Plasma Electrolytic Oxidation of Al-Zn-Mg-Ni-Fe “Nikalin” Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Surface and Cross-Sectional Microstructure of PEO Coatings
3.2. Microhardness of PEO Coatings
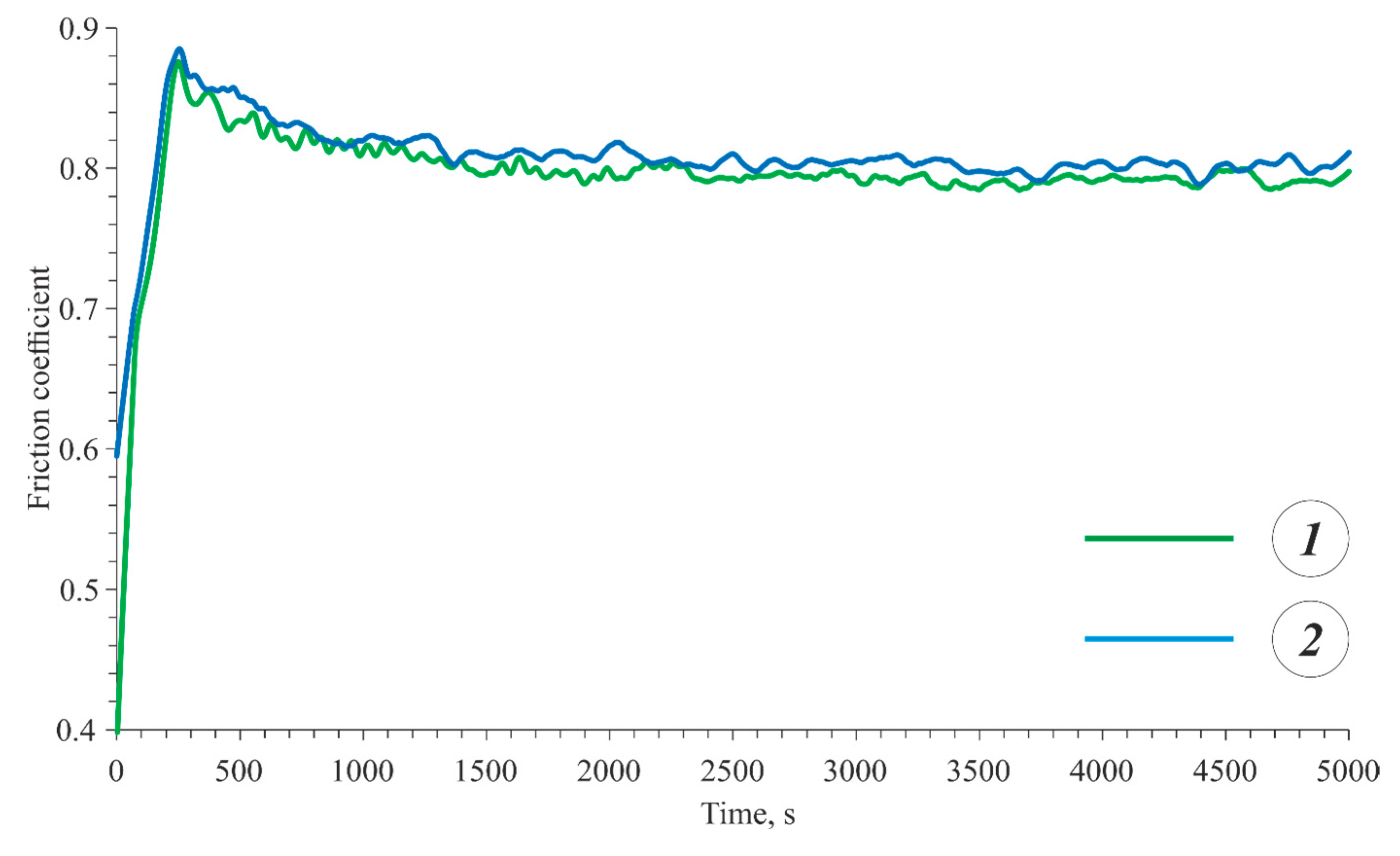
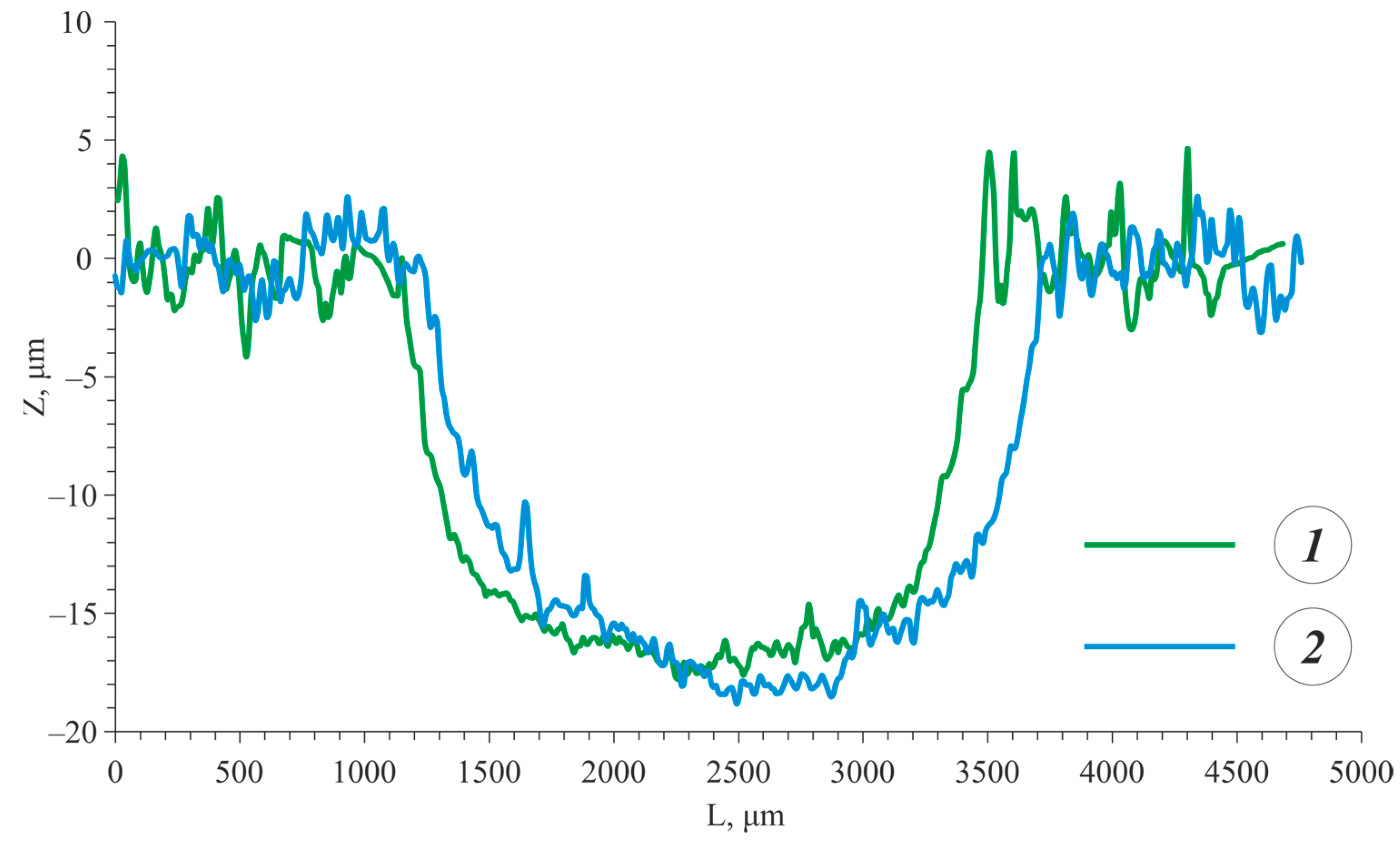
3.3. Wear Resistance
3.4. Electrochemical Behavior of PEO Coatings
4. Discussion
5. Conclusions
- (1)
- The as-cast and heat-treated Al-Zn-Mg-Ni-Fe alloys had compact microstructures consisting of an aluminum matrix (Al), M (MgZn2) precipitates, and eutectic Al9FeNi particles. The hardness of the casting Al5.2Zn1.7Mg0.4Ni0.3Fe and heat-treated Al7.2Zn2.8Mg0.5Ni0.4Fe alloys was 108 and 180 HV, respectively.
- (2)
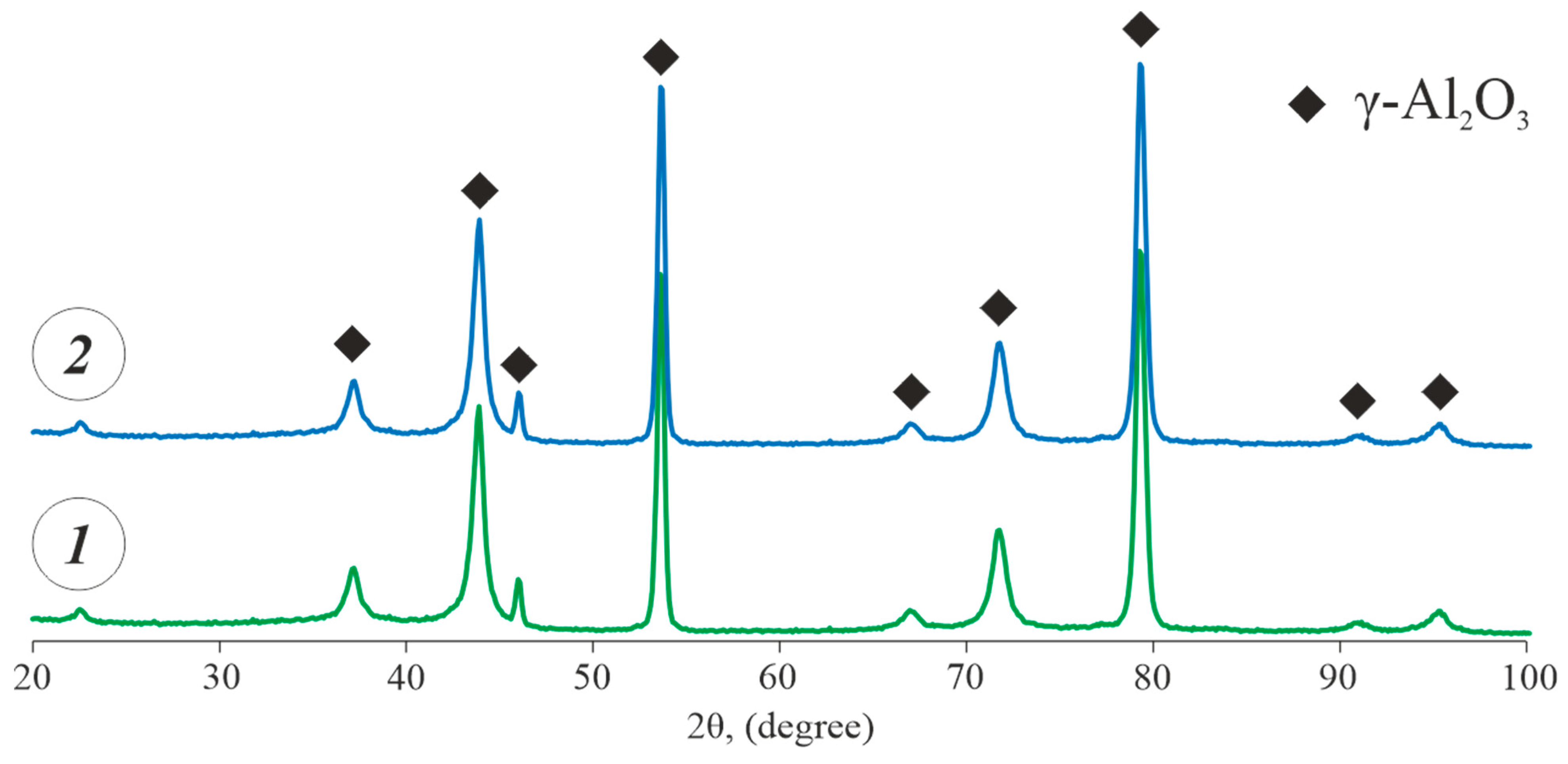
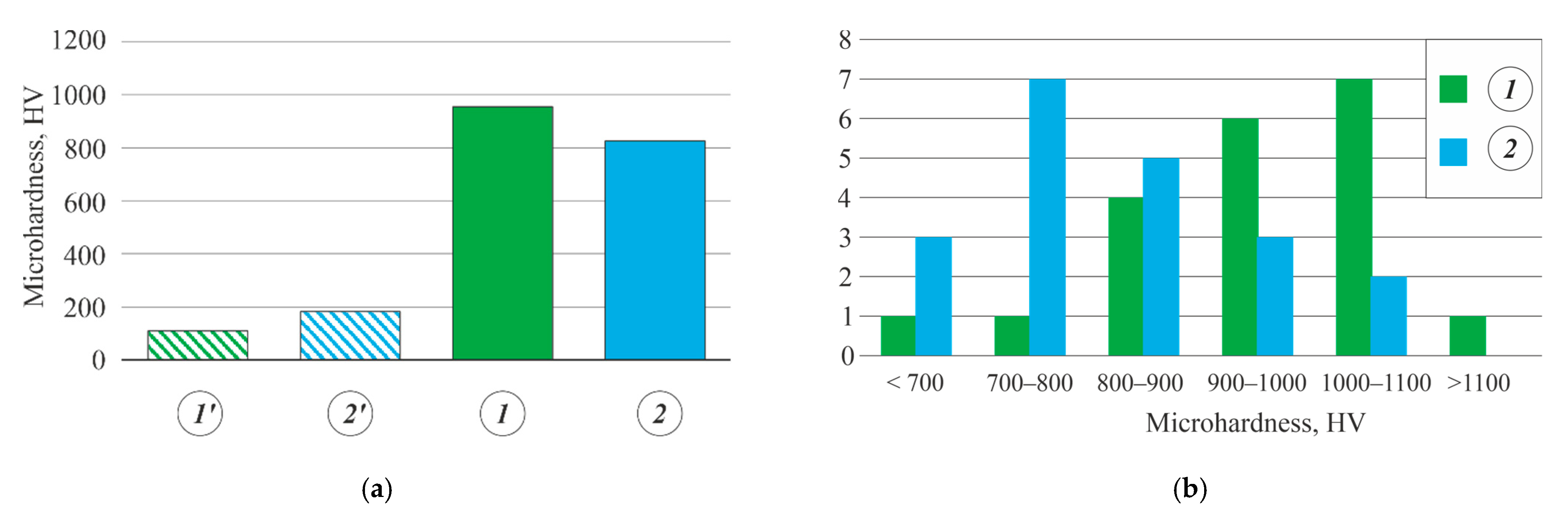
- The PEO coatings formed on the as-cast and heat-treated Al-Zn-Mg-Ni-Fe alloys in a silicate–alkaline electrolyte were uniform and contained a minimum number of defects. Their microhardness varied in the 660–1200 HV range, which was 3.6–11.1 times higher than that of the uncoated base alloys. The predominant phase in the coatings was γ-Al2O3.
- (3)
- The PEO coating formed on the as-cast alloy exhibited a higher wear resistance compared to the heat-treated alloy due to the highest hardness of the PEO coating on the as-cast alloy.
- (4)
- An increase in the fraction of the second phases in the structure of heat-treated Al7.2Zn2.8Mg0.5Ni0.4Fe led to a decrease in the corrosion resistance of the alloy in comparison with as-cast Al5.2Zn1.7Mg0.4Ni0.3Fe. The corrosion rates of the as-cast and heat-treated Al-Zn-Mg-Ni-Fe alloys increased significantly after PEO treatment by about 3.7–5.7 times. The effect on the corrosion resistance was greater for the PEO coatings formed on as-cast Al5.2Zn1.65Mg0.4Ni0.3Fe due to the lower fraction of residual Zn and Mg in the bulk of the PEO coatings.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shin, J.; Kim, T.; Kim, D.E.; Kim, D.; Kim, K. Castability and mechanical properties of new 7xxx aluminum alloys for automotive chassis/body applications. J. Alloys Compd. 2017, 698, 577–590. [Google Scholar] [CrossRef]
- Akopyan, T.K.; Belov, N.A. Approaches to the design of the new high-strength casting aluminum alloys of 7xxx series with high iron content. Non Ferr. Met. 2016, 1, 20–27. [Google Scholar] [CrossRef]
- Mann, V.K.; Alabin, A.N.; Krokhin, A.Y.; Frolov, A.V.; Belov, N.A. New Generation of High Strength Aluminum Casting Alloys. Light Met. Age 2015, 73, 44–47. [Google Scholar]
- Shurkin, P.K.; Belov, N.A.; Musin, A.F.; Aksenov, A.A. Novel High-Strength Casting Al−Zn−Mg−Ca−Fe Aluminum Alloy without Heat Treatment. Russ. J. Non-Ferr. Met. 2020, 61, 179–187. [Google Scholar] [CrossRef]
- Gamin, Y.V.; Belov, N.A.; Akopyan, T.K.; Timofeev, V.N.; Cherkasov, S.O.; Motkov, M.M. Effect of Radial-Shear Rolling on the Structure and Hardening of an Al–8%Zn–3.3%Mg–0.8%Ca–1.1%Fe Alloy Manufactured by Electromagnetic Casting. Materials 2023, 16, 677. [Google Scholar] [CrossRef] [PubMed]
- Shurkin, P.K.; Akopyan, T.K.; Galkin, S.P.; Aleshchenko, A.S. Effect of radial shear rolling on the structure and mechanical properties of a new-generation high-strength aluminum alloy based on the Al-Zn-Mg-Ni-Fe system. Met. Sci. Heat Treat. 2019, 60, 11–12. [Google Scholar] [CrossRef]
- Shurkin, P.; Akopyan, T.; Korotkova, N.; Prosviryakov, A.; Bazlov, A.; Komissarov, A.; Moskovskikh, D. Microstructure and Hardness Evolution of Al8Zn7Ni3Mg Alloy after Casting at very Different Cooling Rates. Metals 2020, 10, 762. [Google Scholar] [CrossRef]
- Rakoch, A.G.; Gladkova, A.A.; Dub, A.V. Plasma Electrolytic Treatment of Aluminum and Titanium Alloys; MISiS PH: Moscow, Russia, 2017; pp. 8–27. [Google Scholar]
- Zhu, L.; Guo, Z.; Zhang, Y.; Li, Z.; Sui, M. A mechanism for the growth of a plasma electrolytic oxide coating on Al. Electrochim. Acta 2016, 208, 296–303. [Google Scholar] [CrossRef]
- Letyagin, N.V.; Sokorev, A.A.; Kokarev, V.N.; Shatrov, A.S.; Tsydenov, A.G.; Finogeev, A.S.; Musin, A.F.; Petrzhik, M.I. The comparative characteristics of the structure and functional properties of coatings formed on aluminum alloys 2xxx and 7xxx series by the method of plasma electrolytic oxidation. Phys. Met. Metallogr. 2023, 124, 238–244. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, W.; Wang, X.; Wang, Y.; Yue, H.; Li, Q.; Yang, X.; Guo, C.; Li, C. Micro-arc oxidation of Al alloys: Mechanism, microstructure, surface properties, and fatigue damage behavior. J. Mater. Res. Technol. 2023, 23, 4307–4333. [Google Scholar] [CrossRef]
- Rodriguez, L.; Paris, J.-Y.; Denape, J.; Delbé, K. Micro-Arcs Oxidation Layer Formation on Aluminium and Coatings Tribological Properties—A Review. Coatings 2023, 13, 373. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Q.; Cao, X.; Cha, L.; Ye, R.; Ramachandran, C.S. Significance of plasma electrolytic oxidation treatment on corrosion and sliding wear performances of selective laser melted AlSi10Mg alloy. Mater. Charact. 2021, 181, 111479. [Google Scholar] [CrossRef]
- Wang, P.; Wu, T.; Xiao, Y.T.; Zhang, L.; Pu, J.; Cao, W.J.; Zhong, X.M. Characterization of micro-arc oxidation coatings on aluminum drill pipes at different current density. Vacuum 2017, 142, 21–28. [Google Scholar] [CrossRef]
- Cerchier, P.; Pezzato, L.; Gennari, C.; Moschin, E.; Moro, I.; Dabal, M. PEO coating containing copper: A promising anticorrosive and antifouling coating for seawater application of AA 7075. Surf. Coat. Technol. 2020, 393, 125774. [Google Scholar] [CrossRef]
- Zhu, M.; Song, Y.; Liu, Z.; Xu, D.; Dong, K.; Han, E.H. Optimization of thermal control and corrosion resistance of PEO coatings on 7075 aluminum alloy by frequency alteration. Surf. Coat. Technol. 2022, 446, 128797. [Google Scholar] [CrossRef]
- Lu, C.; Ding, J.; Shi, P.; Jia, J.; Xie, E.; Sun, Y. Effects of Texture Density on the Tribological Properties of Plasma Electrolytic Oxidation/Polytetrafluoroethylene Coatings Formed on Aluminum Alloys. Macromol. Mater. Eng. 2022, 307, 2100678. [Google Scholar] [CrossRef]
- Li, K.; Zhang, G.; Chen, K.; Li, W.; Zhu, Z.; Huang, Y.; Zhu, W.; Liao, Z.; Wan, B.; Liang, J.; et al. Effects of Ni content on microstructure and performance of MAO coatings on binary Al–Ni alloys. J. Mater. Res. Technol. 2024, 28, 3597–3608. [Google Scholar] [CrossRef]
- Famiyeh, L.; Huang, X. Plasma Electrolytic Oxidation Coatings on Aluminum Alloys: Microstructures, Properties, and Applications. Mod. Concept. Mater. Sci. 2019, 2, 000526. [Google Scholar]
- Wang, C.; Ma, R.; Du, A.; Fan, Y.; Zhao, X.; Cao, X. Growth methods of PEO coatings on 7075 aluminum alloy at two cathodic current densities. Surf. Coat. Technol. 2022, 432, 128099. [Google Scholar] [CrossRef]
- Tillous, K.; Toll-Duchanoy, T.; Bauer-Grosse, E.; Hericher, L.; Geandier, G. Microstructure and phase composition of microarc oxidation surface layers formed on aluminium and its alloys 2214-T6 and 7050-T74. Surf. Coat. Technol. 2009, 203, 2969–2973. [Google Scholar] [CrossRef]
- Ghafaripoora, M.; Raeissia, K.; Santamariab, M.; Hakimizada, A. The corrosion and tribocorrosion resistance of PEO composite coatings containing α-Al2O3 particles on 7075 Al alloy. Surf. Coat. Technol. 2018, 349, 470–479. [Google Scholar] [CrossRef]
- Premchand, C.; Manojkumar, P.; Lokeshkumar, E.; Rama Krishna, L.; Ravisankar, B.; Rameshbabu, N. Surface characteristics of AC PEO coatings fabricated on commercial Al alloys. Surf. Coat. Technol. 2022, 449, 128975. [Google Scholar] [CrossRef]
- Oh, Y.-J.; Mun, J.-I.; Kim, J.-H. Effects of alloying elements on microstructure and protective properties of Al2O coatings formed on aluminum alloy substrates by plasma electrolysis. Surf. Coat. Technol. 2009, 204, 141–148. [Google Scholar] [CrossRef]
- Gulec, A.E.; Gencer, Y.; Tarakci, M. The characterization of oxide based ceramic coating synthesized on Al-Si binary alloys by microarc oxidation. Surf. Coat. Technol. 2015, 269, 100–107. [Google Scholar] [CrossRef]
- Li, K.; Li, W.; Zhang, G.; Zhu, W.; Zheng, F.; Zhang, D.; Wang, M. Effects of Si phase refinement on the plasma electrolytic oxidation of eutectic Al-Si alloy. J. Alloys Compd. 2019, 790, 650–656. [Google Scholar] [CrossRef]
- Rogov, A.B.; Lyu, H.; Matthews, A.; Yerokhin, A. AC plasma electrolytic oxidation of additively manufactured and cast AlSi12 alloys. Surf. Coat. Technol. 2020, 399, 126116. [Google Scholar] [CrossRef]
- Mora-Sanchez, H.; Olmo, R.; Rams, J.; Torres, B.; Mohedano, M.; Matykina, E.; Arrabal, R. Hard Anodizing and Plasma Electrolytic Oxidation of an Additively Manufactured Al-Si alloy. Surf. Coat. Technol. 2021, 420, 127339. [Google Scholar] [CrossRef]
- Cengiz, S. Synthesis of eutectic Al–18Ce alloy and effect of cerium on the PEO coating growth. Mater. Chem. Phys. 2020, 247, 122897. [Google Scholar] [CrossRef]
- Letyagin, N.V.; Akopyan, T.K.; Sokorev, A.A.; Sviridova, T.A.; Cherkasov, S.O.; Mansurov, Y.N. The Characterization of Coatings Formed on As-Cast Al, Al–Si, and Al–Ca Aluminum Substrates by Plasma Electrolytic Oxidation. Metals 2023, 13, 1509. [Google Scholar] [CrossRef]
- Tarakci, M. Plasma electrolytic oxidation coating of synthetic Al-Mg binary alloys. Mater. Char. 2011, 62, 1214–1221. [Google Scholar] [CrossRef]
- Gencer, Y.; Gulec, A.E. The effect of Zn on the microarc oxidation coating behavior of synthetic Al-Zn binary alloys. J. Alloys Compd. 2012, 525, 159–165. [Google Scholar] [CrossRef]
- Oter, Z.C.; Gencer, Y.; Tarakci, M. The characterization of the coating formed by Microarc oxidation on binary Al-Mn alloys. J. Alloys Compd. 2015, 650, 185–192. [Google Scholar] [CrossRef]
- Cengiz, S.; Tarakci, M.; Gencer, Y.; Devecili, A.O.; Azakli, Y. Oxide based ceramic coating on Al-4Cu alloy by microarc oxidation. Acta Phys. Pol. A 2013, 123, 445–448. [Google Scholar] [CrossRef]
- Agureev, L.; Savushkina, S.; Ashmarin, A.; Borisov, A.; Apelfeld, A.; Anikin, K.; Tkachenko, N.; Gerasimov, M.; Shcherbakov, A.; Ignatenko, V.; et al. Study of Plasma Electrolytic Oxidation Coatings on Aluminum Composites. Metals 2018, 8, 459. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Shin, D.; Michi, R.A.; Poplawsky, J.D.; Wang, H.; Yang, Y.; Bahl, S.; Shyam, A.; Plotkowski, A. Effect of microalloying additions on microstructural evolution and thermal stability in cast Al-Ni alloys. J. Alloys Compd. 2024, 997, 174810. [Google Scholar] [CrossRef]
- Czerwinski, F.; Aniolek, M.; Li, J. Strengthening retention and structural stability of the Al-Al3Ni eutectic at high temperatures. Scr. Mater. 2022, 214, 114679. [Google Scholar] [CrossRef]
- Deng, J.W.; Chen, C.; Liu, X.C.; Li, Y.P.; Zhoua, M.; Guo, S.M. A high-strength heat-resistant Al-5.7Ni eutectic alloy with spherical Al3Ni nanoparticles by selective laser melting. Scripta Mater. 2021, 203, 114034. [Google Scholar] [CrossRef]
- Cosan, K.A.; Gunduz, K.O.; Tarakcı, M.; Gencer, Y. Plasma electrolytic oxidation of as-cast and heat-treated binary Al-Ni alloys. Surf. Coat. Technol. 2022, 450, 128998. [Google Scholar] [CrossRef]
- Shelekhov, E.V.; Sviridova, T.A. Programs for X-ray analysis of polycrystals. Met. Sci. Heat Treat. 2000, 42, 309–313. [Google Scholar] [CrossRef]
- ASTM G99-17; Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus. ASTM: West Conshohocken, PA, USA, 2017.
- ISO 4287-2014; Geometrical Product Specifications (GPS). Surface Texture. Profile Method. Terms, Definitions and Surface Texture Pa-rameters. Standartinform: Moscow, Russia, 2015.
- Ikeuba, A.I.; Zhang, B.; Wang, J.; Han, E.-H.; Ke, W.; Okafor, P.C. SVET and SIET Study of Galvanic Corrosion of Al/MgZn2 in Aqueous Solutions at Different pH. J. Electrochem. Soc. 2018, 165, 180–194. [Google Scholar] [CrossRef]
- Shi, J.; Mu, Y.; Shen, H.; Xing, N. Effect of Zn on Corrosion Resistance of 5083 Alloys. J. Phys. Conf. Ser. 2023, 2468, 012018. [Google Scholar] [CrossRef]
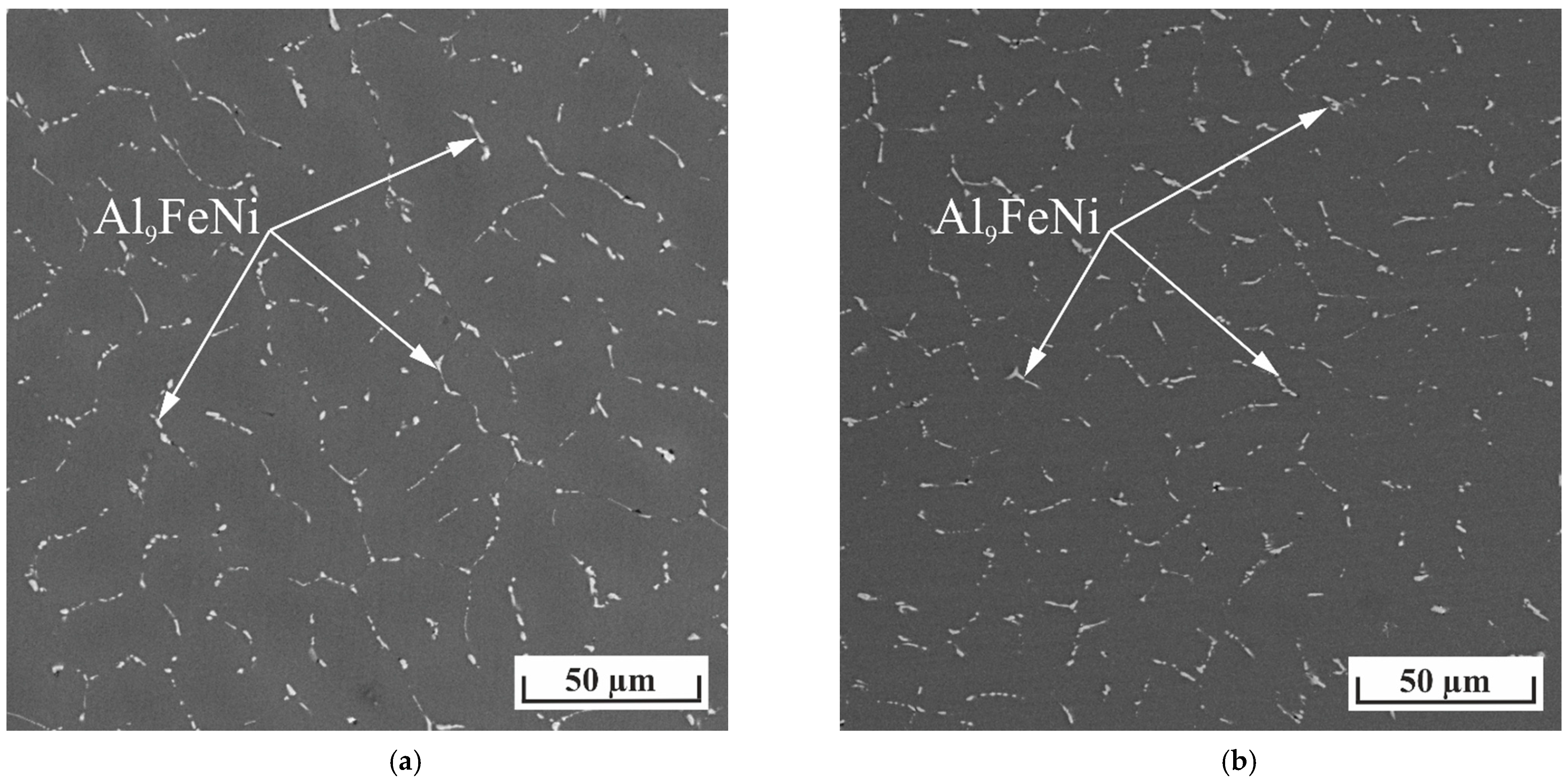

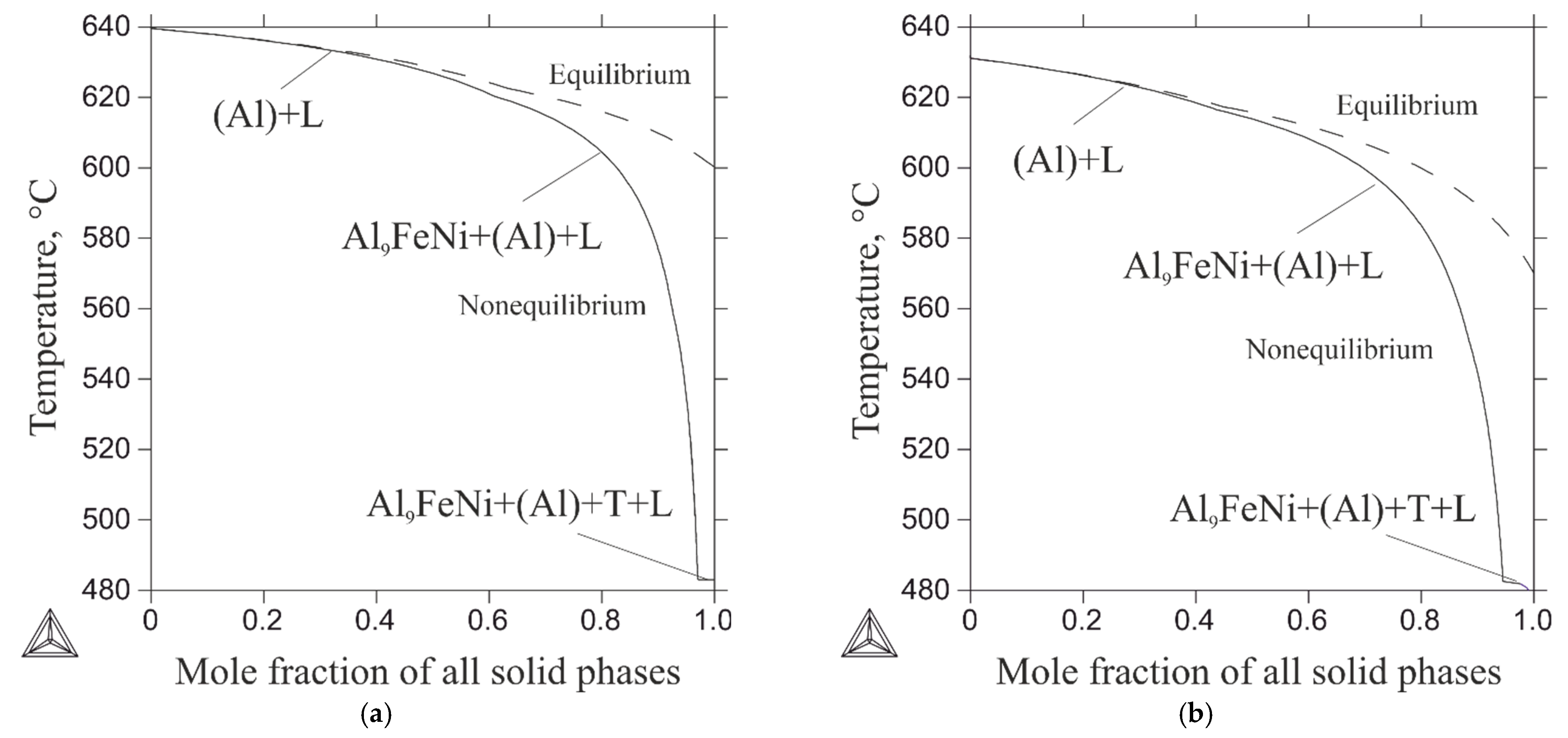
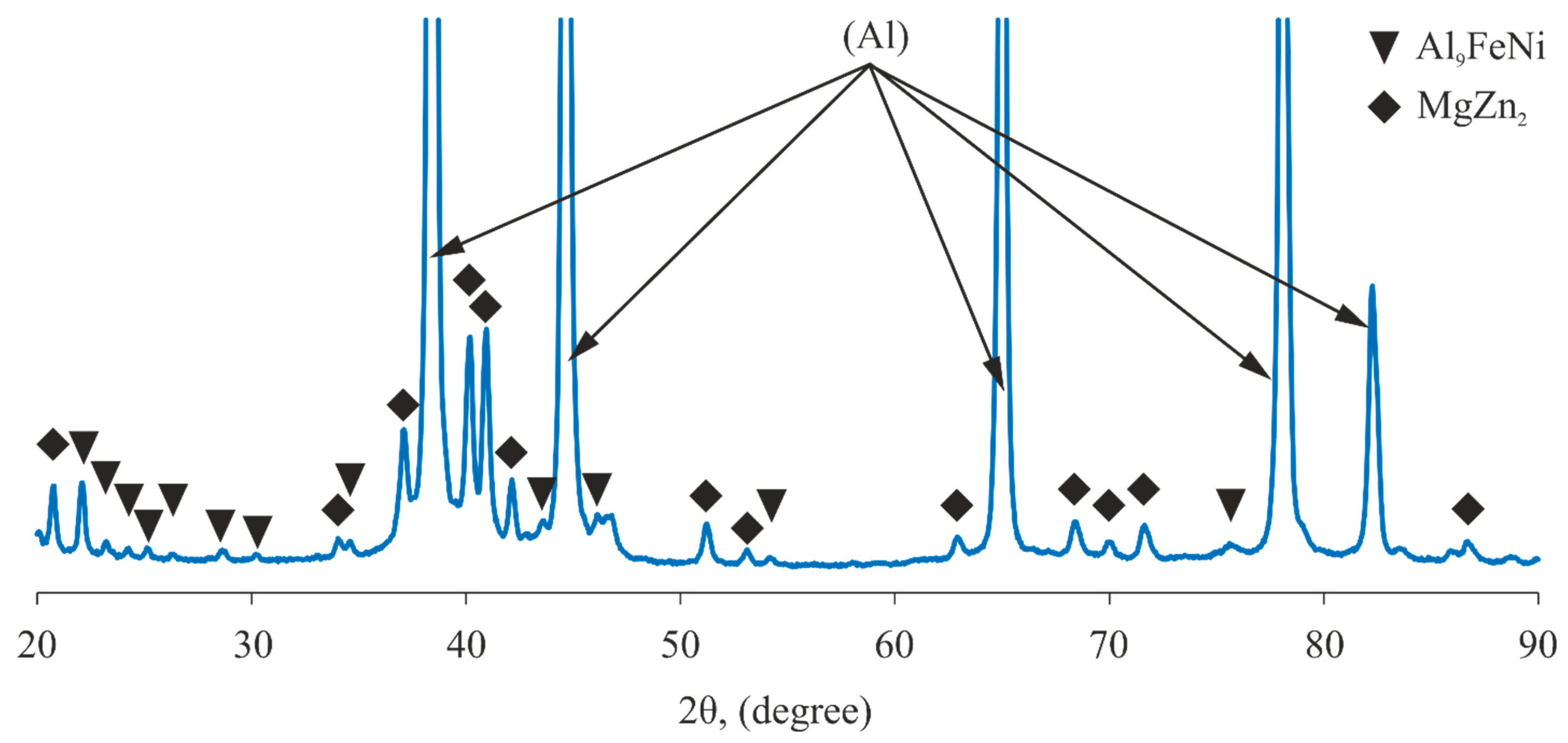
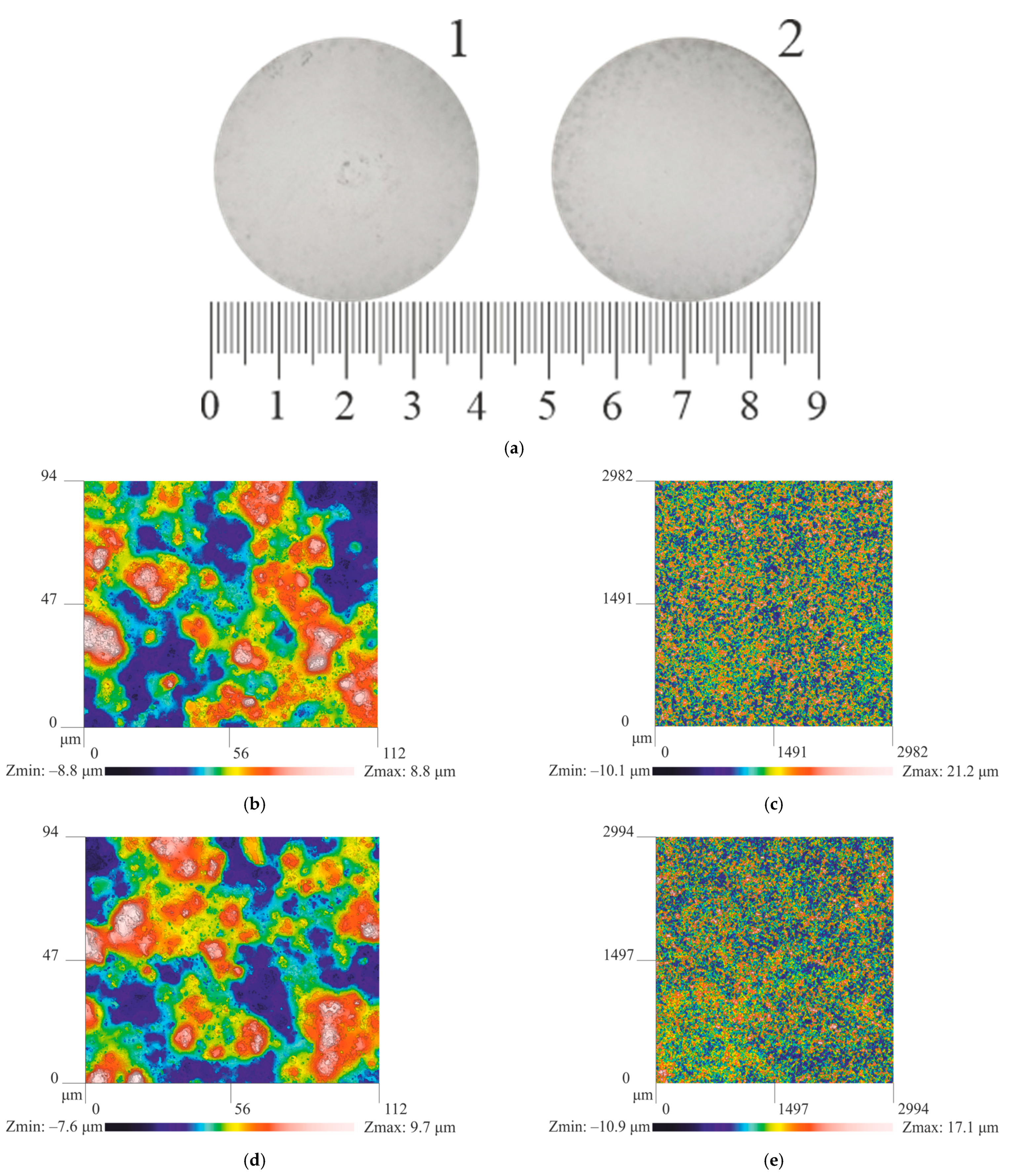


| No | Alloy | Actual Chemical Composition, wt.% | HV | |||||
|---|---|---|---|---|---|---|---|---|
| Al | Zn | Mg | Ni | Fe | Si | |||
| 1 | Al5.2Zn1.7Mg0.4Ni0.3Fe | balance | 5.2 | 1.7 | 0.4 | 0.3 | 0.04 | 108 |
| 2 | Al7.0Zn2.7Mg0.5Ni0.4Fe | balance | 7.0 | 2.7 | 0.6 | 0.4 | 0.06 | 182 |
| No | Alloy | Surface Roughness, μm | Thickness of Oxide Layer, μm | |
|---|---|---|---|---|
| Ra | Rz | |||
| 1 | Al5.2Zn1.65Mg0.4Ni0.3Fe | 2.6 | 18.2 | 50 |
| 2 | Al7.0Zn2.7Mg0.5Ni0.4Fe HT | 2.2 | 17.4 | 58 |
| Alloy | Microhardness, HV |
|---|---|
| Al5.2Zn1.65Mg0.4Ni0.3Fe | 954 (690–1200) |
| Al7.0Zn2.7Mg0.5Ni0.4Fe | 825(660–1040) |
| Sample | Coefficient of Friction | Wear Rate (mm3/N·m) |
|---|---|---|
| as-cast Al5.2Zn1.65Mg0.4Ni0.3Fe + PEO | 0.8 | 2.36 × 10−4 |
| heat-treated Al7.2Zn2.8Mg0.5Ni0.4Fe + PEO | 0.82 | 2.57 × 10−4 |
| Sample | Corrosion Potential, Ecorr. (mV) | Corrosion Current Density, icorr. (mA/cm2) |
|---|---|---|
| as-cast Al5.2Zn1.7Mg0.4Ni0.3Fe | −860 | 14.9 × 10−3 |
| as-cast Al5.2Zn1.7Mg0.4Ni0.3Fe + PEO | −804 | 2.6 × 10−3 |
| heat-treated Al7.0Zn2.7Mg0.5Ni0.4Fe | −932 | 45.1 × 10−3 |
| heat-treated Al7.0Zn2.7Mg0.5Ni0.4Fe + PEO | −920 | 12.2 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Letyagin, N.V.; Akopyan, T.K.; Sokorev, A.A.; Shkaley, I.V.; Cherkasov, S.O.; Doroshenko, V.V.; Sviridova, T.A.; Churyumov, A.Y. Plasma Electrolytic Oxidation of Al-Zn-Mg-Ni-Fe “Nikalin” Alloys. Metals 2024, 14, 680. https://doi.org/10.3390/met14060680
Letyagin NV, Akopyan TK, Sokorev AA, Shkaley IV, Cherkasov SO, Doroshenko VV, Sviridova TA, Churyumov AY. Plasma Electrolytic Oxidation of Al-Zn-Mg-Ni-Fe “Nikalin” Alloys. Metals. 2024; 14(6):680. https://doi.org/10.3390/met14060680
Chicago/Turabian StyleLetyagin, Nikolay V., Torgom K. Akopyan, Alexander A. Sokorev, Ivan V. Shkaley, Stanislav O. Cherkasov, Vitali V. Doroshenko, Tatiana A. Sviridova, and Alexander Yu. Churyumov. 2024. "Plasma Electrolytic Oxidation of Al-Zn-Mg-Ni-Fe “Nikalin” Alloys" Metals 14, no. 6: 680. https://doi.org/10.3390/met14060680







