Correlation between Thermodynamic Studies and Experimental Process for Roasting Cobalt-Bearing Pyrite
Abstract
1. Introduction
2. Materials and Methods
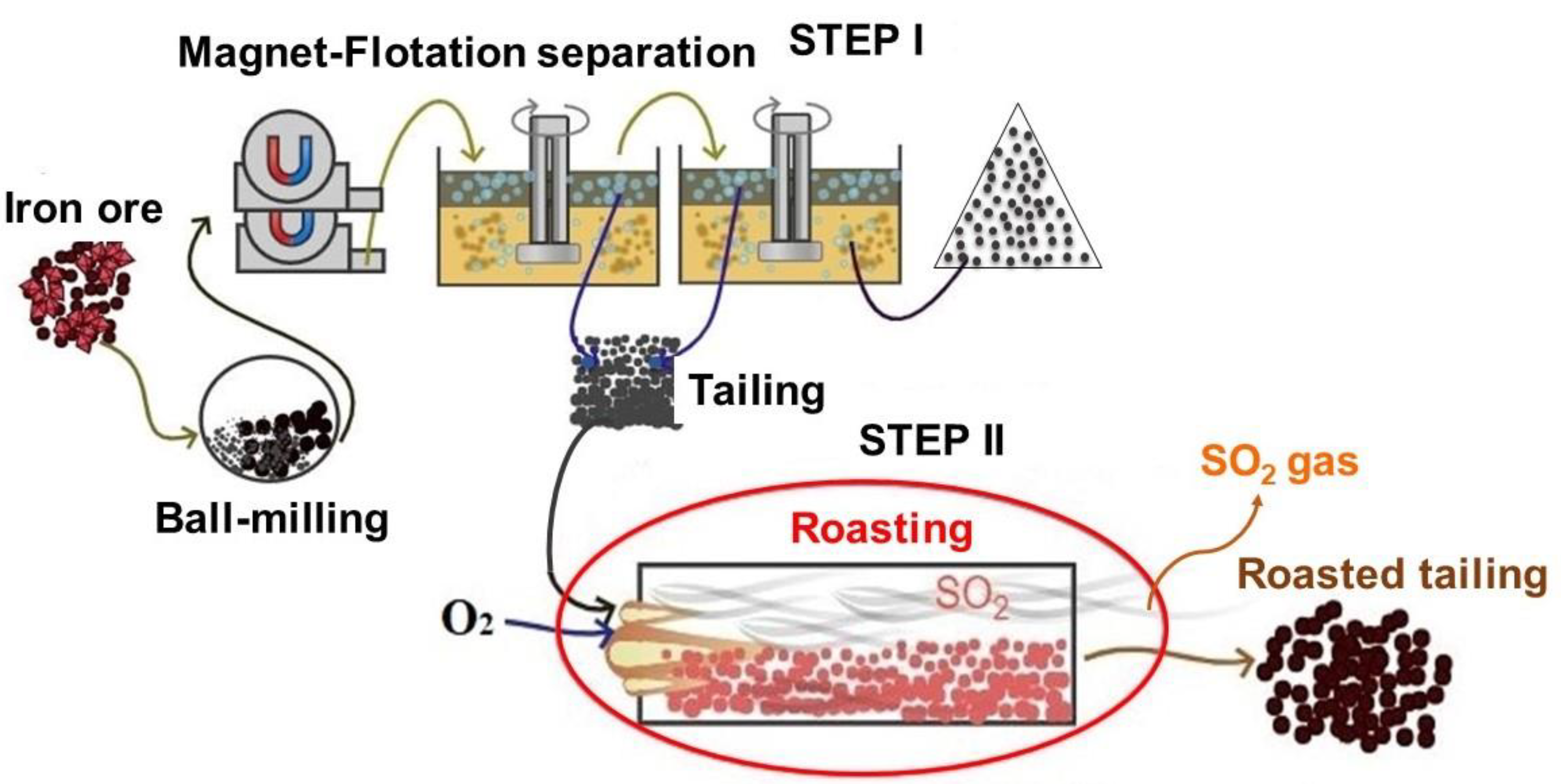
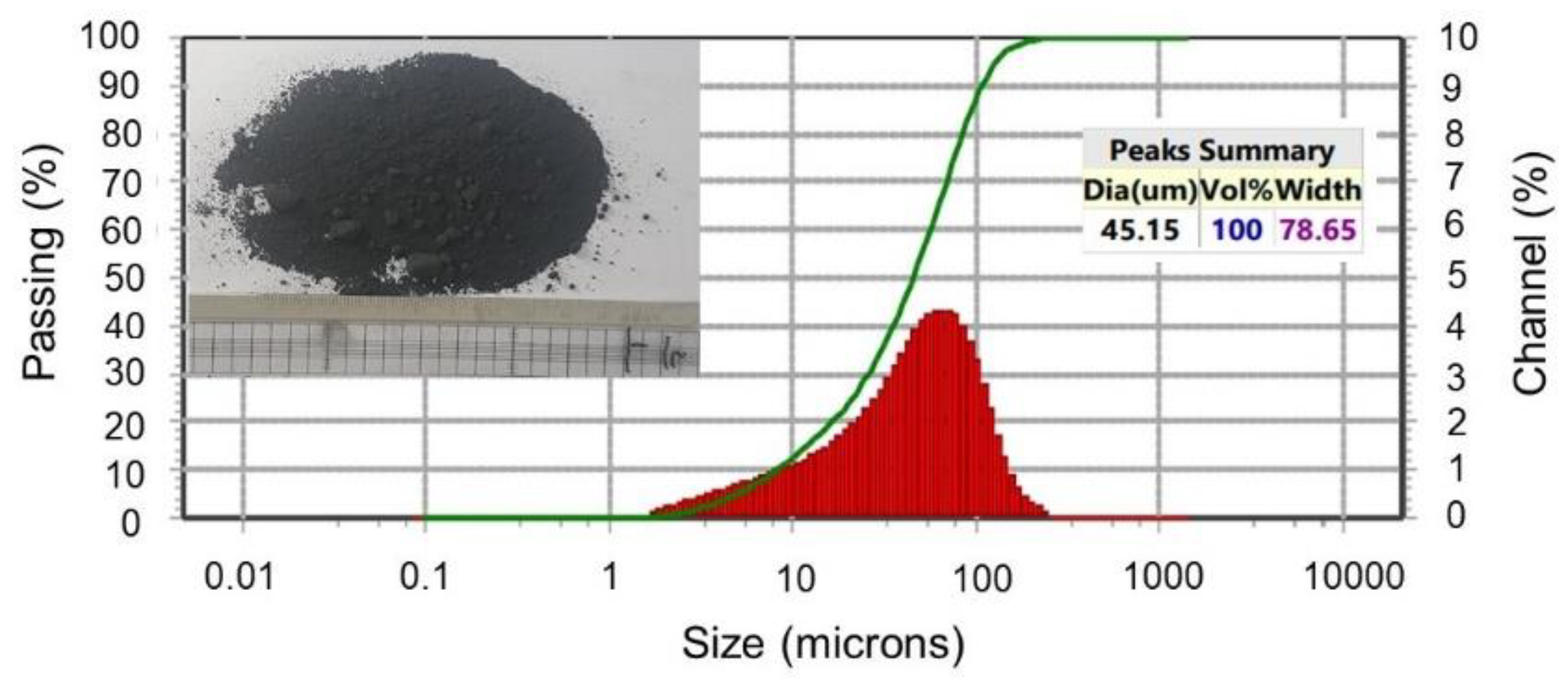
2.1. Experimental Materials
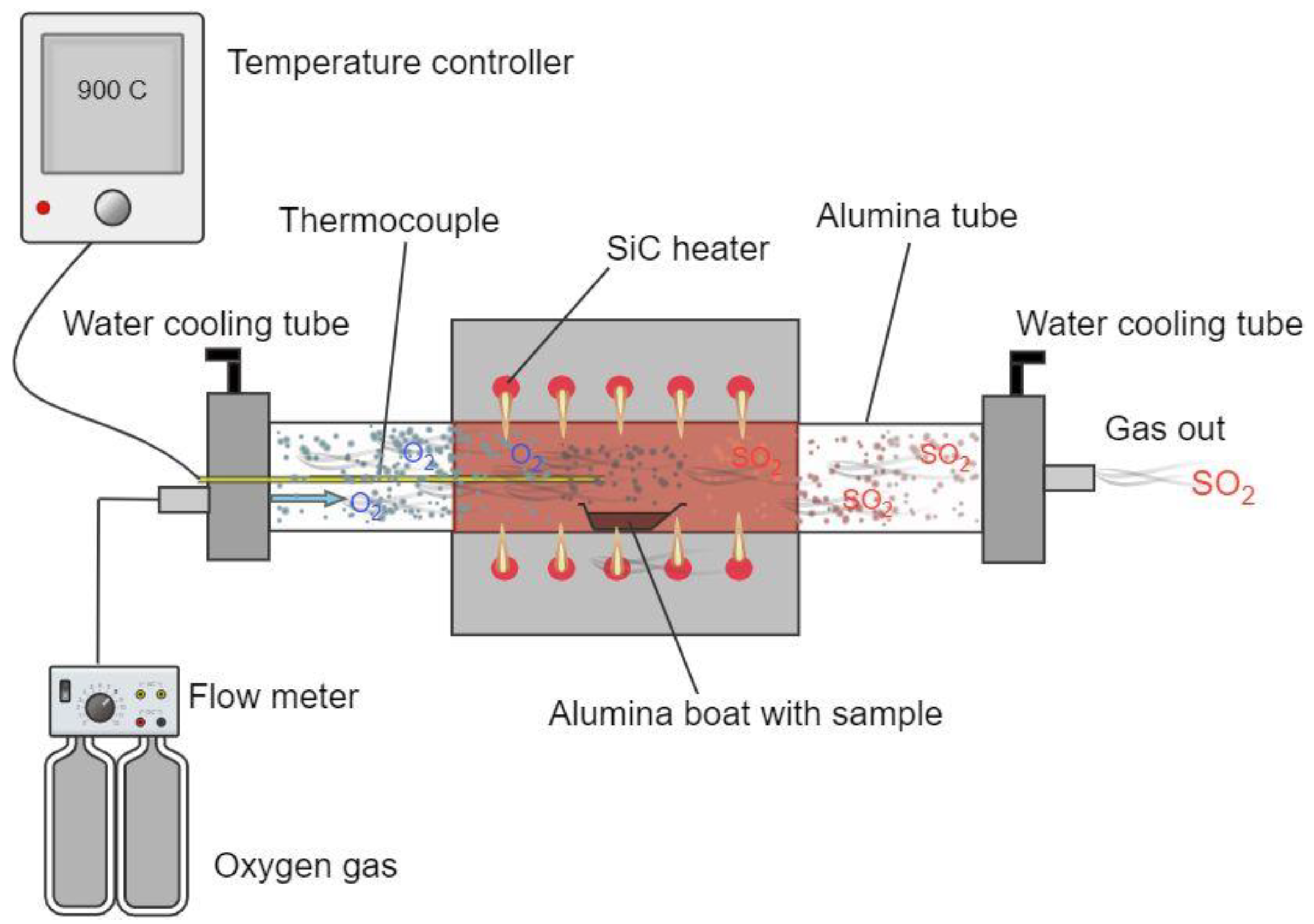
2.2. Experimental Procedures
2.3. Analytical Methods
2.3.1. Thermodynamic Modeling
2.3.2. Calculating the Desulfurization Rate
3. Results and Discussion
3.1. Thermodynamic Modeling for Optimal Roasting
3.2. Efficient Desulfurization during Oxidative Roasting
4. Conclusions
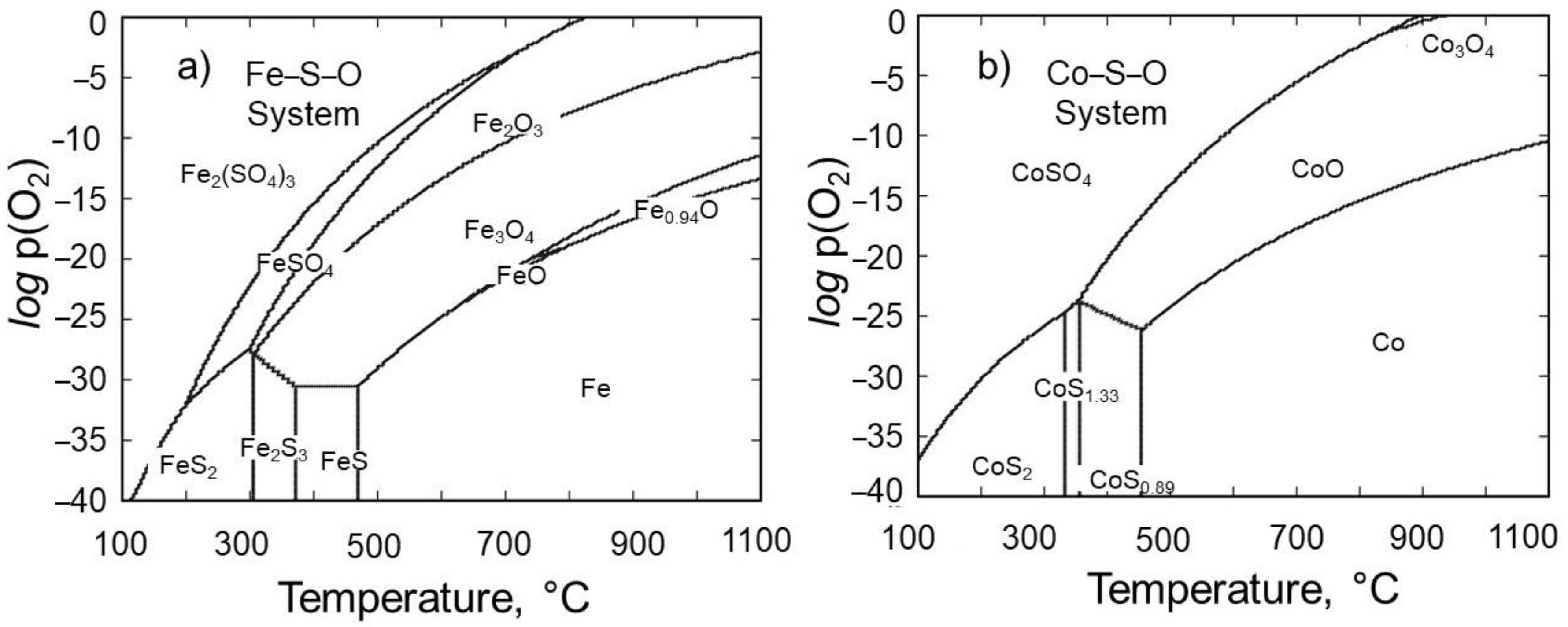
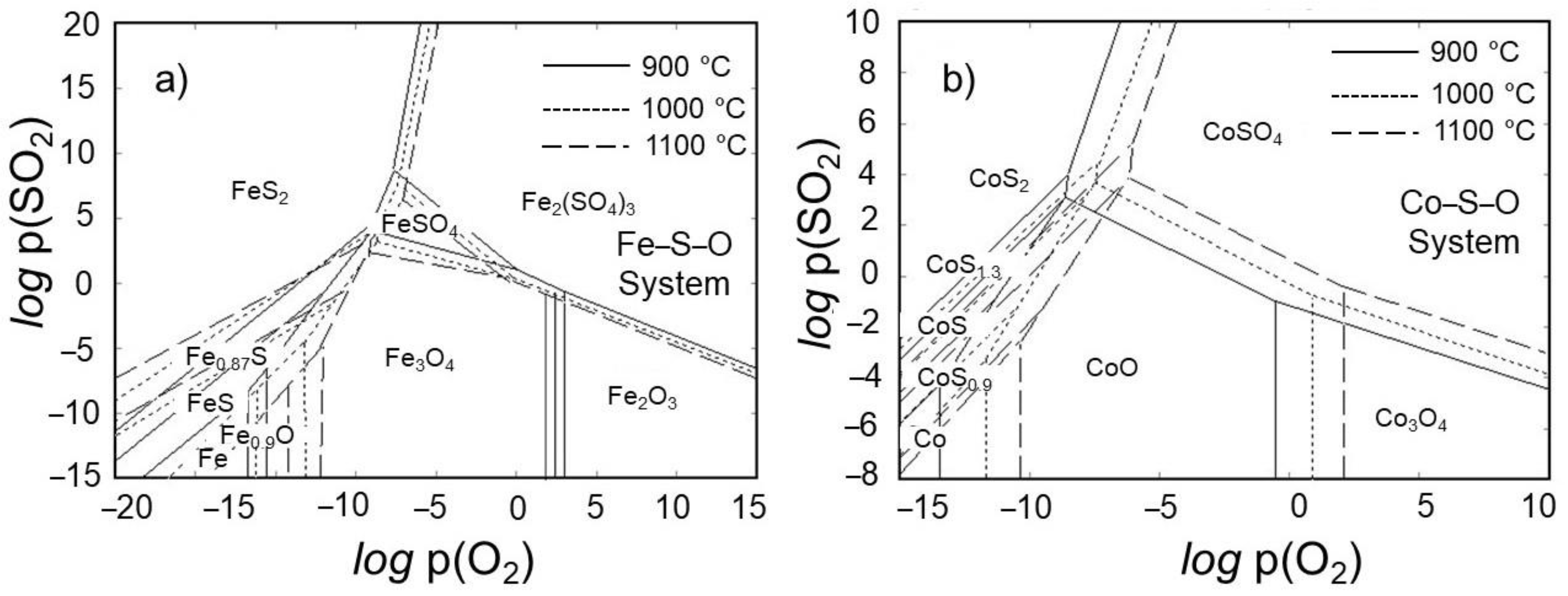
- As a highlight, the poisonous sulfur gas is also eliminated as its oxide during the roasting process, thus allowing a rather environmentally friendly roasting of ore. The thermodynamic modeling in Fe–S–O and Co–S–O systems proved that the iron and cobalt sulfides can be oxidized moderately at relatively high temperatures (>900 °C) under the atmosphere, such as logp(O2) > −5 supplied from outside and logp(SO2) < 0 generated during roasting.
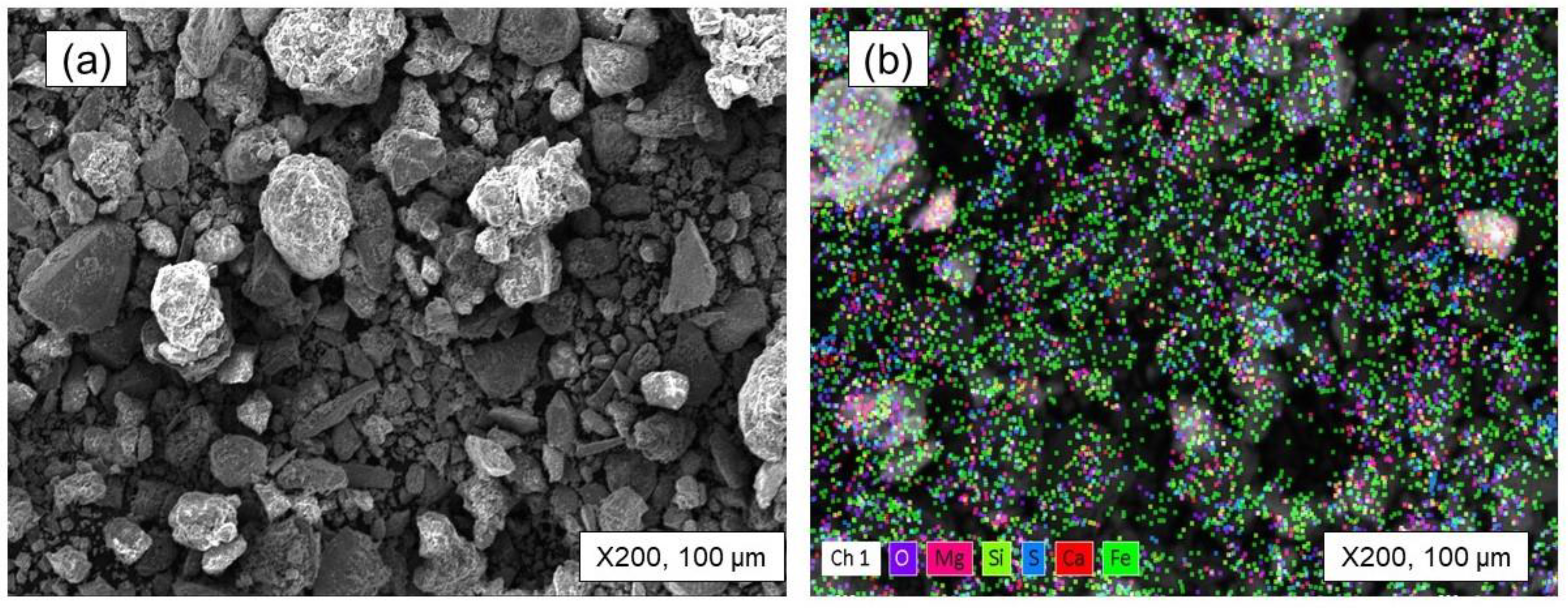
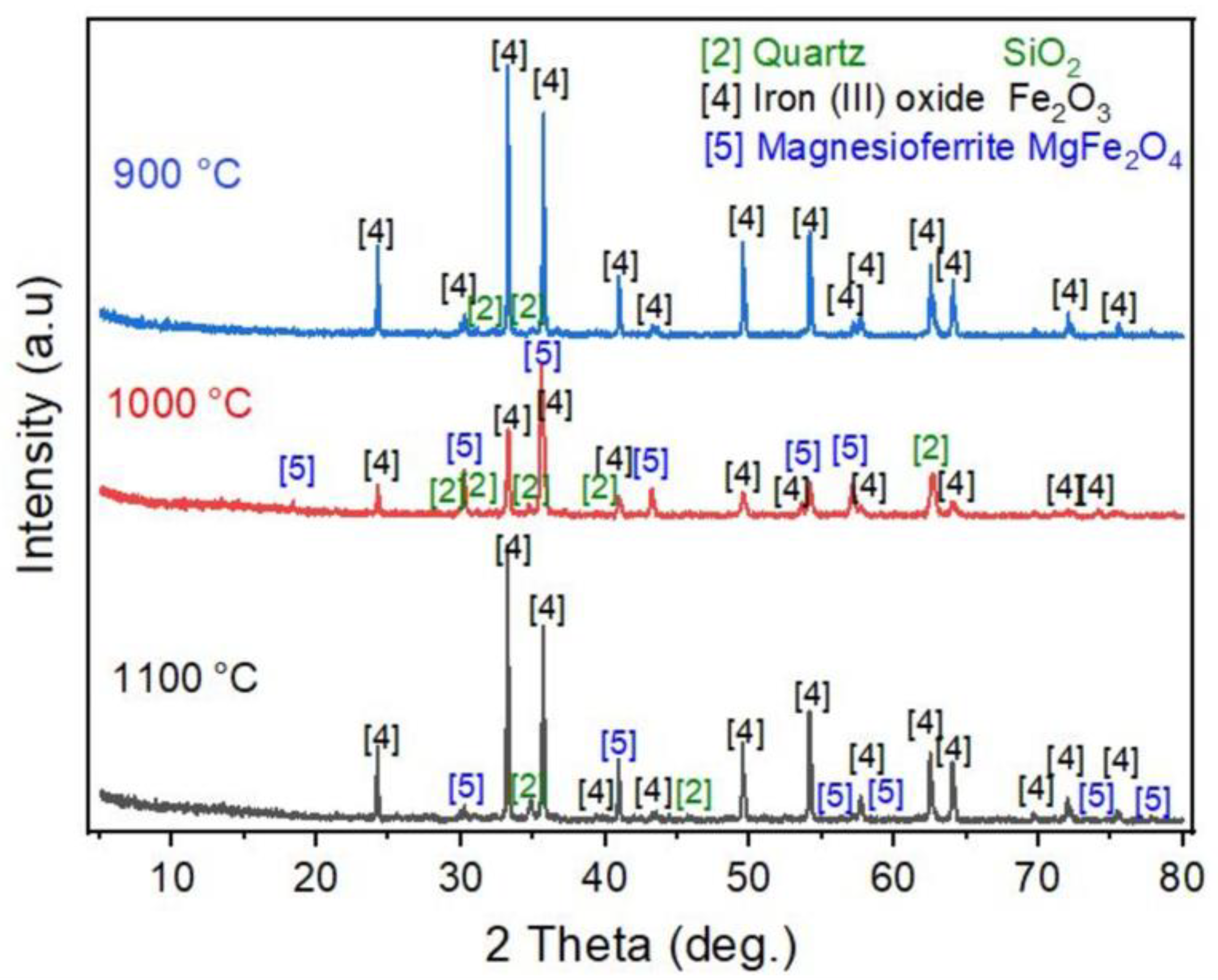
- The equilibrium module was employed in the multi-component system of the tailing to determine the products that are generated during the roasting process. The main target elements in these products are Fe, Co, and S, while impurity elements like Mg and Si are also present. By subjecting iron and cobalt sulfides to roasting within the temperature range of 900 to 1100 °C, it was determined that sulfur can be effectively and entirely eliminated, undergoing transformation into SO2 gas. Furthermore, it should be mentioned that under these temperature conditions, there is a possibility for iron magnetite to undergo a conversion into hematite. Furthermore, it is intriguing to observe that the Fe2O3 that is present in the system can react with MgO, leading to the synthesis of a compound called MgFe2O4. The results of the XRD analysis strongly support the presence of the compound in the roasted tailing, as indicated by the close agreement.
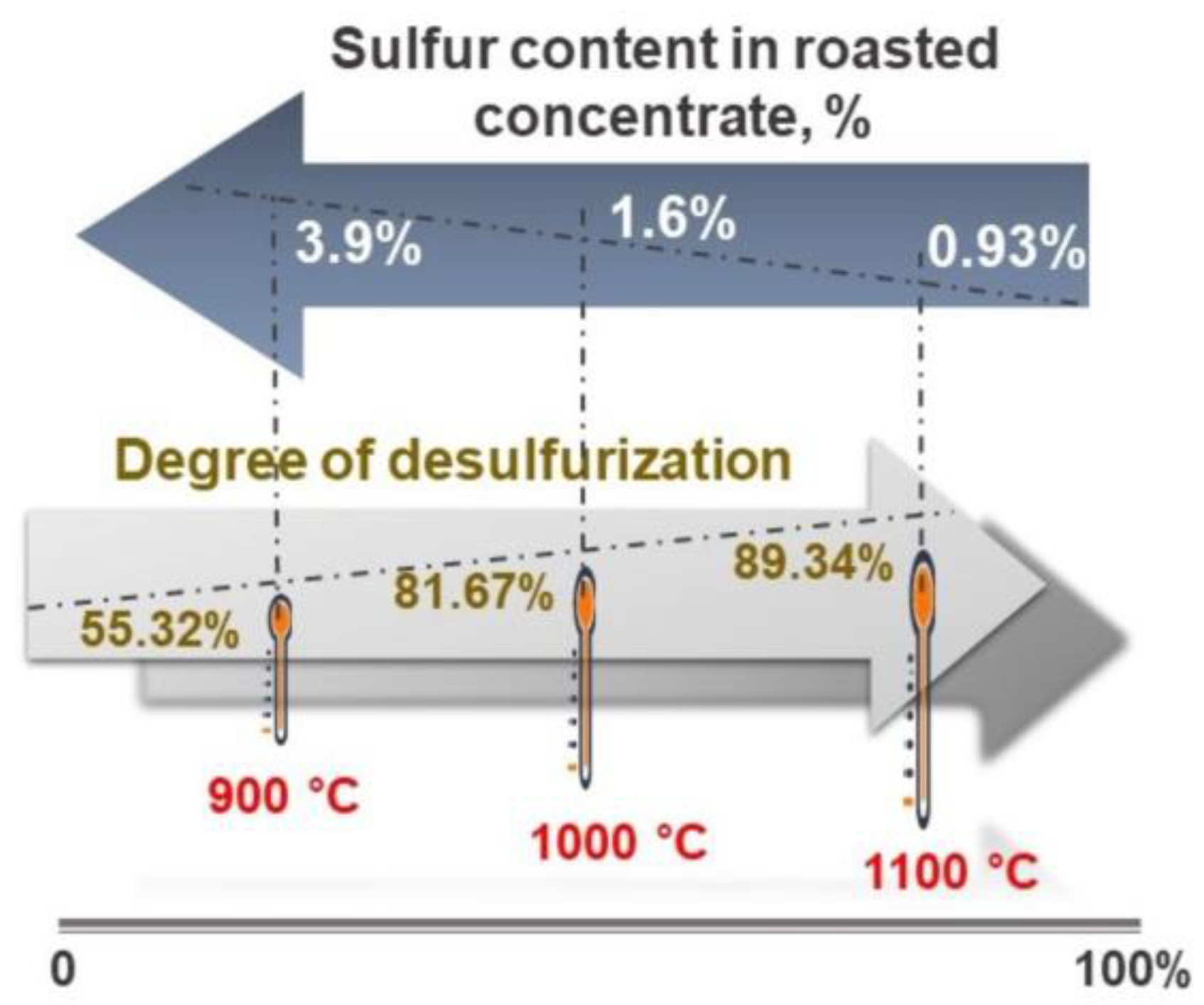
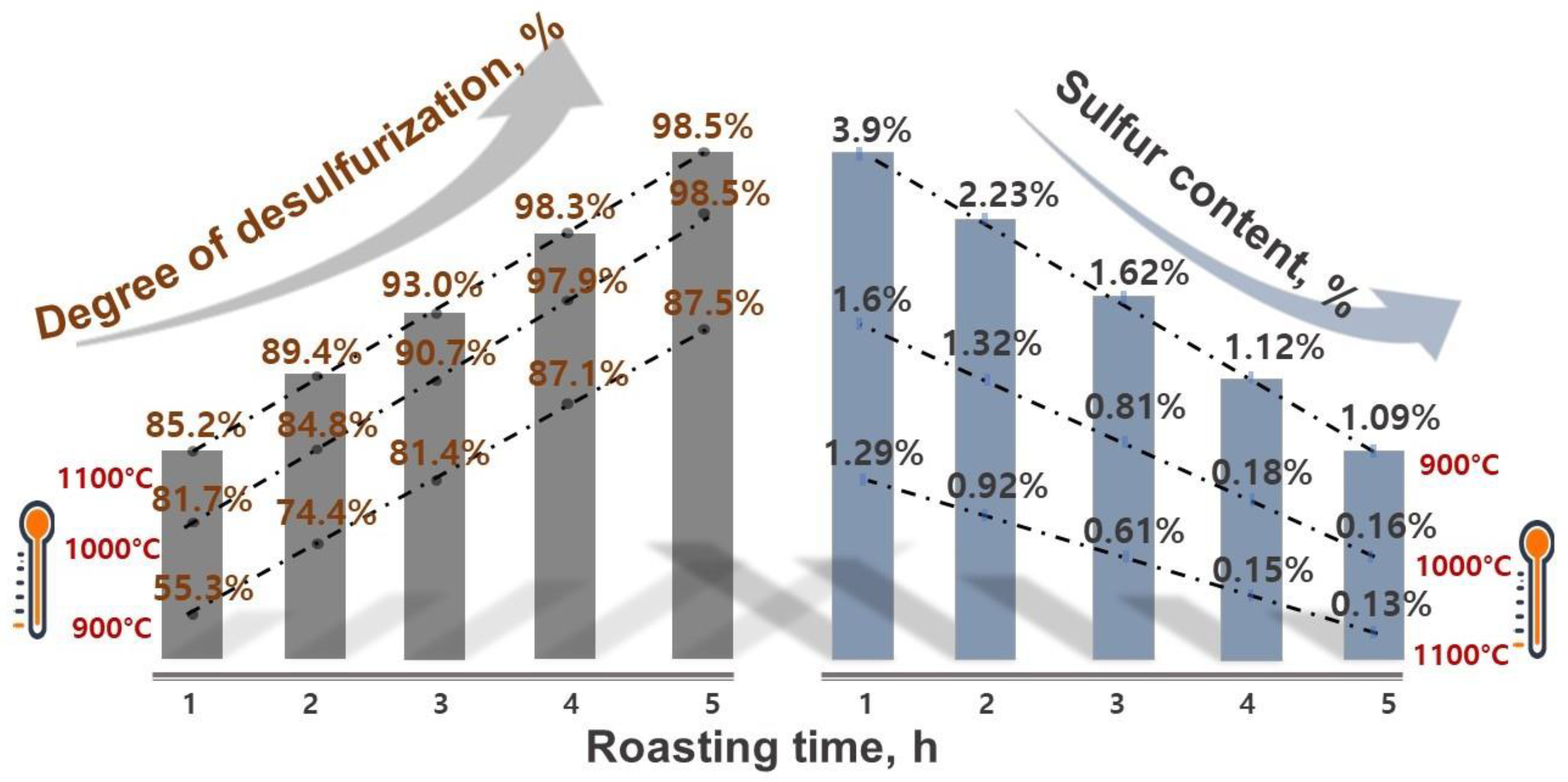
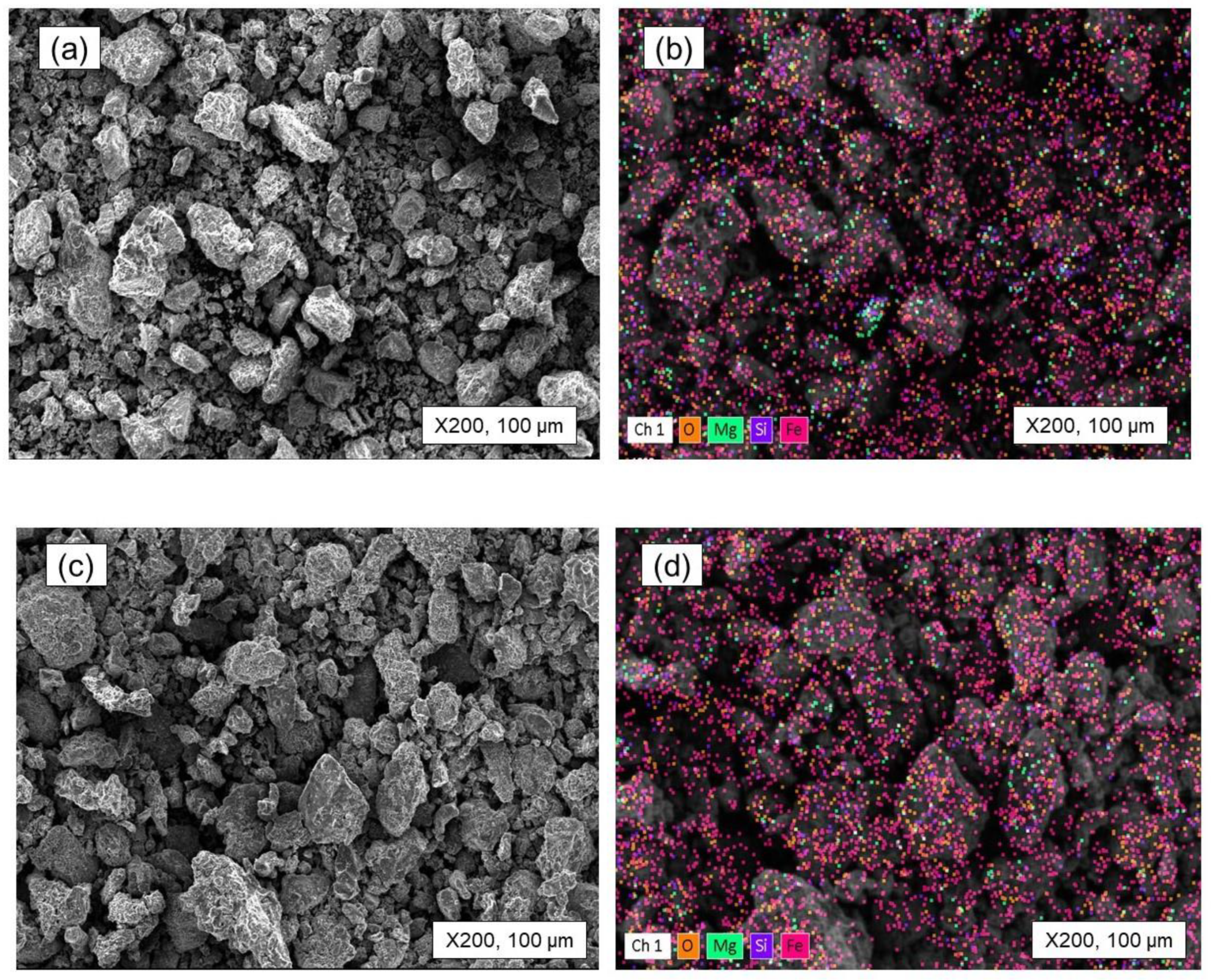
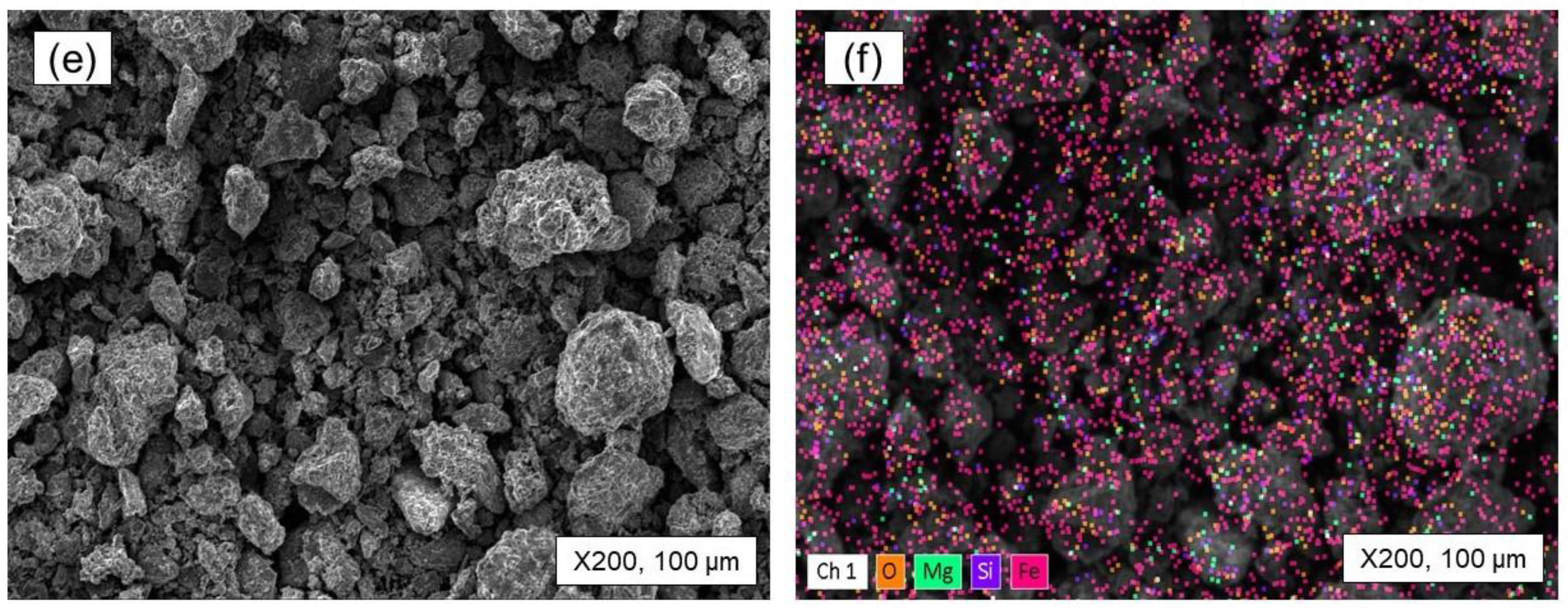
- The roasting experiments performed at 1000 and 1100 °C for 4 and 5 h yielded a 98% degree of desulfurization. The magnetite phases (Fe3O4) in the cobalt-bearing tailing were completely converted to hematite (Fe2O3). The absence of the pyrite phase in the roasted tailing confirms that the oxidation of pyrite was successful, as evident by the XRF and XRD analyses. During the roasting process at 1000 and 1100 °C, the tailing particles underwent a division, resulting in their size being reduced by half. As a result, these particles had an average diameter ranging from 19 to 21 μm, accompanied by a width spanning from 32 to 38 μm. An observation was made that all of the grains trailing behind had a rough surface, suggesting a phase transition of iron oxide and the separation of sulfur from pyrite.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Banu, Y.; Haci, D.; Ersin, Y.Y.; Oktay, C. Extraction of cobalt from a cobaltiferrous pyrite concentrate using H2SO4-NaNO3 lixiviant system. Miner. Eng. 2023, 198, 108077. [Google Scholar] [CrossRef]
- Harry, F. Cobalt Market Report 2022; Cobalt Institute: Guildford, UK, 2023; pp. 6–7. Available online: https://www.cobaltinstitute.org/wp-content/uploads/2023/05/Cobalt-Market-Report-2022_final-1.pdf (accessed on 1 May 2023).
- Ugur, U. Review of the Recovery of Cobalt from Secondary Resources. In Critical and Rare Earth Elements: From Secondary Resources, 1st ed.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- William, E.G.; Grace, M.B.; Kurt, Z.H. The predicted persistence of cobalt in lithium-ion batteries. Nat. Energy 2022, 7, 1132–1142. [Google Scholar] [CrossRef]
- Marcelo, B.M.; Alexandre, S.G.; Martina, P. An Overview on the Recovery of Cobalt from End-of-life Lithium Ion Batteries. Miner. Process. Extr. Metall. 2022, 43, 489–509. [Google Scholar] [CrossRef]
- Shihan, Q.; Daxiong, W.; Yan, D.; Jiaqin, L.; Christopher, W.F.; Colm, O.; Yuezhan, F.; Chuntai, L.; Jianmin, M. Cobalt-based electrode materials for sodium-ion batteries. J. Chem. Eng. 2019, 370, 185–207. [Google Scholar] [CrossRef]
- Lupi, C.; Pasquali, M.; Dell’Era, A. Nickel and cobalt recycling from lithium-ion batteries by electrochemical processes. Waste Manag. 2005, 25, 215–220. [Google Scholar] [CrossRef]
- Rabeeh, G.; Fereshteh, R.; Ehsan, V. Recovery of lithium and cobalt from spent lithium-ion batteries using organic acids: Process optimization and kinetic aspects. Waste Manag. 2017, 64, 244–254. [Google Scholar] [CrossRef]
- Min, Y.; Zehui, Z.; Feng, X.; Bin, Y.; Guanghui, G.; Jianghua, Q. A more simple and efficient process for recovery of cobalt and lithium from spent lithium-ion batteries with citric acid. Sep. Purif. Technol. 2019, 215, 398–402. [Google Scholar] [CrossRef]
- Chandra, M.; Yu, D.; Tian, Q.; Guo, X. Recovery of Cobalt from Secondary Resources: A Comprehensive Review. Miner. Process. Extr. Metall. Rev. 2021, 43, 679–700. [Google Scholar] [CrossRef]
- Yukun, H.; Zhifei, Z.; Yijun, C.; Guihong, H.; Weijun, P.; Xiaofeng, Z.; Ting-an, Z.; Zhihe, D. Overview of cobalt resources and comprehensive analysis of cobalt recovery from zinc plant purification residue- a review. Hydrometallurgy 2020, 193, 105327. [Google Scholar] [CrossRef]
- Burger, M.H.; Schmeda, G.; Long, K.R.; Reyes, T.A.; Karl, N.A. Cobalt Deposits in the United States: U.S. Geological Survey Data Release; U.S. Geological Survey: Reston, VA, USA, 2018. [Google Scholar] [CrossRef]
- Junhui, X.; Yushu, Z. Extraction of Cobalt and Iron from Refractory Co-Bearing Sulfur Concentrate. Processes 2020, 8, 200. [Google Scholar] [CrossRef]
- Shahjadi, H.F.; Nazmul, H.; Parvez Mahmud, M.A. Life cycle assessment of cobalt extraction process. J. Sustain. Min. 2019, 18, 150–161. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Vasyukova, O.V. Constraints on the Genesis of Cobalt Deposits: Part I. Theoretical Considerations. Econ. Geol. 2021, 117, 513–528. [Google Scholar] [CrossRef]
- Quentin, D.; Laurens, T.T.; Hylke, J.G.; Tuomo, T.; Alan, R.B. Geometallurgy of cobalt ores: A review. Miner. Eng. 2021, 160, 106656. [Google Scholar] [CrossRef]
- Mir-Ali, A.M.; Hossein, K.; Kazem, G. Mineral chemistry and formation conditions of calc-silicate minerals of Qozlou Fe skarn deposit, Zanjan Province, NW Iran. Arab. J. Geosci. 2019, 12, 658. [Google Scholar] [CrossRef]
- Mariko, N.; Masahide, A.; Yoji, M. Ore and Skarn Mineralogy of the Yamato Mine, Yamaguchi Prefecture, Japan, with Emphasis on Silver-, Bismuth-, Cobalt-, and Tin-bearing Sulfides. Resour. Geol. 2015, 66, 37–54. [Google Scholar] [CrossRef]
- Huan, X.; Xiaowen, H.; Yumiao, M.; Houmingrui, T.; Liang, Q. Discrimination of Mineralization Types of Skarn Deposits by Magnetite Chemistry. Minerals. 2022, 12, 608. [Google Scholar] [CrossRef]
- Xian, L.; Fang-Yue, W.; Long, Z.; Jun-Wu, Z.; Chang-Shuai, W.; Yu, F.; Xian-Zheng, G.; Tao-Fa, Z.; Ju-Quan, Z.; Qing-Tian, L. Cobalt distribution and enrichment in skarn iron deposits: A case study of the Zhuchong skarn iron deposit, Eastern China. Ore Geol. Rev. 2023, 163, 105778. [Google Scholar] [CrossRef]
- Gerel, O.; Franco, P.; Batkhishig, B.; Jaroslav, D. Mineral Resources of Mongolia; Springer: Singapore, 2021; pp. 247–253. [Google Scholar] [CrossRef]
- Shuo, W.; MingJian, C.; GuangMing, L.; Noreen, J.E.; Wangdui, S.; KeZhang, Q. Tracing the genesis of the Zhibula skarn deposit, Tibet, using Co, Te, Au and Ag occurrence. Ore Geol. Rev. 2023, 160, 105601. [Google Scholar] [CrossRef]
- Shuo, W.; MingJian, C.; GuangMing, L.; Wangdui, S.; PengFei, S.; KeZhang, Q. The occurrence of cobalt and implications for genesis of the Pusangguo cobalt-rich skarn deposit in Gangdese, Tibet. Ore Geol. Rev. 2022, 150, 1051693. [Google Scholar] [CrossRef]
- Peter, L.R.; Feifei, S.; Mohammad, R.; Sarma, V.P. Availability and Estimate of Resources of Cobalt in Pennsylvania. Technical Report. The Pennsylvania State University Center for Critical Minerals. 2020. Available online: https://www.researchgate.net/publication/351094367_Availability_and_Estimate_of_Resources_of_Cobalt_in_Pennsylvania?_tp=eyJjb250ZXh0Ijp7InBhZ2UiOiJwdWJsaWNhdGlvbiIsInByZXZpb3VzUGFnZSI6InB1YmxpY2F0aW9uIn19 (accessed on 1 November 2020).
- Junhui, X.; Yushu, Z. Recovering Cobalt and Sulfur in Low Grade Cobalt-Bearing V–Ti Magnetite Tailings Using Flotation Process. Processes 2019, 8, 536. [Google Scholar] [CrossRef]
- Guobao, C.; Hongying, Y.; Haijun, L.; Linlin, T. Recovery of Cobalt as Cobalt Oxalate from Cobalt Tailings Using Moderately Thermophilic Bioleaching Technology and Selective Sequential Extraction. Minerals 2016, 6, 67. [Google Scholar] [CrossRef]
- Michel, S.; Lutandula, B.M. Recovery of cobalt and copper through reprocessing of tailings from flotation of oxidized ores. J. Environ. Chem. Eng. 2013, 1, 1085–1090. [Google Scholar] [CrossRef]
- Mustafa, O. Cobalt and Copper Recovery from the Ancient Flotation Tailings using Selective Sulfation Roast—Leaching Process. J. Min. Metall. B Metall. 2019, 55, 315–324. [Google Scholar] [CrossRef]
- Harry, R.; Niki, H.; Krystin, R. The quantification and application of handheld energy-dispersive x-ray fluorescence (ED-XRF) in mudrock chemostratigraphy and geochemistry. Chem. Geol. 2012, 324, 122–131. [Google Scholar] [CrossRef]
- Seshadri, S. Fundamentals of Metallurgy; The Institute of Materials, Minerals & Mining: Boca Raton, FL, USA, 2005; ISBN 10: 0-8493-3443-8. [Google Scholar]
- Turkdogan, E.T. Fundamentals of Steelmaking; The Institute of Materials: London, UK, 2010; pp. 13–20. [Google Scholar]
- Tyler, K.; Khagendra, P.B.; Ebin, B.; Bradley, M.M.; Neale, O.H.; Paul, J.R.; Terry, P.B.; Randy, J.E. Majority Carrier Type Control of Cobalt Iron Sulfide (CoxFe1−xS2) Pyrite Nanocrystals. J. Phys. Chem. C 2016, 120, 5706–5713. [Google Scholar] [CrossRef]
- Lehner, S.W.; Savage, K.S.; Ayers, J.C. Vapor growth and characterization of pyrite (FeS2) doped with Co, Ni, and As: Variations in semiconducting properties. J. Cryst. Growth 2006, 286, 306–317. [Google Scholar] [CrossRef]
- Panahi, E.; Abdollahzadeh, A.; Sam, A.; Jahani, M.; Yaghobi, M.M.; Mehrani, A. Desulfurization of Hematitic Concentrate of Iron Ore of the Gol-E-Gohar Mine Using Reverse Flotation Method. Techn. Rep. 2012. [Google Scholar] [CrossRef]
- Jianguo, Y.; Mingrong, H.; Wenqiang, Y.; Juan, A.; Xuejiao, Z.; Wentang, X.; Liwen, H. Roasting Pretreatment of High-Sulfur Bauxite with Low-Median Grade in Chongqing China. In Light Metals 2015; TMS (The Minerals, Metals and Materials Society); Wiley: Hoboken, NJ, USA, 2015; pp. 11–14. [Google Scholar] [CrossRef]
- Yan, Z.; Qian, L.; Xiaoliang, L.; Bin, X.; Yongbin, Y.; Tao, J. A thermodynamic analysis on the roasting of pyrite. Minerals 2019, 9, 220. [Google Scholar] [CrossRef]




| Oxides | Fe2O3 | MgO | SiO2 | Al2O3 | CaO | MnO | TiO2 | SO3 | Co | S |
|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 67.12 | 13.67 | 6.66 | 1.32 | 0.69 | 0.26 | 0.14 | 10.12 | 1.28 | 8.73 |
| Elements | Fe | O | Si | Mg | Ca | S |
|---|---|---|---|---|---|---|
| wt.% | 60.34 | 24.21 | 4.09 | 6.43 | 0.90 | 4.03 |
| Temperature, °C | Element, wt.% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe2O3 | MgO | SiO2 | Al2O3 | CaO | MnO | TiO2 | SO3 | S | |
| 900 | 74.05 | 13.46 | 9.13 | 1.96 | 0.90 | 0.25 | 0.12 | 0.13 | 1.09 |
| 1000 | 78.22 | 10.37 | 8.64 | 1.76 | 0.92 | 0.26 | 0.18 | 0.08 | 0.16 |
| 1100 | 76.42 | 11.37 | 8.71 | 1.65 | 0.99 | 0.29 | 0.14 | - | 0.13 |
| Temperature, °C | Elements, wt.% | ||||
|---|---|---|---|---|---|
| Fe | O | Si | Mg | S | |
| 900 | 75.87 | 14.92 | 3.85 | 5.36 | - |
| 1000 | 78.85 | 16.75 | 2.19 | 2.21 | - |
| 1100 | 78.10 | 14.50 | 3.33 | 4.07 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urtnasan, E.; Kumar, A.; Wang, J.-P. Correlation between Thermodynamic Studies and Experimental Process for Roasting Cobalt-Bearing Pyrite. Metals 2024, 14, 777. https://doi.org/10.3390/met14070777
Urtnasan E, Kumar A, Wang J-P. Correlation between Thermodynamic Studies and Experimental Process for Roasting Cobalt-Bearing Pyrite. Metals. 2024; 14(7):777. https://doi.org/10.3390/met14070777
Chicago/Turabian StyleUrtnasan, Erdenebold, Avneesh Kumar, and Jei-Pil Wang. 2024. "Correlation between Thermodynamic Studies and Experimental Process for Roasting Cobalt-Bearing Pyrite" Metals 14, no. 7: 777. https://doi.org/10.3390/met14070777
APA StyleUrtnasan, E., Kumar, A., & Wang, J.-P. (2024). Correlation between Thermodynamic Studies and Experimental Process for Roasting Cobalt-Bearing Pyrite. Metals, 14(7), 777. https://doi.org/10.3390/met14070777








