A Comprehensive Understanding of Thermal Barrier Coatings (TBCs): Applications, Materials, Coating Design and Failure Mechanisms
Abstract
1. Introduction
2. Brief History of TBCs’ Evolution over Time
3. The Main Application Areas of TBCs
3.1. Aerospace Applications
3.2. Power Generation
3.3. Automotive Applications
3.4. Naval Industry
4. Configuration of TBCs
4.1. Substrate
4.2. Bond Coat
4.3. Thermally Grown Oxide
- Good adhesion to both the metallic substrate and the upper ceramic layer to prevent delamination and spallation;
- Chemical stability at high temperatures to withstand harsh operating conditions;
- Mechanical resistance to static and dynamic loads, ensuring durability under stress;
- Slow, consistent and continuous growth (thickness ≤ 10 µm) to maintain structural integrity;
- The ability to generate suitable oxides to form an effective barrier against oxygen diffusion through the TC.
4.4. Topcoat
- Low thermal conductivity for thermal insulation and a CTE similar to the substrate to reduce thermal stresses and the risk of delamination or cracking;
- Phase stability at high temperatures during prolonged exposure and thermal shock;
- Resistance to chemical attack by various compounds and chemical elements;
- Mechanical and thermodynamic compatibility with the TGO.
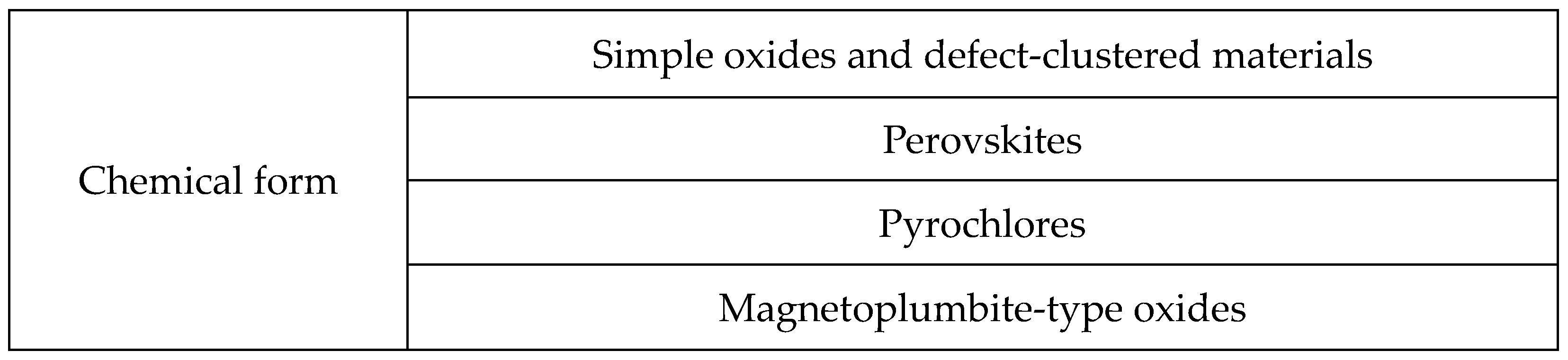
5. Topcoat Materials
- A.
- Simple Oxides and Defect-Clustered Materials
- B.
- Perovskites
- C.
- Pyrochlores
- D.
- Magnetoplumbite-Type Oxides
5.1. Metallic and Rare Earth Oxides
5.2. Zirconates
5.2.1. Zirconia-Based Materials
5.2.1.1. Yttria-Stabilized Zirconia (YSZ/ Y2O3-ZrO2)
5.2.1.2. Calcia-Stabilized Zirconia (Ca-SZ/CaZrO3)
5.2.1.3. Magnesia-Stabilized Zirconia (Mg-SZ/MgZrO3)
5.2.1.4. Ceria-Stabilized Zirconia (Ce-SZ/CeO2-ZrO2)
5.2.2. Rare Earth Zirconates (RE-Zirconates)
5.3. Rare Earth Niobates (RE-Niobates) and Tantalates (RE-Tantalates)
5.4. Other Materials
5.4.1. Mullite (Porcellanite)
5.4.2. Aluminates
5.4.3. Silicates and Phosphates
5.4.4. High-Entropy Coatings (HECs)
6. Design of TBCs
6.1. Coating Techniques
6.1.1. Air Plasma Spray (APS)
6.1.2. Electron Beam–Physical Vapor Deposition (EB-PVD)
6.1.3. Suspension Plasma Spraying (SPS)
6.2. Porosity
6.3. Multi-Layer Topcoat
6.4. Functionally Graded Thermal Barrier Coatings
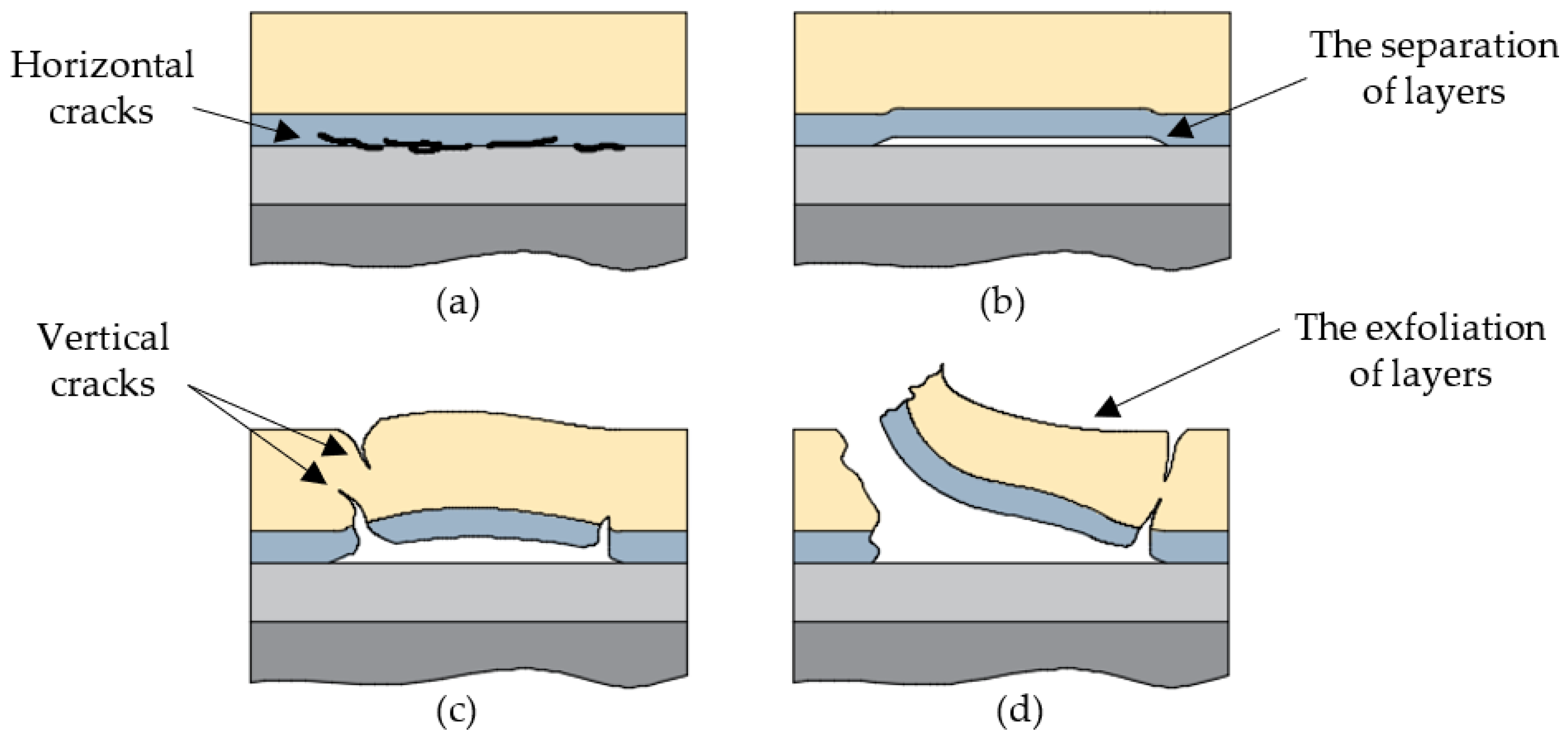
7. Degradation and Failure of TBCs
7.1. TGO Growth
7.2. Aluminum Depletion
7.3. Sulfur Segregation
7.4. Foreign Object Damage and Erosion
7.5. Corrosion and CMAS Attack
7.6. Sintering of the TC
7.7. Creep and Rumpling
8. Study Perspectives
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simic, M.; Alil, A.; Martinović, S.; Vlahović, M.; Savić, A.; Volkov-Husović, T. High temperature materials: Properties, demands and applications. Hem. Ind. 2020, 74, 273–284. [Google Scholar] [CrossRef]
- Chellaganesh, D.; Adam Khan, M.; Winowlin Jappes, J.T. Thermal barrier coatings for high temperature applications—A short review. Mater. Today Proc. 2021, 45, 1529–1534. [Google Scholar] [CrossRef]
- Song, J.; Wang, L.; Yao, J.; Dong, H. Multi-Scale Structural Design and Advanced Materials for Thermal Barrier Coatings with High Thermal Insulation: A Review. Coatings 2023, 13, 343. [Google Scholar] [CrossRef]
- Harrison, W.N.; Moore, D.G.; Richmond, J.C. Review of an Investigation of Ceramic Coatings for Metallic Turbine Parts and Other High-Temperature Applications; TN-1186; National Advisory Committee for Aeronautics: Washington, DC, USA, 1947.
- Garrett, F.B.; Gyorgak, C.A. Adhesive and Protective Characteristics of Ceramic Coating A-417 and Its Effect on Engine Life of Forged Refractaloy-26 (AMS 5760) and Cast Stellite (AMS 5385) Turbine Blades; RM-E52L30; National Advisory Committee for Aeronautics: Washington, DC, USA, 1953.
- Shafer, L.J.; Stepka, F.S.; Brown, W.B. Comparison of Theoretically and Experimentally Determined Effects of Oxide Coatings Supplied by Fuel Additives on Uncooled Turbine-Blade Temperature during Transient Turbojet-Engine Operation; RM-E53A19; National Advisory Committee for Aeronautics: Washington, DC, USA, 1953.
- Miller, R.A. Current status of thermal barrier coatings—An overview. Surf. Coat. Technol. 1987, 30, 1–11. [Google Scholar] [CrossRef]
- Miller, R.A. Thermal barrier coatings for aircraft engines: History and directions. J. Therm. Spray Technol. 1997, 6, 35–42. [Google Scholar] [CrossRef]
- Miller, R.A. History of Thermal Barrier Coatings for Gas Turbine Engines: Emphasizing NASA’s Role from 1942 to 1990. In Proceedings of the Thermal Barrier Coatings II, Engineering Conference International (No. NASA/TM-2009-215459), KlosterIree, Germany, 12–17 August 2007. [Google Scholar]
- Clarke, D.R.; Oechsner, M.; Padture, N.P. Thermal-barrier coatings for more efficient gas-turbine engines. MRS Bull. 2012, 37, 891–898. [Google Scholar] [CrossRef]
- Barwinska, I.; Kopec, M.; Kukla, D.; Senderowski, C.; Kowalewski, Z.L. Thermal Barrier Coatings for High-Temperature Performance of Nickel-Based Superalloys: A Synthetic Review. Coatings 2023, 13, 769. [Google Scholar] [CrossRef]
- Sezavar, A.; Sajjadi, S.A.; Babakhani, A.; Peng, R.L.; Yuan, K. Hot Corrosion Behavior of Micro- and Nanostructured Thermal Barrier Coatings: Conventional Bilayer and Compositionally Graded Layer YSZ. Oxid. Met. 2021, 96, 469–486. [Google Scholar] [CrossRef]
- Patnaik, P.C.; Huang, X.; Singh, J. State of the Art and Future Trends in the Development of Thermal Barrier Coating Systems. In Innovative Missile Systems; Meeting Proceedings RTO-MP-AVT-135; RTO: Neuilly-sur-Seine, France, 2006; pp. 38:1–38:20. [Google Scholar]
- Krishna, V.G.; Parammasivam, K.M. Thermal barrier coated surface modifications for gas turbine film cooling: A review. J. Therm. Anal. Calorim. 2021, 146, 545–580. [Google Scholar] [CrossRef]
- Fiedler, T.; Rösler, J.; Bäker, M.; Hötte, F.; von Sethe, C.; Daub, D.; Haupt, M.; Haidn, O.J.; Esser, B.; Gülhan, A. Mechanical Integrity of Thermal Barrier Coatings: Coating Development and Micromechanics. In Future Space-Transport-System Components under High Thermal and Mechanical Loads; Springer: Berlin/Heidelberg, Germany, 2021; Volume 146, pp. 295–307. [Google Scholar] [CrossRef]
- Fiedler, T.; Schloesser, J.; Rösler, J. Development of a thermal-barrier coating-system for rocket combustion chambers. In Proceedings of the 6th European Conference for Aerospace Sciences, Kraków, Poland, 29 June–3 July 2015. [Google Scholar]
- Greuel, D.; Suslov, D.; Haidn, O.; Fritscher, K. Thermal Barrier Coatings for Cryogenic Rocket Engines. In Proceedings of the 38th AIAA/ASME/SAE/ASEE/JPC Conference and Exhibit, Indianapolis, IN, USA, 7–10 July 2002; p. 4145. [Google Scholar]
- Liu, L.; Yu, H.; Zheng, C.; Ye, D.; He, W.; Wang, S.; Li, J.; Wu, L.; Zhang, Y.; Xie, J.; et al. Nondestructive Thickness Measurement of Thermal Barrier Coatings for Turbine Blades by Terahertz Time Domain Spectroscopy. Photonics 2023, 10, 105. [Google Scholar] [CrossRef]
- Biswas, S.; Ramachandra, S.; Hans, P.; Kumar, S.S.P. Materials for Gas Turbine Engines: Present Status, Future Trends and Indigenous Efforts. J. Indian Inst. Sci. 2022, 102, 297–309. [Google Scholar] [CrossRef]
- ASM Aerospace Specification Metals. Available online: https://asm.matweb.com (accessed on 5 March 2024).
- Zhang, Y.; Liu, P.; Li, Z. Impact of Cooling with Thermal Barrier Coatings on Flow Passage in a Gas Turbine. Energies 2022, 15, 85. [Google Scholar] [CrossRef]
- Kistenmacher, D.A.; Davidson, F.T.; Bogard, D.G. Realistic Trench Film Cooling With a Thermal Barrier Coating and Deposition. In Proceedings of the ASME Turbo Expo 2013: Turbine Technical Conference and Exposition, Volume 3B: Heat Transfer, San Antonio, TX, USA, 3–7 June 2013; p. V03BT13A057. [Google Scholar] [CrossRef]
- Davidson, F.T.; Kistenmacher, D.A.; Bogard, D.G. Film Cooling With a Thermal Barrier Coating: Round Holes, Craters, and Trenches. J. Turbomach. 2014, 136, 041007. [Google Scholar] [CrossRef]
- Ghosh, S.; Saha, M.; Bakshi, S.; Mondal, S. Effect of Thermal Barrier Coating in Sustainable Power production of Gas Turbines. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1187, 012034. [Google Scholar] [CrossRef]
- Ali, Z.A.; Mohammad, T.R. Effects of Thermal Barrier coating (TBC) thickness on temperature distribution of gas turbine blade. In Proceedings of the 3rd Conference on Advances in Mechanical Engineering, Istanbul, Turkey, 19–21 December 2017. [Google Scholar]
- Yang, X.; Zhang, J.; Lu, Z.; Park, H.Y.; Jung, Y.G.; Park, H.; Koo, D.D.; Sinatra, R.; Zhang, J. Removal and repair techniques for thermal barrier coatings: A review. Trans. IMF 2020, 98, 121–128. [Google Scholar] [CrossRef]
- Al-Shami, M.; Mohamed, O.; Abu Elhaija, W. Energy-Efficient Control of a Gas Turbine Power Generation System. Designs 2023, 7, 85. [Google Scholar] [CrossRef]
- Khattak, M.A.; Mohd Ali, N.S.; Abidin, N.H.Z.; Azhar, N.S.; Omar, M.H. Common Type of Turbines in Power Plant: A Review. J. Adv. Res. Appl. Sci. Eng. Technol. 2016, 3, 77–100. [Google Scholar]
- Slade, S.; Palmer, C. Worldwide Gas Turbine Forecast. In Turbomachinery International Handbook; Turbomachinery International: Norwalk, CT, USA, 2019; Volume 59, pp. 34–39. [Google Scholar]
- Slade, S.; Palmer, C. Worldwide Market Report: 2020 proved to be an interesting year for gas turbines. In Turbomachinery International Handbook; Turbomachinery International: Norwalk, CT, USA, 2020; Volume 60, pp. 28–29. [Google Scholar]
- Rao, A. Advanced Brayton Cycles. In The Gas Turbine Handbook; U.S. Department of Energy-National Energy Technology Laboratory: Morgantown, WV, USA, 2006; pp. 115–121. [Google Scholar]
- Pirin, O.T.; Jardón, E.R.; García, J.C.; Mariaca, Y.; Hernández, Y.S.; Ñeco, R.; Dávalos, O. Effect of Thermal Barrier Coating on the Thermal Stress of Gas Microturbine Blades and Nozzles. J. Mech. Eng. 2020, 66, 581–590. [Google Scholar] [CrossRef]
- Jude, S.A.A.; Winowlin Jappes, J.T.; Adamkhan, M. Thermal barrier coatings for high-temperature application on superalloy substrates—A review. Mater. Today Proc. 2022, 60, 1670–1675. [Google Scholar] [CrossRef]
- Safakish, G.R. Temperature Estimation in the Combustion Chamber of an Internal Combustion Engine. Adv. Mech. Eng. 2012, 4, 931584. [Google Scholar] [CrossRef]
- Naval Education and Training Professional Development and Technology Center. Construction Mechanic Basic. Volume 1; NAVEDTRA 14264; Naval Education and Training Professional Development and Technology Center: Pensacola, FL, USA, 1998. [Google Scholar]
- Hayes, T.K.; White, R.A.; Peters, J.E. Combustion Chamber Temperature and Instantaneous Local Heat Flux Measurements in a Spark Ignition Engine; SAE Technical Paper No. 930217; SAE International: Warrendale, PA, USA, 1993. [Google Scholar] [CrossRef]
- Sharma, P.; Dwivedi, V.K.; Kumar, D. A Review on Thermal Barrier Coatings (TBC) Usage and Effect on Internal Combustion Engine. In Advances in Fluid and Thermal Engineering; Springer: Singapore, 2021; pp. 77–85. [Google Scholar] [CrossRef]
- Dahham, R.Y.; Wei, H.; Pan, J. Improving Thermal Efficiency of Internal Combustion Engines: Recent Progress and Remaining Challenges. Energies 2022, 15, 6222. [Google Scholar] [CrossRef]
- Rohini, A.; Prema, S. A review on thermal barrier coating for diesel engine and its characteristics studies. J. Phys. Conf. Ser. 2020, 1473, 012039. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, J.; Deng, X.; Liu, Y.; Sun, D.; Zhang, Y. Research and analysis of a thermal optimisation design method for aluminium alloy pistons in diesel engines. Case Stud. Therm. Eng. 2023, 52, 103667. [Google Scholar] [CrossRef]
- Das, D.; Majumdar, G.; Sen, R.S.; Ghosh, B.B. The Effects of Thermal Barrier Coatings on Diesel Engine Performance and Emission. J. Inst. Eng. India Ser. C 2014, 95, 63–68. [Google Scholar] [CrossRef]
- Raghu; Girishkumar, G.S.; Chandrashekara, K. Experimental Study of the Effect of Thermal Barrier Coating on Diesel Engine Performance. Int. Res. J. Eng. Technol. 2018, 5, 2051–2054. [Google Scholar]
- Karthickeyan, V.; Balamurugan, P. Effect of thermal barrier coating with various blends of pumpkin seed oil methyl ester in DI diesel engine. Heat Mass Transf. 2017, 53, 3141–3154. [Google Scholar] [CrossRef]
- Uchida, N. A review of thermal barrier coatings for improvement in thermal efficiency of both gasoline and diesel reciprocating engines. Int. J. Engine Res. 2022, 23, 3–19. [Google Scholar] [CrossRef]
- Durat, M.; Kapsiz, M.; Nart, E.; Ficici, F.; Parlak, A. The effects of coating materials in spark ignition engine design. Mater. Des. 2012, 36, 540–545. [Google Scholar] [CrossRef]
- Liu, H.; Hong, Q.; Liu, H.; Huang, Z.; Zhang, X.; Chen, W.; Zeng, X.; Pan, S. Effects of Temperature and Additives on NOx Emission From Combustion of Fast-Growing Grass. Front. Energy Res. 2021, 9, 772755. [Google Scholar] [CrossRef]
- Bussman, W.R.; Baukal, C.E. Ambient conditions impact CO and NOx emissions: Part II. Pet. Technol. Q. 2009, 14, 37–41. [Google Scholar]
- Sehili, Y.; Loubar, K.; Lounici, M.S.; Tarabet, L.; Cerdoun, M.; Lacroix, C. Development of knock prediction technique in dual fuel engines and its mitigation with direct water injection. Fuel 2024, 358, 130297. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Wakisaka, Y.; Nishikawa, N.; Kosaka, H.; Yamashita, H.; Yamashita, C.; Iguma, H.; Fukui, K.; Takada, N.; Tomoda, T. Thermo-swing insulation to reduce heat loss from the combustion chamber wall of a diesel engine. Int. J. Engine Res. 2019, 20, 805–816. [Google Scholar] [CrossRef]
- MAN Energy Solutions. Basic Principles of Ship Propulsion. Available online: https://www.man-es.com (accessed on 6 March 2024).
- Jin, Z.; Yang, Y. Research on ship electric propulsion. IOP Conf. Ser. Earth Environ. Sci. 2020, 446, 042057. [Google Scholar] [CrossRef]
- Ozgurluk, Y.; Doleker, K.M.; Karaoglanli, A.C. Hot corrosion behavior of YSZ, Gd2Zr2O7 and YSZ/Gd2Zr2O7 thermal barrier coatings exposed to molten sulfate and vanadate salt. Appl. Surf. Sci. 2018, 438, 96–113. [Google Scholar] [CrossRef]
- Parthiban, K.; Roy, P.; Ghosh, S. Hot Corrosion Behaviour of Three-Layered Functionally Graded Glass–ceramic–YSZ-based Thermal Barrier Coating System. Trans. Indian Inst. Met. 2023, 77, 1407–1412. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, Y.; Li, W.; Liu, W.; Wu, Y.; Zhao, Z. Na2SO4 + NaCl molten salts corrosion mechanism of thermal barrier coatings used in ships. J. Therm. Anal. Calorim. 2021, 144, 2043–2056. [Google Scholar] [CrossRef]
- Hille, T.; Suiker, A.A.; Turteltaub, S.R. Engineering Fracture Mechanics. Microcrack nucleation in thermal barrier coating systems. Eng. Fract. Mech. 2009, 76, 813–825. [Google Scholar] [CrossRef]
- Li, B.; Fan, X.; Li, D.; Jiang, P. Design of Thermal Barrier Coatings Thickness for Gas Turbine Blade Based on Finite Element Analysis. Math. Probl. Eng. 2017, 2017, 2147830. [Google Scholar] [CrossRef]
- Mouritz, A.P. Superalloys for gas turbine engines. In Introduction to Aerospace Materials; Woodhead Publishing Limited: Sawston, UK, 2012; pp. 251–267. [Google Scholar]
- Gurrappa, I.; Yashwanth, I.V.S. The Importance of Corrosion and the Necessity of Applying Intelligent Coatings for Its Control. In Intelligent Coatings for Corrosion Control; Butterworth-Heinemann: Oxford, UK, 2015; pp. 17–58. [Google Scholar] [CrossRef]
- Mukherji, D.; Rösler, J.; Strunz, P.; Gilles, R.; Schumacher, G.; Piegert, S. Beyond Ni-based superalloys: Development of CoRe-based alloys for gas turbine applications at very high temperatures. Int. J. Mater. Res. 2011, 102, 1125–1132. [Google Scholar] [CrossRef]
- Nowotnik, A. Nickel-Based Superalloys. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Yu, J.; Wang, D.; Chen, J.; Yang, C.; Hao, X.; Zhou, J.; Li, D.; Shu, D.; Xiao, C.; Peng, Y. High Temperature Behaviors of a Casting Nickel-Based Superalloy Used for 815 °C. Materials 2021, 14, 716. [Google Scholar] [CrossRef]
- Horke, K.; Meyer, A.; Singer, R.F. Metal injection molding (MIM) of nickel-base superalloys. In Handbook of Metal Injection Molding, 2nd ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 575–608. [Google Scholar] [CrossRef]
- Reed, R.C. The Superalloys. Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2006; pp. 1–28. [Google Scholar]
- Wee, S.; Do, J.; Kim, K.; Lee, C.; Seok, C.; Choi, B.G.; Choi, Y.; Kim, W. Review on Mechanical Thermal Properties of Superalloys and Thermal Barrier Coating Used in Gas Turbines. Appl. Sci. 2020, 10, 5476. [Google Scholar] [CrossRef]
- Kawagishi, K.; Yeh, A.; Yokokawa, T.; Kobayashi, T.; Koizumi, Y.; Harada, H. Development of an Oxidation-Resistant High-Strength Sixth-Generation Single-Crystal Superalloy TMS-238. Superalloys 2012, 9, 189–195. [Google Scholar] [CrossRef]
- Jithin, S.D.; Senthil, K.S.; Vijay Vishaal, K.E.; Sundaramali, G. Comparative Analysis between 5th and 6th Generation Superalloys and Previous Generation Superalloys. Adv. Mater. Sci. Eng. 2022, 2022, 3530689. [Google Scholar] [CrossRef]
- Saltykov, P.; Fabrichnaya, O.; Golczewski, J.; Aldinger, F. Thermodynamic modeling of oxidation of Al–Cr–Ni alloys. J. Alloys Compd. 2004, 381, 99–113. [Google Scholar] [CrossRef]
- Aabid, A.; Khan, S.A. Optimization of Heat Transfer on Thermal Barrier Coated Gas Turbine Blade. IOP Conf. Ser. Mater. Sci. Eng. 2018, 370, 012022. [Google Scholar] [CrossRef]
- Haynes, J.A.; Ferber, M.K.; Porter, W.D. Thermal cycling behavior of plasma-sprayed thermal barrier coatings with various MCrAlX bond coats. J. Therm. Spray Technol. 2000, 9, 38–48. [Google Scholar] [CrossRef]
- Cojocaru, C.V.; Aghasibeig, M.; Irissou, E. NiCoCrAlX (X = Y, Hf and Si) Bond Coats by Cold Spray for High Temperature Applications. J. Therm. Spray Technol. 2022, 31, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y. Oxidation and Corrosion of New MCrAlX Coatings-Modelling and Experiments. Doctoral Thesis, Linköping University, Linköping, Sweden, 2014. ISBN 978-91-7519-247-5. [Google Scholar]
- Nicholls, J.R. Designing oxidation-resistant coatings. JOM 2000, 52, 28–35. [Google Scholar] [CrossRef]
- Abedi, H.R.; Salehi, M.; Shafyei, A. Mechanical and Thermal Propertiesof Double-layer and Triple-layer Thermal Barrier Coatings with DifferentCeramic Top Coats onto Polyimide Matrix Composite. Ceram. Int. 2017, 43, 12770–12780. [Google Scholar] [CrossRef]
- Naumenko, D.; Shemet, V.; Singheiser, L.; Quadakkers, W.J. Failure mechanisms of thermal barrier coatings on MCrAlY-type bondcoats associated with the formation of the thermally grown oxide. J. Mater. Sci. 2009, 44, 1687–1703. [Google Scholar] [CrossRef]
- Naumenko, D.; Pillai, R.; Chyrkin, A.; Quadakkers, W.J. Overview on Recent Developments of Bondcoats for Plasma-Sprayed Thermal Barrier Coatings. J. Therm. Spray Technol. 2017, 26, 1743–1757. [Google Scholar] [CrossRef]
- Ogawa, K.; Ito, K.; Shoji, T.; Seo, D.W.; Tezuka, H.; Kato, H. Effects of Ce and Si additions to CoNiCrAlY bond coat materials on oxidation behavior and crack propagation of thermal barrier coatings. J. Therm. Spray Technol. 2006, 15, 640–651. [Google Scholar] [CrossRef]
- Duan, W.; Li, Y.; Qiang, W. Effect of Hf-Doped MCrAlY Alloy on the Structure and Properties of Thermally Grown Oxide Layer. J. Mater. Eng. Perform. 2023; in press. [Google Scholar] [CrossRef]
- Duan, W.; Huang, B.; Li, Y.; Huang, X.; Zhou, M.; Qiang, W. Hf and Ta co-doping MCrAlY alloy to improve the lifetime of coatings. Surf. Coat. Technol. 2023, 468, 129781. [Google Scholar] [CrossRef]
- Wang, H.X.; Zhang, Y.; Cheng, J.L.; Li, Y.S. High temperature oxidation resistance and microstructure change of aluminized coating on copper substrate. Trans. Nonferrous Met. Soc. China 2015, 25, 184–190. [Google Scholar] [CrossRef]
- Song, P. Influence of Material and Testing Parameters on the Lifetime of TBC Systems with MCrAlY and NiPtAl Bondcoats. Doctoral Thesis, RWTH Aachen University, Aachen, Germany, 2011. ISBN 978-3-89336-783-2. [Google Scholar]
- Marino, K.A.; Hinnemann, B.; Carter, E.A. Atomic-scale insight and design principles for turbine engine thermal barrier coatings from theory. Proc. Natl. Acad. Sci. USA 2011, 108, 5480–5487. [Google Scholar] [CrossRef]
- Zhou, C.G.; Song, Y.X. Oxidation and hot corrosion of thermal barrier coatings (TBCs). In Thermal Barrier Coatings; Woodhead Publishing: Sawston, UK, 2011; pp. 193–214. [Google Scholar] [CrossRef]
- Soare, A.; Csáki, I.; Sohaciu, M.; Oprea, C.; Soare, S.; Costina, I.; Petrescu, M.I. New Bond Coat Materials for Thermal Barrier Coating Systems Processed Via Different Routes. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 012045. [Google Scholar] [CrossRef]
- Li, S.; Qi, H.; Song, J.; Yang, X.; Che, C. Effect of bond-coat surface roughness on failure mechanism and lifetime of air plasma spraying thermal barrier coatings. Sci. China Technol. Sci. 2019, 62, 989–995. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, Q.; Lee, K.I.; Zhu, W.; Wu, L.T.; Wu, R.T. The Effect of Bond Coat Roughness on the CMAS Hot Corrosion Resistance of EB-PVD Thermal Barrier Coatings. Coatings 2022, 12, 596. [Google Scholar] [CrossRef]
- Takahashi, R.J.; Assis, J.M.K.; Neto, F.P.; Reis, D.A.P. Heat treatment for TGO growth on NiCrAlY for TBC application. Mater. Res. Express 2019, 6, 126442. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Y.; Cheng, B.; Tahir, A. Influence of non-uniform feature of thermally grown oxide thickness on the local stress state and cracking behavior in TBC. Surf. Coat. Technol. 2022, 443, 128607. [Google Scholar] [CrossRef]
- Liu, C. Study of Bond Coats and Failure Mechanisms of Thermal Barrier Coatings. Doctoral Thesis, University of Manchester, Manchester, UK, 2019. [Google Scholar]
- Chen, W.R.; Wu, X.; Marple, B.R.; Nagy, D.R.; Patnaik, P.C. TGO growth behaviour in TBCs with APS and HVOF bond coats. Surf. Coat. Technol. 2008, 202, 2677–2683. [Google Scholar] [CrossRef]
- Garcia-Herrera, J.E.; Espinosa-Arbeláez, D.G.; Cáceres-Díaz, L.A.; Mondragón-Rodríguez, G.C.; Ruiz-Luna, H.; González-Hernández, J.; Trápaga-Martínez, L.G.; Muñoz-Saldaña, J.; Alvarado-Orozco, J.M. Effect of pre-oxidation treatments on the structural, microstructural, and chemical properties of β-(Ni,Pt)Al system. Surf. Coat. Technol. 2019, 367, 156–164. [Google Scholar] [CrossRef]
- Hille, T.S. Lifetime Modeling Thermal Barrier Coatings. Doctoral Thesis, Universität Stuttgart, Stuttgart, Germany, 2009. ISBN 978-90-771-7243-8. [Google Scholar]
- Padture, N.P.; Gell, M.; Jordan, E.H. TBCs for Gas-Turbine Engine Applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Moskal, G. Thermal barrier coatings: Characteristics of microstructure and properties, generation and directions of development of bond. J. Achiev. Mater. Manuf. Eng. 2009, 37, 323–331. [Google Scholar]
- Zhu, D.; Miller, R.A. Development of Advanced Low Conductivity Thermal Barrier Coatings. Int. J. Appl. Ceram. Technol. 2004, 1, 86–94. [Google Scholar] [CrossRef]
- Ma, W.; Jarligo, M.; Mack, D.E.; Pitzer, D.; Malzbender, J.; Vaßen, R.; Stöver, D. New Generation Perovskite Thermal Barrier Coating Materials. J. Therm. Spray Technol. 2008, 17, 831–837. [Google Scholar] [CrossRef]
- Mebdoua-Lahmar, Y.; Derbal-Habak, H.; Benkhaled, A. Alternative Materials for Performant TBCs: Short Review. J. Minera Land Mater. Sci. 2023, 4, 1051. [Google Scholar] [CrossRef]
- Vaßen, R.; Jarligo, M.O.; Steinke, T.; Mack, D.E.; Stöver, D. Overview on advanced thermal barrier coatings. Surf. Coat. Technol. 2010, 205, 938–942. [Google Scholar] [CrossRef]
- Shvydyuk, K.O.; Nunes-Pereira, J.; Rodrigues, F.F.; Silva, A.P. Review of Ceramic Composites in Aeronautics and Aerospace: A Multifunctional Approach for TPS, TBC and DBD Applications. Ceramics 2023, 6, 195–230. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Zhang, B.; Jiang, G.; Liu, H.; Yang, J.; Wang, J.; Liu, W. Present status and prospects of nanostructured thermal barrier coatings and their performance improvement strategies: A review. J. Manuf. Processes 2023, 97, 12–34. [Google Scholar] [CrossRef]
- Cao, X.Q.; Vassen, R.; Stoever, D. Ceramic materials for thermal barrier coatings. J. Eur. Ceram. Soc. 2004, 24, 1–10. [Google Scholar] [CrossRef]
- Naraparaju, R.; Pubbysetty, R.P.; Mechnich, P.; Schulz, U. EB-PVD alumina (Al2O3) as a top coat on 7YSZ TBCs against CMAS/VA infiltration: Deposition and reaction mechanisms. J. Eur. Ceram. Soc. 2018, 38, 3333–3346. [Google Scholar] [CrossRef]
- Bolelli, G.; Meschini, D.; Varis, T.; Testa, V.; Morelli, S.; Lusvarghi, L.; Vuoristo, P. Corrosion Properties of Thermally Sprayed Bond Coatings UnderPlasma-Sprayed Chromia Coating in Sulfuric Acid Solutions. J. Therm. Spray Technol. 2020, 29, 270–284. [Google Scholar] [CrossRef]
- Venkadesan, G.; Muthusamy, J. Experimental investigation of Al2O3/8YSZ and CeO2/8YSZ plasma sprayed thermal barrier coating on diesel engine. Ceram. Int. 2019, 45, 3166–3176. [Google Scholar] [CrossRef]
- Kiryc, M.; Kazamer, N.; Kurumlu, D.; Marginean, G. Comparative Study on the Thermal Performance of Cr-CrxOy and YSZ-CoNiCrAlY Coatings Exposed at 900 °C. Materials 2021, 14, 6040. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Moskal, G. Recent Development in Advance Ceramic Materials and Understanding the Mechanisms of Thermal Barrier Coatings Degradation. Arch. Comput. Methods Eng. 2023, 30, 4855–4896. [Google Scholar] [CrossRef]
- Clarke, D.R.; Levi, C.G. Materials Design for The Next Generation Thermal Barrier Coatings. Annu. Rev. Mater. Res. 2003, 33, 383–417. [Google Scholar] [CrossRef]
- Song, X.; Ding, Y.; Zhang, J.; Jiang, C.; Liu, Z.; Lin, C.; Zheng, W.; Zeng, Y. Thermophysical and mechanical properties of cubic, tetragonal and monoclinic ZrO2. J. Mater. Res. Technol. 2023, 23, 648–655. [Google Scholar] [CrossRef]
- Yu, Z.; Qi, T.; Ge, M.; Zhang, W.; Hu, Z.; Sun, X. Microstructures and phase compositions of Y2O3–ZrO2–HfO2 solid solutions. Ceram. Int. 2023, 49, 26119–26128. [Google Scholar] [CrossRef]
- Esmaeilkhanian, A.H.; Sharifianjazi, F.; Ahmadi, E.; Ijadi, A.; Meskher, H.; Zarei, R.; Nili-Ahmadabadi, M.; Irandoost, M.; Karimi, N.; Ghiasvand, A. Thermal barrier coating with improved durability: An overview of doped, nanostructured, multilayered, and gradient-structured zirconia-based thermal barrier coatings. Mater. Today Commun. 2023, 37, 107514. [Google Scholar] [CrossRef]
- Fabris, S.; Paxton, A.T.; Finnis, M.W. A stabilization mechanism of zirconia based on oxygen vacancies only. Acta Mater. 2002, 50, 5171–5178. [Google Scholar] [CrossRef]
- Smialek, J.; Miller, R. Revisiting the Birth of 7YSZ Thermal Barrier Coatings: Stephan Stecura. Coatings 2018, 8, 255. [Google Scholar] [CrossRef]
- MatWeb. Yttria Stabilized Zirconia, YSZ. Available online: https://www.matweb.com (accessed on 10 March 2024).
- Vaßen, R.; Mack, D.E.; Tandler, M.; Sohn, Y.J.; Sebold, D.; Guillon, O. Unique performance of thermal barrier coatings made of yttria-stabilized zirconia at extreme temperatures (>1500 °C). J. Am. Ceram. Soc. 2020, 104, 463–471. [Google Scholar] [CrossRef]
- Chee Hon Cheong, A.; Sivanesan, S. Perspective Chapter: The Application of Yttria-Stabilized Zirconia (YSZ). In Zirconia—New Advances, Structure, Fabrication and Applications; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Borik, M.A.; Kulebyakin, A.V.; Lomonova, E.E.; Milovich, F.O.; Myzina, V.A.; Ryabochkina, P.A.; Tabachkova, N.Y.; Sidorova, N.V.; Chislov, A.S. Partially Yttria-Stabilized Zirconia Crystals Co-Doped with Neodymium, Cerium, Terbium, Erbium or Ytterbium Oxides. Crystals 2021, 11, 1587. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, Y.; Wang, X.; Wang, Q.; Ai, L.; Zhao, L.; Chu, Y.; Guo, S.; Hu, J.; Zhang, Q. Preparation and thermophysical properties of Ti4+ doped zirconia matrix thermal barrier coatings. J. Alloys Compd. 2019, 777, 646–654. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, S.; Zhang, X.; Xiao, S.; Sun, J.; Zhang, J.; Han, G. Controlled synthesis of Bi2O3–YSZ composite powders and their sintering behavior for high-performance electrolytes. Int. J. Appl. Ceram. Technol. 2023, 20, 1398–1407. [Google Scholar] [CrossRef]
- Xing, Y.Z.; Men, Y.N.; Feng, X.; Geng, J.H.; Zou, Z.R.; Chen, F.H. Evolutions in the microstructure and ionic conductivity of CuO-doped yttria-stabilized zirconia. J. Solid State Chem. 2022, 315, 123497. [Google Scholar] [CrossRef]
- Pollet, M.; Marinel, S.; Desgardin, G. CaZrO3, a Ni-co-sinterable dielectric material for base metal-multilayer ceramic capacitor applications. J. Eur. Ceram. Soc. 2004, 24, 119–127. [Google Scholar] [CrossRef]
- Garcia, E.; Cano, C.; Osendi, M.I.; Miranzo, P.; Coyle, T.W. Thermal Behaviour of Thermally Sprayed CaZrO3 Coatings. In Thermal Spray. In Proceedings of the International Thermal Spray Conference, Maastricht, The Netherlands, 2–4 June 2008; pp. 1004–1008. [CrossRef]
- Ejaz, N.; Ali, L.; Ahmad, A.; Mansoor, M.; Asim, M.M.; Rauf, A.; Mehmood, K. Thermo-Physical Properties Measurement of Advanced TBC Materials with Pyrochlore and Perovskite Structures. Key Eng. Mater. 2018, 778, 236–244. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, R.; Sharma, A.K.; Sharma, R.K. Mechanical stresses analysis of a partially ceramic coated cylinder liners using finite element analysis. Mater. Today Proc. 2023, 72, 2369–2377. [Google Scholar] [CrossRef]
- Drazin, J.W.; Castro, R.H.R. Phase Stability in Calcia-Doped Zirconia Nanocrystals. J. Am. Ceram. Soc. 2016, 99, 1778–1785. [Google Scholar] [CrossRef]
- Oerlikon. Material Product Data Sheet. Magnesia-Stabilized Zirconium Oxide Powders. Available online: https://www.oerlikon.com (accessed on 10 March 2024).
- STC Material Solutions. Material Product Data Sheet. Magnesia Stabilized Zirconia (MSZ). Available online: https://ceramics.net (accessed on 10 March 2024).
- Fouad, M.G.; Ghazaly, N.M.; Abd El Tawwab, A.M.; Abd El Gwwad, K.A. Finite Element Thermal Analysis of A Ceramic Coated Si Engine Piston Considering Coating Thickness. Am. J. Eng. Res. 2017, 6, 109–113. [Google Scholar]
- Azhar, A.Z.A.; Mohamad, H.; Ratnam, M.M.; Ahmad, Z.A. The effects of MgO addition on microstructure, mechanical properties and wear performance of zirconia-toughened alumina cutting inserts. J. Alloys Compd. 2010, 497, 316–320. [Google Scholar] [CrossRef]
- Yamagata, C.; Mello-Castanho, S.R.H.; Paschoal, J.O.A. Synthesis and Mechanical Properties of Stabilized Zirconia Ceramics: MgO–ZrO2 and Y2O3–MgO–ZrO2. In Proceedings of the Congresso Brasileiro de Engenharia e Ciência dos Materiais, Cuiaba, MT, Brazil, 9–13 November 2014. [Google Scholar]
- Gul, S.R.; Khan, M.; Zeng, Y.; Wu, B. Understanding the thermodynamic properties of 20% CeO2 stabilized ZrO2 coatings with atomistic modeling and simulations. Mater. Res. Express 2019, 6, 076532. [Google Scholar] [CrossRef]
- Sodeoka, S.; Suzuki, M.; Ueno, K.; Sakuramoto, H.; Shibata, T.; Ando, M. Thermal and mechanical properties of ZrO2-CeO2 plasma-sprayed coatings. J. Therm. Spray Technol. 1997, 6, 361–367. [Google Scholar] [CrossRef]
- Mousavi, B.; Farvizi, M.; Shamsipoor, A.; Rahimipour, M.R.; Keyvani, A. Role of bond coat deposition method on the hot corrosion behavior of CSZ thermal barrier coatings. Emerg. Mater. Res. 2023, 12, 325–334. [Google Scholar] [CrossRef]
- Keyvani, A.; Bahamirian, M. Hot corrosion and mechanical properties of nanostructured Al2O3/CSZ composite TBCs. Surf. Eng. 2017, 33, 433–443. [Google Scholar] [CrossRef]
- Krogstad, J.A.; Lepple, M.; Levi, C.G. Opportunities for improved TBC durability in the CeO2–TiO2–ZrO2 system. Surf. Coat. Technol. 2013, 221, 44–52. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Salavati-Niasari, M.; Sobhani, A.; Zinatloo-Ajabshir, Z. Rare earth zirconate nanostructures: Recent development on preparation and photocatalytic applications. J. Alloys Compd. 2018, 767, 1164–1185. [Google Scholar] [CrossRef]
- Oglezneva, S.A.; Kachenyuk, M.N.; Smetkin, A.A.; Kul’met’eva, V.B. Influence of rare earth elements on the structure and properties of powders based on zirconium dioxide during consolidation. Int. Conf. Mod. Trends Manuf. Technol. Equip. Mech. Eng. Mater. Sci. 2020, 329, 02015. [Google Scholar] [CrossRef]
- Liu, Z.G.; Zhang, W.H.; Ouyang, J.H.; Zhou, Y. Novel thermal barrier coatings based on rare-earth zirconates/YSZ double-ceramic-layer system deposited by plasma spraying. J. Alloys Compd. 2015, 647, 438–444. [Google Scholar] [CrossRef]
- Wang, J.D.; Pan, W.; Xu, Q.; Mori, K.; Torigoe, T. Thermal Conductivity of the New Candidate Materials for Thermal Barrier Coatings. Key Eng. Mater. 2007, 280, 1503–1506. [Google Scholar] [CrossRef]
- Wang, X.; Bai, X.; Xiao, W.; Liu, Y.; Li, X.; Wang, J.; Peng, C.; Wang, L.; Wang, X. Calculation of Thermal Expansion Coefficient of Rare Earth Zirconate System at High Temperature by First Principles. Materials 2022, 15, 2264. [Google Scholar] [CrossRef]
- Zhou, H.; Yi, D. Effect of rare earth doping on thermo-physical properties of lanthanum zirconate ceramic for thermal barrier coatings. J. Rare Earths 2008, 26, 770–774. [Google Scholar] [CrossRef]
- Li, F.; Zhou, L.; Liu, J.X.; Liang, Y.; Zhang, G.J. High-entropy pyrochlores with low thermal conductivity for thermal barrier coating materials. J. Adv. Ceram. 2019, 8, 576–582. [Google Scholar] [CrossRef]
- Liu, D.; Shi, B.; Geng, L.; Wang, Y.; Xu, B.; Chen, Y. High-entropy rare-earth zirconate ceramics with low thermal conductivity for advanced thermal-barrier coatings. J. Adv. Ceram. 2022, 11, 961–973. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Meng, G.H.; Chen, L.; Li, G.R.; Liu, M.J.; Zhang, W.X.; Zhao, L.N.; Zhang, Q.; Zhang, X.D.; Wan, C.L.; et al. Progress in ceramic materials and structure design toward advanced thermal barrier coatings. J. Adv. Ceram. 2022, 11, 985–1068. [Google Scholar] [CrossRef]
- Chen, L.; Li, B.; Feng, J. Rare-earth tantalates for next-generation thermal barrier coatings. Prog. Mater. Sci. 2024, 144, 101265. [Google Scholar] [CrossRef]
- Yang, J.; Qian, X.; Pan, W.; Yang, R.; Li, Z.; Han, Y.; Zhao, M.; Huang, M.; Wan, C. Diffused Lattice Vibration and Ultralow Thermal Conductivity in the Binary Ln–Nb–O Oxide System. Adv. Mater. 2019, 31, 1808222. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pan, W.; Han, Y.; Zhao, M.; Huang, M.; Wan, C. Mechanical properties, oxygen barrier property and chemical stability of RE3NbO7 for thermal barrier coating. J. Am. Ceram. Soc. 2020, 103, 2302–2308. [Google Scholar] [CrossRef]
- Wu, F.; Wu, P.; Zhou, Y.; Chong, X.; Feng, J. The thermo-mechanical properties and ferroelastic phase transition of RENbO4 (RE = Y, La, Nd, Sm, Gd, Dy, Yb) ceramics. J. Am. Ceram. Soc. 2020, 103, 2727–2740. [Google Scholar] [CrossRef]
- Zhang, P.; Feng, Y.; Li, Y.; Pan, W.; Zong, P.; Huang, M.; Han, Y.; Yang, Z.; Chen, H.; Gong, Q.; et al. Thermal and mechanical properties of ferroelastic RENbO4 (RE = Nd, Sm, Gd, Dy, Er, Yb) for thermal barrier coatings. Scr. Mater. 2020, 180, 51–56. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Y.H.; Chong, X.Y.; Feng, J. Synthesis and thermophysical properties of RETa3O9 (RE = Ce, Nd, Sm, Eu, Gd, Dy, Er) as promising thermal barrier coatings. J. Am. Ceram. Soc. 2018, 101, 1266–1278. [Google Scholar] [CrossRef]
- Chen, L.; Hu, M.; Guo, J.; Chong, X.; Feng, J. Mechanical and thermal properties of RETaO4 (RE = Yb, Lu, Sc) ceramics with monoclinic-prime phase. J. Mater. Sci. Technol. 2020, 52, 20–28. [Google Scholar] [CrossRef]
- Majeed, E.A.; Rashid, H.K.; Hussain, M.K. Review of ceramic materials that used as a thermal barrier in diesel engine pistons. J. Phys. Conf. Ser. 2021, 1973, 012125. [Google Scholar] [CrossRef]
- MatWeb. Mullite. Available online: https://www.matweb.com/search/DataSheet.aspx?MatGUID=6ff3fda0bf744c93b4e423806faec494 (accessed on 12 March 2024).
- Xu, H.; Guo, H.; Gong, S. Thermal barrier coatings. In Developments in High Temperature Corrosion and Protection of Materials; Woodhead Publishing: Sawston, UK, 2008; pp. 476–491. [Google Scholar] [CrossRef]
- Weinberg, A.V.; Varona, C.; Chaucherie, X.; Goeuriot, D.; Poirier, J. Corrosion of Al2O3-SiO2 refractories by sodium and sulfur vapors: A case study on hazardous waste incinerators. Ceram. Int. 2017, 43, 5743–5750. [Google Scholar] [CrossRef]
- Syamkumar, K.; Babu, N.; Govindarajan, S.; Arya, S.B. Hot corrosion behaviour of mullite thermal barrier coatings for marine diesel engines. Ceram. Int. 2024, 50, 2808–2818. [Google Scholar] [CrossRef]
- Chu, K.; Zhang, Y.; Zhao, J.; Liu, Y.; Li, Y.; Li, W.; Liu, B. Screening rare-earth aluminates as promising thermal barrier coatings by high-throughput first-principles calculations. J. Am. Ceram. Soc. 2023, 106, 3089–3102. [Google Scholar] [CrossRef]
- Torrez-Herrera, J.J.; Fuentes-Ordoñez, E.G.; Ordoñez, E.G.F.; Korili, S.A.; Gil, A. Evidence for the synthesis of La-hexaaluminate from aluminum-containing saline slag wastes: Correction of structural defects and phase purification at low temperature. Powder Technol. 2021, 377, 80–88. [Google Scholar] [CrossRef]
- Xie, X.; Guo, H.; Gong, S.; Xu, H. Lanthanum–titanium–aluminum oxide: A novel thermal barrier coating material for applications at 1300 °C. J. Eur. Ceram. Soc. 2011, 31, 1677–1683. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, Z.; Fan, X.; Zhao, S.; Cao, X.; He, L. Y4Al2O9 ceramics as a novel thermal barrier coating material for high-temperature applications. Mater. Lett. 2014, 134, 146–148. [Google Scholar] [CrossRef]
- Stokes, J.L.; Harder, B.J.; Wiesner, V.L.; Wolfe, D.E. Crystal structures and thermal expansion of Yb2Si2O7–Gd2Si2O7 solid solutions. J. Solid State Chem. 2022, 312, 123166. [Google Scholar] [CrossRef]
- Guo, L.; Feng, J.; Meng, S. Corrosion resistance of GdPO4 thermal barrier coating candidate in the presence of CMAS + NaVO3 and CMAS. Corros. Sci. 2022, 208, 110628. [Google Scholar] [CrossRef]
- Arshad, M.; Amer, M.; Hayat, Q.; Janik, V.; Zhang, X.; Moradi, M.; Bai, M. High-Entropy Coatings (HEC) for High-Temperature Applications: Materials, Processing, and Properties. Coatings 2022, 12, 691. [Google Scholar] [CrossRef]
- Patel, P.; Roy, A.; Sharifi, N.; Stoyanov, P.; Chromik, R.R.; Moreau, C. Tribological Performance of High-Entropy Coatings (HECs): A Review. Materials 2022, 15, 3699. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yang, F.; Zhao, X. Multicomponent high-entropy Zr-Y-Yb-Ta-Nb-O oxides for next-generation thermal barrier coating applications. J. Am. Ceram. Soc. 2022, 105, 35–43. [Google Scholar] [CrossRef]
- Hsu, W.L.; Murakami, H.; Yeh, J.W.; Yeh, A.C.; Shimoda, K. A Heat-Resistant NiCo0.6Fe0.2Cr1.5SiAlTi0.2 Overlay Coating for High-Temperature Applications. J. Electrochem. Soc. 2016, 163, C752. [Google Scholar] [CrossRef]
- Kumar, V.; Kandasubramanian, B. Processing and design methodologies for advanced and novel thermal barrier coatings for engineering applications. Particuology 2016, 27, 1–28. [Google Scholar] [CrossRef]
- Mehta, A.; Vasudev, H.; Singh, S.; Prakash, C.; Saxena, K.K.; Linul, E.; Buddhi, D.; Xu, J. Processing and Advancements in the Development of Thermal Barrier Coatings: A Review. Coatings 2022, 12, 1318. [Google Scholar] [CrossRef]
- Latka, L. Thermal Barrier Coatings Manufactured by Suspension Plasma Spraying—A Review. Adv. Mater. Sci. 2018, 18, 95–117. [Google Scholar] [CrossRef]
- Miranda, F.; Caliari, F.; Essiptchouk, A.; Pertraconi, G. Atmospheric Plasma Spray Processes: From Micro to Nanostructures. In Atmospheric Pressure Plasma—From Diagnostics to Applications; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Luo, L.; Chen, Y.; Zhou, M.; Shan, X.; Lu, J.; Zhao, X. Progress update on extending the durability of air plasma sprayed thermal barrier coatings. Ceram. Int. 2022, 48, 18021–18034. [Google Scholar] [CrossRef]
- Curry, N.; Leitner, M.; Körner, K. High-Porosity Thermal Barrier Coatings from High-Power Plasma Spray Equipment—Processing, Performance and Economics. Coatings 2020, 10, 957. [Google Scholar] [CrossRef]
- Rabieifar, A.; Mehrizi, V.A.; Haghighi, M.G. Degradation Mechanisms of APS and EB-PVD Thermal Barrier Coatings. J. Environ. Friendly Mater. 2023, 7, 47–63. [Google Scholar]
- Bakan, E.; Mack, D.E.; Mauer, G.; Vaßen, R.; Lamon, J.; Padture, N.P. High-temperature materials for power generation in gas turbines. In Advanced Ceramics for Energy Conversion and Storage; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–62. [Google Scholar] [CrossRef]
- Kubaszek, T.; Goral, M.; Pedrak, P. Influence of air plasma spraying process parameters on the thermal barrier coating deposited with micro- and nanopowders. Mater. Sci.-Pol. 2022, 40, 80–92. [Google Scholar] [CrossRef]
- Peng, H.; Gong, S. Thermal barrier coatings prepared by electron beam-physical vapor deposition (EB-PVD). In Thermal Barrier Coatings, 2nd ed.; Woodhead Publishing: Sawston, UK, 2023; pp. 119–136. [Google Scholar] [CrossRef]
- Lokachari, S.; Leng, K.; Romero, A.R.; Curry, N.; Brewster, G.; Norton, A.; Hussain, T. Processing–Microstructure–Properties of Columns in Thermal Barrier Coatings: A Study of Thermo-Chemico-Mechanical Durability. ACS Appl. Mater. Interfaces 2024, 16, 10646–10660. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.A.; Lim, M.J. A relationship of porosity and mechanical properties of spark plasma sintered scandia stabilized zirconia thermal barrier coating. Results Eng. 2023, 19, 101263. [Google Scholar] [CrossRef]
- Curry, N.; VanEvery, K.; Snyder, T.; Markocsan, N. Thermal Conductivity Analysis and Lifetime Testing of Suspension Plasma-Sprayed Thermal Barrier Coatings. Coatings 2014, 4, 630–650. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Morikawa, M.; Hamaguchi, T.; Habu, Y.; Ohide, Y.; Takagi, K. Relationship between the mechanical properties and structure of a suspension plasma-sprayed thermal barrier coating with columnar microstructure. Surf. Coat. Technol. 2022, 439, 128430. [Google Scholar] [CrossRef]
- Tesar, T.; Musalek, R.; Medricky, J.; Cizek, J. On growth of suspension plasma-sprayed coatings deposited by high-enthalpy plasma torch. Surf. Coat. Technol. 2019, 371, 333–343. [Google Scholar] [CrossRef]
- Bernard, B.T.; Quet, A.; Bianchi, L.; Schick, V.; Joulia, A.; Malié, A.; Rémy, B. Effect of Suspension Plasma-Sprayed YSZ Columnar Microstructure and Bond Coat Surface Preparation on Thermal Barrier Coating Properties. J. Therm. Spray Technol. 2017, 26, 1025–1037. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Y.; Yang, D.; Gao, J. A Novel Plasma-Sprayed Nanostructured Coating with Agglomerated-Unsintered Feedstock. J. Therm. Spray Technol. 2016, 25, 291–300. [Google Scholar] [CrossRef]
- Zhu, J.; Ma, K. Microstructural and mechanical properties of thermal barrier coating at 1400 °C treatment. Theor. Appl. Mech. Lett. 2014, 4, 021008. [Google Scholar] [CrossRef][Green Version]
- Dwivedi, G.; Viswanathan, V.; Sampath, S.; Shyam, A.; Lara-Curzio, E. Fracture toughness of plasma sprayed thermal barrier ceramics: Influence of processing, microstructure, and thermal aging. J. Am. Ceram. Soc. 2014, 97, 2736–2744. [Google Scholar] [CrossRef]
- Mahade, S.; Venkat, A.; Curry, N.; Leitner, M.; Joshi, S. Erosion Performance of Atmospheric Plasma Sprayed Thermal Barrier Coatings with Diverse Porosity Levels. Coatings 2021, 11, 86. [Google Scholar] [CrossRef]
- Boissonnet, G.; Bonnet, G.; Pasquet, A.; Bourhila, N.; Pedraza, F. Evolution of thermal insulation of plasma-sprayed thermal barrier coating systems with exposure to high temperature. J. Eur. Ceram. Soc. 2019, 39, 2111–2121. [Google Scholar] [CrossRef]
- Hu, Z.C.; Liu, B.; Wang, L.; Cui, Y.H.; Wang, Y.W.; Ma, Y.D.; Sun, W.W.; Yang, Y. Research Progress of Failure Mechanism of Thermal Barrier Coatings at High Temperature via Finite Element Method. Coatings 2020, 10, 732. [Google Scholar] [CrossRef]
- Liu, G.; Shen, Z.; He, L.; Mu, R.; Huang, G. LaYZrO/YSZ double ceramic layer thermal barrier coatings by EB-PVD: Thermal performance, morphology and failure behavior. Materialia 2023, 27, 101661. [Google Scholar] [CrossRef]
- Mahade, S.; Li, R.; Curry, N.; Björklund, S.; Markocsan, N.; Nylén, P. Isothermal Oxidation Behavior of Gd2Zr2O7/YSZ Multilayered Thermal Barrier Coatings. Int. J. Appl. Ceram. Technol. 2016, 13, 443–450. [Google Scholar] [CrossRef]
- Mahade, S.; Curry, N.; Björklund, S.; Markocsan, N.; Joshi, S. Durability of Gadolinium Zirconate/YSZ Double-Layered Thermal Barrier Coatings under Different Thermal Cyclic Test Conditions. Materials 2019, 12, 2238. [Google Scholar] [CrossRef]
- Mondal, K.; Nunez, L.; Downey, C.M.; van Rooyen, I.J. Thermal Barrier Coatings Overview: Design, Manufacturing, and Applications in High-Temperature Industries. Ind. Eng. Chem. Res. 2021, 60, 6061–6077. [Google Scholar] [CrossRef]
- Sathish, M.; Radhika, N.; Saleh, B. A critical review on functionally graded coatings: Methods, properties, and challenges. Compos. Part B Eng. 2021, 225, 109278. [Google Scholar] [CrossRef]
- Bhavar, V.; Kattire, P.; Thakare, S.; Patil, S.; Singh, R.K.P. A Review on Functionally Gradient Materials (FGMs) and Their Applications. IOP Conf. Ser. Mater. Sci. Eng. 2017, 229, 012021. [Google Scholar] [CrossRef]
- Kagerer, S.; Hudak, O.E.; Schloffer, M.; Riedl, H.; Mayrhofer, P.H. TGO formation and oxygen diffusion in Al-rich gamma-TiAl PVD-coatings on TNM alloys. Scr. Mater. 2022, 210, 114455. [Google Scholar] [CrossRef]
- Ding, K.; Zhang, T.; Wang, Z.; Yu, J.; Guo, W.; Yang, Y. Effect of Thermal Growth Oxide Composition and Morphology on Local Stresses in Thermal Barrier Coatings. Materials 2022, 15, 8442. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.D.; Toro, A.; Hernández-Ortiz, J.P. Thermal Barrier Coatings for Gas Turbine Applications: Failure Mechanisms and Key Microstructural Features. Dyna 2012, 79, 149–158. [Google Scholar]
- Dong, H.; Yang, G.J.; Li, C.X.; Luo, X.T.; Li, C.J. Effect of TGO Thickness on Thermal Cyclic Lifetime and Failure Mode of Plasma-Sprayed TBCs. J. Am. Ceram. Soc. 2014, 97, 1226–1232. [Google Scholar] [CrossRef]
- Ding, H.; Dong, H.; Li, C.J.; Yang, G.J. Effect of TGO Thickness on Isothermal Cyclic Lifetime of Plasma-Sprayed YSZ Thermal Barrier Coatings. In Proceedings of the International Thermal Spray Conference, Long Beach, CA, USA, 11–14 May 2015; pp. 801–805. [Google Scholar] [CrossRef]
- Torkashvand, K.; Poursaeidi, E.; Mohammadi, M. Effect of TGO thickness on the thermal barrier coatings life under thermal shock and thermal cycle loading. Ceram. Int. 2018, 44, 9283–9293. [Google Scholar] [CrossRef]
- Evans, A.G.; Hutchinson, J.W. On the mechanics of delamination and spalling in compressed films. Int. J. Solids Struct. 1984, 20, 455–466. [Google Scholar] [CrossRef]
- Bhatnagar, H.; Ghosh, S.; Walter, M.E. Parametric studies of failure mechanisms in elastic EB-PVD thermal barrier coatings using FEM. Int. J. Solids Struct. 2006, 43, 4384–4406. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Cai, H.N. Comprehensive effects of TGO growth on the stress characteristic and delamination mechanism in lamellar structured thermal barrier coatings. Ceram. Int. 2020, 46, 2220–2237. [Google Scholar] [CrossRef]
- Yuan, B.; Harvey, C.M.; Thomson, R.C.; Critchlow, G.W.; Rickerby, D.; Wang, S. A new spallation mechanism of thermal barrier coatings and a generalized mechanical model. Compos. Struct. 2019, 227, 111314. [Google Scholar] [CrossRef]
- Eriksson, R. Thermal Barrier Coatings—Durability Assessment and Life Prediction. Doctoral Dissertation, Linköping University, Linköping, Sweden, 2013. ISSN 0345-7524. [Google Scholar]
- Evans, A.G.; He, M.Y.; Hutchinson, J.W. Mechanics-based scaling laws for the durability of thermal barrier coatings. Prog. Mater. Sci. 2001, 46, 249–271. [Google Scholar] [CrossRef]
- Renusch, D.P.; Rudolphi, M.; Schütze, M. Software Tools for Lifetime Assessment of Thermal Barrier Coatings Part II—Bond Coat Aluminum Depletion Failure. J. Solid Mech. Mater. Eng. 2010, 4, 155–166. [Google Scholar] [CrossRef][Green Version]
- Shillington, E.A.G.; Clarke, D.R. Spalling failure of a thermal barrier coating associated with aluminum depletion in the bond-coat. Acta Mater. 1999, 47, 1297–1305. [Google Scholar] [CrossRef]
- Jonnalagadda, K.P. Thermal Barrier Coatings Failure Mechanisms and Life Prediction. Doctoral Thesis, Linköping University, Linköping, Sweden, 2019. ISSN 0345-7524. [Google Scholar]
- Lim, L.Y.; Meguid, S.A. Modeling and characterisation of depletion of aluminium in bond coat and growth of mixed oxides in thermal barrier coatings. Int. J. Mech. Mater. Des. 2020, 16, 667–683. [Google Scholar] [CrossRef]
- Taylor, M.; Evans, H.E.; Gray, S.; Nicholls, J.R. A chromia forming thermal barrier coating system. Mater. Corros. 2011, 62, 668–673. [Google Scholar] [CrossRef]
- Berthod, P. Kinetics of High Temperature Oxidation and Chromia Volatilization for a Binary Ni–Cr Alloy. Oxid. Met. 2005, 64, 235–252. [Google Scholar] [CrossRef]
- Eriksson, R. High-Temperature Degradation of Plasma Sprayed Thermal Barrier Coating Systems. Doctoral Thesis, Linköping University, Linköping, Sweden, 2011. ISSN 0280-7971. [Google Scholar]
- Wang, L.; Zhao, Y.X.; Zhong, X.H.; Tao, S.Y.; Zhang, W.; Wang, Y. Influence of “Island-Like” Oxides in the Bond-Coat on the Stress and Failure Patterns of the Thermal-Barrier Coatings Fabricated by Atmospheric Plasma Spraying During Long-Term High Temperature Oxidation. J. Therm. Spray Technol. 2014, 23, 431–446. [Google Scholar] [CrossRef]
- Zou, Z.; Jia, L.; Yang, L.; Shan, X.; Luo, L.; Guo, F.; Zhao, X.; Xiao, P. Role of internal oxidation on the failure of air plasma sprayed thermal barrier coatings with a double-layered bond coat. Surf. Coat. Technol. 2017, 319, 370–377. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, P.; Moverare, J.; Li, X.H.; Cui, L.; Peng, R.L. Impeding the γ′ depletion during the interdiffusion between bond coatings and superalloys via introduction of tantalum in bond coatings. Mater. Des. 2023, 227, 111792. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, B.; Deng, C.; Li, Q.; Zhang, L.; Deng, P.; Yang, K.; Wu, C.; Liu, M. Microstructure evolution and interdiffusion behaviors of (Ni,Pt)Al coating with and without Re-diffusion barrier on IC21 substrate at 1100 °C. Mater. Charact. 2021, 181, 111450. [Google Scholar] [CrossRef]
- Rouzou, I.; Molins, R.; Rémy, L.; Jomard, F. Study of the Sulfur Segregation for a TBC System. Mater. Sci. Forum 2004, 461–464, 101–108. [Google Scholar] [CrossRef]
- Bai, M.; Jiang, H.; Chen, Y.; Chen, Y.; Grovenor, C.; Zhao, X.; Xiao, P. Migration of sulphur in thermal barrier coatings during heat treatment. Mater. Des. 2016, 97, 364–371. [Google Scholar] [CrossRef]
- Bacos, M.P.; Dorvaux, J.M.; Lavigne, O.; Mévrel, R.; Poulain, M.; Rio, C.; Vidal-Sétif, M.H. Performance and Degradation Mechanisms of Thermal Barrier Coatings for Turbine Blades: A Review of ONERA Activities. Aerosp. Lab 2011, 3, 1–11. [Google Scholar]
- Hou, P.Y. Segregation Behavior at TGO/Bondcoat Interfaces. J. Mater. Sci. 2009, 44, 1711–1725. [Google Scholar] [CrossRef]
- Smialek, J.L.; Pint, B.A. Optimizing Scale Adhesion on Single Crystal Superalloys. Mater. Sci. Forum 2001, 369–372, 459–466. [Google Scholar] [CrossRef]
- Faucett, D.C.; Wright, J.; Ayre, M.; Choi, S.R. Foreign Object Damage (FOD) in Thermal Barrier Coatings. Ceram. Trans. 2012, 234, 245–255. [Google Scholar] [CrossRef]
- Wannman, C. Erosion Behaviour of Thermal Barrier Coatings. Master’s Thesis, Linkoping University, Linköping, Sweden, 2021. [Google Scholar]
- Yu, X.; Li, C.; Chang, S.; Xuan, C.; Peiyuan, L. Foreign Object Damage Performance and Constitutive Modeling of Titanium Alloy Blade. Int. J. Aerosp. Eng. 2020, 2020, 2739131. [Google Scholar] [CrossRef]
- Wellman, R.G.; Deakin, M.J.; Nicholls, J.R. The effect of TBC morphology on the erosion rate of EB PVD TBCs. Wear 2005, 258, 349–356. [Google Scholar] [CrossRef]
- Nicholls, J.R.; Wellman, R.G. Erosion and Foreign Object Damage of Thermal Barrier Coatings. Mater. Sci. Forum 1997, 251–254, 935–948. [Google Scholar] [CrossRef]
- Chen, X.; Hutchinson, J.W. Particle impact on metal substrates with application to foreign object damage to aircraft engines. J. Mech. Phys. Solids 2002, 50, 2669–2690. [Google Scholar] [CrossRef]
- Chen, X.; Wang, R.; Yao, N.; Evans, A.G.; Hutchinson, J.W.; Bruce, R.W. Foreign object damage in a thermal barrier system: Mechanisms and simulations. Mater. Sci. Eng. A 2003, 352, 221–231. [Google Scholar] [CrossRef]
- Pettit, F. Hot Corrosion of Metals and Alloys. Oxid. Met. 2011, 76, 1–21. [Google Scholar] [CrossRef]
- Carlsson, K. A Study of Failure Development in Thick Thermal Barrier Coatings. Master’s Thesis, Linkoping University, Linköping, Sweden, 2007. [Google Scholar]
- Holländer, C.; Kiliani, S.; Stamm, W.; Lüsebrink, O.; Harders, H.; Wessel, E.; Müller, M.; Singheiser, L. Hot corrosion of TBC-coated components uponcombustion of low-sulfur fuels. Mater. Corros. 2021, 72, 1643–1655. [Google Scholar] [CrossRef]
- Ozgurluk, Y.; Doleker, K.M.; Ahlatci, H.; Karaoglanli, A.C. Investigation of hot corrosion behavior of thermal barrier coating (TBC) systems with rare earth contents. Arab. J. Geosci. 2018, 11, 267. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 24436, Sodium Sulfate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-Sulfate (accessed on 20 February 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5234, Sodium Chloride. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-Chloride (accessed on 20 February 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 14814, Vanadium Pentoxide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Vanadium-pentoxide (accessed on 20 February 2024).
- Morelli, S.; Bursich, S.; Testa, V.; Bolelli, G.; Miccichè, A.; Lusvarghi, L. CMAS corrosion and thermal cycling fatigue resistance of alternative thermal barrier coating materials and architectures: A comparative evaluation. Surf. Coat. Technol. 2022, 439, 128433. [Google Scholar] [CrossRef]
- Meng, F.; Ye, F.; Luo, T. The high-temperature CMAS corrosion behavior of high-entropy (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Hf2O7 hafnate thermal barrier coating material with fluorite structure. J. Eur. Ceram. Soc. 2024, 44, 2460–2470. [Google Scholar] [CrossRef]
- Mahade, S. Functional Performance of Gadolinium Zirconate/Yttria Stabilized Zirconia Multi-Layered Thermal Barrier Coatings. Doctoral Thesis, University West, Trollhättan, Sweden, 2018. ISBN 978-91-87531-85-9. [Google Scholar]
- Li, D.; Jiang, P.; Gao, R.; Sun, F.; Jin, X.; Fan, X. Experimental and numerical investigation on the thermal and mechanical behaviours of thermal barrier coatings exposed to CMAS corrosion. J. Adv. Ceram. 2021, 10, 551–564. [Google Scholar] [CrossRef]
- Levi, C.G.; Hutchinson, J.W.; Vidal-Sétif, M.H.; Johnson, C.A. Environmental degradation of thermal-barrier coatings by molten deposits. MRS Bull. 2012, 37, 932–941. [Google Scholar] [CrossRef]
- Yan, J.; Wang, X.; Chen, K.; Lee, K.N. Sintering Modeling of Thermal Barrier Coatings at Elevated Temperatures: A Review of Recent Advances. Coatings 2021, 11, 1214. [Google Scholar] [CrossRef]
- Aygun, A. Novel Thermal Barrier Coatings (TBCs) That Are Resistant to High Temperature Attack by CaO-MgO-Al2O3-SiO2 (CMAS) Glassy Deposits. Doctoral Thesis, The Ohio State University, Columbus, OH, USA, 2008. [Google Scholar]
- Wellman, R.G.; Nicholls, J.R. Erosion, corrosion and erosion–corrosion of EB PVD thermal barrier coatings. Tribol. Int. 2008, 41, 657–662. [Google Scholar] [CrossRef]
- Wang, L.S.; Song, J.B.; Dong, H.; Yao, J.T. Sintering-Induced Failure Mechanism of Thermal Barrier Coatings and Sintering-Resistant Design. Coatings 2022, 12, 1083. [Google Scholar] [CrossRef]
- Tian, H.; Wei, L.; He, L. Phase Composition and Stability, Sintering and Thermal Conductivity of Gd2O3 and Yb2O3 Co-Doped YSZ. Coatings 2023, 13, 53. [Google Scholar] [CrossRef]
- Yi, H.; Che, J.; Liang, G.; Liu, X. Effect of Rare Earth Elements on Stability and Sintering Resistance of Tetragonal Zirconia for Advanced Thermal Barrier Coatings. Crystals 2021, 11, 287. [Google Scholar] [CrossRef]
- Ali, M.Y.; Nusier, S.Q.; Newaz, G.M. Creep effects on early damage initiation in a TBC system. J. Mater. Sci. 2004, 39, 3383–3390. [Google Scholar] [CrossRef]
- Gupta, C.K. Nuclear Reactor Materials. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 6339–6349. [Google Scholar] [CrossRef]
- Scott MacKenzie, D. The mechanism of creep and its stages. In Thermal Processing. Hot Seat; Thermal Processing: Yeager Parkway Pelham, AL, USA, 2024; pp. 24–25. [Google Scholar]
- Pelleg, J. Creep in Ceramics; Springer: Berlin/Heidelberg, Germany, 2017; Volume 241, pp. 41–61. [Google Scholar] [CrossRef]
- Schmidt, U.T.; Vohringer, O.; Lohe, D. The Creep Damage Behavior of the Plasma-Sprayed Thermal Barrier Coating System NiCr22Co12Mo9-NiCoCrAlY-ZrO2/7%Y2O3. J. Eng. Gas Turbines Power 1999, 121, 678–682. [Google Scholar] [CrossRef]
- Yang, L.; Zou, Z.; Kou, Z.; Chen, Y.; Zhao, G.; Zhao, X.; Guo, F.; Xiao, P. High temperature stress and its influence on surface rumpling in NiCoCrAlY bond coat. Acta Mater. 2017, 139, 122–137. [Google Scholar] [CrossRef]
- Xu, B.Q.; Luo, L.R.; Lu, J.; Zhao, X.F.; Xiao, P. Effect of residual stress on the spallation of the thermally-grown oxide formed on NiCoCrAlY coating. Surf. Coat. Technol. 2020, 381, 125112. [Google Scholar] [CrossRef]
- Panat, R.; Zhang, S.; Hsia, K.J. Bond coat surface rumpling in thermal barrier coatings. Acta Mater. 2003, 51, 239–249. [Google Scholar] [CrossRef]
- Mahfouz, L.; Marchand, B.; Guipont, V.; Coudon, F.; Maurel, V. Driving forces in thermal barrier coatings blistering. Materialia 2023, 28, 101728. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Bai, M.; Yang, L.; Li, C.; Wang, L.; Carr, J.A.; Xiao, P. A mechanistic understanding on rumpling of a NiCoCrAlY bond coat for thermal barrier coating applications. Acta Mater. 2017, 128, 31–42. [Google Scholar] [CrossRef]




| Material | CTE (10−6 K−1) | Thermal Conductivity at 1200 °C (Wm−1K−1) |
|---|---|---|
| Lanthanum zirconate (La2Zr2O7, LZ) | 9.1 | 1.98 |
| Samarium Zirconate (Sm2Zr2O7, SZO) | 10.8 | 2.09 |
| Neodymium Zirconate (Nd2Zr2O7, NZO) | 9.5 | 1.83 |
| Gadolinium Zirconate (Gd2Zr2O7, GZO) | 11.6 | 1.91 |
| Compound | CTE (10−6 K−1) | Thermal Conductivity (Wm−1K−1) |
|---|---|---|
| RETaO4 | 5.2–10.7 | 1.27–7.92 |
| RE3TaO7 | 6.1–10.5 | 1.20–1.97 |
| RETa3O9 | 4.1–9.6 | 1.17–2.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdan, M.; Peter, I. A Comprehensive Understanding of Thermal Barrier Coatings (TBCs): Applications, Materials, Coating Design and Failure Mechanisms. Metals 2024, 14, 575. https://doi.org/10.3390/met14050575
Bogdan M, Peter I. A Comprehensive Understanding of Thermal Barrier Coatings (TBCs): Applications, Materials, Coating Design and Failure Mechanisms. Metals. 2024; 14(5):575. https://doi.org/10.3390/met14050575
Chicago/Turabian StyleBogdan, Maria, and Ildiko Peter. 2024. "A Comprehensive Understanding of Thermal Barrier Coatings (TBCs): Applications, Materials, Coating Design and Failure Mechanisms" Metals 14, no. 5: 575. https://doi.org/10.3390/met14050575
APA StyleBogdan, M., & Peter, I. (2024). A Comprehensive Understanding of Thermal Barrier Coatings (TBCs): Applications, Materials, Coating Design and Failure Mechanisms. Metals, 14(5), 575. https://doi.org/10.3390/met14050575









