The Formation Mechanism of Oxide Inclusions in a High-Aluminum Ni-Based Superalloy during the Vacuum Induction Remelting Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Oxide Inclusion Characterization Methods
3. Results
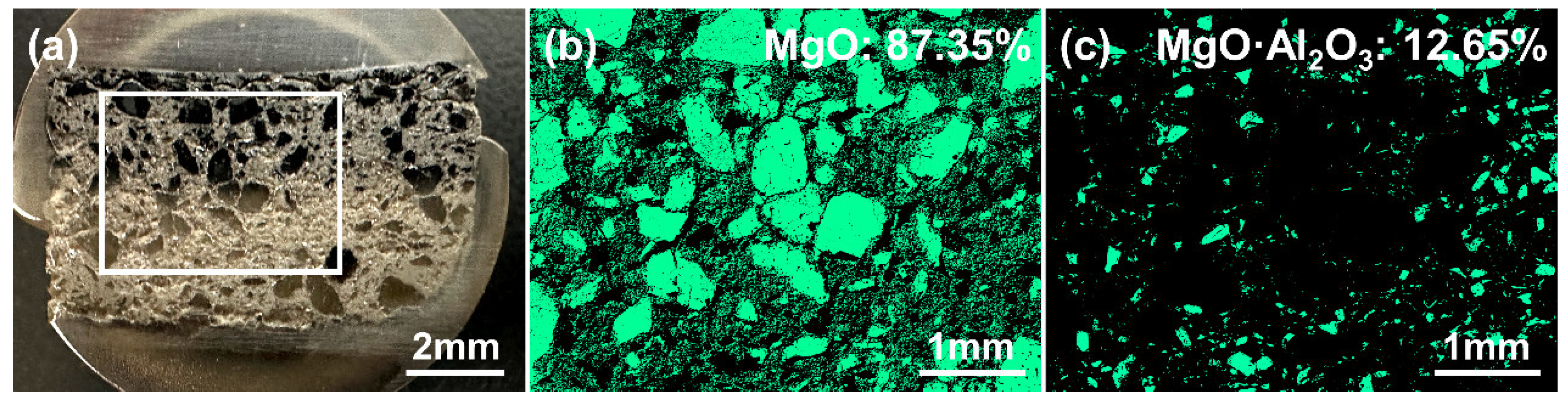
3.1. The Characteristics of the MgO Crucible Used in the VIR Process
3.2. Characteristics of Typical Oxide Inclusions before and after the VIR Process
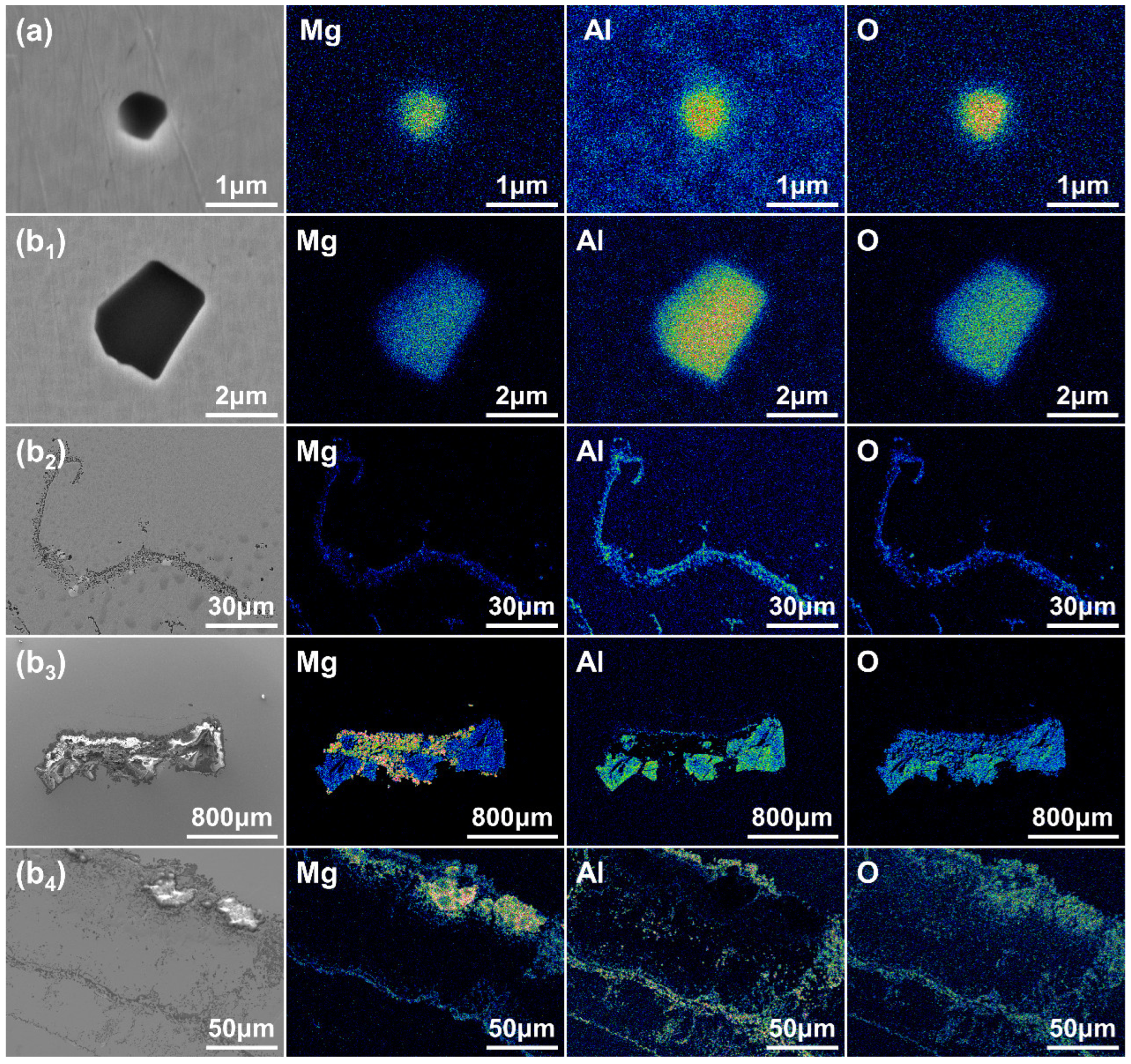
3.2.1. The Morphology of Typical Oxide Inclusions
3.2.2. Types and Number Densities of Typical Oxide Inclusions
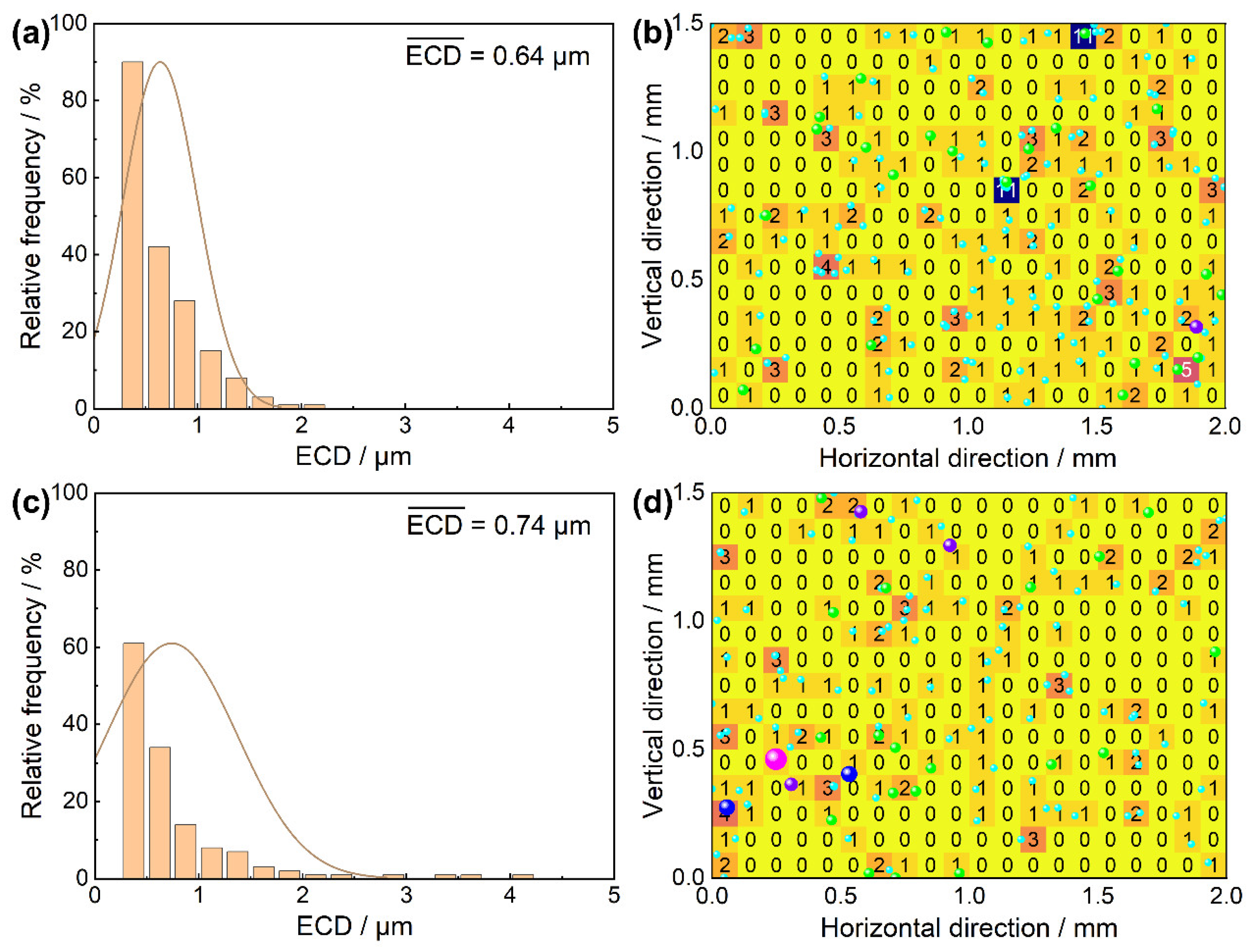
3.2.3. The Size Distribution and Dispersion Degree of Typical Oxide Inclusions
4. Discussion
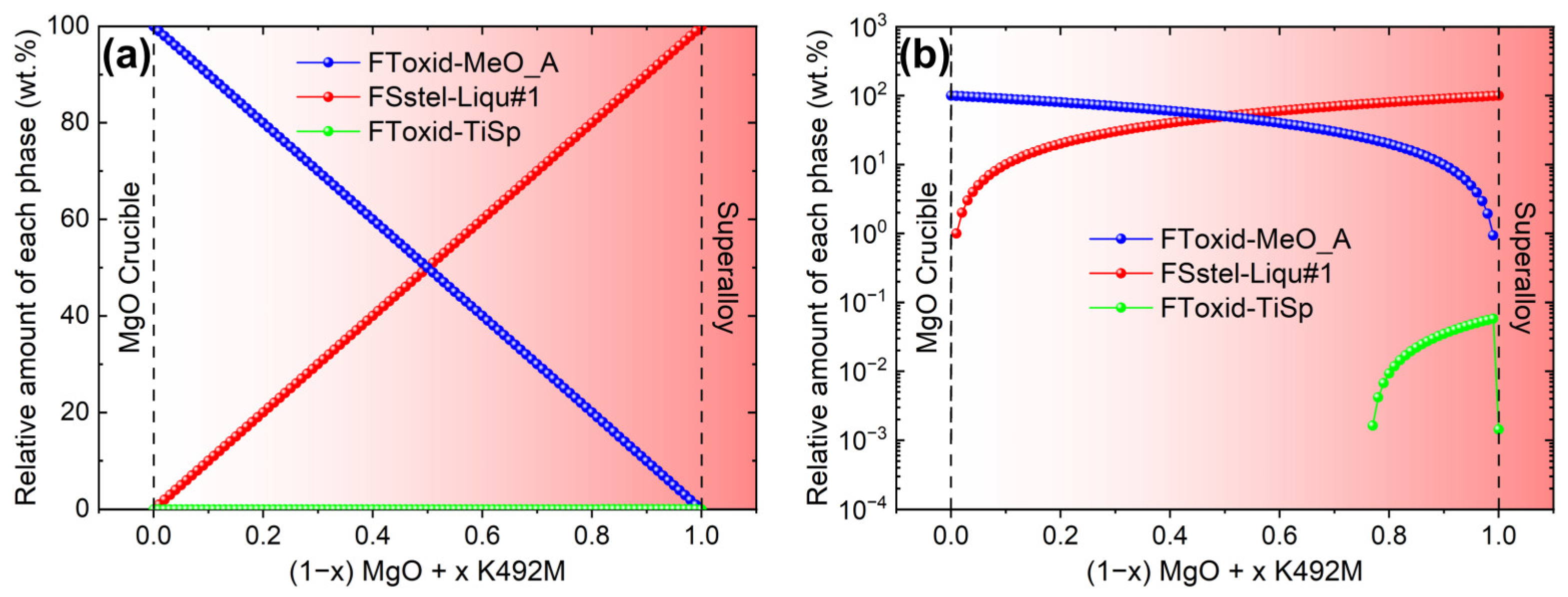
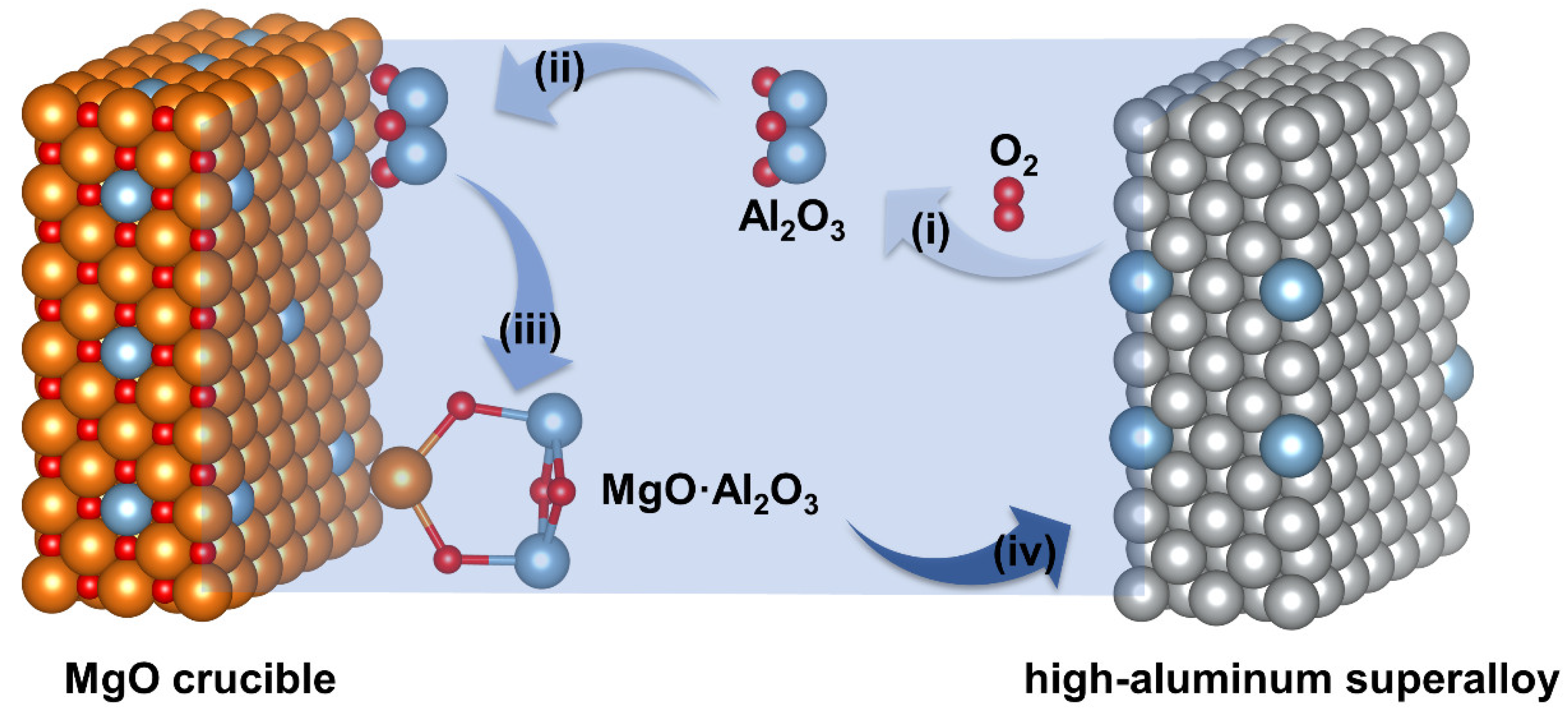
4.1. The Formation Mechanism of Endogenous Irregular MgO·Al2O3 Inclusions after the VIR Process
4.2. The Formation Mechanism of Film-like MgO·Al2O3 Inclusions after the VIR Process
4.3. The Formation Mechanism of Large MgO·Al2O3 and MgO Inclusions after the VIR Process
5. Conclusions
- (1)
- The typical oxide inclusions in the master alloy and high-aluminum Ni-based superalloy after the VIR process are both made up of irregular MgO·Al2O3. The VIR process effectively reduced the number density of the oxide inclusions in the high-aluminum Ni-based superalloy from 62.67 to 45.00 mm−2. Simultaneously, the average oxide inclusion size increases from 0.64 to 0.74 μm, the area fraction of inclusions increases from 0.00264 to 0.00335%, and the standard deviation of the areal density of the oxide inclusions decreases from 1.180 to 0.737, indicating an increase in oxide inclusion uniformity.
- (2)
- While the VIR process demonstrates efficacy in reducing oxide inclusions, it introduces millimeter-scale oxide inclusions, several tens of microns of MgO inclusions, and large micron-scale film inclusions into high-aluminum Ni-based superalloys. It is imperative to rigorously eliminate these introduced inclusions. Consequently, selecting a high-quality crucible during the smelting process is of paramount importance.
- (3)
- The VIR process in high-aluminum Ni-based superalloys involves three primary formation mechanisms for MgO·Al2O3. These mechanisms include the heredity of oxide inclusions, the interfacial reaction between the MgO crucible and the high-aluminum superalloy melt, and the exfoliation and entrapment of the inner wall of the MgO crucible, which adsorbs Al2O3 inclusions and undergoes chemical reactions. The second three mechanisms introduce several hundred micron-thick film-like and bulk millimeter-scale MgO·Al2O3 inclusions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, C.; Hu, R.; Wang, X.; Du, J.; Luo, X.; Bi, Z.; Gan, B. Effect of pre-tensile treatments on the mechanical properties and deformation mechanism of a novel Ni-based superalloy. Mater. Sci. Eng. A 2023, 874, 145063. [Google Scholar] [CrossRef]
- Juillet, C.; Oudriss, A.; Balmain, J.; Feaugas, X.; Pedraza, F. Characterization and oxidation resistance of additive manufactured and forged IN718 Ni-based superalloys. Corros. Sci. 2018, 142, 266–276. [Google Scholar] [CrossRef]
- Detrois, M.; Antonov, S.; Rozman, K.A.; Hawk, J.A.; Jablonski, P.D. Improved creep and tensile properties of a corrosion resistant Ni-Based superalloy using high temperature aging and Nb/Ta additions. Metall. Mater. Trans. A 2022, 53, 2600–2613. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Li, J.; Chen, C.; Yang, S.; Li, X. Effects of aluminum and titanium additions on the formation of nonmetallic inclusions in nickel-based superalloys. J. Alloys Compd. 2022, 906, 164281. [Google Scholar] [CrossRef]
- Bergsmo, A.; Xu, Y.; Poole, B.; Dunne, F.P.E. Twin boundary fatigue crack nucleation in a polycrystalline Nickel superalloy containing non-metallic inclusions. J. Mech. Phys. Solids 2022, 160, 104785. [Google Scholar] [CrossRef]
- Yao, Z.; Hou, J.; Chen, Y.; Xu, W.; Jiang, H.; Dong, J. Effect of micron-sized particles on the crack growth behavior of a Ni-based powder metallurgy superalloy. Mater. Sci. Eng. A 2022, 860, 144242. [Google Scholar] [CrossRef]
- Liu, P.; Jiang, H.; Dong, J.; Chen, Z. Effect of micron-scale nonmetallic inclusions on fatigue crack nucleation in a nickel-based superalloy. Int. J. Solids Struct. 2023, 279, 112368. [Google Scholar] [CrossRef]
- Texier, D.; Stinville, J.C.; Echlin, M.L.P.; Pierret, S.; Villechaise, P.; Pollock, T.M.; Cormier, J. Short crack propagation from cracked non-metallic inclusions in a Ni-based polycrystalline superalloy. Acta Mater. 2019, 165, 241–258. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Chen, C.; Li, J.; Li, X. Effect of a MgO–CaO–ZrO2-based refractory on the cleanliness of a K4169 Ni-based superalloy. Ceram. Int. 2023, 49, 117–125. [Google Scholar] [CrossRef]
- Gao, R.; Li, L.; Chen, C.; Li, J.; Wang, L.; Li, X.; Yang, H. Formation and aggregation behavior of inclusions in Ni-based alloys with different Mg contents. J. Mater. Res. Technol. 2023, 26, 5252–5263. [Google Scholar] [CrossRef]
- Miller, C.F.; Simmons, G.W.; Wei, R.P. Mechanism for oxygen enhanced crack growth in inconel 718. Scr. Mater. 2001, 44, 2405–2410. [Google Scholar] [CrossRef]
- Jiang, R.; Ji, D.; Shi, H.; Hu, X.; Song, Y.; Gan, B. Effects of thermal exposure on high-cycle-fatigue behaviours in Ni-based superalloy GH4169. Mater. Sci. Technol. 2019, 35, 1265–1274. [Google Scholar] [CrossRef]
- Yang, S.; Yang, S.; Qu, J.; Du, J.; Gu, Y.; Zhao, P.; Wang, N. Inclusions in wrought superalloys: A review. J. Iron Steel Res. Int. 2021, 28, 921–937. [Google Scholar] [CrossRef]
- Chan, K.S. A fatigue life model for predicting crack nucleation at inclusions in Ni-based superalloys. Metall. Mater. Trans. A 2020, 51, 1148–1162. [Google Scholar] [CrossRef]
- Akande, I.G.; Oluwole, O.O.; Fayomi, O.S.I.; Odunlami, O.A. Overview of mechanical, microstructural, oxidation properties and high-temperature applications of superalloys. Mater. Today Proc. 2021, 43, 2222–2231. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, L.; Qu, X.; Luan, Y.; Chen, X. Investigation on the formation mechanism of non-metallic inclusions in high-aluminum and titanium-alloyed Ni-based superalloy. Vacuum 2020, 177, 109409. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Wang, E.; Chen, G.; Xu, E.; Zhao, F.; Zhao, Y.; Li, C.; Hou, X. Interaction between CA6-MA crucible and molten wrought Ni-based superalloys. J. Eur. Ceram. Soc. 2023, 43, 1714–1722. [Google Scholar] [CrossRef]
- Song, Q.; Qian, K.; Shu, L.; Chen, B.; Ma, Y.; Liu, K. Interfacial reaction between nickel-based superalloy K417G and oxide refractories. Acta Metall. Sin. 2021, 58, 868–882. [Google Scholar]
- Gao, X.; Zhang, L.; Qu, X.; Chen, X.; Luan, Y. Effect of interaction of refractories with Ni-based superalloy on inclusions during vacuum induction melting. Int. J. Miner. Metall. Mater. 2020, 27, 1551–1559. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Gao, M.; Li, Q.; Liu, H.; Zhang, H. Effect of vacuum level on the interfacial reactions between K417 superalloy and Y2O3 crucibles. Vacuum 2020, 182, 109701. [Google Scholar] [CrossRef]
- Yiliti, Y.; Dong, G.; Liu, X.; You, X.; Han, W.; Dong, L.; Zhao, Y.; Yi, L.; Wang, Y. The high temperature oxidation behavior of a superalloy prepared by vacuum induction melting and electron beam smelting: A comparative study. J. Mater. Res. Technol. 2023, 25, 6977–6991. [Google Scholar] [CrossRef]
- You, X.; Dong, G.; Zhou, H.; Zhang, H.; Tan, Y.; Wang, Y.; Li, P.; You, Q.; Li, Y.; Cui, H.; et al. Removal of oxygen, nitrogen, and inclusions in powder superalloy scraps by electron beam smelting and induced solidification and the purification mechanisms. Sep. Purif. Technol. 2023, 304, 122290. [Google Scholar] [CrossRef]
- Campbell, J.; Tiryakioğlu, M. Bifilm defects in Ni-based alloy castings. Metall. Mater. Trans. B 2012, 43, 902–914. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, J.; Zhang, T.; Dunne, F.P.E.; Britton, T.B. On the mechanistic basis of fatigue crack nucleation in Ni superalloy containing inclusions using high resolution electron backscatter diffraction. Acta Mater. 2015, 97, 367–379. [Google Scholar] [CrossRef]
- Hu, D.; Wang, T.; Ma, Q.; Liu, X.; Shang, L.; Li, D.; Pan, J.; Wang, R. Effect of inclusions on low cycle fatigue lifetime in a powder metallurgy nickel-based superalloy FGH96. Int. J. Fatigue 2019, 118, 237–248. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, G.; Gorley, M.J.; Lee, T.L.; Li, Z.; Xiao, C.; Tang, C.C. Effects of vacuum on gas content, oxide inclusions and mechanical properties of Ni-based superalloy using electron beam button and synchrotron diffraction. Mater. Des. 2021, 207, 109861. [Google Scholar] [CrossRef]



| Sample | Cr | Co | W | Al | Ta | Ti | Mo | C | B | Mg | Zr | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VIM | 11–14 | 7–11 | 3–6 | 2–5 | 3–7 | 3–6 | 1–3 | 0.03–0.1 | trace | trace | trace | bal. |
| VIR | 11–13 | 8–10 | 4–5 | 2–5 | 3–6 | 3–5 | 1–3 | 0.06–0.1 | trace | trace | trace | bal. |
| Process | Type | Number | Area, mm | Number Density, mm−2 |
|---|---|---|---|---|
| VIM | MgO·Al2O3 | 188 | 3 | 62.67 |
| VIR | MgO·Al2O3 | 145 | 3 | 45.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, E.; Xing, W.; Xue, Z.; Fan, W.; Zhao, Y.; Luo, Y.; Ge, C.; Xia, M. The Formation Mechanism of Oxide Inclusions in a High-Aluminum Ni-Based Superalloy during the Vacuum Induction Remelting Process. Metals 2024, 14, 654. https://doi.org/10.3390/met14060654
Zhang L, Liu E, Xing W, Xue Z, Fan W, Zhao Y, Luo Y, Ge C, Xia M. The Formation Mechanism of Oxide Inclusions in a High-Aluminum Ni-Based Superalloy during the Vacuum Induction Remelting Process. Metals. 2024; 14(6):654. https://doi.org/10.3390/met14060654
Chicago/Turabian StyleZhang, Lihui, Erkang Liu, Weijie Xing, Zhaojiang Xue, Wenjie Fan, Yunsong Zhao, Yushi Luo, Changchun Ge, and Min Xia. 2024. "The Formation Mechanism of Oxide Inclusions in a High-Aluminum Ni-Based Superalloy during the Vacuum Induction Remelting Process" Metals 14, no. 6: 654. https://doi.org/10.3390/met14060654






