Separation of Zr and Si in Zirconium Silicate by Sodium Hydroxide Sub-Molten Salt
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
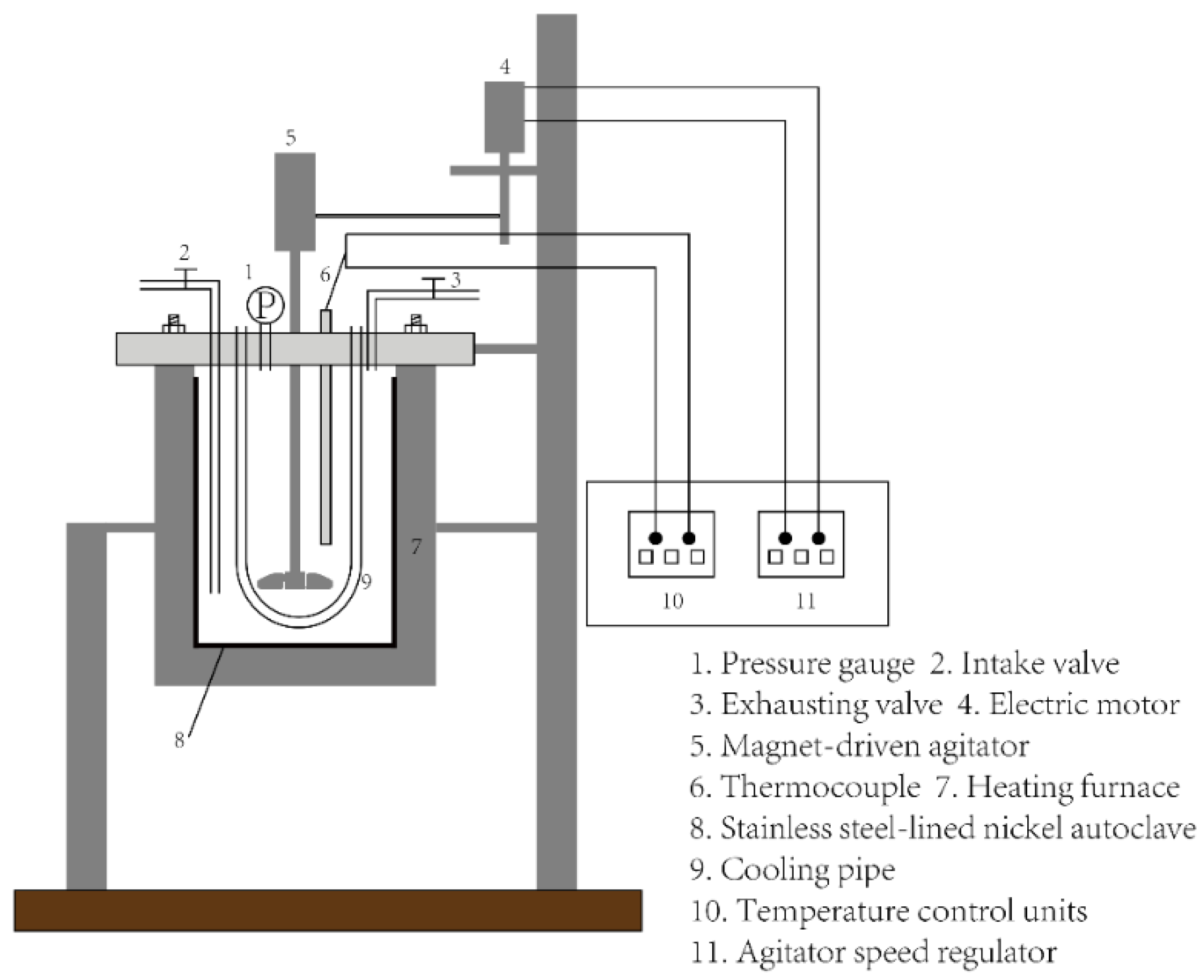
2.2. Experimental Device and Process
3. Results and Discussion
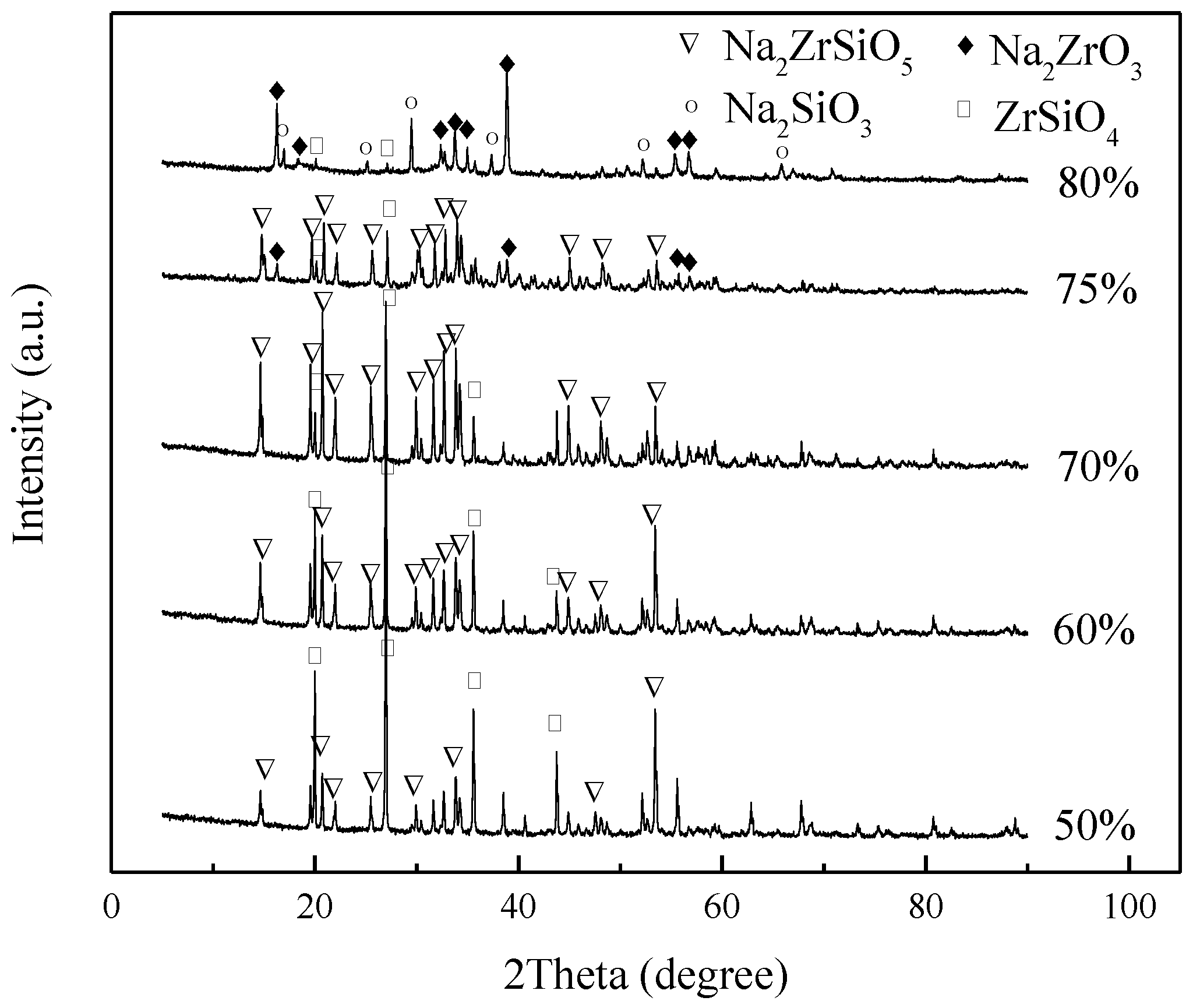
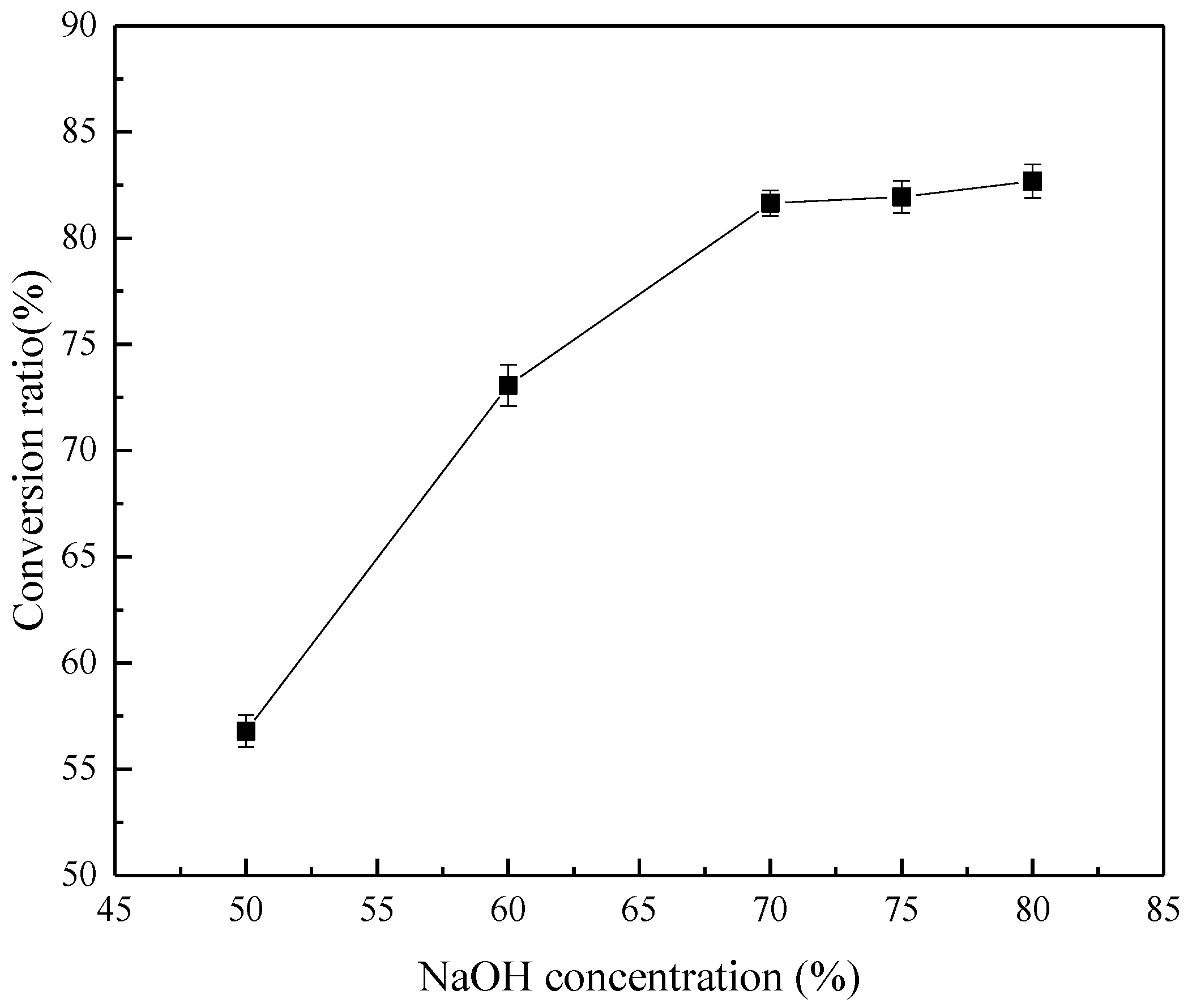
3.1. Effect of Initial NaOH Concentration
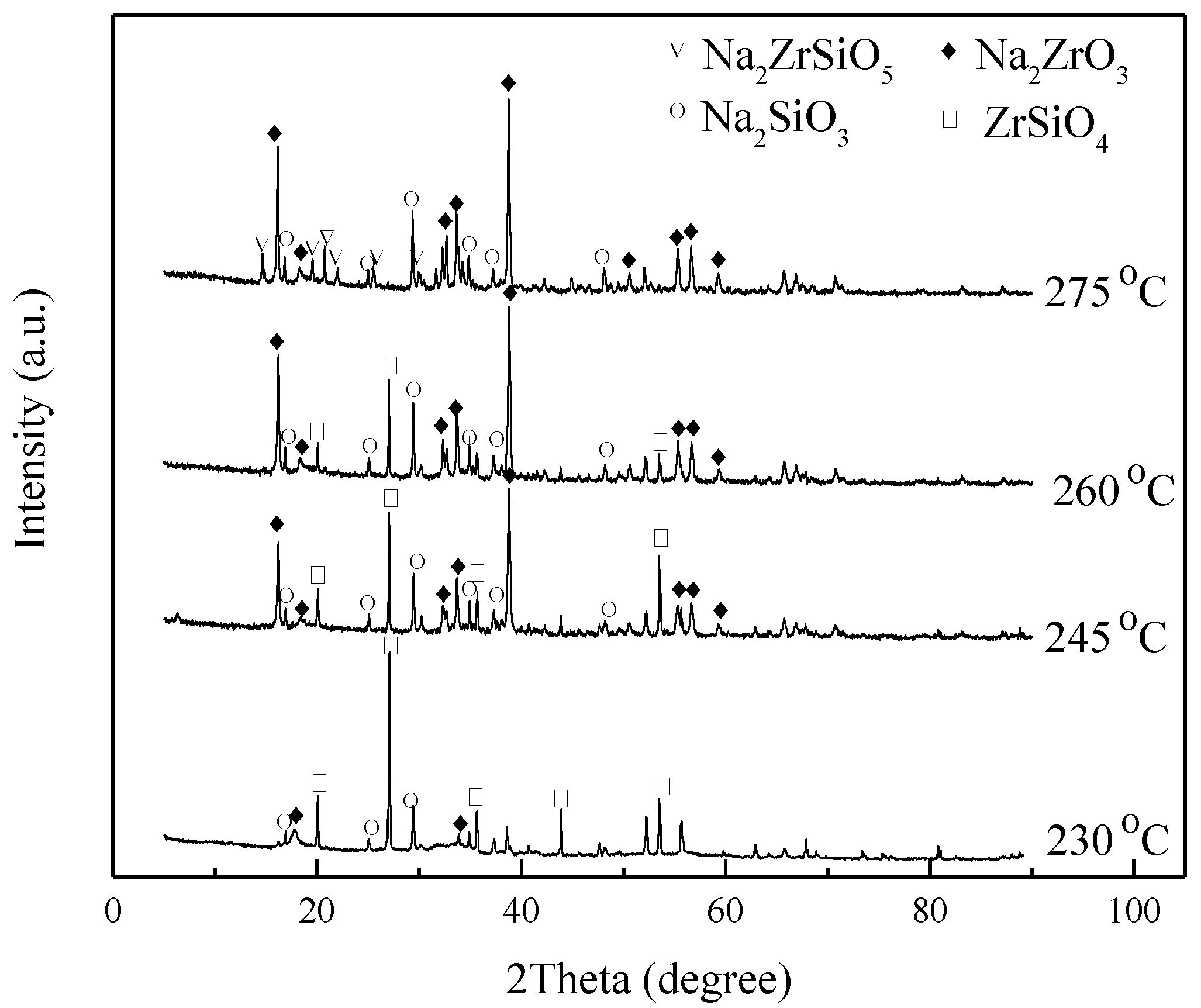
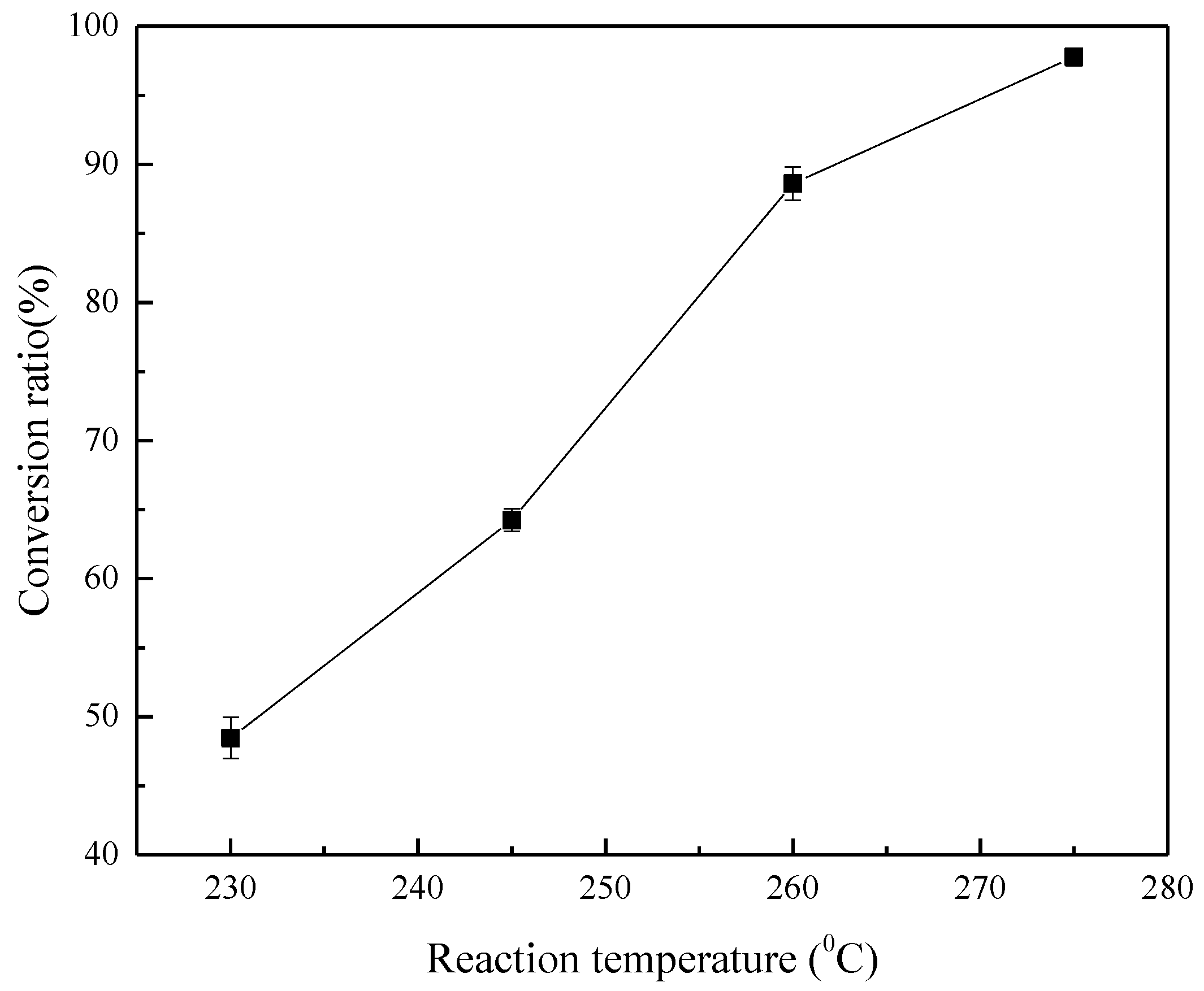
3.2. Effect of Reaction Temperature
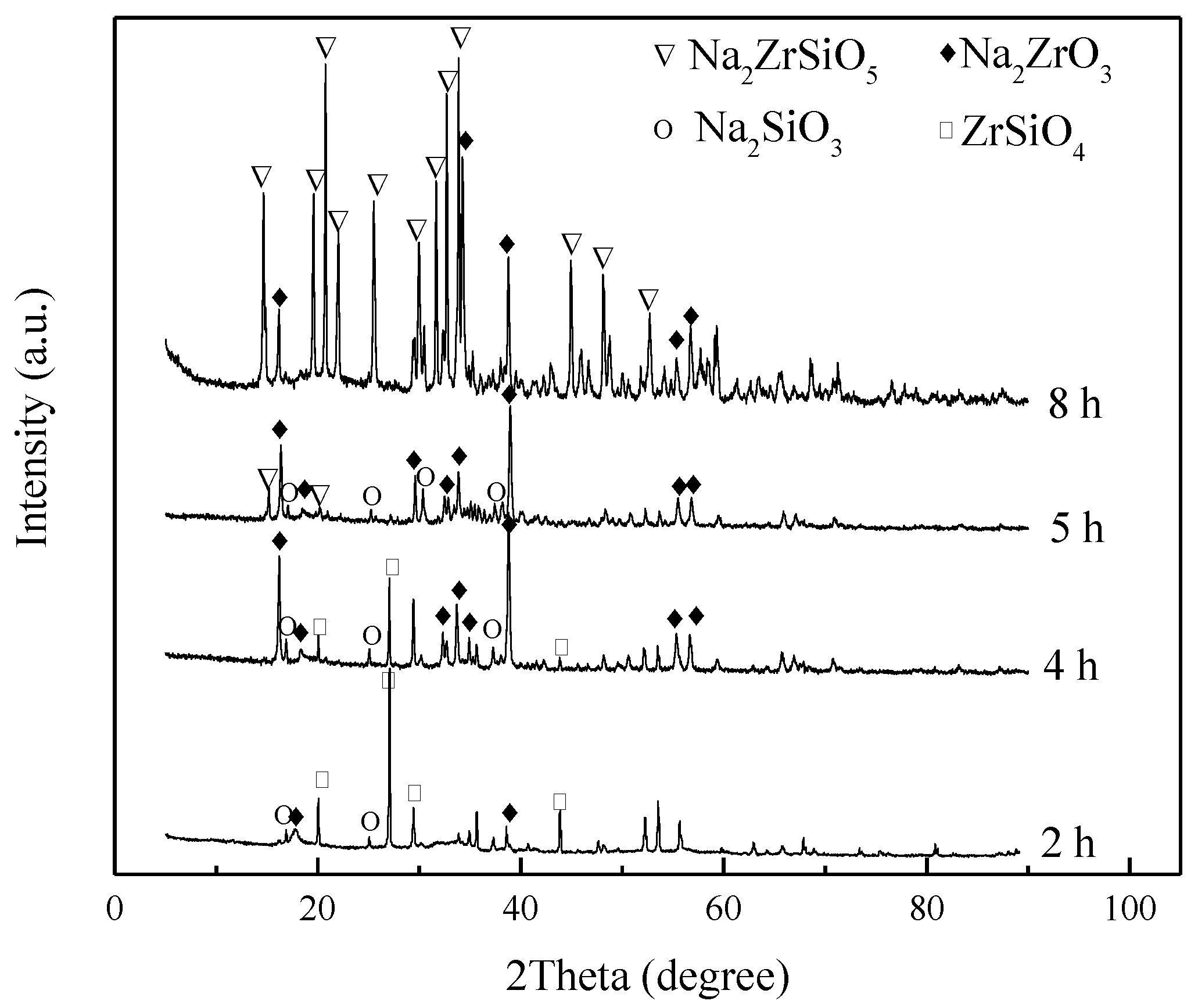
3.3. Effect of Reaction Time
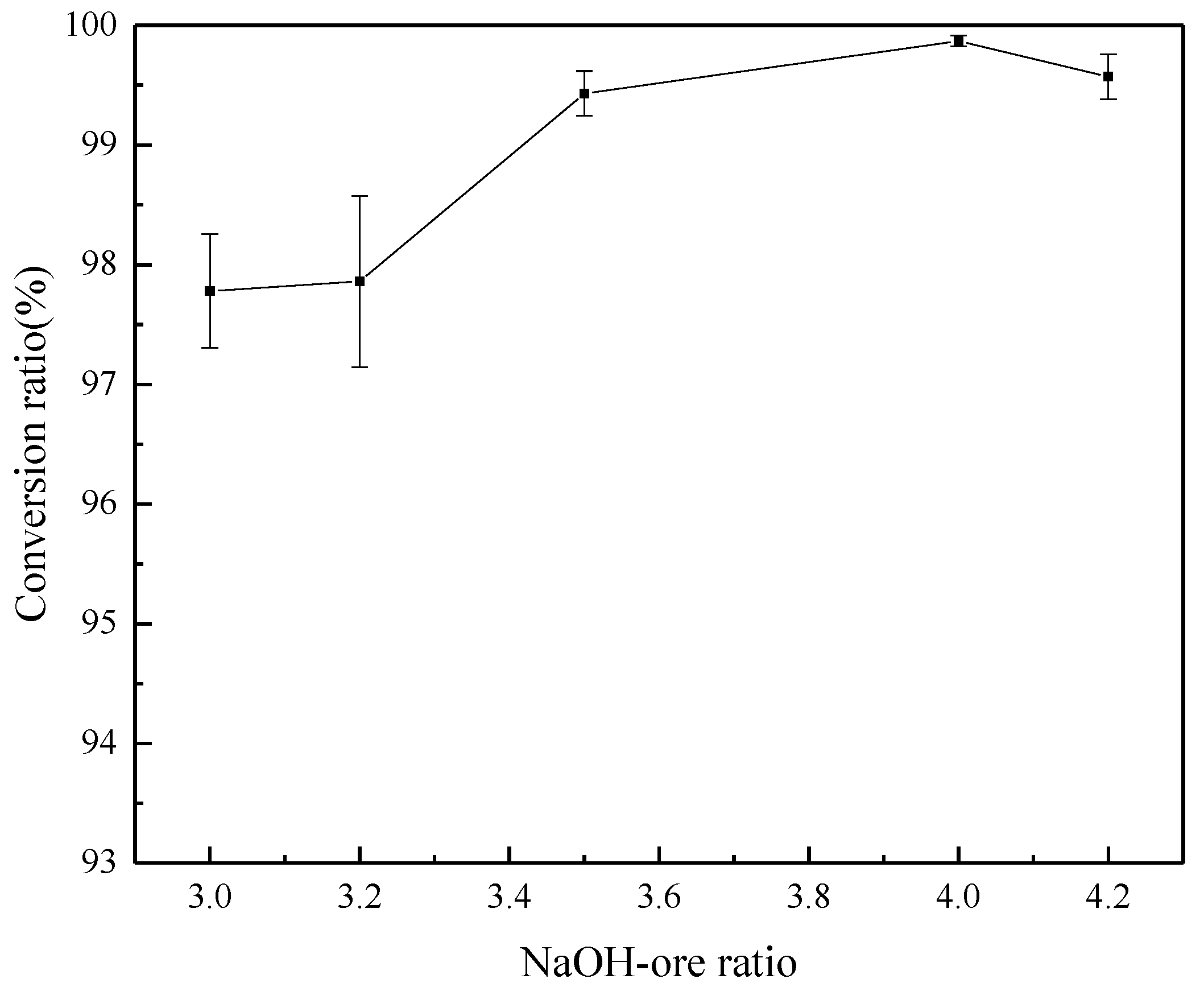
3.4. Effect of NaOH/Ore Mass Ratio
3.5. Optimum Technological Condition
3.6. Mechanism Analysis
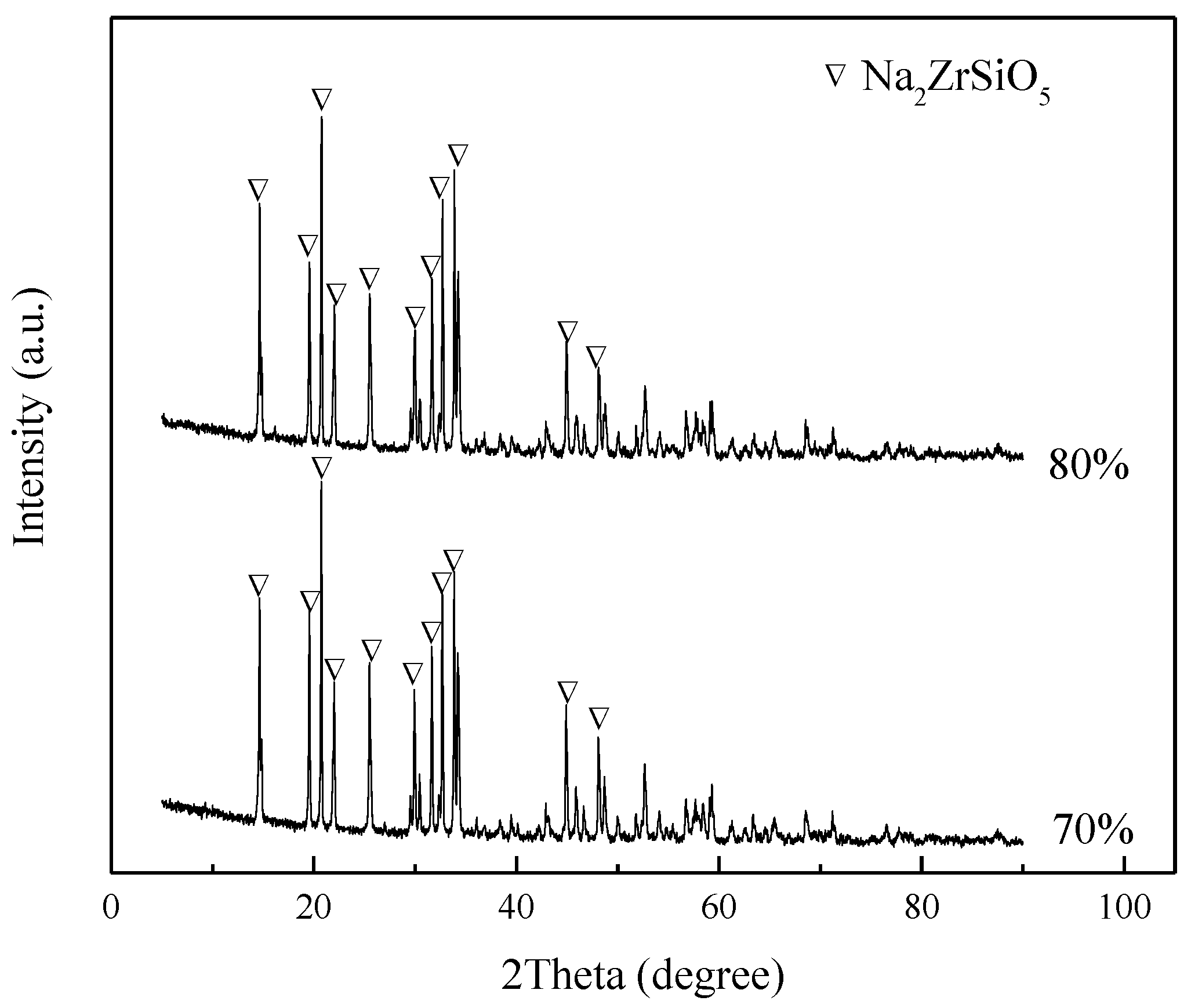
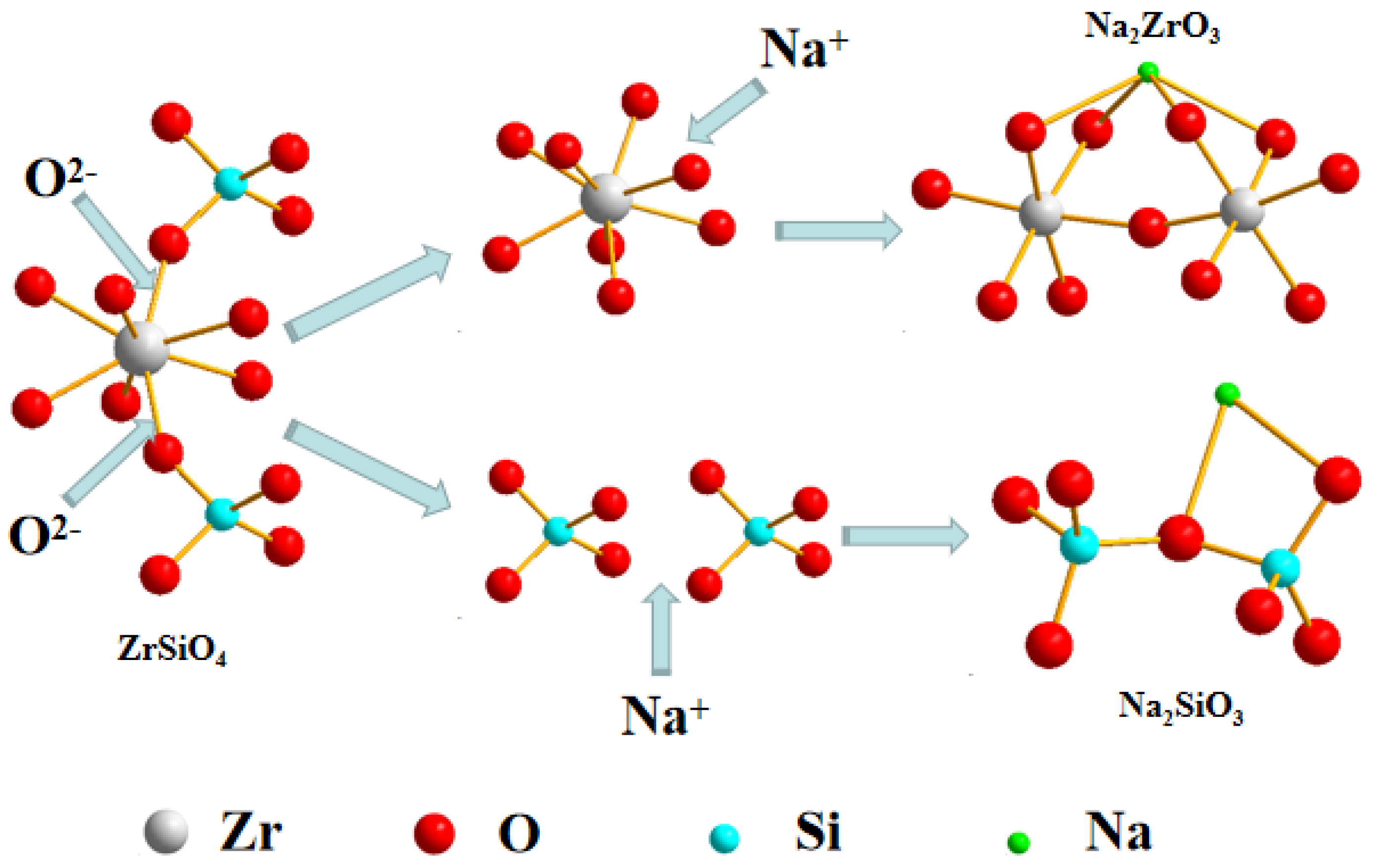
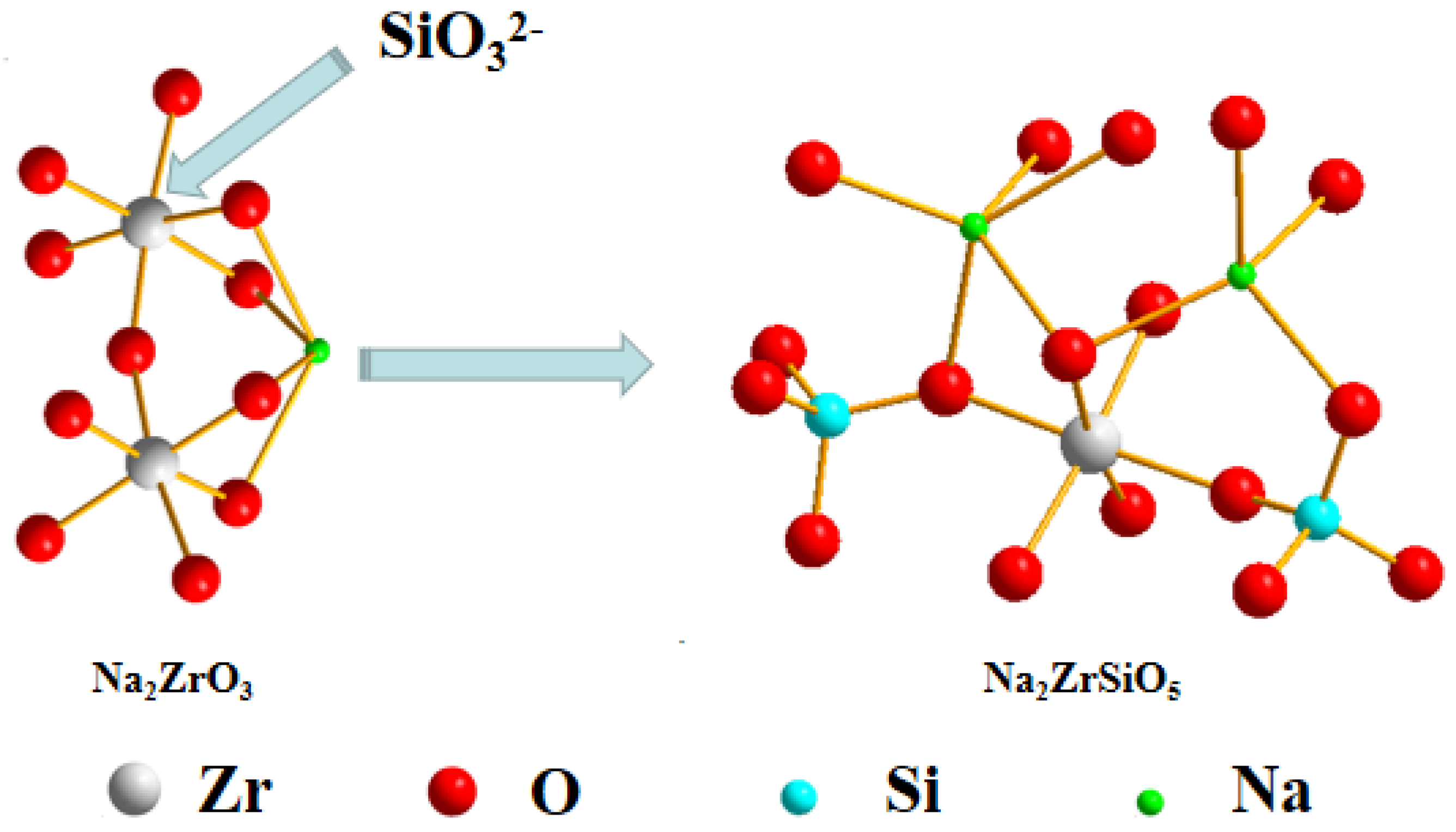
3.6.1. Speculation of Reaction Pathway
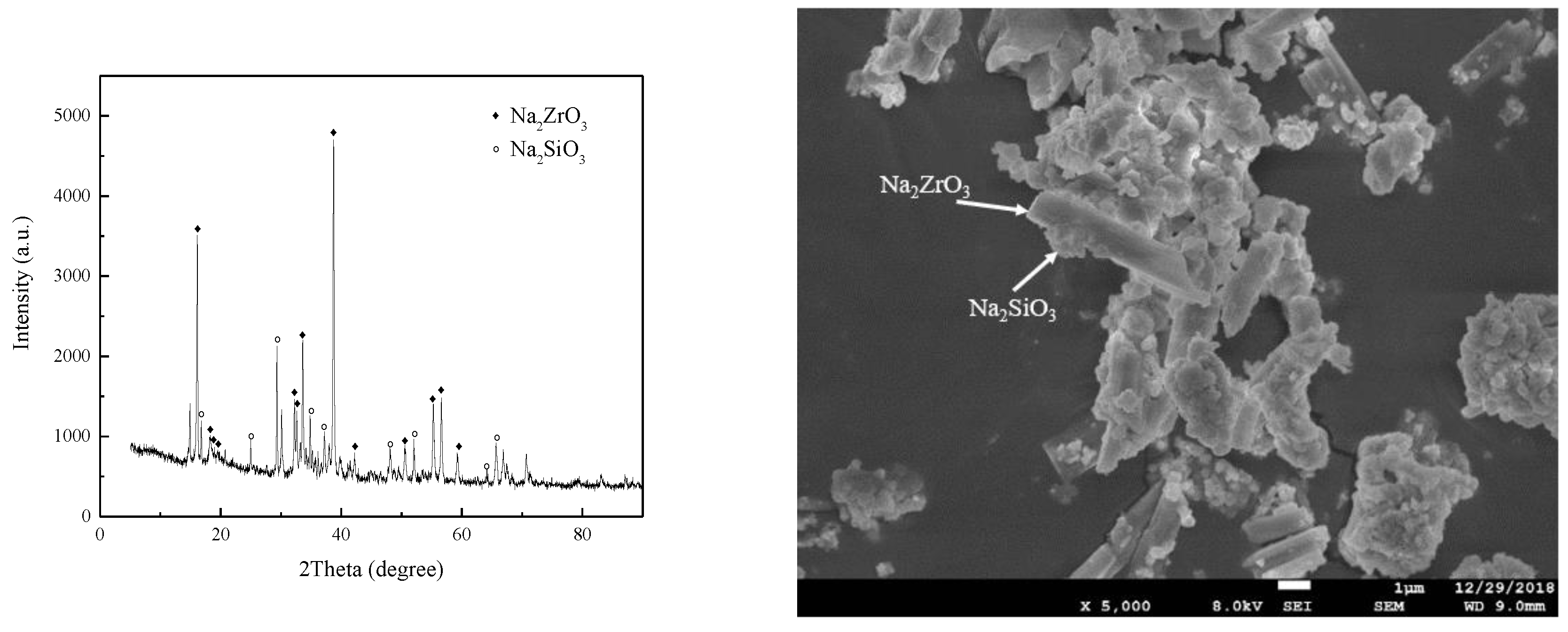
3.6.2. Verification of Reaction Pathway
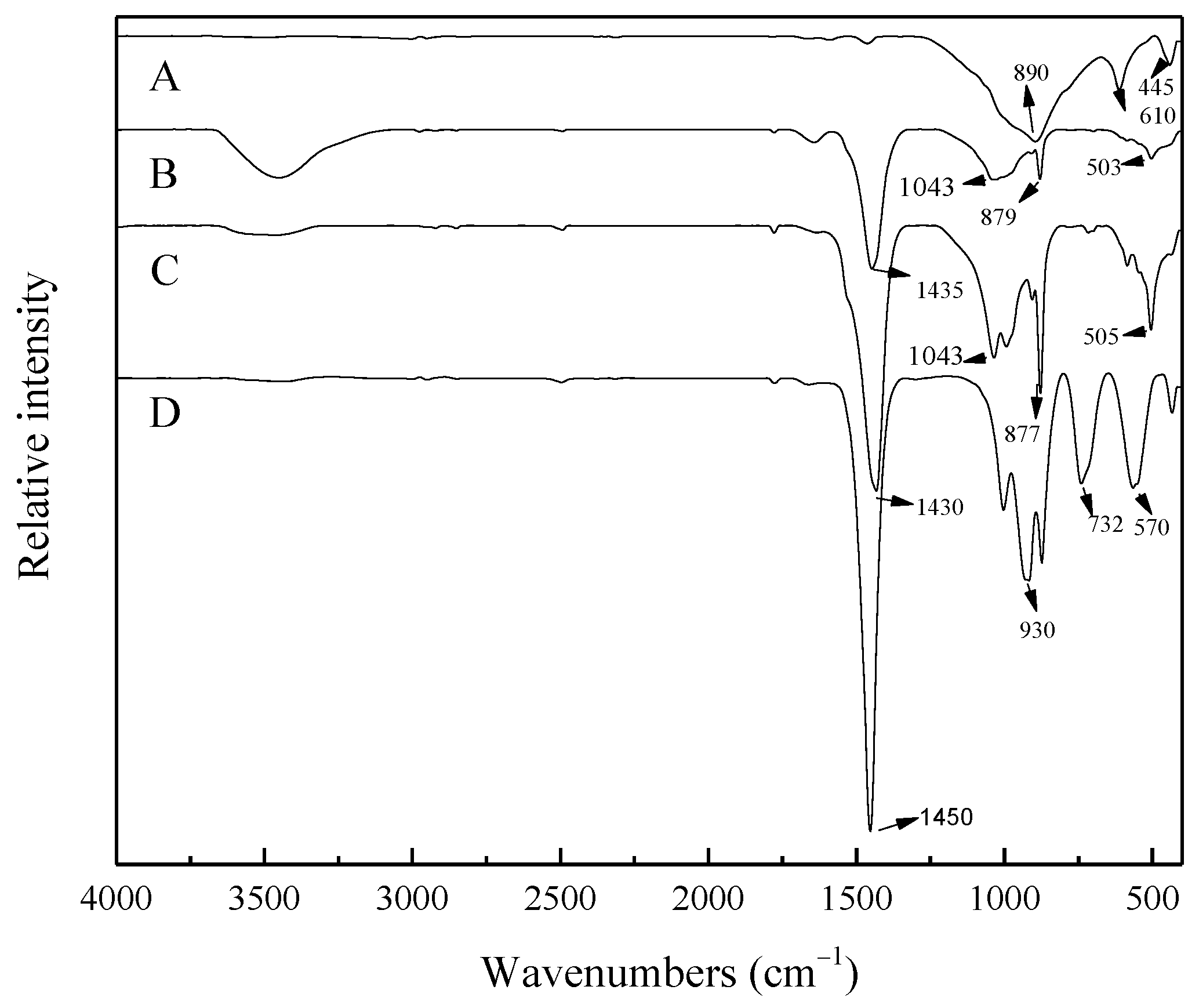
3.6.3. Infrared Spectroscopy
3.6.4. Raman Spectroscopy
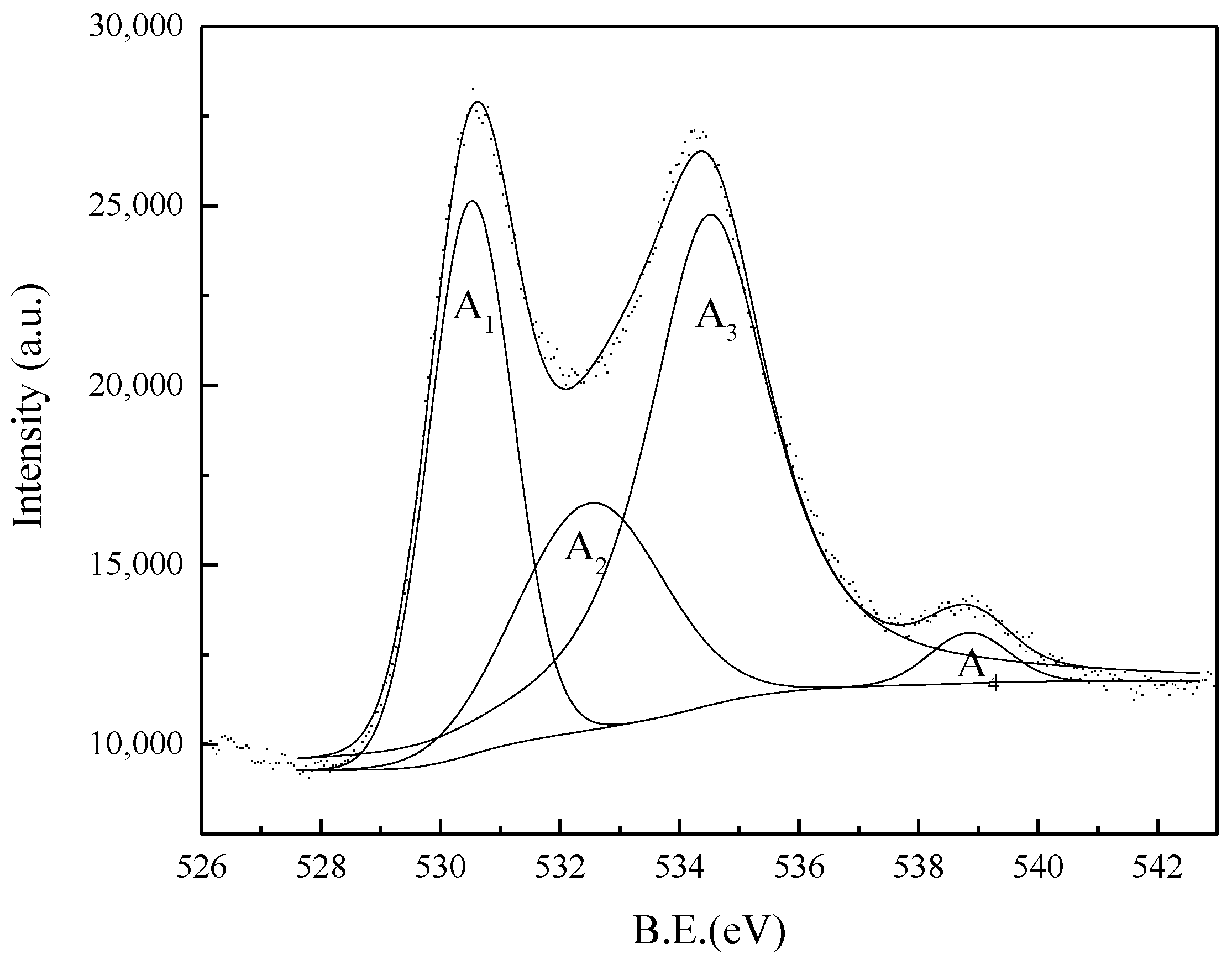
3.6.5. X-ray Photoelectron Spectroscopy
4. Conclusions
- The effects of alkali concentration, temperature, alkali/ore ratio, and reaction time on the product were explored. The product was extremely dependent on NaOH concentration. It was necessary to coordinate the relationship among NaOH concentration, temperature, alkali/ore ratio, and reaction time to strictly control the formation of Na2ZrSiO5.
- The optimum technological conditions were obtained: alkali concentration 80%, reaction temperature 245 °C, alkali/ore ratio 4:1, reaction time 10 h, stirring rate 400 r/min. Under these conditions, the product obtained was ensured to be Na2ZrO3, achieving effective separation of Zr and Si; further, a high decomposition rate of ZrSiO4 can be ensured.
- The reaction mechanism was investigated and verified. The reaction process of ZrSiO4 in sodium hydroxide sub-molten salt was obtained by XRD spectroscopy infrared spectroscopy, Raman spectroscopy, and XPS analysis. It is elucidated that ZiSiO4 is decomposed to Na2ZrO3 and Na2SiO3 by reacting with NaOH, and then the reactions between Na2ZrO3 and Na2SiO3 result in the formation of Na2ZrSiO5.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nikoofar, K.; Shaddel, N.; Jozi, F. Investigation of the Role of Zirconia and Zirconia-containing Systems as Catalysts in Organic Transformations. Curr. Org. Chem. 2024, 28, 433–462. [Google Scholar] [CrossRef]
- Ran, L.; Qu, J.; Jing, S.; Tao, Q.; Du, A. Analysis of water leaching and transition processes in zirconium oxychloride octahydrate production. Ceram. Int. 2014, 40, 1431–1438. [Google Scholar]
- Liu, J.; Jing, S.; Tao, Q.; Zhang, C.; Qu, J. Controlling the formation of Na2ZrSiO5 in alkali fusion process for zirconium oxychloride production. Adv. Powder Technol. 2015, 27, 1–8. [Google Scholar] [CrossRef]
- Irankhah, R.; Mobasherpour, I.; Alizadeh, M.; Nezhad, S.M.M.; Nikzad, L.; Azar, S.S. Carbothermal reduction of ZrSiO4 for in situ formation of ZrO2-based composites using spark plasma sintering. Ceram. Int. 2023, 49, 2681–2688. [Google Scholar] [CrossRef]
- Fernández-González, D.; Piñuela-Noval, J.; Ruiz-Bustinza, I.; González-Gasca, C.; Gómez-Rodríguez, C.; Quiñonez, L.V.G.; Fernández, A.; Verdejaf, L.F. Solar dissociation of zirconium silicate sand: A clean alternative to obtain zirconium dioxide. J. Clean. Prod. 2023, 420, 138371. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Zhao, Y.; Azhati, A.; Wang, X.; Zhang, Z.; Lv, X. First Principles Investigation of C, Cl2 and CO Co-Adsorption on ZrSiO4 Surfaces for Carbochlorination Reaction. Materials 2024, 17, 1500. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.W.; Zhao, P.; Wu, M.Q.; Yan, P.G.; Gao, X.; Li, J.G.; Huang, J.J.; Lin, X.D.; Jiang, Y.M.; Jin, X.Y.; et al. Effect of H2 addition on the preparation of ZrO2 powder from zircon (ZrSiO4) using a plasma torch. Ceram. Int. 2024, 50, 1360–1369. [Google Scholar] [CrossRef]
- Biswas, R.K.; Habib, M.A.; Karmakar, A.K.; Islam, M.R. A novel method for processing of Bangladeshi zircon: Part I: Baking, and fusion with NaOH. Hydrometallurgy 2010, 103, 124–129. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Jiang, D. Decomposition process of zircon sand concentrate with CaO-NaOH. Rare Met. 2012, 31, 410–414. [Google Scholar] [CrossRef]
- Manhique, A.; Kwela, Z.; Focke, W.W. De Wet Process for the Beneficiation of Zircon: Optimization of the Alkali Fusion Step. Ind. Eng. Chem. Res. 2003, 42, 777–783. [Google Scholar] [CrossRef]
- Silva, R.J.F.D.; Dutra, A.J.B.; Afonso, J.C. Alkali fusion followed by a two-step leaching of a Brazilian zircon concentrate. Hydrometallurgy 2012, 117–118, 93–100. [Google Scholar] [CrossRef]
- Wei, J.; Han, B.; Wei, Y.; Li, N.; Miao, Z. Influence of phase evolution and thermal decomposition kinetics on the properties of zircon ceramic. Ceram. Int. 2021, 47, 27285–27293. [Google Scholar] [CrossRef]
- Wei, J.; Han, B.; Wei, Y.; Li, N.; Wang, Y.; Duan, J.; Fu, J. Decomposition mechanism, sintering process and properties of zircon ceramics: Role of CaO, MnO and Cr2O3. Ceram. Int. 2022, 48, 34289–34297. [Google Scholar] [CrossRef]
- Song, J.; Fan, J.F.; Liu, J.C.; Liu, R.; Qu, J.K.; Qi, T. A two-step zircon decomposition method to produce zirconium oxychloride: Alkali fusion and water leaching. Rare Met. 2020, 39, 448–454. [Google Scholar] [CrossRef]
- Zhu, S.; Mei, J.; Zeng, W.W.; Lu, S.Y.; Su, M.H.; Zhan, H.R.; Huang, Y.S.; Yang, S.L.; Liu, Y.Z. Facile synthesis of TiNb2O7 anode material by KOH sub-molten salt method for lithium-ion batteries. Mater. Lett. 2024, 360, 135980. [Google Scholar] [CrossRef]
- Mohamed, N.; Ornek, C.; Timur, S.; Uergen, M. Anodic behavior of nickel in sub-molten KOH and its relevance for the production of electroactive nickel oxides. Surf. Interfaces 2022, 31, 101963. [Google Scholar] [CrossRef]
- Chen, J.; Guo, S.H.; Omran, M.; Gao, L.; Zheng, H.W.; Chen, G. Microwave-assisted preparation of nanocluster rutile TiO2 from titanium slag by NaOH-KOH mixture activation. Adv. Powder Technol. 2022, 33, 103549. [Google Scholar] [CrossRef]
- Li, S.C.; Kang, C.S.; Kim, S.C.; Kim, C.J.; Kang, C.J. The extraction of Ta, Nb and rare earths from fergusonite by using KOH sub-molten salt leaching. Hydrometallurgy 2021, 201, 105358. [Google Scholar] [CrossRef]
- Sun, H.-Q.; Song, J.; Sun, S.; Qu, J.-K.; Lu, W.; Qi, T. Decomposition kinetics of zircon sand in NaOH sub-molten salt solution. Trans. Nonferrous Met. Soc. China 2019, 29, 1948–1955. [Google Scholar] [CrossRef]
- Ongwandee, M.; Morrison, G.C.; Guo, X.; Chusuei, C.C. Adsorption of trimethylamine on zirconium silicate and polyethylene powder surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2007, 310, 62–67. [Google Scholar] [CrossRef]
- Zhang, M.; Salje, E.K.H.; Wang, A.H.; Li, X.J.; Xie, C.S.; Redfern, S.A.T.; Li, R.X. Vibrational spectroscopy of fast-quenched ZrSiO4 melts produced by laser treatments: Local structures and decomposed phases. J. Phys. Condens. Matter 2005, 17, 6363. [Google Scholar] [CrossRef]
- Zhao, C.M.; Wang, G.C.; Sheng-Li, L.I.; Xin-Gang, A.I.; Wang, Z.R.; Zhai, Y.C. Reaction pathway led by silicate structure transformation on decomposition of CaSiO3 in alkali fusion process using NaOH. Trans. Nonferrous Met. Soc. China 2015, 25, 3827–3833. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Singh, S.; Pandey, O.P. Neutron irradiation effects on optical and structural properties of silicate glasses. Mater. Chem. Phys. 2009, 115, 783–788. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Q.; Xu, M.; Wang, S.; You, J. In situ spectroscopic studies of decomposition of ZrSiO4 during alkali fusion process using various hydroxides. Rsc Adv. 2015, 5, 11658–11666. [Google Scholar] [CrossRef]
- Nasdala, L.; Irmer, G.; Wolf, D. The degree of metamictization in zircon: A Raman spectroscopic study. Eur. J. Mineral. 1995, 7, 471–478. [Google Scholar] [CrossRef]
- Kim, B.K.; Hamaguchi, H.O. Mode Assignments of the Raman Spectrum of Monoclinic Zirconia by Isotopic Exchange Technique. Phys. Status Solidi 1997, 203, 557–563. [Google Scholar] [CrossRef]
- Zhang, M.; Salje, E.K.H. Infrared spectroscopic analysis of zircon: Radiation damage and the metamictstate. J. Phys. Condens. Matter 2001, 13, 3057. [Google Scholar] [CrossRef]
- Guittet, M.J.; Crocombette, J.P.; Gautier-Soyer, M. Bonding and XPS chemical shifts in ZrSiO4 versus SiO2 and ZrO2 Charge transfer and electrostatic effects. Phys. Rev. B 2001, 63, 125117. [Google Scholar] [CrossRef]
- Moon, S.C.; Fujino, M.; Yamashita, H.; Anpo, M. Characterization of Zirconium−Silicon Binary Oxide Catalysts Prepared by the Sol−Gel Method and Their Photocatalytic Activity for the Isomerization of 2-Butene. J. Phys. Chem. B 1997, 101, 444–450. [Google Scholar] [CrossRef]
- Vipul, B.; Asad, S.; Bhargava, S.K.; Absar, A.; Murali, S. Zirconia enrichment in zircon sand by selective fungus-mediated bioleaching of silica. Langmuir 2007, 23, 4993–4998. [Google Scholar]
- Balan, E.; Trocellier, P.; Jupille, J.; Fritsch, E.; Muller, J.P.; Calas, G. Surface chemistry of weathered zircons. Chem. Geol. 2001, 181, 13–22. [Google Scholar] [CrossRef]

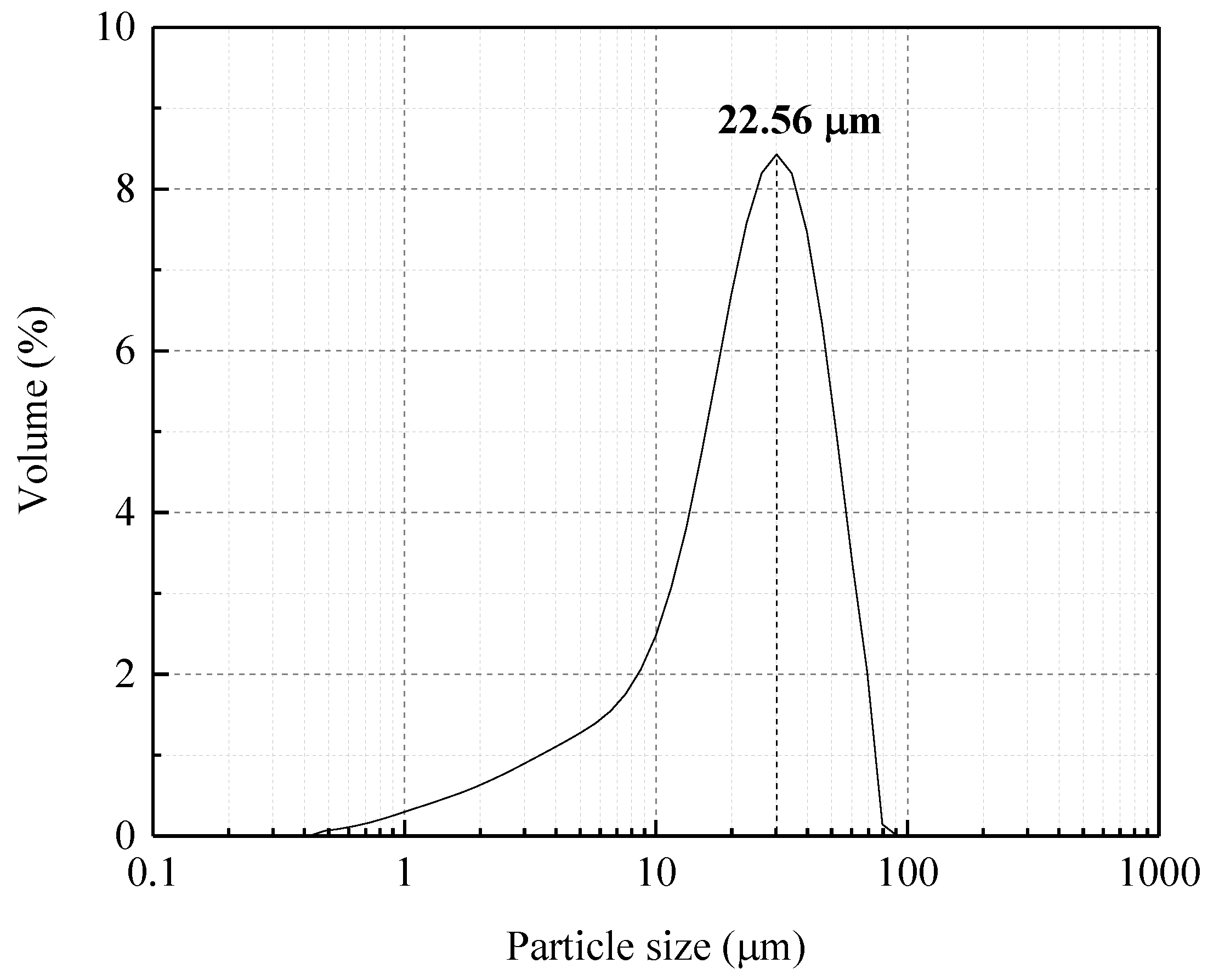







| ZrO2 | HfO2 | SiO2 | Al2O3 | TiO2 | P2O5 | Y2O3 | Fe2O3 | As2O3 | CaO | IrO2 | PbO | Nd2O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 64.53 | 1.41 | 31.46 | 0.73 | 0.45 | 0.32 | 0.23 | 0.17 | 0.16 | 0.16 | 0.14 | 0.12 | 0.12 |
| NaOH Concentration | Reaction Temperature (°C) | Time (h) | Phase | ||
|---|---|---|---|---|---|
| 3:1 | 3.5:1 | 4:1 | |||
| 70% | 260 | 4 | ZS, NZS | ZS, NZS | ZS, NZS |
| 6 | ZS, NZS | ZS, NZS | NZS | ||
| 8 | NZS | NZS | NZS | ||
| 10 | NZS | NZS | NZS | ||
| 80% | 230 | 4 | ZS, NZ | ZS, NZ | ZS, NZ |
| 6 | ZS, NZ | ZS, NZ | ZS, NZ | ||
| 8 | ZS, NZ | ZS, NZ | ZS, NZ | ||
| 10 | ZS, NZ | ZS, NZ | ZS, NZ | ||
| 245 | 4 | ZS, NZ | ZS, NZ | ZS, NZ | |
| 6 | ZS, NZ | ZS, NZ | ZS, NZ | ||
| 8 | ZS, NZ | ZS, NZ | ZS, NZ | ||
| 10 | ZS, NZ | ZS, NZ | NZ | ||
| 260 | 4 | ZS, NZ | ZS, NZ | ZS, NZ | |
| 6 | ZS, NZ, NZS | ZS, NZ | ZS, NZ | ||
| 8 | NZ, NZS | NZ, NZS | NZ, NZS | ||
| 10 | NZS | NZS | NZS | ||
| 275 | 4 | NZ, NZS | NZ, NZS | NZ, NZS | |
| 6 | NZ, NZS | NZ, NZS | NZ, NZS | ||
| 8 | NZ, NZS | NZ, NZS | NZ, NZS | ||
| 10 | NZS | NZS | NZS | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Song, J.; Qi, T. Separation of Zr and Si in Zirconium Silicate by Sodium Hydroxide Sub-Molten Salt. Metals 2024, 14, 630. https://doi.org/10.3390/met14060630
Sun H, Song J, Qi T. Separation of Zr and Si in Zirconium Silicate by Sodium Hydroxide Sub-Molten Salt. Metals. 2024; 14(6):630. https://doi.org/10.3390/met14060630
Chicago/Turabian StyleSun, Hongqian, Jing Song, and Tao Qi. 2024. "Separation of Zr and Si in Zirconium Silicate by Sodium Hydroxide Sub-Molten Salt" Metals 14, no. 6: 630. https://doi.org/10.3390/met14060630
APA StyleSun, H., Song, J., & Qi, T. (2024). Separation of Zr and Si in Zirconium Silicate by Sodium Hydroxide Sub-Molten Salt. Metals, 14(6), 630. https://doi.org/10.3390/met14060630






