Precipitation Thermodynamics in an Al–Zn–Mg Alloy with Different Grain Sizes
Abstract
1. Introduction
2. Experimental Procedures
3. Experimental Results
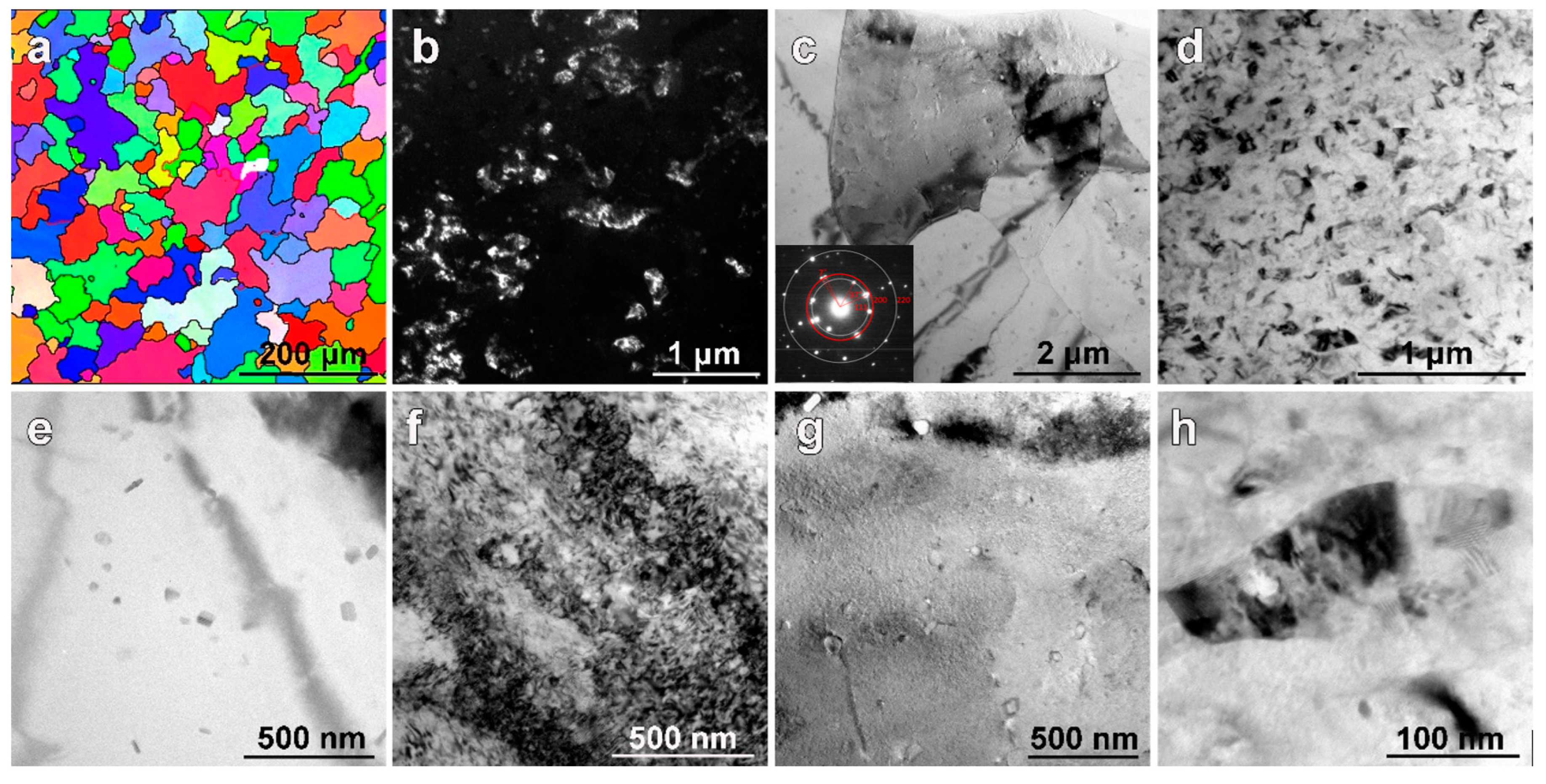
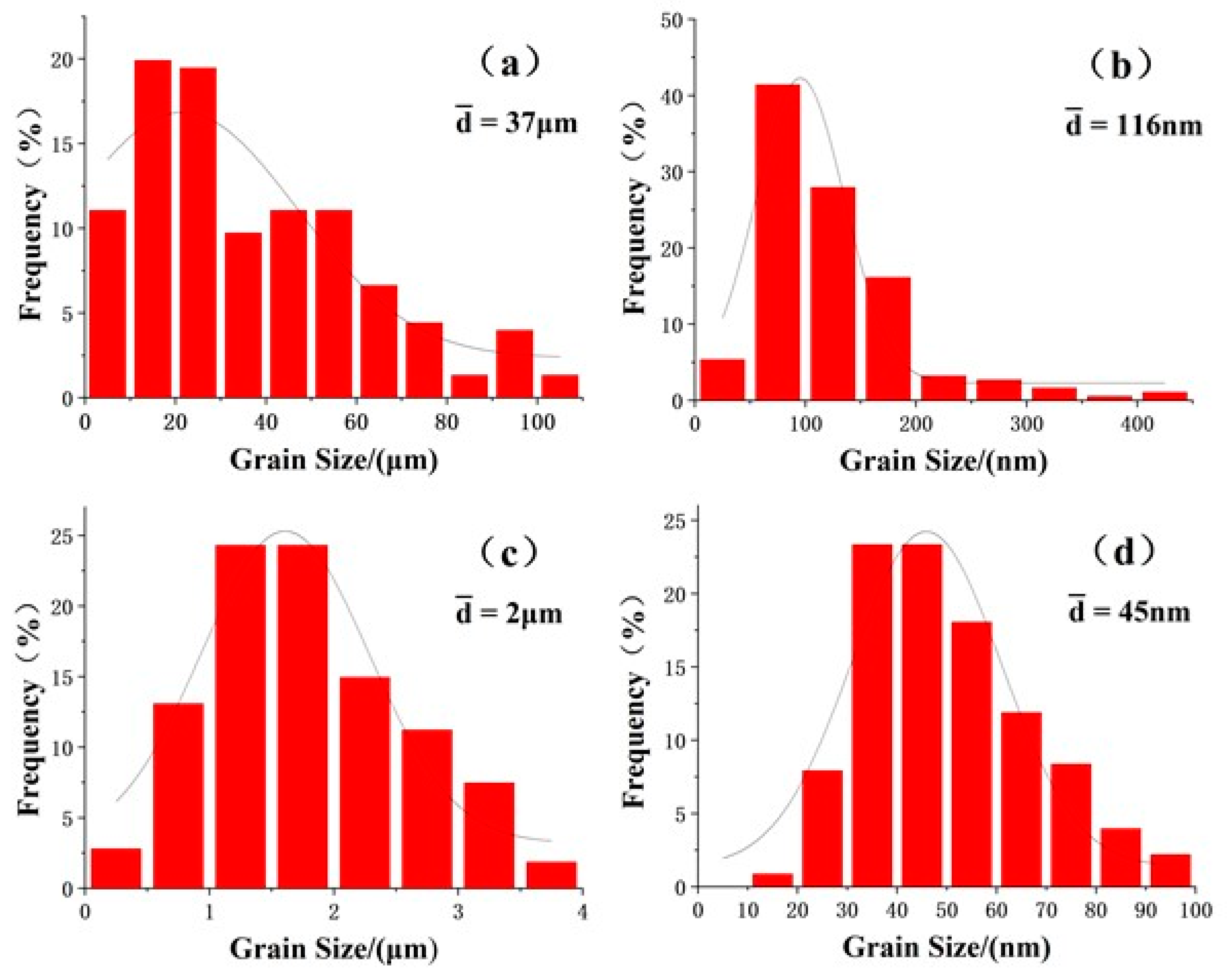
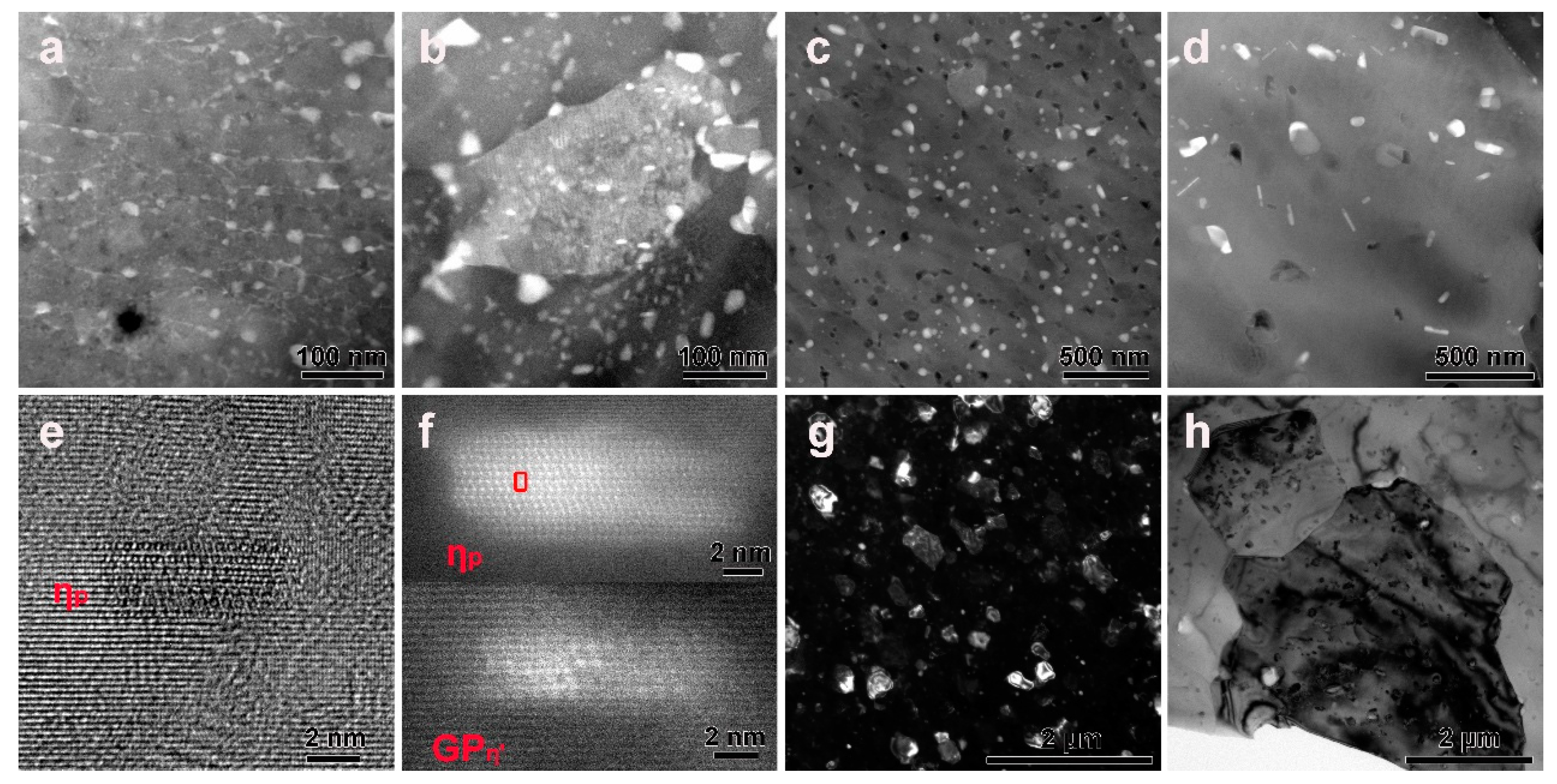
3.1. Microstructures before DSC Analysis
3.2. Thermodynamic Data
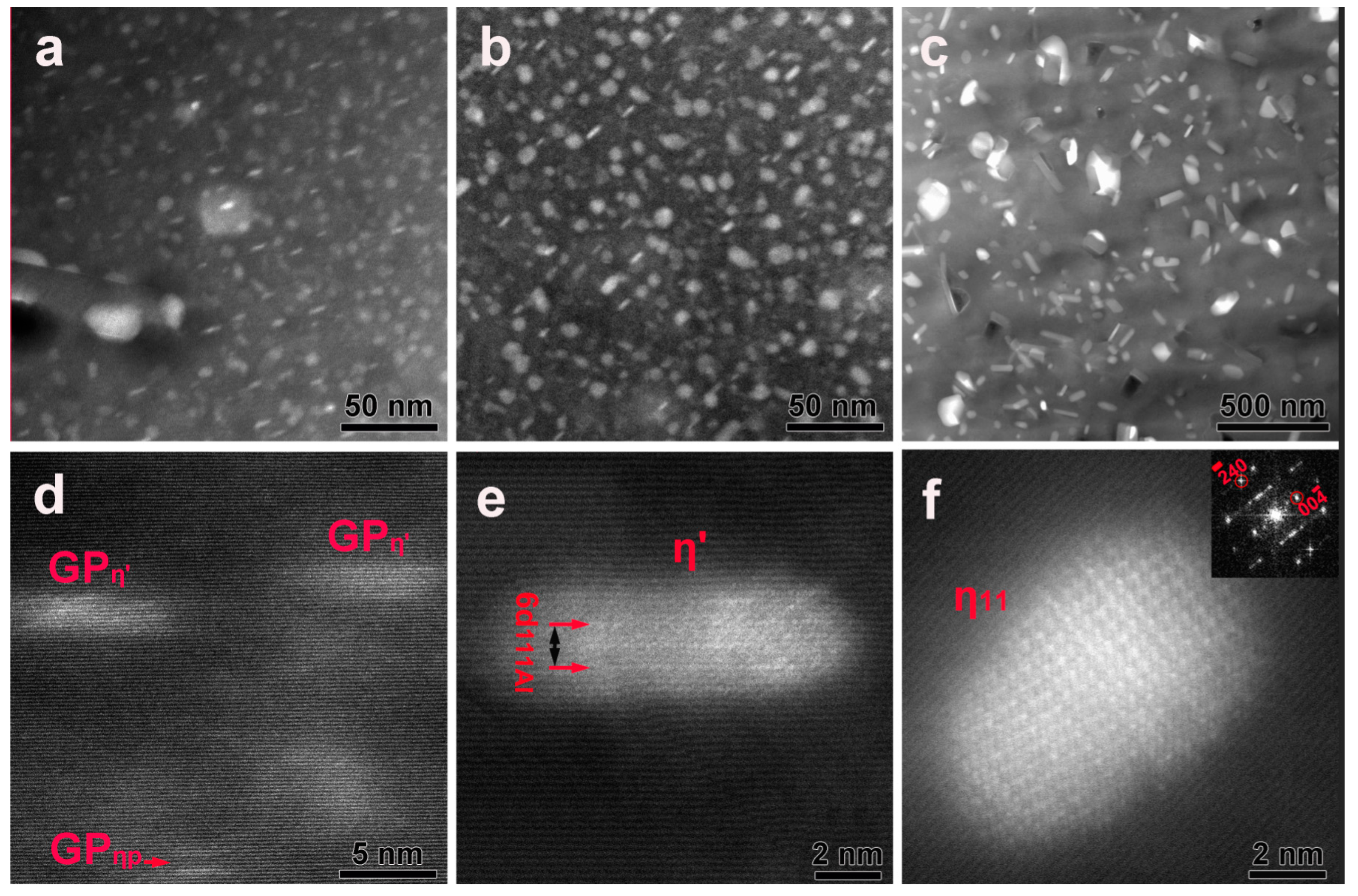
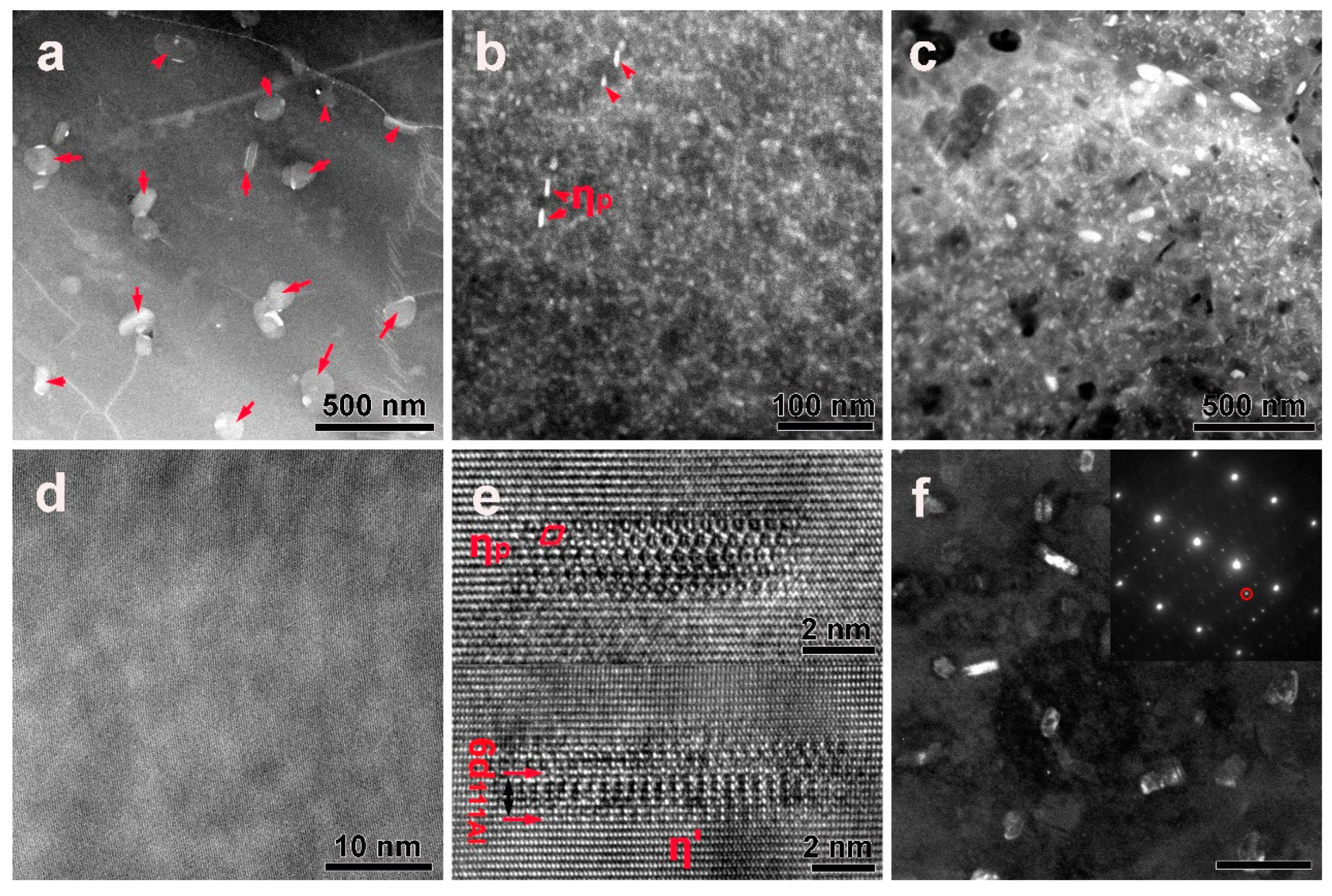
3.3. Precipitates in the Interior of Grains before or after DSC Treatment
4. Discussion
4.1. The Precipitation Thermodynamics in CG and UFG2 Materials
4.2. The Precipitation Thermodynamics in NC and UFG1 Materials
4.3. The Precipitation Thermodynamics Affected by ECAP and HPT Processes
5. Conclusions
- (1)
- Homogenous nucleation is conformed to be the main precipitation mechanism in the CG and UFG2 materials, and the formation of GP zones is highly dependent on the excess vacancy concentration. The three steps during the decomposition of the supersaturated solid solution can be revealed by three clear exothermic peaks on the DSC curves for the CG materials.
- (2)
- The nucleation of the GP zones in the UFG2 materials has been suppressed significantly at the first exothermic peak temperature due to the fewer vacancies remaining and the lower supersaturation in grain interiors. These disappeared vacancies and solute atoms have been absorbed by a higher volume of grain boundaries. Thus, the nucleation will occur at high temperature and make contributions to the second exothermic peak, along with the phase transformation from the GP zones to metastable phases.
- (3)
- The whole decomposition processes of the solid solution have been nearly completed in the first exothermic peak; the other two exothermic peaks are more attributed to the recovery and recrystallization of the UFGs and NGs in the UFG1 and NC materials. The defect-assisted heterogeneous nucleation is confirmed to be the main precipitation mechanism, and these defects involve dislocations, lattice distortions, and grain boundaries.
- (4)
- It is found that the more excess vacancies remaining in the grain interiors, the lower the temperature of the nucleation of the GP zones.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.-Q.; Xu, J.-B.; Pan, S.-P.; Li, N.-B.; Ou, C.-G.; Liu, W.-H.; Song, Y.-F.; Tan, X.-R.; Liu, Y. Effect of T6I6 treatment on dynamic mechanical behaviour of Al-Si-Mg-Cu cast alloy and impact resistance of its cast motor shell. J. Cent. South Univ. 2022, 29, 924–936. [Google Scholar] [CrossRef]
- Hornbogen, E. Hundred years of precipitation hardening. J. Light Met. 2001, 1, 127–132. [Google Scholar] [CrossRef]
- Matsuda, K.; Yasumoto, T.; Bendo, A.; Tsuchiya, T.; Lee, S.; Nishimura, K.; Nunomura, N.; Marioara, C.D.; Levik, A.; Holmestad, R.; et al. Effect of Copper Addition on Precipitation Behavior near Grain Boundary in Al-Zn-Mg Alloy. Mater. Trans. 2019, 60, 1688–1696. [Google Scholar] [CrossRef]
- Cassell, A.M.; Robson, J.D.; Race, C.P.; Eggeman, A.; Hashimoto, T.; Besel, M. Dispersoid composition in zirconium containing Al-Zn-Mg-Cu (AA7010) aluminium alloy. Acta Mater. 2019, 169, 135–146. [Google Scholar] [CrossRef]
- Zhao, H.; De Geuser, F.; da Silva, A.K.; Szczepaniak, A.; Gault, B.; Ponge, D.; Raabe, D. Segregation assisted grain boundary precipitation in a model Al-Zn-Mg-Cu alloy. Acta Mater. 2018, 156, 318–329. [Google Scholar] [CrossRef]
- Santos, J.F.D.; Staron, P.; Fischer, T.; Robson, J.D.; Kostka, A.; Colegrove, P.; Wang, H.; Hilgert, J.; Bergmann, L.; Hütsch, L.L.; et al. Understanding precipitate evolution during friction stir welding of Al-Zn-Mg-Cu alloy through in-situ measurement coupled with simulation. Acta Mater. 2018, 148, 163–172. [Google Scholar] [CrossRef]
- Gumbmann, E.; De Geuser, F.; Sigli, C.; Deschamps, A.; Mg, I.O. Ag and Zn minor solute additions on the precipitation kinetics and strengthening of an Al-Cu-Li alloy. Acta Mater. 2017, 133, 172–185. [Google Scholar] [CrossRef]
- Deschamps, A.; Fribourg, G.; Bréchet, Y.; Chemin, J.L.; Hutchinson, C.R. In situ evaluation of dynamic precipitation during plastic straining of an Al–Zn–Mg–Cu alloy. Acta Mater. 2012, 60, 1905–1916. [Google Scholar] [CrossRef]
- Liu, J.Z.; Chen, J.H.; Yang, X.B.; Ren, S.; Wu, C.L.; Xu, H.Y.; Zou, J. Revisiting the precipitation sequence in Al–Zn–Mg-based alloys by high-resolution transmission electron microscopy. Scr. Mater. 2010, 63, 1061–1064. [Google Scholar] [CrossRef]
- Liddicoat, P.V.; Liao, X.-Z.; Zhao, Y.; Zhu, Y.; Murashkin, M.Y.; Lavernia, E.J.; Valiev, R.Z.; Ringer, S.P. Nanostructural hierarchy increases the strength of aluminium alloys. Nat. Commun. 2010, 1, 63. [Google Scholar] [CrossRef]
- Zha, M.; Li, Y.; Mathiesen, R.H.; Bjørge, R.; Roven, H.J. Microstructure evolution and mechanical behavior of a binary Al–7Mg alloy processed by equal-channel angular pressing. Acta Mater. 2015, 84, 42–54. [Google Scholar] [CrossRef]
- Mulyukov, R.R.; Imayev, R.M.; Nazarov, A.A. Production, properties and application prospects of bulk nanostructured materials. J. Mater. Sci. 2008, 43, 7257. [Google Scholar] [CrossRef]
- Du, C.; Jin, S.; Fang, Y.; Li, J.; Hu, S.; Yang, T.; Zhang, Y.; Huang, J.; Sha, G.; Wang, Y.; et al. Ultrastrong nanocrystalline steel with exceptional thermal stability and radiation tolerance. Nat. Commun. 2018, 9, 5389. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Feng, Z.; Zhu, L.; Xu, J.; Miyagi, L.; Dong, H.; Sheng, H.; Wang, Y.; Li, Q.; Ma, Y.; et al. High-pressure strengthening in ultrafine-grained metals. Nature 2020, 579, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhou, H.; Lu, Q.; Gao, H.; Lu, L. Extra strengthening and work hardening in gradient nanotwinned metals. Science 2018, 362, eaau1925. [Google Scholar] [CrossRef] [PubMed]
- Ovid, I.A.; Valiev, R.Z.; Zhu, Y.T. Review on superior strength and enhanced ductility of metallic nanomaterials. Prog. Mater. Sci. 2018, 94, 462–540. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Estrin, Y.; Horita, Z.; Langdon, T.G.; Zehetbauer, M.J.; Zhu, Y.T. Fundamentals of Superior Properties in Bulk NanoSPD Materials. Mater. Res. Lett. 2016, 4, 1–21. [Google Scholar] [CrossRef]
- Wu, X.L.; Zhu, Y.T.; Wei, Y.G.; Wei, Q. Strong Strain Hardening in Nanocrystalline Nickel. Phys. Rev. Lett. 2009, 103, 205504. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Wen, H.; Hu, T.; Topping, T.D.; Isheim, D.; Seidman, D.N.; Lavernia, E.J.; Schoenung, J.M. Mechanical behavior and strengthening mechanisms in ultrafine grain precipitation-strengthened aluminum alloy. Acta Mater. 2014, 62, 141–155. [Google Scholar] [CrossRef]
- Ma, K.; Hu, T.; Yang, H.; Topping, T.; Yousefiani, A.; Lavernia, E.J.; Schoenung, J.M. Coupling of dislocations and precipitates: Impact on the mechanical behavior of ultrafine grained Al–Zn–Mg alloys. Acta Mater. 2016, 103, 153–164. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, Y.; Marceau, R.; Wang, L.; Zhang, Q.; Gao, X.; Hutchinson, C. Precipitation strengthening of aluminum alloys by room-temperature cyclic plasticity. Science 2019, 363, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.-S.; He, T.; Song, M.; Huo, Y.-M.; Du, X.-Y.; Vereshchaka, A.; Li, J.; Hu, H.-Y. Microstructure evolution of 7050 Al alloy fasteners during cold upsetting after equal channel angular pressing. J. Cent. South Univ. 2023, 30, 3682–3695. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Liao, X.Z.; Cheng, S.; Ma, E.; Zhu, Y.T. Simultaneously Increasing the Ductility and Strength of Nanostructured Alloys. Adv. Mater. 2006, 18, 2280–2283. [Google Scholar] [CrossRef]
- Huo, W.; Guo, M.; Hou, L.; Cui, H.; Sun, T.; Zhuang, L.; Zhang, J. Progress on Research of Advanced Thermo-mechanical Treatment of Aluminum Alloy. J. Mater. Sci. Eng. 2014, 32, 284. [Google Scholar]
- Zhang, S.; Hu, W.; Berghammer, R.; Gottstein, G. Microstructure evolution and deformation behavior of ultrafine-grained Al–Zn–Mg alloys with fine η′ precipitates. Acta Mater. 2010, 58, 6695–6705. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Liao, X.Z.; Jin, Z.; Valiev, R.Z.; Zhu, Y.T. Microstructures and mechanical properties of ultrafine grained 7075 Al alloy processed by ECAP and their evolutions during annealing. Acta Mater. 2004, 52, 4589–4599. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, S.; Trimby, P.W.; Liao, X.; Murashkin, M.Y.; Valiev, R.Z.; Liu, J.; Cairney, J.M.; Ringer, S.P.; Sha, G. Dynamic precipitation, segregation and strengthening of an Al-Zn-Mg-Cu alloy (AA7075) processed by high-pressure torsion. Acta Mater. 2019, 162, 19–32. [Google Scholar] [CrossRef]
- Hu, T.; Ma, K.; Topping, T.D.; Schoenung, J.M.; Lavernia, E.J. Precipitation phenomena in an ultrafine-grained Al alloy. Acta Mater. 2013, 61, 2163–2178. [Google Scholar] [CrossRef]
- Kirchheim, R. Grain coarsening inhibited by solute segregation. Acta Mater. 2002, 50, 413–419. [Google Scholar] [CrossRef]
- Sauvage, X.; Wilde, G.; Divinski, S.V.; Horita, Z.; Valiev, R.Z. Grain boundaries in ultrafine grained materials processed by severe plastic deformation and related phenomena. Mater. Sci. Eng. A 2012, 540, 1–12. [Google Scholar] [CrossRef]
- Löffler, H.; Kovács, I.; Lendvai, J. Decomposition processes in Al-Zn-Mg alloys. J. Mater. Sci. 1983, 18, 2215–2240. [Google Scholar] [CrossRef]
- Liu, J.Z.; Chen, J.H.; Liu, Z.R.; Wu, C.L. Fine precipitation scenarios of AlZnMg(Cu) alloys revealed by advanced atomic-resolution electron microscopy study Part II: Fine precipitation scenarios in AlZnMg(Cu) alloys. Mater. Charact. 2015, 99, 142–149. [Google Scholar] [CrossRef]
- Liu, J.Z.; Chen, J.H.; Yuan, D.W.; Wu, C.L.; Zhu, J.; Cheng, Z.Y. Fine precipitation scenarios of AlZnMg(Cu) alloys revealed by advanced atomic-resolution electron microscopy study Part I: Structure determination of the precipitates in AlZnMg(Cu) alloys. Mater. Charact. 2015, 99, 277–286. [Google Scholar] [CrossRef]
- Komura, Y.; Tokunaga, K. Structural studies of stacking variants in Mg-base Friauf-Laves phases. Acta Crystallogr. Sect. B 1980, 36, 1548–1554. [Google Scholar] [CrossRef]
- Chung, T.-F.; Yang, Y.-L.; Shiojiri, M.; Hsiao, C.-N.; Li, W.-C.; Tsao, C.-S.; Shi, Z.; Lin, J.; Yang, J.-R. An atomic scale structural investigation of nanometre-sized η precipitates in the 7050 aluminium alloy. Acta Mater. 2019, 174, 351–368. [Google Scholar] [CrossRef]
- Li, X.Z.; Hansen, V.; GjØnnes, J.; Wallenberg, L.R. HREM study and structure modeling of the η′ phase, the hardening precipitates in commercial Al–Zn–Mg alloys. Acta Mater. 1999, 47, 2651–2659. [Google Scholar] [CrossRef]
- Ma, Y.; Ahmed, A.; Ji, G.; Zhang, M.X.; Williams, L.; Chen, Z.; Ji, V. Atomic-scale investigation of the interface precipitation in a TiB2 nanoparticles reinforced Al–Zn–Mg–Cu matrix composite. Acta Mater. 2020, 185, 287–299. [Google Scholar] [CrossRef]
- Liu, J.; Hu, R.; Zheng, J.; Zhang, Y.; Ding, Z.; Liu, W.; Zhu, Y.; Sha, G. Formation of solute nanostructures in an Al–Zn–Mg alloy during long-term natural aging. J. Alloys Compd. 2020, 821, 153572. [Google Scholar] [CrossRef]
- Lervik, A.; Thronsen, E.; Friis, J.; Marioara, C.D.; Wenner, S.; Bendo, A.; Matsuda, K.; Holmestad, R.; Andersen, S.J. Atomic structure of solute clusters in Al–Zn–Mg alloys. Acta Mater. 2021, 205, 116574. [Google Scholar] [CrossRef]
- Razazi, H.A.; Paidar, M.; Ojo, O.O. Effect of Mn and Cr on structure and mechanical properties of Al-10%Mg-0.1%Ti alloy. Vacuum 2018, 155, 619–630. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, L.; Wu, X.; Lin, X.; Yao, T.; Peng, L. Multi-alloying effect of Ti, Mn, Cr, Zr, Er on the cast Al-Zn-Mg-Cu alloys. Mater. Charact. 2023, 201, 112984. [Google Scholar] [CrossRef]
- Fattahi, M.; Hsu, C.Y.; Ali, A.O.; Mahmoud, Z.H.; Dang, N.P.; Kianfar, E. Severe plastic deformation: Nanostructured materials; metal-based; polymer-based nanocomposites: A review. Heliyon 2023, 9, e22559. [Google Scholar] [CrossRef] [PubMed]
- Sha, G.; Wang, Y.B.; Liao, X.Z.; Duan, Z.C.; Ringer, S.P.; Langdon, T.G. Influence of equal-channel angular pressing on precipitation in an Al–Zn–Mg–Cu alloy. Acta Mater. 2009, 57, 3123–3132. [Google Scholar] [CrossRef]
- Deschamps, A.; De Geuser, F.; Horita, Z.; Lee, S.; Renou, G. Precipitation kinetics in a severely plastically deformed 7075 aluminium alloy. Acta Mater. 2014, 66, 105–117. [Google Scholar] [CrossRef]
- Jiang, X.J.; Tafto, J.; Noble, B.; Holme, B.; Waterloo, G. Differential scanning calorimetry and electron diffraction investigation on low-temperature aging in Al-Zn-Mg alloys. Metall. Mater. Trans. A 2000, 31, 339–348. [Google Scholar] [CrossRef]
- Richard, D.; Adler, P.N. Calorimetric studies of 7000 series aluminum alloys: I. Matrix precipitate characterization of 7075. Metall. Trans. A 1977, 8, 1177–1183. [Google Scholar] [CrossRef]
- Gubicza, J.; Schiller, I.; Chinh, N.Q.; Illy, J.; Horita, Z.; Langdon, T.G. The effect of severe plastic deformation on precipitation in supersaturated Al–Zn–Mg alloys. Mater. Sci. Eng. A 2007, 460–461, 77–85. [Google Scholar] [CrossRef]
- Legros, M.; Dehm, G.; Arzt, E.; Balk, T.J. Observation of Giant Diffusivity Along Dislocation Cores. Science 2008, 319, 1646–1649. [Google Scholar] [CrossRef]
- Wolverton, C. Crystal structure and stability of complex precipitate phases in Al–Cu–Mg–(Si) and Al–Zn–Mg alloys. Acta Mater. 2001, 49, 3129–3142. [Google Scholar] [CrossRef]
- Berg, L.K.; Gjønnes, J.; Hansen, V.; Li, X.Z.; Knutson-Wedel, M.; Waterloo, G.; Schryvers, D.; Wallenberg, L.R. GP-zones in Al–Zn–Mg alloys and their role in artificial aging. Acta Mater. 2001, 49, 3443–3451. [Google Scholar] [CrossRef]
- Embury, J.D.; Nicholson, R.B. The nucleation of precipitates: The system Al-Zn-Mg. Acta Metall. 1965, 13, 403–417. [Google Scholar] [CrossRef]
- Sha, G.; Cerezo, A. Early-stage precipitation in Al–Zn–Mg–Cu alloy (7050). Acta Mater. 2004, 52, 4503–4516. [Google Scholar] [CrossRef]
- Sha, G.; Cerezo, A. Kinetic Monte Carlo simulation of clustering in an Al–Zn–Mg–Cu alloy (7050). Acta Mater. 2005, 53, 907–917. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Huang, S.; Zhang, W.; Li, S.; Liu, J. Precipitation Thermodynamics in an Al–Zn–Mg Alloy with Different Grain Sizes. Metals 2024, 14, 625. https://doi.org/10.3390/met14060625
Wang Z, Huang S, Zhang W, Li S, Liu J. Precipitation Thermodynamics in an Al–Zn–Mg Alloy with Different Grain Sizes. Metals. 2024; 14(6):625. https://doi.org/10.3390/met14060625
Chicago/Turabian StyleWang, Zhen, Siqi Huang, Wenkai Zhang, Shunqiang Li, and Jizi Liu. 2024. "Precipitation Thermodynamics in an Al–Zn–Mg Alloy with Different Grain Sizes" Metals 14, no. 6: 625. https://doi.org/10.3390/met14060625
APA StyleWang, Z., Huang, S., Zhang, W., Li, S., & Liu, J. (2024). Precipitation Thermodynamics in an Al–Zn–Mg Alloy with Different Grain Sizes. Metals, 14(6), 625. https://doi.org/10.3390/met14060625





