Design and Mechanical Performance Evaluation of WE43 Magnesium Alloy Biodegradable Stents via Finite Element Analysis
Abstract
:1. Introduction
2. Materials and Models
2.1. Structural Design of Stent
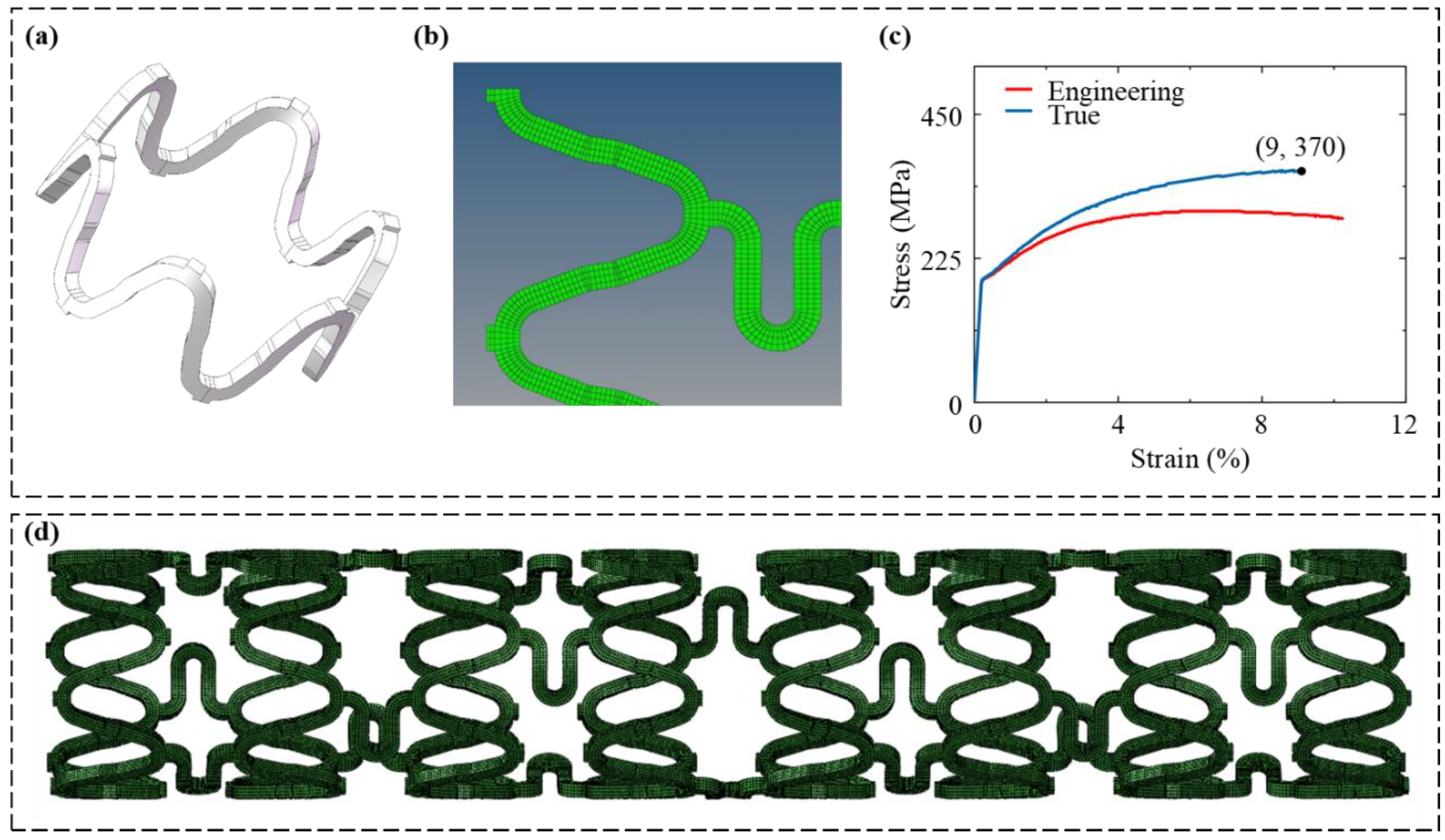
2.2. Geometry Model of Stent
2.3. Material Properties
2.4. Meshing
2.5. Boundary Conditions and Load Settings
2.5.1. Crimping and Expansion Process
- (1)
- Crimping: The radial displacement load is applied to the crimping shell, compressing the stent from an outer diameter of 3 mm to 2 mm. Constraints are applied to the crimping shell to prevent rigid body displacement and rotation, allowing only radial deformation.
- (2)
- Recoil: Set the radial displacement load of the crimping shell to x = 0 mm, allowing the stent to elastically recover, and the crimping shell returns to its initial position.
- (3)
- Expansion: The radial displacement load is applied to the expansion shell, expanding the stent from an outer diameter of 2 mm to 4 mm. The constraints are similar to those during the crimping process, and the contact elements between the crimping shell and the outer surface of the stent are deactivated.
- (4)
- Expansion unloading: Set the radial displacement load of the expansion shell to x = 0 mm, allowing the stent to elastically recover.
2.5.2. Radial Support Testing Process
- (1)
- Planar compression: The radial displacement is applied to 12 planar compression plates, crimping the magnesium alloy vascular stent to 2 mm [46]. The rigid body displacements and rotations of the planar compression plates are constrained in all directions except for the radial direction, thus allowing only the radial displacement loads. Circumferential constraints are applied at both ends of the stent to prevent rotation, and axial constraints are applied at the center of the stent to prevent axial rigid body displacement.
- (2)
- Planar compression unloading: The radial displacement load r = 0 mm is applied to the planar compression plates to allow the elastic recovery of the stent while the planar compression plates return to their initial positions.
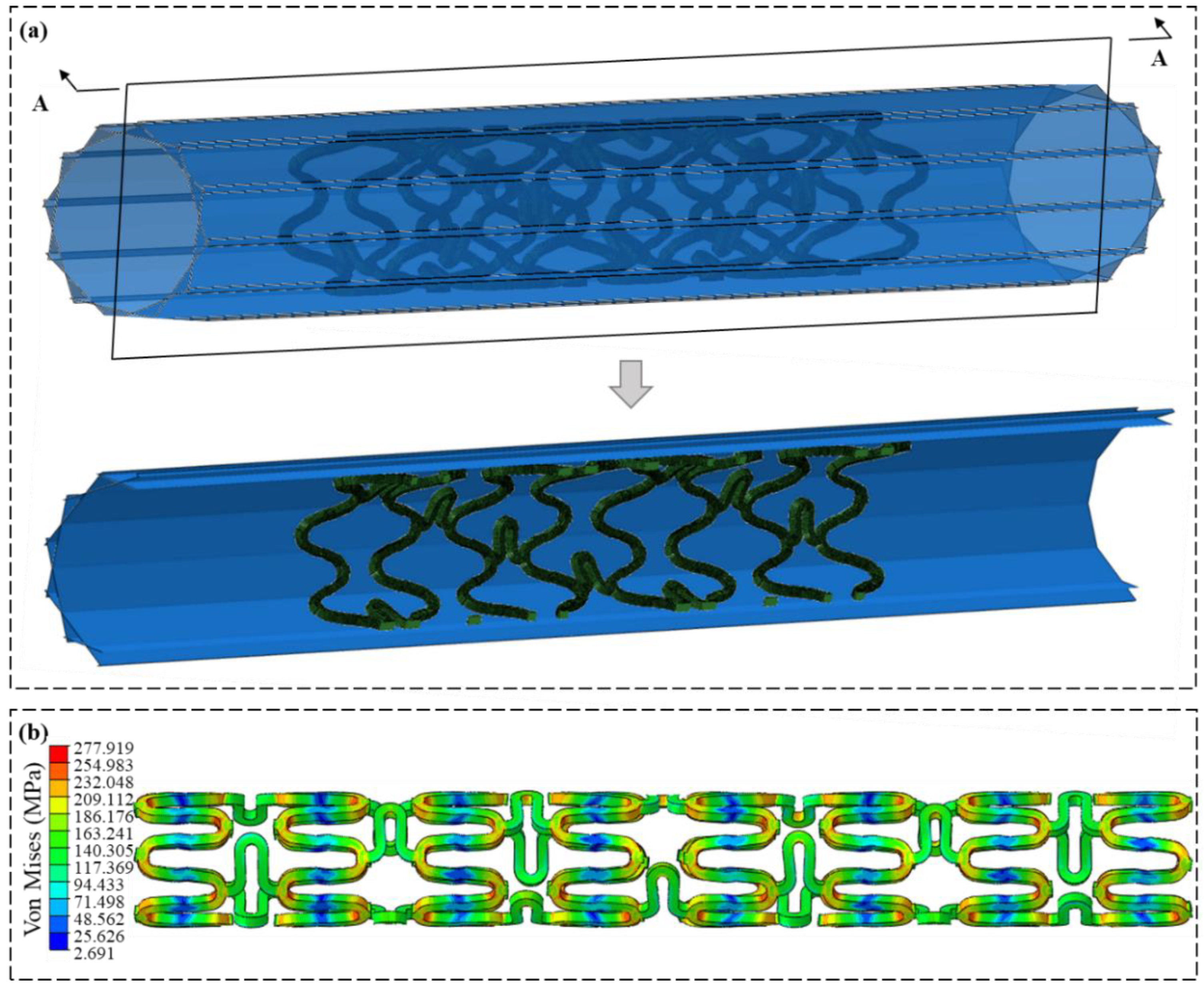
2.5.3. Flexibility Analysis Process
2.5.4. Fatigue Performance Process
3. Analysis of the Mechanical Properties of the Stent
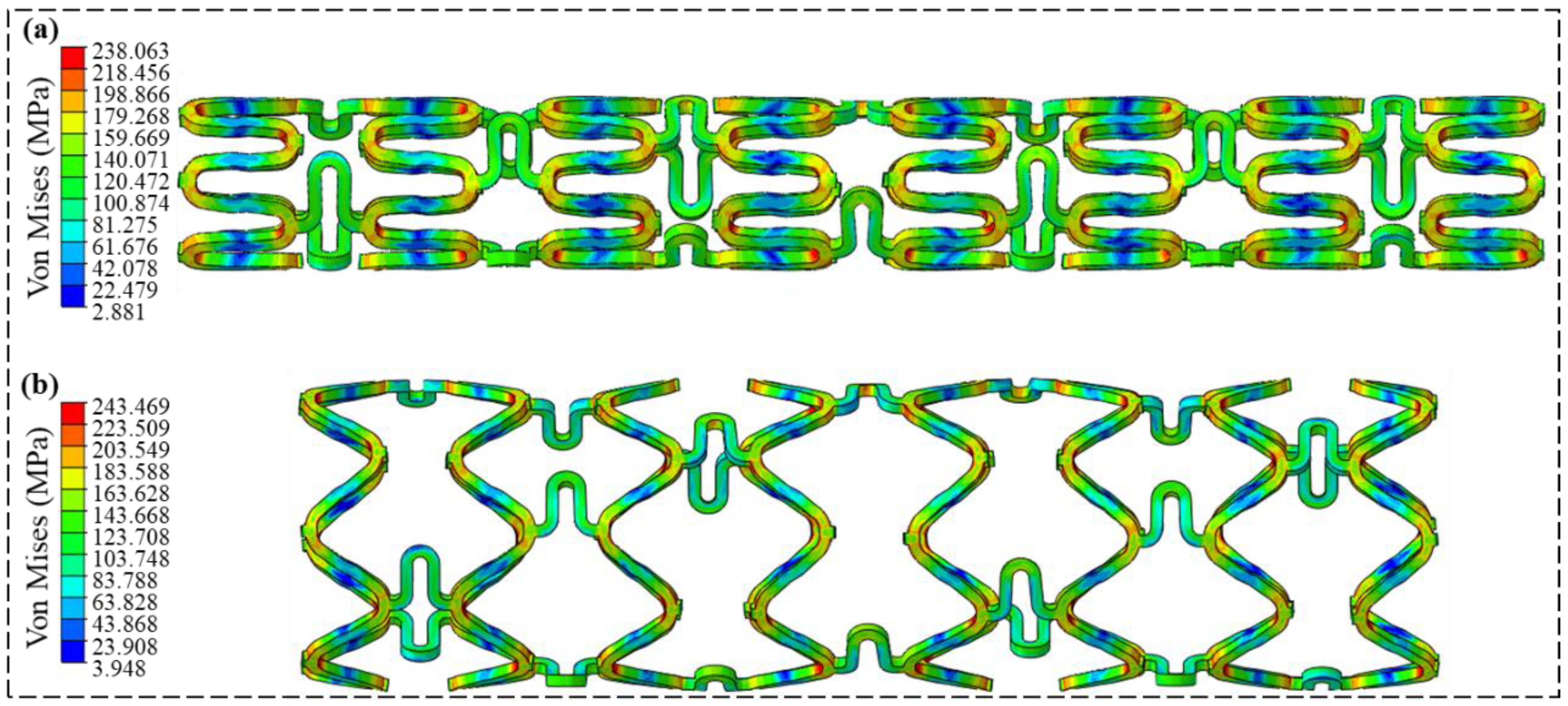
3.1. Crimping and Expansion Analysis
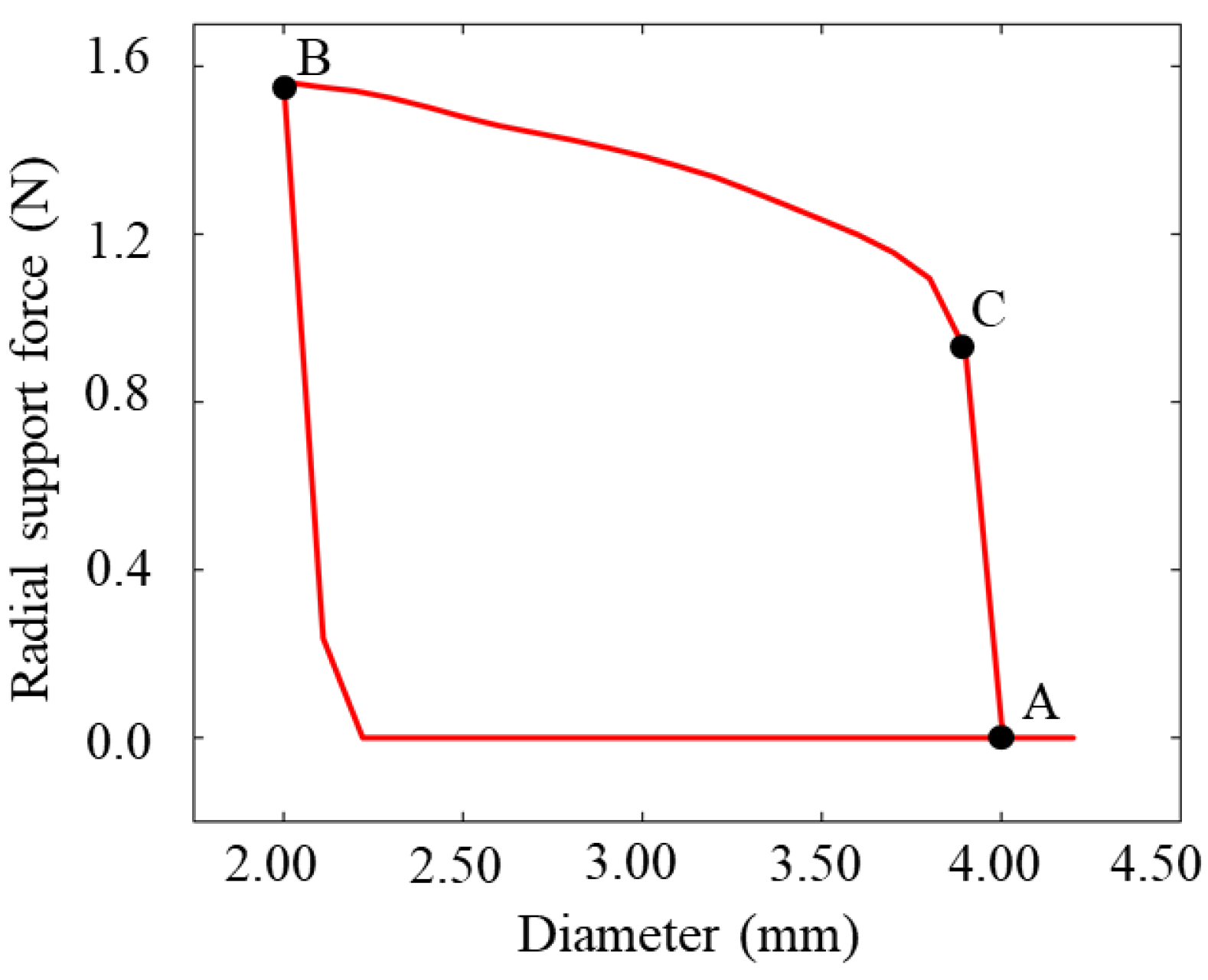
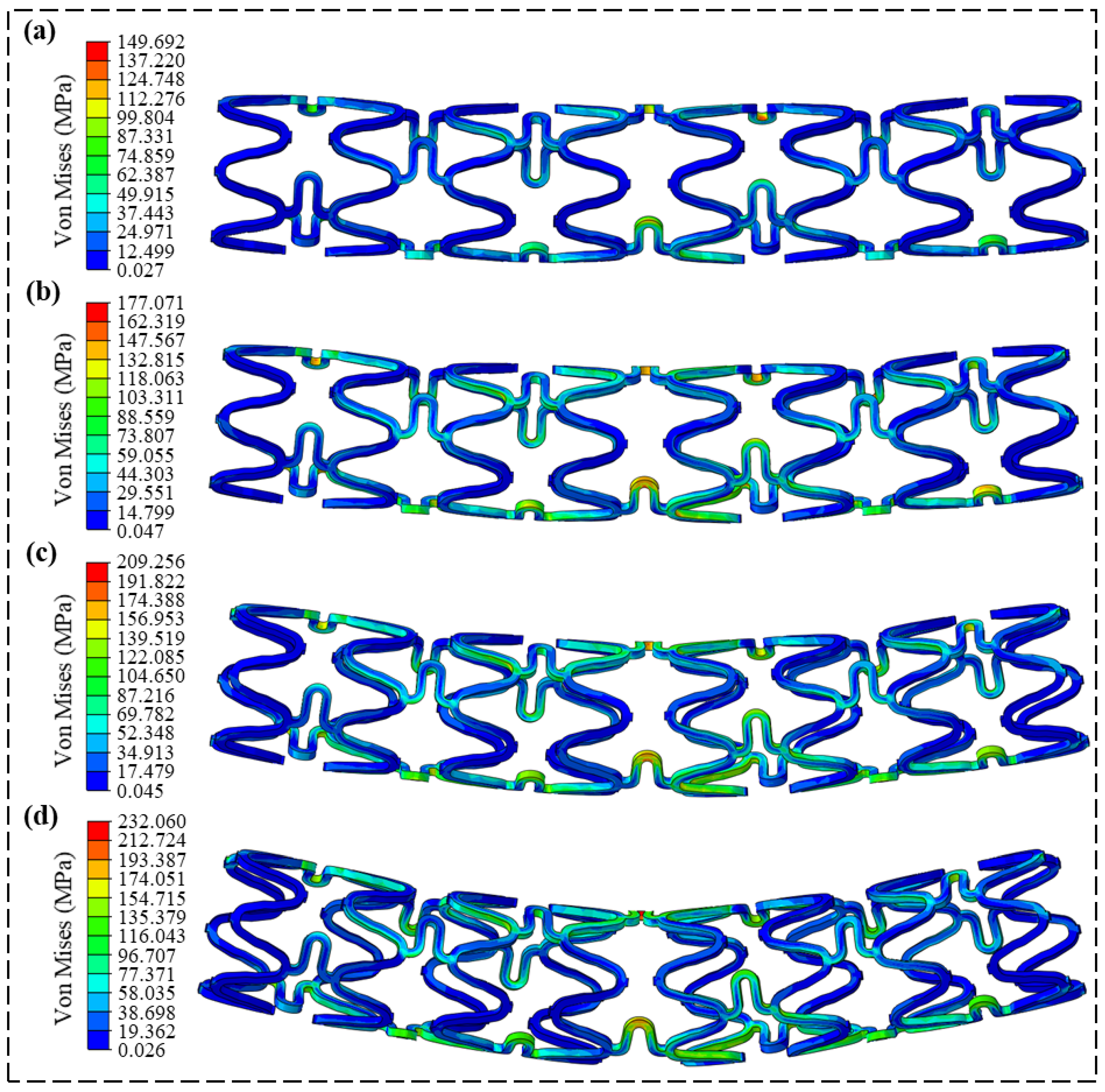
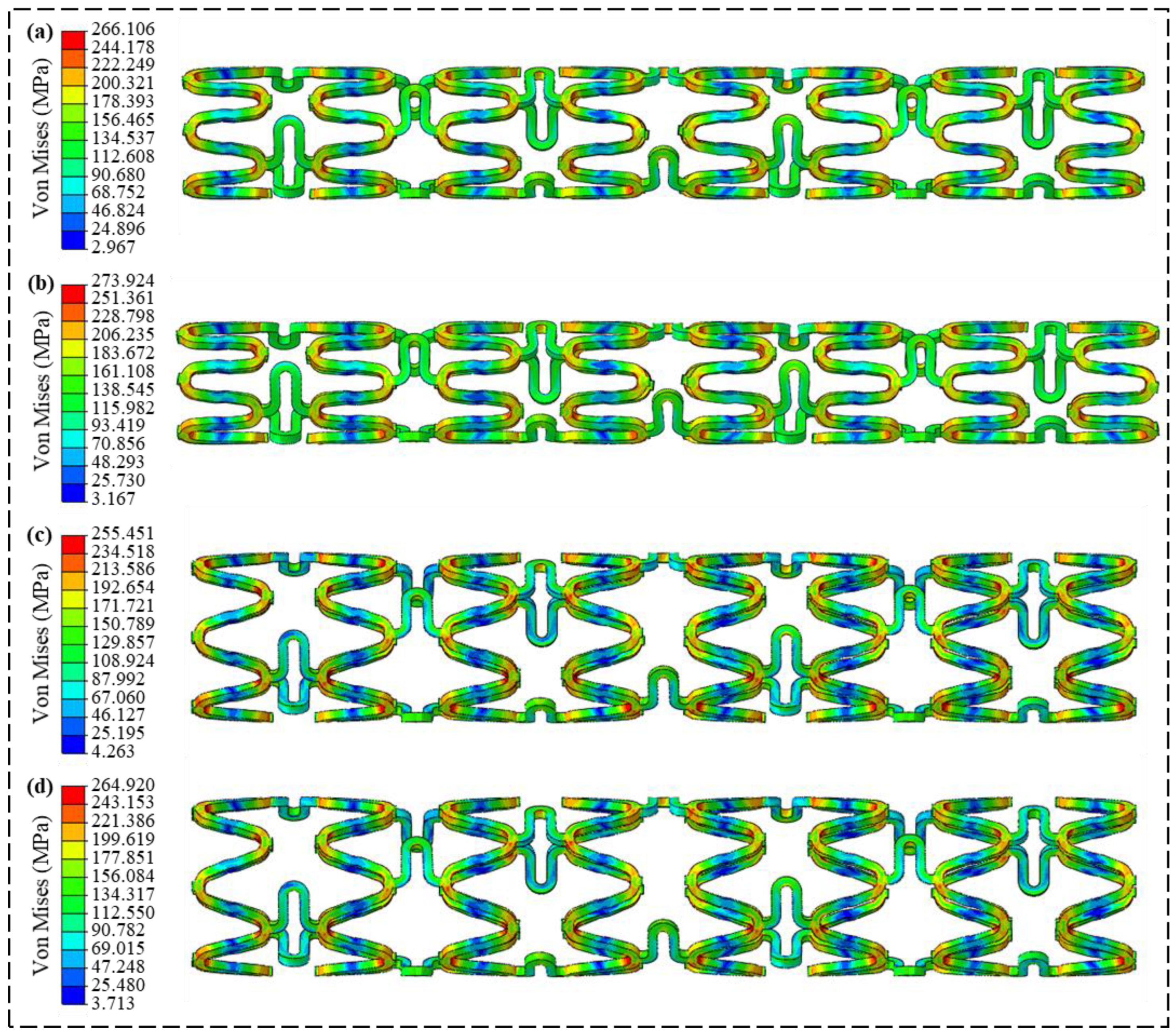
3.2. Radial Support Analysis
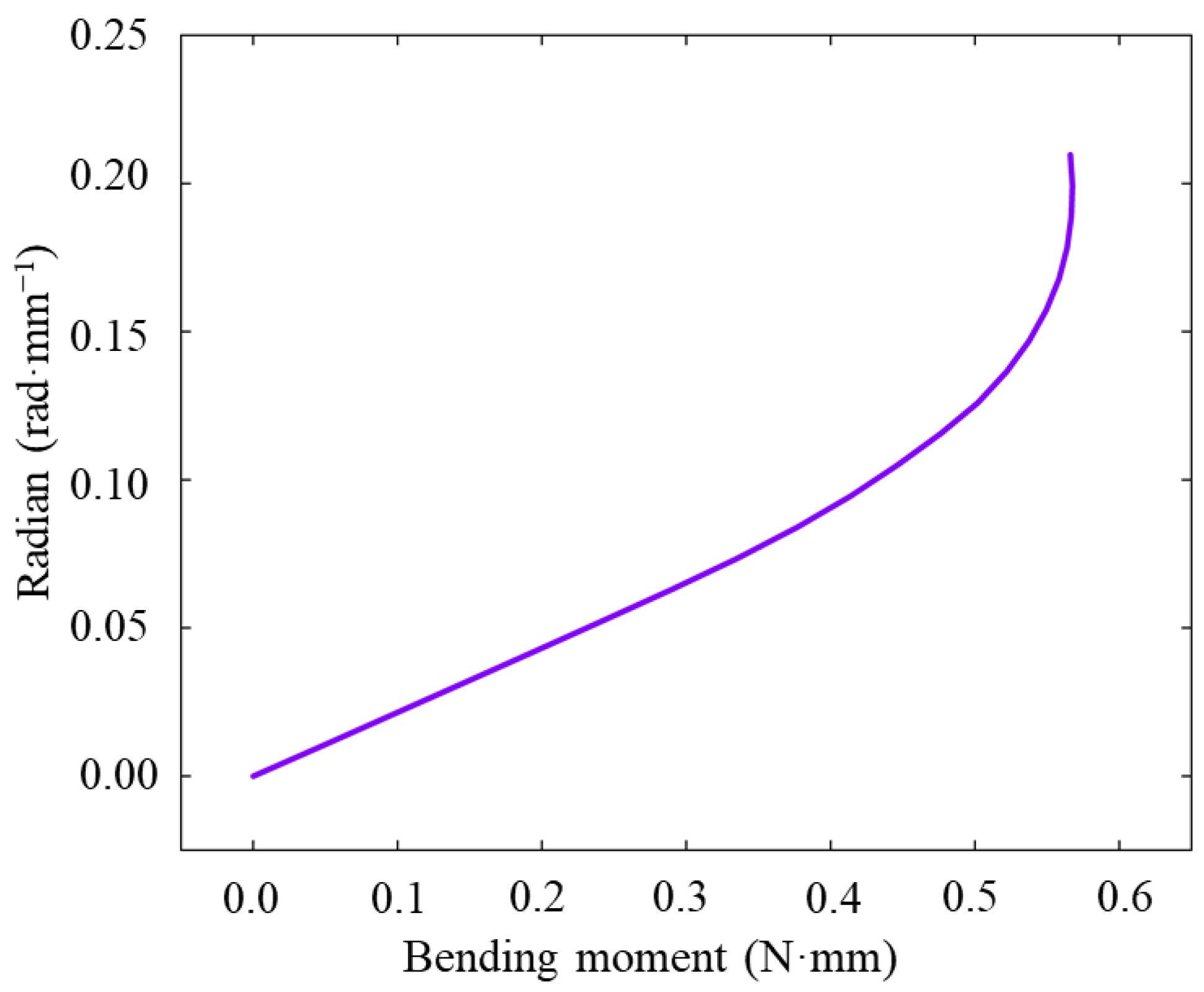
3.3. Flexibility Analysis
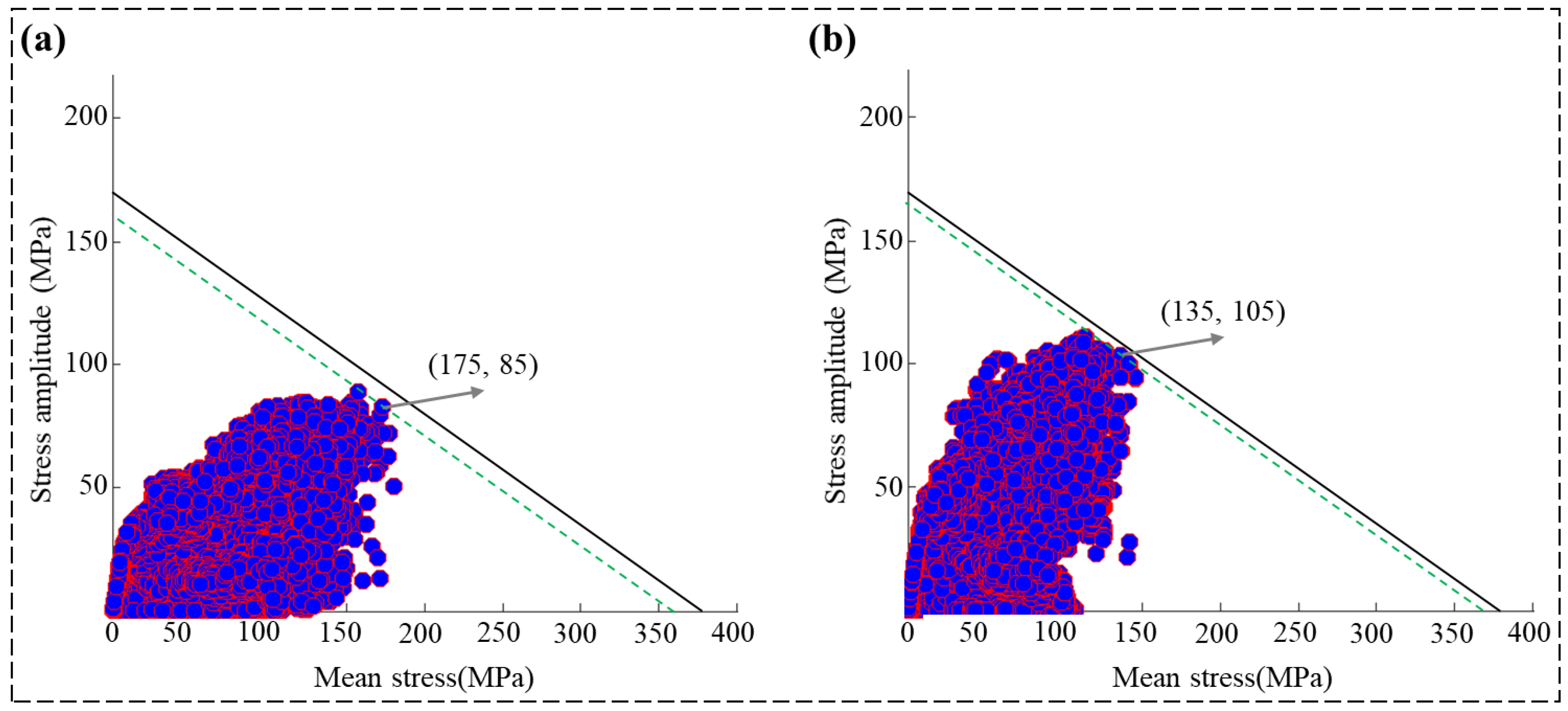
3.4. Fatigue Performance Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naghavi, M.; Wang, H.; Lozano, R.; Davis, A.; Liang, X.; Zhou, M.; Vollset, S.E.; Ozgoren, A.A.; Abdalla, S.; Abd Allah, F.; et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015, 372, 1333–1341. [Google Scholar] [CrossRef]
- Aldea, G.S.; Bakaeen, F.G.; Pal, J.; Fremes, S.; Head, S.J.; Sabik, J.; Rosengart, T.; Kappetein, A.P.; Thourani, V.H.; Firestone, S.; et al. The society of thoracic surgeons clinical practice guidelines on arterial conduits for coronary artery bypass grafting. Ann. Thorac. Surg. 2016, 101, 801–809. [Google Scholar] [CrossRef]
- Gaudino, M.; Benedetto, U.; Fremes, S.; Biondi-Zoccai, G.; Sedrakyan, A.; Puskas, J.D.; Angelini, G.D.; Buxton, B.; Frati, G.; Hare, D.L.; et al. Radial-artery or saphenous-vein grafts in coronary-artery bypass surgery. N. Engl. J. Med. 2018, 378, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Mäkikallio, T.; Holm, N.R.; Lindsay, M.; Spence, M.S.; Erglis, A.; Menown, I.B.A.; Trovik, T.; Eskola, M.; Romppanen, H.; Kellerth, T.; et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): A prospective, randomised, open-label, non-inferiority trial. Lancet 2017, 389, 1102. [Google Scholar] [CrossRef] [PubMed]
- Bønaa, K.H.; Mannsverk, J.; Wiseth, R.; Aaberge, L.; Myreng, Y.; Nygård, O.; Nilsen, D.W.; Kløw, N.-E.; Uchto, M.; Trovik, T.; et al. Drug-eluting or Bare-metal stents for coronary artery disease. N. Engl. J. Med. 2016, 375, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, G.G.; Holmes, D.R., Jr. Drug-eluting coronary-artery stents. N. Engl. J. Med. 2013, 368, 254–265. [Google Scholar] [CrossRef]
- Köster, R.; Vieluf, D.; Kiehn, M.; Sommerauer, M.; Kähler, J.; Baldus, S.; Meinertz, T.; Hamm, C.W. Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet 2000, 356, 1895–1897. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.P.; Sefton, M.V. The influence of biomaterials on endothelial cell thrombogenicity. Biomaterials 2007, 28, 2547–2571. [Google Scholar] [CrossRef]
- Scott, N.A. Restenosis following implantation of bare metal coronary stents: Pathophysiology and pathways involved in the vascular response to injury. Adv. Drug Deliv. Rev. 2006, 58, 358–376. [Google Scholar] [CrossRef]
- Welt, F.G.P.; Rogers, C. Inflammation and restenosis in the stent era. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1769–1776. [Google Scholar] [CrossRef]
- Boland, E.L.; Shine, C.J.; Kelly, N.; Sweeney, C.A.; McHugh, P.E. A Review of Material Degradation Modelling for the Analysis and Design of Bioabsorbable Stents. Ann. Biomed. Eng. 2015, 44, 341–356. [Google Scholar] [CrossRef]
- Gogas, B.D. Bioresorbable scaffolds for percutaneous coronary interventions. Glob. Cardiol. Sci. Pract. 2015, 2014, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, J.; Nef, H.M.; Hamm, C.W. Current Status of Bioresorbable Scaffolds in the Treatment of Coronary Artery Disease. J. Am. Coll. Circardiol. 2014, 64, 2541–2551. [Google Scholar] [CrossRef]
- Hubbert, L.; Baranowski, J.; Delshad, B.; Ahn, H. Left Atrial Pressure Monitoring with an Implantable Wireless Pressure Sensor After Implantation of a Left Ventricular Assist Device. ASAIO J. 2017, 63, e60–e65. [Google Scholar] [CrossRef]
- Raj, L.M.; Saxon, L.A. Haemodynamic monitoring devices in heart failure: Maximising benefit with digitally enabled patient centric care. Arrhythmia Electrophysiol. Rev. 2018, 7, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Schmier, J.K.; Ong, K.L.; Fonarow, G.C. Cost-effectiveness of remote cardiac monitoring with the cardiomems heart failure sys-tem. Clin. Cardiol. 2017, 40, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, H.; Dube, D.; Mantovani, D. Developments in metallic biodegradable stents. Acta Biomater. 2010, 6, 1693–1697. [Google Scholar] [CrossRef]
- Niklason, L.; Dai, G.H. Arterial venous differentiation for vascular bioengineering. Annu. Rev. Biomed. Eng. 2018, 20, 431–447. [Google Scholar] [CrossRef]
- Iqbal, J.; Onuma, Y.; Ormiston, J.; Abizaid, A.; Waksman, R.; Serruys, P. Bioresorbable scaffolds: Rationale, current status, challenges, and future. Eur. Heart J. 2013, 35, 765–776. [Google Scholar] [CrossRef]
- Yamaji, K.; Ueki, Y.; Souteyrand, G.; Daemen, J.; Wiebe, J.; Nef, H.; Adriaenssens, T.; Loh, J.P.; Lattuca, B.; Wykrzykowska, J.J.; et al. Mechanisms of very late bioresorbable scaffold thrombosis the invest registry. J. Am. Coll. Cardiol. 2017, 70, 2330–2344. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Torii, S.; Sakamoto, A.; Kolodgie, F.D.; Virmani, R.; Finn, A.V. Fully bioresorbable vascular scaffolds: Lessons learned and future directions. Nat. Rev. Cardiol. 2019, 16, 286–304. [Google Scholar] [CrossRef]
- Wang, P.J.; Ferralis, N.; Conway, C.; Grossman, J.C.; Edelman, E.R. Strain-induced accelerated asymmetric spatial degradation of polymeric vascular scaffolds. Proc. Natl. Acad. Sci. USA 2018, 115, 2640–2645. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.J.; Nezami, F.R.; Gorji, M.B.; Berti, F.; Petrini, L.; Wierzbicki, T.; Migliavacca, F.; Edelman, E.R. Effect of working environment and procedural strategies on mechanical performance of bioresorbable vascular scaffolds. Acta Biomater. 2018, 82, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Guillory, R.J., 2nd; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable Metals for Cardiovascular Stents: From Clinical Concerns to Recent Zn-Alloys. Adv. Healthc. Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Mao, L.; Shen, L.; Chen, J.H.; Wu, Y.; Kwak, M.; Lu, Y.; Xue, Q.; Pei, J.; Zhang, L.; Yuan, G.; et al. Enhanced bioactivity of Mg-Nd-Zn-Zr alloy achieved with nanoscale MgF2 surface for vascular stent application. ACS Appl. Mater. Interfaces 2015, 7, 5320–5330. [Google Scholar] [CrossRef]
- Mao, L.; Shen, L.; Niu, J.; Zhang, J.; Ding, W.; Wu, Y.; Fan, R.; Yuan, G. Nanophasic biodegradation enhances the durability and biocompatibility of magnesium alloys for the next-generation vascular stents. Nanoscale 2013, 5, 9517–9522. [Google Scholar] [CrossRef]
- Heublein, B.; Rohde, R.; Kaese, V.; Niemeyer, M.; Hartung, W.; Haverich, A. Biocorrosion of magnesium alloys: A new principle in cardiovascular implant technology? Heart 2003, 89, 651–656. [Google Scholar] [CrossRef]
- DI Mario, C.; Griffiths, H.; Goktekin, O.; Peeters, N.; Verbist, J.; Bosiers, M.; Deloose, K.; Heublein, B.; Rohde, R.; Kasese, V.; et al. Drug-Eluting Bioabsorbable Magnesium Stent. J. Interv. Cardiol. 2004, 17, 391–395. [Google Scholar] [CrossRef]
- Waksman, R.; Pakala, R.; Kuchulakanti, P.K.; Baffour, R.; Hellinga, D.; Seabron, R.; Tio, F.O.; Wittchow, E.; Hartwig, S.; Harder, C.; et al. Safety and efficacy of bioabsorbable magnesium alloy stents in porcine coronary arteries. Catheter. Cardiovasc. Interv. 2006, 68, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Di Mario, C.; Bartunek, J.; Bonnier, J.; de Bruyne, B.; Eberli, F.R.; Erne, P.; Haude, M.; Heublein, B.; Horrigan, M.; et al. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: A prospective, non-randomised multicentre trial. Lancet 2007, 369, 1869–1875. [Google Scholar] [CrossRef]
- Haude, M.; Erbel, R.; Erne, P.; Verheye, S.; Degen, H.; Böse, D.; Vermeersch, P.; Wijnbergen, I.; Weissman, N.; Prati, F.; et al. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet 2013, 381, 836–844. [Google Scholar] [CrossRef]
- Peeters, P.; Bosiers, M.; Verbist, J.; Deloose, K.; Heublein, B. Preliminary Results After Application of Absorbable Metal Stents in Patients with Critical Limb Ischemia. J. Endovasc. Ther. 2005, 12, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Erbel, R.; Di Mario, C.; Bartunek, J.; de Bruyne, B.; Eberli, F.R.; Erne, P.; Haude, M.; Horrigan, M.; Ilsley, C.; et al. Early- and long-term intravascular ultrasound and angiographic findings after bioab-sorbable magnesium stent implantation in human coronary arteries. JACC Cardiovasc. Interv. 2009, 2, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lara, J.; Radu, M.; Brugaletta, S.; Farooq, V.; Diletti, R.; Onuma, Y.; Windecker, S.; Thuesen, L.; McClean, D.; Koolen, J.; et al. Serial Analysis of the Malapposed and Uncovered Struts of the New Generation of Everolimus-Eluting Bioresorbable Scaffold with Optical Coherence Tomography. JACC Cardiovasc. Interv. 2011, 4, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zheng, Y.F. Novel magnesium alloys developed for biomedical application: A review. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Zhang, H.B.; Zhang, Y.R.; Wu, H.; Wei, L.; Zhou, G.; Zhang, Y.; Deng, L.; Cheng, Y.; Li, M.; et al. Endovascular metal devices for the treatment of cerebrovascular diseases. Adv. Mater. 2019, 31, 44. [Google Scholar] [CrossRef]
- Antoniadis, A.P.; Mortier, P.; Kassab, G.; Dubini, G.; Foin, N.; Murasato, Y.; Giannopoulos, A.A.; Tu, S.; Iwasaki, K.; Hikichi, Y.; et al. Biomechanical modeling to improve coronary artery bifurcation stenting expert review document on techniques and clinical implementation. JACC Cardiovasc. Interv. 2015, 8, 1281–1296. [Google Scholar] [CrossRef]
- ISO 25539-2:2020; Cardiovascular Implants—Endovascular Devices—Part 2. Vascular Stents. International Organization for Standardization (ISO): Geneva, Switzerland, 2020.
- YY/T 0693-2008; Passive Implants-Intravascular Catheters. National Medical Products Administration (NMPA): Beijing, China, 2008.
- Cai, Y.; Wei, J.; Yan, H.; Chen, Y.; Chen, R. Low-cycle fatigue behavior of solutionized and aged WE43 magnesium alloys at room temperature. J. Magnes. Alloys, 2022; in press. [Google Scholar] [CrossRef]
- Kubásek, J.; Dvorský, D.; Čavojský, M.; Roudnická, M.; Vojtech, D. WE43 magnesium alloy-material for challenging applications. Met. Mater. 2019, 57, 159–165. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, G.; Zhao, Y.H.; Zhou, J. Improvement of mechanical performance of bioresorbable magnesium alloy coronary artery stents through stent pattern redesign. Appl. Sci. 2018, 8, 22. [Google Scholar] [CrossRef]
- Green, S.R.; Kwon, R.S.; Elta, G.H.; Gianchandani, Y.B. In vivo and in situ evaluation of a wireless magnetoelastic sensor array for plastic biliary stent monitoring. Biomed. Microdevices 2013, 15, 509–517. [Google Scholar] [CrossRef]
- Harvey, S.M. Nitinol Stent Fatigue in a Peripheral Human Artery Subjected to Pulsatile and Articulation Loading. J. Mater. Eng. Perform. 2011, 20, 697–705. [Google Scholar] [CrossRef]
- Lootz, D.; Surber, B.; Wintsch, D.; Riedmueller, J. Stent Having Radially Expandable Main Body. U.S. Patent No. 12/057,806, 25 June 2013. [Google Scholar]
- Kwiecinski, J.; Cheng, C.P.; Uberoi, R.; Hadi, M.; Hempel, P.; Degel, C.; You, Z. Thoracic aortic parallel stent-graft behaviour when subjected to radial loading. J. Mech. Behav. Biomed. Mater. 2021, 118, 104407. [Google Scholar] [CrossRef]
- Schmidt, W.; Behrens, P.; Brandt-Wunderlich, C.; Siewert, S.; Grabow, N.; Schmitz, K.-P. In vitro performance investigation of bioresorbable scaffolds—Standard tests for vascular stents and beyond. Cardiovasc. Revasc. Med. 2016, 17, 375–383. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Non-Clinical Engineering Tests and Recommended Labeling for Intravascular Stents and Associated Delivery Systems: Guidance for Industry and FDA Staff; US Department of Health and Human Services, Food and Drug Administration, Centre for Devices and Radiological Health: Silver Spring, MD, USA, 2010.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Dong, F.; Liu, S. Design and Mechanical Performance Evaluation of WE43 Magnesium Alloy Biodegradable Stents via Finite Element Analysis. Metals 2024, 14, 704. https://doi.org/10.3390/met14060704
Chen J, Dong F, Liu S. Design and Mechanical Performance Evaluation of WE43 Magnesium Alloy Biodegradable Stents via Finite Element Analysis. Metals. 2024; 14(6):704. https://doi.org/10.3390/met14060704
Chicago/Turabian StyleChen, Jiaxuan, Fang Dong, and Sheng Liu. 2024. "Design and Mechanical Performance Evaluation of WE43 Magnesium Alloy Biodegradable Stents via Finite Element Analysis" Metals 14, no. 6: 704. https://doi.org/10.3390/met14060704



