Research on Atmospheric Corrosion of 45# Steel in Low-Latitude Coastal Areas of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Corrosion Test
2.3. Characterization
3. Results and Discussion
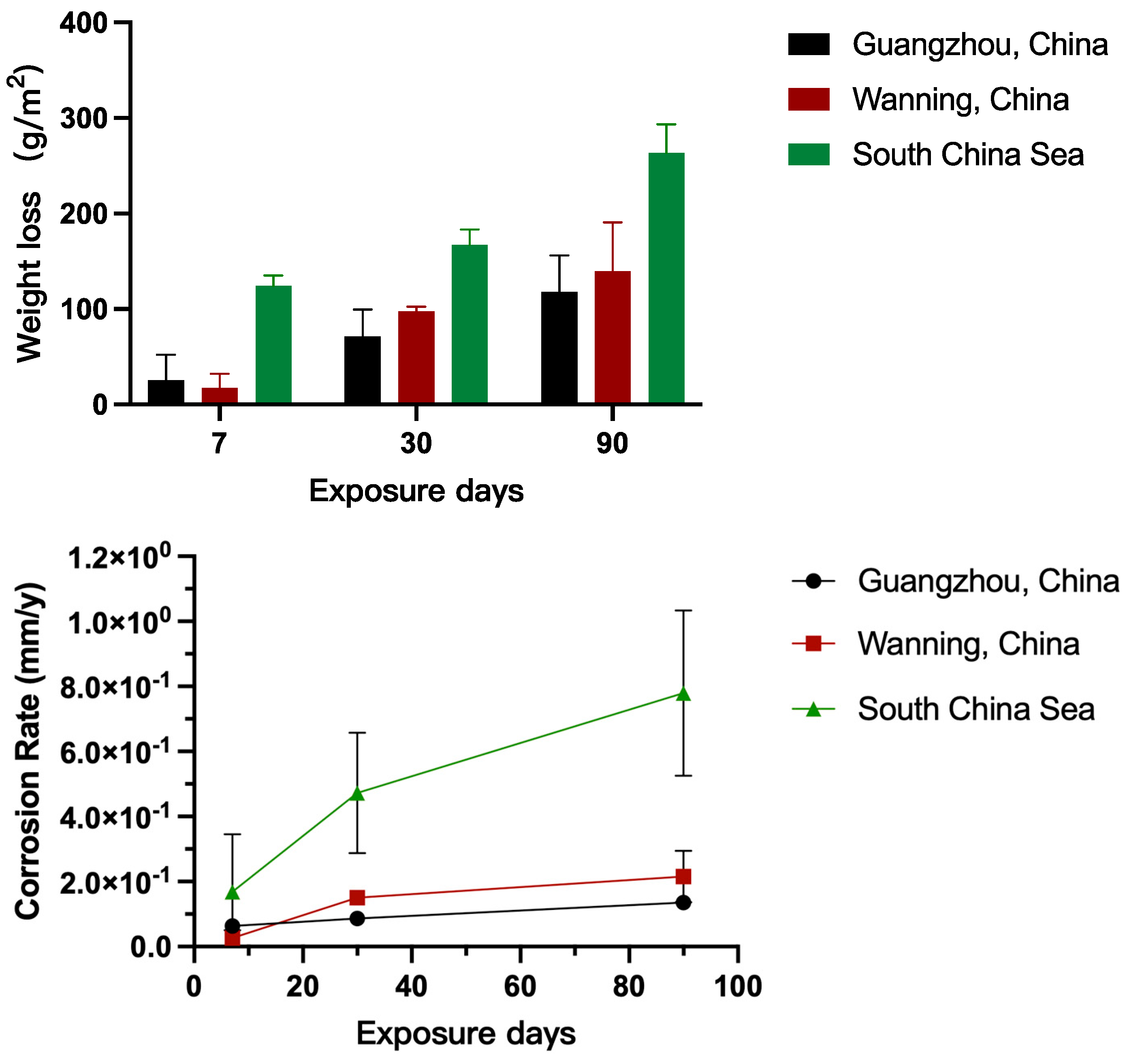
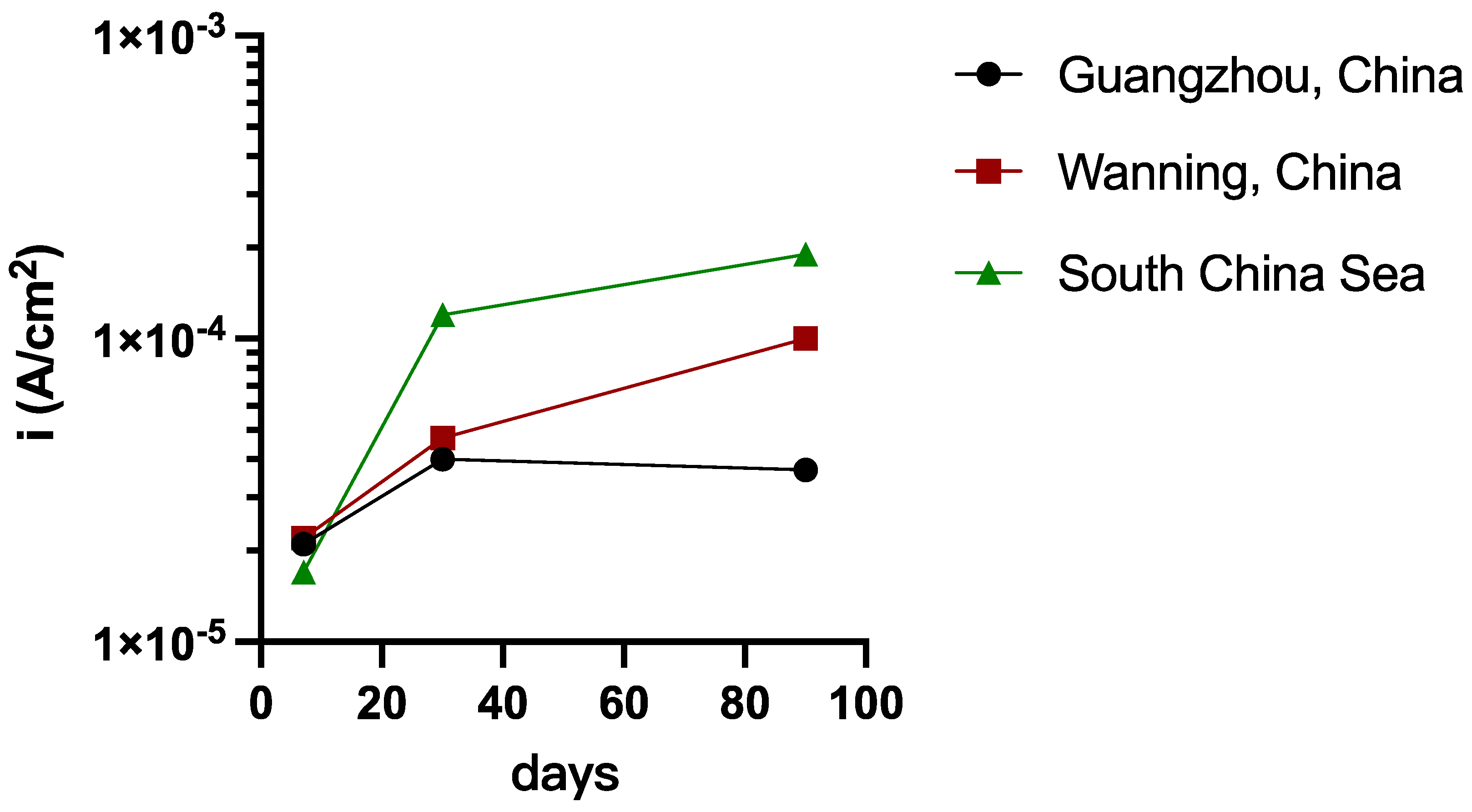
3.1. Atmospheric Corrosion of 45# Steel
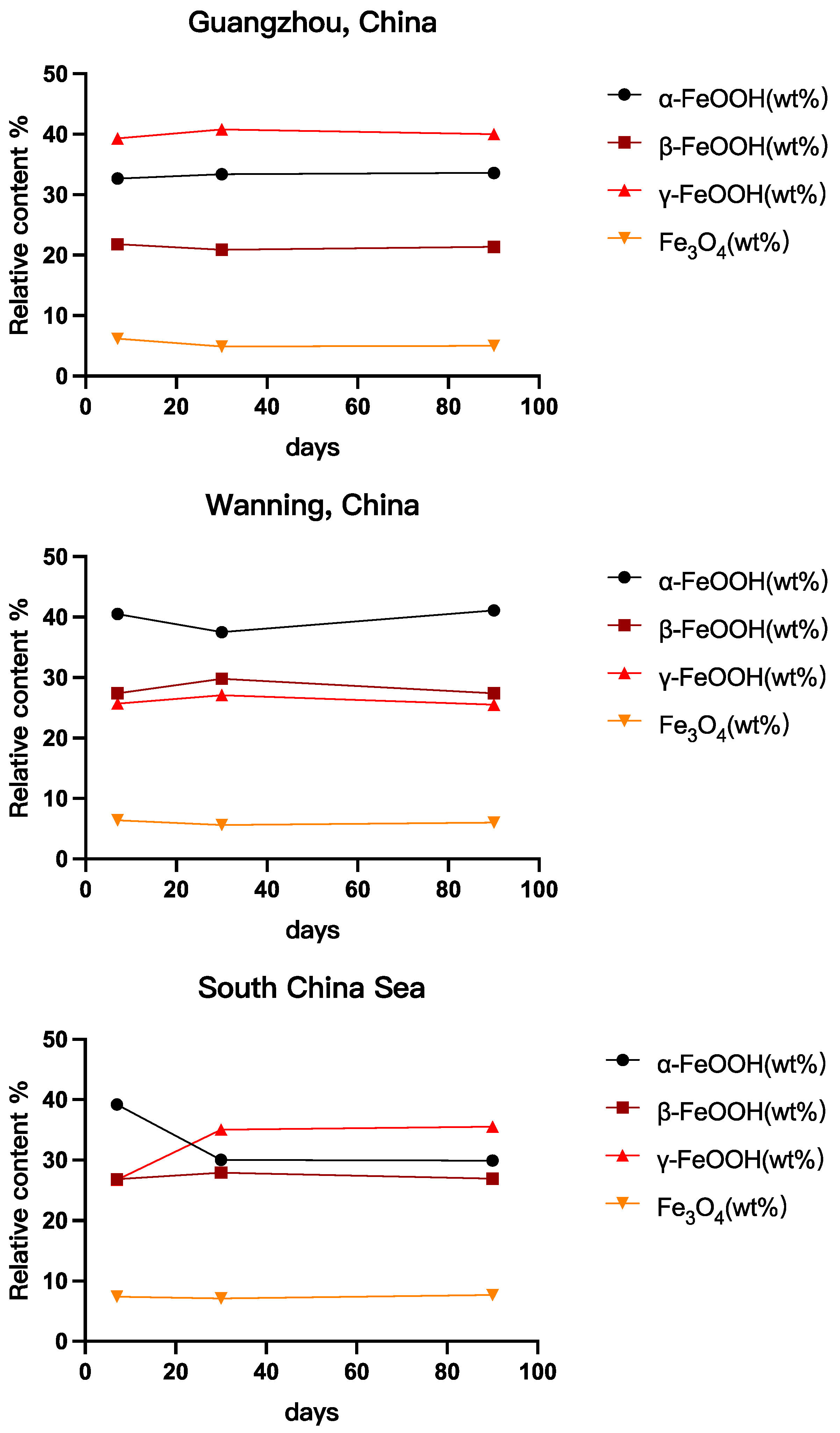
3.2. Surface Characterization and Mechanism Analysis of 45# Steel
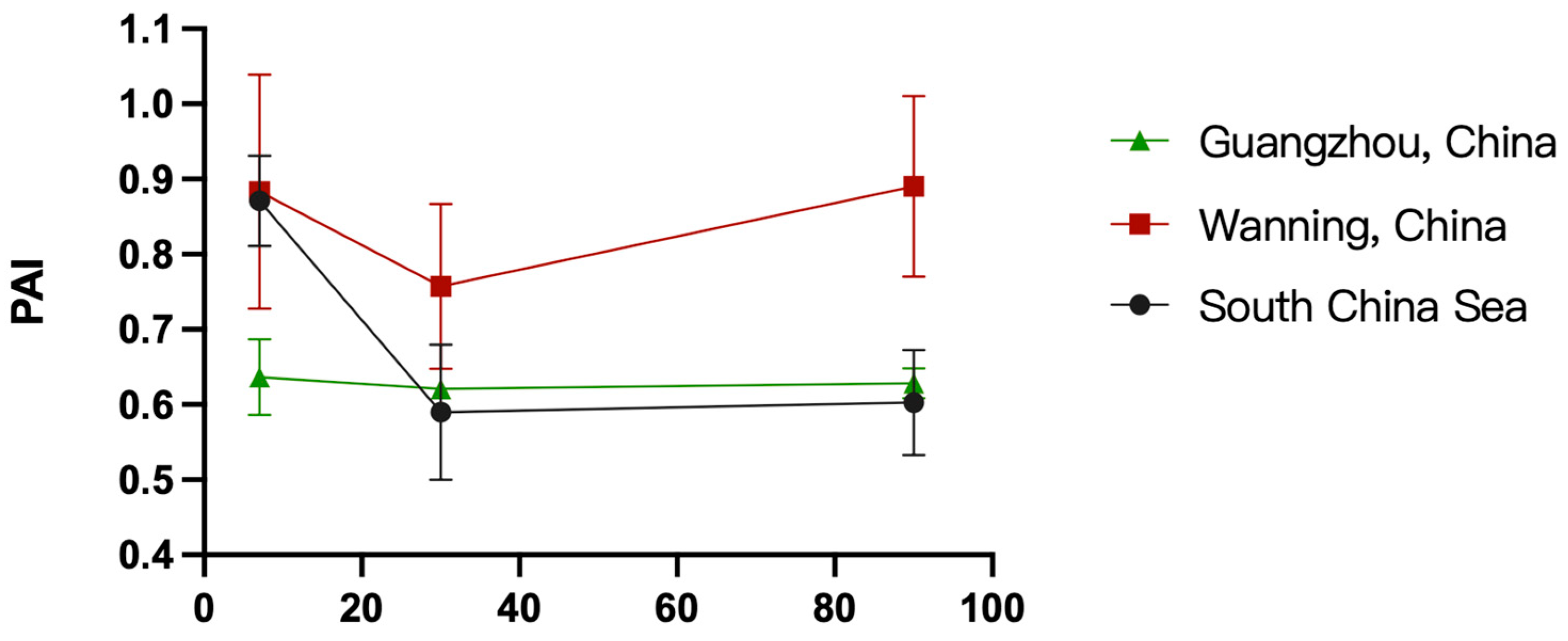
3.3. Atmospheric Corrosion Mechanism of 45# Steel
4. Conclusions
- Corrosion Rate and Geographical Influence:
- 2.
- Corrosion Rust Penetration in Defective Coatings:
- 3.
- Predominant Corrosion Products:
- 4.
- Protection Ability Index (PAI):
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, J.; Li, N.; Li, Q.; Jiang, Z.; Wang, B.; He, Y.; Liu, F.; Liu, C. Effect of Ca and Sb on the Corrosion Resistance of E690 Steel in Marine Atmosphere Environment. Metals 2023, 13, 826. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.; Wang, X.; Jiang, Q.; Li, Y.; Huang, Y.; Yang, L. Research on Dynamic Marine Atmospheric Corrosion Behavior of AZ31 Magnesium Alloy. Metals 2022, 12, 1886. [Google Scholar] [CrossRef]
- He, X.; Zhou, X.; Shang, T.; Liu, W.; Jiang, G.; Liu, C.; Cheng, X.; Zhang, X.; Li, X. Influence Mechanism of Different Elements and Alloy Phases on the Corrosion Resistance of Zn-Al-Mg Coated Steel in the Atmospheric Environment: A Review. Corros. Commun. 2024, 13, 49–59. [Google Scholar] [CrossRef]
- Mathew, Z.P.; Shamnamol, G.K.; Greeshma, K.P.; John, S. Insight on the Corrosion Inhibition of Nanocomposite Chitosan/Boron Nitride Integrated Epoxy Coating System against Mild Steel. Corros. Commun. 2023, 9, 36–43. [Google Scholar] [CrossRef]
- Li, Z.; Fu, D.; Pei, Z. Modeling Research of Initial Atmospheric Corrosion of Q235 Carbon Steel Based on Electrical Resistance Probe. Anti-Corros. Methods Mater. 2021, 68, 45–52. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Lu, X.; Wang, Z. Effect of Temperature and Ultraviolet Radiation on Corrosion Behavior of Carbon Steel in High Humidity Tropical Marine Atmosphere. Mater. Chem. Phys. 2022, 277, 124962. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Q.; Wang, J.; Chen, N.; Chen, J.; Song, J.; Zhang, X.; Xiao, K. Study of the Role of Aluminium and Corrosion Mechanism in Galvalume Coating in the Marine Atmospheric Environment. Anti-Corros. Methods Mater. 2024, 71, 286–294. [Google Scholar] [CrossRef]
- Li, S.; Hihara, L.H. Aerosol Salt Particle Deposition on Metals Exposed to Marine Environments: A Study Related to Marine Atmospheric Corrosion. J. Electrochem. Soc. 2014, 161, C268–C275. [Google Scholar] [CrossRef]
- Lin, C.; Chen, S. Atmospheric Corrosion Behavior of Mild Steel in the Initial Stage under Different Relative Humidity. Int. J. Georesources Environ. 2018, 4, 33–39. [Google Scholar] [CrossRef]
- Ahmad, Z. Atmospheric Corrosion. In Principles of Corrosion Engineering and Corrosion Control; Elsevier: Amsterdam, The Netherlands, 2006; pp. 550–575. [Google Scholar]
- Cole, I.S.; Paterson, D.A.; Ganther, W.D. Holistic Model for Atmospheric Corrosion Part 1–Theoretical Framework for Production, Transportation and Deposition of Marine Salts. Corros. Eng. Sci. Technol. 2003, 38, 129–134. [Google Scholar] [CrossRef]
- Santana, J.J.; Santana, J.; González, J.E.; de la Fuente, D.; Chico, B.; Morcillo, M. Atmospheric Corrosivity Map for Steel in Canary Isles. Br. Corros. J. 2001, 36, 266–271. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Wu, J.S.; Wang, J.J.; Zheng, W.L.; Chen, J.G.; Li, A.B. Corrosion Behavior of Weathering Steel in Marine Atmosphere. Mater. Chem. Phys. 2003, 77, 603–608. [Google Scholar] [CrossRef]
- Askey, A.; Lyon, S.B.; Thompson, G.E.; Johnson, J.B.; Wood, G.C.; Cooke, M.; Sage, P. The Corrosion of Iron and Zinc by Atmospheric Hydrogen Chloride. Corros. Sci. 1993, 34, 233–247. [Google Scholar] [CrossRef]
- ISO 9223:2012; Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Classification, Determination and Estimation. ISO: Geneva, Switzerland, 2012.
- Daneshian, B.; Höche, D.; Knudsen, O.Ø.; Skilbred, A.W.B. Effect of Climatic Parameters on Marine Atmospheric Corrosion: Correlation Analysis of on-Site Sensors Data. Npj Mater. Degrad. 2023, 7, 10. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. Corrosion of Low Carbon Steel in Atmospheric Environments of Different Chloride Content. Corros. Sci. 2009, 51, 997–1006. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; Otero, E.; Mariaca, L. Effect of Marine Aerosol on Atmospheric Corrosio. Mater. Perform. 1999, 38, 72–77. [Google Scholar]
- Yoo, Y.R.; Choi, S.H.; Kim, Y.S. Atmospheric Corrosion Behavior of Carbon Steel by the Outdoor Exposure Test for 10 Years in Korea. Corros. Sci. Technol. 2022, 21, 184–199. [Google Scholar]
- Liu, Y.; Zhao, H.; Wang, Z.; Wei, Y.; Pan, C.; Lv, C. Corrosion Behavior of Low-Carbon Steel and Weathering Steel in a Coastal Zone of the Spratly Islands: A Tropical Marine Atmosphere. Int. J. Electrochem. Sci. 2020, 15, 6464–6477. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, Y.; Li, E.; Wang, T.; Xie, W. Characteristic Identification of Steel Corrosion Products Based on Terahertz Transmission Spectroscopy. J. Build. Eng. 2023, 80, 107996. [Google Scholar] [CrossRef]
- Krivy, V.; Kubzova, M.; Kreislova, K.; Urban, V. Characterization of Corrosion Products on Weathering Steel Bridges Influenced by Chloride Deposition. Metals 2017, 7, 336. [Google Scholar] [CrossRef]
- Veneranda, M.; Aramendia, J.; Bellot-Gurlet, L.; Colomban, P.; Castro, K.; Madariaga, J.M. FTIR Spectroscopic Semi-Quantification of Iron Phases: A New Method to Evaluate the Protection Ability Index (PAI) of Archaeological Artefacts Corrosion Systems. Corros. Sci. 2018, 133, 68–77. [Google Scholar] [CrossRef]
- Dillmann, P.; Mazaudier, F.; Hœrlé, S. Advances in Understanding Atmospheric Corrosion of Iron. I. Rust Characterisation of Ancient Ferrous Artefacts Exposed to Indoor Atmospheric Corrosion. Corros. Sci. 2004, 46, 1401–1429. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Zhang, J.; Lu, X.; Liu, M.-R.; Wang, Z.-Y. Effect of Metal Cations on Corrosion Behavior and Surface Structure of Carbon Steel in Chloride Ion Atmosphere. Acta Metall. Sin. 2020, 33, 1302–1310. [Google Scholar] [CrossRef]
- Misawa, T.; Asami, K.; Hashimoto, K.; Shimodaira, S. The Mechanism of Atmospheric Rusting and the Protective Amorphous Rust on Low Alloy Steel. Corros. Sci. 1974, 14, 279–289. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Tzeng, H.J.; Wei, L.I.; Wang, L.H.; Oung, J.C.; Shih, H.C. Corrosion Resistance and Mechanical Properties of Low-Alloy Steels under Atmospheric Conditions. Corros. Sci. 2005, 47, 1001–1021. [Google Scholar] [CrossRef]
- Misawa, T.; Hashimoto, K.; Shimodaira, S. The Mechanism of Formation of Iron Oxide and Oxyhydroxides in Aqueous Solutions at Room Temperature. Corros. Sci. 1974, 14, 131–149. [Google Scholar] [CrossRef]




| Location | Guangzhou | Wanning | South China Sea |
| Latitude | 23° N | 18° N | 16° N |
| Climatic type | Subtropical monsoon climate | Tropical Rainforest climate | Tropical Marine climate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhang, B.; Liu, G.; Wang, L.; Li, J.; Yuan, P.; Yang, Z.; Feng, Z. Research on Atmospheric Corrosion of 45# Steel in Low-Latitude Coastal Areas of China. Metals 2024, 14, 674. https://doi.org/10.3390/met14060674
Liu L, Zhang B, Liu G, Wang L, Li J, Yuan P, Yang Z, Feng Z. Research on Atmospheric Corrosion of 45# Steel in Low-Latitude Coastal Areas of China. Metals. 2024; 14(6):674. https://doi.org/10.3390/met14060674
Chicago/Turabian StyleLiu, Lihong, Bo Zhang, Guoqiang Liu, Liyan Wang, Jiao Li, Peng Yuan, Zi Yang, and Zhiyuan Feng. 2024. "Research on Atmospheric Corrosion of 45# Steel in Low-Latitude Coastal Areas of China" Metals 14, no. 6: 674. https://doi.org/10.3390/met14060674







