Quantitative Study on Hydrogen Concentration–Hydrogen Embrittlement Sensitivity of X80 Pipeline Steel Based on Hydrogen Permeation Kinetics
Abstract
1. Introduction
2. Materials and Methods
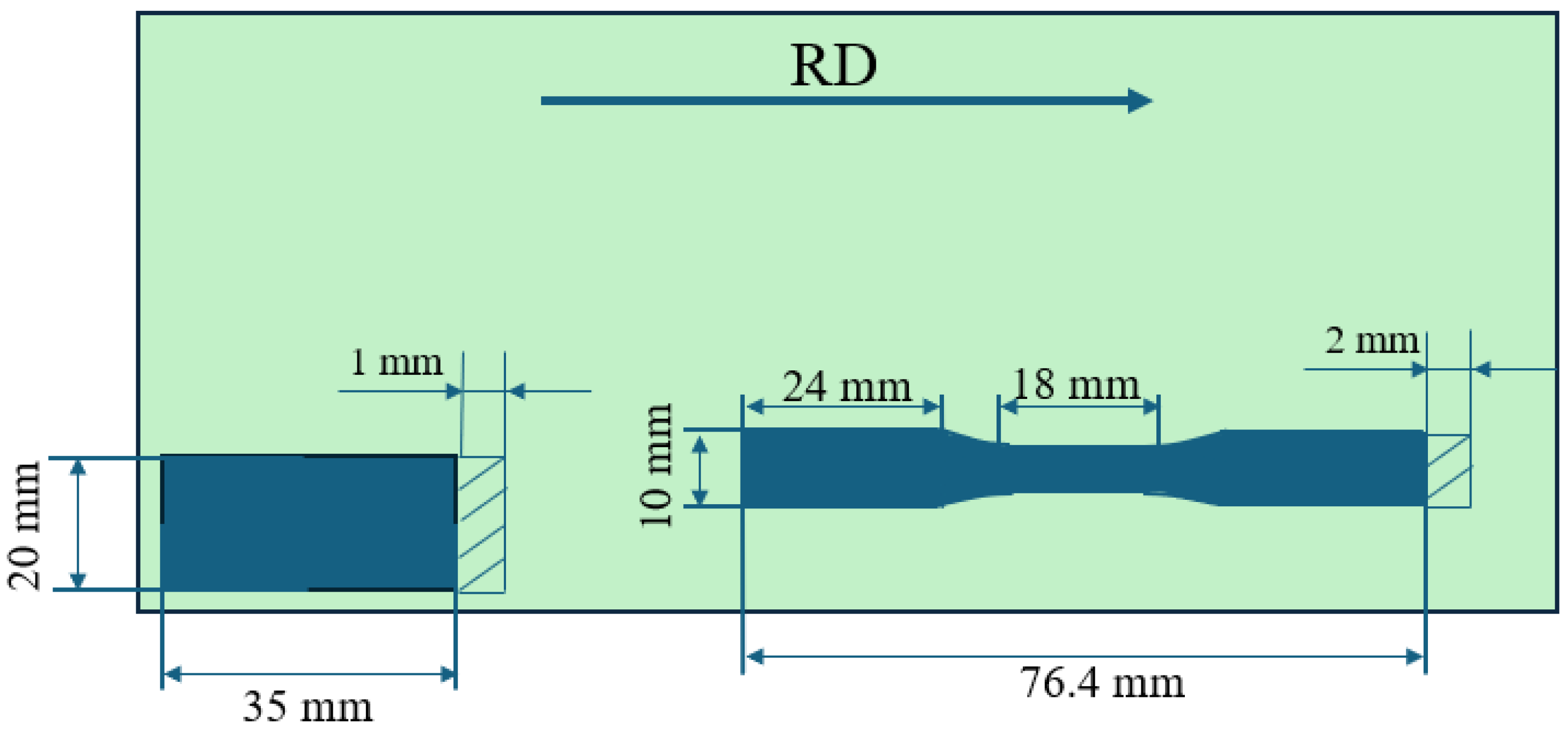
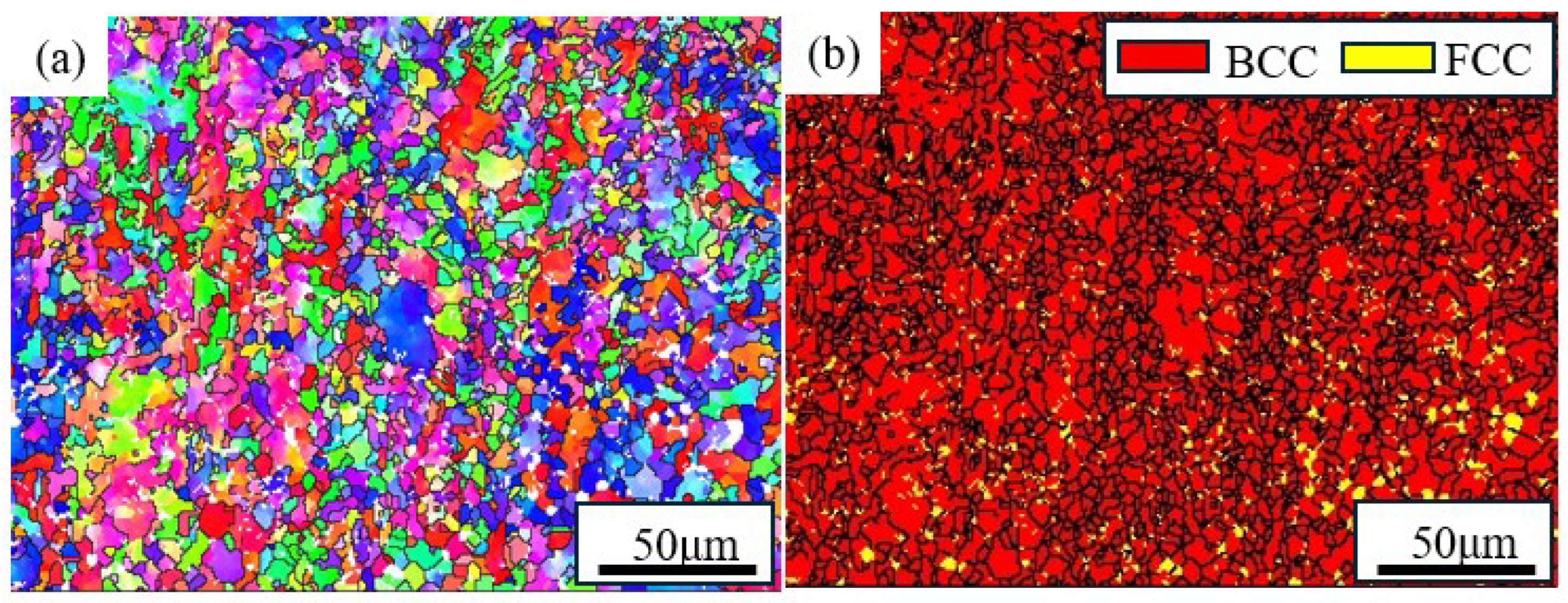
2.1. Materials and Samples Preparation
2.2. Hydrogen Permeation Experiments
2.3. Quantitative Testing for Hydrogen Concentration
2.4. SSRT Experiments
3. Results and Discussion
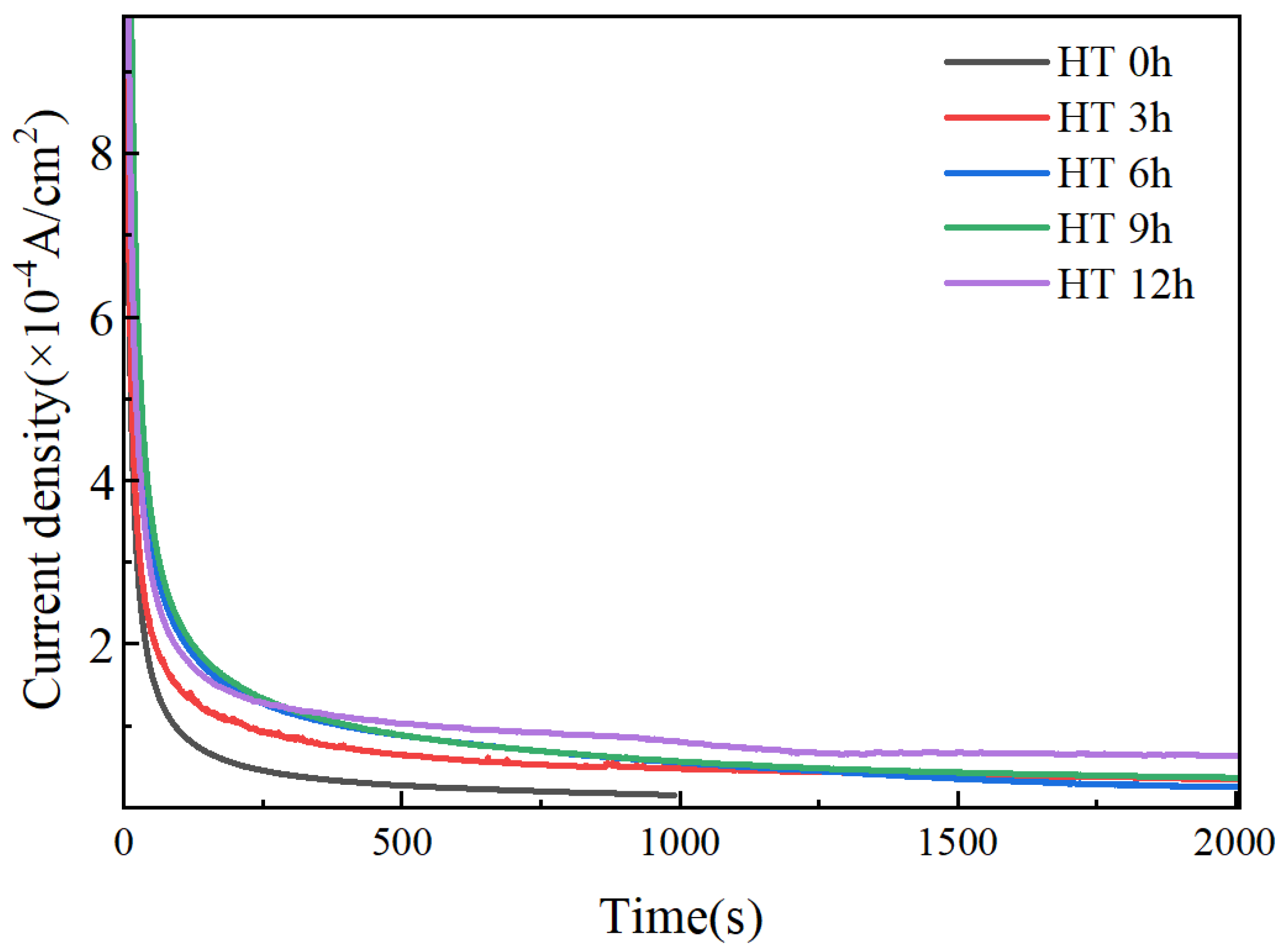
3.1. Hydrogen Permeation Kinetics of X80 Pipeline Steel
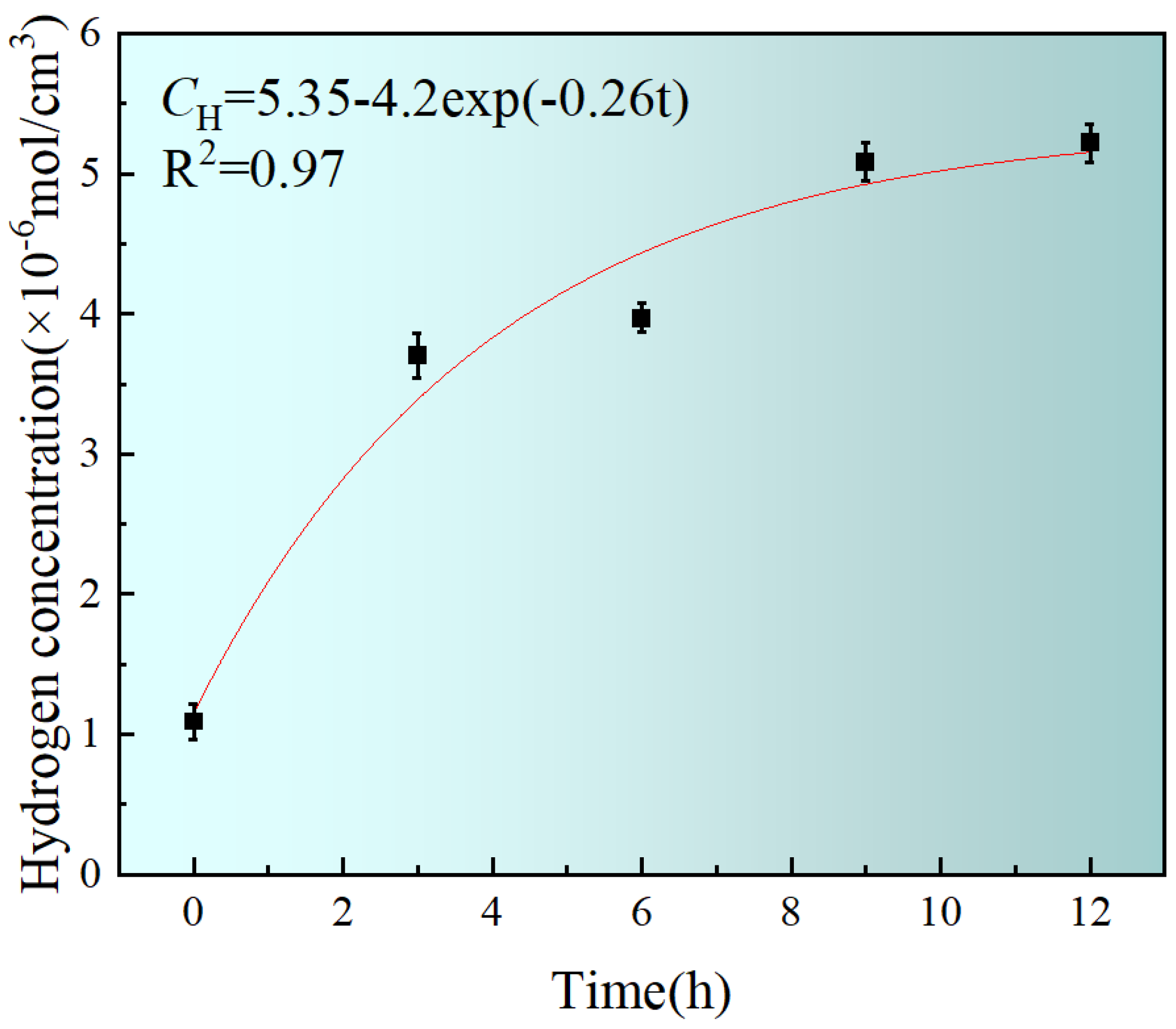
3.2. Calculation of Hydrogen Concentration in X80 Steel
3.3. Quantitative Relationship between Hydrogen Concentration and Mechanical Properties
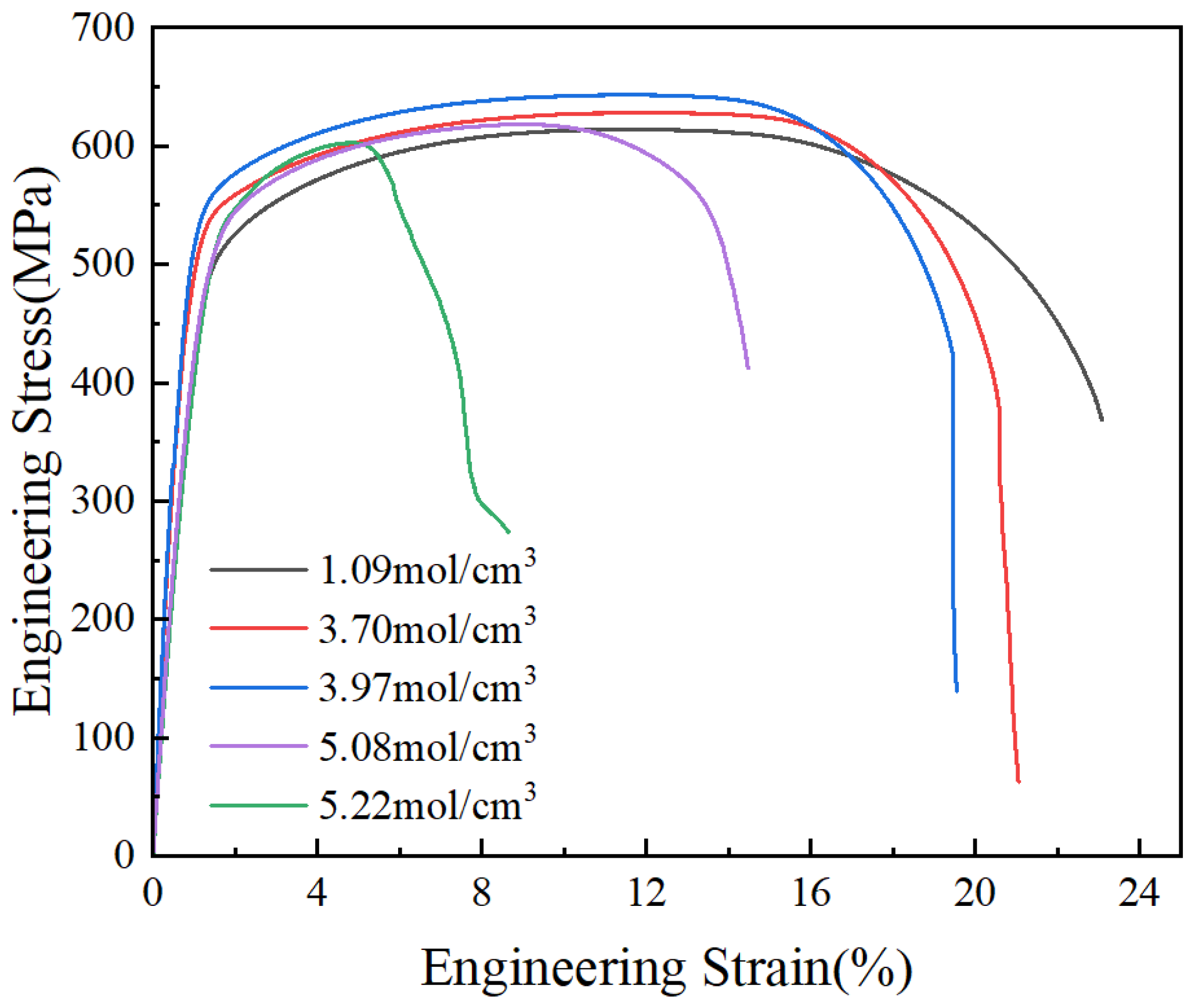
3.3.1. Stress–Strain Curve and Analysis of Mechanical Properties
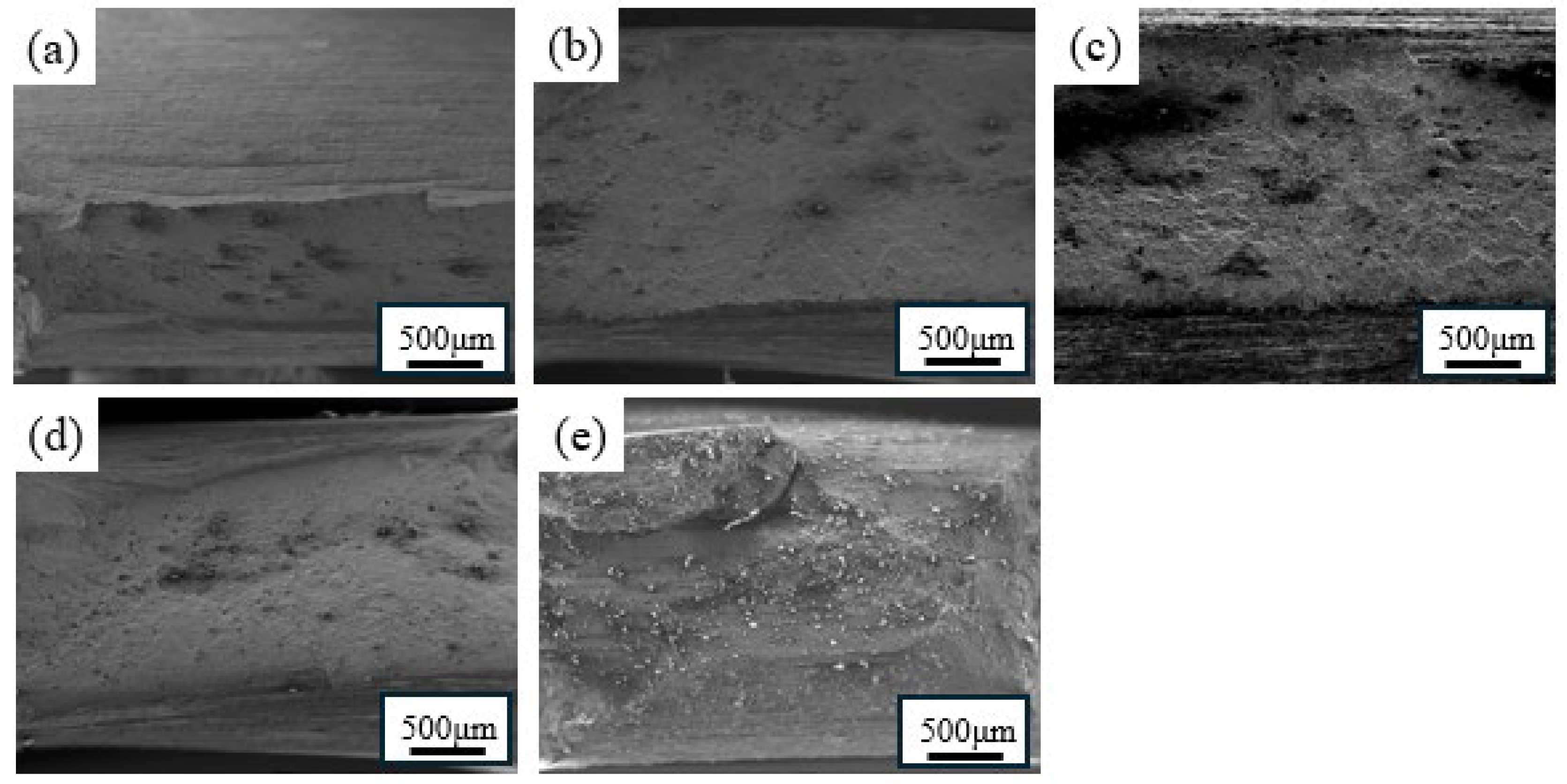
3.3.2. Fracture Morphologies
4. Conclusions
- (1)
- The saturation hydrogen concentration of X80 steel is 5.42 × 10−5 mol/cm3. Assuming that the steady-state maximum hydrogen diffusion flux approximates the hydrogen diffusion flux during electrochemical hydrogen charging, the hydrogen concentration in the steel reaches saturation after 12.24 h of electrochemical hydrogen charging.
- (2)
- By conducting electrochemical hydrogen concentration tests, the variation in the hydrogen concentration in X80 pipeline steel with the charging time is obtained. The variation follows the equation: CH = 5.35 − 4.2 exp (−0.26t). When the charging time approaches infinity, the maximum hydrogen concentration in X80 steel is 5.35 mol/cm3.
- (3)
- The influence of the hydrogen concentration on the strength of X80 steel is minimal, but the plasticity decreases sharply with an increasing hydrogen concentration. The relationship between the hydrogen concentration and the HE index in X80 pipeline steel follows the formula . According to the formula, when the hydrogen concentration in the steel reaches saturation, the hydrogen embrittlement index of the sample is 88.6%.
- (4)
- The concentration of hydrogen atoms has little effect on the strength of steel, but it has a significant effect on the plasticity of steel. The elongation at break of steel decreases sharply with a gradual increase in the hydrogen concentration in the steel.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Veziroglu, T.N.; Sahin, S. 21st Century’s energy: Hydrogen energy system. Energy Convers. Manag. 2008, 49, 1820–1831. [Google Scholar] [CrossRef]
- Witkowski, A.; Rusin, A.; Majkut, M.; Stolecka, K. Analysis of compression and transport of the methane/hydrogen mixture in existing natural gas pipelines. Int. J. Press. Vessel. Pip. 2018, 166, 24–34. [Google Scholar]
- Edwards, R.L.; Font-Palma, C.; Howe, J. The status of hydrogen technologies in the UK: A multi-disciplinary review. Sustain. Energy Technol. 2021, 43, 100901. [Google Scholar] [CrossRef]
- Isaac, T. HyDeploy: The UK’s First Hydrogen Blending Deployment Project. Clean Energy 2019, 3, 114–125. [Google Scholar] [CrossRef]
- Cristello, J.B.; Yang, J.M.; Hugo, R.; Lee, Y.S.; Park, S.S. Feasibility analysis of blending hydrogen into natural gas networks. Int. J. Hydrogen Energy 2023, 48, 17605–17629. [Google Scholar] [CrossRef]
- Zapffe, C.A.; Sims, C.E. Hydrogen embrittlement, internal stress and defects in steel. Trans. Metall. Soc. AIME 1941, 145, 225–261. [Google Scholar]
- Dwivedi, S.K.; Vishwakarma, M. Effect of hydrogen in advanced high strength steel materials. Int. J. Hydrogen Energy 2019, 44, 28007–28030. [Google Scholar] [CrossRef]
- Oriani, R. Mechanistic Theory of Hydrogen Embrittlement of Steels. Berichte Bunsen-Gesellscjaft Phys. Chenue 1972, 76, 848. [Google Scholar] [CrossRef]
- Wan, L.; Geng, W.T.; Ishii, A.; Du, J.P.; Mei, Q.S.; Ishikawa, N.; Kimizuka, H.; Qgata, S. Hydrogen embrittlement controlled by reaction of dislocation with grain boundary in alpha-iron. Int. J. Plast. 2019, 112, 206–219. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Tao, X.; Antonov, S.; Zhong, Y.; Su, Y.J. Hydrogen embrittlement behavior of 13Cr-5Ni-2Mo supermartensitic stainless steel. Corrosion. Sci. 2020, 176, 109046. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Liu, C.; Hu, Q.; Zhang, R.; Xu, X.; Yang, H.; Ning, Y.; Li, Y. Study on hydrogen embrittlement susceptibility of X80 steel through in-situ gaseous hydrogen permeation and slow strain rate tensile tests. Int. J. Hydrogen Energy 2023, 48, 243–256. [Google Scholar] [CrossRef]
- Bae, D.-S.; Baek, U.-B.; Nahm, S.-H.; Jo, I. Effect of Electrochemical Hydrogen Charging Time on Hydrogen Embrittlement of the Hot-Rolled and Accelerated Cooling Treated API X70 Steel. Met. Mater. Int. 2022, 28, 466–474. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Zhang, X.; Zhang, T.; Tang, J.; Sun, Z.; Sui, Y.; Han, X. Irreversible hydrogen embrittlement study of B1500HS high strength boron steel. Mater. Design 2021, 199, 109404. [Google Scholar] [CrossRef]
- Hu, S.; Tian, Z.; Wang, Y.; Hu, H.; Li, X.; Liu, Q.; Liu, D.; Li, Y.; Cheng, G. Combined impact of elastic stress, prestrain and electrochemical charging on the hydrogen-induced cracking of high-strength steel. Int. J. Hydrogen Energy 2023, 48, 14923–14929. [Google Scholar] [CrossRef]
- Zhou, C.; Ye, B.; Song, Y.; Cui, T.; Xu, P.; Zhang, L. Effects of internal hydrogen and surface-absorbed hydrogen on the hydrogen embrittlement of X80 pipeline steel. Int. J. Hydrogen Energy 2019, 44, 22547–22558. [Google Scholar] [CrossRef]
- Cheng, W.; Song, B.; Mao, J. Effect of Ce content on the hydrogen induced cracking of X80 pipeline steel. Int. J. Hydrogen Energy 2023, 48, 15303–15316. [Google Scholar] [CrossRef]
- Wu, C.; Yan, C.; Zhang, S.; Zhou, L.; Shen, M.; Tian, Z. Research on Hydrogen-Induced Induced Cracking Sensitivity of X80 Pipeline Steel under Different Heat Treatments. Materials 2024, 17, 1953. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, C.; Wang, D.; Di, X. Effect of cyclic plastic deformation on hydrogen diffusion behavior and embrittlement susceptibility of reeling-pipeline steel weldments. Int. J. Hydrogen Energy 2021, 46, 30158–30172. [Google Scholar] [CrossRef]
- Yin, C.; Chen, J.; Ye, D.; Xu, Z.; Zhou, H. Hydrogen Concentration Distribution in 2.25Cr-1Mo-0.25V Steel under the Electrochemical Hydrogen Charging and Its Influence on the Mechanical Properties. Materials 2020, 13, 2263. [Google Scholar] [CrossRef]
- Bellemare, J.; Laliberte-Riverin, S.; Menard, D.; Brochu, M.; Sirois, F. Subtleties Behind Hydrogen Embrittlement of Cadmium-Plated 4340 Steel Revealed by Thermal Desorption Spectroscopy and Sustained-Load Tests. Metall. Mater Trans. A 2020, 51, 3054–3065. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lu, H.; Liang, J.; Rosenthal, A.; Liu, H.; Sneddon, G.; McCarroll, I.; Zhao, Z.; Li, W.; Guo, A.M.; et al. Observation of hydrogen trapping at dislocations, grain boundaries, and precipitates. Science 2020, 367, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, T.; Zhao, Y.; Sun, J.; Wang, Y. Hydrogen permeation and embrittlement susceptibility of X80 welded joint under high-pressure coal gas environment. Corrosion. Sci. 2016, 111, 84–97. [Google Scholar] [CrossRef]
- Konert, F.; Wieder, F.; Nietzke, J.; Meinel, D.; Bollinghaus, T.; Sobol, O. Evaluation of the impact of gaseous hydrogen on pipeline steels utilizing hollow sample technique and μCT. Int. J. Hydrogen Energy 2024, 59, 874–879. [Google Scholar] [CrossRef]
- Yadav, S.; Cabezas, J.F.A.; Davani, R.K.Z.; Szpunar, J.; Zhang, J.M. Synergistic effect of different testing environments on the hydrogen-induced mechanical degradation to establish the role of microstructural features on the failure of X70 pipeline steel. Int. J. Hydrogen Energy 2023, 48, 36866–36882. [Google Scholar] [CrossRef]
- Aiello, F.; Beghini, M.; Belardini, C.M.; Bertini, L.; Macoretta, G.; Monelli, B.D.; Valentini, R. Proposal of a hydrogen embrittlement index for a martensitic advanced high-strength steel. Corrosion. Sci. 2023, 222, 111357. [Google Scholar] [CrossRef]
- Zheng, S.; Qin, Y.; Li, W.; Huang, F.; Qiang, Y.; Yang, S.; Wen, L.; Jin, Y. Effect of hydrogen traps on hydrogen permeation in X80 pipeline steel—A joint experimental and modelling study. Int. J. Hydrogen Energy 2023, 48, 4773–4788. [Google Scholar] [CrossRef]
- Wang, A.; Deng, Y.; Li, K.; Li, W.; Yu, D.; Cui, G.; Liu, J.; Li, Z. Study on hydrogen permeation behavior of X80 steel under AC stray current interference. Anti-Corros. Method Mater. 2021, 68, 64–76. [Google Scholar] [CrossRef]
- Thomas, A.; Szpunar, J.A. Hydrogen diffusion and trapping in X70 pipeline steel. Int. J. Hydrogen Energy 2020, 45, 2390–2404. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, W.; Li, T.; Zhao, Y.; Deng, Q.; Wang, Y.; Jiang, W. Comparison of hydrogen embrittlement susceptibility of three cathodic protected subsea pipeline steels from a point of view of hydrogen permeation. Corrosion. Sci. 2018, 131, 104–115. [Google Scholar] [CrossRef]
- Li, L.; Song, B.; Cheng, J.; Yang, Z.; Cai, Z. Effects of vanadium precipitates on hydrogen trapping efficiency and hydrogen induced cracking resistance in X80 pipeline steel. Int. J. Hydrogen Energy 2018, 43, 17353–17363. [Google Scholar] [CrossRef]
- Zhao, W.; Du, T.; Li, X.; Sun, H.; Li, B.; Yuan, S. Effects of multiple welding thermal cycles on hydrogen permeation parameters of X80 steel. Corrosion Sci. 2021, 192, 109797. [Google Scholar] [CrossRef]
- Capelle, J.; Dmytrakh, I.; Pluvinage, G. Comparative assessment of electrochemical hydrogen absorption by pipeline steels with different strength. Corrosion Sci. 2010, 52, 1554–1559. [Google Scholar] [CrossRef]
- Yan, M.; Weng, Y. Study on hydrogen absorption of pipeline steel under cathodic charging. Corrosion Sci. 2006, 48, 432–444. [Google Scholar] [CrossRef]
- Momotani, Y.; Shibata, A.; Tsuji, N. Hydrogen embrittlement behaviors at different deformation temperatures in as-quenched low-carbon martensitic steel. Int. J. Hydrogen Energy 2022, 47, 3131–3140. [Google Scholar] [CrossRef]
- Shen, L.; Su, Y.; He, J.; Chu, W.; Qiao, L. Hydrogen-induced cracking and hydrogen embrittlement of Ni2MnGa FSMAs. J. Phys. D Appl. Phys. 2010, 43, 055001. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, H.; Liu, C.; Wang, C.; Fan, X.; Cheng, F.; Li, Y. Effects of defect on the hydrogen embrittlement behavior of X80 pipeline steel in hydrogen-blended natural gas environments. Int. J. Hydrogen Energy 2024, 58, 158–173. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Liu, G.; Liu, Y.; Xu, D. Research on the hydrogen assisted fatigue damage in X80 pipeline steel welded joint. Mater. Today Commun. 2022, 31, 103524. [Google Scholar] [CrossRef]
- Park, C.; Kang, N.; Liu, S.; Lee, J.; Chun, E.; Yoo, S.J. Effect of Prestrain on Hydrogen Embrittlement Susceptibility of EH 36 Steels Using In Situ Slow-Strain-Rate Testing. Met. Mater. Int. 2019, 25, 584–593. [Google Scholar] [CrossRef]
- Fangnon, E.; Yagodzinskyy, Y.; Malictki, E.; Mehtonen, S.; Virolainen, E.; Vilacam, P. Determination of Critical Hydrogen Concentration and Its Effect on Mechanical Performance of 2200 MPa and 600 HBW Martensitic Ultra-High-Strength Steel. Metals 2021, 11, 984. [Google Scholar] [CrossRef]
- Bai, P.; Zhou, J.; Luo, B.; Zheng, S.; Wang, P.; Tian, Y. Hydrogen embrittlement of X80 pipeline steel in H2S environment: Effect of hydrogen charging time, hydrogen-trapped state and hydrogen charging-releasing-recharging cycles. Int. J. Min. Met. Mater. 2020, 27, 63–73. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, H.; Zhang, C.; Wang, G.; Lu, S.; Chen, Z.; Zhao, A. Effect of hydrogen charging intensities and times on hydrogen embrittlement of Q&P980 steel. Mater. Res. Express 2024, 11, 016504. [Google Scholar]





| Element | C | Si | Mn | P | S | Ni | Cr | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Content | 0.05 | 0.25 | 1.68 | 0.01 | 0.001 | 0.11 | 0.17 | 0.013 | Balance |
| Group | (mol/(cm2·s)) | D (cm2/s) | C0 (mol/cm3) | NT (cm−3) |
|---|---|---|---|---|
| Exp 1 | 2.88 × 10−9 | 6.61 × 10−6 | 4.52 × 10−5 | 1.66 × 1020 |
| Exp 2 | 2.65 × 10−9 | 5.29 × 10−6 | 5.30 × 10−5 | 2.47 × 1020 |
| Exp 3 | 2.46 × 10−9 | 5.35 × 10−6 | 5.42 × 10−5 | 2.49 × 1020 |
| Average | 2.66 × 10−9 | 5.75 × 10−6 | 5.08 × 10−5 | 2.66 × 1020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Ai, S.; Long, M.; Wan, L.; Li, Y.; Jia, D.; Duan, H.; Chen, D. Quantitative Study on Hydrogen Concentration–Hydrogen Embrittlement Sensitivity of X80 Pipeline Steel Based on Hydrogen Permeation Kinetics. Metals 2024, 14, 763. https://doi.org/10.3390/met14070763
Zhang R, Ai S, Long M, Wan L, Li Y, Jia D, Duan H, Chen D. Quantitative Study on Hydrogen Concentration–Hydrogen Embrittlement Sensitivity of X80 Pipeline Steel Based on Hydrogen Permeation Kinetics. Metals. 2024; 14(7):763. https://doi.org/10.3390/met14070763
Chicago/Turabian StyleZhang, Rundong, Songyuan Ai, Mujun Long, Lihua Wan, Yifan Li, Danbin Jia, Huamei Duan, and Dengfu Chen. 2024. "Quantitative Study on Hydrogen Concentration–Hydrogen Embrittlement Sensitivity of X80 Pipeline Steel Based on Hydrogen Permeation Kinetics" Metals 14, no. 7: 763. https://doi.org/10.3390/met14070763
APA StyleZhang, R., Ai, S., Long, M., Wan, L., Li, Y., Jia, D., Duan, H., & Chen, D. (2024). Quantitative Study on Hydrogen Concentration–Hydrogen Embrittlement Sensitivity of X80 Pipeline Steel Based on Hydrogen Permeation Kinetics. Metals, 14(7), 763. https://doi.org/10.3390/met14070763






