Effect of Ag and Cu Content on the Properties of Zn-Ag-Cu-0.05Mg Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Microstructure Analysis
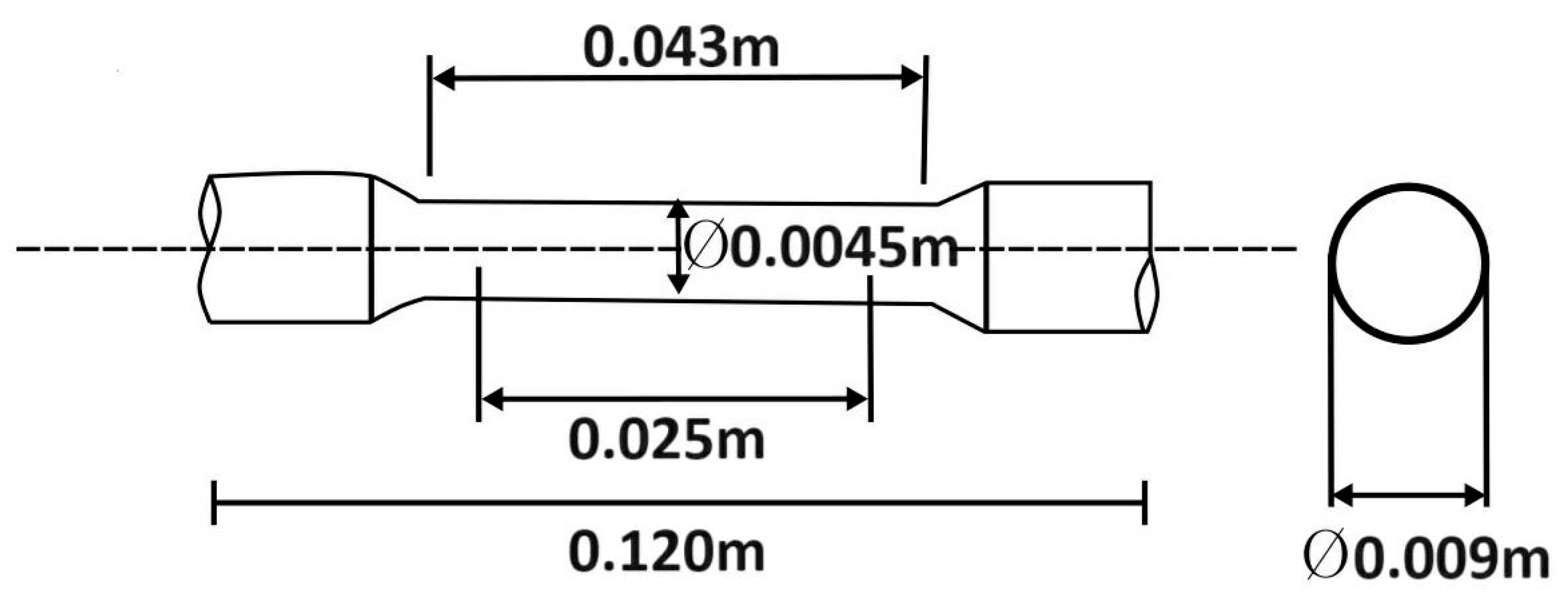
2.3. Mechanical Properties Testing
2.4. Corrosion Rate
2.5. Antibacterial Tests
2.6. Cytotoxicity Tests
3. Results
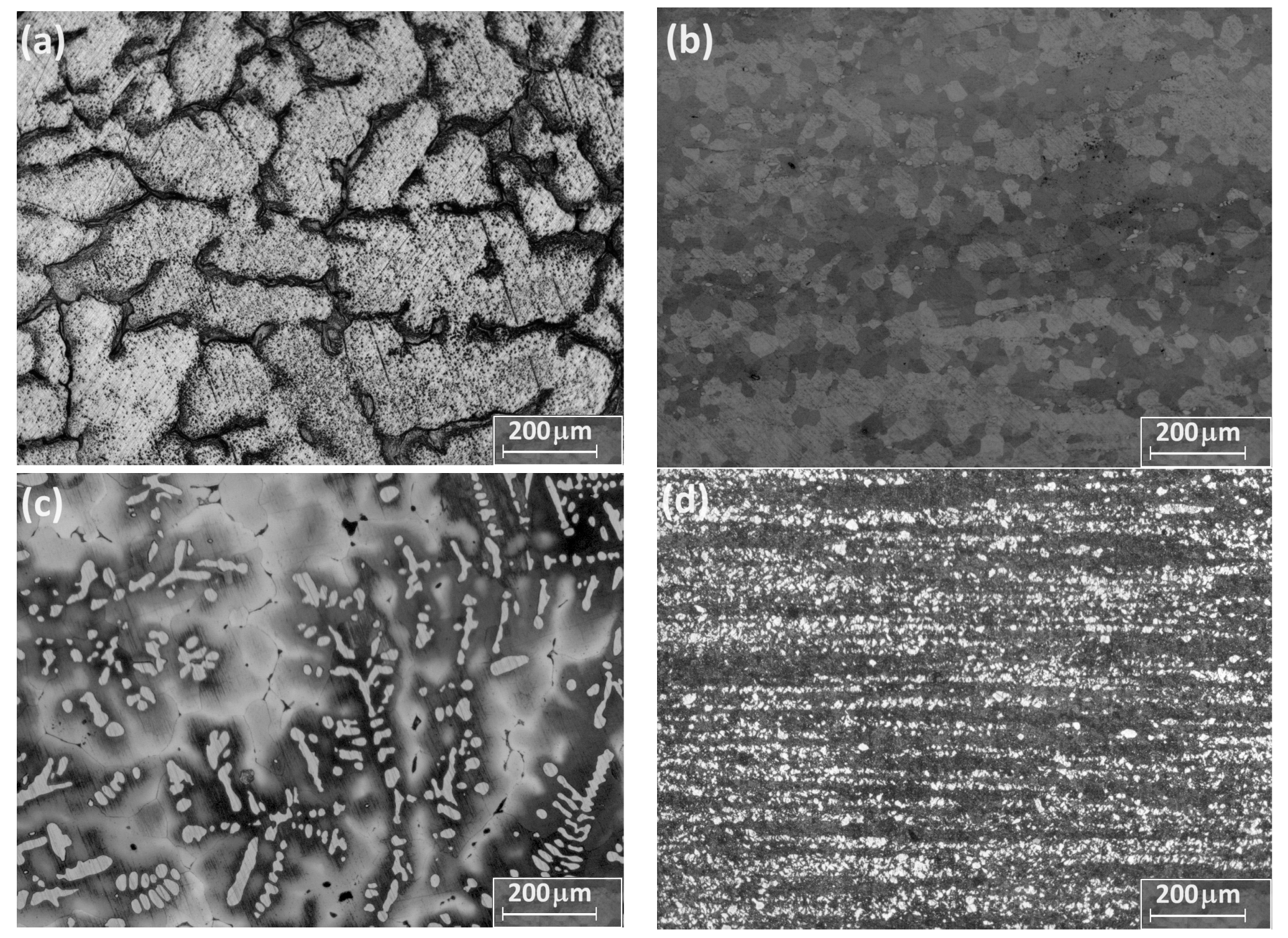
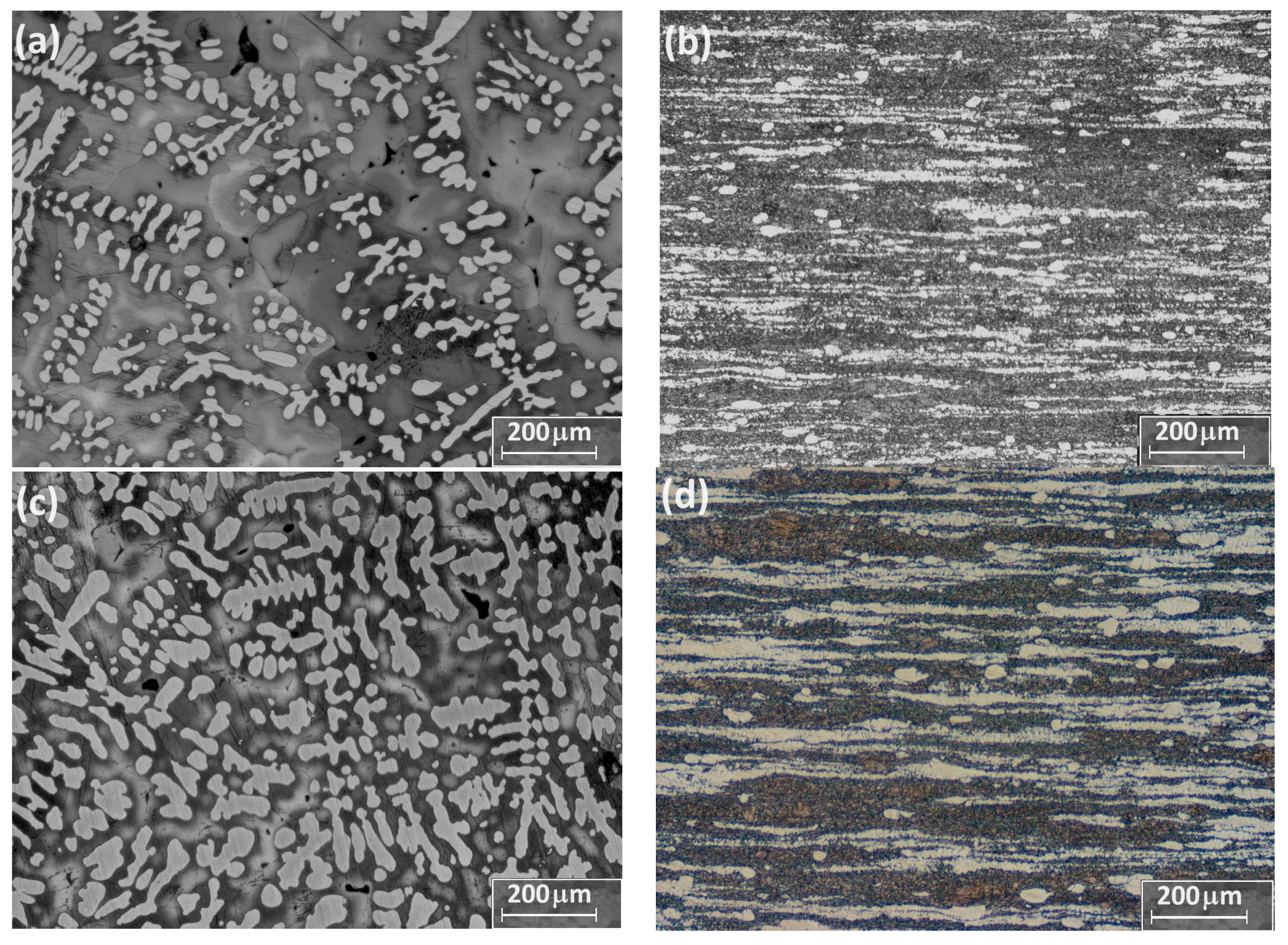
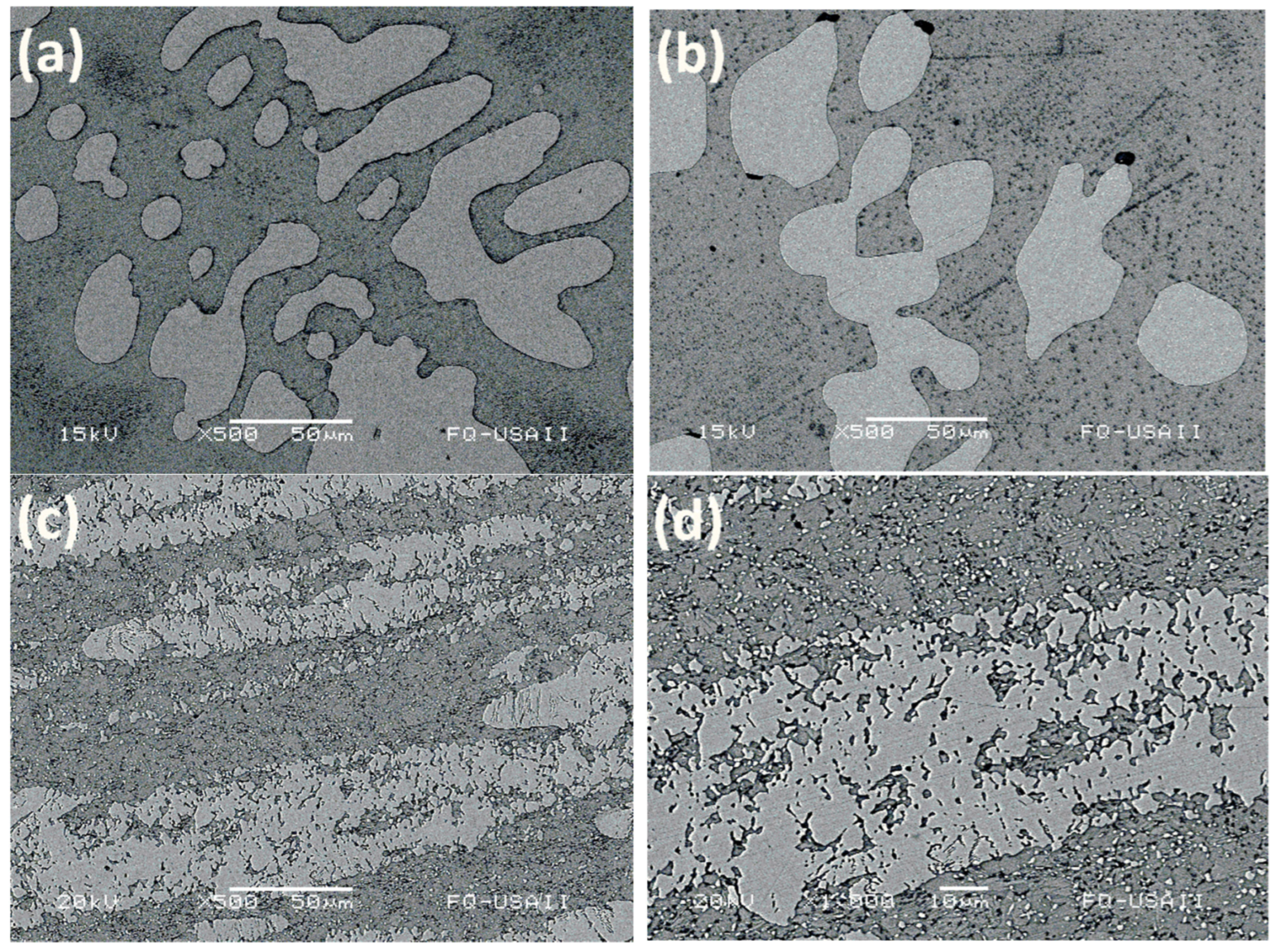
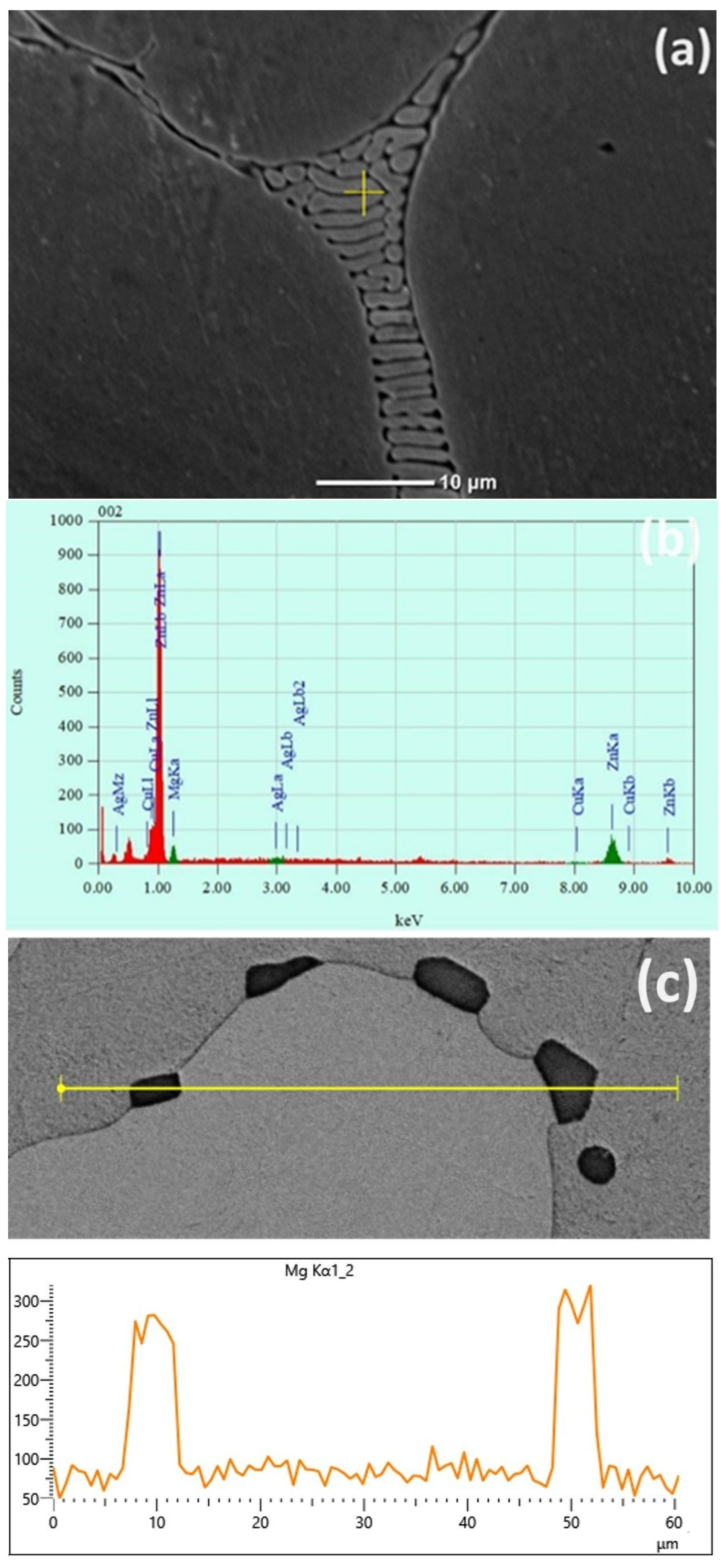
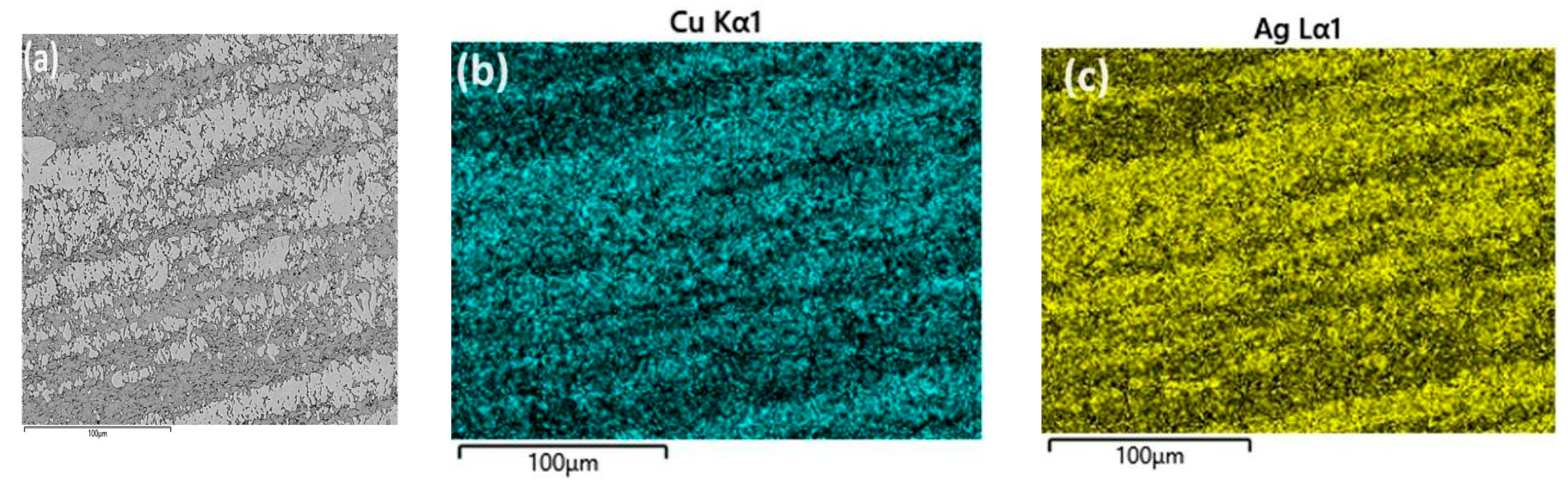
3.1. Microstructural Characterization
3.2. Corrosion Rate
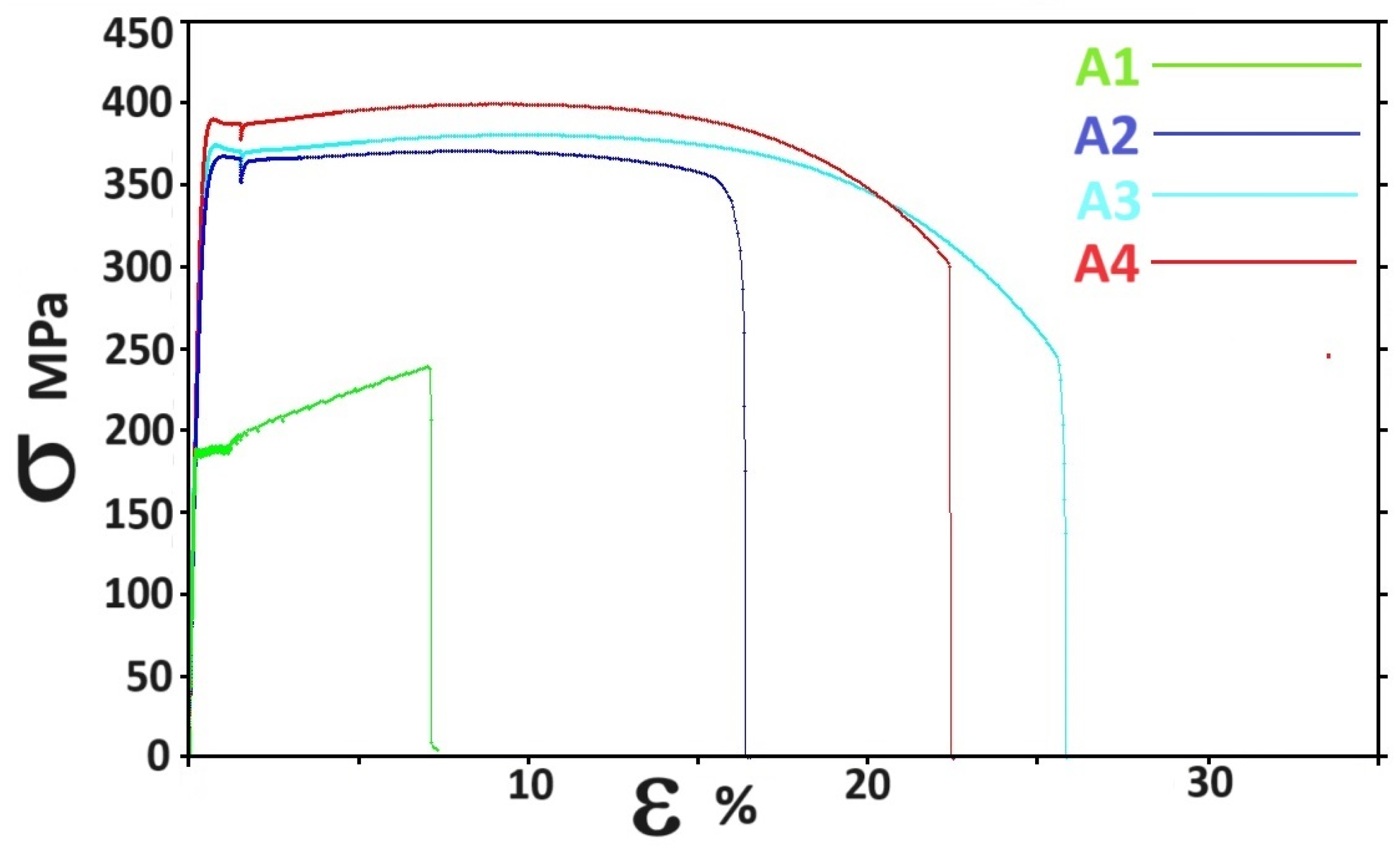
3.3. Mechanical Properties
3.4. Evaluation of Antibacterial Properties
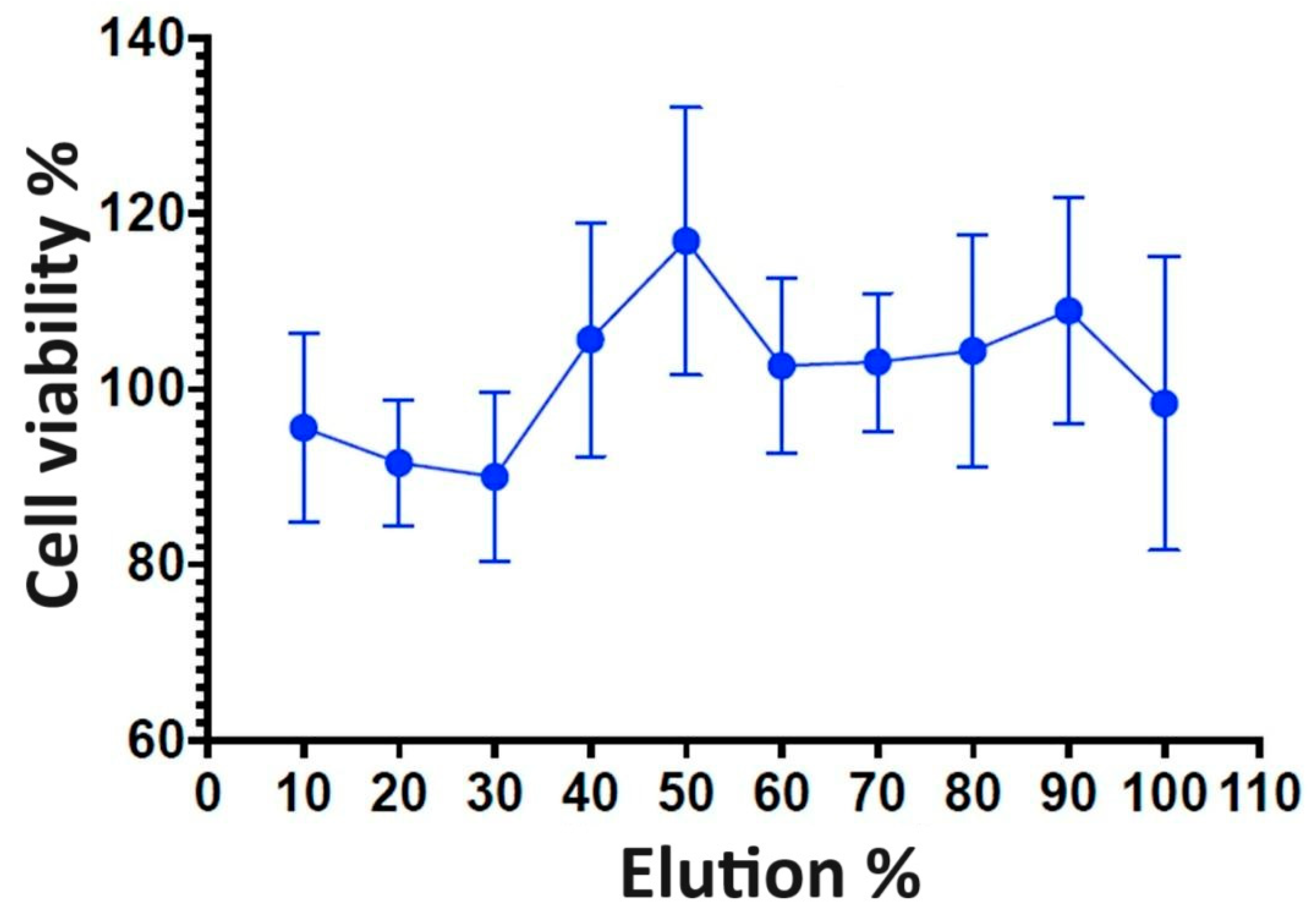
3.5. Cytotoxicity
4. Conclusions
- An increment in the Ag and Cu contents increases the amount of the second phase (Ag, Cu)Zn4, improves the mechanical properties, and increases the corrosion rate.
- Experimental alloys showing the second phase exhibit good antibacterial properties against S. aureus and a mechanical behavior similar to that reported for biphasic hetero-materials with heterogeneous lamella.
- The alloy with the highest Ag and Cu contents showed very low cytotoxicity.
- The results suggest that ZnAgCu-0.05 wt% Mg alloys, with Ag and Cu contents in intervals shown by A2, A3, and A4 experimental alloys, show good potential for biomedical applications.
- It seems possible to tune the properties of the ZnAgCu-0.05 wt% Mg alloys by changing the Ag and Cu contents between the experiment’s composition intervals.
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Z.Z.; Gao, X.X.; Zhang, H.J.; Liu, X.F.; Li, H.Y.; Zhou, C.; Yin, Y.X.; Wang, L.N. Design biodegradable Zn alloys: Second phases and their significant influences on alloy properties. Bioact. Mater. 2020, 5, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yang, H.; Jia, B.; Wang, M.; Yue, B.; Zheng, Y.; Dai, K. Zinc alloy-based bone internal fixation screw with antibacterial and anti-osteolytic properties. Bioact. Mater. 2021, 6, 4607–4624. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Venezuela, J.; Almathami, S.; Dargusch, M. Zinc-nutrient element based alloys for absorbable wound closure devices fabrication: Current status, challenges, and future prospects. Biomaterials 2022, 280, 121301. [Google Scholar] [CrossRef] [PubMed]
- Di, T.; Xu, Y.; Liu, D.; Sun, X. Microstructure, mechanical performance and anti-bacterial activity of degradable Zn-Cu-Ag alloy. Metals 2022, 12, 1444. [Google Scholar] [CrossRef]
- Nie, Y.J.; Dai, J.W.; Zhang, X.B. Effect of Ag Addition on Microstructure, Mechanical and Corrosion Properties of Mg–Nd–Zn–Zr Alloy for Orthopedic Application. Acta Metall. Sin. 2023, 36, 295–309. [Google Scholar] [CrossRef]
- Sikora-Jasinska, M.; Mostaed, E.; Mostaed, A.; Beanland, R.; Mantovani, D.; Vedani, M. Fabrication, mechanical properties and in vitro degradation behavior of newly developed ZnAg alloys for degradable implant applications. Mater. Sci. Eng. C 2017, 77, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Niu, J.; Huang, H.; Zhang, H.; Pei, J.; Ou, J.; Yuan, G. Potential biodegradable Zn-Cu binary alloys developed for cardiovascular implant applications. J. Mech. Behav. Biomed. Mater. 2017, 72, 182–191. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, D.; Wang, F.; Taylor, J.A.; Zhang, M. The grain refining mechanism of cast zinc through silver inoculation. Acta Mater. 2014, 79, 315–326. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, D.; Wang, F.; Taylor, J.A.; Zhang, M. Crystallography of grain refinement in cast zinc–copper alloys. J. Appl. Crystallogr. 2015, 48, 890–900. [Google Scholar] [CrossRef]
- Venezuela, J.; Dargusch, M.S. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef]
- Yue, R.; Huang, H.; Ke, G.; Zhang, H.; Pei, J.; Xue, G.; Yuan, G. Microstructure, mechanical properties and in vitro degradation behavior of novel Zn-Cu-Fe alloys. Mater. Charact. 2017, 134, 114–122. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, H.; Niu, J.; Zhang, L.; Zhang, H.; Pei, J.; Tan, J.; Yuan, G. Design and characterizations of novel biodegradable Zn-Cu-Mg alloys for potential biodegradable implants. Mater. Des. 2017, 117, 84–94. [Google Scholar] [CrossRef]
- Lin, J.; Tong, X.; Wang, K.; Shi, Z.; Li, Y.; Dargusch, M.; Wen, C. Biodegradable Zn–3Cu and Zn–3Cu–0.2 Ti alloys with ultrahigh ductility and antibacterial ability for orthopedic applications. J. Mater. Sci. Technol. 2021, 68, 76–90. [Google Scholar] [CrossRef]
- Wątroba, M.; Bednarczyk, W.; Kawałko, J.; Lech, S.; Wieczerzak, K.; Langdon, T.G.; Bała, P. A novel high-strength Zn-3Ag-0.5 Mg alloy processed by hot extrusion, cold rolling, or high-pressure torsion. Metall. Mater. Trans. A 2020, 51, 3335–3348. [Google Scholar] [CrossRef]
- Wątroba, M.; Bednarczyk, W.; Kawałko, J.; Bała, P. Fine-tuning of mechanical properties in a Zn–Ag–Mg alloy via cold plastic deformation process and post-deformation annealing. Bioact. Mater. 2021, 6, 3424–3436. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Li, W.; Chen, L.; Lai, Y.; Guo, H.; Xing, Q.; Yang, X. Effects of Ag Content and Hot-Rolling on Microstructure and Mechanical Properties of Zn-Cu-xAg-Zr Alloy. J. Mater. Eng. Perform. 2022, 31, 5964–5972. [Google Scholar] [CrossRef]
- Jin, H.; Yang, L.; Lai, Y.; Liu, Y. The effects of Zr content and hot rolling on the microstructure and mechanical properties of Zn-1.5 Cu-1.0 Ag-xZr alloys. J. Alloys Compd. 2022, 912, 165116. [Google Scholar] [CrossRef]
- Niu, K.; Zhang, D.; Qi, F.; Lin, J.; Dai, Y. The effects of Cu and Mn on the microstructure, mechanical, corrosion properties and biocompatibility of Zn–4Ag alloy. J. Mater. Res. Technol. 2022, 21, 4969–4981. [Google Scholar] [CrossRef]
- Guillory, I.I.R.J.; Mostaed, E.; Oliver, A.A.; Morath, L.M.; Earley, E.J.; Flom, K.L.; Kolesar, T.M.; Mostaed, A.; Summers, H.D.; Kwesiga, M.P.; et al. Improved biocompatibility of Zn–Ag-based stent materials by microstructure refinement. Acta Biomater. 2022, 145, 416–426. [Google Scholar] [CrossRef]
- Yilmazer, H.; Basit, S.; Sen, A.; Yilmazer, Y.; Dalbayrak, B.; Arisan, E.D.; Arisan, S.; Islamgaliev, R.K.; Dikici, B. A comprehensive study on microstructure, in-vitro biodegradability, bacterial sensitivity, and cellular interactions of novel ternary Zn-Cu-xAg alloys for urological applications. J. Alloys Compd. 2023, 965, 171290. [Google Scholar] [CrossRef]
- Heiss, A.; Thatikonda, V.S.; Richter, A.; Schmitt, L.Y.; Park, D.; Klotz, U.E. Development, Processing and Aging of Novel Zn-Ag-Cu Based Biodegradable Alloys. Materials 2023, 16, 3198. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yue, R.; Zhang, J.; Huang, H.; Niu, J.; Yuan, G. Biodegradable Zn-1.5 Cu-1.5 Ag alloy with anti-aging ability and strain hardening behavior for cardiovascular stents. Mater. Sci. Eng. C 2020, 116, 111172. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ge, W.; Zhao, L.; Kong, L.; Yang, H.; Zhang, X.; Gu, X.; Zhu, C.; Fan, Y. Endowing biodegradable Zinc implants with dual-function of antibacterial ability and osteogenic activity by micro-addition of Mg and Ag (≤0.1 wt.%). Acta Biomater. 2023, 157, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhao, S.; Guillory, R.; Bowen, P.K.; Yin, Z.; Griebel, A.; Schaffer, J.; Earley, E.J.; Goldman, J.; Drelich, J.W. Novel high-strength, low-alloys Zn-Mg (<0.1 wt% Mg) and their arterial biodegradation. Mater. Sci. Eng. C 2018, 4, 67–79. [Google Scholar]
- Ardakani, M.S.; Mostaed, E.; Sikora-Jasinska, M.; Kampe, S.L.; Drelich, J.W. The effects of alloying with Cu and Mn and thermal treatments on the mechanical instability of Zn-0.05 Mg alloy. Mater. Sci. Eng. A 2020, 770, 138529. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fan, G. An overview of tailoring strain delocalization for strength-ductility synergy. Prog. Mater. Sci. 2020, 113, 100675. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, X. Heterostructured materials. Prog. Mater. Sci. 2023, 131, 101019. [Google Scholar] [CrossRef]
- Zhuo, X.; Wu, Y.; Ju, J.; Liu, H.; Jiang, J.; Hu, Z.; Bai, J.; Xue, F. Recent progress of novel biodegradable zinc alloys: From the perspective of strengthening and toughening. J. Mater. Res. Technol. 2022, 17, 244–269. [Google Scholar] [CrossRef]
- Petzow, G.; Effenberg, G. Ag (Silver) Ternary Alloy Phase Diagrams. In Alloy Phase Diagrams; Okamoto, H., Schlesinger, M.E., Mueller, E.M., Eds.; ASM International: Novelty, OH, USA, 2016; Volume 3, pp. 1501–1502. [Google Scholar]
- Xiao, C.; Wang, L.; Ren, Y.; Sun, S.; Zhang, E.; Yan, C.; Liu, Q.; Sun, X.; Shou, F.; Duan, J.; et al. Indirectly extruded biodegradable Zn-0.05 wt% Mg alloy with improved strength and ductility: In vitro and in vivo studies. J. Mater. Sci. Technol. 2018, 34, 1618–1627. [Google Scholar] [CrossRef]
- ASTME8-04; Test Methods for Tension Testing of Metallic Materials. American Society for Testing and Materials: West Conshohocken, PN, USA, 2004.
- ISO 20645:2004; Textile Fabrics—Determination of the Antibacterial Activity—Agar Diffusion Plate Test. International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 10993:2009; Biological evaluation of medical devices Part 5: Tests for in vitro cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Xiao, X.; Liu, E.; Shao, J.; Ge, S. Advances on biodegradable zinc-silver-based alloys for biomedical applications. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211062407. [Google Scholar] [CrossRef]
- Palai, D.; Roy, T.; Prasad, P.S.; Hazra, C.; Dhara, S.; Sen, R.; Das, S.; Das, K. Influence of copper on the microstructural, mechanical, and biological properties of commercially pure Zn-based alloys for a potential biodegradable implant. ACS Biomater. Sci. Eng. 2022, 8, 1443–1463. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Ueji, R.; Inoue, T. Yield-Point Phenomenon and Plastic Bands in Ferrite–Pearlite Steels. Materials 2022, 16, 195. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Fan, G.H.; Geng, L.; Cao, G.J.; Du, Y.; Wu, H.; Zhang, T.T.; Kang, H.J.; Wang, T.M.; Du, G.H.; et al. Revealing extraordinary tensile plasticity in layered Ti-Al metal composite. Sci. Rep. 2016, 6, 38461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Z.; Wang, Y.; Ma, G.; Huang, T.; Wu, G.; Jensen, D.J. Fabricating interstitial-free steel with simultaneous high strength and good ductility with homogeneous layer and lamella structure. Scr. Mater. 2017, 141, 111–114. [Google Scholar] [CrossRef]
- Rowlands, B.S.; Rae, C.; Galindo-Nava, E. The Portevin-Le Chatelier effect in nickel-base superalloys: Origins, consequences and comparison to strain ageing in other alloy systems. Prog. Mater. Sci. 2023, 132, 101038. [Google Scholar] [CrossRef]


| Alloy/wt% | Cu | Mg | Ag | Zn |
|---|---|---|---|---|
| A1 | 0.9 | 0.05 | 1.8 | 97.3 |
| A2 | 1.8 | 0.05 | 3.9 | 94.3 |
| A3 | 2.1 | 0.04 | 5.1 | 92.8 |
| A4 | 3.2 | 0.05 | 5.3 | 91.5 |
| Alloy | As-Cast | Extruded |
|---|---|---|
| A1 | - | - |
| A2 | 13.9 ± 1 | 13.0 ± 1 |
| A3 | 19.2 ± 1 | 17.9 ± 1 |
| A4 | 33.9 ± 2 | 31.5 ± 2 |
| Alloy | Vcorr (mm/Year) |
|---|---|
| A1 | 0.012 ± 0.01 |
| A2 | 0.032 ± 0.01 |
| A3 | 0.074 ± 0.01 |
| A4 | 0.120 ± 0.03 |
| Alloy | YS (MPa) | UTS (MPa) | El (%) |
|---|---|---|---|
| A1 | 186 ± 4 | 237 ± 9 | 7.3 ± 1 |
| A2 | 345 ± 5 | 366 ± 9 | 18.4 ± 3 |
| A3 | 360 ± 4 | 372 ± 8 | 26.6 ± 1 |
| A4 | 375 ± 4 | 398 ± 4 | 22.6 ± 1 |
| Alloy | IZD (mm) |
|---|---|
| A2 | 5.6 ± 0.1 |
| A3 | 4.9 ± 0.7 |
| A4 | 4.8 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jara-Chávez, G.; Amaro-Villeda, A.; Campillo-Illanes, B.; Ramírez-Argáez, M.; González-Rivera, C. Effect of Ag and Cu Content on the Properties of Zn-Ag-Cu-0.05Mg Alloys. Metals 2024, 14, 740. https://doi.org/10.3390/met14070740
Jara-Chávez G, Amaro-Villeda A, Campillo-Illanes B, Ramírez-Argáez M, González-Rivera C. Effect of Ag and Cu Content on the Properties of Zn-Ag-Cu-0.05Mg Alloys. Metals. 2024; 14(7):740. https://doi.org/10.3390/met14070740
Chicago/Turabian StyleJara-Chávez, Gloria, Adrián Amaro-Villeda, Bernardo Campillo-Illanes, Marco Ramírez-Argáez, and Carlos González-Rivera. 2024. "Effect of Ag and Cu Content on the Properties of Zn-Ag-Cu-0.05Mg Alloys" Metals 14, no. 7: 740. https://doi.org/10.3390/met14070740





