Leaching of Rare Earths from End-of-Life NdFeB Magnets with Citric Acid Using Full Factorial Design, Response Surface Methodology, and Artificial Neural Network Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Characterization
2.2. Procedure
2.3. Design of Experiments and RSM
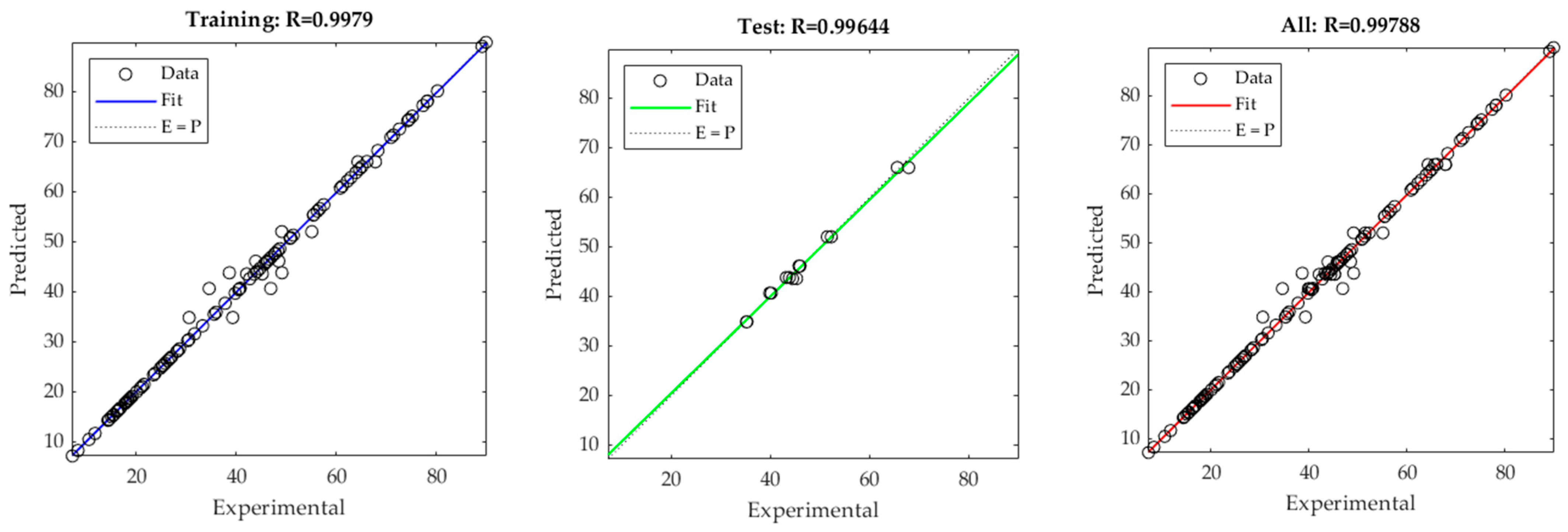
2.4. Artificial Neural Network
3. Results
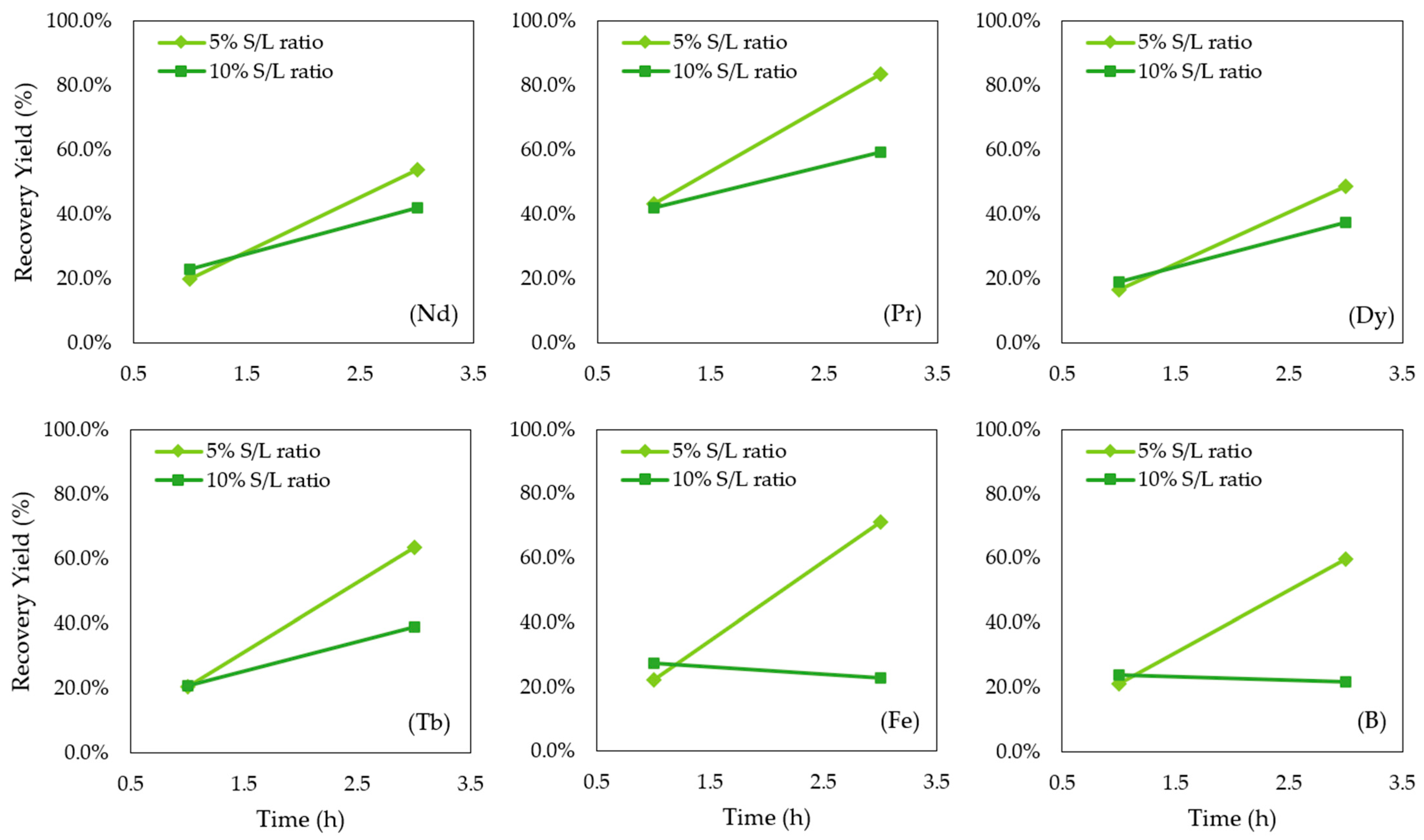
3.1. Leaching Results
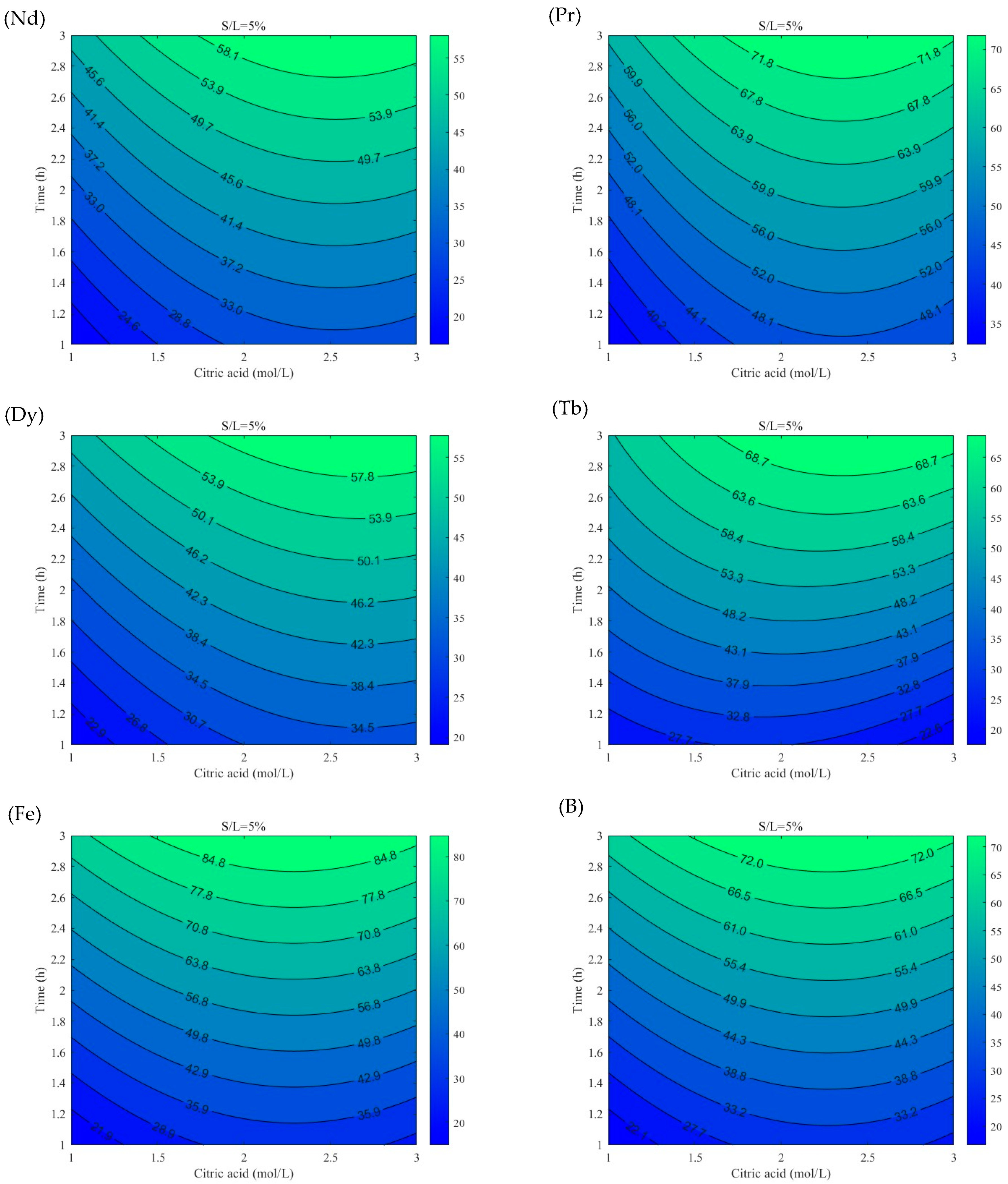
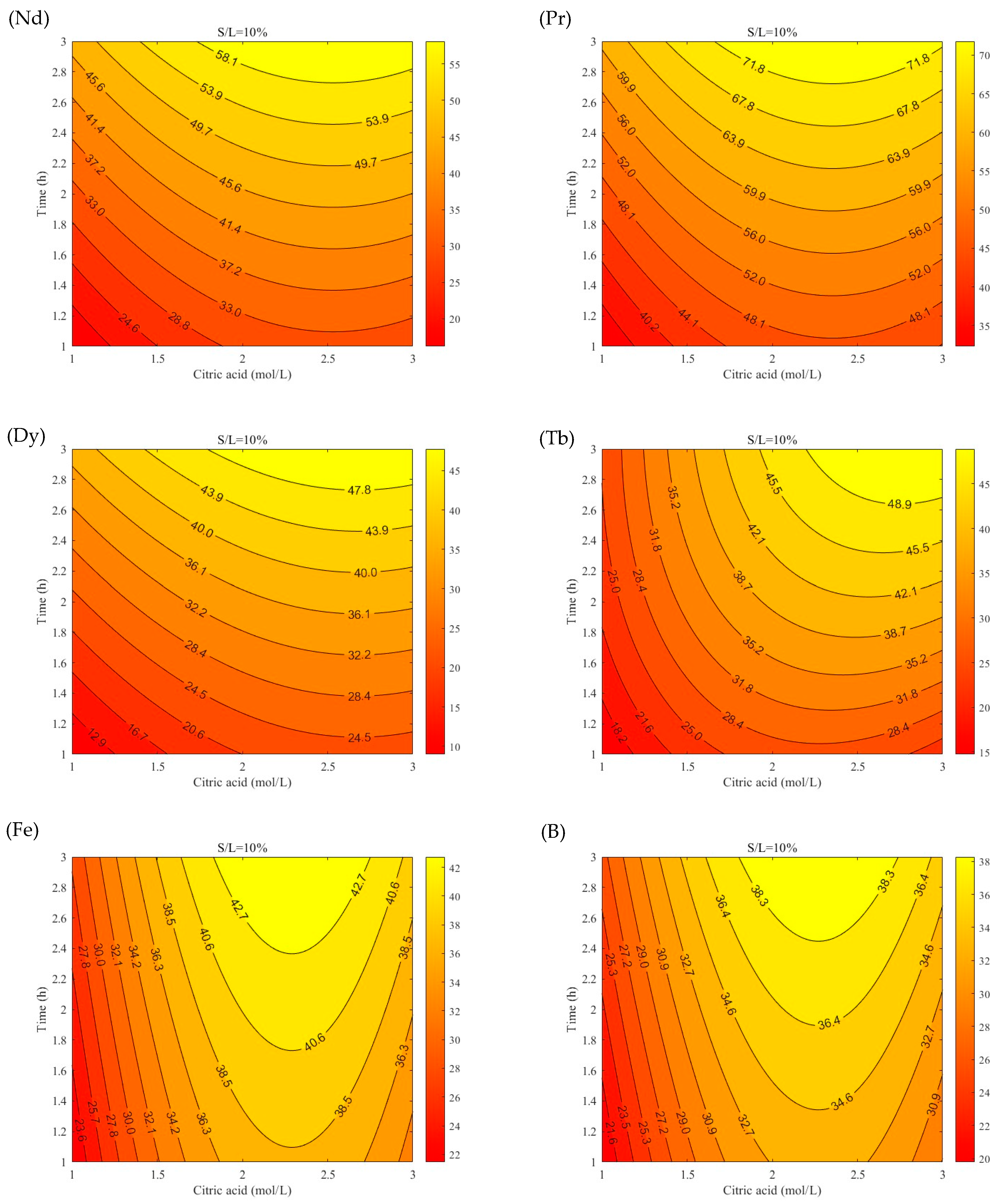
3.2. Leaching Efficiency Models Using RSM
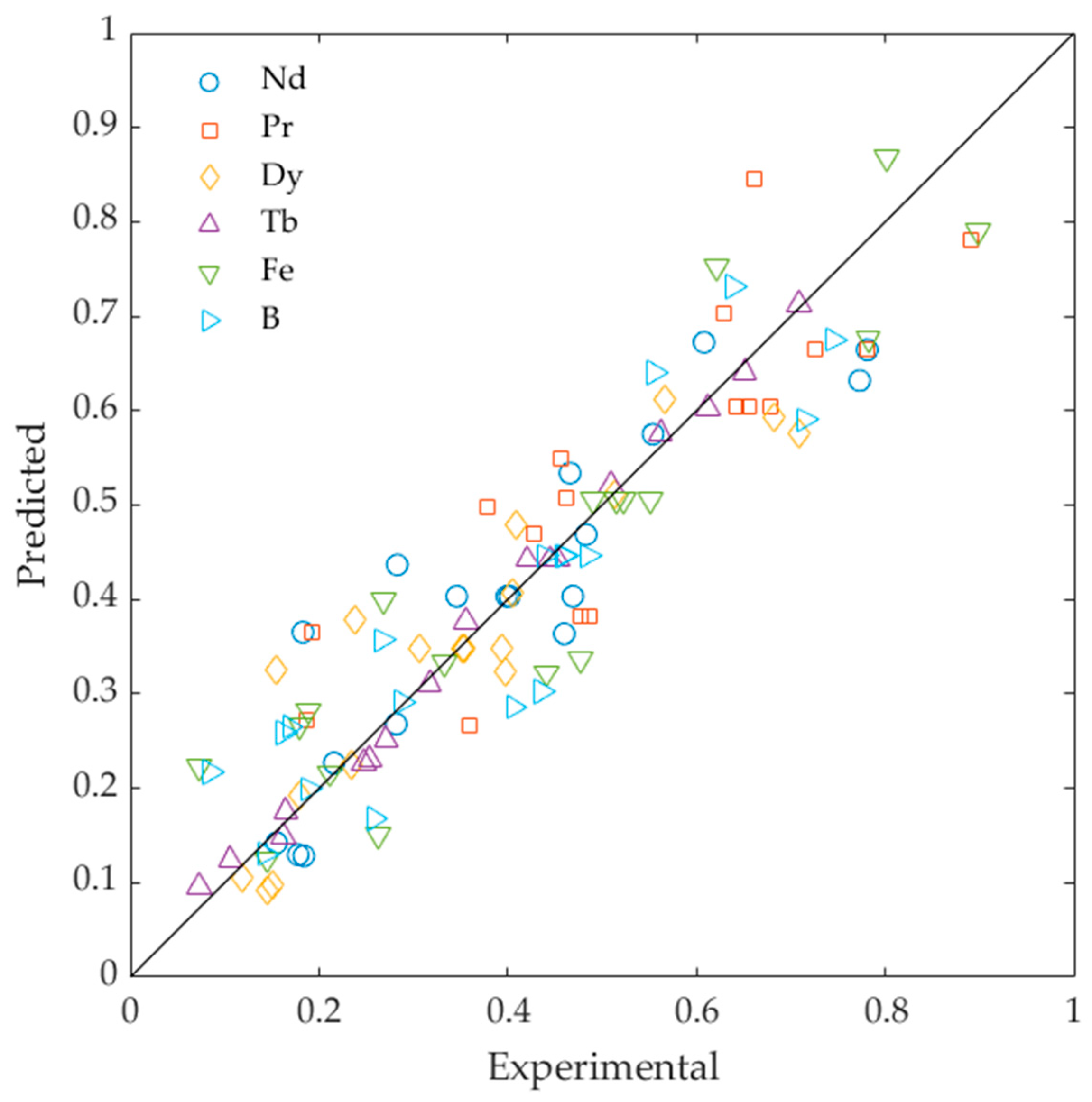
3.3. Artificial Neural Networks
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorzin, H.; Ghaemi, A.; Hemmati, A.; Maleki, A. Equilibrium and kinetics of praseodymium and neodymium extraction from NdFeB magnet-leaching solutions with [R4N+][NO3−] using single drop column. J. Mol. Liq. 2020, 318, 114376. [Google Scholar] [CrossRef]
- Ni, A.C.; Wang, Y.-F.; Chen, S.-W.; You, S.-J. Recovery of rare earth elements from waste permanent magnet (WPMs) via selective leaching using the Taguchi method. J. Taiwan Inst. Chem. Eng. 2019, 97, 137–145. [Google Scholar]
- Gergoric, M.; Ekberg, C.; Foreman, M.R.S.J.; Steenari, B.-M.; Retegan, T. Characterization and leaching of neodymium magnet waste and solvent extraction of the rare-earth elements using TODGA. J. Sustain. Metall. 2017, 3, 638–645. [Google Scholar] [CrossRef]
- Papagianni, S.; Moschovi, A.M.; Sakkas, K.M.; Chalaris, M.; Yakoumis, I. Preprocessing and Leaching Methods for Extraction of REE from Permanent Magnets: A Scoping Review. AppliedChem 2022, 2, 14. [Google Scholar] [CrossRef]
- Belfqueh, S.; Seron, A.; Chapron, S.; Arrachart, G.; Menad, N. Evaluating organic acids as alternative leaching reagents for rare earth elements recovery from NdFeB magnets. J. Rare Earths 2023, 41, 621–631. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Reisdörfer, G.; Bertuol, D.; Tanabe, E.H. Recovery of neodymium from the magnets of hard disk drives using organic acids. Miner. Eng. 2019, 143, 105938. [Google Scholar] [CrossRef]
- Liu, F.; Porvali, A.; Halli, P.; Wilson, B.P.; Lundström, M. Comparison of different leaching media and their effect on REEs recovery from spent Nd-Fe-B magnets. JOM 2020, 72, 806–815. [Google Scholar] [CrossRef]
- Xiao, F.; Hu, W.; Zhao, J.; Zhu, H. Technologies of Recycling REEs and Iron from NdFeB Scrap. Metals 2023, 13, 779. [Google Scholar] [CrossRef]
- Kumari, A.; Sinha, M.K.; Pramanik, S.; Sahu, S.K. Recovery of rare earths from spent NdFeB magnets of wind turbine: Leaching and kinetic aspects. Waste Manag. 2018, 75, 486–498. [Google Scholar] [CrossRef]
- Parhi, P.K.; Misra, P.K.; Jyothi, R.K. Integrated hydrometallurgical investigation for extraction and recovery of neodymium (Nd) from waste permanent Nd-Fe-B magnet. Inorg. Chem. Commun. 2023, 148, 110251. [Google Scholar] [CrossRef]
- Klemettinen, A.; Adamski, Z.; Chojnacka, I.; Leśniewicz, A.; Rycerz, L. Recovery of Rare Earth Elements from the Leaching Solutions of Spent NdFeB Permanent Magnets by Selective Precipitation of Rare Earth Oxalates. Minerals 2023, 13, 846. [Google Scholar] [CrossRef]
- Wu, R.; Liu, R.; Liu, X.; Zhang, J.; Xue, W.; Yang, Y. P350-N235 synergistic extraction system used for the recovery of Nd (III) from waste NdFeB magnets. Sep. Purif. Technol. 2023, 319, 124042. [Google Scholar] [CrossRef]
- Chung, H.; Prasakti, L.; Stopic, S.R.; Feldhaus, D.; Cvetković, V.S.; Friedrich, B. Recovery of Rare Earth Elements from Spent NdFeB Magnets: Metal Extraction by Molten Salt Electrolysis (Third Part). Metals 2023, 13, 559. [Google Scholar] [CrossRef]
- Wu, J.; Wang, D.; Ye, C.; Wang, Z.; Hu, X. Selective extraction and separation of REEs from NdFeB magnets scrap using co-chlorination and water leaching. Sep. Purif. Technol. 2023, 306, 122452. [Google Scholar] [CrossRef]
- Rahmati, S.; Adavodi, R.; Hosseini, M.R.; Veglio’, F. Efficient Metal Extraction from Dilute Solutions: A Review of Novel Selective Separation Methods and Their Applications. Metals 2024, 14, 605. [Google Scholar] [CrossRef]
- Emil-Kaya, E.; Polat, B.; Stopic, S.; Gürmen, S.; Friedrich, B. Recycling of NdFeB magnets employing oxidation, selective leaching, and iron precipitation in an autoclave. RSC Adv. 2023, 13, 1320–1332. [Google Scholar] [CrossRef] [PubMed]
- Gergoric, M.; Ravaux, C.; Steenari, B.-M.; Espegren, F.; Retegan, T. Leaching and recovery of rare-earth elements from neodymium magnet waste using organic acids. Metals 2018, 8, 721. [Google Scholar] [CrossRef]
- Rücker, C.; Mahmoud, W.M.; Schwartz, D.; Kümmerer, K. Biodegradation tests of mercaptocarboxylic acids, their esters, related divalent sulfur compounds and mercaptans. Environ. Sci. Pollut. Res. 2018, 25, 18393–18411. [Google Scholar] [CrossRef]
- Erust, C.; Akcil, A.; Tuncuk, A.; Deveci, H.; Yazici, E. A multi-stage process for recovery of neodymium (Nd) and dysprosium (Dy) from spent hard disc drives (HDDs). Miner. Process. Extr. Metall. Rev. 2021, 42, 90–101. [Google Scholar] [CrossRef]
- del Mundo Dacera, D.; Babel, S. Use of citric acid for heavy metals extraction from contaminated sewage sludge for land application. Water Sci. Technol. 2006, 54, 129–135. [Google Scholar] [CrossRef][Green Version]
- Mohanty, C.; Behera, S.; Marandi, B.; Tripathy, S.; Parhi, P.; Sanjay, K. Citric acid mediated leaching kinetics study and comprehensive investigation on extraction of vanadium (V) from the spent catalyst. Sep. Purif. Technol. 2021, 276, 119377. [Google Scholar] [CrossRef]
- Punt, T.; Akdogan, G.; Bradshaw, S.; van Wyk, P. Development of a novel solvent extraction process using citric acid for lithium-ion battery recycling. Miner. Eng. 2021, 173, 107204. [Google Scholar] [CrossRef]
- Behera, S.; Parhi, P. Leaching kinetics study of neodymium from the scrap magnet using acetic acid. Sep. Purif. Technol. 2016, 160, 59–66. [Google Scholar] [CrossRef]
- Birloaga, I.; Vegliò, F. Hydrometallurgical method for the treatment of permanent magnets. IT102018 2018:000005178. WO2019215583A1, 2019. [Google Scholar]
- Romano, P.; Rahmati, S.; Adavodi, R.; Birloaga, I.; Vegliò, F. Leaching of rare earth elements from permanent magnet swarf in citric acid: Effects of acid concentration on extraction kinetics. Metals 2023, 13, 1801. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Diwa, R.R.; Tabora, E.U.; Haneklaus, N.H.; Ramirez, J.D. Rare earths leaching from Philippine phosphogypsum using Taguchi method, regression, and artificial neural network analysis. J. Mater. Cycles Waste Manag. 2023, 25, 3316–3330. [Google Scholar] [CrossRef]
- Hoseinian, F.S.; Abdollahzade, A.; Mohamadi, S.S.; Hashemzadeh, M. Recovery prediction of copper oxide ore column leaching by hybrid neural genetic algorithm. Trans. Nonferrous Met. Soc. China 2017, 27, 686–693. [Google Scholar] [CrossRef]
- Kasongo, K.B.; Mwanat, H.-M. Application of Taguchi method and artificial neural network model for the prediction of reductive leaching of cobalt (III) from oxidised low-grade ores. S. Afr. J. Sci. 2021, 117, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Al-Thyabat, S. On the optimization of froth flotation by the use of an artificial neural network. J. China Univ. Min. Technol. 2008, 18, 418–426. [Google Scholar] [CrossRef]
- Zhang, W. Evaluation of susceptibility to hydrogen embrittlement—A rising step load testing method. Mater. Sci. Appl. 2016, 7, 389. [Google Scholar] [CrossRef]
- Habibzadeh, A.; Kucuker, M.A.; Go, M. Review on the parameters of recycling NdFeB magnets via a hydrogenation process. ACS Omega 2023, 8, 17431–17445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yue, Z.; Ma, Y.; He, J.; Li, X.; Gong, W.; Huang, Y. Effect of Nb on microstructure and magnetic decay of sintered NdFeB magnets in NaCl solution. J. Alloys Compd. 2023, 969, 172391. [Google Scholar] [CrossRef]
- Romano, P.; Birloaga, I.; Vegliò, F. Recovery of Platinum and Palladium from Spent Automotive Catalysts: Study of a New Leaching System Using a Complete Factorial Design. Minerals 2023, 13, 479. [Google Scholar] [CrossRef]
- Adavodi, R.; Dini, G. Benzotriazoium Bis (2-Ethylhexyl) Phosphate Ionic Liquid as a Catalyst and Multifunctional Lubricant Additive: Synthesis, Optimization, Characterization, and Tribological Evaluation. Arab. J. Sci. Eng. 2024, 49, 7995–8010. [Google Scholar] [CrossRef]
- Rahmati, S.; Birloaga, I.; Romano, P.; Vegliò, F. Optimization of Rare Earth Magnet Recovery Processes Using Oxalic Acid in Precipitation Stripping: Insights from Experimental Investigation and Statistical Analysis. Heliyon 2024, 10, E34811. [Google Scholar] [CrossRef]
- Majdi, A.; Beiki, M. Evolving neural network using a genetic algorithm for predicting the deformation modulus of rock masses. Int. J. Rock Mech. Min. Sci. 2010, 47, 246–253. [Google Scholar] [CrossRef]


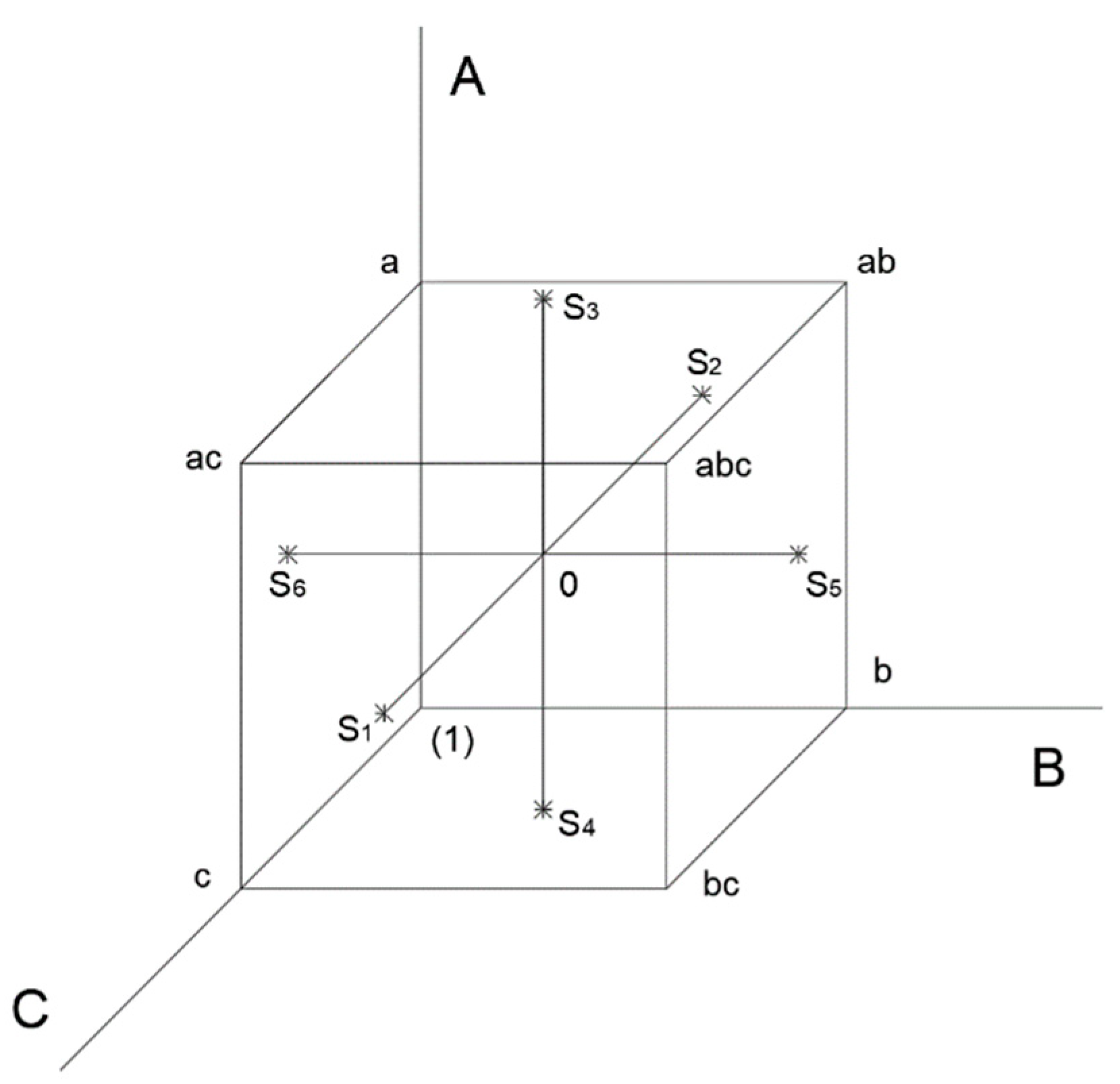
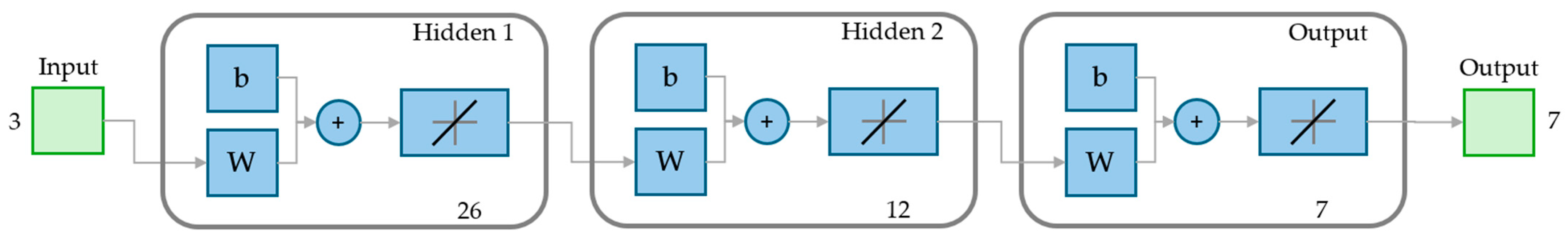
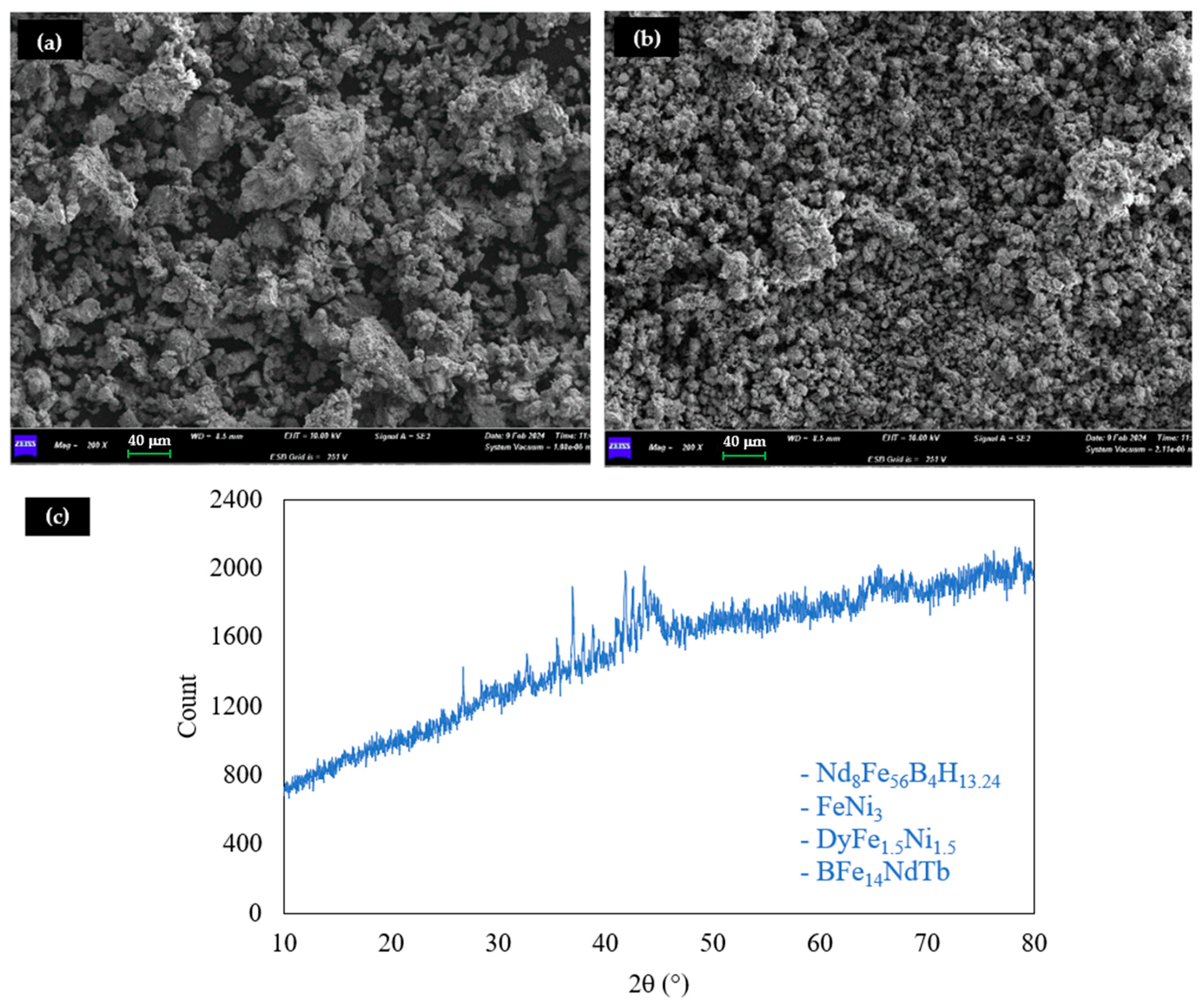
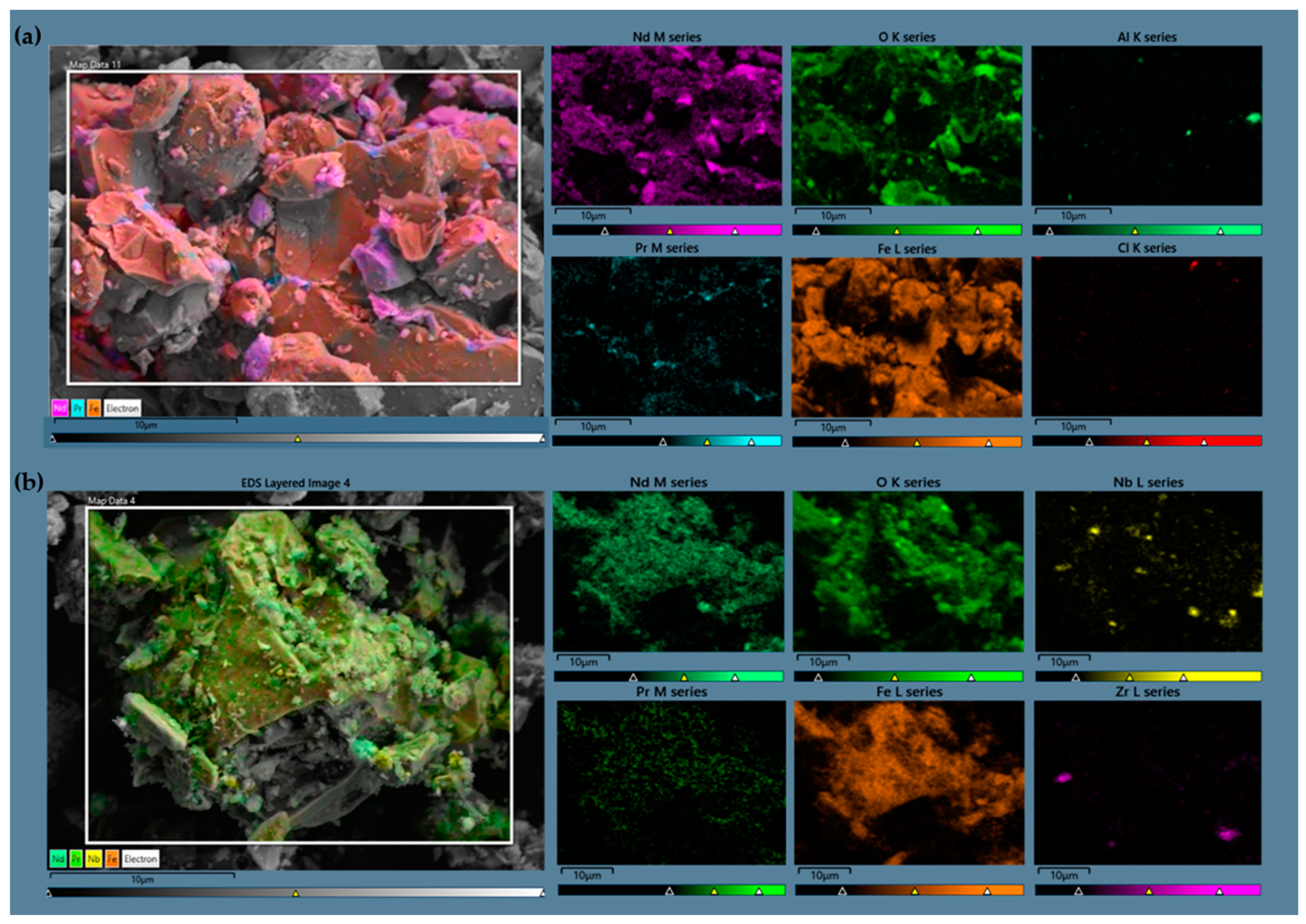


| Element | Fe | Pr | Tb | Dy | Nd | B | Other * |
|---|---|---|---|---|---|---|---|
| Composition (%wt.) | 56.80 ± 1.28 | 4.21 ± 0.14 | 0.33 ± 0.11 | 2.92 ± 0.18 | 21.17 ± 1.36 | 0.91 ± 0.01 | 13.66 ± 1.88 |
| Level | Factors | ||
|---|---|---|---|
| A—Citric Acid Concentration | B—Reaction Time | C—S/L Ratio | |
| Low (−1) | 1 M | 1 h | 5.0% |
| Medium (0) | 2 M | 2 h | 7.5% |
| High (1) | 3 M | 3 h | 10.0% |
| Run | A—Citric Acid | B–Time | C—S/L Ratio | Recovery Yield (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (mol/L) | (h) | (%wt./vol.) | Nd | Pr | Dy | Tb | Fe | B | TREE | |
| 1 | 1 | 1 | 5% | 21.6% | 48.7% | 17.9% | 24.8% | 26.3% | 25.8% | 25.3% |
| a | 3 | 1 | 5% | 18.3% | 37.8% | 15.4% | 16.5% | 18.0% | 16.3% | 20.9% |
| b | 1 | 3 | 5% | 46.6% | 78.1% | 40.8% | 56.2% | 62.2% | 55.5% | 50.8% |
| ab | 3 | 3 | 5% | 60.8% | 89.1% | 56.7% | 70.9% | 80.2% | 63.9% | 64.6% |
| c | 1 | 1 | 10% | 17.8% | 36.0% | 14.6% | 16.2% | 21.2% | 18.9% | 20.1% |
| ac | 3 | 1 | 10% | 28.2% | 47.8% | 23.5% | 25.4% | 33.3% | 28.7% | 30.6% |
| bc | 1 | 3 | 10% | 28.3% | 45.6% | 23.8% | 27.1% | 18.9% | 16.9% | 30.3% |
| abc | 3 | 3 | 10% | 55.4% | 72.6% | 51.4% | 50.9% | 26.8% | 26.7% | 57.5% |
| 0 | 2 | 2 | 7.5% | 40.2% | 67.9% | 35.3% | 45.3% | 51.5% | 45.8% | 43.9% |
| 0 | 2 | 2 | 7.5% | 39.9% | 65.6% | 35.2% | 44.5% | 52.3% | 46.0% | 43.2% |
| 0 | 2 | 2 | 7.5% | 34.6% | 64.3% | 30.6% | 42.1% | 49.1% | 43.9% | 38.6% |
| 0 | 2 | 2 | 7.5% | 46.9% | 67.8% | 39.3% | 45.2% | 55.1% | 48.5% | 49.1% |
| S1 | 2 | 2 | 11.7% | 48.3% | 46.2% | 40.5% | 35.6% | 47.7% | 43.6% | 47.0% |
| S2 | 2 | 2 | 3.3% | 77.3% | 62.9% | 70.9% | 61.2% | 78.2% | 71.4% | 74.3% |
| S3 | 3.7 | 2 | 7.5% | 46.0% | 42.7% | 39.8% | 31.7% | 44.1% | 40.6% | 44.7% |
| S4 | 0.3 | 2 | 7.5% | 18.4% | 18.7% | 15.1% | 10.6% | 14.5% | 14.4% | 18.1% |
| S5 | 2 | 3.7 | 7.5% | 78.1% | 66.1% | 68.3% | 65.1% | 89.9% | 74.5% | 75.2% |
| S6 | 2 | 0.3 | 7.5% | 15.5% | 19.2% | 11.8% | 7.3% | 7.3% | 8.4% | 15.6% |
| Source | SS | df | MS | F-Value | p-Value | R2 | Adj. R2 | |
|---|---|---|---|---|---|---|---|---|
| Nd | Model | 4480.32 | 3 | 1493.44 | 10.99 | 0.0006 | 0.702 | 0.638 |
| A—Citric Acid | 658.3 | 1 | 658.3 | 4.84 | 0.045 | |||
| B—Time | 3243.93 | 1 | 3243.93 | 23.87 | 0.0002 | |||
| A2 | 578.09 | 1 | 578.09 | 4.25 | 0.0582 | |||
| Pr | Model | 4134.52 | 3 | 1378.17 | 8.61 | 0.0017 | 0.648 | 0.573 |
| A—Citric Acid | 460.03 | 1 | 460.03 | 2.87 | 0.1122 | |||
| B—Time | 2755.15 | 1 | 2755.15 | 17.2 | 0.001 | |||
| A2 | 919.33 | 1 | 919.33 | 5.74 | 0.0311 | |||
| Dy | Model | 4577.4 | 5 | 915.48 | 11.33 | 0.0003 | 0.825 | 0.752 |
| A—Citric Acid | 612.24 | 1 | 612.24 | 7.58 | 0.0175 | |||
| B—Time | 2822.18 | 1 | 2822.18 | 34.92 | <0.0001 | |||
| C—S/L ratio | 344.85 | 1 | 344.85 | 4.27 | 0.0611 | |||
| A2 | 322.81 | 1 | 322.81 | 3.99 | 0.0688 | |||
| C2 | 337.13 | 1 | 337.13 | 4.17 | 0.0637 | |||
| Tb | Model | 6095.32 | 9 | 677.26 | 116.41 | <0.0001 | 0.992 | 0.984 |
| A—Citric Acid | 410.63 | 1 | 410.63 | 70.58 | <0.0001 | |||
| B—Time | 3524.95 | 1 | 3524.95 | 605.9 | <0.0001 | |||
| C—S/L ratio | 617.8 | 1 | 617.8 | 106.19 | <0.0001 | |||
| AB | 176.72 | 1 | 176.72 | 30.38 | 0.0006 | |||
| AC | 88.44 | 1 | 88.44 | 15.2 | 0.0046 | |||
| BC | 305.05 | 1 | 305.05 | 52.43 | <0.0001 | |||
| A2 | 788.43 | 1 | 788.43 | 135.52 | <0.0001 | |||
| B2 | 83.81 | 1 | 83.81 | 14.41 | 0.0053 | |||
| C2 | 38.27 | 1 | 38.27 | 6.58 | 0.0334 | |||
| Fe | Model | 8462.6 | 5 | 1692.52 | 13.57 | 0.0001 | 0.850 | 0.787 |
| A—Citric Acid | 462.57 | 1 | 462.57 | 3.71 | 0.0782 | |||
| B—Time | 3813.66 | 1 | 3813.66 | 30.57 | 0.0001 | |||
| C—S/L ratio | 1390.32 | 1 | 1390.32 | 11.14 | 0.0059 | |||
| BC | 1428.45 | 1 | 1428.45 | 11.45 | 0.0054 | |||
| A2 | 1367.6 | 1 | 1367.6 | 10.96 | 0.0062 | |||
| B | Model | 5579.06 | 5 | 1115.81 | 10.89 | 0.0004 | 0.820 | 0.744 |
| A—Citric Acid | 286.61 | 1 | 286.61 | 2.8 | 0.1202 | |||
| B—Time | 2491.63 | 1 | 2491.63 | 24.33 | 0.0003 | |||
| C—S/L ratio | 1003.28 | 1 | 1003.28 | 9.8 | 0.0087 | |||
| BC | 826.21 | 1 | 826.21 | 8.07 | 0.0149 | |||
| A2 | 971.34 | 1 | 971.34 | 9.48 | 0.0095 | |||
| TREE | Model | 4699.06 | 4 | 1174.77 | 17.75 | 0.0001 | 0.809 | 0.750 |
| A—Citric Acid | 617.55 | 1 | 617.55 | 7.23 | 0.0186 | |||
| B—Time | 3123.46 | 1 | 3123.46 | 36.55 | <0.0001 | |||
| C—S/L ratio | 348.75 | 1 | 348.75 | 4.08 | 0.0645 | |||
| A2 | 609.30 | 1 | 609.30 | 7.13 | 0.0193 |
| Pros | Cons | |
|---|---|---|
| Response Surface Methodology |
|
|
| Artificial Neural Network |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, P.; Zuffranieri, A.; Rahmati, S.; Adavodi, R.; Ferella, F.; Vegliò, F. Leaching of Rare Earths from End-of-Life NdFeB Magnets with Citric Acid Using Full Factorial Design, Response Surface Methodology, and Artificial Neural Network Analysis. Metals 2024, 14, 932. https://doi.org/10.3390/met14080932
Romano P, Zuffranieri A, Rahmati S, Adavodi R, Ferella F, Vegliò F. Leaching of Rare Earths from End-of-Life NdFeB Magnets with Citric Acid Using Full Factorial Design, Response Surface Methodology, and Artificial Neural Network Analysis. Metals. 2024; 14(8):932. https://doi.org/10.3390/met14080932
Chicago/Turabian StyleRomano, Pietro, Adriana Zuffranieri, Soroush Rahmati, Roshanak Adavodi, Francesco Ferella, and Francesco Vegliò. 2024. "Leaching of Rare Earths from End-of-Life NdFeB Magnets with Citric Acid Using Full Factorial Design, Response Surface Methodology, and Artificial Neural Network Analysis" Metals 14, no. 8: 932. https://doi.org/10.3390/met14080932
APA StyleRomano, P., Zuffranieri, A., Rahmati, S., Adavodi, R., Ferella, F., & Vegliò, F. (2024). Leaching of Rare Earths from End-of-Life NdFeB Magnets with Citric Acid Using Full Factorial Design, Response Surface Methodology, and Artificial Neural Network Analysis. Metals, 14(8), 932. https://doi.org/10.3390/met14080932









