Efficient Metal Extraction from Dilute Solutions: A Review of Novel Selective Separation Methods and Their Applications
Abstract
1. Introduction
2. Research Methodology
3. Conventional Methods
3.1. Chemical Precipitation
3.2. Surface Adsorption
3.3. Ion Exchange
4. Recently Developed Approaches
4.1. Immobilized Ligands
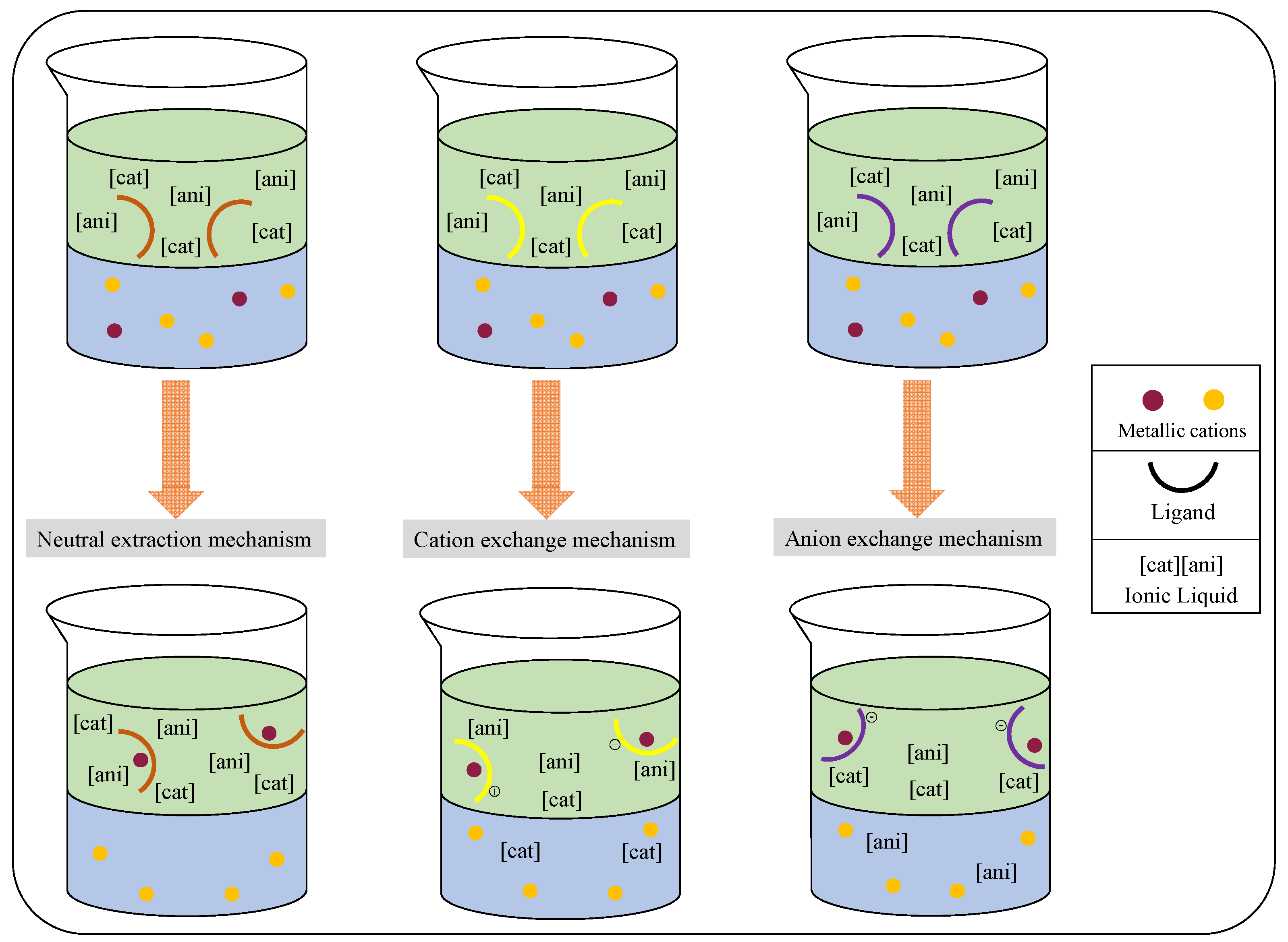
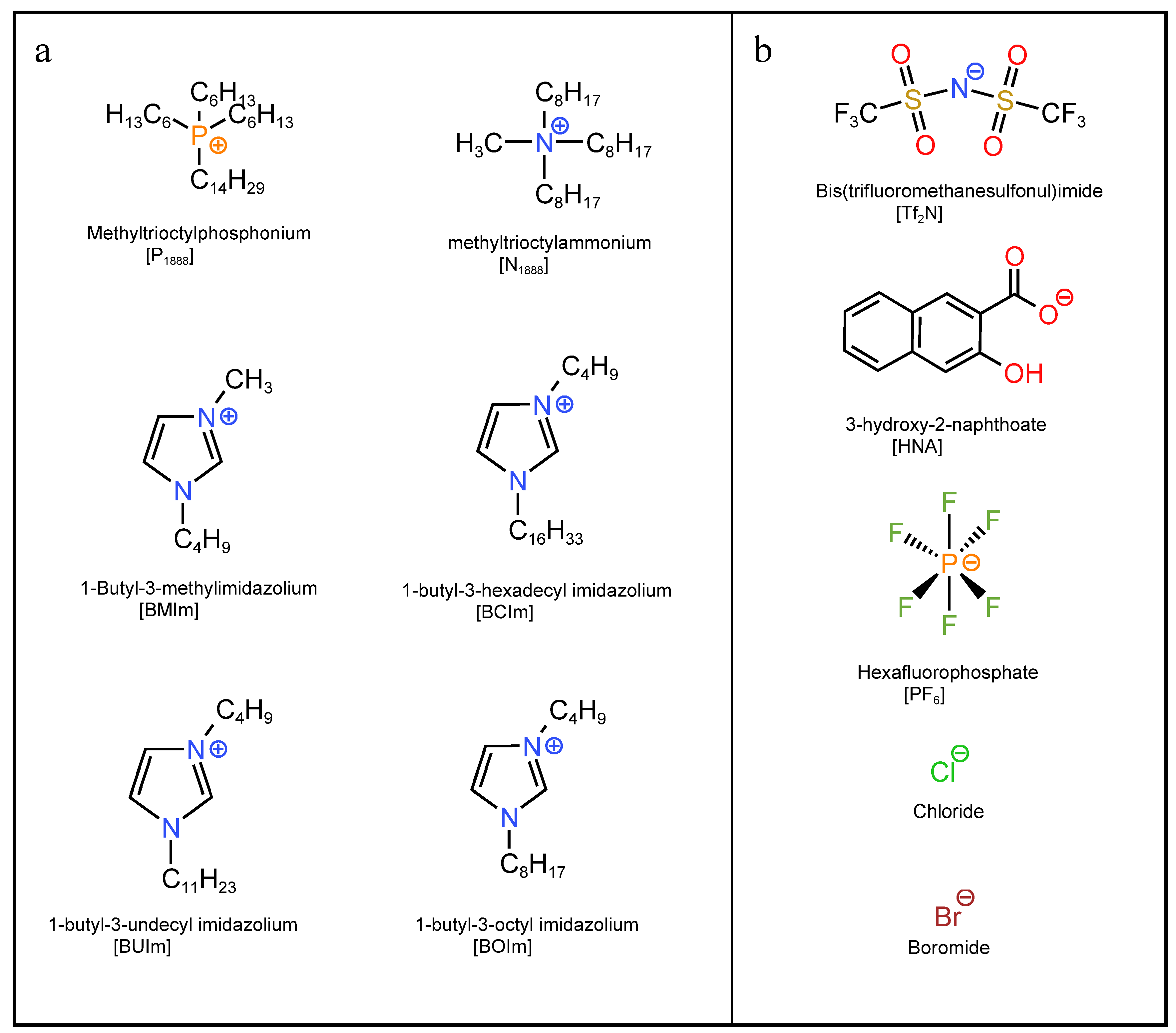
4.2. Ionic Liquid
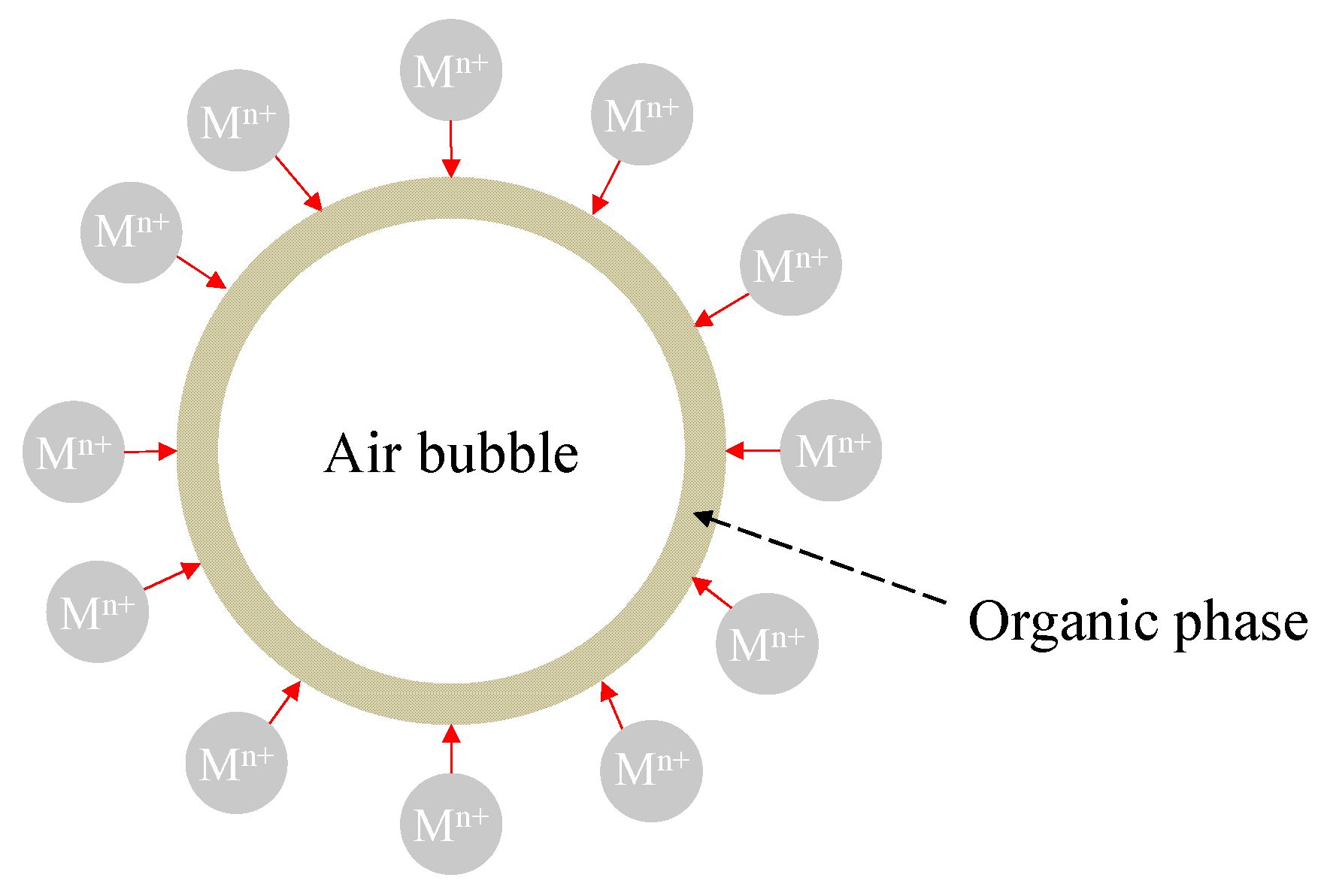
4.3. Solvent-Coated Bubbles
5. Summary and Future Research Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid mine drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Egiebor, N.O.; Oni, B. Acid rock drainage formation and treatment: A review. Asia-Pac. J. Chem. Eng. 2007, 2, 47–62. [Google Scholar] [CrossRef]
- Parbhakar-Fox, A.; Lottermoser, B.G. A critical review of acid rock drainage prediction methods and practices. Miner. Eng. 2015, 82, 107–124. [Google Scholar] [CrossRef]
- Han, G.; Wang, Y.; Liu, B.; Huang, Y.; Su, S.; Sun, H.; Yang, S. Rhenium extraction from dilute solution by precipitation flotation and oxidative volatilization techniques. J. Environ. Chem. Eng. 2023, 11, 111457. [Google Scholar] [CrossRef]
- Lightfoot, E.; Cockrem, M. What are dilute solutions? Sep. Sci. Technol. 1987, 22, 165–189. [Google Scholar] [CrossRef]
- Xanthopoulos, P. Recovery of metal ions from dilute aqueous solutions by ion flotation. In Proceedings of the (Re)Mining Extractive Waste, a New Business? Mechelen, Belgium, 17–18 May 2022. [Google Scholar]
- Yudaev, P.; Chistyakov, E. Chelating Extractants for Metals. Metals 2022, 12, 1275. [Google Scholar] [CrossRef]
- Masindi, V.; Foteinis, S.; Renforth, P.; Ndiritu, J.; Maree, J.P.; Tekere, M.; Chatzisymeon, E. Challenges and avenues for acid mine drainage treatment, beneficiation, and valorisation in circular economy: A review. Ecol. Eng. 2022, 183, 106740. [Google Scholar] [CrossRef]
- Aghaei, E.; Wang, Z.; Tadesse, B.; Tabelin, C.B.; Quadir, Z.; Alorro, R.D. Performance Evaluation of Fe-Al Bimetallic Particles for the Removal of Potentially Toxic Elements from Combined Acid Mine Drainage-Effluents from Refractory Gold Ore Processing. Minerals 2021, 11, 590. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, C.; Su, P.; Tang, Y.; Huang, Z.; Ma, T. A review of acid mine drainage: Formation mechanism, treatment technology, typical engineering cases and resource utilization. Process Saf. Environ. Prot. 2023, 170, 1240–1260. [Google Scholar] [CrossRef]
- Fan, R.; Qian, G.; Li, Y.; Short, M.D.; Schumann, R.C.; Chen, M.; Smart, R.S.C.; Gerson, A.R. Evolution of pyrite oxidation from a 10-year kinetic leach study: Implications for secondary mineralisation in acid mine drainage control. Chem. Geol. 2022, 588, 120653. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. The microbiology of acidic mine waters. Res. Microbiol. 2003, 154, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Khorasanipour, M.; Tangestani, M.H.; Naseh, R.; Hajmohammadi, H. Hydrochemistry, mineralogy and chemical fractionation of mine and processing wastes associated with porphyry copper mines: A case study from the Sarcheshmeh mine, SE Iran. Appl. Geochem. 2011, 26, 714–730. [Google Scholar] [CrossRef]
- León-Venegas, E.; Vilches-Arenas, L.F.; Fernández-Baco, C.; Arroyo-Torralvo, F. Potential for water and metal recovery from acid mine drainage by combining hybrid membrane processes with selective metal precipitation. Resour. Conserv. Recycl. 2023, 188, 106629. [Google Scholar] [CrossRef]
- Jergensen, G.V. Copper Leaching, Solvent Extraction, and Electrowinning Technology; SME: Southfield, MI, USA, 1999. [Google Scholar]
- Ihsanullah; Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Liu, Y.; Ke, X.; Zhu, H.; Chen, R.; Chen, X.; Zheng, X.; Jin, Y.; Van der Bruggen, B. Treatment of raffinate generated via copper ore hydrometallurgical processing using a bipolar membrane electrodialysis system. Chem. Eng. J. 2020, 382, 122956. [Google Scholar] [CrossRef]
- Arroyo Torralvo, F.; Alvarez-Martin, F.; Moreno Bermejo, N.; Luna Galiano, Y.; Leiva, C.; Vilches, L.F. Effluent valorization in copper hydrometallurgy plant. Int. J. Miner. Process. 2017, 169, 70–78. [Google Scholar] [CrossRef]
- Ruan, R.; Zou, G.; Zhong, S.; Wu, Z.; Chan, B.; Wang, D. Why Zijinshan copper bioheapleaching plant works efficiently at low microbial activity—Study on leaching kinetics of copper sulfides and its implications. Miner. Eng. 2013, 48, 36–43. [Google Scholar] [CrossRef]
- Rezazadeh, L.; Sharafi, S.; Schaffie, M.; Ranjbar, M. Synthesis and characterization of magnetic nanoparticles from raffinate of industrial copper solvent extraction plants. Mater. Chem. Phys. 2019, 229, 372–379. [Google Scholar] [CrossRef]
- Akhgar, B.N.; Kavanlouei, M.; Kazemi, H.; Ghavidel, S.; Dardman, S. Effect of synthesis routes on the microstructural properties of nano zero-valent metals of Fe and Cu synthesized from the industrial copper solvent extraction process. Mater. Chem. Phys. 2023, 302, 127704. [Google Scholar] [CrossRef]
- Cuevas, F.; Murray, C.; Platzer, W.; Heimsath, A. Large scale solar plants integration in electro-winning copper recuperation process. Energy Procedia 2015, 70, 605–614. [Google Scholar] [CrossRef]
- Habashi, F. A short history of hydrometallurgy. Hydrometallurgy 2005, 79, 15–22. [Google Scholar] [CrossRef]
- Kentish, S.E.; Stevens, G.W. Innovations in separations technology for the recycling and re-use of liquid waste streams. Chem. Eng. J. 2001, 84, 149–159. [Google Scholar] [CrossRef]
- El Ouardi, Y.; Virolainen, S.; Massima Mouele, E.S.; Laatikainen, M.; Repo, E.; Laatikainen, K. The recent progress of ion exchange for the separation of rare earths from secondary resources—A review. Hydrometallurgy 2023, 218, 106047. [Google Scholar] [CrossRef]
- Han, G.; Wang, J.; Sun, H.; Liu, B.; Huang, Y. A Critical Review on the Removal and Recovery of Hazardous Cd from Cd-Containing Secondary Resources in Cu-Pb-Zn Smelting Processes. Metals 2022, 12, 1846. [Google Scholar] [CrossRef]
- Estay, H.; Barros, L.; Troncoso, E. Metal Sulfide Precipitation: Recent Breakthroughs and Future Outlooks. Minerals 2021, 11, 1385. [Google Scholar] [CrossRef]
- Fei, Y.; Hu, Y.H. Recent progress in removal of heavy metals from wastewater: A comprehensive review. Chemosphere 2023, 335, 139077. [Google Scholar] [CrossRef]
- Cerrillo-Gonzalez, M.d.M.; Villen-Guzman, M.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Metal Recovery from Wastewater Using Electrodialysis Separation. Metals 2024, 14, 38. [Google Scholar] [CrossRef]
- Tripathy, D.B.; Gupta, A. Nanomembranes-Affiliated Water Remediation: Chronology, Properties, Classification, Challenges and Future Prospects. Membranes 2023, 13, 713. [Google Scholar] [CrossRef]
- Samavati, Z.; Samavati, A.; Goh, P.S.; Ismail, A.F.; Abdullah, M.S. A comprehensive review of recent advances in nanofiltration membranes for heavy metal removal from wastewater. Chem. Eng. Res. Des. 2023, 189, 530–571. [Google Scholar] [CrossRef]
- Birniwa, A.H.; Habibu, S.; Abdullahi, S.S.a.; Mohammad, R.E.A.; Hussaini, A.; Magaji, H.; Al-dhawi, B.N.S.; Noor, A.; Jagaba, A.H. Membrane technologies for heavy metals removal from water and wastewater: A mini review. Case Stud. Chem. Environ. Eng. 2023, 9, 100538. [Google Scholar] [CrossRef]
- León, L.; León, G.; Senent, J.; Guzmán, M.A. Kinetic Study of Copper(II) Simultaneous Extraction/Stripping from Aqueous Solutions by Bulk Liquid Membranes Using Coupled Transport Mechanisms. Metals 2016, 6, 212. [Google Scholar] [CrossRef]
- Zawierucha, I.; Nowik-Zajac, A.; Malina, G. Selective Removal of As(V) Ions from Acid Mine Drainage Using Polymer Inclusion Membranes. Minerals 2020, 10, 909. [Google Scholar] [CrossRef]
- Kumkum, P.; Kumar, S. A Review on Biochar as an Adsorbent for Pb(II) Removal from Water. Biomass 2024, 4, 243–272. [Google Scholar] [CrossRef]
- Yefremova, S.; Kablanbekov, A.; Satbaev, B.; Zharmenov, A. Rice Husk-Based Adsorbents for Removal of Metals from Aqueous Solutions. Materials 2023, 16, 7353. [Google Scholar] [CrossRef]
- Mahlangu, D.; Mphahlele, K.; De Paola, F.; Mthombeni, N.H. Microalgae-Mediated Biosorption for Effective Heavy Metals Removal from Wastewater: A Review. Water 2024, 16, 718. [Google Scholar] [CrossRef]
- Alguacil, F.J.; López, F.A. Adsorption Processing for the Removal of Toxic Hg(II) from Liquid Effluents: Advances in the 2019 Year. Metals 2020, 10, 412. [Google Scholar] [CrossRef]
- Wang, L.; Hu, G.; Lyu, F.; Yue, T.; Tang, H.; Han, H.; Yang, Y.; Liu, R.; Sun, W. Application of Red Mud in Wastewater Treatment. Minerals 2019, 9, 281. [Google Scholar] [CrossRef]
- Villen-Guzman, M.; Jiménez, C.; Rodriguez-Maroto, J.M. Batch and Fixed-Bed Biosorption of Pb (II) Using Free and Alginate-Immobilized Spirulina. Processes 2021, 9, 466. [Google Scholar] [CrossRef]
- Zawierucha, I.; Nowik-Zajac, A.; Girek, T.; Lagiewka, J.; Ciesielski, W.; Pawlowska, B.; Biczak, R. Arsenic(V) Removal from Water by Resin Impregnated with Cyclodextrin Ligand. Processes 2022, 10, 253. [Google Scholar] [CrossRef]
- Juang, R.-S.; Huang, W.-Z.; Fu, C.-C. Separation of heavy metals from dilute solutions using D2EHPA carrier fixed onto the outer surface of coaxially electrospun core-sheath fibers. Sep. Purif. Technol. 2024, 348, 127735. [Google Scholar] [CrossRef]
- Rahmati, S.; Ahmadi, A.; Hosseini, M.R.; Nasab, M.M. Effect of liquid and air flowrates on the efficiency of a two-stage air-assisted solvent extraction system in pilot scale. Miner. Eng. 2020, 153, 106376. [Google Scholar] [CrossRef]
- Finch, J.; How Kuan, S. Air-assisted solvent extraction: Scale-up attempt with the Jameson downcomer. In Proceedings of the Procemin 2013, Santiago, Chile, 15–18 October 2013. [Google Scholar]
- Tarkan, H.; Finch, J. Air-assisted solvent extraction: Towards a novel extraction process. Miner. Eng. 2005, 18, 83–88. [Google Scholar] [CrossRef]
- Goutham, R.; Rohit, P.; Vigneshwar, S.S.; Swetha, A.; Arun, J.; Gopinath, K.P.; Pugazhendhi, A. Ionic liquids in wastewater treatment: A review on pollutant removal and degradation, recovery of ionic liquids, economics and future perspectives. J. Mol. Liq. 2022, 349, 118150. [Google Scholar] [CrossRef]
- Cholico-Gonzalez, D.; Chagnes, A.; Cote, G.; Avila-Rodriguez, M. Separation of Co (II) and Ni (II) from aqueous solutions by bis (2, 4, 4-trimethylpentyl) phosphinic acid (Cyanex 272) using trihexyl (tetradecyl) phosphonium chloride (Cyphos IL 101) as solvent. J. Mol. Liq. 2015, 209, 203–208. [Google Scholar] [CrossRef]
- Deng, H.; Liu, C.; Xu, X.; Wu, Y.; Chen, M.; Huang, Z. Separation of palladium from alkaline cyanide solutions through microemulsion extraction using imidazolium ionic liquids. Int. J. Mol. Sci. 2023, 24, 10709. [Google Scholar] [CrossRef]
- El-Ashgar, N.M. Column extraction and separation of some metal ions by diethylenetriamine polysiloxane immobilized ligand system. J. Chem. 2008, 5, 107–113. [Google Scholar] [CrossRef]
- Awual, M.R.; Yaita, T. Rapid sensing and recovery of palladium(II) using N,N-bis(salicylidene)1,2-bis(2-aminophenylthio)ethane modified sensor ensemble adsorbent. Sens. Actuators B Chem. 2013, 183, 332–341. [Google Scholar] [CrossRef]
- El-Ashgar, N.M.; El-Nahhal, I.M.; Chehimi, M.M.; Delamar, M.; Babonneau, F.; Livage, J. Extraction of metal ions (Fe3+, Co2+, Ni2+, Cu2+ and Zn2+) using immobilized-polysiloxane iminobis(n-2-aminophenylacetamide) ligand system. J. Sol-Gel Sci. Technol. 2007, 41, 3–10. [Google Scholar] [CrossRef]
- Jia, L.-p.; Huang, J.-j.; Ma, Z.-l.; Liu, X.-h.; Chen, X.-y.; Li, J.-t.; He, L.-h.; Zhao, Z.-w. Research and development trends of hydrometallurgy: An overview based on Hydrometallurgy literature from 1975 to 2019. Trans. Nonferrous Met. Soc. China 2020, 30, 3147–3160. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, B.; Han, G.; Wang, M.; Huang, Y.; Su, S.; Xue, Y.; Wang, Y. Clean separation and purification for strategic metals of molybdenum and rhenium from minerals and waste alloy scraps–A review. Resour. Conserv. Recycl. 2022, 181, 106232. [Google Scholar] [CrossRef]
- Krishnan, S.; Zulkapli, N.S.; Kamyab, H.; Taib, S.M.; Din, M.F.B.M.; Majid, Z.A.; Chaiprapat, S.; Kenzo, I.; Ichikawa, Y.; Nasrullah, M.; et al. Current technologies for recovery of metals from industrial wastes: An overview. Environ. Technol. Innov. 2021, 22, 101525. [Google Scholar] [CrossRef]
- Opare, E.O.; Struhs, E.; Mirkouei, A. A comparative state-of-technology review and future directions for rare earth element separation. Renew. Sustain. Energy Rev. 2021, 143, 110917. [Google Scholar] [CrossRef]
- BrbootI, M.M.; Abid, B.A.; Al-ShuwaikI, N.M. Removal of heavy metals using chemicals precipitation. Eng. Technol. J. 2011, 29, 595–612. [Google Scholar]
- Thevarajah, B.; Nishshanka, G.K.S.H.; Premaratne, M.; Wasath, W.A.J.; Nimarshana, P.H.V.; Malik, A.; Ariyadasa, T.U. Cyanobacterial pigment production in wastewaters treated for heavy metal removal: Current status and perspectives. J. Environ. Chem. Eng. 2023, 11, 108999. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, A.; Panesar, P.S. A review on the industrial wastewater with the efficient treatment techniques. Chem. Pap. 2023, 77, 4131–4163. [Google Scholar] [CrossRef]
- Lejwoda, P.; Świnder, H.; Thomas, M. Evaluation of the stability of heavy metal-containing sediments obtained in the wastewater treatment processes with the use of various precipitating agents. Environ. Monit. Assess. 2023, 195, 442. [Google Scholar] [CrossRef]
- Benalia, M.C.; Youcef, L.; Bouaziz, M.G.; Achour, S.; Menasra, H. Removal of heavy metals from industrial wastewater by chemical precipitation: Mechanisms and sludge characterization. Arab. J. Sci. Eng. 2022, 47, 5587–5599. [Google Scholar] [CrossRef]
- Trus, I.; Halysh, V.; Gomelya, M.; Benatov, D.; Ivanchenko, A. Techno-economic feasibility for water purification from copper ions. Ecol. Eng. Environ. Technol. 2021, 22, 27–34. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I. Chemical treatment for removal of heavy metals from industrial wastewater. Egypt. J. Chem. 2015, 58, 1–12. [Google Scholar]
- Ain Zainuddin, N.; Azwan Raja Mamat, T.; Imam Maarof, H.; Wahidah Puasa, S.; Rohana Mohd Yatim, S. Removal of nickel, zinc and copper from plating process industrial raw effluent via hydroxide precipitation versus sulphide precipitation. In Proceedings of the IOP Conference Series: Materials Science and Engineering, 2019; IOP Publishing: Bristol, UK, 2019; p. 012122. [Google Scholar]
- Ayres, D.M.; Davis, A.P.; Gietka, P.M. Removing heavy metals from wastewater. Eng. Res. Cent. Rep. 1994, 90, 1–21. [Google Scholar]
- Zhang, X.; Zeng, L.; Wang, Y.; Tian, J.; Wang, J.; Sun, W.; Han, H.; Yang, Y. Selective separation of metals from wastewater using sulfide precipitation: A critical review in agents, operational factors and particle aggregation. J. Environ. Manag. 2023, 344, 118462. [Google Scholar] [CrossRef]
- KARTAL, L.; ŞEN, A.; TIMUR, S. The removal of heavy metals from refinery effluents by sulfide precipitation. Acta Montan. Slovaca 2023, 28, 214–225. [Google Scholar]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Yatim, S.R.M.; Kasmuri, S.N.H.; Syahjidan, H.N.; Mokhtar, N.S.; Zainuddin, N.A. Removing copper, chromium and nickel in industrial effluent using hydroxide precipitation versus sulphide precipitation. Heal. Off. Res. Book Fac. Health Sci. UiTM 2020, 3, 54–60. [Google Scholar]
- Kim, B.; Gaines, W.; Szafranski, M.; Bernath, E.; Miles, A. Removal of heavy metals from automotive wastewater by sulfide precipitation. J. Environ. Eng. 2002, 128, 612–623. [Google Scholar] [CrossRef]
- Kumar, M.; Nandi, M.; Pakshirajan, K. Recent advances in heavy metal recovery from wastewater by biogenic sulfide precipitation. J. Environ. Manag. 2021, 278, 111555. [Google Scholar] [CrossRef]
- Prokkola, H.; Nurmesniemi, E.-T.; Lassi, U. Removal of Metals by Sulphide Precipitation Using Na2S and HS−-Solution. ChemEngineering 2020, 4, 51. [Google Scholar] [CrossRef]
- Oh, M.; Lee, K.; Jeon, M.K.; Foster, R.I.; Lee, C.-H. Chemical precipitation–based treatment of acidic wastewater generated by chemical decontamination of radioactive concrete. J. Environ. Chem. Eng. 2023, 11, 110306. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Kavand, M.; Eslami, P.; Razeh, L. The adsorption of cadmium and lead ions from the synthesis wastewater with the activated carbon: Optimization of the single and binary systems. J. Water Process Eng. 2020, 34, 101151. [Google Scholar] [CrossRef]
- Eletta, O.A.A.; Ayandele, F.O.; Ighalo, J.O. Adsorption of Pb(II) and Fe(II) by mesoporous composite activated carbon from Tithonia diversifolia stalk and Theobroma cacao pod. Biomass Convers. Biorefinery 2023, 13, 9831–9840. [Google Scholar] [CrossRef]
- Raninga, M.; Mudgal, A.; Patel, V.K.; Patel, J.; Kumar Sinha, M. Modification of activated carbon-based adsorbent for removal of industrial dyes and heavy metals: A review. Mater. Today Proc. 2023, 77, 286–294. [Google Scholar] [CrossRef]
- Wang, B.; Lan, J.; Bo, C.; Gong, B.; Ou, J. Adsorption of heavy metal onto biomass-derived activated carbon. RSC Adv. 2023, 13, 4275–4302. [Google Scholar] [CrossRef]
- Fahim Chyad, T.; Fahim Chyad Al-Hamadani, R.; Ageel Hammood, Z.; Abd Ali, G. Removal of Zinc (II) ions from industrial wastewater by adsorption on to activated carbon produced from pine cone. Mater. Today Proc. 2023, 80, 2706–2711. [Google Scholar] [CrossRef]
- Song, X.; Gunawan, P.; Jiang, R.; Leong, S.S.J.; Wang, K.; Xu, R. Surface activated carbon nanospheres for fast adsorption of silver ions from aqueous solutions. J. Hazard. Mater. 2011, 194, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Marthi, R.; Twinney, J.; Ghahreman, A. A review on adsorption mechanism of gold cyanide complex onto activation carbon. J. Ind. Eng. Chem. 2022, 111, 35–42. [Google Scholar] [CrossRef]
- Xia, J.; Mahandra, H.; Ghahreman, A. Efficient gold recovery from cyanide solution using magnetic activated carbon. ACS Appl. Mater. Interfaces 2021, 13, 47642–47649. [Google Scholar] [CrossRef]
- Martínez-Peñuñuri, R.; Parga-Torres, J.R.; Valenzuela-García, J.L.; Díaz-Galaviz, H.J.; González-Zamarripa, G.; García-Alegría, A.M. Thermodynamic and Kinetic Aspects of Gold Adsorption in Micrometric Activated Carbon and the Impact of Their Loss in Adsorption, Desorption, and Reactivation Plants. Materials 2023, 16, 4961. [Google Scholar] [CrossRef]
- Firmansyah, M.L.; Rizki, I.N.; Amalina, I.; Abdul Jalil, A.; Ullah, N. Recovery of precious metals from mobile phone waste: Studies on leaching and adsorption by functionalized activated carbon. Results Eng. 2024, 22, 102011. [Google Scholar] [CrossRef]
- Zounia, M.; Yazdi, M.R.S.; Hakimi, M.; Zare, H.; Amiri, A. Silver adsorption from gold leaching cyanide wastewater by a green functionalized magnetic graphene oxide Nano-adsorbent. Hydrometallurgy 2024, 225, 106265. [Google Scholar] [CrossRef]
- Zietzschmann, F.; Stützer, C.; Jekel, M. Granular activated carbon adsorption of organic micro-pollutants in drinking water and treated wastewater—Aligning breakthrough curves and capacities. Water Res. 2016, 92, 180–187. [Google Scholar] [CrossRef]
- Orha, C.; Pode, R.; Manea, F.; Lazau, C.; Bandas, C. Titanium dioxide-modified activated carbon for advanced drinking water treatment. Process Saf. Environ. Prot. 2017, 108, 26–33. [Google Scholar] [CrossRef]
- Fu, H.; Li, X.; Wang, J.; Lin, P.; Chen, C.; Zhang, X.; Suffet, I.H. Activated carbon adsorption of quinolone antibiotics in water: Performance, mechanism, and modeling. J. Environ. Sci. 2017, 56, 145–152. [Google Scholar] [CrossRef]
- Anirudhan, T.; Sreekumari, S. Adsorptive removal of heavy metal ions from industrial effluents using activated carbon derived from waste coconut buttons. J. Environ. Sci. 2011, 23, 1989–1998. [Google Scholar] [CrossRef]
- Kamboj, V.; Tiwari, D. Removal of heavy metal (Cu, Cr, and Ni) ions from aqueous solution using derived activated carbon from water hyacinth. Biomass Convers. Biorefinery 2024, 14, 5075–5083. [Google Scholar] [CrossRef]
- Alkherraz, A.M.; Ali, A.K.; Elsherif, K.M. Removal of Pb (II), Zn (II), Cu (II) and Cd (II) from aqueous solutions by adsorption onto olive branches activated carbon: Equilibrium and thermodynamic studies. Chem. Int. 2020, 6, 11–20. [Google Scholar]
- Zhang, Z.; Wang, T.; Zhang, H.; Liu, Y.; Xing, B. Adsorption of Pb(II) and Cd(II) by magnetic activated carbon and its mechanism. Sci. Total Environ. 2021, 757, 143910. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, J.I.; Cortés, S.; Maluenda, P.; Soto, I. Biosorption of Heavy Metals with Algae: Critical Review of Its Application in Real Effluents. Sustainability 2023, 15, 5521. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Farjadfard, S.; Esmaeili, H.; Saberi, M.; Sahebi, S.; Dobaradaran, S.; Ramavandi, B. Characteristics and performance of Cd, Ni, and Pb bio-adsorption using Callinectes sapidus biomass: Real wastewater treatment. Environ. Sci. Pollut. Res. 2019, 26, 6336–6347. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.R. Bio-sorption of toxic metals from industrial wastewater by algae strains Spirulina platensis and Chlorella vulgaris: Application of isotherm, kinetic models and process optimization. Sci. Total Environ. 2021, 755, 142654. [Google Scholar] [CrossRef]
- Senthilkumaar, S.; Bharathi, S.; Nithyanandhi, D.; Subburam, V. Biosorption of toxic heavy metals from aqueous solutions. Bioresour. Technol. 2000, 75, 163–165. [Google Scholar] [CrossRef]
- Samarth, D.P.; Chandekar, C.J.; Bhadekar, R.K. Biosorption of heavy metals from aqueous solution using Bacillus licheniformis. Int. J. Pure Appl. Sci. Technol. 2012, 10, 12. [Google Scholar]
- Li, W.; Mu, B.; Yang, Y. Feasibility of industrial-scale treatment of dye wastewater via bio-adsorption technology. Bioresour. Technol. 2019, 277, 157–170. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.-L.; Akhtar, F. Adsorption of heavy metals on natural zeolites: A review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- Natarajan, B.; Kannan, P.; Rather, J.A.; Sheikh, R.A. Recent developments in metal nanoparticles functionalized nanocomposite adsorbents for heavy metals removal from wastewaters. J. Taiwan Inst. Chem. Eng. 2023, 147, 104942. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Ayalew, A.A. Chapter 10—Application of metal-based nanoparticles for metal removal for treatments of wastewater—A review. In Emerging Techniques for Treatment of Toxic Metals from Wastewater; Ahmad, A., Kumar, R., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 183–231. [Google Scholar]
- Varvara, S.; Popa, M.; Bostan, R.; Damian, G. Preliminary considerations on the adsorption of heavy metals from acidic mine drainage using natural zeolite. J. Environ. Prot. Ecol. 2013, 14, 1506–1514. [Google Scholar]
- Gaikwad, R.W.; Sonawane, A.V.; Hakke, V.S.; Sonawane, S.H.; Gaikwad, M.S.; Lakhera, S.K.; Babu, G.V.; Warade, A.R.; Urgunde, A.B.; Sapkal, V.S. Application of apophyllite and thomsonite natural zeolite as modified adsorbents for the removal of zinc from acid mine drainage. Chemosphere 2024, 350, 141095. [Google Scholar] [CrossRef]
- Thacker, B.; Vadodaria, G.; Priyadarshi, G.; Trivedi, M. Biopolymer-based fly ash-activated zeolite for the removal of chromium from acid mine drainage. Sci. Temper 2023, 14, 1217–1226. [Google Scholar] [CrossRef]
- Ferrel-Luna, R.; García-Arreola, M.E.; González-Rodríguez, L.M.; Loredo-Cancino, M.; Escárcega-González, C.E.; De Haro-Del Río, D.A. Reducing toxic element leaching in mine tailings with natural zeolite clinoptilolite. Environ. Sci. Pollut. Res. 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Elboughdiri, N. The use of natural zeolite to remove heavy metals Cu (II), Pb (II) and Cd (II), from industrial wastewater. Cogent Eng. 2020, 7, 1782623. [Google Scholar] [CrossRef]
- Zanin, E.; Scapinello, J.; de Oliveira, M.; Rambo, C.L.; Franscescon, F.; Freitas, L.; de Mello, J.M.M.; Fiori, M.A.; Oliveira, J.V.; Dal Magro, J. Adsorption of heavy metals from wastewater graphic industry using clinoptilolite zeolite as adsorbent. Process Saf. Environ. Prot. 2017, 105, 194–200. [Google Scholar] [CrossRef]
- Hernández-Montoya, V.; Pérez-Cruz, M.A.; Mendoza-Castillo, D.I.; Moreno-Virgen, M.R.; Bonilla-Petriciolet, A. Competitive adsorption of dyes and heavy metals on zeolitic structures. J. Environ. Manag. 2013, 116, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Ok, Y.S.; Yang, J.E.; Zhang, Y.-S.; Kim, S.-J.; Chung, D.-Y. Heavy metal adsorption by a formulated zeolite-Portland cement mixture. J. Hazard. Mater. 2007, 147, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Motsi, T.; Rowson, N.A.; Simmons, M.J.H. Adsorption of heavy metals from acid mine drainage by natural zeolite. Int. J. Miner. Process. 2009, 92, 42–48. [Google Scholar] [CrossRef]
- Daneshvar, M.; Hosseini, M.R. Kinetics, isotherm, and optimization of the hexavalent chromium removal from aqueous solution by a magnetic nanobiosorbent. Environ. Sci. Pollut. Res. 2018, 25, 28654–28666. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.K.; Ali, D.; Khan, S.H.; Gnanamoorthy, G.; Choudhary, N.; Yadav, K.K.; Thai, V.N.; Hussain, S.A.; Manhrdas, S. Synthesis and Characterization of Amorphous Iron Oxide Nanoparticles by the Sonochemical Method and Their Application for the Remediation of Heavy Metals from Wastewater. Nanomaterials 2020, 10, 1551. [Google Scholar] [CrossRef] [PubMed]
- Almomani, F.; Bhosale, R.; Khraisheh, M.; Kumar, A.; Almomani, T. Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP). Appl. Surf. Sci. 2020, 506, 144924. [Google Scholar] [CrossRef]
- Jabbar, K.Q.; Barzinjy, A.A.; Hamad, S.M. Iron oxide nanoparticles: Preparation methods, functions, adsorption and coagulation/flocculation in wastewater treatment. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100661. [Google Scholar] [CrossRef]
- Adeli, M.; Yamini, Y.; Faraji, M. Removal of copper, nickel and zinc by sodium dodecyl sulphate coated magnetite nanoparticles from water and wastewater samples. Arab. J. Chem. 2017, 10, S514–S521. [Google Scholar] [CrossRef]
- Baseri, H.; Tizro, S. Heavy metals removal from wastewater by using different kinds of magnetite nanoadsorbents: Effects of different organic and inorganic coatings on the removal of copper and lead ions. J. Adv. Mater. Process. 2016, 4, 15–29. [Google Scholar]
- Karami, H. Heavy metal removal from water by magnetite nanorods. Chem. Eng. J. 2013, 219, 209–216. [Google Scholar] [CrossRef]
- Nikoloski, A.N.; Ang, K.-L. Review of the application of ion exchange resins for the recovery of platinum-group metals from hydrochloric acid solutions. Miner. Process. Extr. Metall. Rev. 2014, 35, 369–389. [Google Scholar] [CrossRef]
- Van Deventer, J. Selected ion exchange applications in the hydrometallurgical industry. Solvent Extr. Ion Exch. 2011, 29, 695–718. [Google Scholar] [CrossRef]
- Saleh, T.A.; Mustaqeem, M.; Khaled, M. Water treatment technologies in removing heavy metal ions from wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100617. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of heavy metals from industrial wastewaters: A review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Ergunova, O.; Ferreira, R.; Ignatenko, A.; Lizunkov, V.G.; Malushko, E. Use of ion exchange filters in wastewater treatment. In Proceedings of the European Proceedings of Social & Behavioural Sciences (EpSBS). Vol. 26: Responsible Research and Innovation (RRI 2016), Nicosia, Cyprus, 7–10 November 2017; Volume 262016, pp. 541–549. [Google Scholar]
- Kansara, N.; Bhati, L.; Narang, M.; Vaishnavi, R. Wastewater treatment by ion exchange method: A review of past and recent researches. ESAIJ (Environ. Sci. Indian J.) 2016, 12, 143–150. [Google Scholar]
- Swanckaert, B.; Geltmeyer, J.; Rabaey, K.; De Buysser, K.; Bonin, L.; De Clerck, K. A review on ion-exchange nanofiber membranes: Properties, structure and application in electrochemical (waste) water treatment. Sep. Purif. Technol. 2022, 287, 120529. [Google Scholar] [CrossRef]
- Danko, B.; Dybczyński, R.S.; Samczyński, Z.; Gajda, D.; Herdzik-Koniecko, I.; Zakrzewska-Kołtuniewicz, G.; Chajduk, E.; Kulisa, K. Ion exchange investigation for recovery of uranium from acidic pregnant leach solutions. Nukleonika 2017, 62, 213–221. [Google Scholar] [CrossRef]
- Ladeira, A.C.Q.; Morais, C.A. Uranium recovery from industrial effluent by ion exchange—Column experiments. Miner. Eng. 2005, 18, 1337–1340. [Google Scholar] [CrossRef]
- Lee, H.-K.; Park, W.; Chang, S.; Jeon, H.; Park, S. Uranium Recovery from Sulfate-Based Acidic Soil Washing Effluent Using Ion-Exchange Resins. Water Air Soil Pollut. 2022, 233, 453. [Google Scholar] [CrossRef]
- Amphlett, J.T.M.; Choi, S.; Parry, S.A.; Moon, E.M.; Sharrad, C.A.; Ogden, M.D. Insights on uranium uptake mechanisms by ion exchange resins with chelating functionalities: Chelation vs. anion exchange. Chem. Eng. J. 2020, 392, 123712. [Google Scholar] [CrossRef]
- Hubicki, Z.; Wawrzkiewicz, M.; Wołowicz, A. Application of ion exchange methods in recovery of Pd (II) ions—A review. Chem. Anal. (Wars.) 2008, 53, 759–784. [Google Scholar]
- Mpinga, C.N.; Eksteen, J.J.; Aldrich, C.; Dyer, L. A conceptual hybrid process flowsheet for platinum group metals (PGMs) recovery from a chromite-rich Cu-Ni PGM bearing ore in oxidized mineralization through a single-stage leach and adsorption onto ion exchange resin. Hydrometallurgy 2018, 178, 88–96. [Google Scholar] [CrossRef]
- Yahorava, V.; Kotze, M. Ion exchange technology for the efficient recovery of precious metals from waste and low-grade streams. J. S. Afr. Inst. Min. Metall. 2014, 114, 173–181. [Google Scholar]
- Choi, J.-W.; Song, M.-H.; Bediako, J.K.; Yun, Y.-S. Sequential recovery of gold and copper from bioleached wastewater using ion exchange resins. Environ. Pollut. 2020, 266, 115167. [Google Scholar] [CrossRef]
- Janin, A.; Coudert, L.; Mercier, G.; Blais, J.-F. Copper extraction and recovery from alkaline copper quaternary and copper azole treated wood using sulfuric acid leaching and ion exchange or electrodeposition. J. Clean. Prod. 2021, 279, 123687. [Google Scholar] [CrossRef]
- Yalçin, S.; Apak, R.; Hizal, J.; Afşar, H. RECOVERY OF COPPER (II) AND CHROMIUM (III,VI) FROM ELECTROPLATING-INDUSTRY WASTEWATER BY ION EXCHANGE. Sep. Sci. Technol. 2001, 36, 2181–2196. [Google Scholar] [CrossRef]
- Avdibegović, D.; Barbier, E.; Jaklič, B.; Škapin, S.D.; Spreitzer, M.; Binnemans, K. Removal of copper and iron from ethanolic solutions by an anion exchange resin and its implication to rare-earth magnet recycling. Chemosphere 2023, 330, 138603. [Google Scholar] [CrossRef]
- Elfeghe, S.; Anwar, S.; James, L.; Zhang, Y. Adsorption of Cu (II) ions from aqueous solutions using ion exchange resins with different functional groups. Can. J. Chem. Eng. 2023, 101, 2128–2138. [Google Scholar] [CrossRef]
- Kononova, O.N.; Bryuzgina, G.L.; Apchitaeva, O.V.; Kononov, Y.S. Ion exchange recovery of chromium (VI) and manganese (II) from aqueous solutions. Arab. J. Chem. 2019, 12, 2713–2720. [Google Scholar] [CrossRef]
- Leonard, J.; Sivalingam, S.; Srinadh, R.V.; Mishra, S. Efficient removal of hexavalent chromium ions from simulated wastewater by functionalized anion exchange resin: Process optimization, isotherm and kinetic studies. Environ. Chem. Ecotoxicol. 2023, 5, 98–107. [Google Scholar] [CrossRef]
- Thiripelu, P.; Manjunathan, J.; Revathi, M.; Ramasamy, P. Removal of hexavalent chromium from electroplating wastewater by ion-exchange in presence of Ni(II) and Zn(II) ions. J. Water Process Eng. 2024, 58, 104815. [Google Scholar] [CrossRef]
- Bekchanov, D.; Mukhamediev, M.; Babojonova, G.; Lieberzeit, P.; Su, X. Anion exchange material based on polyvinylchloride and urea for the removal of chromium (vi) ions from aqueous solutions. CLEAN–Soil Air Water 2023, 51, 2200411. [Google Scholar] [CrossRef]
- Feng, D.; Aldrich, C.; Tan, H. Treatment of acid mine water by use of heavy metal precipitation and ion exchange. Miner. Eng. 2000, 13, 623–642. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Zorpas, A.A.; Loizidou, M.D.; Grigoropoulou, H.P. The effect of competitive cations and anions on ion exchange of heavy metals. Sep. Purif. Technol. 2005, 46, 202–207. [Google Scholar] [CrossRef]
- Agrawal, A.; Sahu, K. Influence of temperature on the exchange of alkaline earth and transition metals on iminodiacetate resin. Solvent Extr. Ion Exch. 2005, 23, 265–287. [Google Scholar] [CrossRef]
- Hubicki, Z.; Kołodyńska, D. Selective removal of heavy metal ions from waters and waste waters using ion exchange methods. Ion Exch. Technol. 2012, 7, 193–240. [Google Scholar]
- Juang, R.-S.; Lin, S.-H.; Wang, T.-Y. Removal of metal ions from the complexed solutions in fixed bed using a strong-acid ion exchange resin. Chemosphere 2003, 53, 1221–1228. [Google Scholar] [CrossRef]
- AlOthman, Z.A.; Alam, M.M.; Naushad, M. Heavy toxic metal ion exchange kinetics: Validation of ion exchange process on composite cation exchanger nylon 6,6 Zr(IV) phosphate. J. Ind. Eng. Chem. 2013, 19, 956–960. [Google Scholar] [CrossRef]
- Bilbao, L.; Ortueta, M.; Mijangos, F. Effect of concentration and temperature on mass transfer in metal ion exchange. Ind. Eng. Chem. Res. 2016, 55, 7287–7295. [Google Scholar] [CrossRef]
- Ariga, K.; Ito, H.; Hill, J.P.; Tsukube, H. Molecular recognition: From solution science to nano/materials technology. Chem. Soc. Rev. 2012, 41, 5800–5835. [Google Scholar] [CrossRef]
- James, L. Nobel Laureates in Chemistry, 1901–1992; Chemical Heritage Foundation: Philadelphia, PA, USA, 1993; Volume 1. [Google Scholar]
- Izatt, S.R.; Bruening, R.L.; Izatt, N.E.; Dale, J.B. The Application of Molecular Recognition Technology (MRT) in the Nuclear Power Cycle: From Uranium Mining and Refining to Power Plant Waste Separation and Recovery, as well as Element Analysis and Isotope Purification-9075. In Proceedings of the WM2009 Conference, Phoenix, AZ, USA, 1–5 March 2009. [Google Scholar]
- Bradshaw, J.S.; Izatt, R.M. Crown ethers: The search for selective ion ligating agents. Acc. Chem. Res. 1997, 30, 338–345. [Google Scholar] [CrossRef]
- Rahman, I.M.M.; Begum, Z.A.; Hasegawa, H. Selective separation of elements from complex solution matrix with molecular recognition plus macrocycles attached to a solid-phase: A review. Microchem. J. 2013, 110, 485–493. [Google Scholar] [CrossRef]
- Izatt, R.M.; Pawlak, K.; Bradshaw, J.S.; Bruening, R.L. Thermodynamic and kinetic data for macrocycle interactions with cations and anions. Chem. Rev. 1991, 91, 1721–2085. [Google Scholar] [CrossRef]
- Weber, E. Crown Ethers and Analogs; John Wiley & Sons: Hoboken, NJ, USA, 1989; Volume 76. [Google Scholar]
- Alexandratos, S.D.; Stine, C.L. Synthesis of ion-selective polymer-supported crown ethers: A review. React. Funct. Polym. 2004, 60, 3–16. [Google Scholar] [CrossRef]
- Gokel, G.W. Cation Binding by Crown Ethers. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Ilioudis, C.A.; Steed, J.W. Organic macrocyclic polyamine-based receptors for anions. J. Supramol. Chem. 2001, 1, 165–187. [Google Scholar] [CrossRef]
- Bazzicalupi, C.; Bencini, A.; Bianchi, A.; Danesi, A.; Faggi, E.; Giorgi, C.; Santarelli, S.; Valtancoli, B. Coordination properties of polyamine-macrocycles containing terpyridine units. Coord. Chem. Rev. 2008, 252, 1052–1068. [Google Scholar] [CrossRef]
- Guo, R.; McGrath, J.E. 5.17—Aromatic Polyethers, Polyetherketones, Polysulfides, and Polysulfones. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 377–430. [Google Scholar]
- Diehnelt, C.W.; Peterman, S.M.; Budde, W.L. Liquid chromatography–tandem mass spectrometry and accurate m/z measurements of cyclic peptide cyanobacteria toxins. TrAC Trends Anal. Chem. 2005, 24, 622–634. [Google Scholar] [CrossRef]
- Blackburn, C.; Kates, S.A. [9] Solid-phase synthesis of cyclic homodetic peptides. In Methods in Enzymology; Gregg, B.F., Ed.; Academic Press: Cambridge, MA, USA, 1997; Volume 289, pp. 175–198. [Google Scholar]
- Lécaillon, J.; Gilles, P.; Subra, G.; Martinez, J.; Amblard, M. Synthesis of cyclic peptides via O–N-acyl migration. Tetrahedron Lett. 2008, 49, 4674–4676. [Google Scholar] [CrossRef]
- Granata, G.; Consoli, G.M.L.; Sciuto, S.; Geraci, C. Polymer supported calixarene derivative useful for solid-phase synthesis application. Tetrahedron Lett. 2010, 51, 6139–6142. [Google Scholar] [CrossRef]
- Wieser, C.; Dieleman, C.B.; Matt, D. Calixarene and resorcinarene ligands in transition metal chemistry. Coord. Chem. Rev. 1997, 165, 93–161. [Google Scholar] [CrossRef]
- Morisaki, Y.; Chujo, Y. Cyclophane-containing polymers. Prog. Polym. Sci. 2008, 33, 346–364. [Google Scholar] [CrossRef]
- Hayashida, O.; Uchiyama, M. Cyclophane-based tetra(resorcinarene) as a host for both histone and hydrophobic molecular guests. Tetrahedron Lett. 2006, 47, 4091–4094. [Google Scholar] [CrossRef]
- Liu, M.; Li, L.-S.; Da, S.-L.; Feng, Y.-Q. High performance liquid chromatography with cyclodextrin and calixarene macrocycle bonded silica stationary phases for separation of steroids. Talanta 2005, 66, 479–486. [Google Scholar] [CrossRef]
- Takeda, Y. The solvent extraction of metal ions by crown compounds. In Host Guest Complex Chemistry III; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–38. [Google Scholar]
- Tsukube, H. Double armed crown ethers and armed macrocycles as a new series of metal-selective reagents: A review. Talanta 1993, 40, 1313–1324. [Google Scholar] [CrossRef]
- Rahman, I.M.; Furusho, Y.; Begum, Z.; Sabarudin, A.; Motomizu, S.; Maki, T.; Hasegawa, H. Selective separation of some ecotoxic transition metal ions from aqueous solutions using immobilized macrocyclic material containing solid phase extraction system. Cent. Eur. J. Chem. 2011, 9, 1019–1026. [Google Scholar] [CrossRef]
- Yamini, Y.; Alizadeh, N.; Shamsipur, M. Solid phase extraction and determination of ultra trace amounts of mercury(II) using octadecyl silica membrane disks modified by hexathia-18-crown-6-tetraone and cold vapour atomic absorption spectrometry. Anal. Chim. Acta 1997, 355, 69–74. [Google Scholar] [CrossRef]
- Izatt, S.; Bruening, R.; Izatt, N.; Dale, J. A review of the application of molecular recognition technology (MRT) for Ni/Cu/Co hydrometallurgical process separations and for the purification of cobalt streams. In Proceedings of the Base Metals Conference, Mowana Lodge, Kasane, Botswana, 27–31 July 2009. [Google Scholar]
- Izatt, R.M.; Izatt, S.R.; Izatt, N.E.; Bruening, R.L.; Krakowiak, K.E. Green chemistry molecular recognition processes applied to metal separations in ore beneficiation, element recycling, metal remediation, and elemental analysis. Tools Green Chem. 2017, 10, 10. [Google Scholar]
- Hojamberdiev, M.; Daminova, S.S.; Kadirova, Z.C.; Sharipov, K.T.; Mtalo, F.; Hasegawa, M. Ligand-immobilized spent alumina catalyst for effective removal of heavy metal ions from model contaminated water. J. Environ. Chem. Eng. 2018, 6, 4623–4633. [Google Scholar] [CrossRef]
- Salimi, M.; Behbahani, M.; Sobhi, H.R.; Ghambarian, M.; Esrafili, A. Trace measurement of lead and cadmium ions in wastewater samples using a novel dithizone immobilized metal–organic framework-based μ-dispersive solid-phase extraction. Appl. Organomet. Chem. 2020, 34, e5715. [Google Scholar] [CrossRef]
- Awual, M.R.; Khaleque, M.A.; Ferdows, M.; Chowdhury, A.M.S.; Yaita, T. Rapid recognition and recovery of gold(III) with functional ligand immobilized novel mesoporous adsorbent. Microchem. J. 2013, 110, 591–598. [Google Scholar] [CrossRef]
- Awual, M.R.; Ismael, M.; Yaita, T.; El-Safty, S.A.; Shiwaku, H.; Okamoto, Y.; Suzuki, S. Trace copper(II) ions detection and removal from water using novel ligand modified composite adsorbent. Chem. Eng. J. 2013, 222, 67–76. [Google Scholar] [CrossRef]
- Awual, M.R.; Yaita, T.; Shiwaku, H. Design a novel optical adsorbent for simultaneous ultra-trace cerium(III) detection, sorption and recovery. Chem. Eng. J. 2013, 228, 327–335. [Google Scholar] [CrossRef]
- Awual, M.R.; Kobayashi, T.; Miyazaki, Y.; Motokawa, R.; Shiwaku, H.; Suzuki, S.; Okamoto, Y.; Yaita, T. Selective lanthanide sorption and mechanism using novel hybrid Lewis base (N-methyl-N-phenyl-1,10-phenanthroline-2-carboxamide) ligand modified adsorbent. J. Hazard. Mater. 2013, 252–253, 313–320. [Google Scholar] [CrossRef]
- Awual, M.R.; Ismael, M.; Yaita, T. Efficient detection and extraction of cobalt(II) from lithium ion batteries and wastewater by novel composite adsorbent. Sens. Actuators B Chem. 2014, 191, 9–18. [Google Scholar] [CrossRef]
- Rahman, I.M.M.; Furusho, Y.; Begum, Z.A.; Izatt, N.; Bruening, R.; Sabarudin, A.; Hasegawa, H. Separation of lead from high matrix electroless nickel plating waste solution using an ion-selective immobilized macrocycle system. Microchem. J. 2011, 98, 103–108. [Google Scholar] [CrossRef]
- Izatt, R.M.; Izatt, S.R.; Izatt, N.E.; Krakowiak, K.E.; Bruening, R.L.; Navarro, L. Industrial applications of molecular recognition technology to separations of platinum group metals and selective removal of metal impurities from process streams. Green Chem. 2015, 17, 2236–2245. [Google Scholar] [CrossRef]
- Adavodi, R.; Dini, G. Benzotriazoium Bis (2-Ethylhexyl) Phosphate Ionic Liquid as a Catalyst and Multifunctional Lubricant Additive: Synthesis, Optimization, Characterization, and Tribological Evaluation. Arab. J. Sci. Eng. 2024, 1–16. [Google Scholar] [CrossRef]
- Soylak, M.; Jagirani, M.S. Ionic Liquids for Analysis of Heavy Metals in Waters. In Ionic Liquids for Environmental Issues; Royal Society of Chemistry: London, UK, 2023. [Google Scholar]
- Llaver, M.; Fiorentini, E.F.; Quintas, P.Y.; Oviedo, M.N.; Arenas, M.B.B.; Wuilloud, R.G. Task-specific ionic liquids: Applications in sample preparation and the chemistry behind their selectivity. Adv. Sample Prep. 2022, 1, 100004. [Google Scholar] [CrossRef]
- Yudaev, P.A.; Chistyakov, E.M. Ionic liquids as components of systems for metal extraction. ChemEngineering 2022, 6, 6. [Google Scholar] [CrossRef]
- Chen, R. Ionic liquids-mediated recovery of metals from spent batteries. J. Ion. Liq. 2023, 3, 100070. [Google Scholar] [CrossRef]
- Lee, J.-c.; Kurniawan, K.; Kim, S.; Nguyen, V.T.; Pandey, B.D. Ionic liquids-assisted solvent extraction of precious metals from chloride solutions. Sep. Purif. Rev. 2023, 52, 242–261. [Google Scholar] [CrossRef]
- Cai, C.; Hanada, T.; Fajar, A.T.; Goto, M. An ionic liquid extractant dissolved in an ionic liquid diluent for selective extraction of Li (I) from salt lakes. Desalination 2021, 509, 115073. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Ionic liquids and deep-eutectic solvents in extractive metallurgy: Mismatch between academic research and industrial applicability. J. Sustain. Metall. 2023, 9, 423–438. [Google Scholar] [CrossRef]
- Hawker, R.R.; Haines, R.S.; Harper, J.B. Variation of the cation of ionic liquids: The effects on their physicochemical properties and reaction outcome. Targets Heterocycl. Syst. Prop 2015, 18, 141–213. [Google Scholar] [CrossRef]
- Cocalia, V.A.; Holbrey, J.D.; Gutowski, K.E.; Bridges, N.J.; Roqers, R.D. Separations of metal ions using ionic liquids: The challenges of multiple mechanisms. Tsinghua Sci. Technol. 2006, 11, 188–193. [Google Scholar] [CrossRef]
- Janssen, C.H.; Macías-Ruvalcaba, N.A.; Aguilar-Martínez, M.; Kobrak, M.N. Metal extraction to ionic liquids: The relationship between structure, mechanism and application. Int. Rev. Phys. Chem. 2015, 34, 591–622. [Google Scholar] [CrossRef]
- Arrachart, G.; Couturier, J.; Dourdain, S.; Levard, C.; Pellet-Rostaing, S. Recovery of rare earth elements (REEs) using ionic solvents. Processes 2021, 9, 1202. [Google Scholar] [CrossRef]
- Platzer, S.; Leyma, R.; Wolske, S.; Kandioller, W.; Heid, E.; Schröder, C.; Schagerl, M.; Krachler, R.; Jirsa, F.; Keppler, B.K. Thioglycolate-based task-specific ionic liquids: Metal extraction abilities vs acute algal toxicity. J. Hazard. Mater. 2017, 340, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Leyma, R.; Platzer, S.; Jirsa, F.; Kandioller, W.; Krachler, R.; Keppler, B.K. Novel thiosalicylate-based ionic liquids for heavy metal extractions. J. Hazard. Mater. 2016, 314, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Platzer, S.; Kar, M.; Leyma, R.; Chib, S.; Roller, A.; Jirsa, F.; Krachler, R.; MacFarlane, D.R.; Kandioller, W.; Keppler, B.K. Task-specific thioglycolate ionic liquids for heavy metal extraction: Synthesis, extraction efficacies and recycling properties. J. Hazard. Mater. 2017, 324, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Tsuchida, Y.; Matsumiya, M.; Uchino, Y.; Yanagi, I. Separation of tungsten and cobalt from WC-Co hard metal wastes using ion-exchange and solvent extraction with ionic liquid. Miner. Eng. 2018, 128, 224–229. [Google Scholar] [CrossRef]
- Fuerhacker, M.; Haile, T.M.; Kogelnig, D.; Stojanovic, A.; Keppler, B. Application of ionic liquids for the removal of heavy metals from wastewater and activated sludge. Water Sci. Technol. 2012, 65, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Villemin, D.; Didi, M.A. Extraction of rare earth and heavy metals, using ionic solvents as extraction medium (A Review). Orient. J. Chem. 2013, 29, 1267–1284. [Google Scholar] [CrossRef]
- Lertlapwasin, R.; Bhawawet, N.; Imyim, A.; Fuangswasdi, S. Ionic liquid extraction of heavy metal ions by 2-aminothiophenol in 1-butyl-3-methylimidazolium hexafluorophosphate and their association constants. Sep. Purif. Technol. 2010, 72, 70–76. [Google Scholar] [CrossRef]
- Fischer, L.; Falta, T.; Koellensperger, G.; Stojanovic, A.; Kogelnig, D.; Galanski, M.S.; Krachler, R.; Keppler, B.K.; Hann, S. Ionic liquids for extraction of metals and metal containing compounds from communal and industrial waste water. Water Res. 2011, 45, 4601–4614. [Google Scholar] [CrossRef]
- Turanov, A.N.; Karandashev, V.K.; Baulin, V.E. Extraction of Lanthanides (III) from Aqueous Nitric Acid Solutions with Tetra (n-octyl) diglycolamide into Methyltrioctylammonium Bis (trifluoromethanesulfonul) imide Ionic Liquid and Its Mixtures with Molecular Organic Diluents. Minerals 2023, 13, 736. [Google Scholar] [CrossRef]
- Diabate, P.D.; Dupont, L.; Boudesocque, S.; Mohamadou, A. Novel task specific ionic liquids to remove heavy metals from aqueous effluents. Metals 2018, 8, 412. [Google Scholar] [CrossRef]
- Do-Thanh, C.-L.; Luo, H.; Dai, S. A perspective on task-specific ionic liquids for the separation of rare earth elements. RSC Sustain. 2023, 1, 1168–1176. [Google Scholar] [CrossRef]
- Messadi, A.; Mohamadou, A.; Boudesocque, S.; Dupont, L.; Guillon, E. Task-specific ionic liquid with coordinating anion for heavy metal ion extraction: Cation exchange versus ion-pair extraction. Sep. Purif. Technol. 2013, 107, 172–178. [Google Scholar] [CrossRef]
- Pirkwieser, P.; López-López, J.A.; Kandioller, W.; Keppler, B.K.; Moreno, C.; Jirsa, F. Novel 3-hydroxy-2-naphthoate-based task-specific ionic liquids for an efficient extraction of heavy metals. Front. Chem. 2018, 6, 172. [Google Scholar] [CrossRef] [PubMed]
- Subodh; Mogha, N.K.; Chaudhary, K.; Kumar, G.; Masram, D.T. Fur-imine-functionalized graphene oxide-immobilized copper oxide nanoparticle catalyst for the synthesis of xanthene derivatives. ACS Omega 2018, 3, 16377–16385. [Google Scholar] [CrossRef] [PubMed]
- Imdad, S.; Dohare, R.K. A critical review on heavy metals removal using ionic liquid membranes from the industrial wastewater. Chem. Eng. Process.-Process Intensif. 2022, 173, 108812. [Google Scholar] [CrossRef]
- Khalid, A.; Zulfiqar, S.; Tabassum, N.; Khan, A.S.; Abid, M.A.; Akhtar, M.S.; Al-Misned, F.; Aljuwayid, A.M.; Zahmatkesh, S.; Asif, S. Biocompatible cellulose acetate supported ammonium based ionic liquid membranes; way forward to remediate water pollution. Chemosphere 2023, 322, 138151. [Google Scholar] [CrossRef]
- Yu, H.-Z.; Bencherif, S.; Pham-Truong, T.-N.; Ghilane, J. Immobilization of molecule-based ionic liquids: A promising approach to improve elecrocatalyst performance towards the hydrogen evolution reaction. New J. Chem. 2022, 46, 454–464. [Google Scholar] [CrossRef]
- Taggart, A.F. Handbook of Ore Dressing; John and Wiley & Sons: Hoboken, NJ, USA, 1927. [Google Scholar]
- Misra, M.; Anazia, I. Ultrafine coal flotation by gas phase transport of atomized reagents. Miner. Metall. Process. 1987, 233, 233–236. [Google Scholar] [CrossRef]
- Peng, F.; Li, R. Oil-Coated Air Bubble Flotation to Improve Coal Flotation Rate and Recovery. Preprint 1991, 91, 25–28. [Google Scholar]
- Doungdeethaveeratana, D.; Sohn, H. The kinetics of extraction in a novel solvent extraction process with bottom gas injection without moving parts. Hydrometallurgy 1998, 49, 229–254. [Google Scholar] [CrossRef]
- Chen, F.; Finch, J.; Distin, P.; Gomez, C. Air assisted solvent extraction. Can. Metall. Q. 2003, 42, 277–280. [Google Scholar] [CrossRef]
- Garrett, P.R. Preliminary considerations concerning the stability of a liquid heterogeneity in a plane-parallel liquid film. J. Colloid Interface Sci. 1980, 76, 587–590. [Google Scholar] [CrossRef]
- Pugh, R.J. Foaming, foam films, antifoaming and defoaming. Adv. Colloid Interface Sci. 1996, 64, 67–142. [Google Scholar] [CrossRef]
- Tarkan, H.; Kuan, S.; Finch, J. Studies on Air-Assisted Solvent Extraction. In Separation Technologies for Minerals, Coal, and Earth Resources; Society for Mining, Metallurgy, and Exploration, Inc. (SME): Englewood, CO, USA, 2012. [Google Scholar]
- Li, C.-W.; Chen, Y.-M.; Hsiao, S.-T. Compressed air-assisted solvent extraction (CASX) for metal removal. Chemosphere 2008, 71, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Tarkan, H.; Finch, J. Foaming properties of solvents for use in air-assisted solvent extraction. Colloids Surf. A Physicochem. Eng. Asp. 2005, 264, 126–132. [Google Scholar] [CrossRef]
- Tarkan, H.; Gelinas, S.; Finch, J. Measurement of thickness and composition of a solvent film on a bubble. J. Colloid Interface Sci. 2006, 297, 732–737. [Google Scholar] [CrossRef]
- Tarkan, H.; Gélinas, S.; Aspinall, C.; Finch, J. Solvent Layer Thickness on Bubbles of Different Sizes Blown in Air. Can. Metall. Q. 2007, 46, 259–263. [Google Scholar] [CrossRef]
- Tarkan, H.M. Air-Assisted Solvent Extraction; McGill University (Canada): Montreal, QC, Canada, 2006. [Google Scholar]
- Li, C.-W.; Chiu, C.-H.; Lee, Y.-C.; Chang, C.-H.; Lee, Y.-H.; Chen, Y.-M. Integration of ceramic membrane and compressed air-assisted solvent extraction (CASX) for metal recovery. Water Sci. Technol. 2010, 62, 1274–1280. [Google Scholar] [CrossRef]
- Lee, P.-C.; Li, C.-W.; Chen, S.-S.; Chiu, C.-H. Compressed Air-Assisted Solvent Extraction (CASX) for Chromate Removal: Regeneration and Recovery. Sep. Sci. Technol. 2009, 44, 3911–3922. [Google Scholar] [CrossRef]
- Kuan, S.H. Collapsing Bubble Bed in a Downcomer with the Introduction of Solvent. Ph.D. Thesis, McGill University, Montreal, QC, Canada, 2013. [Google Scholar]
- Tavakoli Mohammadi, M.; Koleini, S.J.; Abolghasemi, H.; Abdollahy, M. DESIGN FEATURES OF A COLUMN FLOTOEXTRACTION (CFE) SYSTEMInternational Mineral Processing Congress. In Proceedings of the 16th International Mineral Processing Congress, New Dehli, India, 24–28 September 2012; pp. 2503–2511. [Google Scholar]
- Mohammadi, M.R.T.; Koleini, S.M.J.; Javanshir, S.; Abolghasemi, H.; Abdollahy, M. Solvent extraction of rubidium from gold waste using conventional SX and new CFE methods. Rare Met. 2015, 34, 818–828. [Google Scholar] [CrossRef]
- Mohammadi, M.T.; Koleini, S.J.; Abolghasemi, H.; Abdollahy, M.; Sedaghat, B. Performance and selectivity of the new column flotoextraction method. Miner. Eng. 2015, 71, 27–33. [Google Scholar] [CrossRef]
- Koleini, S.J.; Tavakoli Mohammadi, M.; Abdollahy, M. Evaluation the Influence of Performance Mode Dissolved Nitrogen Predispersed Solvent Extraction Method on Selectivity of Copper Solvent Extraction. In Proceedings of the 22nd International Mining Congress and Exhibition of Turkey, Ankara, Turkey, 11–13 May 2011; pp. 331–342. [Google Scholar]
- Koleini, S.J.; Tavakoli Mohammadi, M.; Abdollahy, M. Comparison the Performance of Dissolved Nitrogen Predispersed Solvent Extraction and Conventional SX Methods in Synthetic Dilute and Dense Copper Solutions. In Proceedings of the 22nd International Mining Congress and Exhibition of Turkey, Ankara, Turkey, 11–13 May 2011; pp. 319–330. [Google Scholar]
- Tavakoli Mohammadi, M.; Koleini, S.J.; Abdollahy, M. New dissolved nitrogen predispersed solvent extraction method, 1: Performance. Ind. Eng. Chem. Res. 2013, 52, 3842–3851. [Google Scholar] [CrossRef]
- Tavakoli Mohammadi, M.; Koleini, S.J.; Abdollahy, M. New dissolved nitrogen predispersed solvent extraction method, 2: Selectivity. Ind. Eng. Chem. Res. 2013, 52, 3852–3857. [Google Scholar] [CrossRef]
- Mohammadi, M.R.T.; Koleini, S.M.J.; Abdollahy, M. Features and Capabilities of New DNPDSE Method. J. Tethys 2014, 2, 357–374. [Google Scholar]
- Rahmati, S.; Ahmadi, A.; Nasab, M.M. Metal Extraction Using AASX Continous-Mode from Wastewaters. Iranian Patent 94540, 2017. [Google Scholar]
- Rahmati, S.; Ahmadi, A.; Hosseini, M.R.; Nasab, M.M. Surface phenomena in air-assisted solvent extraction. Hydrometallurgy 2018, 177, 168–177. [Google Scholar] [CrossRef]
- Rahmati, S.; Ahmadi, A.; Hosseini, M.R.; Nasab, M.M. Optimization of continuous air-assisted solvent extraction for treating dilute Cu leach solutions using response surface methodology. Miner. Eng. 2019, 131, 154–163. [Google Scholar] [CrossRef]
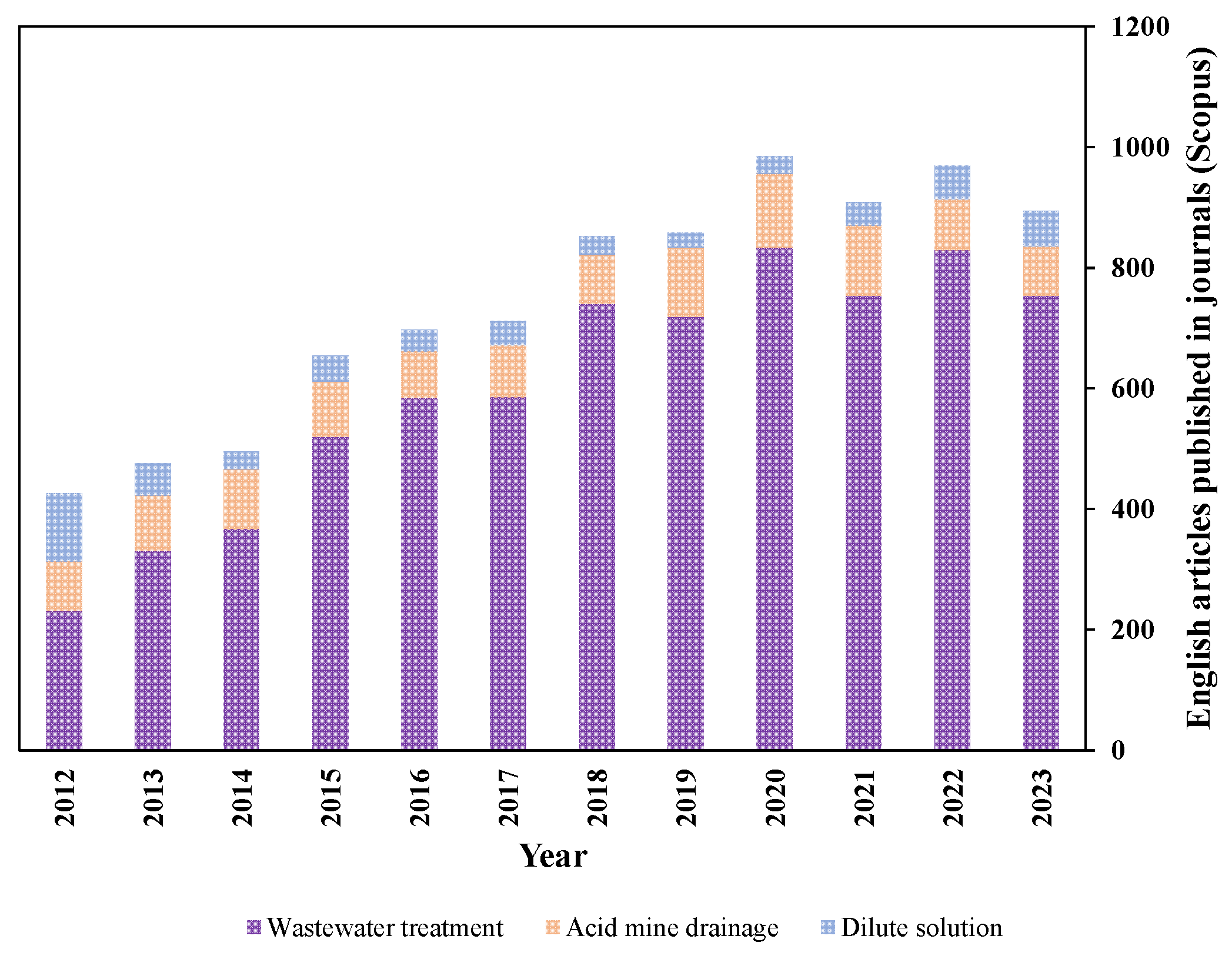


| Site | Country | pH | Eh | Cu | Ni | Zn | Al | Fe | Mn |
|---|---|---|---|---|---|---|---|---|---|
| Storwartz mine | Norway | 6.5 | - | 0.06 | - | 2.13 | 0.03 | 1.6 | 1.35 |
| Bullhouse | England | 5.9 | 257 | - | - | - | 1.2 | 61 | 15 |
| Wheal Jane | England | 3.4 | 462 | 1.2 | - | 132 | 27 | 290 | 8 |
| Killingdal (mine dump) | Norway | 2.8 | - | 5.65 | - | 61.1 | 38.3 | 265 | 4.24 |
| King’s mine stream | Norway | 2.7 | - | 15.8 | - | 25.4 | 22.5 | 172 | 0.78 |
| Parys mine | Wales | 2.5 | 685 | 40 | - | 60 | 70 | 650 | 10 |
| Iberian Pyrite Belt (AMD) | Spain | 2.6 | 100 | 1.5 | 200 | 700 | 3900 | 60 | |
| Rio Tinto | Spain | 2.2 | 450 | 109 | - | 225 | - | - | - |
| Iron Mountain | USA | 0.5–1 | - | 120–650 | - | 700–2600 | 1400–6700 | 13,000–19,000 | 17–120 |
| Sarcheshmeh (AMD) | Iran | 4.4 | - | 22.780 | 0.606 | 10.857 | 29.040 | 0.242 | 55.262 |
| Sarcheshmeh (ARD) | Iran | 3.2 | - | 25.000 | 1.140 | 6.230 | 39.300 | 13.700 | 27.900 |
| Plant Name | Country | pH | Cu (mg/L) |
|---|---|---|---|
| Zijinshan copper mine | China | - | 1470 |
| Pasminco Metals-BHAS | Australia | - | 1100 |
| El Abra | Chile | - | 500–1000 |
| Sociedad Contractual mineral El Abra | Chile | 1.2 | 690 |
| Empresa Minera de Mantos Blancos, Mantos Mine | Chile | 0.8 | 500 |
| Empresa Minera de Mantos Blancos, Mantoverda Mine | Chile | 1.1 | 500 |
| Griilambone Copper Co. | Australia | 1.5 | 100–500 |
| Cerro Colorado | Chile | - | 400 |
| Phelps Dodge Morenci, Metcalf SX | USA | 1.4 | 340 |
| Compania Minera Cerro Colorado Ltd. | Chile | 1.3 | 340 |
| Phelps Dodge Morenci, Modoc SX Plant | USA | 1.4 | 330 |
| Mexicana de Cobre | Mexico | 1.7 | 300 |
| Phelps Dodge Morenci, Central SX Plant | USA | 1.3 | 270 |
| Zaldivar | Chile | - | 250 |
| Great Australia Mining Co. | Australia | 1.5 | 250 |
| Phelps Dodge Morenci, Southwest SX Plant | USA | 1.7 | 250 |
| Compania Minera Cameron de Andacollo | Chile | 0.8 | 240 |
| Sarcheshmeh | Iran | 1.3 | 63–219 |
| Aberfoyle Resources Ltd. | Australia | 1.5 | 200 |
| Zambia CCM Tailings Leach Plant | Zambia | 1.2 | 200 |
| Compania Minera Quebrada Balanca | Chile | 1.4 | 200 |
| Compania Minera Zaldivar | Chile | - | 200 |
| Burro Chief (Phelps Dodge), Santa Rita | USA | 1.6 | 200 |
| Southern Peru Limited | Peru | 1.8 | 160 |
| Zijin Minging Group Co. | China | 1.4 | 135 |
| Burro Chief Copper Co. (Phelps Dodge), Tyrone | USA | 1.6 | 110 |
| Mount Isa Mines Ltd. | Australia | 1.6–1.8 | 100 |
| Method | Advantages | Disadvantages |
|---|---|---|
| IX | Low energy requirement Effectiveness < 100 ppm | Adsorption of organics Chemical regeneration requirements Organic contamination from the resin Expensive resins Prone to fouling in mixed waste streams |
| SA | Wide range of commercial products and a wide variety of target contaminants Rapid kinetics Simple metal removal process Adaptable to many treatment formats Low adsorbent cost Effective for solution < 100 ppm | Requirement for several types of adsorbents Low selectivity Recurring cost of new adsorbent Disposal cost of spent adsorbent |
| CP | Well established Low detention time requirements | High reagent consumption Large quantities of sludge being generated Ineffective in the removal of the metal ions at low concentration Low selectivity Several unit operations |
| SX (mixer-settler) | Effective in extracting/removing metal ions in solutions Produces high-purity solutions and compounds Continuous mode/esed commercially Highly selective | Efficient in high-concentration solutions Capital costs Losing organic phase (emissions to raffinate) High solvent requirement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmati, S.; Adavodi, R.; Hosseini, M.R.; Veglio’, F. Efficient Metal Extraction from Dilute Solutions: A Review of Novel Selective Separation Methods and Their Applications. Metals 2024, 14, 605. https://doi.org/10.3390/met14060605
Rahmati S, Adavodi R, Hosseini MR, Veglio’ F. Efficient Metal Extraction from Dilute Solutions: A Review of Novel Selective Separation Methods and Their Applications. Metals. 2024; 14(6):605. https://doi.org/10.3390/met14060605
Chicago/Turabian StyleRahmati, Soroush, Roshanak Adavodi, Mohammad Raouf Hosseini, and Francesco Veglio’. 2024. "Efficient Metal Extraction from Dilute Solutions: A Review of Novel Selective Separation Methods and Their Applications" Metals 14, no. 6: 605. https://doi.org/10.3390/met14060605
APA StyleRahmati, S., Adavodi, R., Hosseini, M. R., & Veglio’, F. (2024). Efficient Metal Extraction from Dilute Solutions: A Review of Novel Selective Separation Methods and Their Applications. Metals, 14(6), 605. https://doi.org/10.3390/met14060605








