Preparation and Characterization of Duplex PEO/UV-Curable Powder Coating on AZ91 Magnesium Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Material
2.2. Synthesis of Conversion Coatings
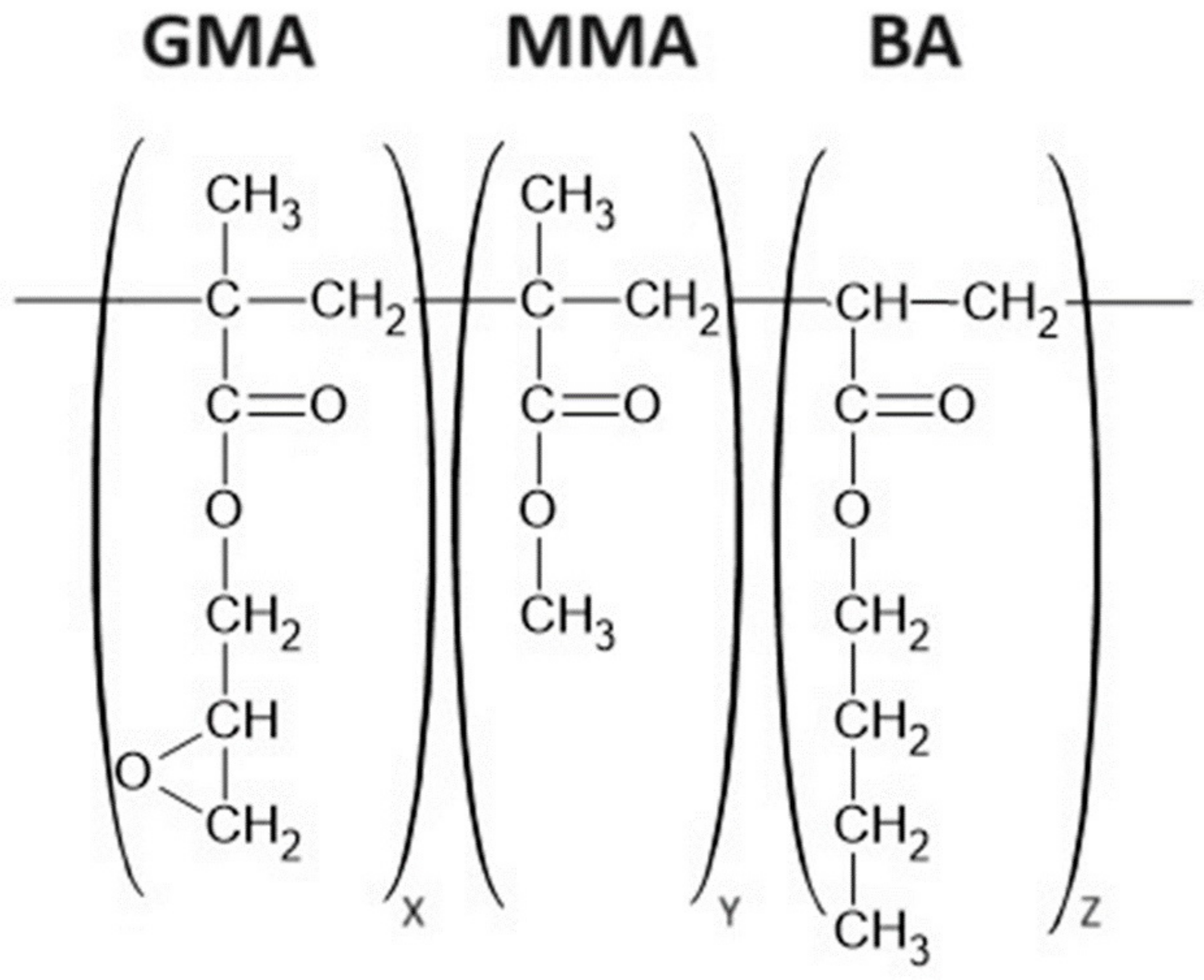
2.3. Synthesis of Powder Composition
2.4. Preparation of UV-Cured Powder Coating
2.5. Coatings Characterization
3. Results and Discussion
3.1. Morphologies of Coatings
3.2. Thickness and Roughness of Coatings
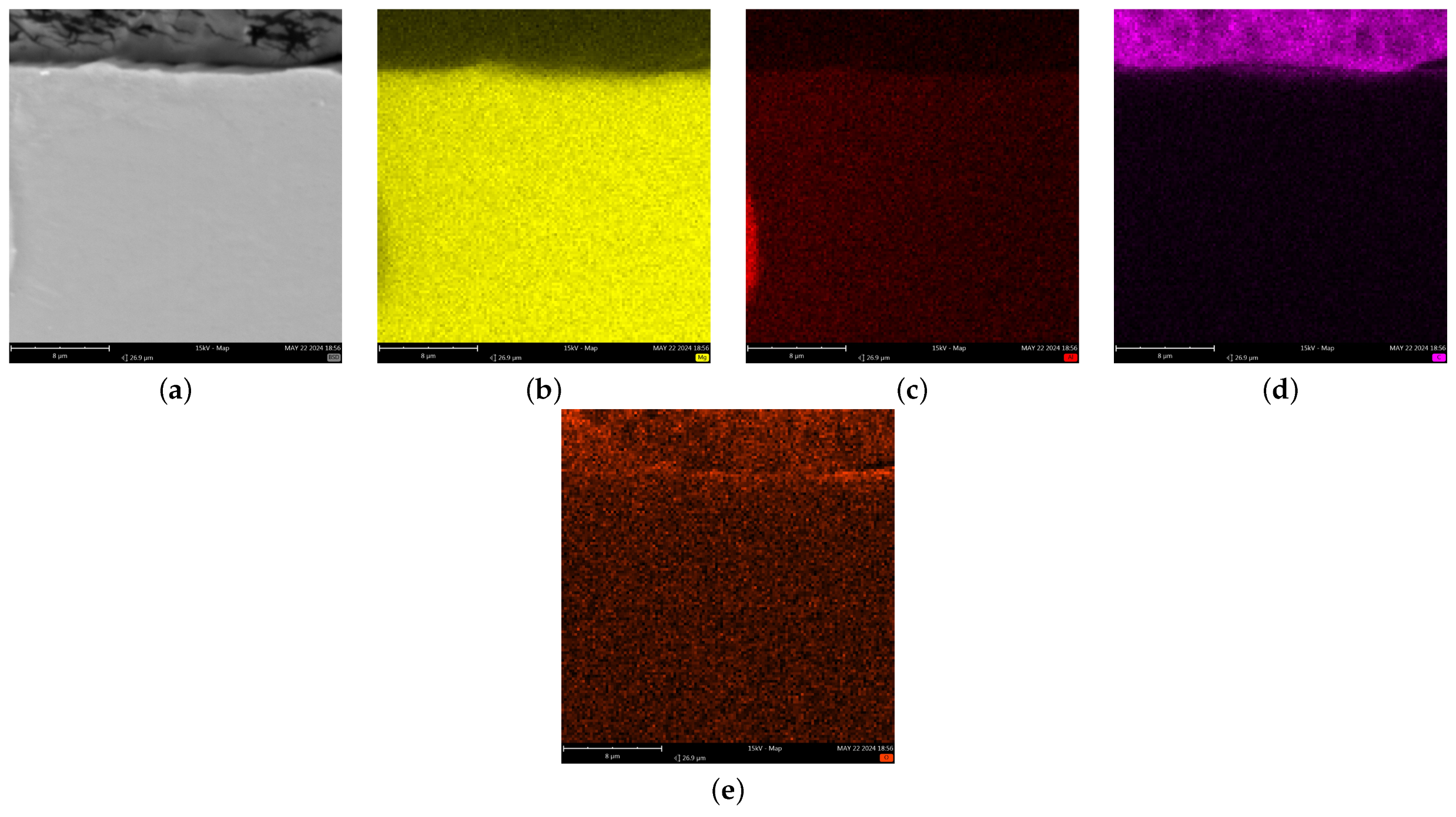
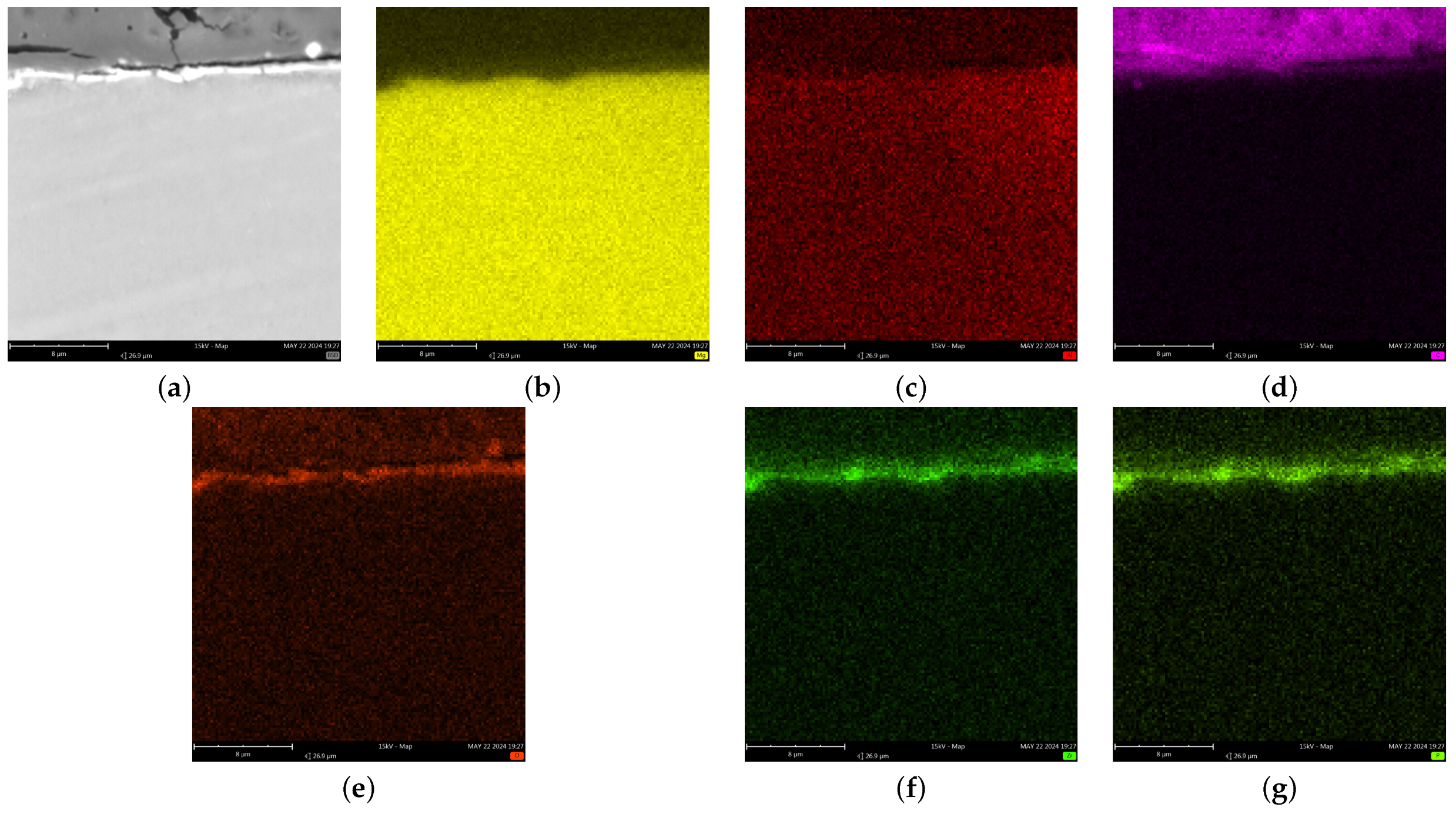
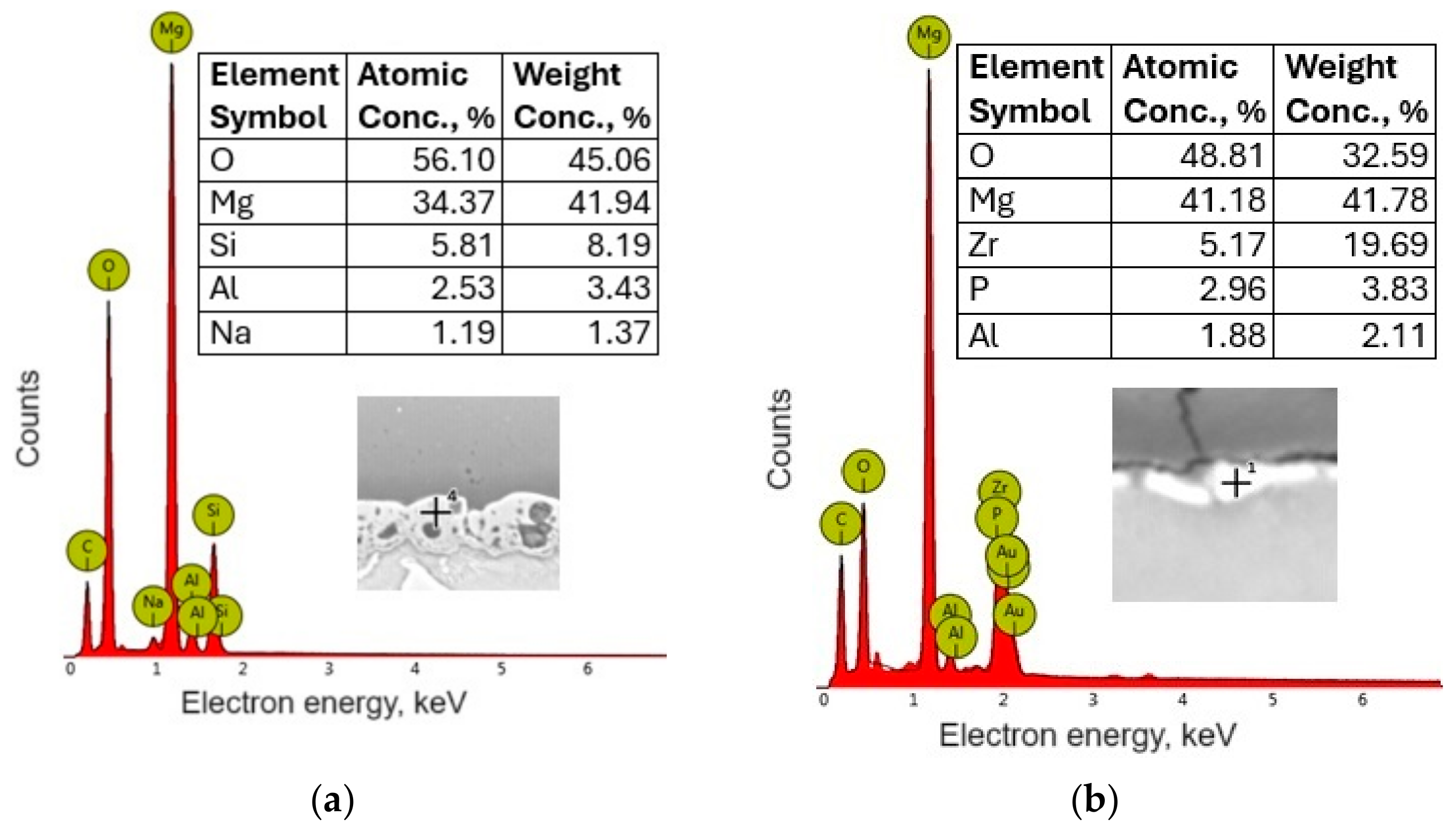
3.3. Chemical Compound of Coatings
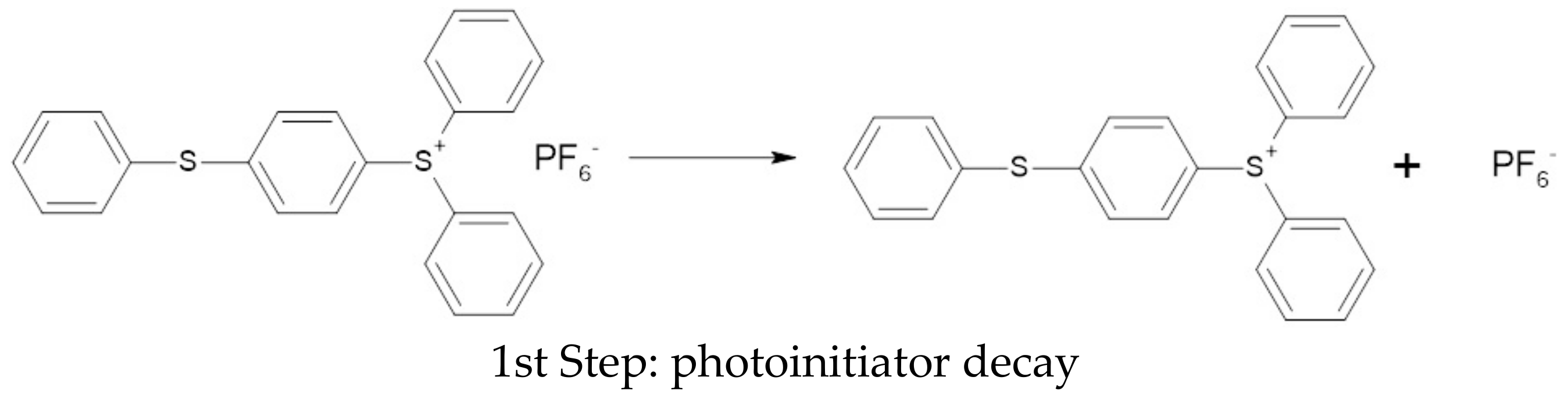
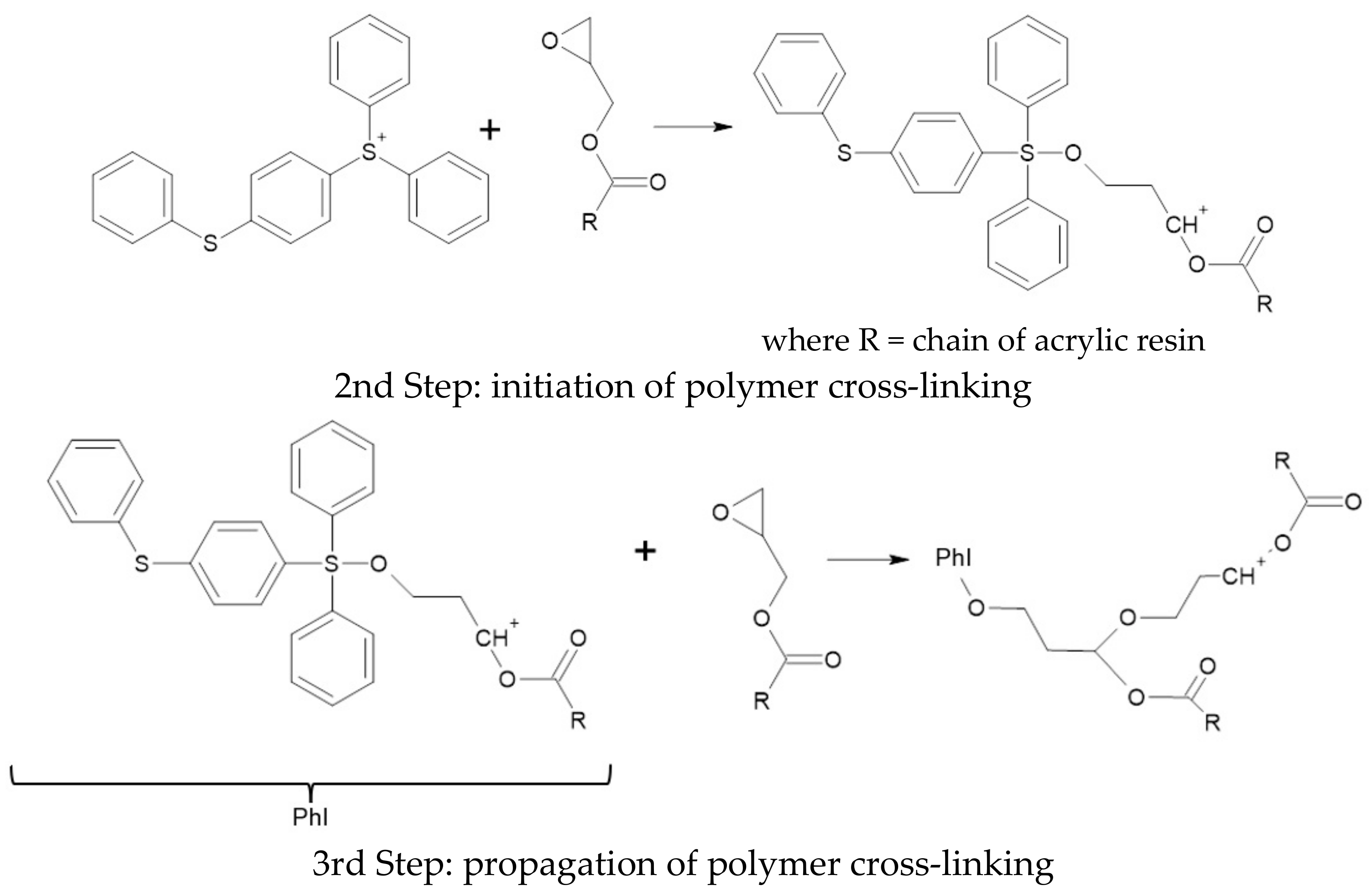
3.4. Polymerization Test of Organic Coating
3.5. Adhesive of Organic Coating
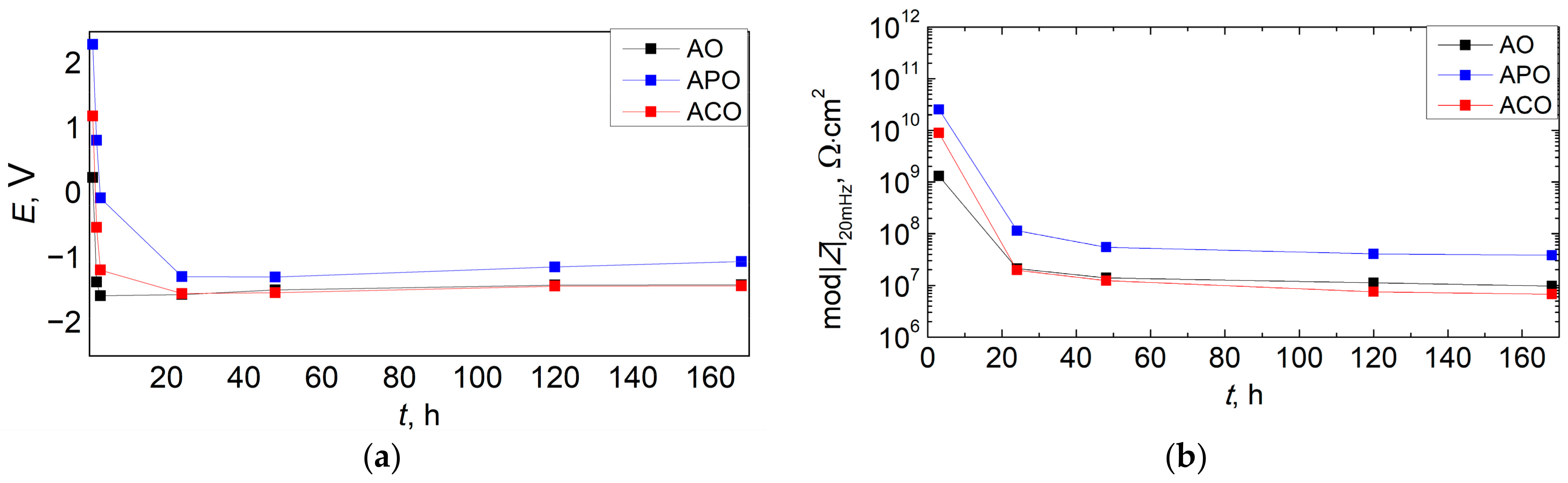
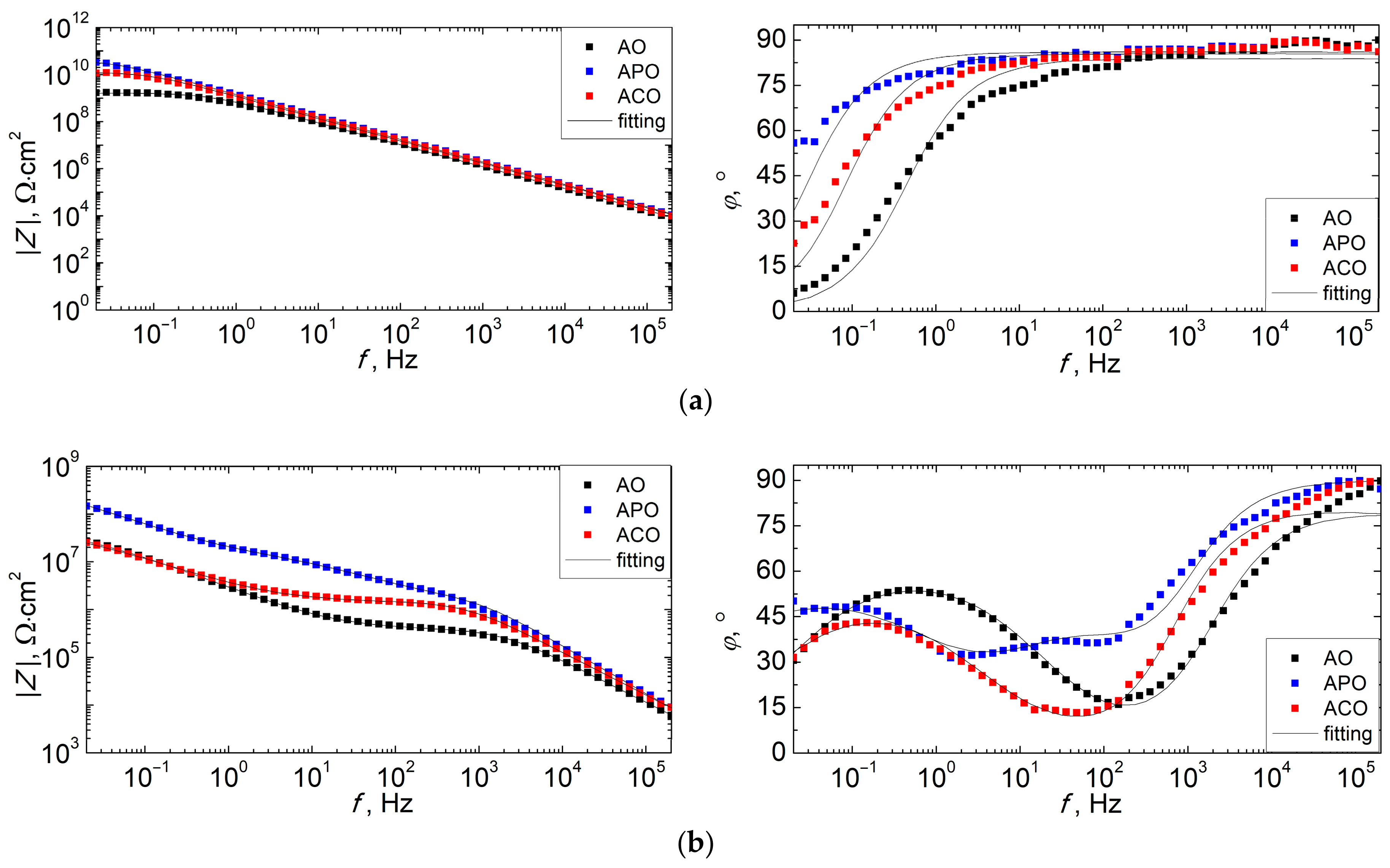
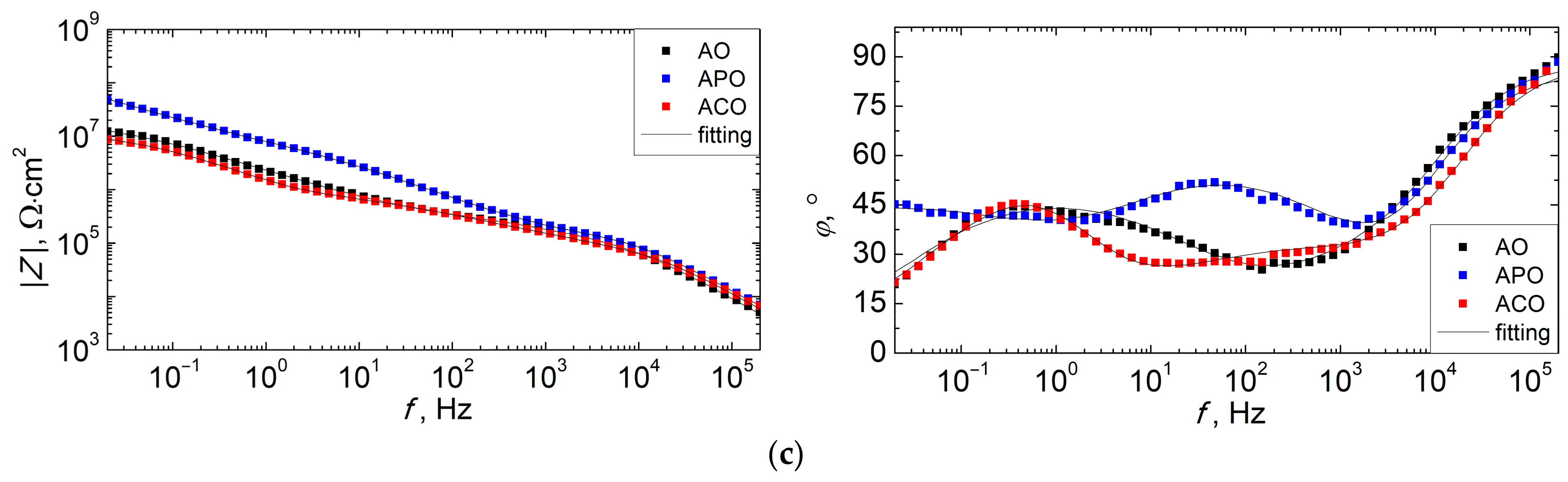
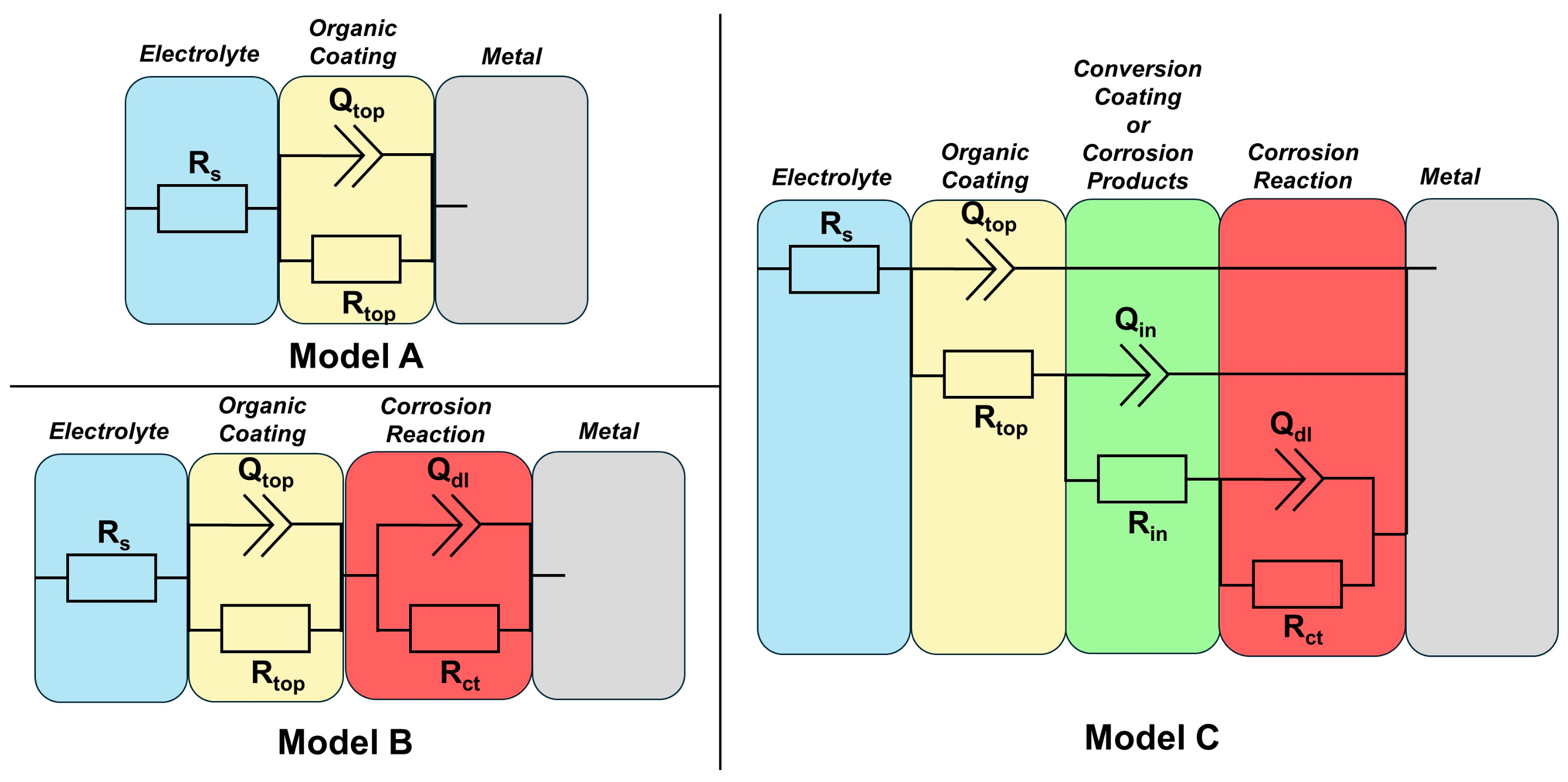
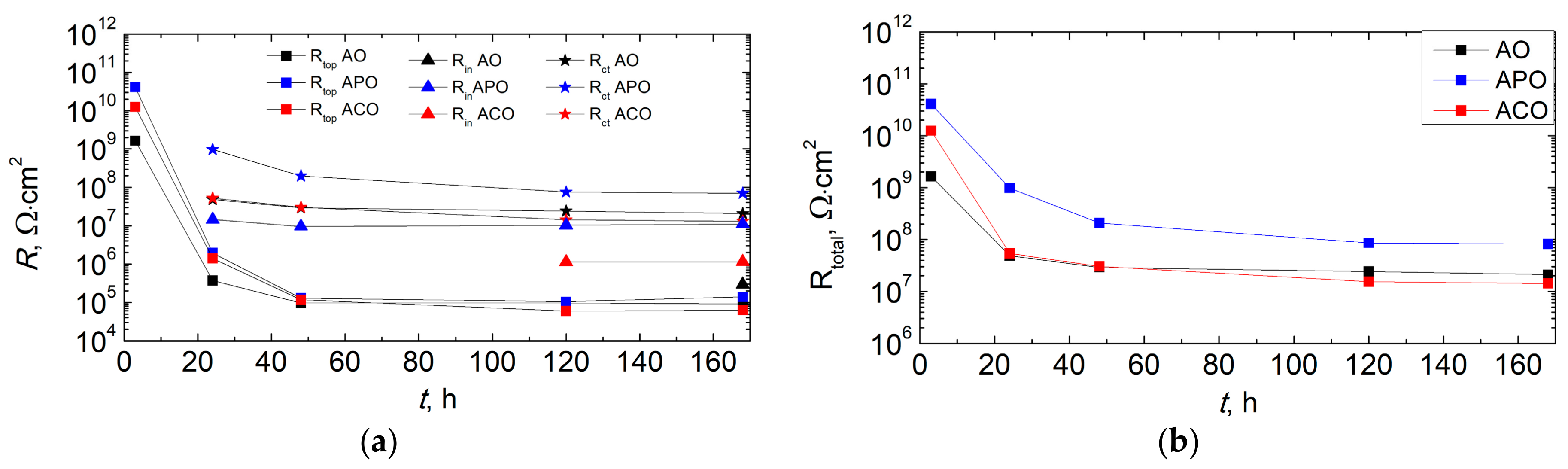
3.6. Corrosion Resistance of Coatings
4. Conclusions
- Regardless of the presence or absence of a conversion coating on the magnesium alloy, the powder coating was successfully cured by UV radiation. The organic coating adhered well to all surfaces, its thickness was in the range of 120–180 μm, and its roughnesses were similar in all cases.
- The synthesis of conversion coatings was successful. The coating produced in the PEO process had a thickness of approximately 12–13 μm, was continuous, and had a characteristic crater-like surface morphology. It was composed mainly of Mg, O, and Si. The conversion coating produced from the zircon phosphate bath had a thickness of 0.2–0.3 μm and cracked in places. It was composed of Mg, O, P, and Zr.
- The formation of a conversion coating in the PEO process significantly increased the adhesion of the powder coating to the magnesium substrate. The use of chemical conversion had a negligible effect.
- The duplex PEO/UV-curable powder coating showed significantly improved anticorrosion properties compared to other systems (chemical conversion + powder coating and only powder coating). Chemical conversion from the zircon phosphate bath initially increased the impedance of the sample (compared to the coating produced directly on the magnesium alloy), but in the long term (168 h), it did not have a positive effect on the anticorrosion properties.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys worldwide in 2022. J. Magnes. Alloys 2023, 11, 2611–2654. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Zhang, X.; Yang, Q.; Zhang, J.; Li, X. Development and application of magnesium alloy parts for automotive OEMs: A review. J. Magnes. Alloys 2023, 11, 15–47. [Google Scholar] [CrossRef]
- Bai, J.; Yang, Y.; Wen, C.; Chen, J.; Zhou, G.; Jiang, B.; Peng, X.; Pan, F. Applications of magnesium alloys for aerospace: A review. J. Magnes. Alloys 2023, 11, 3609–3619. [Google Scholar] [CrossRef]
- Zan, R.; Shen, S.; Huang, Y.; Yu, H.; Liu, Y.; Yang, S.; Zheng, B.; Gong, Z.; Wang, W.; Zhang, X.; et al. Research hotspots and trends of biodegradable magnesium and its alloys. Smart Mater. Med. 2023, 4, 468–479. [Google Scholar] [CrossRef]
- Sharma, S.K.; Saxena, K.K.; Malik, V.; Mohammed, K.A.; Prakash, C.; Buddhi, D.; Dixit, S. Significance of Alloying Elements on the Mechanical Characteristics of Mg-Based Materials for Biomedical Applications. Crystals 2022, 12, 1138. [Google Scholar] [CrossRef]
- Rong-Chang, Z.; Zheng-Zheng, Y.; Xiao-Bo, C.; Dao-Kui, X. Corrosion Types of Magnesium Alloys. In Magnesium Alloys; Tomasz, T., Wojciech, B., Mariusz, K., Eds.; IntechOpen: Rijeka, Croatia, 2018; p. Ch. 3. [Google Scholar]
- Xu, L.; Liu, X.; Sun, K.; Fu, R.; Wang, G. Corrosion Behavior in Magnesium-Based Alloys for Biomedical Applications. Materials 2022, 15, 2613. [Google Scholar] [CrossRef] [PubMed]
- Song, G.-L.; Atrens, A. Recently deepened insights regarding Mg corrosion and advanced engineering applications of Mg alloys. J. Magnes. Alloys 2023, 11, 3948–3991. [Google Scholar] [CrossRef]
- Liu, H.; Cao, F.; Song, G.-L.; Zheng, D.; Shi, Z.; Dargusch, M.S.; Atrens, A. Review of the atmospheric corrosion of magnesium alloys. J. Mater. Sci. Technol. 2019, 35, 2003–2016. [Google Scholar] [CrossRef]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Predko, P.; Rajnovic, D.; Grilli, M.L.; Postolnyi, B.O.; Zemcenkovs, V.; Rijkuris, G.; Pole, E.; Lisnanskis, M. Promising Methods for Corrosion Protection of Magnesium Alloys in the Case of Mg-Al, Mg-Mn-Ce and Mg-Zn-Zr: A Recent Progress Review. Metals 2021, 11, 1133. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, K. Corrosion and Protection of Magnesium Alloys: Recent Advances and Future Perspectives. Coatings 2023, 13, 1533. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloys 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Florczak, Ł.; Nawrat, G.; Kwolek, P.; Sieniawski, J.; Sobkowiak, A. Plasma electrolytic oxidation as a method for protection against corrosion of magnesium and its alloys. Przemysł Chem. 2018, 97, 2145–2153. (In Polish) [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Yao, W.; Wu, L.; Wang, J.; Jiang, B.; Zhang, D.; Serdechnova, M.; Shulha, T.; Blawert, C.; Zheludkevich, M.L.; Pan, F. Micro-arc oxidation of magnesium alloys: A review. J. Mater. Sci. Technol. 2022, 118, 158–180. [Google Scholar] [CrossRef]
- Yang, C.; Chen, P.; Wu, W.; Sheng, L.; Zheng, Y.; Chu, P.K. A Review of Corrosion-Resistant PEO Coating on Mg Alloy. Coatings 2024, 14, 451. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Vaghefinazari, B.; Mohedano, M.; Visser, P.; Posner, R.; Blawert, C.; Zheludkevich, M.; Lamaka, S.; Matykina, E.; Arrabal, R. Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: Part II—PEO and Anodizing. Materials 2022, 15, 8515. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, amending DIRECTIVE 1999/45/EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Available online: https://eur-lex.europa.eu/eli/reg/2006/1907/oj (accessed on 16 June 2024).
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma Electrolytic Oxidation (PEO) Process—Processing, Properties, and Applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Alateyah, A.I.; Aljohani, T.A.; Alawad, M.O.; Elkatatny, S.; El-Garaihy, W.H. Improving the Corrosion Behavior of Biodegradable AM60 Alloy through Plasma Electrolytic Oxidation. Metals 2021, 11, 953. [Google Scholar] [CrossRef]
- Florczak, Ł.; Nawrat, G.; Darowicki, K.; Ryl, J.; Sieniawski, J.; Wierzbińska, M.; Raga, K.; Sobkowiak, A. The Effect of Sodium Tetrafluoroborate on the Properties of Conversion Coatings Formed on the AZ91D Magnesium Alloy by Plasma Electrolytic Oxidation. Processes 2022, 10, 2089. [Google Scholar] [CrossRef]
- Lujun, Z.; Hongzhan, L.; Qingmei, M.; Jiangbo, L.; Zhengxian, L. The mechanism for tuning the corrosion resistance and pore density of plasma electrolytic oxidation (PEO) coatings on Mg alloy with fluoride addition. J. Magnes. Alloys 2023, 11, 2823–2832. [Google Scholar] [CrossRef]
- Florczak, Ł.; Kościelniak, B.; Kramek, A.; Sobkowiak, A. The Influence of Potassium Hexafluorophosphate on the Morphology and Anticorrosive Properties of Conversion Coatings Formed on the AM50 Magnesium Alloy by Plasma Electrolytic Oxidation. Materials 2023, 16, 7573. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Blawert, C.; Serdechnova, M.; Konchakova, N.; Shulha, T.; Wu, T.; Zheludkevich, M.L. Effect of low concentration electrolytes on the formation and corrosion resistance of PEO coatings on AM50 magnesium alloy. J. Magnes. Alloys 2024, 12, 1386–1405. [Google Scholar] [CrossRef]
- Hu, R.-G.; Zhang, S.; Bu, J.-F.; Lin, C.-J.; Song, G.-L. Recent progress in corrosion protection of magnesium alloys by organic coatings. Prog. Org. Coat. 2012, 73, 129–141. [Google Scholar] [CrossRef]
- Directive 2004/42/EC of the European Parliament and of the Council of 21 April 2004 on the Limitation of Emissions of volatile Organic Compounds due to the Use of Organic Solvents in Certain Paints and Varnishes and Vehicle Refinishing Products and Amending Directive 1999/13/EC. Available online: https://eur-lex.europa.eu/eli/dir/2004/42/oj (accessed on 16 June 2024).
- Spyrou, E. Powder Coatings Chemistry and Technology; Vincentz Network: Hannover, Germany, 2014. [Google Scholar]
- Poth, U.; Schwalm, R.; Schwartz, M.; Baunstark, R. Acrylic Resins; Vincentz Network: Hanover, Germany, 2011. [Google Scholar]
- Pojnar, K.; Pilch-Pitera, B.; Patil, R. Progress in the development of acrylic resin-based powder coatings—An overview. Polimery 2024, 69, 143–158. [Google Scholar] [CrossRef]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Deng, L.; Tang, L.; Qu, J. Synthesis and photopolymerization of novel UV-curable macro-photoinitiators. Prog. Org. Coat. 2020, 141, 105546. [Google Scholar] [CrossRef]
- Czachor-Jadacka, D.; Pilch-Pitera, B.; Kisiel, M.; Gumieniak, J. Hydrophobic UV-Curable Powder Clear Coatings: Study on the Synthesis of New Crosslinking Agents Based on Raw Materials Derived from Renewable Sources. Materials 2021, 14, 4710. [Google Scholar] [CrossRef] [PubMed]
- Hammer, T.J.; Mehr, H.M.S.; Pugh, C.; Soucek, M.D. Urethane methacrylate reactive diluents for UV-curable polyester powder coatings. J. Coat. Technol. Res. 2021, 18, 333–348. [Google Scholar] [CrossRef]
- Pojnar, K.; Pilch-Pitera, B.; Kisiel, M.; Zioło, A.; Kędzierski, M. UV-curable powder transparent coatings based on oligo(meth)acrylic resins. Polimery 2024, 69, 11–24. [Google Scholar] [CrossRef]
- Rawat, R.S.; Chouhan, N.; Talwar, M.; Diwan, R.K.; Tyagi, A.K. UV coatings for wooden surfaces. Prog. Org. Coat. 2019, 135, 490–495. [Google Scholar] [CrossRef]
- Lukac, P.; Trojanova, Z. Mechanical Properties of Az91 Magnesium Alloys. Commun. Sci. Lett. Univ. Zilina 2004, 6, 26–29. [Google Scholar] [CrossRef]
- Xiulan, A.; Gaofeng, Q. The Recent Research on Properties of Anti-High Temperature Creep of AZ91 Magnesium Alloy. In Magnesium Alloys; Frank, C., Ed.; IntechOpen: Rijeka, Croatia, 2011; p. Ch. 16. [Google Scholar]
- Zimina, M.; Malek, P.; Bohlen, J.; Letzig, D.; Kurz, G.; Cieslar, M. Mechanical properties of homogenized twin-roll cast and conventionally cast AZ31 magnesium alloys. Mater. Eng. 2015, 22, 8–15. [Google Scholar]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Filonina, V.S.; Ustinov, A.Y.; Gnedenkov, S.V. Hybrid Coatings for Active Protection against Corrosion of Mg and Its Alloys. Polymers 2023, 15, 3035. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Liang, W.; Huang, G.; Jiang, B.; Atrens, A.; Pan, F. Superhydrophobic coatings for corrosion protection of magnesium alloys. J. Mater. Sci. Technol. 2020, 52, 100–118. [Google Scholar] [CrossRef]
- Farshid, S.; Kharaziha, M. Micro and nano-enabled approaches to improve the performance of plasma electrolytic oxidation coated magnesium alloys. J. Magnes. Alloys 2021, 9, 1487–1504. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R.; Babaei, K. Impressive strides in amelioration of corrosion and wear behaviors of Mg alloys using applied polymer coatings on PEO porous coatings: A review. J. Magnes. Alloys 2022, 10, 1171–1190. [Google Scholar] [CrossRef]
- Li, N.; Ling, N.; Fan, H.; Wang, L.; Zhang, J. Self-healing and superhydrophobic dual-function composite coating for active protection of magnesium alloys. Surf. Coat. Technol. 2023, 454, 129146. [Google Scholar] [CrossRef]
- ASTM B94; Standard Specification for Magnesium-Alloy Die Castings. ASTM International: West Conshohocken, PA, USA, 2009.
- Bellot, P. Specifications for a Quality Label for Liquid and Powder Coatings on Aluminium for Architectural Applications; QUALICOAT Specifications: Zurich, Switzerland, 2024. [Google Scholar]
- PN-EN ISO 2409; Paints and Varnishes—Cross-Cut Testing. International Organization for Standardization: Geneva, Switzerland, 2013. (In Polish)
- Liu, J.; Lu, Y.; Jing, X.; Yuan, Y.; Zhang, M. Characterization of plasma electrolytic oxidation coatings formed on Mg–Li alloy in an alkaline silicate electrolyte containing silica sol. Mater. Corros. 2009, 60, 865–870. [Google Scholar] [CrossRef]
- Aktuğ, S.L.; Durdu, S.; Kutbay, I.; Usta, M. Effect of Na2SiO3·5H2O concentration on microstructure and mechanical properties of plasma electrolytic oxide coatings on AZ31 Mg alloy produced by twin roll casting. Ceram. Int. 2016, 42, 1246–1253. [Google Scholar] [CrossRef]
- Hafeez, M.A.; Farooq, A.; Zang, A.; Saleem, A.; Deen, K.M. Phosphate chemical conversion coatings for magnesium alloys: A review. J. Coat. Technol. Res. 2020, 17, 827–849. [Google Scholar] [CrossRef]
- Yi, A.; Du, J.; Wang, J.; Mu, S.; Zhang, G.; Li, W. Preparation and characterization of colored Ti/Zr conversion coating on AZ91D magnesium alloy. Surf. Coat. Technol. 2015, 276, 239–247. [Google Scholar] [CrossRef]
- Vaghefinazari, B.; Wierzbicka, E.; Visser, P.; Posner, R.; Arrabal, R.; Matykina, E.; Mohedano, M.; Blawert, C.; Zheludkevich, M.; Lamaka, S. Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: PART I—Pre-Treatment and Conversion Coating. Materials 2022, 15, 8676. [Google Scholar] [CrossRef] [PubMed]
- Fandi, M.; Li, L. Electrochemical Evaluation Technologies of Organic Coatings. In Coatings and Thin-Film Technologies; Jaime Andres, P.-T., Alba, G.A.B., Eds.; IntechOpen: Rijeka, Croatia, 2018; p. Ch. 3. [Google Scholar]
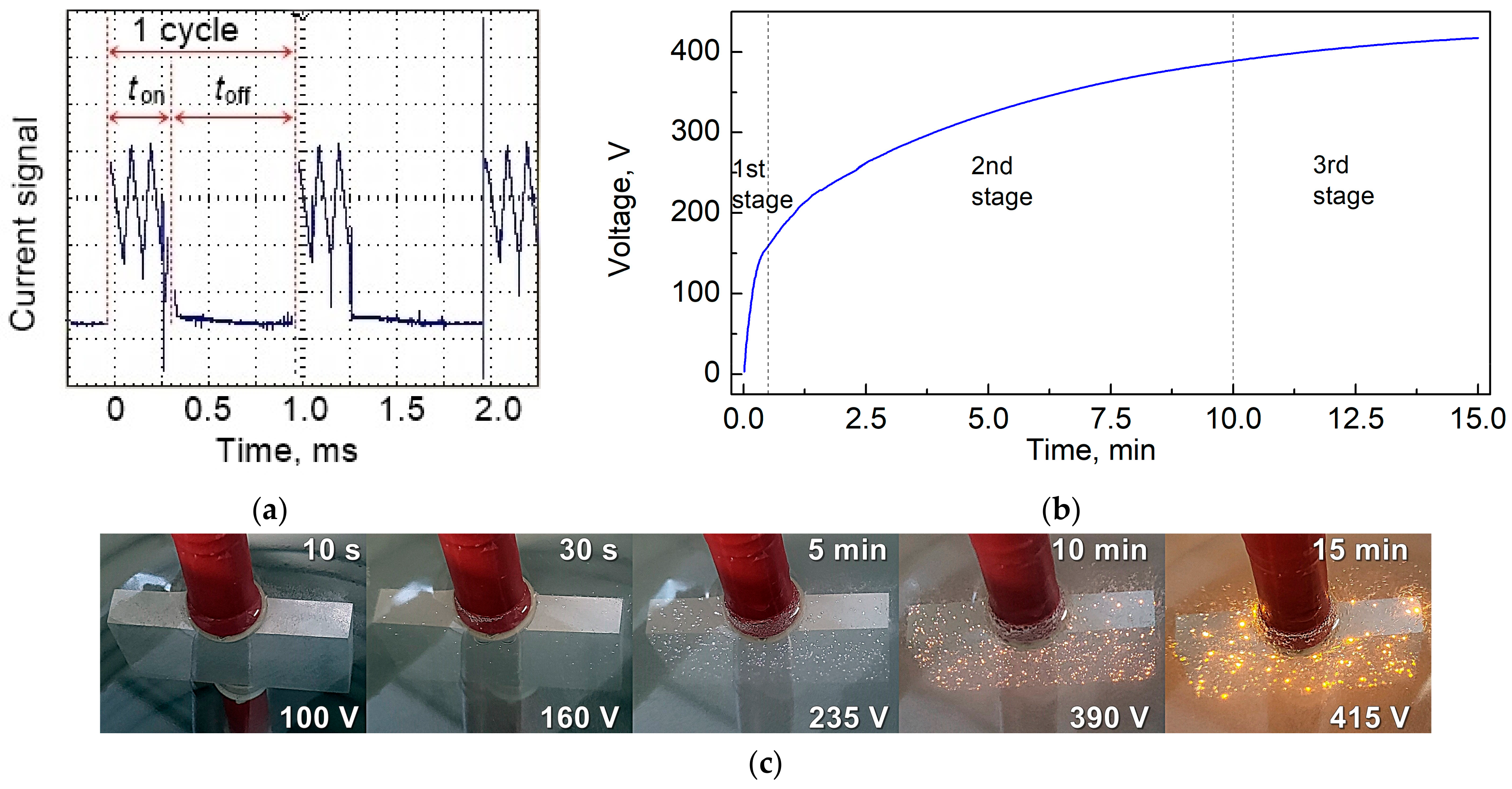
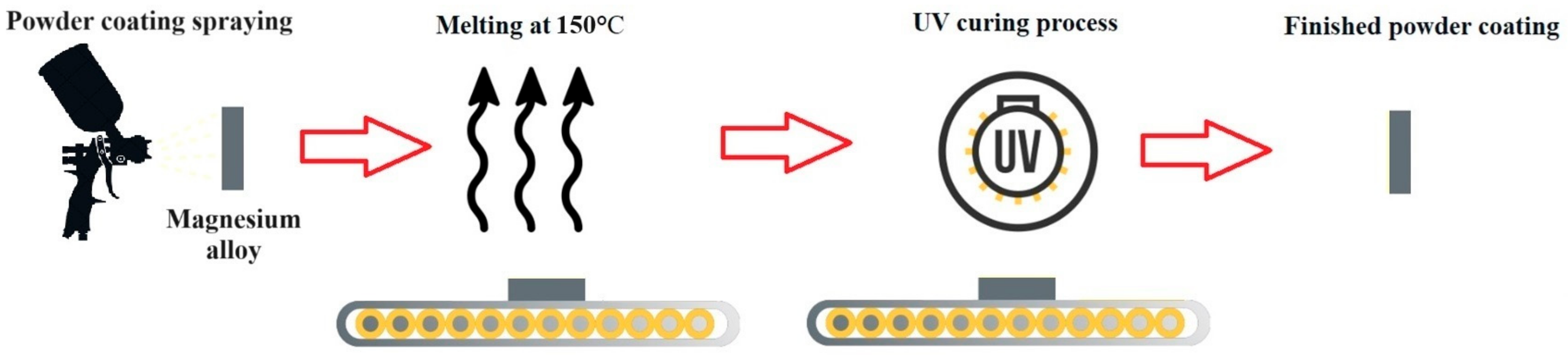
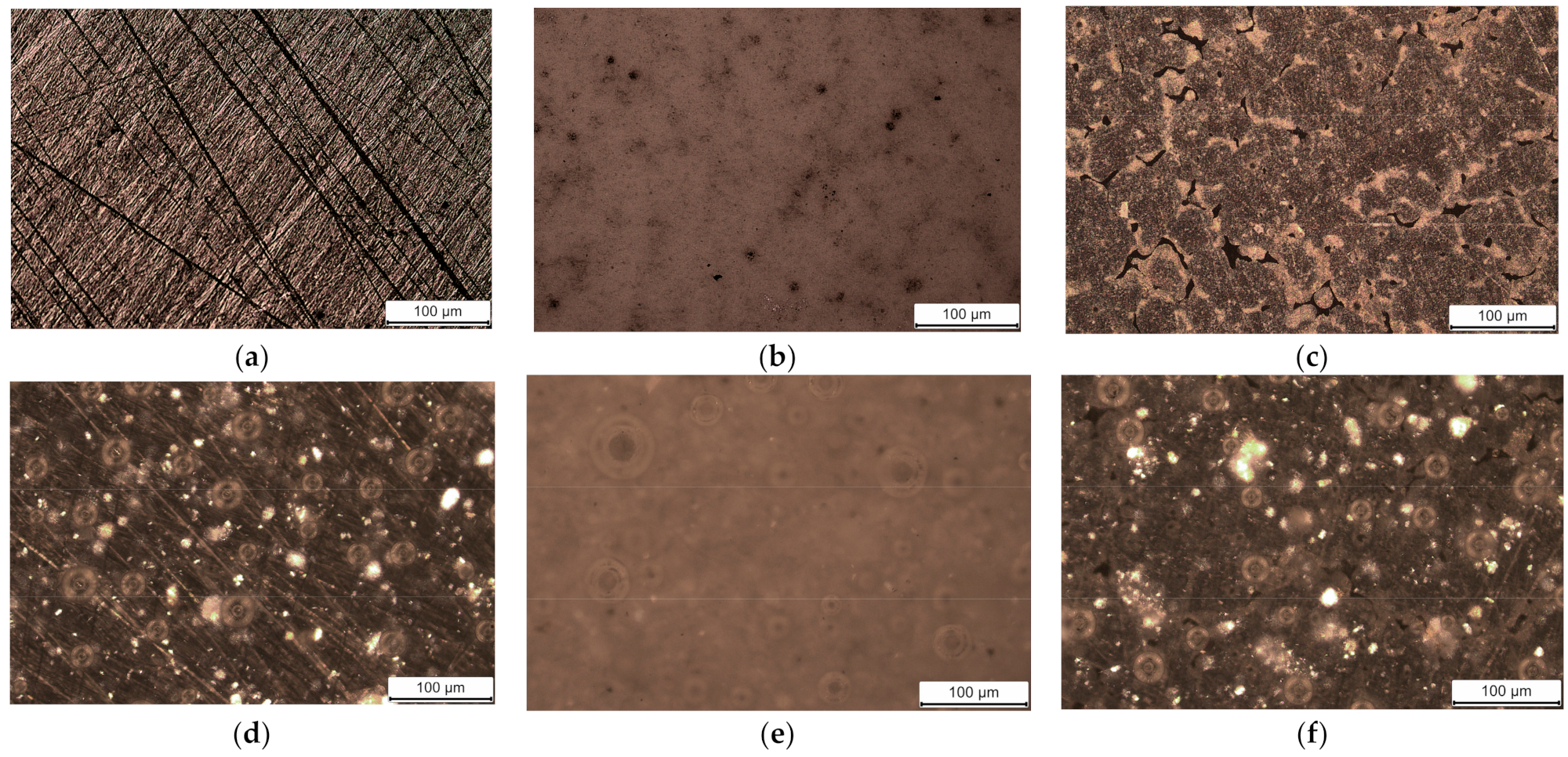

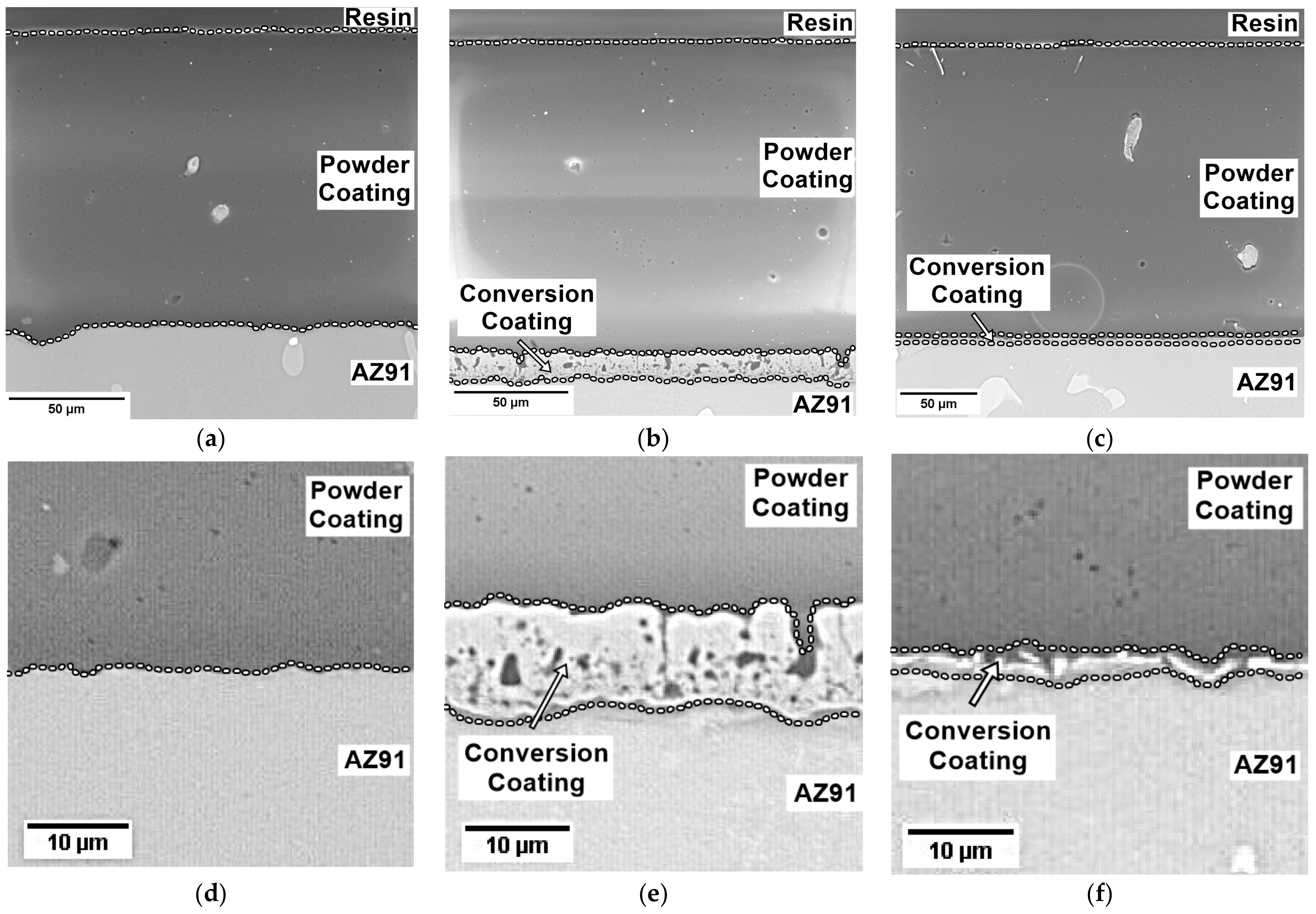

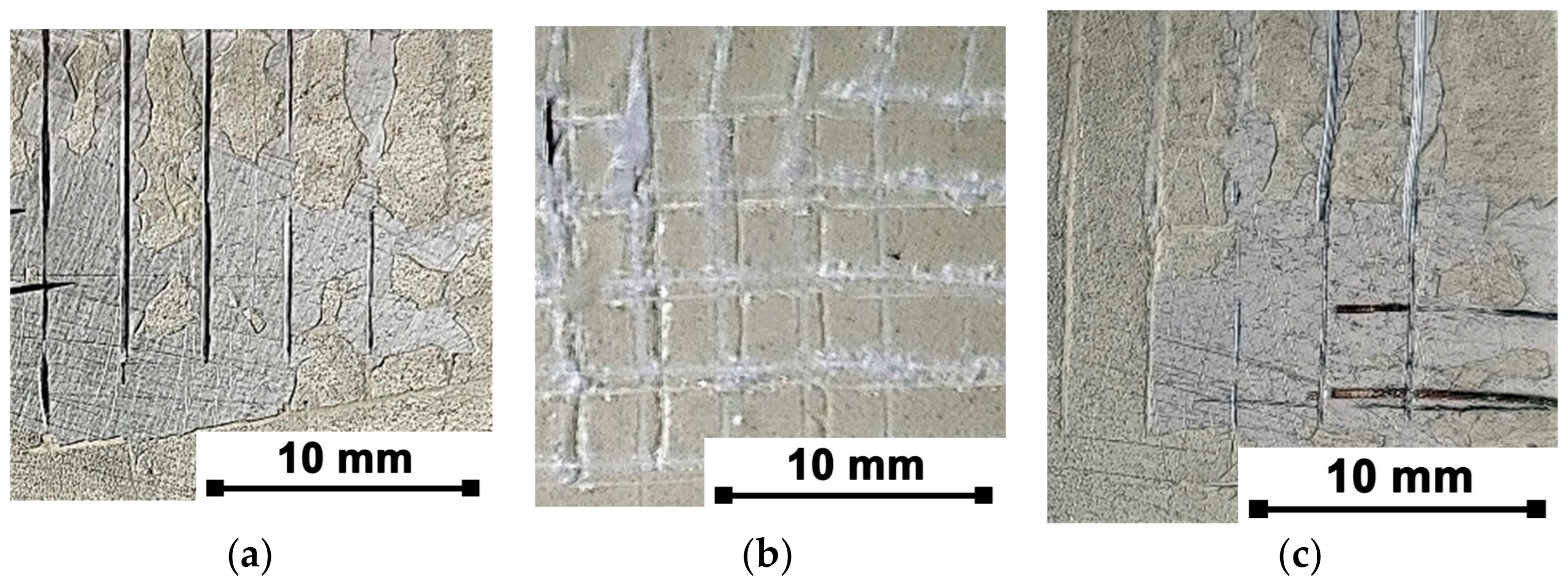

| Code Name | Substrate | Treatment |
|---|---|---|
| A | AZ91 | Uncoated |
| AP | Electrochemical conversion (in PEO process) | |
| AC | Chemical conversion (in Eskaphor Z 2000 C solution) | |
| AO | Organic coating (in UV-curable powder coating) | |
| APO | Electrochemical conversion + organic coating | |
| ACO | Chemical conversion + organic coating |
| Sample | d1 [μm] | d2 [μm] | Ra [μm] | Rz [μm] |
|---|---|---|---|---|
| A | - | - | 0.19 ± 0.02 | 1.79 ± 0.22 |
| AP | 12.2 ± 0.3 | 13.12 ± 0.94 | 0.71 ± 0.02 | 5.24 ± 0.40 |
| AC | 0.2 ± 0.1 | 0.33 ± 0.03 | 0.24 ± 0.02 | 2.14 ± 0.23 |
| AO | 126.0 ± 5.0 | 120.40 ± 1.43 | 0.25 ± 0.04 | 1.54 ± 0.57 |
| APO | 138.0 ± 29.0 | 149.30 ± 2.83 | 0.25 ± 0.05 | 1.47 ± 0.34 |
| ACO | 178.0 ± 24.0 | 187.10 ± 1.10 | 0.27 ± 0.06 | 1.72 ± 0.42 |
| Sample | Time, h | Qtop, Fn/cm2 | ntop | Rtop, Ω·cm2 | Qin, Fn/cm2 | nin | Rin, Ω·cm2 | Qdl, Fn/cm2 | ndl | Rct, Ω·cm2 |
|---|---|---|---|---|---|---|---|---|---|---|
| AO | 3 | 2.36 × 10−10 | 0.931 | 1.64 × 109 | ||||||
| 24 | 5.75 × 10−10 | 0.901 | 3.73 × 105 | 9.94 × 10−8 | 0.688 | 4.85 × 107 | ||||
| 168 | 2.25 × 10−10 | 0.980 | 9.06 × 104 | 3.75 × 10−8 | 0.575 | 2.96 × 105 | 1.09 × 10−7 | 0.598 | 2.08 × 107 | |
| APO | 3 | 1.22 × 10−10 | 0.955 | 4.10 × 1010 | ||||||
| 24 | 9.17 × 10−11 | 0.952 | 1.96 × 106 | 5.24 × 10−9 | 0.662 | 1.46 × 107 | 1.82 × 10−8 | 0.626 | 9.68 × 108 | |
| 168 | 2.37 × 10−10 | 0.951 | 1.38 × 105 | 2.06 × 10−8 | 0.658 | 1.11 × 107 | 4.22 × 10−8 | 0.724 | 7.04 × 107 | |
| ACO | 3 | 1.45 × 10−10 | 0.949 | 1.25 × 1010 | ||||||
| 24 | 3.25 × 10−10 | 0.919 | 1.40 × 106 | 1.10 × 10−7 | 0.634 | 5.25 × 107 | ||||
| 168 | 1.74 × 10−10 | 0.982 | 6.26 × 104 | 1.20 × 10−7 | 0.484 | 1.15 × 106 | 1.01 × 10−7 | 0.855 | 1.30 × 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florczak, Ł.; Pojnar, K.; Kościelniak, B.; Pilch-Pitera, B. Preparation and Characterization of Duplex PEO/UV-Curable Powder Coating on AZ91 Magnesium Alloys. Metals 2024, 14, 733. https://doi.org/10.3390/met14060733
Florczak Ł, Pojnar K, Kościelniak B, Pilch-Pitera B. Preparation and Characterization of Duplex PEO/UV-Curable Powder Coating on AZ91 Magnesium Alloys. Metals. 2024; 14(6):733. https://doi.org/10.3390/met14060733
Chicago/Turabian StyleFlorczak, Łukasz, Katarzyna Pojnar, Barbara Kościelniak, and Barbara Pilch-Pitera. 2024. "Preparation and Characterization of Duplex PEO/UV-Curable Powder Coating on AZ91 Magnesium Alloys" Metals 14, no. 6: 733. https://doi.org/10.3390/met14060733
APA StyleFlorczak, Ł., Pojnar, K., Kościelniak, B., & Pilch-Pitera, B. (2024). Preparation and Characterization of Duplex PEO/UV-Curable Powder Coating on AZ91 Magnesium Alloys. Metals, 14(6), 733. https://doi.org/10.3390/met14060733







