Abstract
Waste-conductive silver pastes are considered an important secondary resource. The recovery of metals from waste-conductive silver pastes have high economic value. The traditional cyanidation method has serious environmental pollution, while the thiosulfate method is green, environmentally friendly, and has become a viable alternative for metal extraction. The exposure of thiosulfate complexes to ultraviolet (UV) light has been found to generate metal sulfides, and this can be used to realize the recovery of valuable metals. In this study, the extraction of silver and copper from conductive silver pastes was systematically performed using sodium thiosulfate, and the effects of sodium thiosulfate concentration, solid-to-liquid ratio, and extraction and photolytic process temperatures were investigated. The photolytic products were characterized using X-ray diffraction, scanning electron microscopy, energy dispersive X-ray spectroscopy, transmission electron microscopy, and X-ray photoelectron spectroscopy. After 4 h of UV irradiation with a wavelength of 254 nm, 87% of silver and 49% of copper were recovered and transformed into silver and copper sulfide, respectively. This study demonstrates that thiosulfate can be applied in combination with UV photolysis technology to recover valuable metals in an environmentally friendly manner.
1. Introduction
As society develops and people’s living conditions improve, the consumption of silver rises year after year, along with the increase in the quantity and diversity of silver-containing wastes. Due to the limited geological reserves of Ag, along with the complexity and high cost of the Ag production process, it is crucial to expand the Ag source by recovering silver from various silver-containing wastes. Conductive Ag pastes play an indispensable role in the electronics sector. Their industrial application surpasses that of other precious metals. Therefore, in the recycling of silver-containing wastes, waste-conductive Ag pastes account for a significant portion of it. Waste-conductive Ag pastes are not only derived from electronic wastes but are also produced in electronics manufacturing. These waste-conductive Ag pastes, if left untreated, can lead to environmental pollution and pose a risk to human health [1].
Conductive silver pastes, specialized electronic material crucial for various electronic products, play a pivotal role in component packaging, electrode fabrication, and interconnect assembly. They are widely used in aviation, construction, chemical engineering, communications, and military applications [2,3]. These pastes are composed of a conductive phase, a bonded phase, and an organic carrier. Ag has excellent electrical properties and good oxidation resistance [4]. The rapid development of the conductive Ag paste industry in China, demonstrated by its substantial market size, is primarily driven by the expanding photovoltaic sector, particularly the solar cell industry. However, the high costs limit the further development of conductive Ag pastes. Cu and Ag have similar conductivities and resistivities; however, Cu is relatively inexpensive. Therefore, to reduce production costs, the incorporation of Cu into Ag pastes offers a promising solution. By doping Cu into Ag powder, manufacturers can create mixed or composite powders, effectively reducing the overall Ag content while maintaining conductivity and enhancing cost-efficiency. Moreover, the Ag and Cu in the conductive Ag pastes can be recycled and reused.
Initially, the recycling methods used were often rudimentary, including manual dismantling [5], incineration [6], and strong acid extraction [7]. In the strong acid extraction process, HNO3, HClO4, and aqua regia [8,9,10] have been commonly used to recover metals from e-waste; however, this method is associated with significant drawbacks, including toxic pollution, particularly, environmental contamination, and a low recovery rate [11]. New and advanced recycling technologies, such as mechanical treatment [12] and pyrometallurgical [13], bio- [14], and hydrometallurgical technology [15], have now been proposed. Alvarado-Macías et al. [16] utilized the S2O32−-NO2−-SO32−-Cu2+ system to extract pure metallic silver. Through a thermodynamic study of various system variables, at 0.1 mol/L S2O32−, 1.5 mol/L NO2−, 0.05 mol/L Cu2+, pH = 7.27, and room temperature, they achieved the highest extraction rate of 96% for Ag extraction. Ficeriová et al. [17] investigated the extraction of waste circuit boards at 0.5 mol/L (NH4)2S2O3, 1 mol/L NH3, 0.2 mol/LCuSO4·5H2O, a solid-liquid ratio of 80 g/L, pH = 9, and a temperature of 40 °C for 48 h. The extraction rates of Au and Ag reached 98% and 93%, respectively. Cui et al. [18] investigated the extraction of Ag using the Cu2+-S2O32− solution system. The results indicated that in the absence of ammonium ions, the Cu2+-S2O32− solution system exhibited significant efficacy. The extraction rate of Ag can reach 95% when the concentration of S2O32− is 0.12 mol/L and the concentration of Cu2+ is 0.048 mol/L.
A comparison of several recycling techniques reveals that mechanical treatment technology has high energy consumption, complex processes, and low recovery rates. Pyrometallurgical technology generates a large amount of harmful gases that excessively pollute the environment. Biotechnology is still in the research stage, with limited available microbial species and stringent environmental requirements. Conversely, hydrometallurgical technology is easy to implement, with high metal recovery and low pollution; therefore, it is applied as an effective method for metal extraction [19]. Cyanide is the earliest and most common extraction agent; however, it has high toxicity, making it not only dangerous to use but also harmful to health. With the continuous enhancement of people’s awareness of environmental protection, cyanide is no longer suitable for application in the extraction process; so far, more than 30 alternative extraction agents have been developed [20]. However, only a few have been put to use, and thiosulfate has become one of the most promising alternatives, owing to its non-toxic, efficient, and environmentally friendly characteristics [21,22]. Additionally, the recovery of metals from extraction solutions is essential for resource recycling. Traditional recovery technologies include activated carbon adsorption [23,24], electrodeposition [25], resin adsorption [26], and solvent extraction [27,28,29]. Most heavy metal-thiosulfate complex solutions exhibit intense absorption in the ultraviolet (UV) region (200–280 nm), enabling them to decompose into the corresponding heavy metal sulfides. For example, mercuric-thiosulfate complexes obtained via extraction from mercury-containing soil can be spontaneously decomposed to mercuric sulfide by a disproportionation reaction [30]. Wang [31] studied the decomposition process of mercury-thiosulfate complexes under UV light conditions, determined that the decomposition product was mercury sulfide, and found that most heavy metal sulfides generated were on the scale of nanometer to micrometer.
In this study, the traditional idea of recovering metal from an extraction solution is changed, and ultraviolet decomposition technology is introduced to investigate the ability of thiosulfate combined with UV photolysis to recover Ag and Cu from waste-conductive Ag pastes. The effects of experimental parameters, including the thiosulfate concentration, solid-to-liquid ratio, and temperature, on the extraction and recovery processes were systematically investigated. Based on the results, a discussion of the feasibility of metal recovery from e-waste and a detailed analysis are presented.
2. Experimental
2.1. Materials
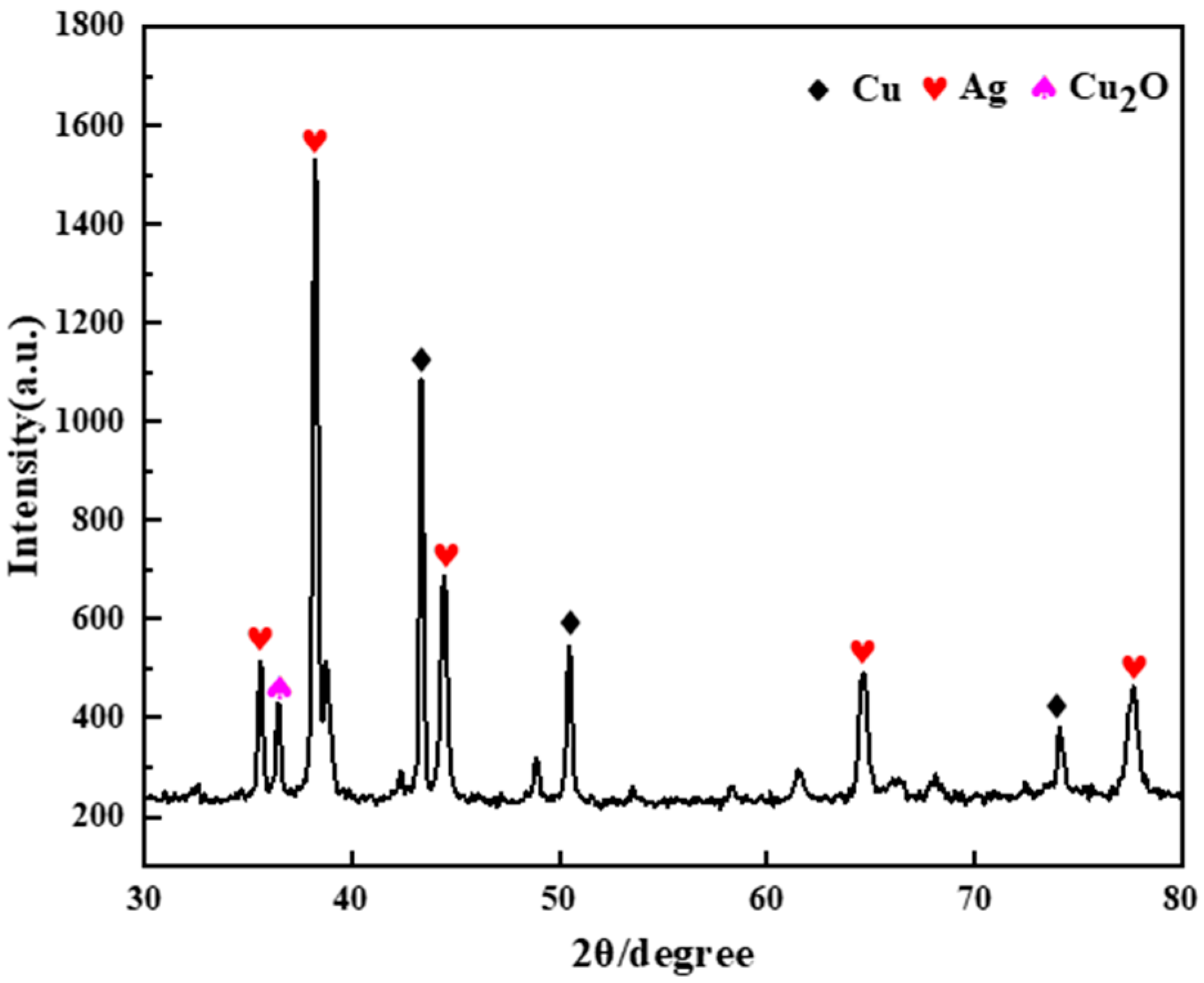
The conductive Ag pastes used for this study were obtained from Shenzhen Baoan District Shajing Xindasheng Instrument Electronic Accessories Company (Shenzhen, China). Before conducting the analysis and experiment, the sample was first poured into a surface dish and placed in a drying oven at 70 °C for 3 h to obtain the cured conductive Ag pastes. Then, the cured conductive Ag pastes were placed in a heating furnace at a high temperature of 500 °C for 2 h. Finally, the conductive Ag pastes were ground into powder form to serve as the experimental raw material for later use. Figure 1 shows the X-ray diffraction (XRD) pattern of the conductive paste. The detected phases reveal that the conductive paste mainly contained Ag, Cu, and Cu2O. Conductive Ag paste contains 21.89% Ag and 53.00% Cu.
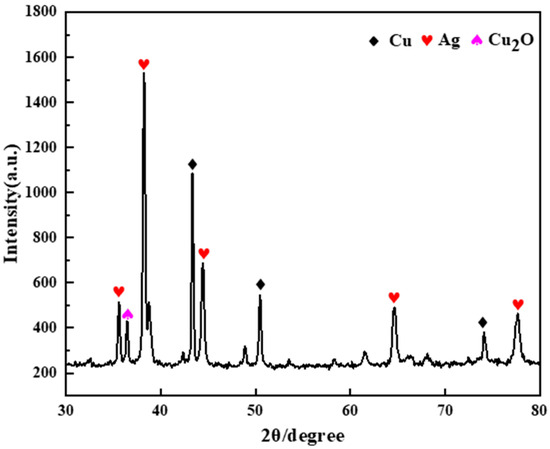
Figure 1.
XRD pattern of the conductive paste.
Analytical-grade reagents used in this study included sodium thiosulfate pentahydrate (Na2S2O3-5H2O (≥99.0%)), nitric acid, and hydrochloric acid. Deionized water produced from a high-purity water system with a typical resistivity of 18.24 MΩ·cm was used in all experiments. Standard Ag and Cu solutions were prepared via the stepwise dilution of a standard stock solution (1000 mg/L Ag as AgNO3 and 1000 mg/L Cu as Cu(NO3)2).
2.2. Extraction Experiments
The conductive Ag pastes were extracted using a KQ3200DE 6 L (Quanzhou Tongbai Technology Co., Ltd., Quanzhou, China) model constant-temperature magnetic stirrer. First, M grams of sodium thiosulfate were weighed in a beaker on an electronic balance. A certain amount of deionized water was added, and the mixture was stirred with a glass rod until it was thoroughly dissolved. The solution in the beaker was poured into a 250 mL volumetric flask to fix the volume. N g of conductive paste was weighed and poured into the 250 mL conical flask, then the sodium thiosulfate solution after the constant volume was poured into the conical bottle. The conical flask containing the solution was placed on the constant-temperature magnetic stirrer, and the corresponding temperature, shaking speed, and time were set. Then, 10 mL of the extraction solution was taken at regular intervals for filtration and dilution, and the Ag and Cu concentrations were determined using an inductively coupled plasma-emission optical spectrometer ICP-OES (PerkinElmer Optima 8000, PerkinElmer, Inc., Singapore); subsequently, the extraction rates (%) of Ag and Cu were calculated.
2.3. Recycling Experiments
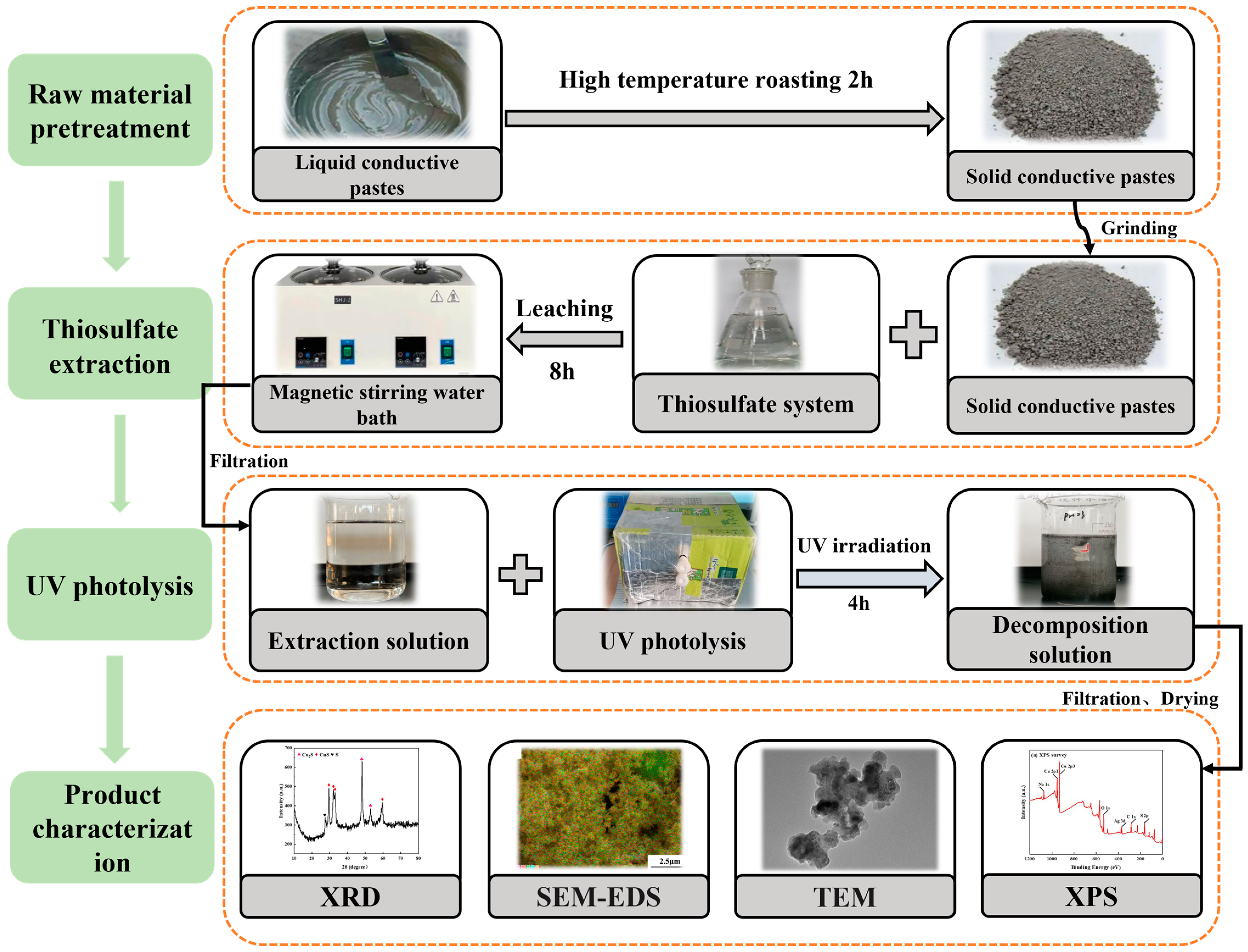
The extraction solution was filtered and poured into a quartz beaker. Then, a UV lamp with a wavelength of 254 nm was turned on (operating parameters of the UV lamp: mode of connection: single-ended four wire; power: 12 W; wavelength: 254 nm; voltage: 42 V; current: 380 mA; length/OD: 287 mm/15 mm; radiation intensity: 31 µW/cm2; lamp quantity: 2; the distance between lamp and solution: 5 cm) and applied for 4 h. The solution was taken at 10 mL intervals, and after filtration and dilution, its concentration was determined using an ICP-OES; subsequently, the decomposition (%) of Ag and Cu was calculated. The flowchart diagram for the experiment is shown in Figure 2.
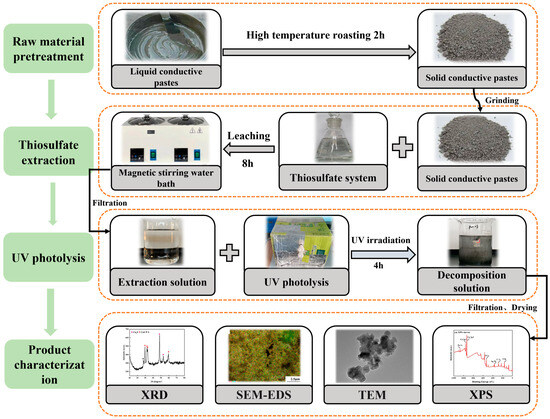
Figure 2.
Flowchart diagram for the experiment.
2.4. Analytical Methods
The Ag and Cu concentrations in the aqueous samples were analyzed using the aforementioned ICP-OES, and the Ag and Cu concentrations were calculated to determine the Ag and Cu precipitates. The phase composition of the precipitate was analyzed via XRD using a Bruker D8 X-ray diffractometer (Bruker, Inc., Karlsruhe, Germany). The diffractometer was applied at 40 mA and 40 kV with Cu Kα (λ = 0.154 nm) radiation; the detector was rotated from 20° to 80° at a scan speed of 0.1°/s. The XRD data were compared and analyzed using standard cards in Jade 6 software; the surface morphology of each of the products was characterized using field-emission scanning electron microscopy (SEM, JSM-5610LV, JEOL, Beijing, China), energy-dispersive X-ray spectrometry (EDS), and transmission electron microscopy (TEM). The solid products on the surface of the photolytic products were investigated using X-ray photoelectron spectroscopy (XPS, ESCALAB 250XI, Thermo Fisher Scientific, Inc., Waltham, MA, USA) methods. The XPS analysis was performed by applying monochromatic Al Kα x-radiation at the take-off angle of 90° with no sputtering or charge neutralization. The C 1s (284.8 eV) line was used for binding energy calibration. The XPS data were fitted using Advantage (v5.9921) software.
3. Results and Discussion
3.1. Extraction of Ag and Cu from Conductive Ag Pastes Using Thiosulfate
The ligand S2O32− in thiosulfate extraction solution can establish strong σ bonds with metal ions such as Ag and Cu through the S atoms at the end. Additionally, the Ag and Cu in the thiosulfate extraction conductive paste mainly exist in the form of Ag-thiosulfate complex ions Ag(S2O3)n(2n−1)− and Cu-thiosulfate complex ions Cu(S2O3)n(2n−1)−, respectively. Ag-thiosulfate complex ions mainly exist in Ag(S2O3)−, Ag(S2O3)23−, and Ag(S2O3)35− forms. The form of the Ag complex ions also differs according to the concentration of S2O32− in the solution; the amount of S2O32− complexed by Ag+ increases with an increase in the concentration of S2O32− in the solution. Cu-thiosulfate complex ions are Cu(S2O3)−, Cu(S2O3)23−, and Cu(S2O3)35−; the presence of Cu in the extraction solution is similar to that of Ag [32].
3.1.1. Effects of Different Sodium Thiosulfate Concentrations
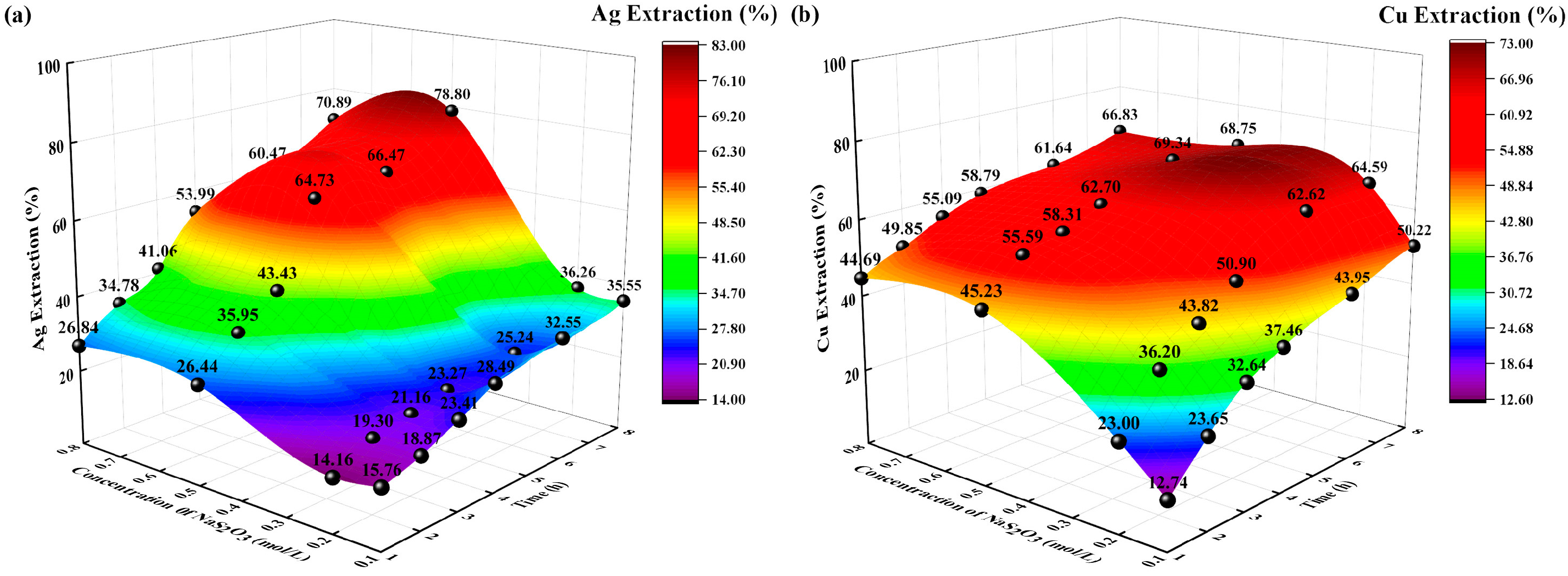
The reaction conditions were as follows: a temperature of 35 °C, a solid-to-liquid ratio of 0.1:250 g/mL, an extraction time of 8 h, and sodium thiosulfate concentrations of 0.1, 0.2, and 0.5 mol/L. The changes in the extraction rates (%) of Ag and Cu with time were examined, and the experimental results are shown in Figure 3.
Figure 3.
Effects of sodium thiosulfate concentration on extraction rate (%). (a) Ag extraction rate (%) vs. time, sodium thiosulfate concentration; (b) Cu extraction rate (%) vs. time, sodium thiosulfate concentration.
The extraction rates (%) of Ag and Cu increased with increasing sodium thiosulfate concentration and time. When the concentration of sodium thiosulfate was 0.5 mol/L and the extraction time was 8 h, the extraction rates of Ag and Cu reached their maxima of 78.80% and 68.75%, respectively. This is because Ag, Cu, and S2O32− form Ag(S2O3)23− and Cu(S2O3)23− in alkaline solutions, coinciding with gradual increases in the extraction rates of Ag and Cu. From the perspective of the law of chemical equilibrium, this phenomenon occurred because the increase in S2O32− ions in the solution increased the contact opportunity of thiosulfate, which improved the complexation ability of Ag and thiosulfate and prompted the equilibrium to move in the direction of the reaction. From the perspective of reaction kinetics, it was beneficial to increase the concentration of reactants to increase the reaction rate and minimize the time required for the reaction to reach equilibrium. When the concentration of sodium thiosulfate was 0.8 mol/L, the extraction rate of Ag and Cu decreased. This decrease was attributed to the decomposition of thiosulfate. Therefore, the optimal concentration of sodium thiosulfate for the extraction of Ag and Cu is 0.5 mol/L.
3.1.2. Effects of Different Solid-to-Liquid Ratios
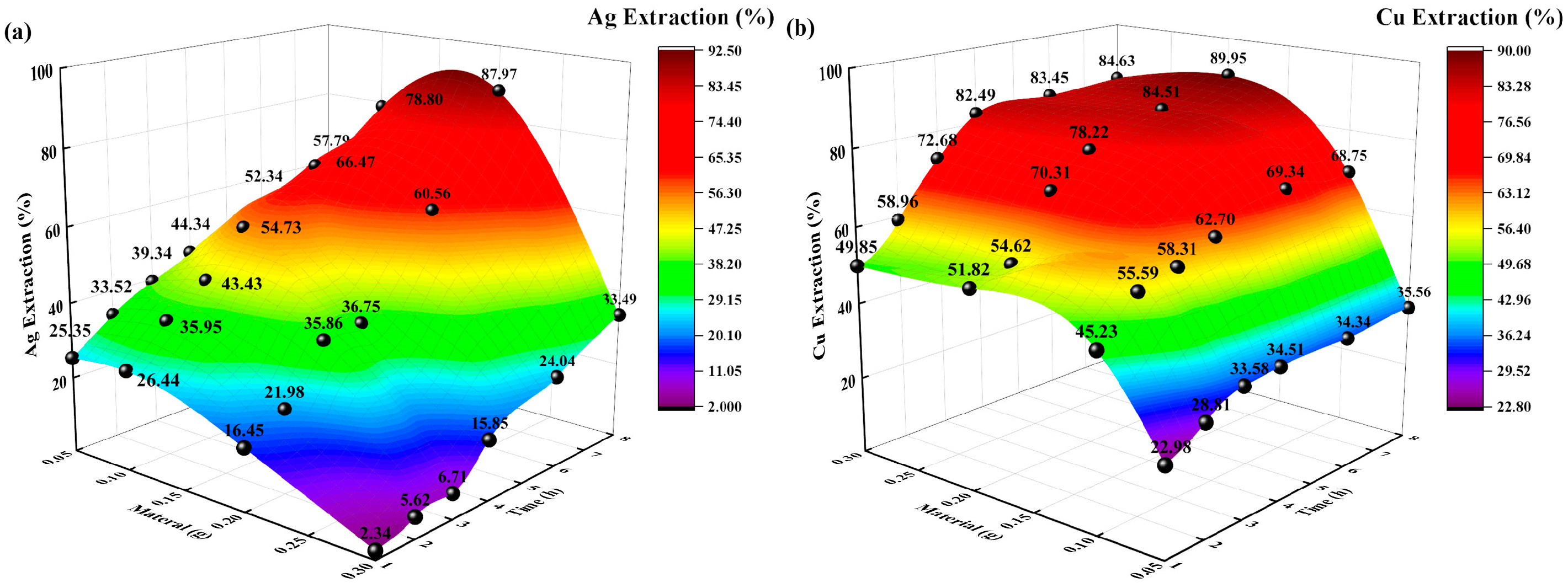
The reaction conditions were as follows: a temperature of 35 °C, a sodium thiosulfate concentration of 0.5 mol/L, an extraction time of 8 h, and solid-to-liquid ratios of 0.05:250, 0.1:250, 0.2:250 g/mL. The changes in the Ag and Cu extraction rates (%) over time were investigated, and the experimental results are shown in Figure 4.
Figure 4.
Effects of solid-to-liquid ratio on extraction rate (%). (a): Ag extraction rate (%) vs. time, solid-to-liquid ratio; (b) Cu extraction rate (%) vs. time, solid-to-liquid ratio.
The Ag and Cu extraction rates (%) increased with increasing time and solid-liquid ratio. When the solid-liquid ratio was 0.05:250 g/mL, the Ag and Cu extraction rates were 57.79% and 35.56%, respectively. When the solid-liquid ratio was 0.2:250 g/mL, the Ag and Cu extraction rates were 87.97% and 89.95%, respectively. Different solid-to-liquid ratios indicate that the total content of Ag and Cu differed for the same extraction solution concentration, that is, the initial concentration of sodium thiosulfate was fixed, thereby changing the concentration of the reactants to allow thiosulfate to react with Ag and Cu. Owing to the low weight of the entire sample and the sufficient concentration of thiosulfate, the solid powder could make direct contact with the thiosulfate ions; thus, the higher the solid-to-liquid ratio, the higher the Ag and Cu extraction rates. Due to the limited concentration of thiosulfate, an excess of solid powder leads to reduced contact with thiosulfate and lower extraction rates. Given these comprehensive results, the best solid-to-liquid ratio for Ag and Cu extraction was found to be 0.1:250 g/mL.
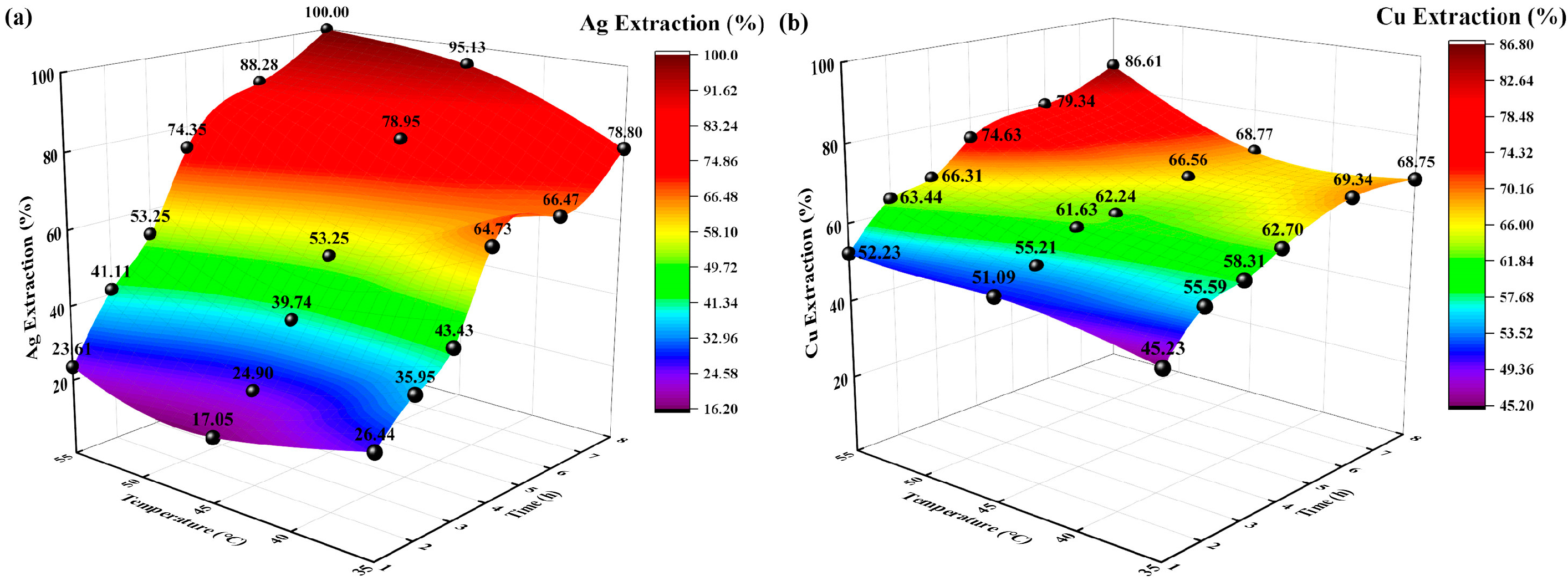
3.1.3. Effects of Different Temperatures
The reaction conditions were as follows: sodium thiosulfate concentration of 0.5 mol/L, solid-to-liquid ratio of 0.1:250 g/mL, extraction time of 8 h, and temperatures of 35, 45, and 55 °C. The changes in the extraction rates (%) of Ag and Cu with time were investigated, and the experimental results are shown in Figure 5.
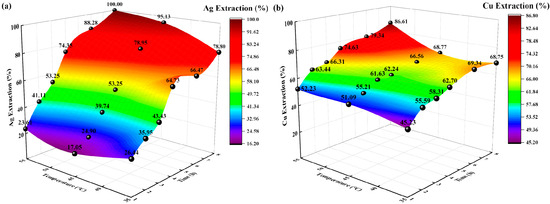
Figure 5.
Effects of temperature on extraction rate (%). (a) Ag extraction rate (%) vs. time, temperature; (b) Cu extraction rate (%) vs. time, temperature.
The extraction rates (%) of Ag and Cu increased with increasing temperature and time. At temperatures of 35 °C and 45 °C, the extraction rate (%) of Cu increased slowly, reaching 68% at 8 h. However, the extraction rates of Ag and Cu at 55 °C are significantly higher than those at 35 °C and 45 °C; the highest extraction rates were 100% and 86.61%, respectively. According to diffusion theory and the thermodynamic analysis of activation energy [33], as the temperature increased, the diffusion coefficient increased, the rate of diffusion of solution ions accelerated, the activation energy required for the reaction decreased, and the likelihood of a complexation reaction increased, thus increasing the Ag and Cu extraction rates. Therefore, the best temperature for Ag and Cu extraction is 55 °C.
3.2. UV Photolysis
According to previous research [34,35,36], the photolysis of Ag-thiosulfate complexes occurs as follows. The complex has strong adsorption in UV-C that varies according to the charge transport spectra. Upon absorption of the energy of UV-C (200–280 nm) radiation, the Ag-thiosulfate complex ions are activated to form Ag2S2O3⁎, entering an unstable excited state, as shown in Equation (1). Through the participation of water molecules in the solution, activated Ag-thiosulfate complex ions are transformed into Ag-hydroxide complexes and thiosulfate ions by the hydroxide ions in the solution. The activated Ag-thiosulfate complex ions can react with hydroxide because the S-S bond is the weakest in the thiosulfate ion; furthermore, the S-S bond in the metal thiosulfate is evidently easy to beak upon excitation. UV irradiation can accelerate the electron transfer, causing the dissociation of S-S bonds and leading to the oxidation of thiosulfate ions (S2O32− → SO42−) and reduction of Ag ions (Ag+ → Ag0). Equations (2) and (3) describe the secondary reactions of the photochemical process, and the total reaction (Equation (4)) is obtained using Equations (1)–(3), as follows:
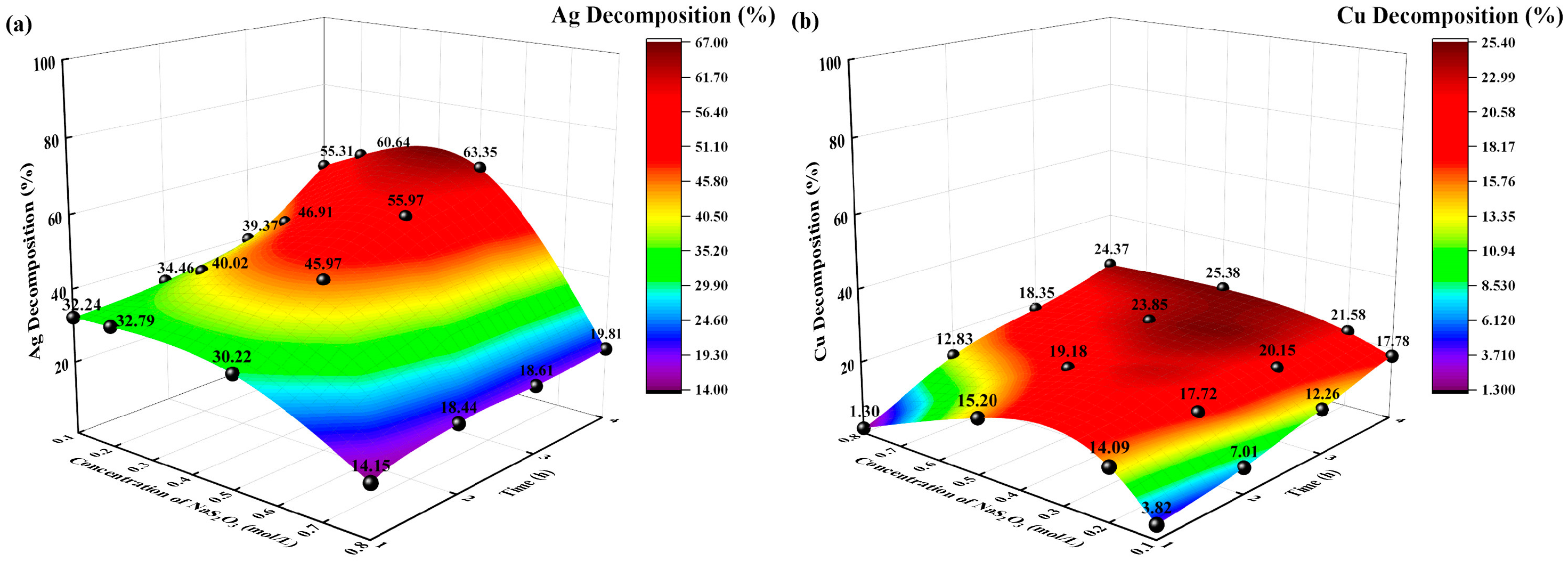
3.2.1. Effects of Different Sodium Thiosulfate Concentrations
The reaction conditions were as follows: temperature of 35 °C, solid-to-liquid ratio of 0.1:250 g/mL, extraction time of 8 h, and sodium thiosulfate concentrations of 0.1, 0.2, and 0.5 mol/L. The changes in the decomposition rate (%) of Ag and Cu with time were investigated, and the experimental results are shown in Figure 6.
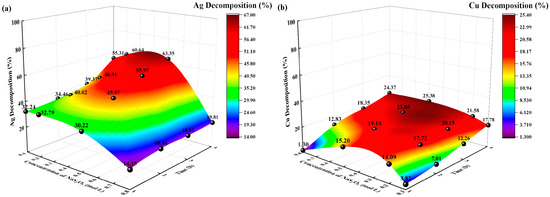
Figure 6.
Effects of sodium thiosulfate concentration on decomposition rate (%). (a) Ag decomposition rate (%) vs. time, sodium thiosulfate concentration; (b): Cu decomposition rate (%) vs. time, sodium thiosulfate concentration.
The decomposition rates (%) of Ag and Cu increased with increasing sodium thiosulfate concentration and time. When the concentration of sodium thiosulfate was 0.1 mol/L, the decomposition rates (%) of Ag and Cu were 55.31% and 17.78%, respectively. When the concentration of sodium thiosulfate was 0.5 mol/L, the decomposition rates (%) of Ag and Cu were 63.35% and 25.38%, respectively. When the concentration of sodium thiosulfate was 0.8 mol/L, the decomposition rates of Ag and Cu decreased to 19.81% and 24.37%, respectively. The different concentrations of sodium thiosulfate resulted in different concentrations of Ag-, Cu-, and Na-thiosulfate complexes in the initial decomposition solution. Figure 6 illustrates that the decomposition rate of Ag and Cu increases as the concentration of sodium thiosulfate increases. However, excessively high concentrations of sodium thiosulfate lead to a decrease in the decomposition rate.
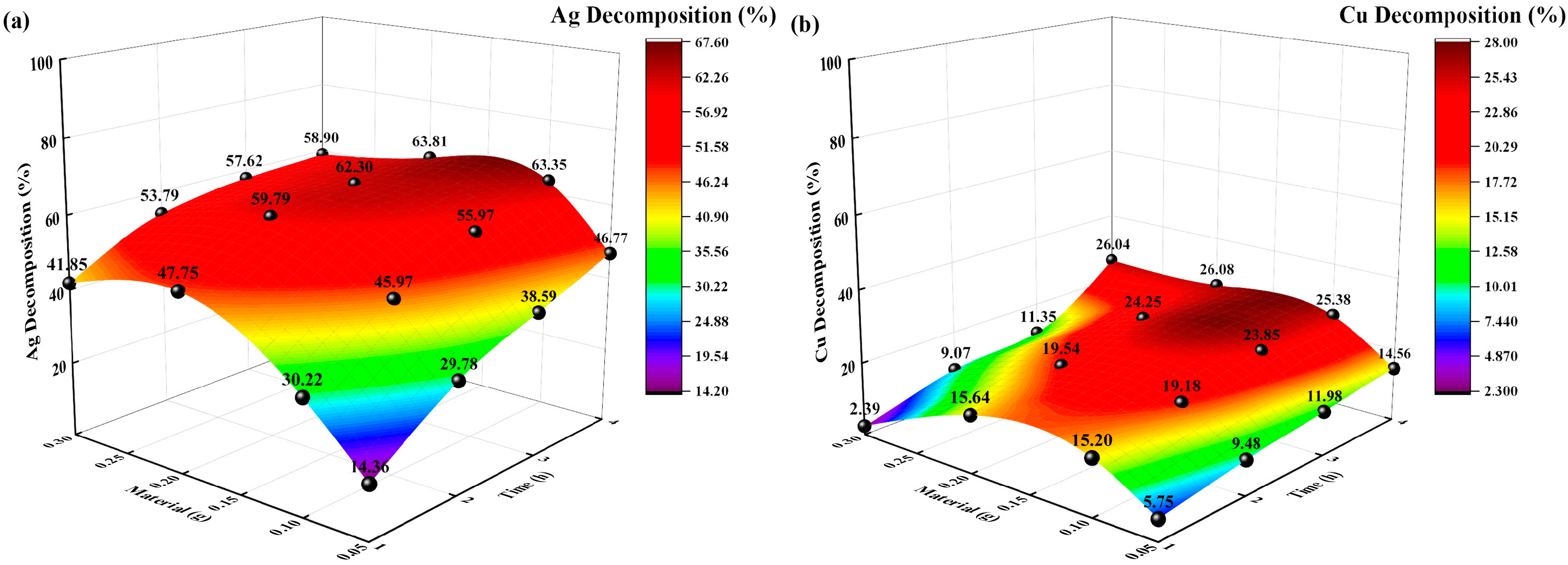
3.2.2. Effects of Different Solid-to-Liquid Ratios
The reaction conditions were as follows: temperature of 35 °C, sodium thiosulfate concentration of 0.5 mol/L, extraction time of 8 h, and solid-to-liquid ratios of 0.05:250, 0.1:250, and 0.2:250 g/mL. The changes in Ag and Cu extraction rates (%) over time were investigated, and the experimental results are shown in Figure 7.
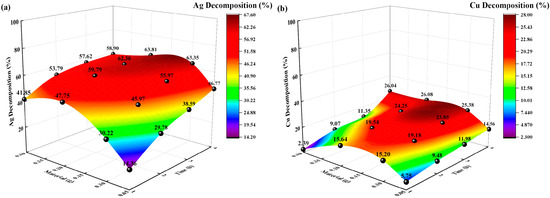
Figure 7.
Effects of solid-to-liquid ratio on decomposition rate (%). (a) Ag decomposition rate (%) vs. time, solid-to-liquid ratio; (b): Cu decomposition (%) vs. time, solid-to-liquid ratio.
The Ag and Cu decomposition rates (%) increased with increasing solid-to-liquid ratio and time. When the solid-liquid ratio was 0.05:250 g/mL, and the decomposition time was 4 h, the highest decomposition rates (%) of Ag and Cu were 46.77% and 14.56%, respectively. However, when the solid-liquid ratio was 0.2:250 g/mL, and the decomposition time was 4 h, the decomposition rates (%) of Ag and Cu increased to 63.81% and 26.08%, respectively. However, when the solid-liquid ratio continued to increase to 0.3:250 g/mL, the decomposition rate of Ag and Cu decreased to 58.9% and 26.04%, respectively. The different solid-liquid ratios resulted in different concentrations of Ag- and Cu-thiosulfate complexes in the initial decomposition solution, as well as different decomposition rates under UV irradiation. The solid-liquid ratio is too high, which will decrease the decomposition rate of Ag and Cu.
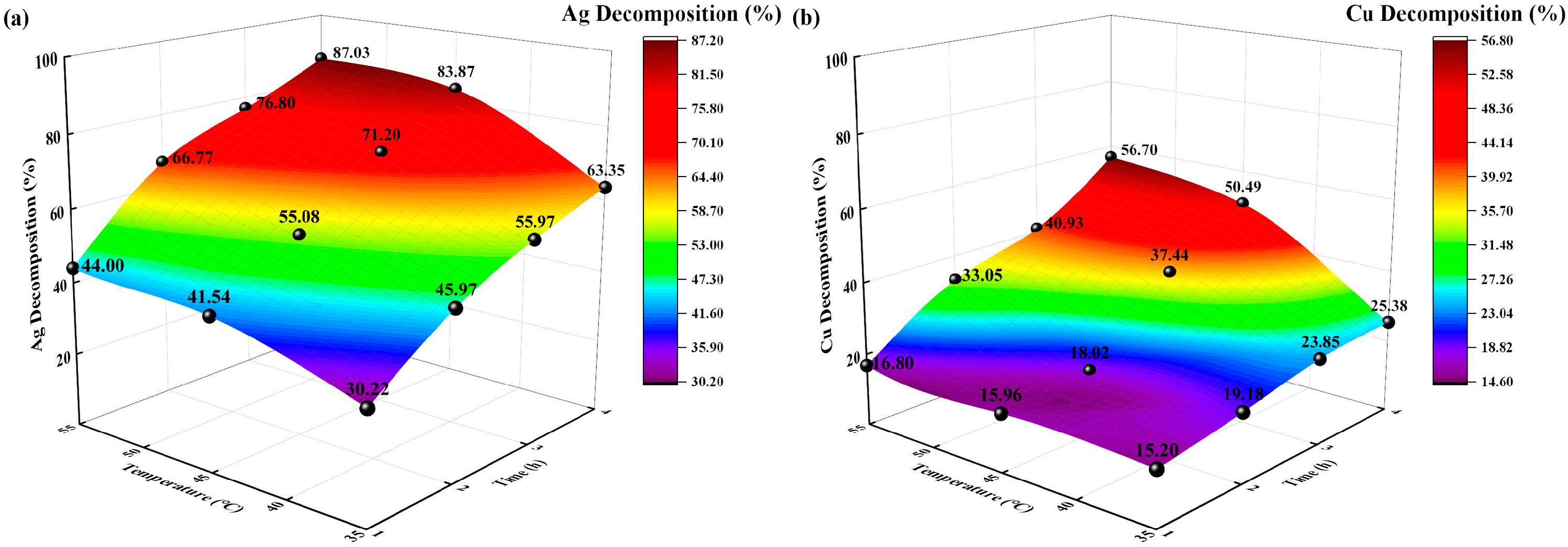
3.2.3. Effects of Different Temperatures
The reaction conditions were as follows: sodium thiosulfate concentration of 0.5 mol/L, solid-to-liquid ratio of 0.1:250 g/mL, extraction time of 8 h, and temperatures of 35, 45, and 55 °C. The changes in the Ag and Cu extraction rates (%) with time were investigated, and the experimental results are shown in Figure 8.
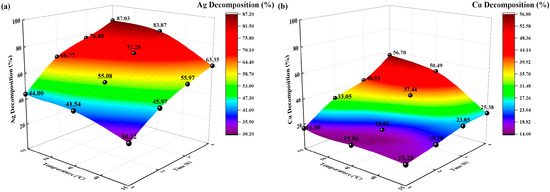
Figure 8.
Effects of temperature on decomposition rate (%). (a) Ag decomposition rate (%) vs. time, temperature; (b) Cu decomposition rate (%) vs. time, temperature.
The decomposition rates (%) of Ag and Cu increased with increasing temperature and time. At 35 °C, the Ag and Cu decomposition rates (%) were 63.35% and 25.38%, respectively, after 4 h of irradiation. At 55 °C, the Ag and Cu decomposition rates (%) reached their maximum after 4 h of irradiation, corresponding to 87.03% and 56.70%, respectively. Different temperatures corresponded to different concentrations of Ag-, Cu-, and Na-thiosulfate complexes in the initial decomposition solution. For Ag and Cu, the higher the temperature, the higher the decomposition rate.
3.3. Characterization of Photolytic Products
To confirm the composition of the photolytic products, XRD was used to investigate the phases of the products. The morphology of the products was analyzed by applying SEM-EDS and TEM, and the surface element composition and chemical valence states of the products were analyzed by applying XPS.
3.3.1. Phase of Photolytic Products
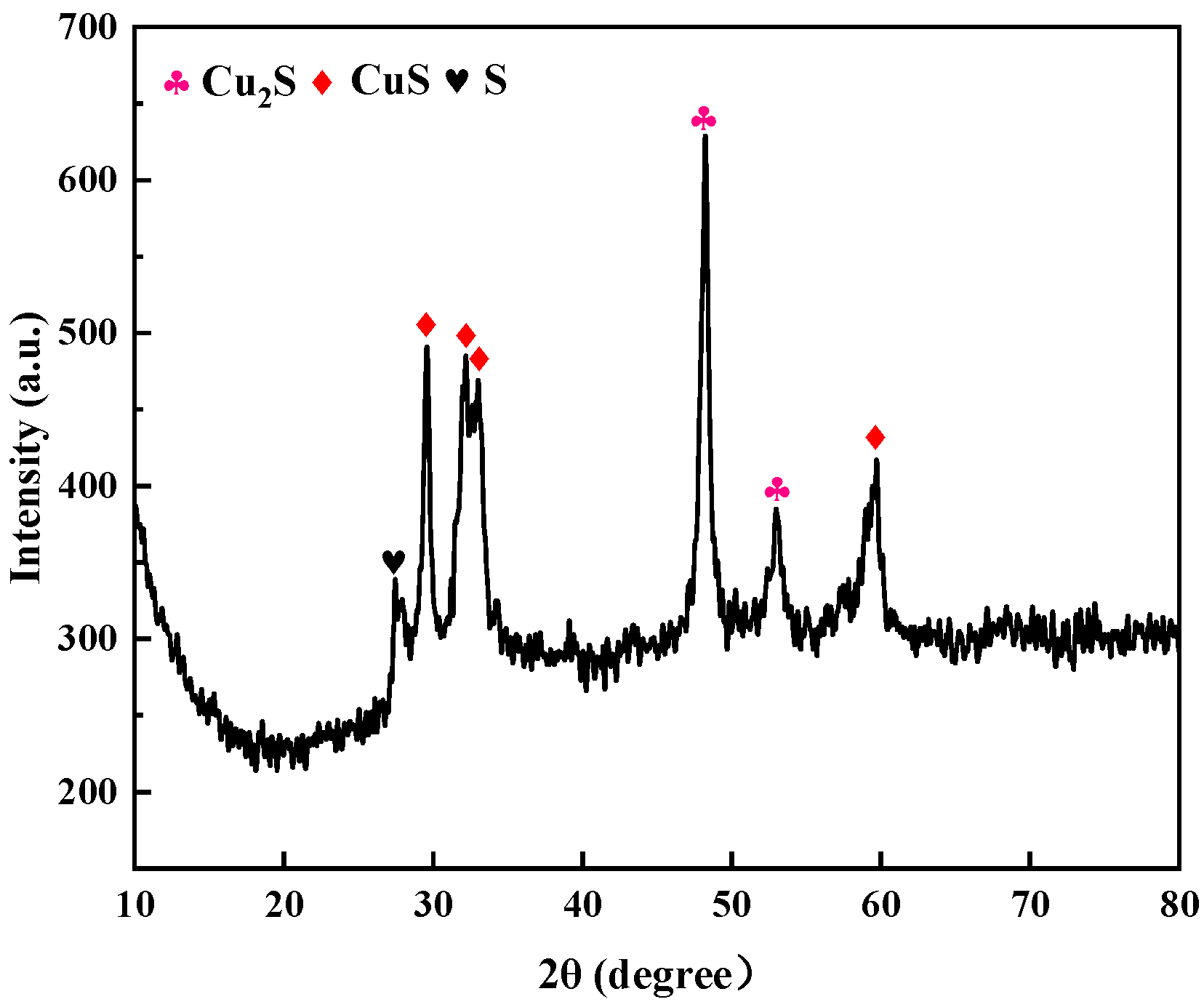
Figure 9 shows the XRD pattern of the photolytic products. In the detection range 2θ = 10–80°, the diffraction peaks located at 2θ = 48.164° and 53.557° corresponded to (103) and (112) crystal plane diffraction of the hexagonal-phase Cu2S (JCPDS card no. 99-0028) structure, respectively; the diffraction peaks located at 2θ = 29.288°, 31.793°, and 32.856° corresponded to (102), (103), and (006) crystal plane diffraction of the orthorhombic CuS (JCPDS card no. 99-0037) structure, respectively; and the diffraction peak at 2θ = 27.699° corresponded to (206) plane diffraction of the orthorhombic S (JCPDS card number: 99-0066) structure. Other miscellaneous peaks can be seen in the figure, indicating that the crystallinity of the product was poor; furthermore, there was no peak corresponding to Ag2S, indicating that there was less Ag2S in the product. SEM-EDS and TEM were used to determine the composition of the generated product.
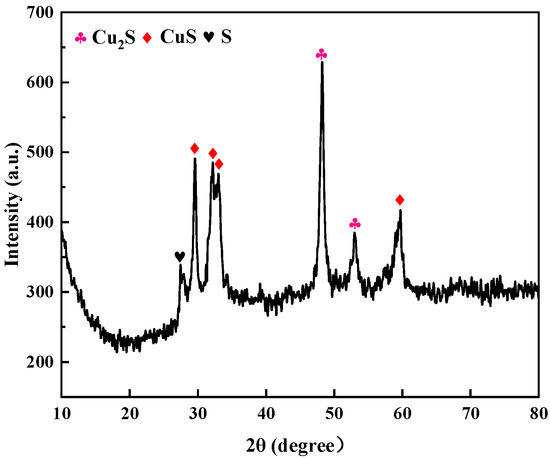
Figure 9.
XRD pattern of photolytic products ([S2O32− = 0.5 mol/L, 308 K, uncontrolled pH]).
3.3.2. Morphology of Photolytic Products
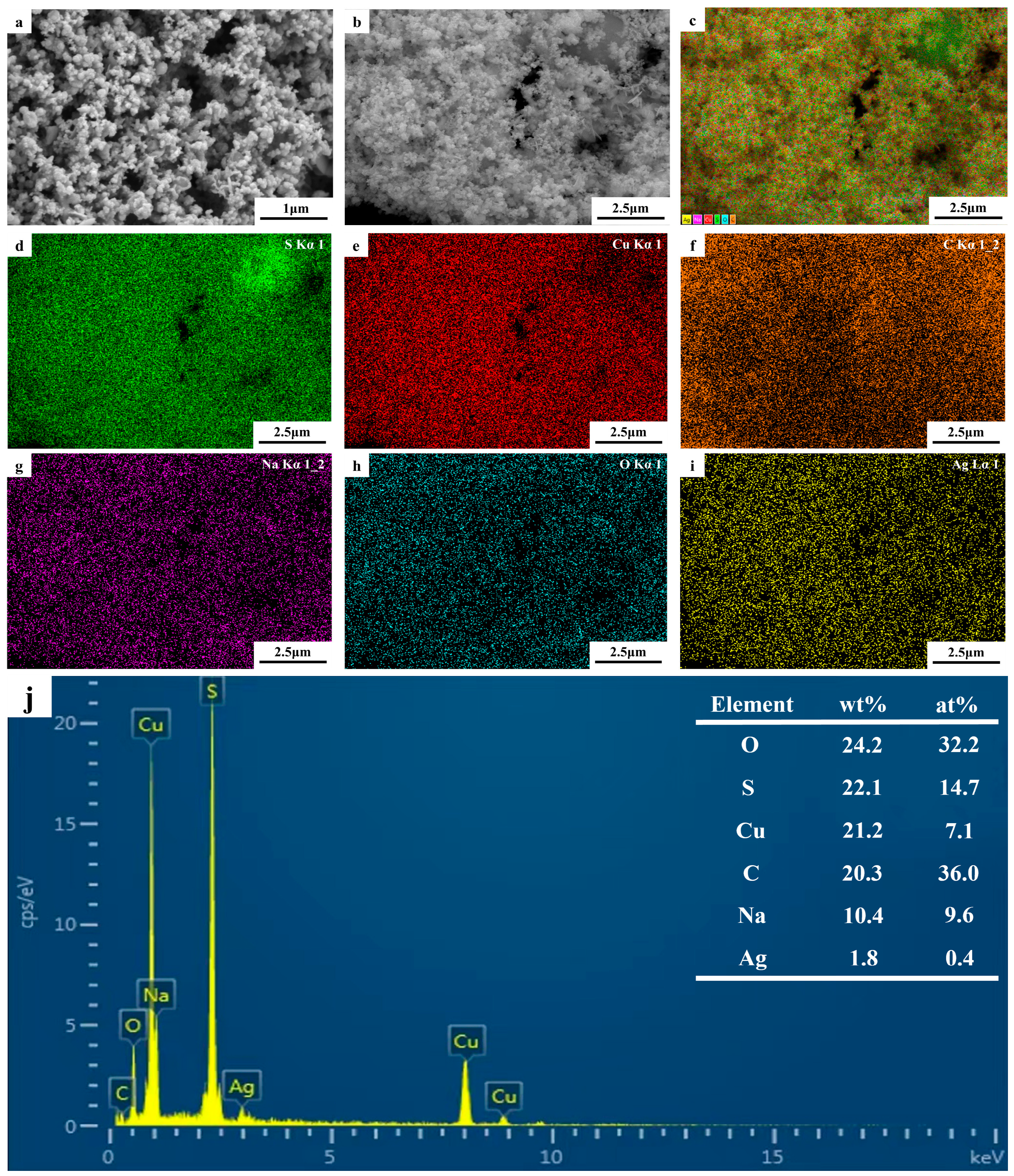
The SEM images of the photolytic products are shown in Figure 10a,b. The product size was not uniform, the powder was relatively agglomerated, and the particles were spherical. To identify the types and distributions of the elements, an EDS analysis was conducted; the results are shown in Figure 10c–i. Figure 10c shows the EDS diagram for the electronic image, and Figure 10e-i shows the EDS data for S, Cu, C, Na, O, and Ag in the electronic image. The atomic ratio of S to Cu in Figure 10j was approximately 2, indicating that the aqueous solution of the thiosulfate complex can be decomposed into cuprous sulfide by UV light. However, owing to the limitations of SEM, determining the morphology and particle size of the product was difficult; therefore, it was further analyzed using TEM.
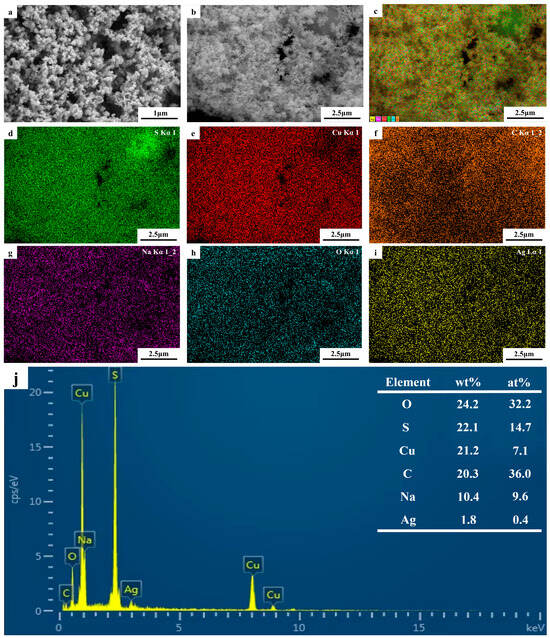
Figure 10.
(a,b) SEM images of photolytic products; (c–i) EDS elemental maps for (b); (j) corresponding EDS data for (b).
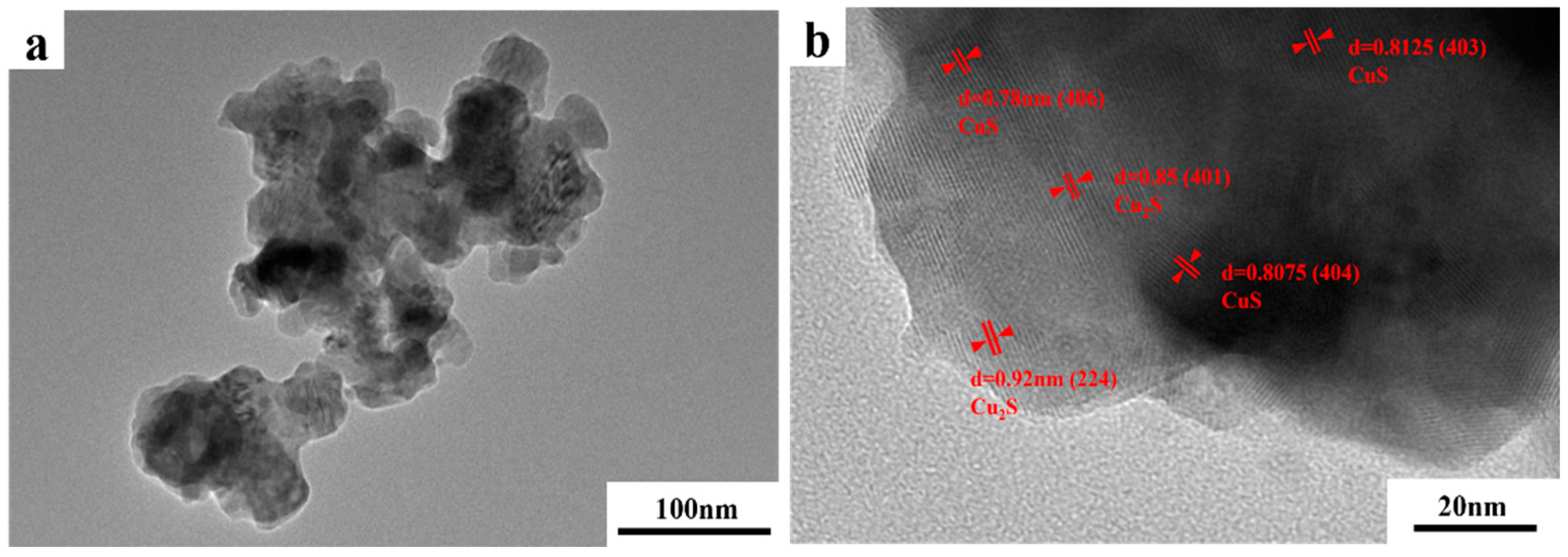
TEM images of the photodecomposition products are shown in Figure 11a. The products were spherical granular structures with different particle size distributions. The powders agglomerated and exhibited poor dispersion. Given the scale of the figure, most particles were within the 50–200 nm diameter range, which is consistent with the nanoscale. In Figure 11b, “d” represents the lattice spacing of decomposition products. The lattice spacings of 0.78, 0.8075, and 0.8125 nm corresponded to the crystal faces of the CuS cubic phases (406), (404), and (403), respectively. The lattice spacings of 0.86 nm and 0.92 nm corresponded to the (401) and (224) crystal faces of the Cu2S cubic phase, respectively. This indicates that the thiosulfate complexes in the extraction solution decomposed under UV irradiation and produced a precipitate containing CuS and Cu2S nanoparticles.
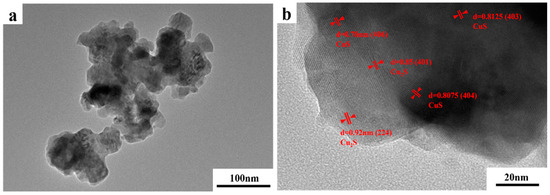
Figure 11.
(a) TEM and (b) high-resolution TEM images of photolytic products.
3.3.3. Chemical Composition of Photolytic Products
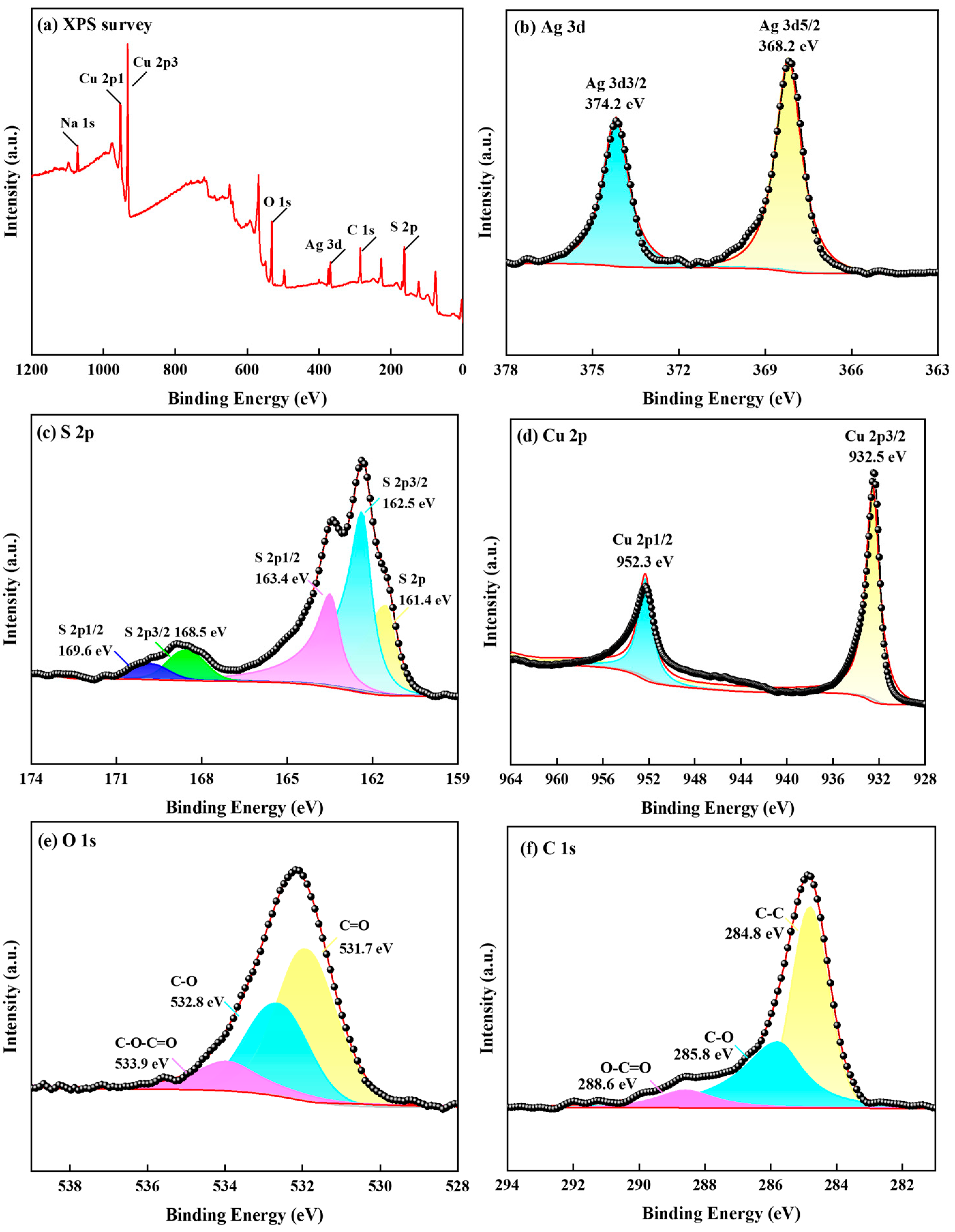
The S 2p, Ag 3d, and Cu 2p energy spectra of the photolytic products are shown in Figure 12.
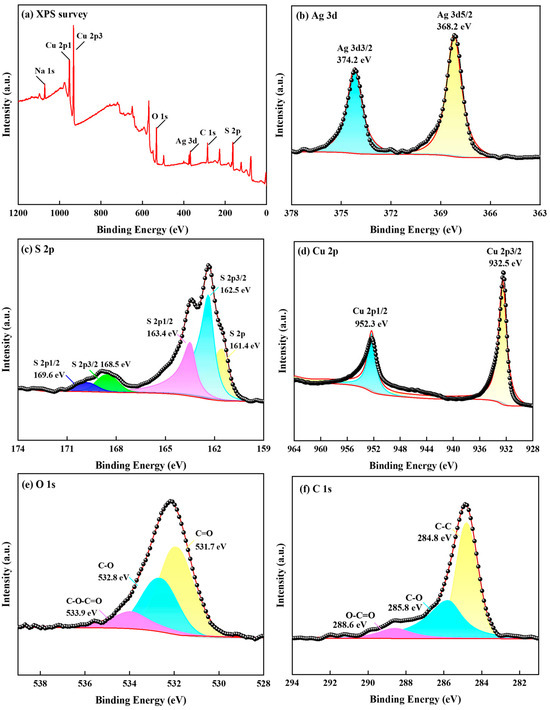
Figure 12.
Deconvoluted XPS spectra for the photoproduct. (a) Survey spectrum; (b) Ag 3d spectrum; (c) S 2p spectrum; (d) Cu 2p spectrum; (e) O 1s spectrum; (f) C 1s spectrum ([S2O32− = 0.5 mol/L, 308 K, uncontrolled pH]).
The XPS spectra in Figure 12a indicate the coexistence of Ag, S, Cu, O, and Na. In the high-resolution XPS spectrum of Ag 3d (Figure 12b, contaminated carbon internal standard C 1s: 284.8 eV), the two broad independent peaks at 368.2 and 374.2 eV were attributed to Ag2S and Ag 3d3/2, which are associated with silver sulfide groups [37]. This indicates that Ag components existed as Ag2S and Ag on the surface of the photoproducts. The spectrum shown in Figure 12c reveals that S 2p had two peaks in the detection range. The S 2p peaks were characterized by S 2p3/2 and S 2p1/2 double peaks. The binding energy values shown in the figure are generally consistent with those reported in the literature for Ag 3d and S 2p as silver sulfide [38]. Furthermore, the binding energy of 162.5 eV may be attributable to Cu2S 2p3/2 and CuS 2p3/2, whereas, at 163.4 eV, it may be attributable to Ag2S 2p1/2 and S0. The junction energies of 168.5 and 169.6 eV may be attributable to Na2S2O3 2p3/2 and Na2S2O3 2p1/2. Additionally, the Cu 2p peak was observed in the photolytic products (Figure 12d). The binding energy of Cu 2p3/2 was found to be 932.5 eV, which is consistent with the Cu ion in the literature and may be attributable to Cu2S; moreover, the binding energy of Cu 2p1/2 was found to be 952.3 eV, possibly attributable to CuS and Cu2S [39]. Figure 12e shows the spectrum of O 1s with binding energies of 531.7, 532.8, and 533.9 eV, which are peaks associated with C=O, C-O, and C-O-C=O groups, respectively [40]. Figure 12f shows the spectrum of C 1s, in which the peaks at binding energies of 284.8, 285.8, and 288.6 eV correspond to C-C, C-O, and O-C=O groups, respectively [41].
4. Conclusions
In this study, we explored a novel method to recover valuable metals from waste conductive silver pastes, i.e., thiosulfate extraction combined with UV photolysis technology, to further optimize the extraction process. In addition, through comparative analysis across various parameters, we observed that extraction and decomposition rates increase with higher concentrations of sodium thiosulfate, greater solid-liquid ratios, and elevated temperatures, and at a sodium thiosulfate concentration of 0.5 mol/L, temperature of 55 °C, and solid-to-liquid ratio of 0.1:250 g/mL, the Ag and Cu extraction rates (%) reached 100% and 86.61%, respectively. Additionally, under UV light, the Ag and Cu decomposition rates (%) reached 87.03% and 56.70%, respectively. The photolytic products were characterized using various methods. The photodecomposition products were characterized using XRD, SEM-EDS, TEM, and XPS, revealing the presence of CuS, Cu2S, Ag2S, and S. The powders existed as spherical particles with diameters in the nanometer range but tended to agglomerate easily. These results demonstrate that the use of thiosulfate in combination with UV photolysis technology can feasibly improve the metal recovery process. The use of this method can broaden the development prospects for industrial thiosulfate systems, solve the problem of environmental governance, and provide economic and environmental benefits.
Author Contributions
Conceptualization, C.H.; methodology, C.H. and Q.T.; resources, Q.T.; writing—original draft preparation, Q.T.; writing—review and editing, C.H., Q.J. and G.W.; supervision, Q.J. and G.W. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the National Natural Science Foundation of China (grant number 52104349), Henan Provincial Science and Technology R & D Plan Joint Fund Project (grant number 232103810032), and the Funds for HAUST Young Cadre Teacher (grant number 400213450022).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We thank Jiaqi Shi and Longwei Zhao for their help with the experiment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lingling, Z.; Yangyang, Y.; Ning, W.; Mingli, L.; Shaomin, S.; Wei, B.; Choi, M.M.F. Sulfur-doped graphitic carbon nitride nanosheets as a sensitive fluorescent probe for detecting environmental and intracellular Ag. Methods Appl. Fluoresc. 2022, 10, 045001. [Google Scholar]
- Wan, A.B.; Kukjoo, K.; Mijung, K.; So-Yun, K.; Seung-Hyun, H.; Jang-Ung, P. Direct printing of reduced graphene oxide on planar or highly curved surfaces with high resolutions using electrohydrodynamics. Small 2015, 11, 2263–2268. [Google Scholar]
- Michael, G.; Alexander, K.; Florentina, M.C.; Florin, A.D.; Shlomo, M. Conductive inks with a “built-in” mechanism that enables sintering at room temperature. ACS Nano 2011, 5, 3354–3359. [Google Scholar]
- Huang, G.-L.; Chiu, P.-H.; Fujino, M.; Song, J.-M. Interconnect Fabrication on Polymer Substrate using Submicron/Nano Silver Particles with the Assistance of Low-Density Irradiations. JOM 2019, 71, 3057–3065. [Google Scholar] [CrossRef]
- Xiu, F.-R.; Qi, Y.; Zhang, F.-S. Leaching of Au, Ag, and Pd from waste printed circuit boards of mobile phone by iodide lixiviant after supercritical water pre-treatment. Waste Manag. 2015, 41, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xiao, Y.; Sietsma, J.; Agterhuis, H.; Yang, Y. A Cleaner Process for Selective Recovery of Valuable Metals from Electronic Waste of Complex Mixtures of End-of-Life Electronic Products. Environ. Sci. Technol. 2015, 49, 7981–7988. [Google Scholar] [CrossRef] [PubMed]
- Click, N.; Teknetzi, I.; Tam, E.P.L.; Tao, M.; Ebin, B. Innovative recycling of high purity silver from silicon solar cells by acid leaching and ultrasonication. Sol. Energy Mater. Sol. Cells 2024, 270, 112834. [Google Scholar] [CrossRef]
- Abdel-Aal, E.A.; Farghaly, F.E. Preparation of silver powders in micron size from used photographic films via leaching-cementation technique. Powder Technol. 2007, 178, 51–55. [Google Scholar] [CrossRef]
- Petter, P.M.H.; Veit, H.M.; Bernardes, A.M. Evaluation of gold and silver leaching from printed circuit board of cellphones. Waste Manag. 2014, 34, 475–482. [Google Scholar] [CrossRef]
- Joda, N.N.; Rashchi, F. Recovery of ultra fine grained silver and copper from PC board scraps. Sep. Purif. Technol. 2012, 92, 36–42. [Google Scholar] [CrossRef]
- Jadhao, P.R.; Ahmad, E.; Pant, K.K.; Nigam, K.D.P. Advancements in the field of electronic waste Recycling: Critical assessment of chemical route for generation of energy and valuable products coupled with metal recovery. Sep. Purif. Technol. 2022, 289, 120773. [Google Scholar] [CrossRef]
- Blumbergs, E.; Shishkin, A.; Markus, K.; Serga, V.; Goljandin, D.; Klauson, A.; Abramovskis, V.; Baronins, J.; Zarkov, A.; Pankratov, V. Economic Aspects of Mechanical Pre-Treatment’s Role in Precious Metals Recovery from Electronic Waste. Metals 2024, 14, 95. [Google Scholar] [CrossRef]
- Schwartz, E.; He, H.; Frost, K.; Nguyen, B.H.; Ogunseitan, O.A.; Schoenung, J.M. Comparative life cycle assessment of copper and gold recovery from waste printed circuit boards: Pyrometallurgy, chemical leaching and bioleaching. J. Hazard. Mater. 2024, 473, 134545. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Oberholster, P.J.; Erasmus, M. Bioleaching of Metals from E-Waste Using Microorganisms: A Review. Minerals 2023, 13, 828. [Google Scholar] [CrossRef]
- Deblina, D.; Rahul, R.; Srinivas, G.L.K.; Debajyoti, K.; Pooja, S.; Mamta, T.; Sunil, K. A review on recovery processes of metals from E-waste: A green perspective. Sci. Total Environ. 2022, 859, 160391. [Google Scholar]
- Alvarado-Macías, G.; Fuentes-Aceituno, J.C.; Nava-Alonso, F. Silver leaching with the thiosulfate-nitrite-sulfite-copper alternative system. Hydrometallurgy 2015, 152, 120–128. [Google Scholar] [CrossRef]
- Ficeriová, J.; Baláž, P.; Gock, E. Leaching of gold, silver and accompanying metals from circuit boards (PCBs) waste. Acta Montan. Slovaca 2011, 16, 128–131. [Google Scholar]
- Cui, Y.; Tong, X.; Lopez-Valdivieso, A. Silver sulfide leaching with a copper-thiosulfate solution in the absence of ammonia. Rare Met. 2011, 30, 105–109. [Google Scholar] [CrossRef]
- Kumari, S.; Panda, R.; Prasad, R.; Alorro, R.D.; Jha, M.K. Sustainable Process to Recover Metals from Waste PCBs Using Physical Pre-Treatment and Hydrometallurgical Techniques. Sustainability 2024, 16, 418. [Google Scholar] [CrossRef]
- Graham, J.S.; Woodcock, J.T. Cyanide and other lixiviant leaching systems for gold with some practical applications. Miner. Process. Extr. Metall. Rev. 1995, 14, 193–247. [Google Scholar]
- Zhang, G.; Hou, L.; Chen, P.; Zhang, Q.; Chen, Y.; Zainiddinovich, N.Z.; Wu, C.; Alejandro, L.V.; Jia, F. Efficient and stable leaching of gold in a novel ethydiaminedhephen acetic-thiosulfate system. Miner. Eng. 2024, 209, 108639. [Google Scholar] [CrossRef]
- Nan, C.J.; Feng, X.; Wei, W.; Yan, F.; Jian, W.; Bin, X. Leaching of silver sulfide with copper sulfate-tartrate-thiosulfate solutions. J. Cent. South Univ. 2023, 30, 677–690. [Google Scholar]
- Ke, L.; Qian, L.; Yan, Z.; Yongbin, Y.; Tao, J. Effect of metal ions on gold adsorption onto activated carbon in the acidic thiourea solution: Experiment study and DFT calculation. J. Mol. Liq. 2023, 387, 122691. [Google Scholar]
- Tanısalı, E.; Özer, M.; Burat, F. Precious Metals Recovery from Waste Printed Circuit Boards by Gravity Separation and Leaching. Miner. Process. Extr. Metall. Rev. 2020, 42, 24–37. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Z.M.; Song, Q.M. Selective Extraction of Silver and Palladium in Leachate Based on EDTA Complexation: Electrodeposition, Nucleation Mechanism, and Kinetic Analysis. Acs Sustain. Chem. Eng. 2022, 10, 16647–16656. [Google Scholar] [CrossRef]
- Lampinen, M.; Laari, A.; Turunen, I. Ammoniacal thiosulfate leaching of pressure oxidized sulfide gold concentrate with low reagent consumption. Hydrometallurgy 2015, 151, 1–9. [Google Scholar] [CrossRef]
- Bozejewicz, D.; Kaczorowska, M.A.; Witt, K. Recent advances in the recovery of precious metals (Au, Ag, Pd) from acidic and WEEE solutions by solvent extraction and polymer inclusion membrane processes-a mini-review. Desalination Water Treat. 2022, 246, 12–24. [Google Scholar] [CrossRef]
- Seyed, H.B.; Shahram, R.; Ahmad, A.A. Study of silver extraction from Ag2S containing concentrate in the presence of copper sulfate, sodium thiosulfate, sodium metabisulfite, and ascorbic acid. Miner. Eng. 2022, 183, 107607. [Google Scholar]
- Khaobang, C.; Kathongthung, N.; Phitsuwan, P.; Sitthichirachat, P.; Wibowo, H.; Areeprasert, C. In-situ adsorptive pyrolysis of e-waste using coal and rice husk fly ash as alternative adsorbents for energy and precious metal recovery by solvent extraction. J. Anal. Appl. Pyrolysis 2024, 179, 106465. [Google Scholar] [CrossRef]
- Han, C.; Wang, G.; Zou, M.; Shi, C. Separation of Ag and Cu from Their Aqueous Thiosulfate Complexes by UV-C Irradiation. Metals 2019, 9, 1178. [Google Scholar] [CrossRef]
- Wang, W.; Han, C.; Xie, F. Efficient mercury recovery from mercuric-thiosulfate solutions by ultraviolet photolysis. Environ. Chem. Lett. 2018, 16, 1049–1054. [Google Scholar] [CrossRef]
- Cai, X. Mechanistic Study of Zinc Replacement of Silver in Ammonia-Free Thiosulfate Precious Liquid. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2021. [Google Scholar]
- Zhu, Y. The Study on Wet-Efficient Extracting Silver from Components of Waste Mobile Phones. Master’s Thesis, South China University of Technology, Guangzhou, China, 2017. [Google Scholar]
- Han, C.; Wang, G.; Cheng, C.; Shi, C.; Yang, Y.; Zou, M. A kinetic and mechanism study of silver-thiosulfate complex photolysis by UV-C irradiation. Hydrometallurgy 2020, 191, 105212. [Google Scholar] [CrossRef]
- Egorov, N.B. Investigation of Lead Thiosulfate Photolysis in Aqueous Solutions. High Energy Chem. 2014, 48, 37–41. [Google Scholar] [CrossRef]
- Egorov, N.B. A study of photolysis products of aqueous Na6[Pb(S2O3)4] solutions. High Energy Chem. 2016, 50, 311–314. [Google Scholar] [CrossRef]
- Alexander, R.; Maxim, L.; Yuri, M. X-ray Photoelectron Spectroscopy (XPS) Study of the Products Formed on Sulfide Minerals Upon the Interaction with Aqueous Platinum (IV) Chloride Complexes. Minerals 2018, 8, 578. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. Identifying chemisorption in the interaction of thiol collectors with sulfide minerals by XPS: Adsorption of xanthate on silver and silver sulfide. Colloids Surf. A Physicochem. Eng. Asp. 1995, 104, 295–305. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, B.; Zheng, Y.; Li, Q.; Yang, Y.; Liu, X.; Jiang, T.; Lyu, X. Hexaamminecobalt(III) catalyzed thiosulfate leaching of gold from a concentrate calcine and gold recovery from its pregnant leach solution via resin adsorption. Miner. Eng. 2021, 171, 107079. [Google Scholar] [CrossRef]
- Terzyk, A.P. The influence of activated carbon surface chemical composition on the adsorption of acetaminophen (paracetamol) in vitro: The temperature dependence of adsorption at the neutral pH. Colloids Surf. A Physicochem. Eng. Asp. 2001, 177, 23–45. [Google Scholar] [CrossRef]
- Terzyk, A.P.; Rychlicki, G. The influence of activated carbon surface chemical composition on the adsorption of acetaminophen (paracetamol) in vitro: Part II. TG, FTIR, and XPS analysis of carbons and the temperature dependence of adsorption kinetics at the neutral pH. Colloids Surf. A Physicochem. Eng. Asp. 2000, 163, 135–150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).