Effect of Electro-Pulse on Microstructure of Al-Cu-Mn-Zr-V Alloy during Aging Treatment and Mechanism Analysis
Abstract
:1. Introduction
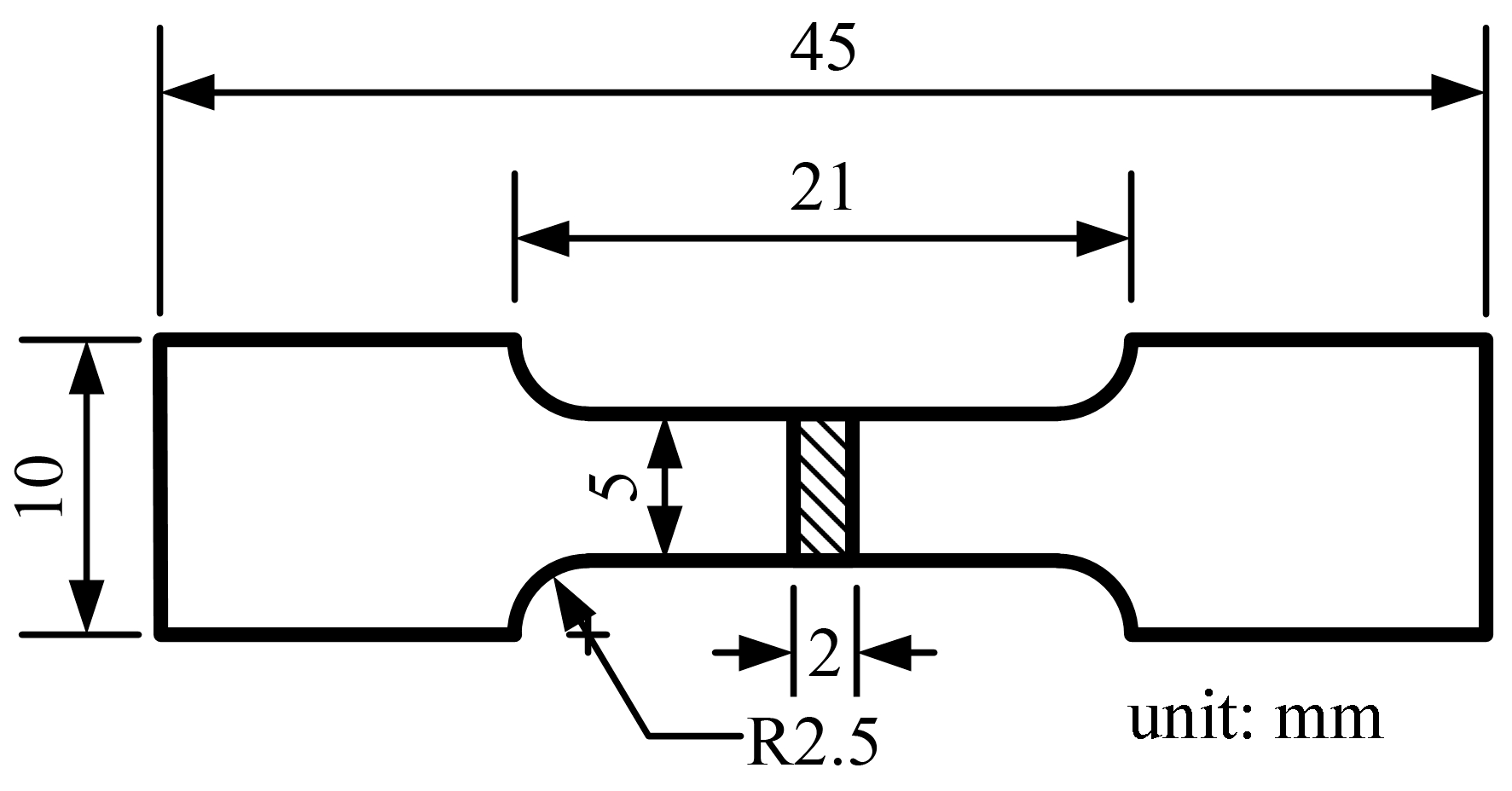
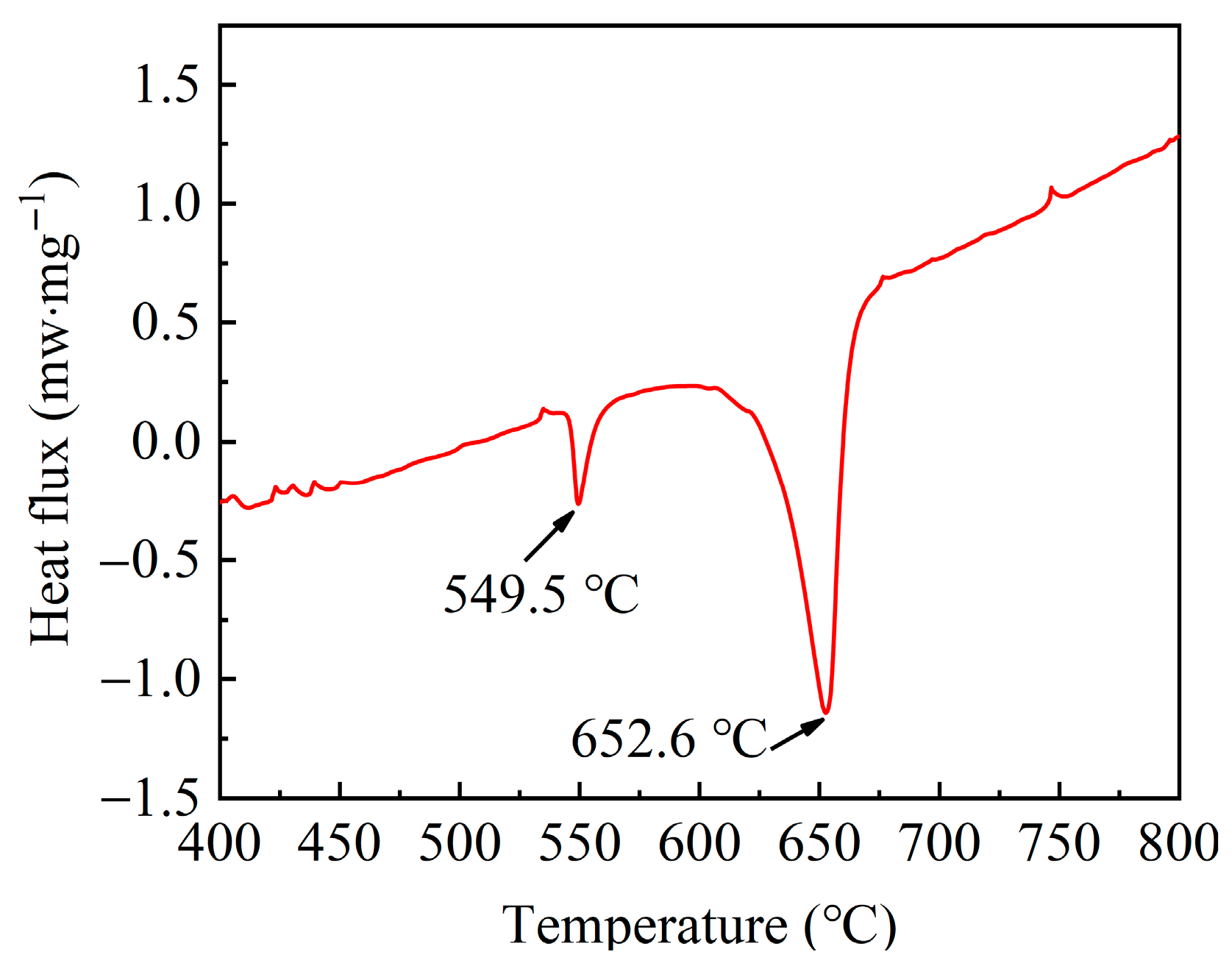
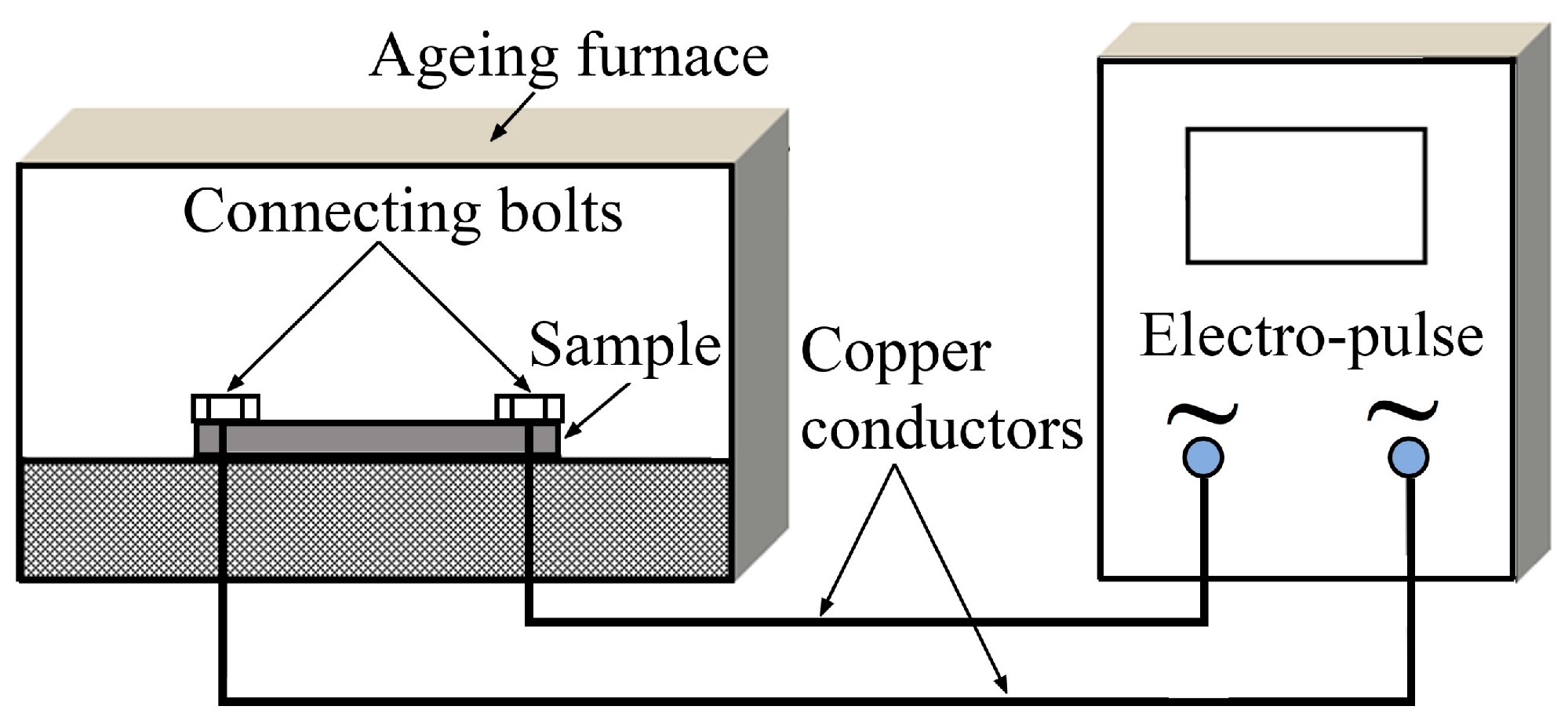
2. Materials and Methods
3. Results and Analysis
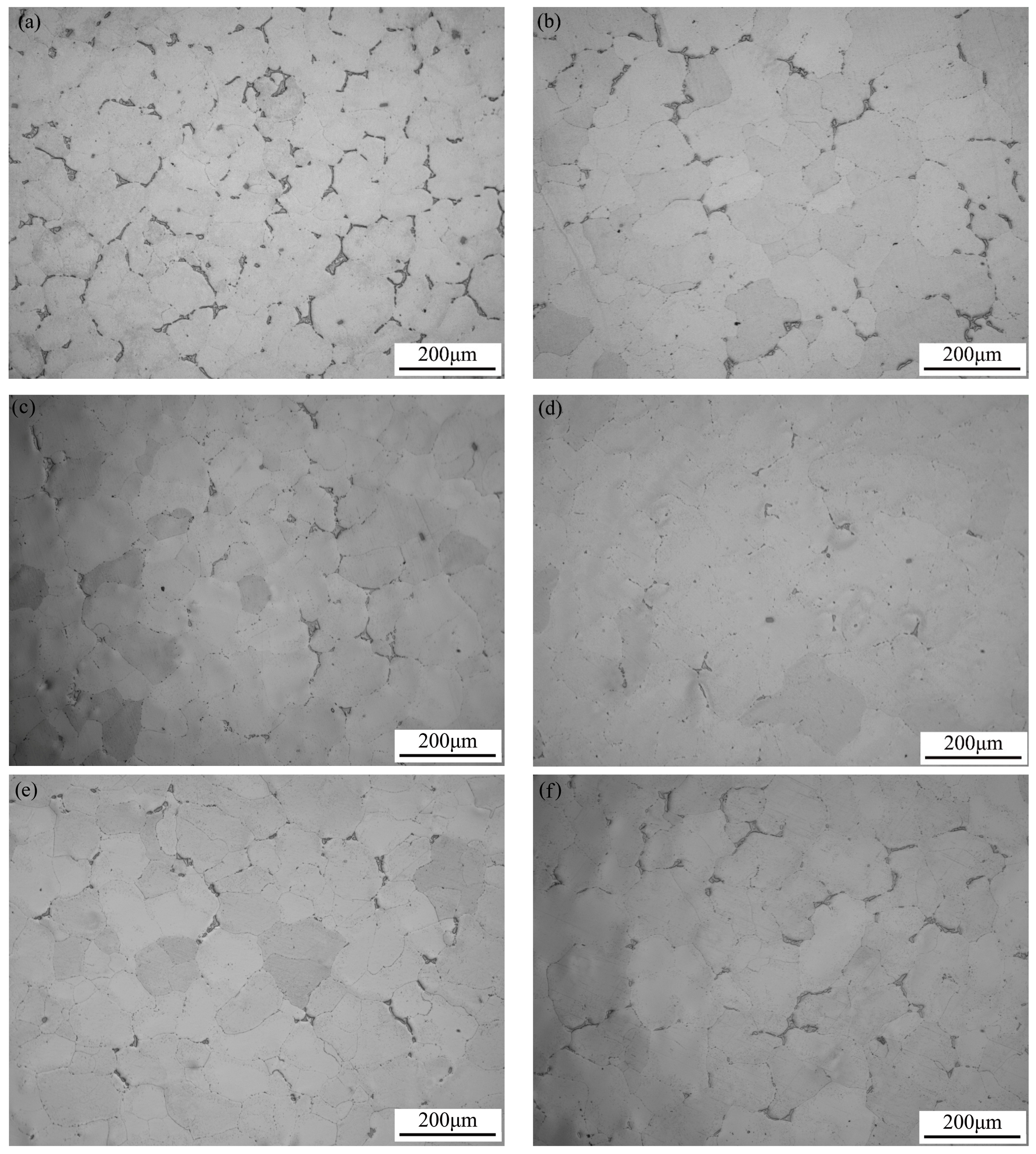
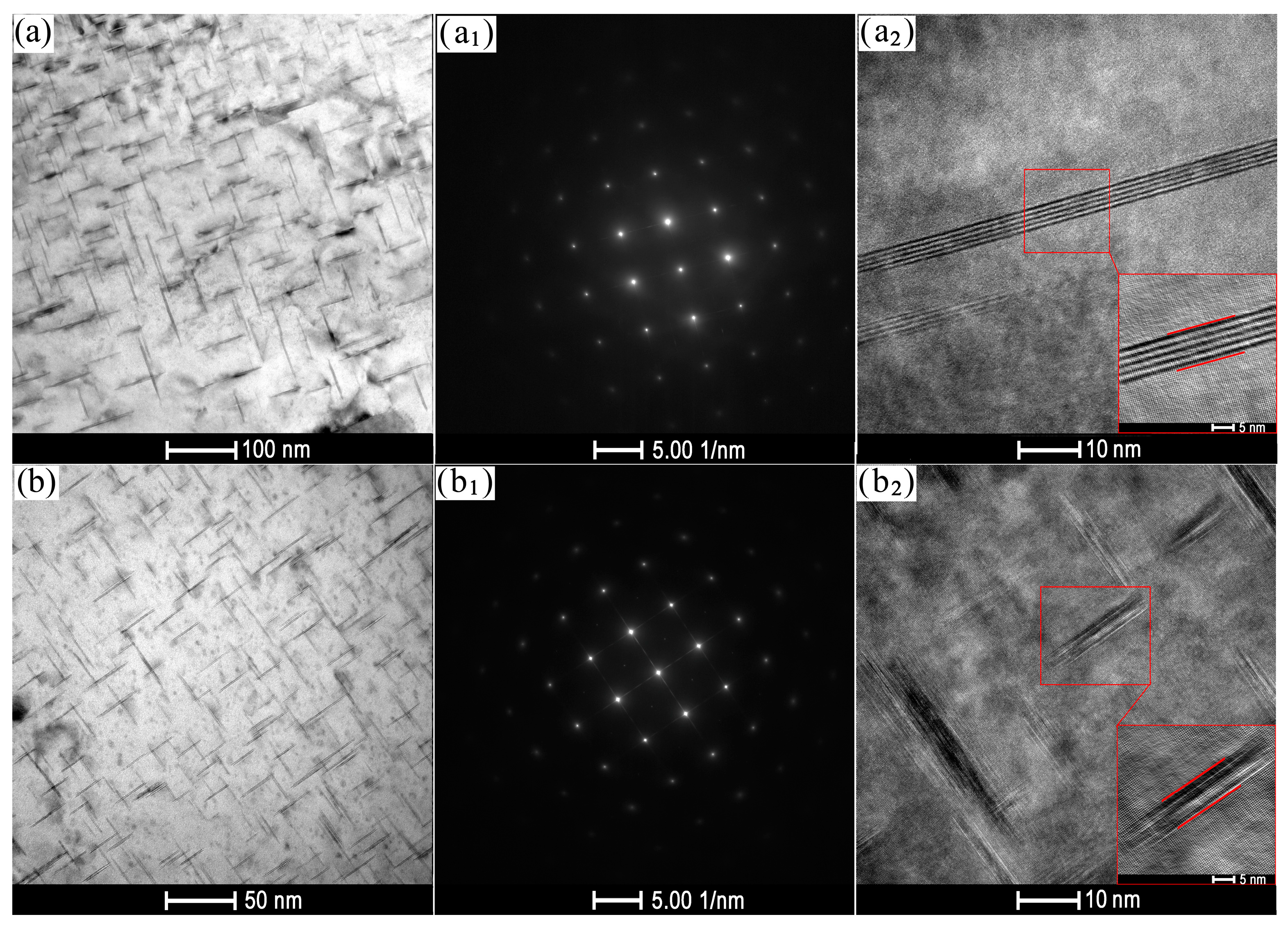
3.1. Microstructure Analysis
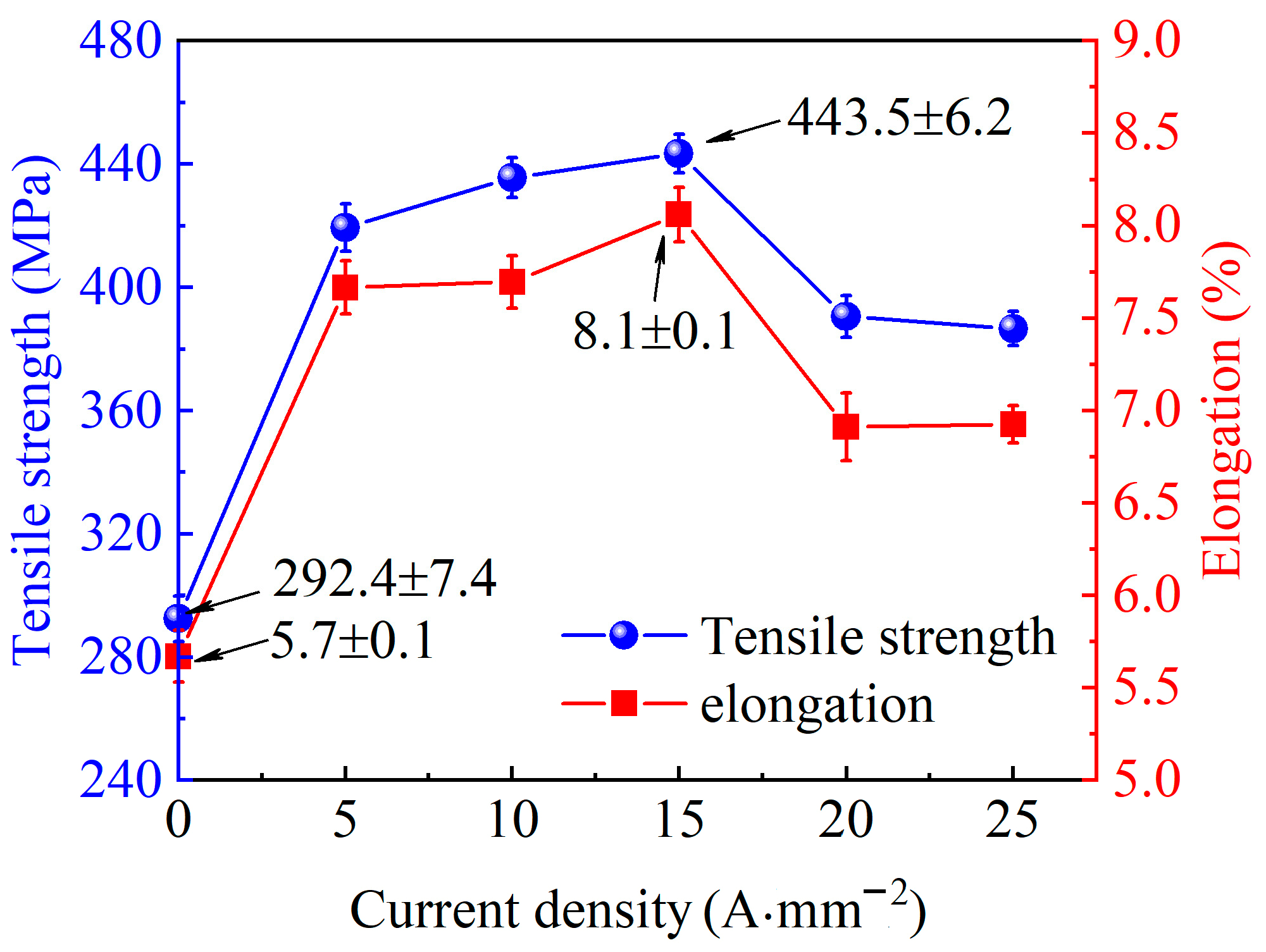
3.2. Mechanical Properties Analysis
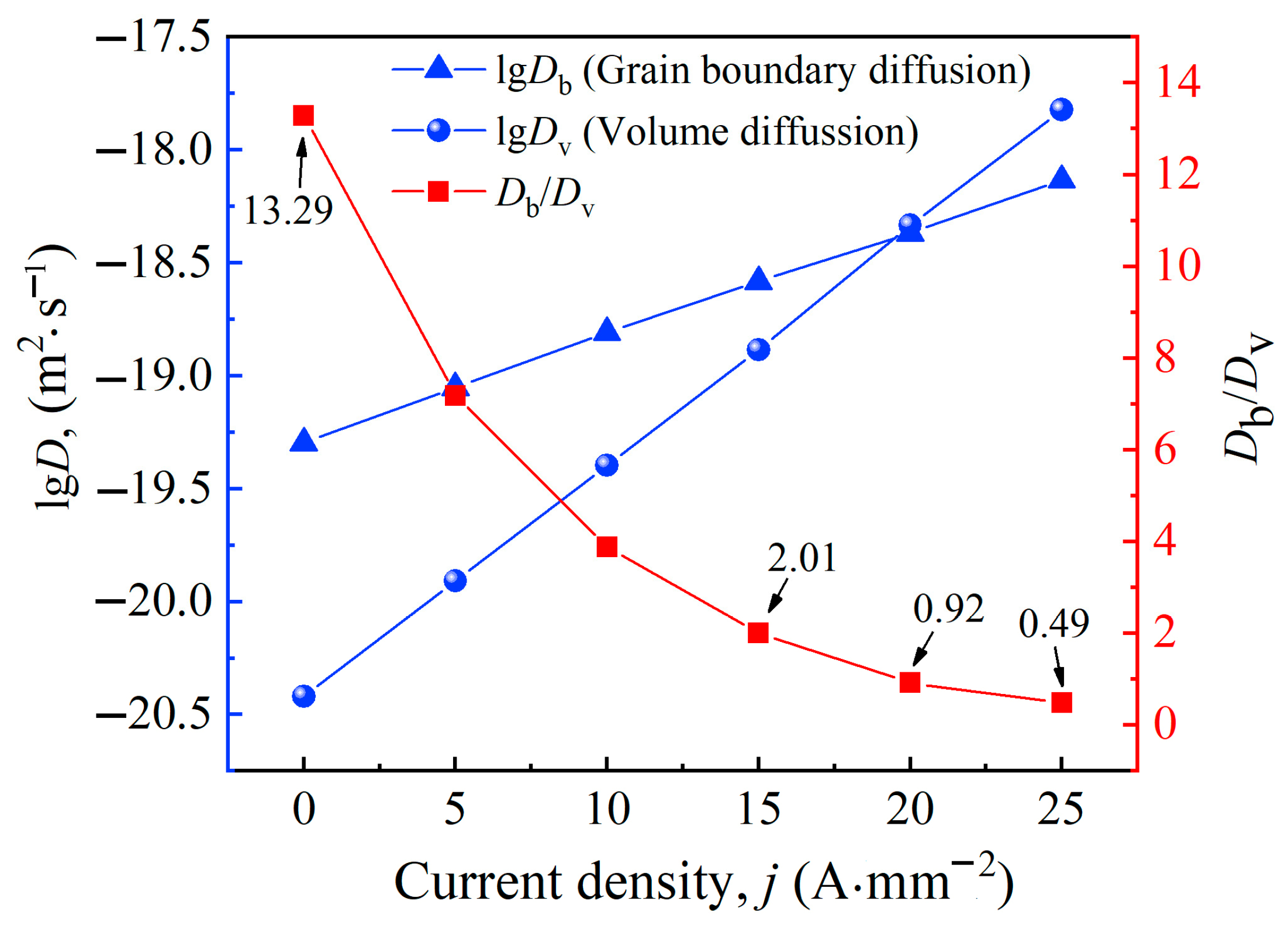
4. Mechanism Analysis of Electro-Pulse on Ageing Treatment
4.1. Thermodynamic Analysis
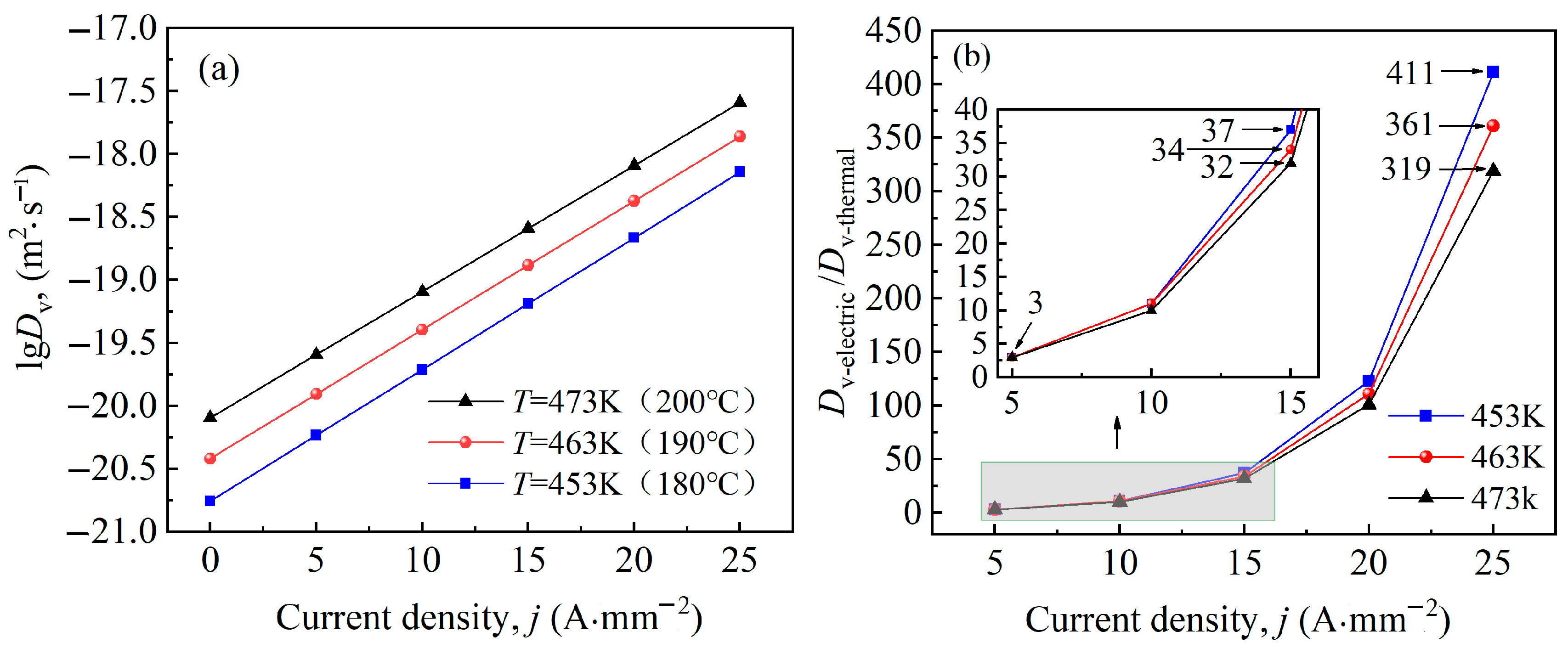
4.2. Kinetic Analysis
5. Conclusions
- (1)
- Electro-pulse has a significant influence on the size and quantity of precipitates in the aged Al-Cu-Mn-Zr-V alloy. As the current density increases, the size and quantity of precipitates continue to decrease, gradually transforming from continuous aggregated distribution at grain boundaries to dispersed distribution. But when the current density exceeds 15 A·mm−2, the size and quantity of precipitates begin to increase again and aggregate at grain boundaries.
- (2)
- Electro-pulse significantly improves the mechanical properties of Al-Cu-Mn-Zr-V alloy. With the increase of current density, the mechanical properties increase, and when the current density is 15 A·mm−2, the mechanical properties reach a peak, with a tensile strength of 443.5 MPa and an elongation of 8.1%, which are 51.7% and 42.1% higher than conventional ageing treatment, respectively.
- (3)
- Electro-pulse provides thermodynamic and kinetic conditions for ageing precipitation of the second phases in the form of additional electrical free energy. The negative electrical free energy is the driving force for phase transformation. The higher the current density, the better the thermodynamic basis for the alloy to precipitate the second phases at a lower ageing temperature.
- (4)
- Electro-pulse enhances the diffusion coefficient, nucleation rate, and grain growth rate of Al-Cu-Mn-Zr-V alloy during ageing treatment. At the temperature of 463 K, the diffusion coefficient at 15 A·mm−2 is approximately 34 times that of thermal field. Electro-pulse improves the nucleation rate by increasing the diffusion coefficient and reducing the critical nucleation activation energy, as well as reduces the critical nucleation radius of the second phases. However, it also accelerates the growth and coarsening of grains, making the second phases coarser than before. There is an appropriate current density of electro-pulse, which is 15 A·mm−2 for this study, that allows the second phases to more rapidly nucleate while growing into smaller particles.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, A.A.; Sachdev, A.K.; Apelian, D. Alloy development and process innovations for light metals casting. J. Mater. Process. Technol. 2022, 360, 117606. [Google Scholar] [CrossRef]
- Su, R.M.; Jia, Y.X.; Xiao, J.; Li, G.L.; Qu, Y.D.; Li, R.D. Effect of secondary aging on microstructure and properties of cast Al-Cu-Mg alloy. China Foundry 2023, 20, 71–77. [Google Scholar] [CrossRef]
- Sun, T.; Geng, J.; Bian, Z.; Wu, Y.; Wang, M.; Chen, D.; Ma, N.; Wang, H. Enhanced thermal stability and mechanical properties of high-temperature resistant Al-Cu alloy with Zr and Mn micro-alloying. Trans. Nonferrous Met. Soc. China 2022, 32, 64–78. [Google Scholar] [CrossRef]
- Koshmin, A.; Cherkasov, S.; Fortuna, A.; Gamin, Y.; Churyumov, A. Optimization of flat-rolling parameters for thermally stable alloy of Al-Cu-Mn System with micro additions of Si and Zr. Metals 2023, 13, 2019. [Google Scholar] [CrossRef]
- Li, D.; Liu, K.; Rakhmonov, J.; Chen, X.G. Enhanced thermal stability of precipitates and elevated-temperature properties via microalloying with transition metals (Zr, V and Sc) in Al-Cu 224 cast alloys. Mater. Sci. Eng. A 2021, 827, 142090. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, H.; Wang, R.; Peng, C.; Feng, Y.; Wang, X. Microstructure and mechanical properties of the extruded Al-Cu-Mn-Sc-Zr alloy during single-stage and two-stage aging. J. Mater. Eng. Perform. 2023, 32, 185–198. [Google Scholar] [CrossRef]
- Michi, R.A.; Sisco, K.; Bahl, S.; Allard, L.F.; Wagner, K.B.; Poplawsky, J.D.; Leonard, D.N.; Dehoff, R.R.; Plotkowski, A.; Shyam, A. Microstructural evolution and strengthening mechanisms in a heat-treated additively manufactured Al-Cu-Mn-Zr alloy. Mater. Sci. Eng. A 2022, 840, 142928. [Google Scholar] [CrossRef]
- Yakovtseva, O.A.; Mochugovskiy, A.G.; Prosviryakov, A.S.; Bazlov, A.I.; Emelina, N.B.; Mikhaylovskaya, A.V. The microstructure and properties of Al-Mn-Cu-Zr alloy after high-energy ball milling and hot-press sintering. Metals 2024, 14, 310. [Google Scholar] [CrossRef]
- Wang, J.; Qi, J.; Zhao, Z.; Guo, H.; Zhao, T. Effects of electric pulse modification on liquid structure of Al-5%Cu alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 2792–2796. [Google Scholar] [CrossRef]
- Li, N.; Zhang, L.; Zhang, R.; Yin, P.; Xing, H.; Wu, H. Research on grain refinement in hypoeutectic Al-Si alloy during solidification under an alternating electric current pulse. Metals 2019, 9, 571. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Huang, M.H.; Ma, Z.Y.; Zhan, L.H. Influence of the low-density pulse current on the ageing behavior of AA2219 aluminum alloy. J. Alloys Compd. 2016, 673, 358–363. [Google Scholar] [CrossRef]
- Chen, Z.; Li, B.; Huang, Q.; Zhang, L.; Liu, J. The effect of the electric pulse treatment on the microstructure and mechanical performance of the Al-Zn alloy. Mater. Sci. Eng. A 2020, 796, 140016. [Google Scholar] [CrossRef]
- Bian, T.J.; Li, H.; Lei, C.; Wu, C.H.; Zhang, L.W. Microstructures and properties evolution of Al-Zn-Mg-Cu alloy under electrical pulse assisted creep aging. Adv. Manuf. 2022, 10, 596–609. [Google Scholar] [CrossRef]
- Kang, K.J.; Li, D.Y.; Shi, D.Q.; Gao, G.L.; Xu, Z.Y.; Wang, L. Evolution of microstructure and mechanical properties in Al-Zn-Mg-Cu alloy by electric pulse aging treatment. Trans. Indian Inst. Met. 2021, 74, 2835–2842. [Google Scholar] [CrossRef]
- Jia, Z.H.; Couzinie, J.P.; Cherdoudi, N. Precipitation behaviour of Al3Zr precipitate in Al-Cu-Zr and Al-Cu-Zr-Ti-V alloys. Trans. Nonferrous Met. Soc. 2012, 22, 1860–1865. [Google Scholar] [CrossRef]
- Meng, F.S.; Wang, Z.; Zhao, Y.L.; Zhang, D.; Zhang, W. Microstructures and properties evolution of Al-Cu-Mn alloy with addition of Vanadium. Metals 2016, 7, 10. [Google Scholar] [CrossRef]
- Askari-paykani, M.; Meratian, M.; Shayan, M.; Raeissi, K. Effects of heat treatment parameters on microstructural changes and corrosion behavior of Al 7075 alclad alloy. Anti-Corros. Methods Mater. 2012, 59, 231–238. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, A.; Dwivedi, D.; Ahn, B.; Manoj, M.K. Influence of various heat treatment procedures on the corrosion behavior of Al-Zn-Mg-Cu alloys. Mater. Res. Express 2019, 6, 126554. [Google Scholar] [CrossRef]
- Jiang, L.; Rouxel, B.; Langan, T.; Dorin, T. Coupled segregation mechanisms of Sc, Zr and Mn at θ′ interfaces enhances the strength and thermal stability of Al-Cu alloys. Acta Mater. 2021, 206, 116634. [Google Scholar] [CrossRef]
- Buyukdogan, S.; Gunduz, S.; Turkmen, M. Influence of aging treatment on microstructure, mechanical properties and adhesive wear behaviour of 6063 aluminium alloy. Ind. Lubr. Tribol. 2014, 66, 520–524. [Google Scholar] [CrossRef]
- Gao, L.; Li, K.; Ni, S. The growth mechanisms of θ’ precipitate phase in an Al-Cu alloy during aging treatment. J. Mater. Sci. Technol. 2021, 61, 25–32. [Google Scholar] [CrossRef]
- Takeda, M.; Komatsu, A.; Ohta, M.; Shirai, T.; Endo, T. The influence of Mn on precipitation behavior in Al-Cu. Scr. Mater. 1998, 39, 1295–1300. [Google Scholar] [CrossRef]
- Duan, S.W.; Matsuda, K.; Wang, T. Microstructures and mechanical properties of a cast Al-Cu-Li alloy during heat treatment procedure. Rare Met. 2021, 40, 1897–1906. [Google Scholar] [CrossRef]
- Psyk, V.; Risch, D.; Kinsey, B.L.; Tekkaya, A.E.; Kleiner, M. Electromagnetic forming—A review. J. Mater. Process. Technol. 2011, 211, 787–829. [Google Scholar] [CrossRef]
- Aksoz, S.; Ocak, Y.; Marasli, N.; Cadirli, E.; Kaya, H.; Boyuk, U. Dependency of the thermal and electrical conductivity on the temperature and composition of cu in the Al based Al-Cu alloys. Exp. Therm. Fluid. Sci. 2010, 34, 1507–1516. [Google Scholar] [CrossRef]
- Guo, J.D.; Wang, X.L.; Dai, W.B. Microstructure evolution in metals induced by high density electric current pulses. Mater. Sci. Technol. 2015, 31, 1545–1554. [Google Scholar] [CrossRef]
- Qin, R.S.; Bhowmik, A. Computational thermodynamics in electric current metallurgy. Mater. Sci. Technol. 2015, 31, 1560–1563. [Google Scholar] [CrossRef]
- Belov, N.A.; Akopyan, T.K.; Korotkova, N.O.; Timofeev, V.N.; Shurkin, P.K. Effect of cold rolling and annealing temperature on structure, hardness and electrical conductivity of rapidly solidified alloy of Al-Cu-Mn-Zr system. Mater. Lett. 2021, 30, 130199. [Google Scholar] [CrossRef]
- Ada, P.; Cieslar, M.; Vostr, P.; Beva, F.; Prochazka, I. Electrical resistivity and positron lifetime studies of precipitation effects in Al-Cu alloy. Acta Phys. Pol. A 1995, 88, 111–117. [Google Scholar] [CrossRef]
- Haslam, A.J.; Phillpot, S.R.; Wolf, D.; Moldovan, D.; Gleiter, H. Mechanisms of grain growth in nanocrystalline FCC metals by molecular-dynamics simulation. Mater. Sci. Eng. A 2001, 318, 293–312. [Google Scholar] [CrossRef]
- Gupta, R.P. The electron wind force in electromigration. J. Phys. Chem. Solids 1986, 47, 1057–1066. [Google Scholar] [CrossRef]
- Lodder, A. The driving force in electromigration. Physica A 1989, 158, 723–739. [Google Scholar] [CrossRef]
- Kresse, T.; Borchers, C.; Kirchheim, R. Vacancy-carbon complexes in BCC iron: Correlation between carbon content, vacancy concentration and diffusion coefficient. Scr. Mater. 2013, 69, 690–693. [Google Scholar] [CrossRef]
- Du, Y.; Chang, Y.A.; Huang, B.; Gong, W.; Jin, Z.; Xu, H.; Yuan, Z.; Liu, Y.; He, Y.; Xie, F.Y. Diffusion coefficients of some solutes in FCC and liquid Al: Critical evaluation and correlation. Mater. Sci. Eng. A 2003, 363, 140–151. [Google Scholar] [CrossRef]
- Styles, M.J.; Marceau, R.K.W.; Bastow, T.J.; Brand, H.E.A.; Gibson, M.A.; Hutchinson, C.R. The competition between metastable and equilibrium S (Al2CuMg) phase during the decomposition of Al-Cu-Mg alloys. Acta Mater. 2015, 98, 64–80. [Google Scholar] [CrossRef]
- Fu, S.; Liu, H.; Qi, N.; Wang, B.; Jiang, Y.; Chen, Z.; Hu, T.; Yi, D. On the electrostatic potential assisted nucleation and growth of precipitates in Al-Cu alloy. Scr. Mater. 2018, 150, 13–17. [Google Scholar] [CrossRef]
- Clementi, E.; Raimondi, D.L.; Reinhardt, W.P. Atomic screening constants from SCF functions. II. atoms with 37 to 86 electrons. J. Chem. Phys. 1967, 47, 1300–1307. [Google Scholar] [CrossRef]
- Csiszar, G.; Misra, A.; Ungar, T. Burgers vector types and the dislocation structures in sputter-deposited Cu-Nb multilayers. Mater. Sci. Eng. A 2011, 528, 6887–6895. [Google Scholar] [CrossRef]
- Brandt, R.; Neuer, G. Electrical resistivity and thermal conductivity of pure aluminum and aluminum alloys up to and above the melting temperature. Int. J. Thermophys. 2007, 28, 1429–1446. [Google Scholar] [CrossRef]
- Fatmi, M.; Ghebouli, B.; Ghebouli, M.A.; Chihi, T.; Ouakdi, E.H.; Heiba, Z.A. Study of precipitation kinetics in Al-3.7wt% Cu alloy during non-isothermal and isothermal ageing. Chin. J. Physiol. 2013, 51, 1019–1032. [Google Scholar]
- Ceresara, S.A. step annealing procedure for the determination of diffusion coefficients in metals by the resistometric method-application to the diffusion of Cu in Al. Phys. Stat. Sol. 1968, 27, 517–520. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, Y.; Zhang, Z. Theoretical and experimental study of precipitation and coarsening kinetics of θ’ phase in Al-Cu alloy. Vacuum 2021, 189, 110263. [Google Scholar] [CrossRef]
- Ho, C.Y.; Ackerman, M.W.; Wu, K.Y.; Havill, T.N.; Bogaard, R.H.; Matula, R.A.; Oh, S.G.; James, H.M. Electrical resistivity of ten selected binary alloy systems. J. Phys. Chem. Ref. Data 1983, 12, 183–322. [Google Scholar] [CrossRef]
- Lalpoor, M.; Dzwonczyk, J.S.; Hort, N.; Offerman, S.E. Nucleation mechanism of Mg17Al12-precipitates in binary Mg-7wt.% Al alloy. J. Alloys Compd. 2013, 557, 73–76. [Google Scholar] [CrossRef]
- Wu, Y.F.; Wang, M.P.; Li, Z.; Xia, F.Z.; Xia, C.D.; Lei, Q.; Yu, H.C. Effects of Pre-aging treatment on subsequent artificial aging characteristics of Al-3.95Cu-(1.32Mg)-0.52Mn-0.11Zr alloys. J. Cent. South Univ. 2015, 22, 1–7. [Google Scholar] [CrossRef]
- Perez, M.; Dumont, M.; Acevedo-Reyes, D. Implementation of classical nucleation and growth theories for precipitation. Acta Mater. 2008, 56, 2119–2132. [Google Scholar] [CrossRef]
- Du, Q.; Poole, W.J.; Wells, M.A. A mathematical model coupled to CALPHAD to predict precipitation kinetics for multicomponent aluminum alloys. Acta Mater. 2012, 60, 3830–3839. [Google Scholar] [CrossRef]
- Holmedal, B.; Osmundsen, E.; Du, Q. Precipitation of non-spherical particles in aluminum alloys part I: Generalization of the Kampmann-Wagner numerical model. Metall. Mater. Trans. A 2016, 47, 581–588. [Google Scholar] [CrossRef]
- Jiang, L.; Li, J.K.; Cheng, P.M.; Liu, G.; Wang, R.H.; Chen, B.A.; Zhang, J.Y.; Sun, J.; Yang, M.X.; Yang, G. Experiment and modeling of ultrafast precipitation in an ultrafine-grained Al-Cu-Sc alloy. Mater. Sci. Eng. A 2014, 607, 596–604. [Google Scholar] [CrossRef]
- Qin, R.S.; Su, S.X.; Guo, J.D.; He, G.H.; Zhou, B.L. Suspension Effect of nanocrystalline grain growth under electropulsing. Nanostruct. Mater. 1998, 10, 71–76. [Google Scholar] [CrossRef]
- Baldan, A. Review progress in Ostwald ripening theories and their applications to nickel-base superalloys part I: Ostwald ripening theories. J. Mater. Sci. 2002, 37, 2171–2202. [Google Scholar] [CrossRef]
- Lifshitz, I.M.; Slyozov, V.V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Streitenberger, P. Generalized Lifshitz-Slyozov Theory of grain and particle coarsening for arbitrary cut-off parameter. Scr. Mater. 1998, 39, 1719–1724. [Google Scholar] [CrossRef]
- Philippe, T.; Voorhees, P.W. Ostwald ripening in multicomponent alloys. Acta Mater. 2013, 61, 4237–4244. [Google Scholar] [CrossRef]
- Senkov, O.N. Particle size distributions during diffusion controlled growth and coarsening. Scr. Mater. 2008, 59, 171–174. [Google Scholar] [CrossRef]
- Conrad, H. Effects of electric current on solid state phase transformations in metals. Mater. Sci. Eng. A 2000, 287, 227–237. [Google Scholar] [CrossRef]
- Jiang, Y.; Tang, G.; Guan, L.; Wang, S.; Xu, Z.; Shek, C.; Zhu, Y. Effect of electropulsing treatment on solid solution behavior of an aged Mg alloy AZ61 strip. J. Mater. Res. 2008, 23, 2685–2691. [Google Scholar] [CrossRef]
- Xiao, H.; Jiang, S.; Shi, C.; Zhang, L.; Lu, Z.; Jiang, J. Study on the microstructure evolution and mechanical properties of an Al-Mg-Li alloy aged by electropulsing assisted ageing processing. Mater. Sci. Eng. A 2019, 756, 442–454. [Google Scholar] [CrossRef]

| Element | Cu | Mn | Zr | V | Ti | Fe | Si | Al |
|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 5.02 | 0.33 | 0.15 | 0.11 | 0.09 | 0.04 | 0.01 | Bal. |
| No. | Current Density (A·mm−2) | Duty Ratio (%) | Frequency (Hz) |
|---|---|---|---|
| 1 | 0 | 0 | 0 |
| 2 | 5 | 1 | 100 |
| 3 | 10 | 1 | 100 |
| 4 | 15 | 1 | 100 |
| 5 | 20 | 1 | 100 |
| 6 | 25 | 1 | 100 |
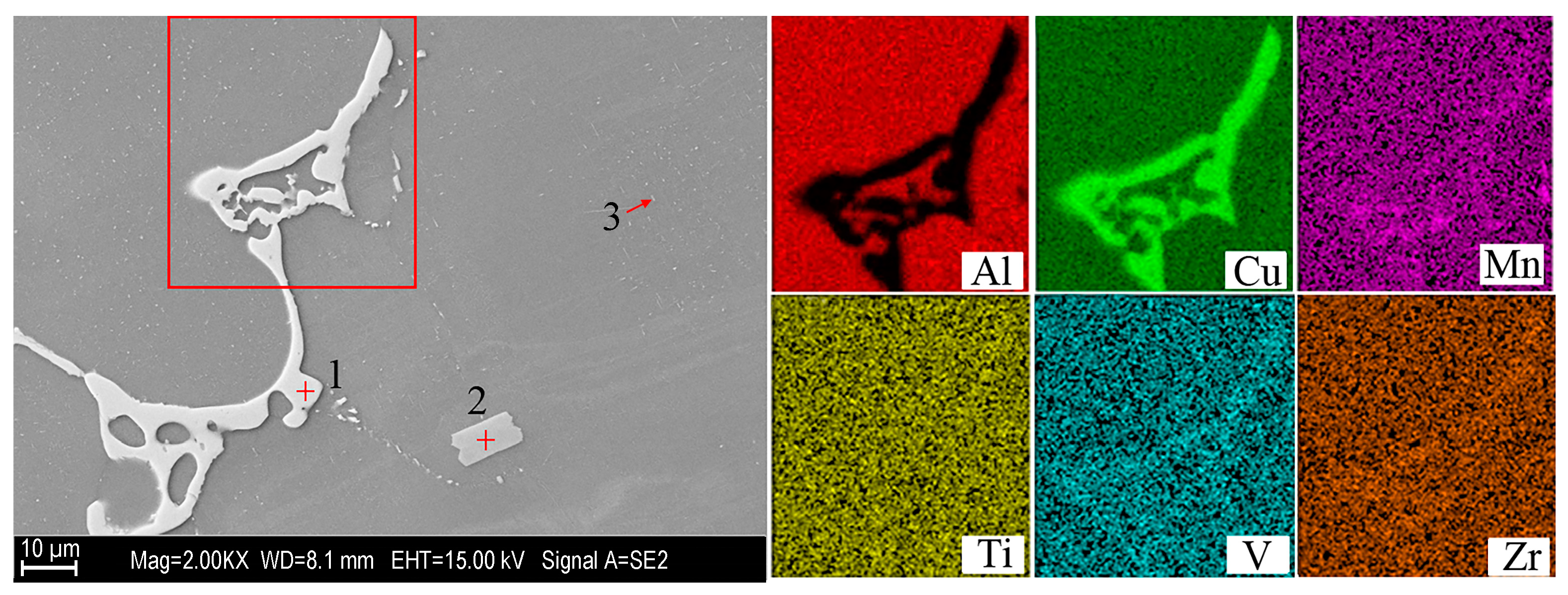
| Points | Elements | |||||
|---|---|---|---|---|---|---|
| Al | Cu | Mn | Zr | V | Ti | |
| 1 | 66.4 | 33.6 | 0 | 0 | 0 | 0 |
| 2 | 76.7 | 0 | 0 | 3.2 | 4.8 | 15.3 |
| 3 | 89.6 | 5.7 | 4.7 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, D.; Yu, W.; Gao, G.; Kang, K. Effect of Electro-Pulse on Microstructure of Al-Cu-Mn-Zr-V Alloy during Aging Treatment and Mechanism Analysis. Metals 2024, 14, 648. https://doi.org/10.3390/met14060648
Shi D, Yu W, Gao G, Kang K. Effect of Electro-Pulse on Microstructure of Al-Cu-Mn-Zr-V Alloy during Aging Treatment and Mechanism Analysis. Metals. 2024; 14(6):648. https://doi.org/10.3390/met14060648
Chicago/Turabian StyleShi, Dequan, Wenbo Yu, Guili Gao, and Kaijiao Kang. 2024. "Effect of Electro-Pulse on Microstructure of Al-Cu-Mn-Zr-V Alloy during Aging Treatment and Mechanism Analysis" Metals 14, no. 6: 648. https://doi.org/10.3390/met14060648
APA StyleShi, D., Yu, W., Gao, G., & Kang, K. (2024). Effect of Electro-Pulse on Microstructure of Al-Cu-Mn-Zr-V Alloy during Aging Treatment and Mechanism Analysis. Metals, 14(6), 648. https://doi.org/10.3390/met14060648





