 900 | 30 | 0.05 ± 0.008 |
| C2 | 150 | 60 | 900 | 30 | 0.13 ± 0.017 |
| Alloy | (%) | VEC | /1000 | ||
|---|---|---|---|---|---|
| C1 | −1.54 R | −12.46 | 1.28 | 8.36 | 1.685 |
| C2 | −1.58 R | −14.21 | 1.3 | 8.11 | 1.575 |
| Threshold values | >1.5 R [5] | −11.6 < < 3.2 [33] | <6.6 [33] | >8 for FCC [34] | ≥1.1 [32] |
| C1 | C2 | |||||
|---|---|---|---|---|---|---|
| Temperature (°C) | YS (MPa) | UTS (MPa) | e (%) | YS (MPa) | UTS (MPa) | e (%) |
| RT | 491.6 ± 22.85 | 663 ± 12.8 | 35.4 ± 0.03 | 539.6 ± 16.2 | 752 ± 14.26 | 37.6 ± 0.02 |
| 700 | 370.67 ± 36.85 | 483.3 ± 51.33 | 15.7 ± 4.73 | 430.3 ± 26.27 | 576.7 ± 20.2 | 15.67 ± 4.73 |
| 800 | 275 ± 49.95 | 379.3 ± 42.6 | 17 ± 5.57 | 295 ± 8.66 | 403.3 ± 7.23 | 18.33 ± 5.13 |
| 900 | 186 ± 12.73 | 218.5 ± 6.36 | 7 ± 1.41 | 203.5 ± 3.54 | 272 ± 4.24 | 27 ± 5.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesh Kumaran, S.; Torralba, J.M. Laser Powder Bed Fusion Processing of Low Cost CoCrFeNiMoxNby High Entropy Alloys with Promising High-Temperature Properties via In Situ Alloying Commercial Powders. Metals 2024, 14, 500. https://doi.org/10.3390/met14050500
Venkatesh Kumaran S, Torralba JM. Laser Powder Bed Fusion Processing of Low Cost CoCrFeNiMoxNby High Entropy Alloys with Promising High-Temperature Properties via In Situ Alloying Commercial Powders. Metals. 2024; 14(5):500. https://doi.org/10.3390/met14050500
Chicago/Turabian StyleVenkatesh Kumaran, S., and José Manuel Torralba. 2024. "Laser Powder Bed Fusion Processing of Low Cost CoCrFeNiMoxNby High Entropy Alloys with Promising High-Temperature Properties via In Situ Alloying Commercial Powders" Metals 14, no. 5: 500. https://doi.org/10.3390/met14050500
APA StyleVenkatesh Kumaran, S., & Torralba, J. M. (2024). Laser Powder Bed Fusion Processing of Low Cost CoCrFeNiMoxNby High Entropy Alloys with Promising High-Temperature Properties via In Situ Alloying Commercial Powders. Metals, 14(5), 500. https://doi.org/10.3390/met14050500






