Insight into the Hot Corrosion Behavior of FeMnCrSi/TiC Coatings at 900 °C
Abstract
1. Introduction
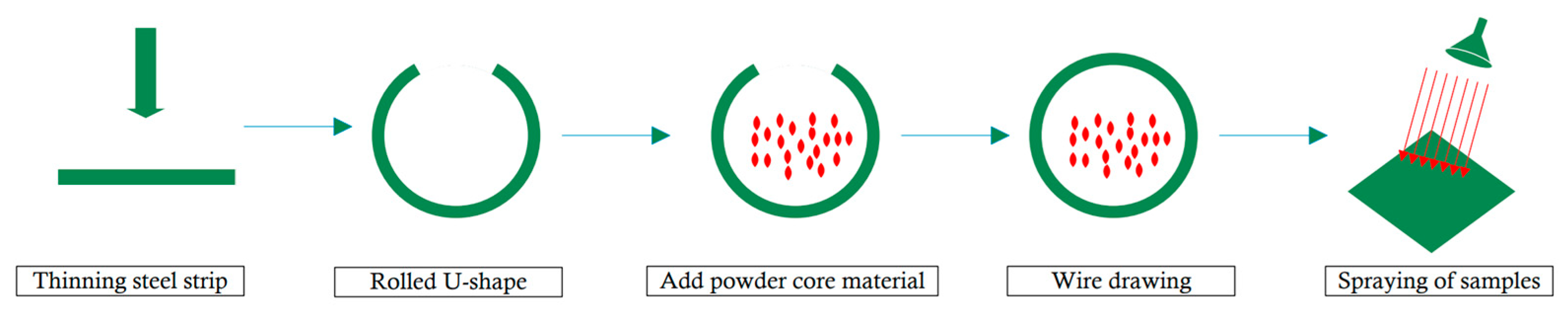
2. Materials and Methods
3. Results
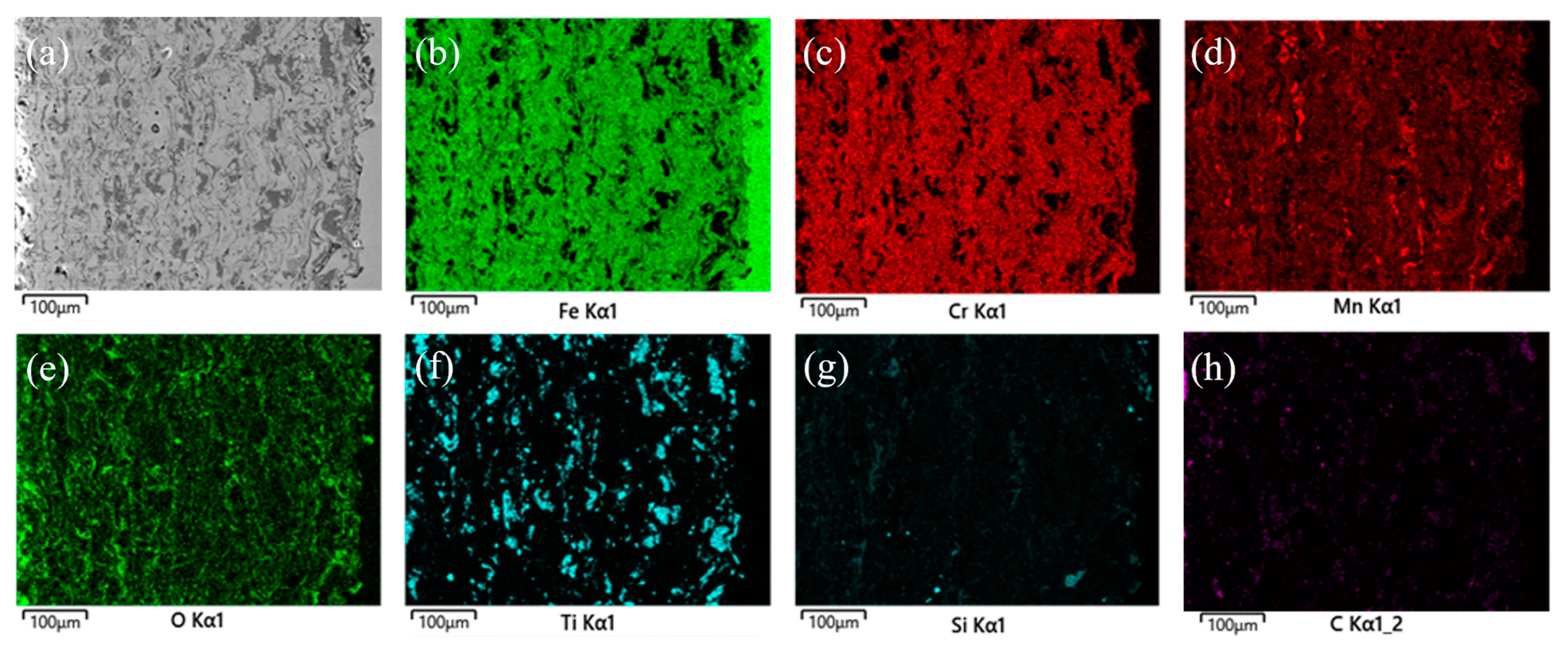
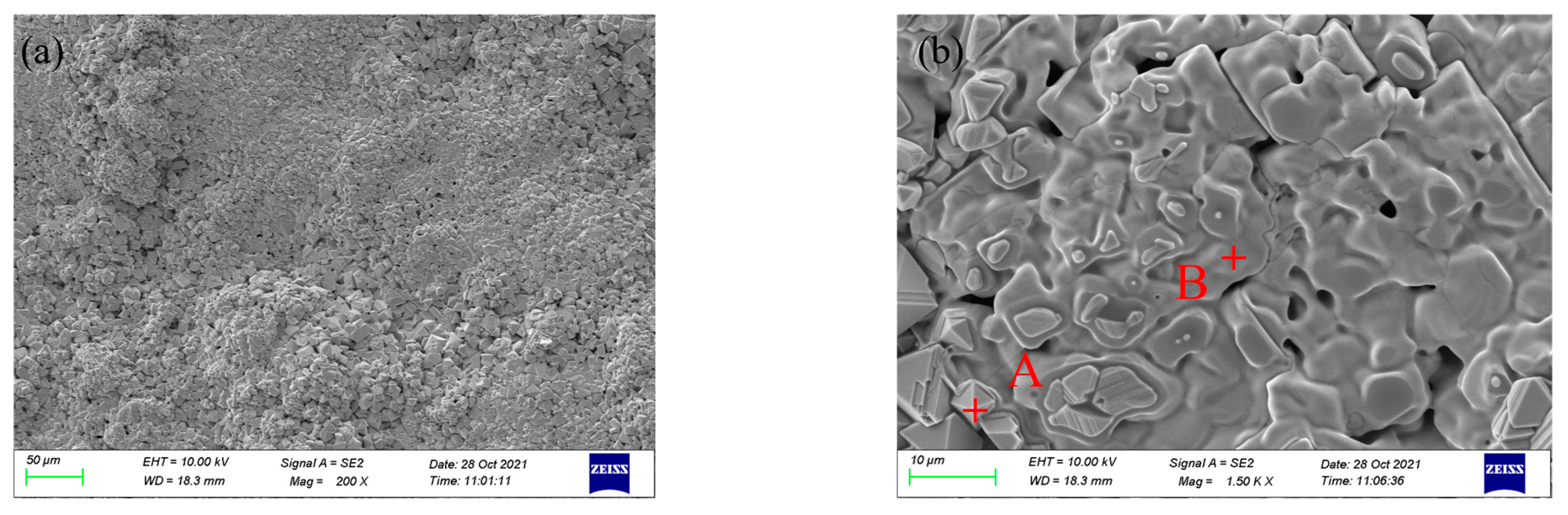
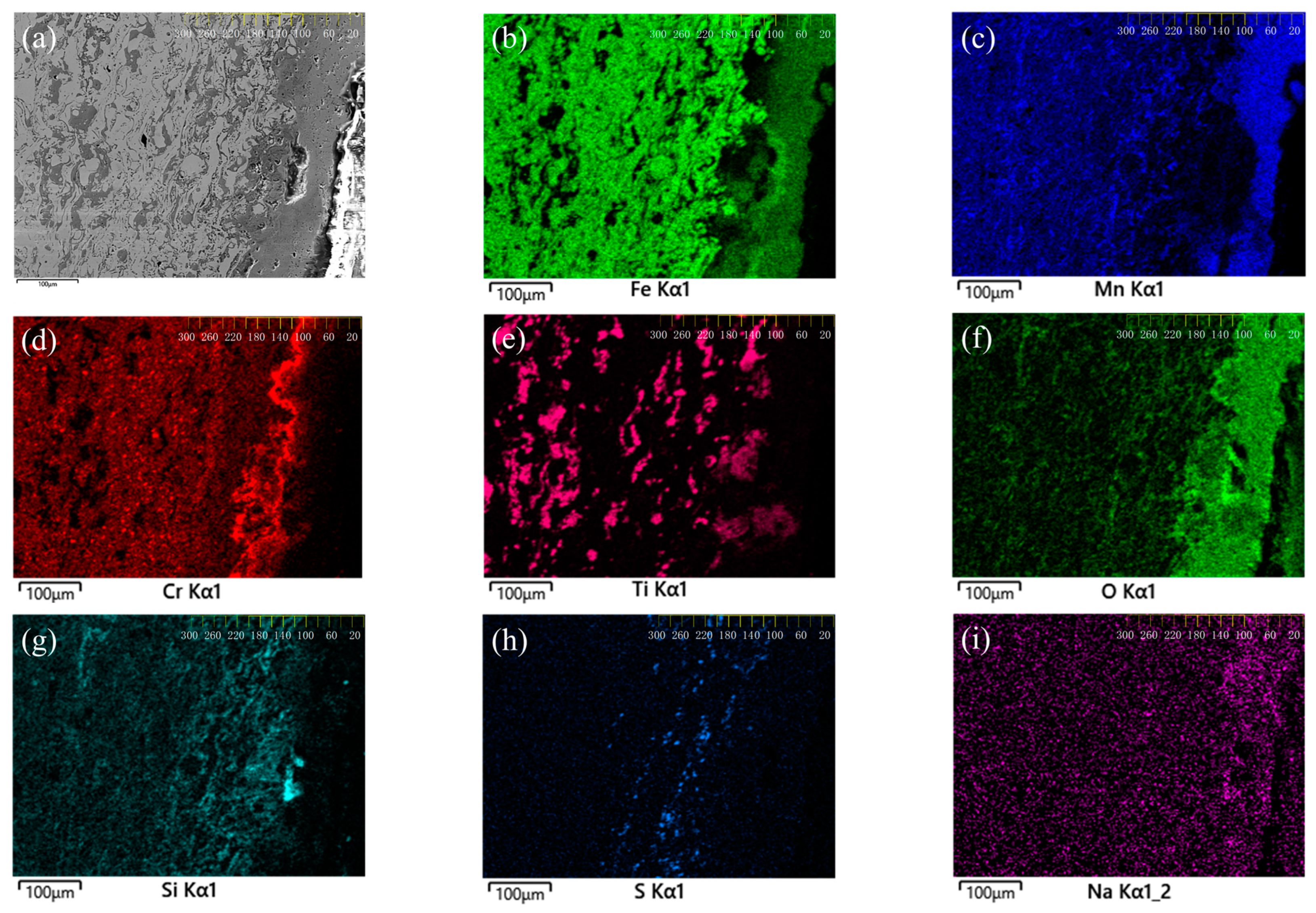
3.1. Microstructure and Phase Analysis of Coating
3.2. Hot Corrosion Kinetic Analysis
3.3. Surface Morphology and Phase Analysis of Coating after Hot Corrosion
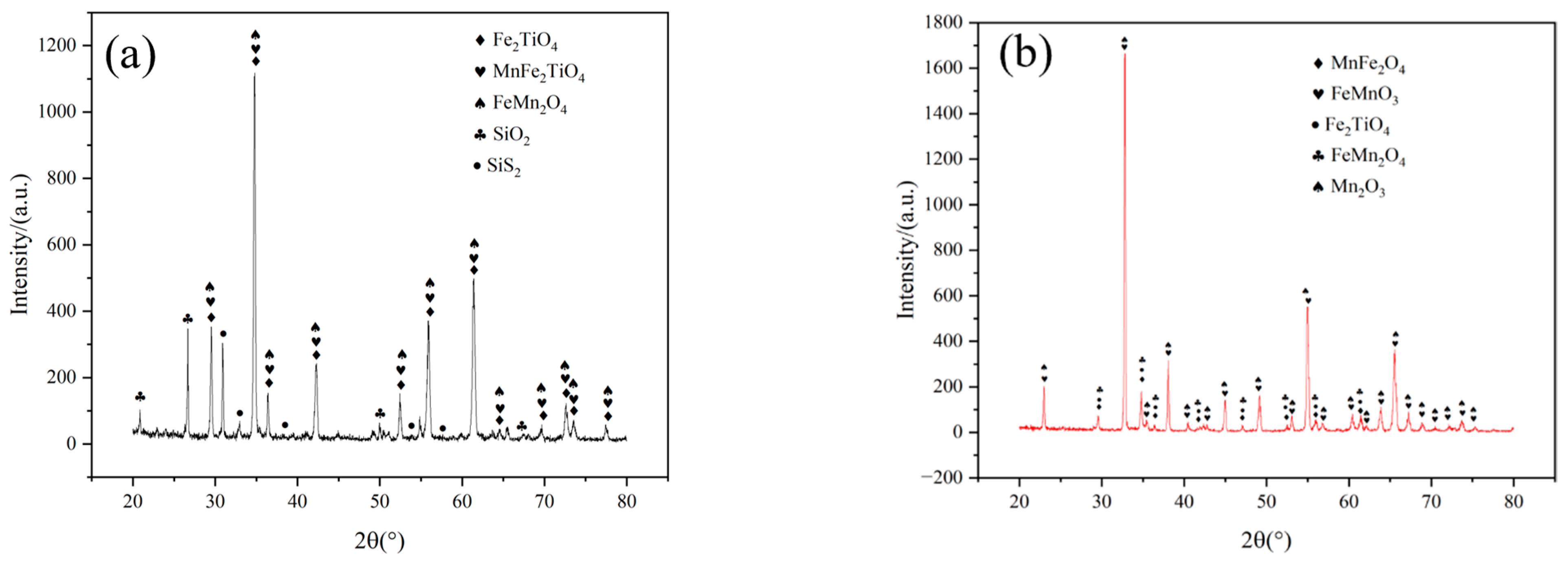
3.4. Cross-Sectional Morphology and Phase Analysis of Coating after Hot Corrosion
4. Discussion of Hot Corrosion Behavior
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prashar, G.; Vasudev, H. Hot corrosion behavior of super alloys. Mater. Today Proc. 2020, 26, 1131–1135. [Google Scholar] [CrossRef]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. npj Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Hu, S.; Finklea, H.; Liu, X. A review on molten sulfate salts induced hot corrosion. J. Mater. Sci. Technol. 2021, 90, 243–254. [Google Scholar] [CrossRef]
- Muthu, S.; Arivarasu, M.; Arivazhagan, N. Investigation of hot corrosion resistance of bare and Ni-20% Cr coated superalloy 825 to Na2SO4-60% V2O5 environment at 900 °C. Procedia Struct. Integr. 2019, 14, 290–303. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Grimm, M.; Rymer, L.-M.; Lampke, T. Influence of Aluminum and Molybdenum on the Microstructure and Corrosion Behavior of Thermally Sprayed High-Entropy Alloy Coatings. J. Therm. Spray Technol. 2022, 31, 1366–1374. [Google Scholar] [CrossRef]
- Patel, N.S.; Pavlík, V.; Boča, M. High-temperature corrosion behavior of superalloys in molten salts—A review. Crit. Rev. Solid State Mater. Sci. 2017, 42, 83–97. [Google Scholar] [CrossRef]
- Yang, L.; Ouyang, J.; Wang, Z.-M.; Song, G.-L. The Early-Stage Propagation of Localized Corrosion of a Sintered NdFeB Alloy in Aqueous Environments. J. Mater. Eng. Perform. 2023. [Google Scholar] [CrossRef]
- Cheng, J.; Wu, Y.; Shen, W.; Hong, S.; Qiao, L.; Wang, Y. A study on hot corrosion performance of high velocity arc-sprayed FeCrNiAlMnB/Cr3C2 coating exposed to Na2SO4 + K2SO4 and Na2SO4 + NaCl. Surf. Coat. Technol. 2020, 397, 126015. [Google Scholar] [CrossRef]
- Prashar, G.; Vasudev, H. Application of thermal spraying techniques used for the surface protection of boiler tubes in power plants: Thermal spraying to combat hot corrosion. In Advanced Surface Coating Techniques for Modern Industrial Applications; IGI Global: Hershey, PA, USA, 2021; pp. 112–134. [Google Scholar]
- Liu, C.; Lu, H.; Qin, E.; Ye, L.; Wu, S. The FeCr-based coating by on-site twin-wire arc spraying for proactive maintenance of power plant components. J. Therm. Spray Technol. 2021, 30, 959–967. [Google Scholar] [CrossRef]
- Galedari, S.A.; Mahdavi, A.; Azarmi, F.; Huang, Y.; McDonald, A. A comprehensive review of corrosion resistance of thermally-sprayed and thermally-diffused protective coatings on steel structures. J. Therm. Spray Technol. 2019, 28, 645–677. [Google Scholar] [CrossRef]
- Tian, H.; Wang, C.; Guo, M.; Tang, Z.; Wei, S.; Xu, B. Study of the frictional-wear performance and abrasion resistance mechanism of a high-speed arc-sprayed FeNiCrAl coating. Surf. Coat. Technol. 2019, 370, 320–330. [Google Scholar] [CrossRef]
- Li, R.; He, D.; Zhou, Z.; Wang, Z.; Song, X. Wear and high temperature oxidation behaviour of wire arc sprayed iron based coatings. Surf. Eng. 2014, 30, 784–790. [Google Scholar] [CrossRef]
- Luo, L.; Liu, S.; Li, J.; Yucheng, W. Oxidation behavior of arc-sprayed FeMnCrAl/Cr3C2–Ni9Al coatings deposited on low-carbon steel substrates. Surf. Coat. Technol. 2011, 205, 3411–3415. [Google Scholar] [CrossRef]
- Abdallah, I.; Kim, T.; Wu, X.; Bailly-Salins, L.; Elbakhshwan, M.; Carroll, M.; Tonks, M.R.; Perepezko, J.H.; Couet, A. Oxidation kinetics and microstructure evolution of high Mn stainless-steel alloy in CO2 at 700 °C. Corros. Sci. 2022, 195, 110013. [Google Scholar] [CrossRef]
- Ndumia, J.N.; Kang, M.; Gbenontin, B.V.; Lin, J.; Nyambura, S.M. A review on the wear, corrosion and high-temperature resistant properties of wire arc-sprayed Fe-based coatings. Nanomaterials 2021, 11, 2527. [Google Scholar] [CrossRef]
- Luo, L.-M.; Liu, S.-G.; Jia, Y.; Juan, L.; Jian, L. Effect of Al content on high temperature erosion properties of arc-sprayed FeMnCrAl/Cr3C2 coatings. Trans. Nonferrous Met. Soc. China 2010, 20, 201–206. [Google Scholar] [CrossRef]
- Boulos, M.I.; Fauchais, P.L.; Heberlein, J.V.R. Industrial Applications of Thermal Spray Technology. In Thermal Spray Fundamentals: From Powder to Part; Boulos, M.I., Fauchais, P.L., Heberlein, J.V.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 997–1096. [Google Scholar]
- Mahajan, S.; Chhibber, R. Hot corrosion studies of boiler steels exposed to different molten salt mixtures at 950 °C. Eng. Fail. Anal. 2019, 99, 210–224. [Google Scholar] [CrossRef]
- Silva, R.; Vacchi, G.; Santos, I.; de Sousa Malafaia, A.; Kugelmeier, C.; Mendes Filho, A.; Pascal, C.; Sordi, V.; Rovere, C. Insights into high-temperature oxidation of Fe-Mn-Si-Cr-Ni shape memory stainless steels and its relationship to alloy chemical composition. Corros. Sci. 2020, 163, 108269. [Google Scholar] [CrossRef]
- da Cruz Passos, J.G.; Rabelo, L.F.P.; de Freitas, B.X.; da Silva, R.; Della Rovere, C.A.; de Sousa Malafaia, A.M. Effects of silicon and manganese content on the oxidation behavior of FeMnSiCrNi alloys and the correlation between Mn-depleted zone, surface roughness and oxidation resistance. Corros. Sci. 2021, 191, 109724. [Google Scholar] [CrossRef]
- de Sousa Malafaia, A.M.; Latu-Romain, L.; Wouters, Y. Initial stages of FeMnSiCrNi shape memory stainless steels oxidation mechanism at 800 °C. Corros. Sci. 2021, 181, 109255. [Google Scholar] [CrossRef]
- Matthews, S.; James, B.; Hyland, M. High temperature erosion–oxidation of Cr3C2–NiCr thermal spray coatings under simulated turbine conditions. Corros. Sci. 2013, 70, 203–211. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Y.; Sun, Y.; Wei, Y.; Wang, X.; Liu, D. Coarse TiC particles reinforced H13 steel matrix composites produced by laser cladding. Surf. Coat. Technol. 2020, 395, 125867. [Google Scholar] [CrossRef]
- Zhu, H.; Ouyang, M.; Hu, J.; Zhang, J.; Qiu, C. Design and development of TiC-reinforced 410 martensitic stainless steel coatings fabricated by laser cladding. Ceram. Int. 2021, 47, 12505–12513. [Google Scholar] [CrossRef]
- GB/T 29037-2012; Thermal Spraying—Coatings for Protection against Corrosion and Oxidation at Elevated Temperatures. The Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- Sun, S.; Zhang, Y.; Xue, Z.; Lin, J.; Chen, X. Deformation Mechanism in Fe61Mn18S11Cr10 Medium Entropy Alloy Under Different Strain Rates. Acta Metall. Sin. (Engl. Lett.) 2021, 34, 1109–1119. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, S.; Ding, J.; Chong, Y.; Jia, T.; Ophus, C.; Asta, M.; Ritchie, R.O.; Minor, A.M. Short-range order and its impact on the CrCoNi medium-entropy alloy. Nature 2020, 581, 283–287. [Google Scholar] [CrossRef]
- Su, M.; Zhao, J.; Gu, C. Investigation of the high-temperature oxidation behavior of Fe-14Cr-9Mn-2.5Ni austenitic stainless steel in N2-21 vol%O2 environment. Corros. Sci. 2023, 220, 111294. [Google Scholar] [CrossRef]
- Katranidis, V.; Gu, S.; Cox, D.C.; Whiting, M.J.; Kamnis, S. FIB-SEM sectioning study of decarburization products in the microstructure of HVOF-sprayed WC-Co coatings. J. Therm. Spray Technol. 2018, 27, 898–908. [Google Scholar] [CrossRef]
- Du, J.; Li, F.; Li, Y.; Lu, H.; Qi, X.; Yang, B.; Li, C.; Yu, P.; Cao, Y. High temperature oxidation behavior and interface diffusion of Cr3C2-NiCrCoMo/nano-CeO2 composite coatings. J. Alloys Compd. 2022, 905, 164177. [Google Scholar] [CrossRef]
- Chandra-ambhorn, S.; Saranyachot, P.; Thublaor, T. High temperature oxidation behaviour of Fe–15.7 wt.% Cr–8.5 wt.% Mn in oxygen without and with water vapour at 700 °C. Corros. Sci. 2019, 148, 39–47. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Influence of processing conditions on the properties of thermal sprayed coating: A review. Surf. Eng. 2021, 37, 1339–1372. [Google Scholar] [CrossRef]
- Ma, R.; Peng, H.; Wen, Y.; Zhang, L.; Zhao, K. Oxidation behavior of an austenitic stainless FeMnSiCrNi shape memory alloy. Corros. Sci. 2013, 66, 269–277. [Google Scholar] [CrossRef]
- Leong, A.; Yang, Q.; McAlpine, S.W.; Short, M.P.; Zhang, J. Oxidation behavior of Fe-Cr-2Si alloys in high temperature steam. Corros. Sci. 2021, 179, 109114. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Wu, M.; Pei, Y.; Gong, S.; Zhang, H. Effects of Different surface native pre-oxides on the hot corrosion properties of nickel-based single crystal superalloys. Materials 2020, 13, 5774. [Google Scholar] [CrossRef] [PubMed]
- Jin-tao, L.; Yan, L.; Zhen, Y.; Jin-yang, H.; Ming, Z.; Gu, Y. Effect of Sulfur and Chlorine on Fireside Corrosion Behavior of Inconel 740 H Superalloy. High Temp. Mater. Process. 2018, 37, 245–251. [Google Scholar] [CrossRef]
- Souza, V.F.d.; Araújo, A.J.; Santos, J.L.d.N.; Rovere, C.A.D.; Malafaia, A.M.d.S. Kinetics oxidation and characterization of cyclically oxidized layers at high temperatures for FeMnSiCrNiCe and FeSiCrNi alloys. Mater. Res. 2017, 20, 365–373. [Google Scholar] [CrossRef]
- Lehmusto, J.; Sattari, M.; Halvarsson, M.; Hupa, L. Should the oxygen source be considered in the initiation of KCl-induced high-temperature corrosion? Corros. Sci. 2021, 183, 109332. [Google Scholar] [CrossRef]
- Inoue, Y.; Hiraide, N.; Hayashi, A.; Ushioda, K. Effect of Mn addition on scale structure of Nb containing ferritic stainless steel. ISIJ Int. 2018, 58, 1850–1859. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Liu, X.; Baker, A. Studies on elements diffusion of Mn/Co coated ferritic stainless steel for solid oxide fuel cell interconnects application. Int. J. Hydrogen Energy 2013, 38, 5075–5083. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, H.; Du, X.; He, Y.; Luo, H.; Song, G.; Mao, L.; Zhou, T.; Wang, L. Corrosion resistance enhancement of CoCrFeMnNi high-entropy alloy fabricated by additive manufacturing. Corros. Sci. 2020, 177, 108954. [Google Scholar] [CrossRef]
- Birbilis, N.; Choudhary, S.; Scully, J.R.; Taheri, M.L. A perspective on corrosion of multi-principal element alloys. npj Mater. Degrad. 2021, 5, 14. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Du, Y.; Yu, D.; Liang, D. Atomic mobilities, uphill diffusion and proeutectic ferrite growth in Fe–Mn–C alloys. Calphad 2009, 33, 614–623. [Google Scholar] [CrossRef]
- de Sousa Malafaia, A.M.; Latu-Romain, L.; Wouters, Y. High temperature oxidation resistance improvement in an FeMnSiCrNi alloy by Mn-depletion under vacuum annealing. Mater. Lett. 2019, 241, 164–167. [Google Scholar] [CrossRef]







| FeMnCrSi/TiC | TiC | Mn | Cr | Si | Fe |
| 5% | 20% | 15% | 11% | 47% |
| Steel | Cr | Mn | Si | C | P | S | Fe |
|---|---|---|---|---|---|---|---|
| 430 steel strips (Saky Steel, Shanghai, China) | 16% | ≤1.00% | 0.5% | ≤0.12% | ≤0.03% | ≤0.04% | Bal. |
| 45 steels | ≤0.25% | 0.6% | 0.25% | 0.45% | ≤0.035% | ≤0.035% | Bal. |
| Voltage/V | Current/A | Atomized Air Pressure/MPa | Spray Distance/mm |
|---|---|---|---|
| 30~35 | 220~240 | 0.65~0.75 | 160~180 |
| Coating | Porosity (%) | Average Value (%) | ||||
|---|---|---|---|---|---|---|
| FeMnCrSi/TiC | 5.55 | 4.43 | 4.55 | 4.94 | 4.82 | 4.86 |
| Coating | Point | Element (at%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Fe | Mn | Cr | Si | Ti | C | O | ||
| FeMnCrSi/TiC | A | 61.62 | 20.32 | 11.29 | 3.95 | 0.37 | 2.45 | 0.01 |
| B | 0.79 | 11.73 | 0.35 | 2.09 | 51.88 | 7.60 | 25.56 | |
| FeMnCrSi/TiC | Point | Element (wt.%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Mn | Cr | Si | Ti | C | O | S | Na | K | ||
| 10 h | A | 33.29 | 39.41 | 1.29 | 0.31 | 0.56 | 0.87 | 22.67 | — | 1.52 | — |
| 10 h | B | 35.84 | 38.1 | 1.28 | 0.48 | 2.77 | 0.66 | 20.17 | — | 0.66 | — |
| 100 h | C | 31.87 | 36.24 | 0.15 | — | 0.23 | 1.36 | 29.81 | — | — | — |
| 100 h | D | 32.25 | 30.68 | 0.13 | — | 0.92 | 1.52 | 34.39 | — | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Li, L.; Huang, Z.; Zhou, J.; Qin, Y.; Zhao, S.; Liu, D.; Xu, Z.; Zeng, J.; Tang, H.; et al. Insight into the Hot Corrosion Behavior of FeMnCrSi/TiC Coatings at 900 °C. Metals 2024, 14, 504. https://doi.org/10.3390/met14050504
Tang X, Li L, Huang Z, Zhou J, Qin Y, Zhao S, Liu D, Xu Z, Zeng J, Tang H, et al. Insight into the Hot Corrosion Behavior of FeMnCrSi/TiC Coatings at 900 °C. Metals. 2024; 14(5):504. https://doi.org/10.3390/met14050504
Chicago/Turabian StyleTang, Xu, Lei Li, Ze Huang, Jieji Zhou, Yonghuang Qin, Shiyang Zhao, Da Liu, Zhengbing Xu, Jianmin Zeng, Hongqun Tang, and et al. 2024. "Insight into the Hot Corrosion Behavior of FeMnCrSi/TiC Coatings at 900 °C" Metals 14, no. 5: 504. https://doi.org/10.3390/met14050504
APA StyleTang, X., Li, L., Huang, Z., Zhou, J., Qin, Y., Zhao, S., Liu, D., Xu, Z., Zeng, J., Tang, H., & Wang, Y. (2024). Insight into the Hot Corrosion Behavior of FeMnCrSi/TiC Coatings at 900 °C. Metals, 14(5), 504. https://doi.org/10.3390/met14050504





