Experimental Investigations of the AC-Conductivity in NaTaO3 Ceramic Materials Doped with Cu and Al Metal Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization Techniques
3. Results
3.1. X-ray Diffraction Analysis
3.2. Scanning Electron Microscopy
3.3. UV-VIS Spectroscopic Analysis
3.4. Electrical Properties
3.4.1. Complex Impedance
3.4.2. DC and AC Conductivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, C.; Alonso, J.A.; Bian, J. Recent Advances in Perovskite-Type Oxides for Energy Conversion and Storage Applications. Adv. Energy Mater. 2020, 2000459, 21. [Google Scholar] [CrossRef]
- AbuSandal, A.S.; Elhadi, M.M.; Suliman, A.H.; Elbadawi, A.A. Comparative study of electrical and optical properties of SrPrFeTiO6 double perovskite ceramic: A potential candidate for solar cells applications. IOSR J. Appl. Phys. (IOSR-JAP) 2022, 14, 16–24. [Google Scholar]
- Temerov, F.; Baghdadi, Y.; Rattner, E.; Eslava, S. A Review on Halide Perovskite-Based Photocatalysts: Key Factors and Challenges. ACS Appl. Energy Mater. 2022, 5, 14605–14637. [Google Scholar] [CrossRef]
- Bati, A.S.R.; Zhong, Y.L.; Burn, P.L.; Nazeeruddin, M.K.; Shaw, P.E.; Batmunkh, M. Next-generation applications for integrated perovskite solar cells. Commun. Mater. 2023, 4, 2. [Google Scholar] [CrossRef]
- Deshmukh, V.; Harini, H.; Nagaswarupa, H.; Naik, R.; Ravikumar, C. Development of novel Co3+ doped LaMnO3 perovskite electrodes for supercapacitors and sensors: Mechanism of electrochemical energy storage and oxygen intercalation. J. Energy Storage 2023, 68, 107805. [Google Scholar] [CrossRef]
- Park, Y.; Lee, J.-S. Metal Halide Perovskite-Based Memristors for Emerging Memory Applications. J. Phys. Chem. Lett. 2022, 13, 5638–5647. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, C.; Zulfikar, A.J.; Mehrez, S.; Huynen, I.; Mahariq, I.; Elbadawy, I. Tuning layer thickness and layer arrangement in a GdMnO3 and GdMnO3-MoSe2 bi-layer absorber to cover the S, C, and X band frequency range. Surf. Interf. 2023, 36, 102507. [Google Scholar] [CrossRef]
- Singh, C.; Kumar, A. Investigation on ferroelectric, dielectric and pressure sensing properties of samarium substituted lead magnesium niobate-lead titanate oxide. Phys. B Cond. Matter 2023, 659, 414869. [Google Scholar] [CrossRef]
- Hoye, R.L.Z.; Hidalgo, J.; Jagt, R.A.; Correa-Baena, J.-P.; Fix, T.; MacManus-Driscoll, J.L. The Role of Dimensionality on the Optoelectronic Properties of Oxide and Halide Perovskites, and their Halide Derivatives. Adv. Energy Mater. 2022, 12, 2100499. [Google Scholar] [CrossRef]
- Rendón, R.C.; Salvo, C.; Sepúlveda, E.; Arulraj, A.; Sanhueza, F.; Rodríguez, J.J.; Mangalaraja, R.V. Evaluation of Structural and Functional Properties of La0.6Sr0.4MnO3 Perovskite Prepared by the Fast Solution Combustion Approach. Catalysts 2022, 12, 1636. [Google Scholar] [CrossRef]
- Park, Y.; Kim, S.H.; Lee, D.; Lee, J.S. Designing zero-dimensional dimer-type all-inorganic perovskites for ultra-fast switching memory. Nat. Commun. 2021, 12, 3527. [Google Scholar] [CrossRef]
- Choi, J.; Han, J.S.; Hong, K.; Kim, S.Y.; Jang, H.W. Organic–inorganic hybrid halide perovskites for memories, transistors, and artificial synapses. Adv. Mater. 2018, 30, 1704002. [Google Scholar] [CrossRef]
- Kato, H.; Asakura, K.; Kudo, A. Highly Efficient Water Splitting into H2 and O2 over Lanthanum-Doped NaTaO3 Photocatalysts with High Crystallinity and Surface Nanostructure. J. Am. Chem. Soc. 2003, 125, 3082–3089. [Google Scholar] [CrossRef]
- Ghrib, T.; Al-Otaibi, A.; Ercan, F.; Manda, A.A.; Ozcelik, B.; Ercan, I. Structural, optical and photocatalytic properties of cerium doped Ba2TiMoO6 double perovskite. Phys. B Cond. Matter 2023, 649, 414454. [Google Scholar] [CrossRef]
- Boulahouache, A.; Benlembarek, M.; Salhi, N.; Djaballah, A.M.; Rabia, C.; Trari, M. Preparation, characterization and electronic properties of LaFeO3 perovskite as photocatalyst for hydrogen production. Int. J. Hydrogen Energy 2023, 48, 14650–14658. [Google Scholar] [CrossRef]
- Wunderlich, W. NaTaO3 composite ceramics—A new thermoelectric material for energy generation. J. Nuclear Mater. 2009, 389, 57–61. [Google Scholar] [CrossRef]
- Wunderlich, W.; Mori, T.; Sologub, O. SPS-sintered NaTaO3–Fe2O3 composite exhibits enhanced Seebeck coefficient and electric current. Mater. Renew. Sustain. Energy 2014, 3, 1–6. [Google Scholar] [CrossRef]
- Vousden, P. A study of the unit-cell dimensions and symmetry of certain ferroelectric compounds of niobium and tantalum at room temperature. Acta Crystallogr. 1951, 4, 373–376. [Google Scholar] [CrossRef]
- Mohanty, S.; Choudharyn, R.N.P.; Pafhee, R.; Parida, B.N. Dielectric and impedance spectroscopy of BiFeO3-NaTaO3 multiferoics. Ceram. Int. 2014, 40, 9017–9025. [Google Scholar] [CrossRef]
- Iwasaki, M.; Hara, M.; Kawada, H.; Tada, H.; Ito, S. Cobalt Ion-Doped TiO2 Photocatalyst Response to Visible Light. J. Colloid Interface Sci. 2000, 224, 202–204. [Google Scholar] [CrossRef]
- Sfirloaga, P.; Marin, C.N.; Malaescu, I.; Vlazan, P. The electrical performance of ceramics materials with perovskite structure doped with metallic ions. Ceram. Int. 2016, 42, 18960–18964. [Google Scholar] [CrossRef]
- Bhoyar, D.N.; Kounsalye, J.S.; Khirade, P.P.; Pandit, A.A.; Jadhav, K.M. Doping effect of Fe ions on the structural, electrical, and magnetic properties of SrTiO3 nanoceramic matrix. J. Supercond. Novel Magn. 2019, 32, 1395–1406. [Google Scholar] [CrossRef]
- Farooq, U.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Self-Assembled Interwoven Nanohierarchitectures of NaNbO3 and NaNb1–xTaxO3 (0.05 ≤ x ≤ 0.20): Synthesis, Structural Characterization, Photocatalytic Applications, and Dielectric Properties. ACS Omega 2022, 7, 16952–16967. [Google Scholar] [CrossRef]
- Kim, J.S.; Heo, J.-M.; Park, G.-S.; Woo, S.-J.; Cho, C.; Yun, H.J.; Kim, D.-H.; Park, J.; Lee, S.-C.; Park, S.-H. Ultra-bright, efficient and stable perovskite light-emitting diodes. Nature 2022, 611, 688–694. [Google Scholar] [CrossRef]
- Lungu, A.; Malaescu, I.; Marin, C.N.; Vlazan, P.; Sfirloaga, P. The electrical properties of manganese ferrite powders prepared by two different methods. Phys. B Cond. Matter 2015, 462, 80–85. [Google Scholar] [CrossRef]
- ASTM D150-98; Standard Test Methods for AC Loss Characteristics and Permittivity (Dielectric Constant) of Solid Electrical Insulation. ASTM: West Conshohocken, PA, USA, 2017.
- Altomare, A.; Camalli, M.; Cuocci, C.; Giacovazzo, C.; Moliternia, A.; Rizzia, R. EXPO2009: Structure solution by powder data in direct and reciprocal space. J. Appl. Cryst. 2009, 42, 1197–1202. [Google Scholar] [CrossRef]
- Ahtee, M.; Unonius, L. The structure of NaTaO3 by X-ray powder diffraction. Acta Cryst. A 1977, 33, 150–154. [Google Scholar] [CrossRef]
- Lin, W.H.; Cheng, C.; Hu, C.C.; Teng, H. NaTaO3 photocatalysts of different crystalline structures for water splitting into H2 and O2. Appl. Phys. Lett. 2006, 89, 211904. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Liu, D.R.; Li, F.F.; Yang, D.F.; Jiang, Y.S. Preparation, characterization and photocatalytic activity of N-doped NaTaO3 nanocubes. Powder Technol. 2011, 214, 155–160. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yang, C.L.; Wang, M.S.; Ma, X.G.; Yi, Y.G. Te-doped perovskite NaTaO3 as a promising photocatalytic material for hydrogen production from water splitting driven by visible light. Mater. Res. Bull. 2018, 107, 125–131. [Google Scholar] [CrossRef]
- Onishi, H. Sodium Tantalate Photocatalysts Doped with Metal Cations: Why Are They Active for Water Splitting? ChemSusChem 2019, 12, 1825–1834. [Google Scholar] [CrossRef]
- Gharbi, I.; Oueslati, A.; Ates, A.; Mahmoud, A.; Zaghrioui, M.; Gargouri, M. Investigation of structural, morphological, and electrical conductivity study for understanding transport mechanisms of perovskite CH3NH3HgCl3. RSC Adv. 2023, 13, 10036–10050. [Google Scholar] [CrossRef]
- Mott, N.F.; Davis, E.A. Electronic Processes in Non-Crystalline Materials; Clarendon: Oxford, UK, 1979. [Google Scholar]
- Elliot, S.R. A.c. conduction in amorphous chalcogenide and pnictide semiconductors. Adv. Phys. 1987, 36, 135–217. [Google Scholar] [CrossRef]
- Debye, P. Polar Molecules; Chemical Catalog Company Inc.: New York, NY, USA, 1929. [Google Scholar]
- Malaescu, I.; Sfirloaga, P.; Marin, C.N.; Bunoiu, O.M.; Vlazan, P. Experimental Investigations on the Electrical Conductivity and Complex Dielectric Permittivity of ZnxMn1−xFe2O4 (x = 0 and 0.4) Ferrites in a Low-Frequency Field. Crystals 2024, 14, 437. [Google Scholar] [CrossRef]
- Zahrin, A.; Azhar, N.A.; Ibrahim, N.; Mohamed, Z. Structural, Magnetic, and Electrical Properties and Magnetoresistance of Monovalent K-Substituted La0.7Ba0.3−xKxMnO3 (x = 0 and 0.04) Manganite. Condens. Matter 2022, 7, 51. [Google Scholar] [CrossRef]
- Jonscher, A.K. Universal Relaxation Law, 1st ed.; Chelsea Dielectrics Press: London, UK, 1996; pp. 198–200. [Google Scholar]
- Ali, H.; Karim, S.; Rafiq, M.A.; Maaz, K.; Rahman, A.U.; Nisar, A.; Ahmad, M. Electrical conduction mechanism in ZnS nanoparticles. J. Alloys Compd. 2014, 612, 64–68. [Google Scholar] [CrossRef]
- Montgomery, M.J.; Sugak, N.; Yang, K.R.; Rogers, J.M.; Kubec, S.A.; Ratinov, A.C.; Schroers, J.; Batista, V.S.; Pfefferle, L.D. Semiconductor-to-conductor transition in 2D copper (II) oxide nanosheets through surface sulfur-functionalization. Nanoscale 2020, 12, 14549–14559. [Google Scholar] [CrossRef]
- Hill, R.M. Poole-Frenkel conduction in amorphous solids. Philos. Mag. 1971, 24, 1307–1325. [Google Scholar] [CrossRef]
- Brindusoiu, S.; Poienar, M.; Marin, C.N.; Sfirloaga, P.; Vlazan, P.; Malaescu, I. The electrical conductivity of Fe3(PO4)2·8H2O materials. J. Mater. Sci. Mater. Electron. 2019, 30, 15693–15699. [Google Scholar] [CrossRef]
- Malaescu, I.; Lungu, A.; Marin, C.N.; Sfirloaga, P.; Vlazan, P.; Brindusoiu, S.; Poienar, M. Temperature dependence of the dynamic electrical properties of Cu1+xMn1−xO2 (x = 0 and 0.06) crednerite materials. Ceram. Int. 2018, 44, 11610–11616. [Google Scholar] [CrossRef]
- Chithambaram, V.; Das, S.J.; Krishnan, S. Synthesis, optical and dielectric studies on novel semiorganic nonlinear optical crystal by solution growth technique. J. Alloys Compd. 2011, 509, 4543–4546. [Google Scholar] [CrossRef]
- Mahmoud, K.H.; Abdel-Rahim, F.M.; Atef, K.; Saddeek, Y.B. Dielectric Dispersion in Lithium-Bismuth-Borate Glasses. Curr. Appl. Phys. 2011, 11, 55–60. [Google Scholar] [CrossRef]
- Wunderlich, W.; Baufeld, B. Development of Thermoelectric Materials Based On NaTaO3—Composite ceramics. In Ceramic Materials; Wunderlich, W., Ed.; Intech Publisher: Rijeka, Croatia, 2010; Chapter 1; pp. 1–28. [Google Scholar]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiments and Applications; Wiley: New York, NY, USA, 2005. [Google Scholar]
- Wubbenhorst, M.; van Turnhout, J. Analysis of complex dielectric spectra. I. One-dimensional derivative techniques and three-dimensional modelling. J. Non-Cryst. Solids 2002, 305, 40–49. [Google Scholar] [CrossRef]
- Mesrar, M.; Elbasset, A.; Echatoui, N.-S.; Abdi, F.; Lamcharfi, T. Studies of Structural, Dielectric, and Impedance Spectroscopy of KBT-Modified Sodium Bismuth Titanate Lead-Free Ceramics. ACS Omega 2022, 7, 37142–37163. [Google Scholar] [CrossRef]
- Rani, S.; Ahlawat, N.; Sangwan, K.M.; Rani, S.; Punia, R.; Malik, J. Structural investigation and giant dielectric response of CaCu3Ti4O12 ceramic by Nd/Zr co-doping for energy storage applications. J. Mater. Sci. Mater. Electron. 2018, 29, 10825–10833. [Google Scholar] [CrossRef]
- Ma, H.; Lin, K.; Fan, L.; Rong, Y.; Chen, J.; Deng, J.; Liu, L.; Kawaguchi, S.; Kato, K.; Xing, X. Structure and Oxide Ion Conductivity in Tetragonal Tungsten Bronze BaBiNb5O15. RSC Adv. 2015, 5, 71890–71895. [Google Scholar] [CrossRef]
- El Heda, I.; Dhahri, R.J.; Massoudi, J.; Dhahri, E.; Bahri, F.; Khirouni, K.; Costa, B.F.O. Study of the structural, electrical, dielectric properties and transport mechanisms of Cu0.5Fe2.5O4 ferrite nanoparticles for energy storage, photocatalytic and microelectronic applications. Heliyon 2023, 9, e17403. [Google Scholar] [CrossRef]
- Casut, C.; Malaescu, I.; Marin, C.N.; Miclau, M. The Effect of Bi2O3 and Fe2O3 Impurity Phases in BiFeO3 Perovskite Materials on Some Electrical Properties in the Low-Frequency Field. Materials 2022, 15, 4764. [Google Scholar] [CrossRef]
- Hiti, M.A. Dielectric behavior and ac electrical conductivity of Zn-substituted Ni-Mg ferrites. J. Magn. Magn. Mater. 1996, 164, 187–196. [Google Scholar]
- Lipare, A.Y.; Vasambekar, P.N.; Vaingankar, A.S. Dielectric behavior and a.c. resistivity study of humidity sensing ferrites. Mater. Chem. Phys. 2003, 81, 108–115. [Google Scholar] [CrossRef]
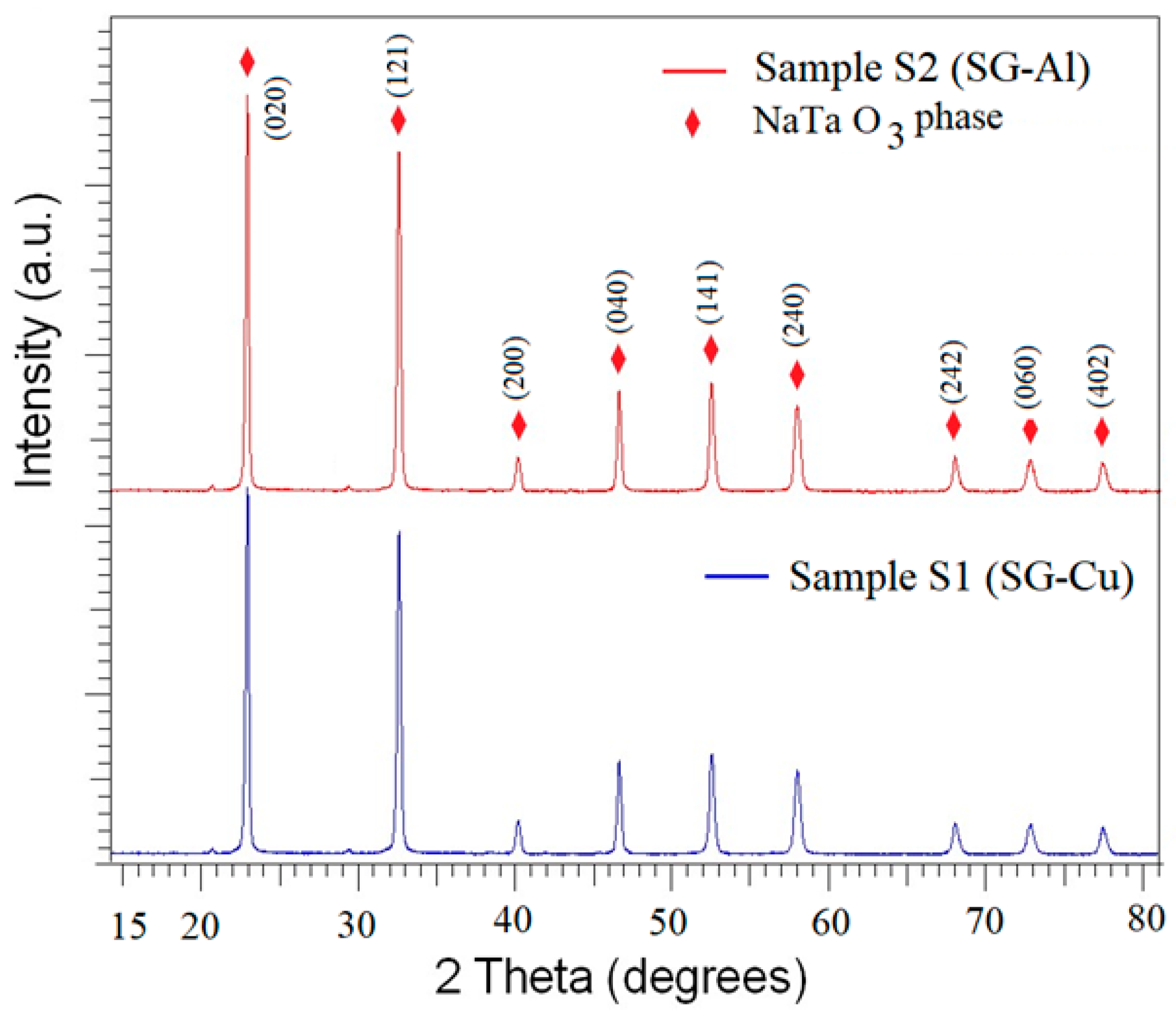
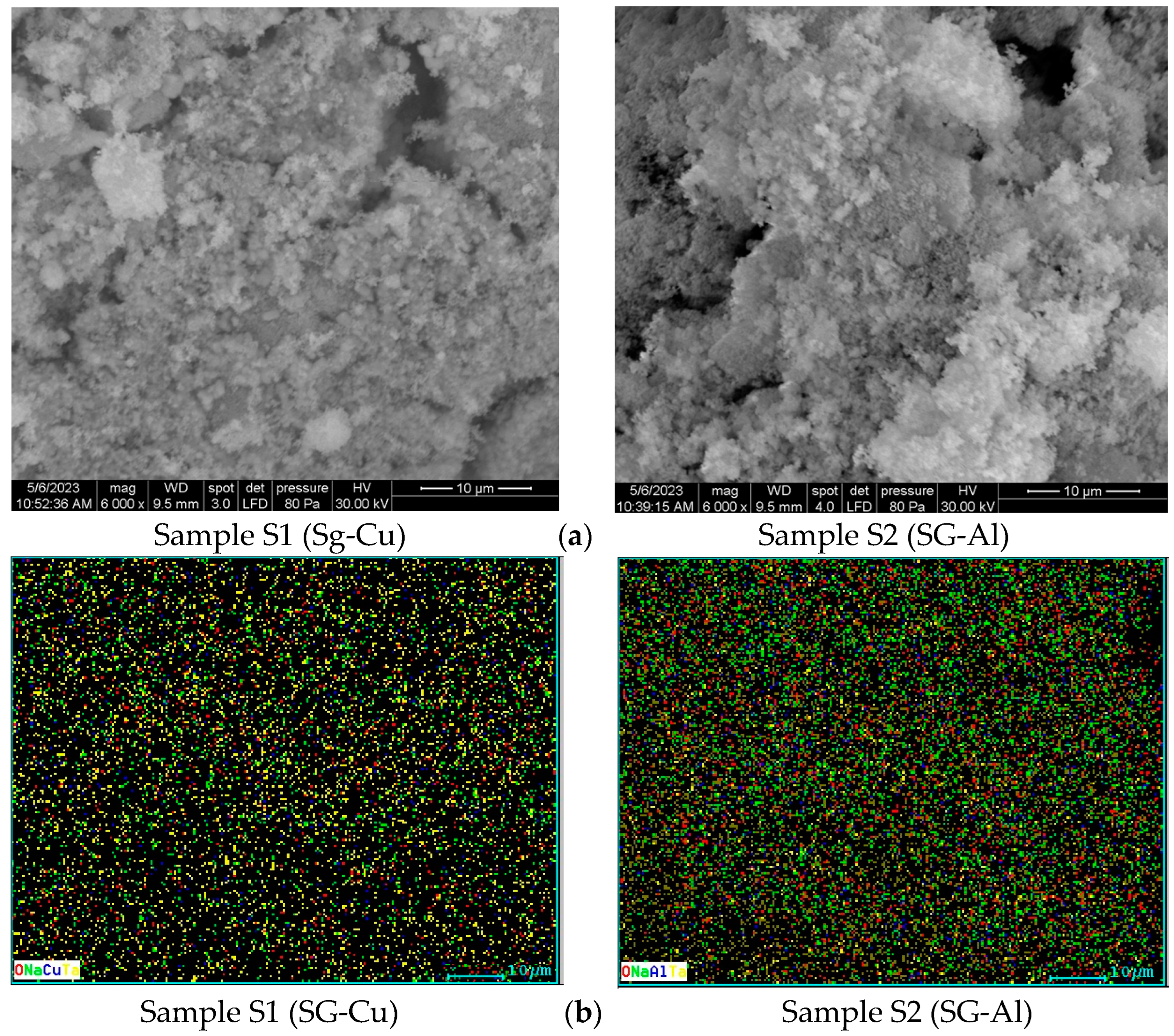
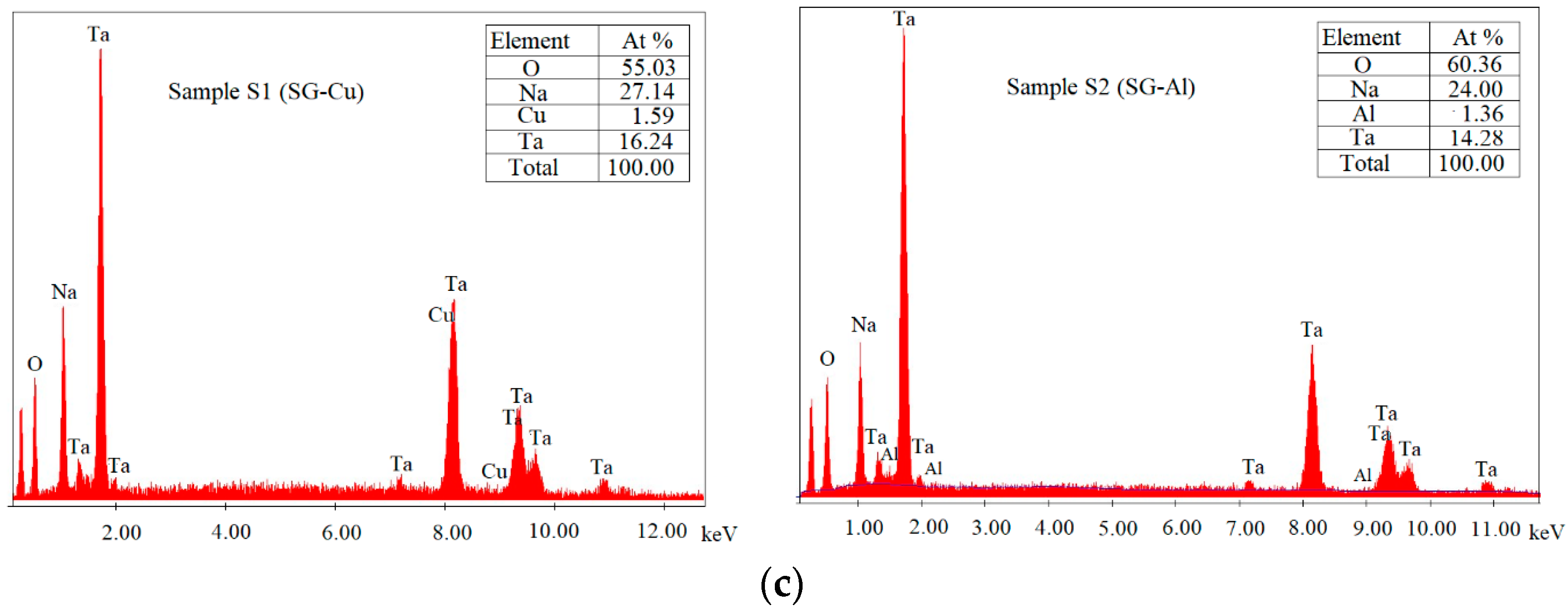
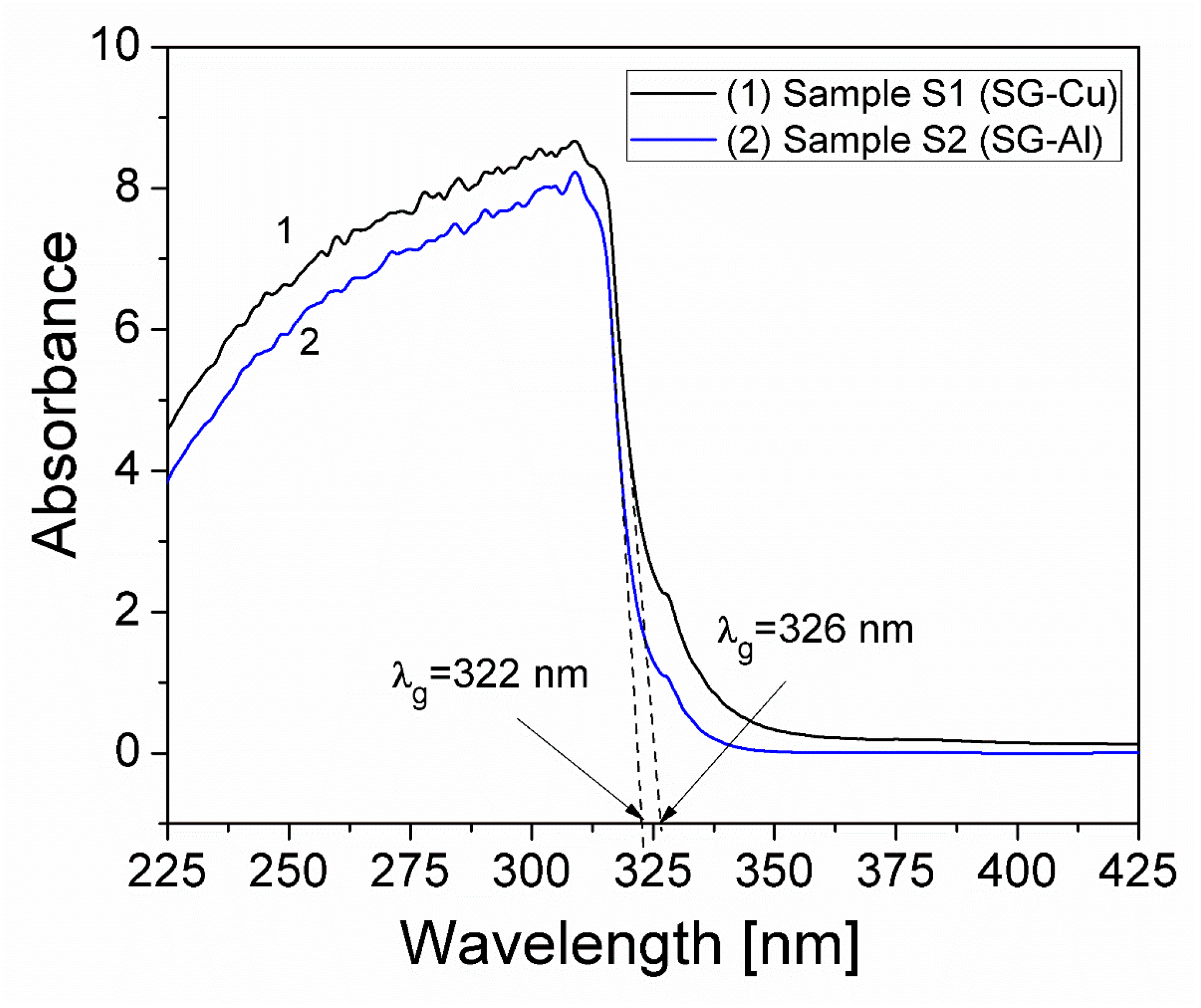

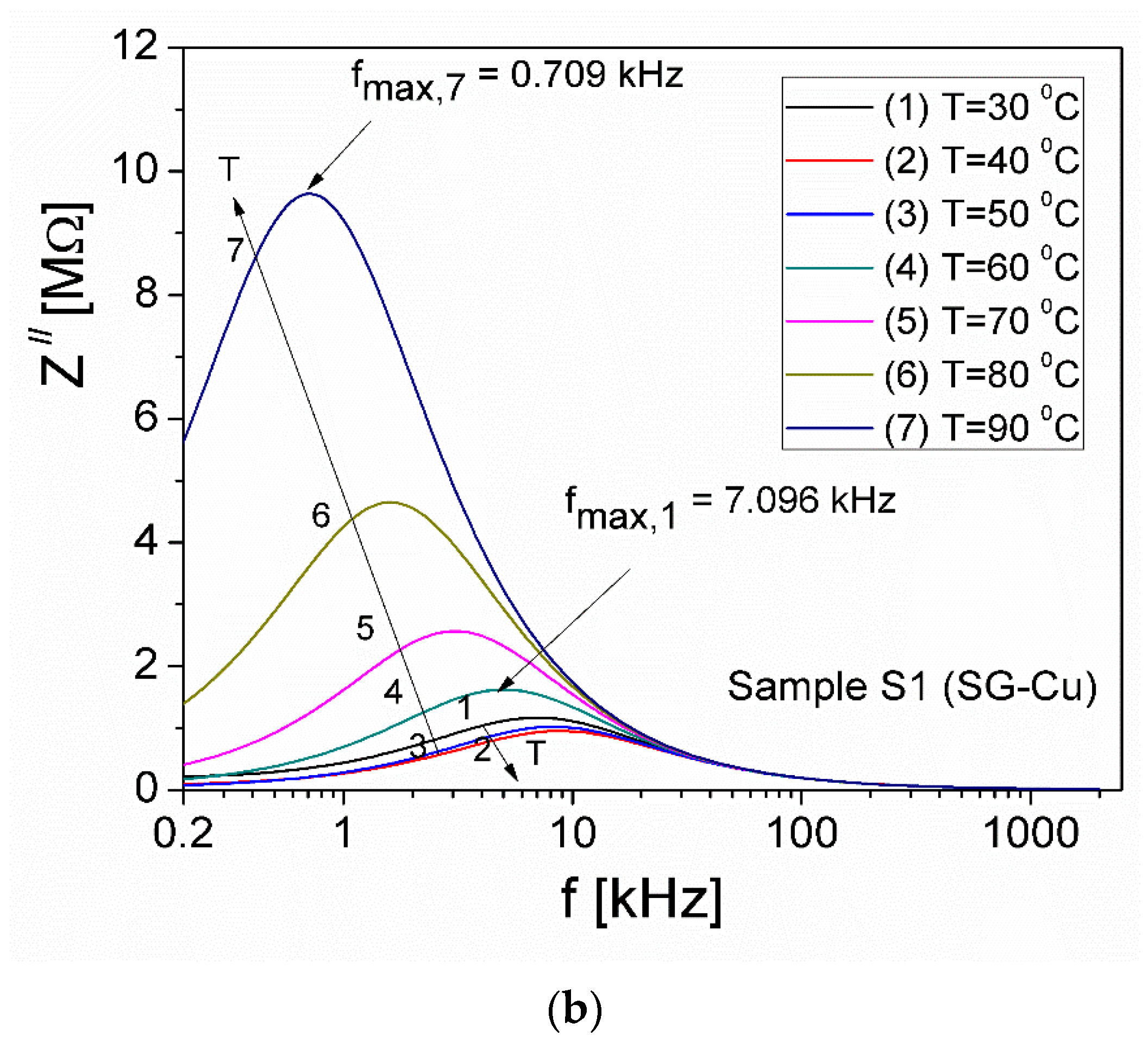
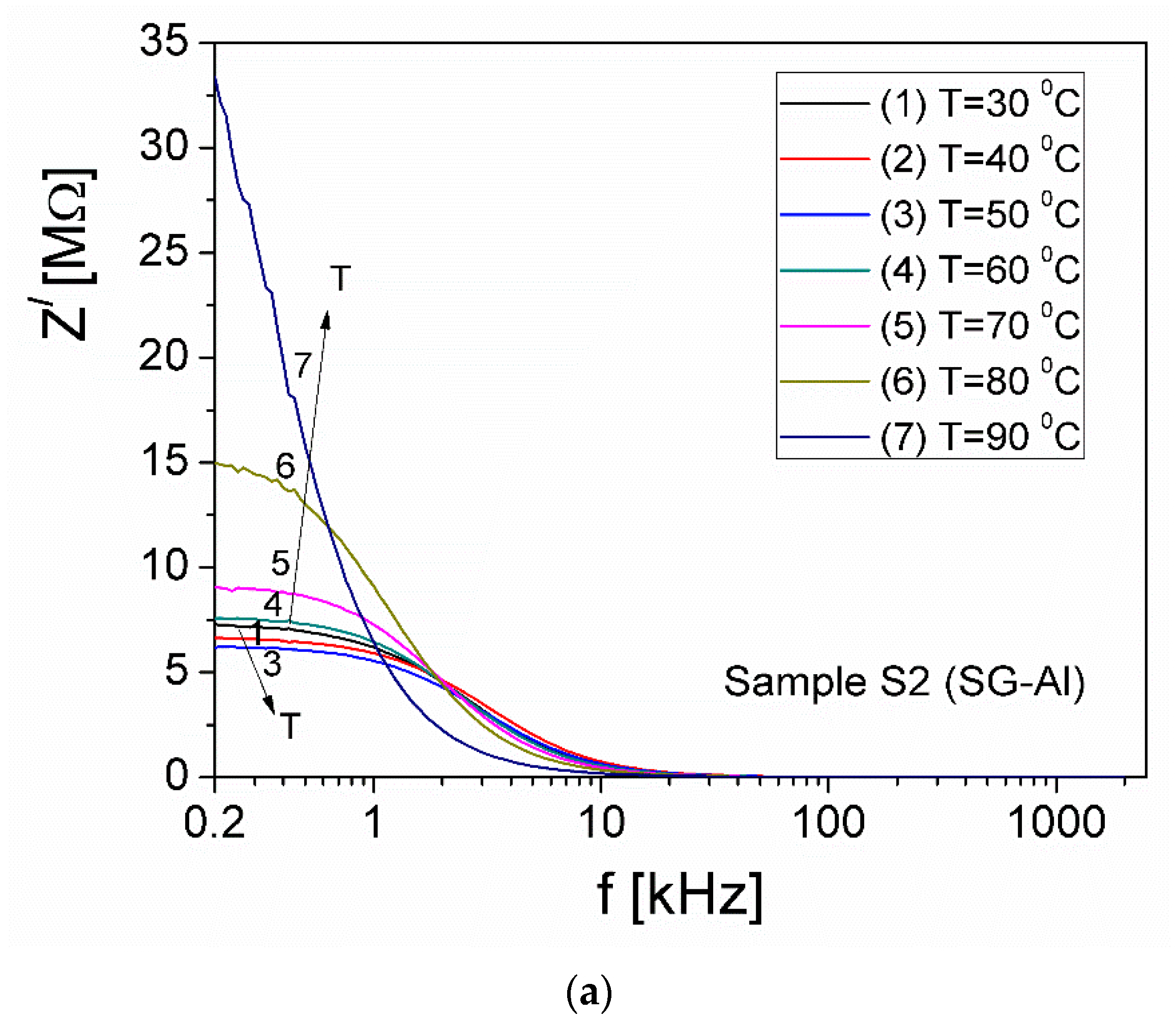
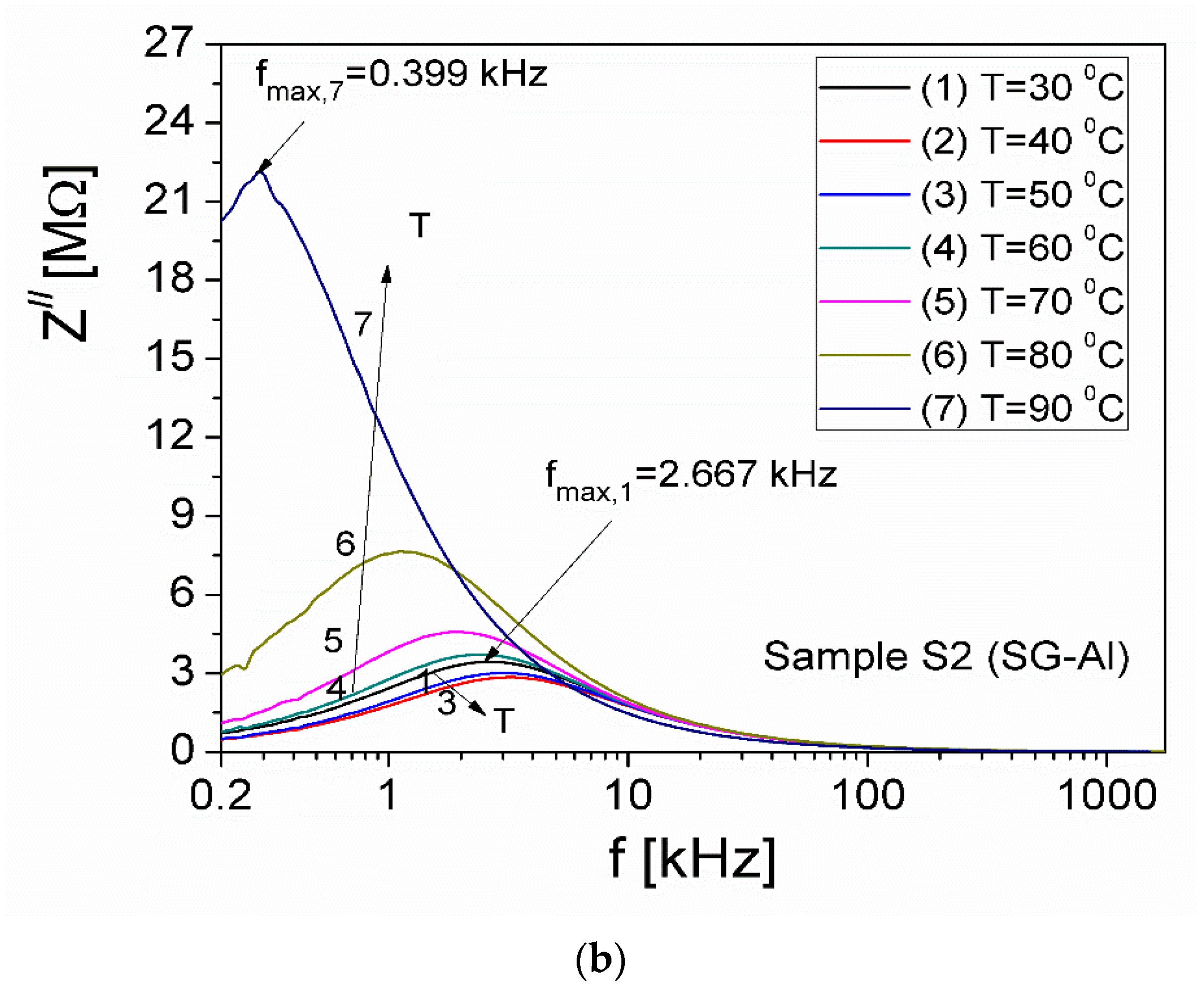
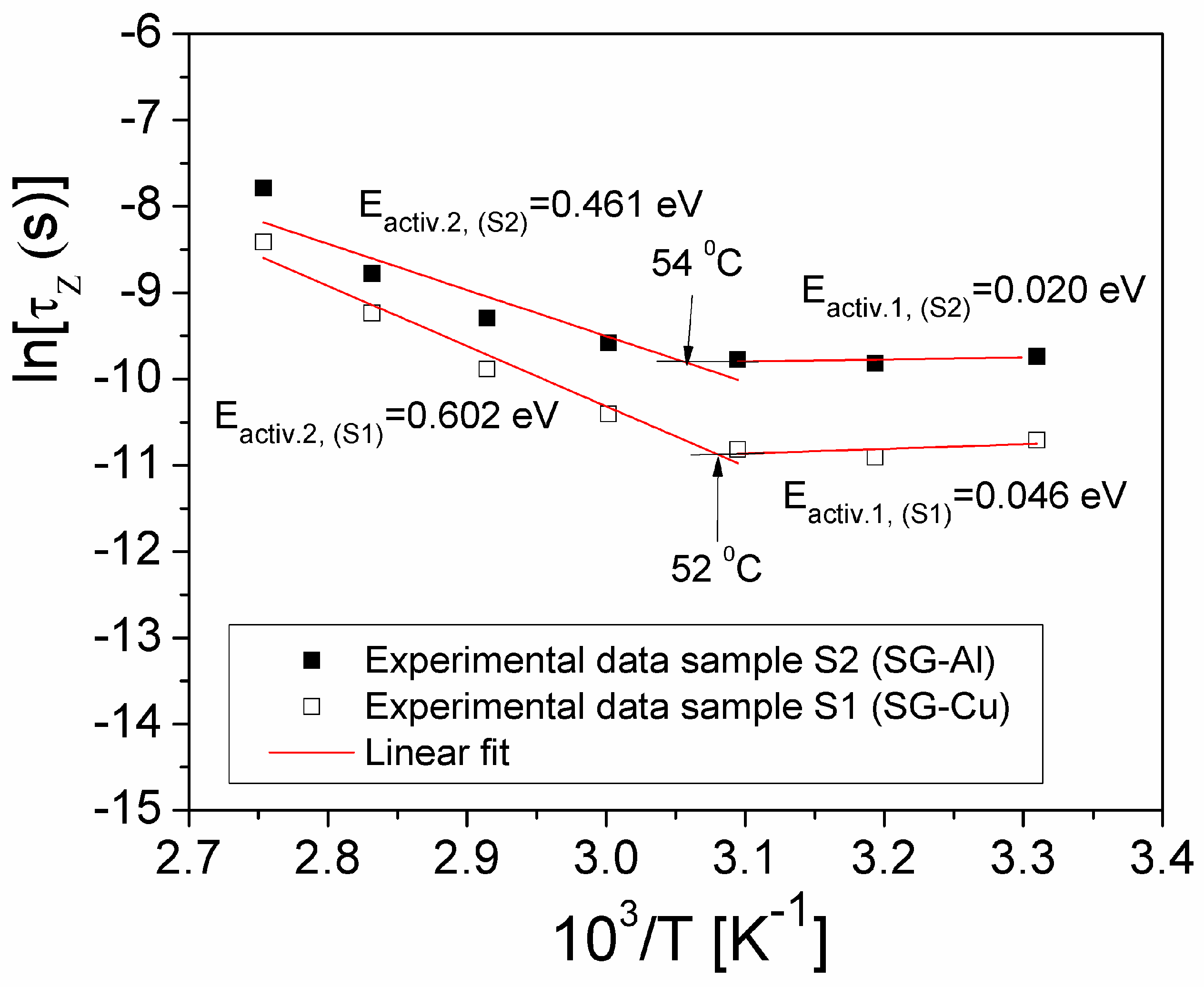
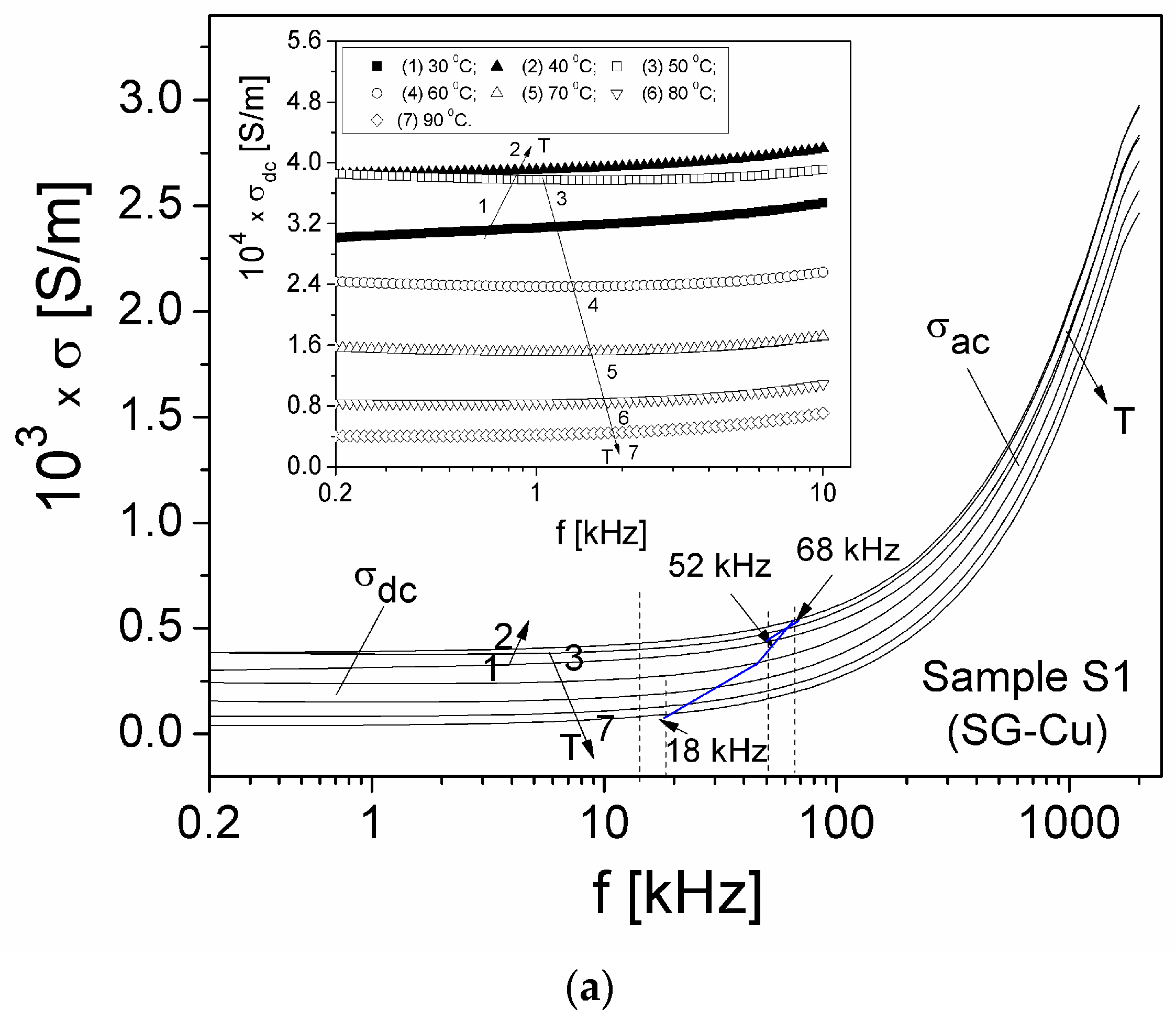
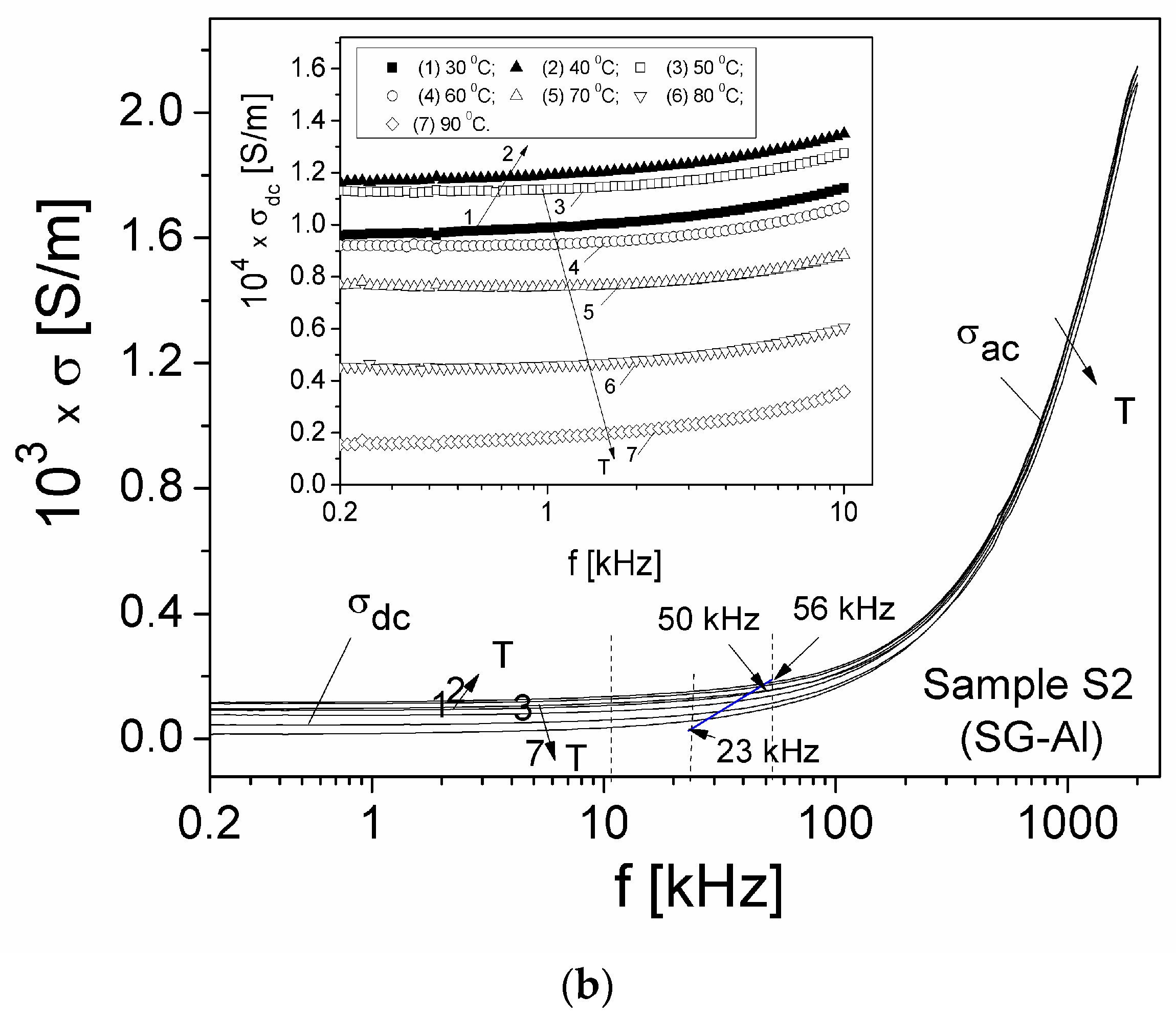
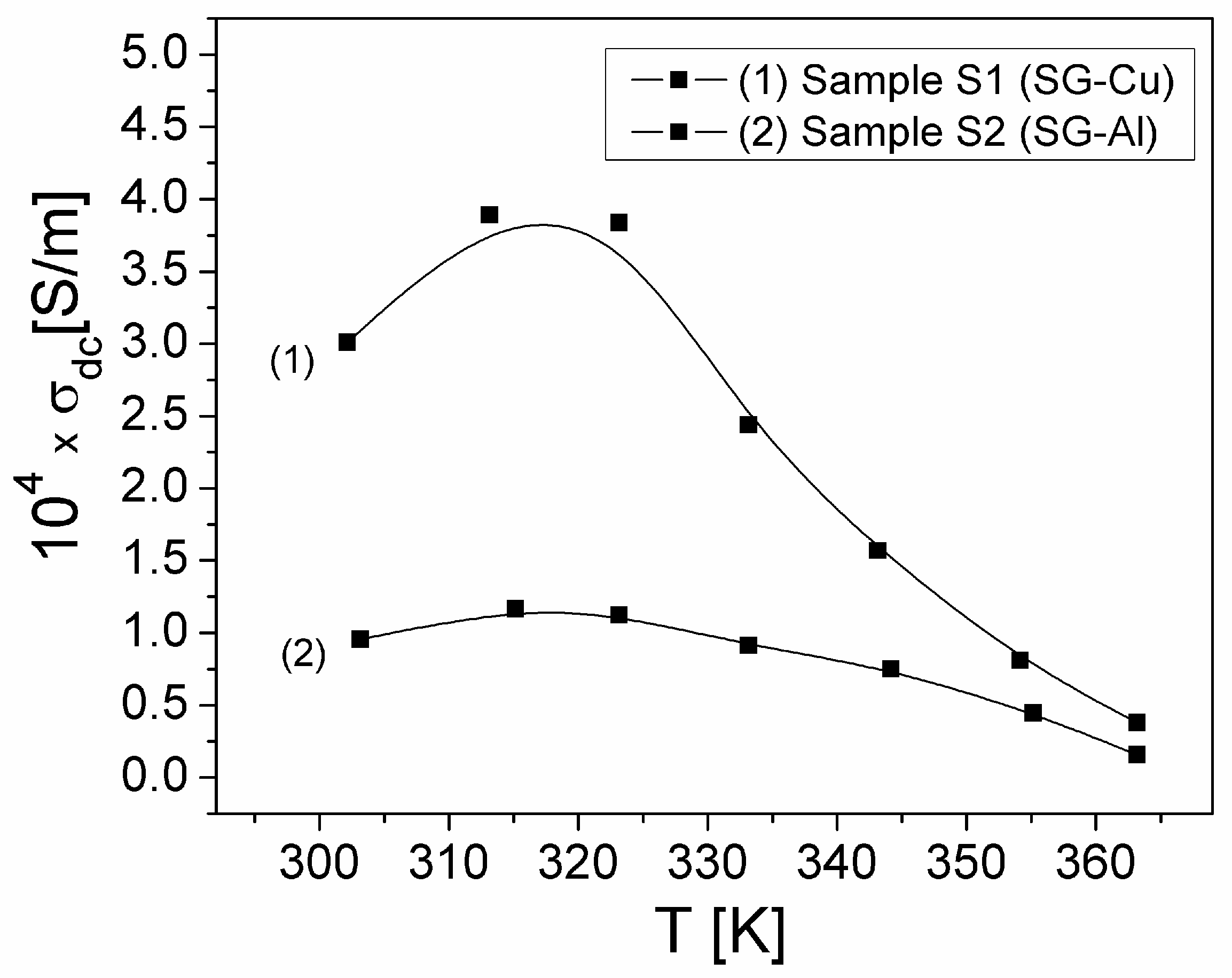
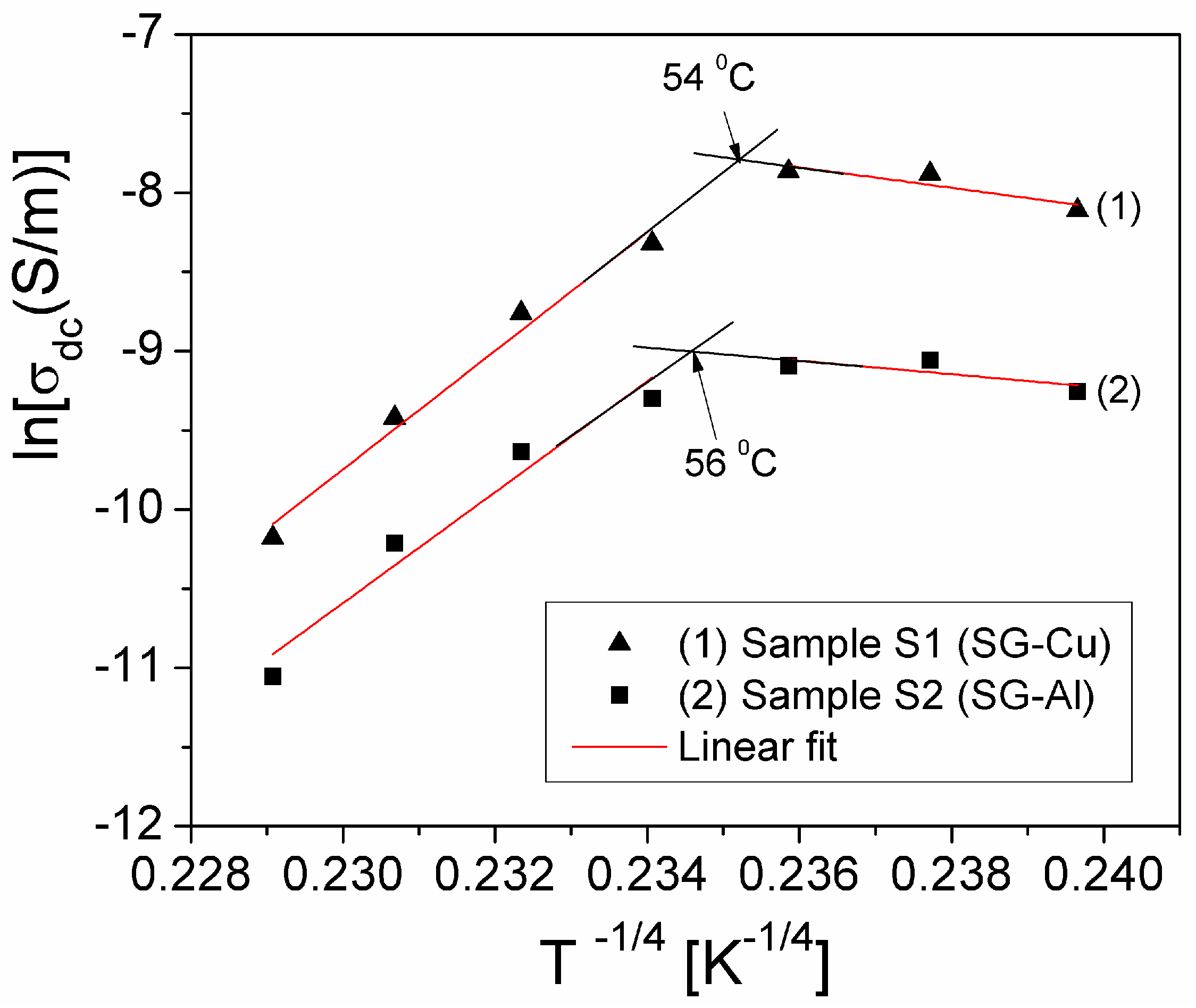
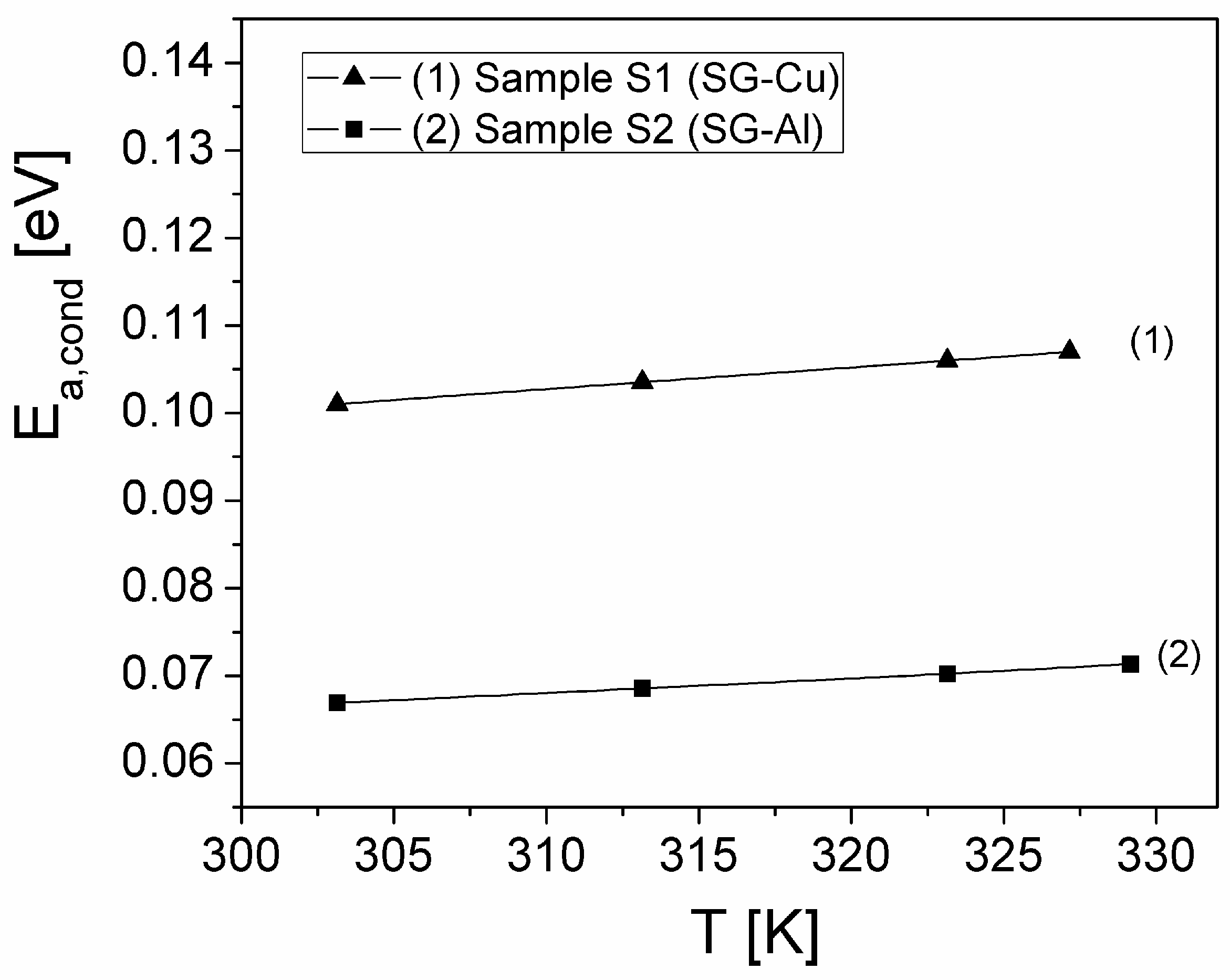
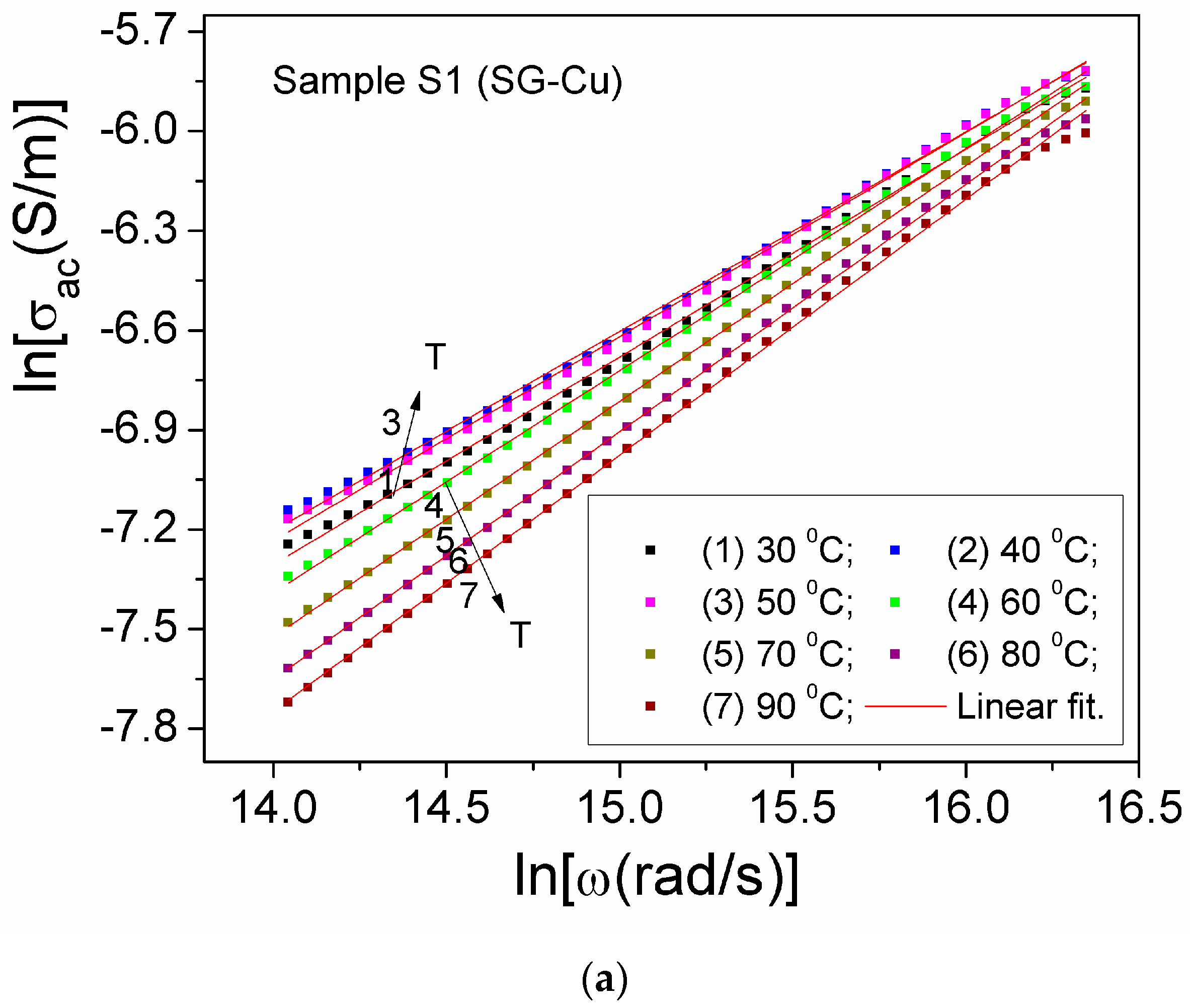
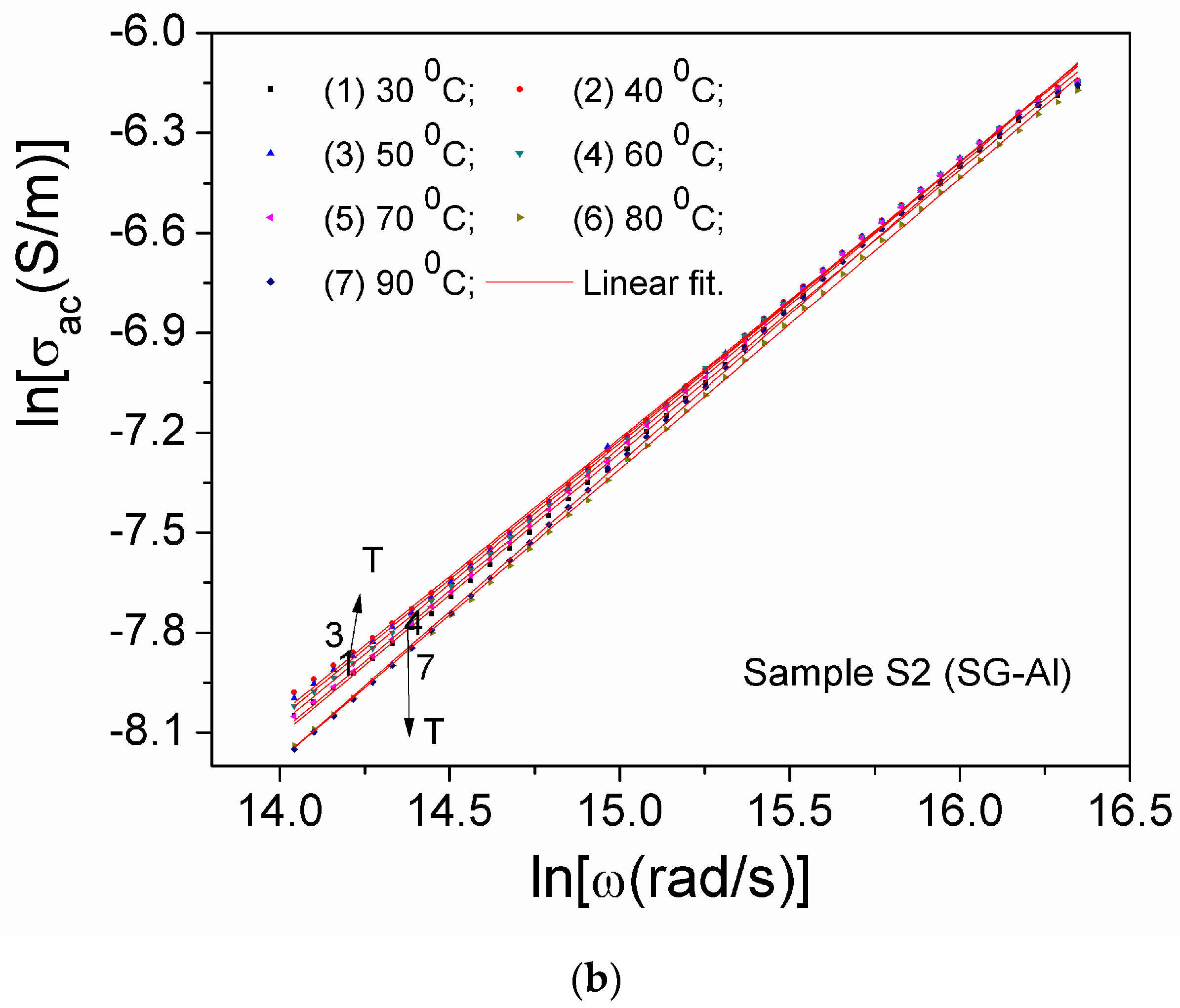
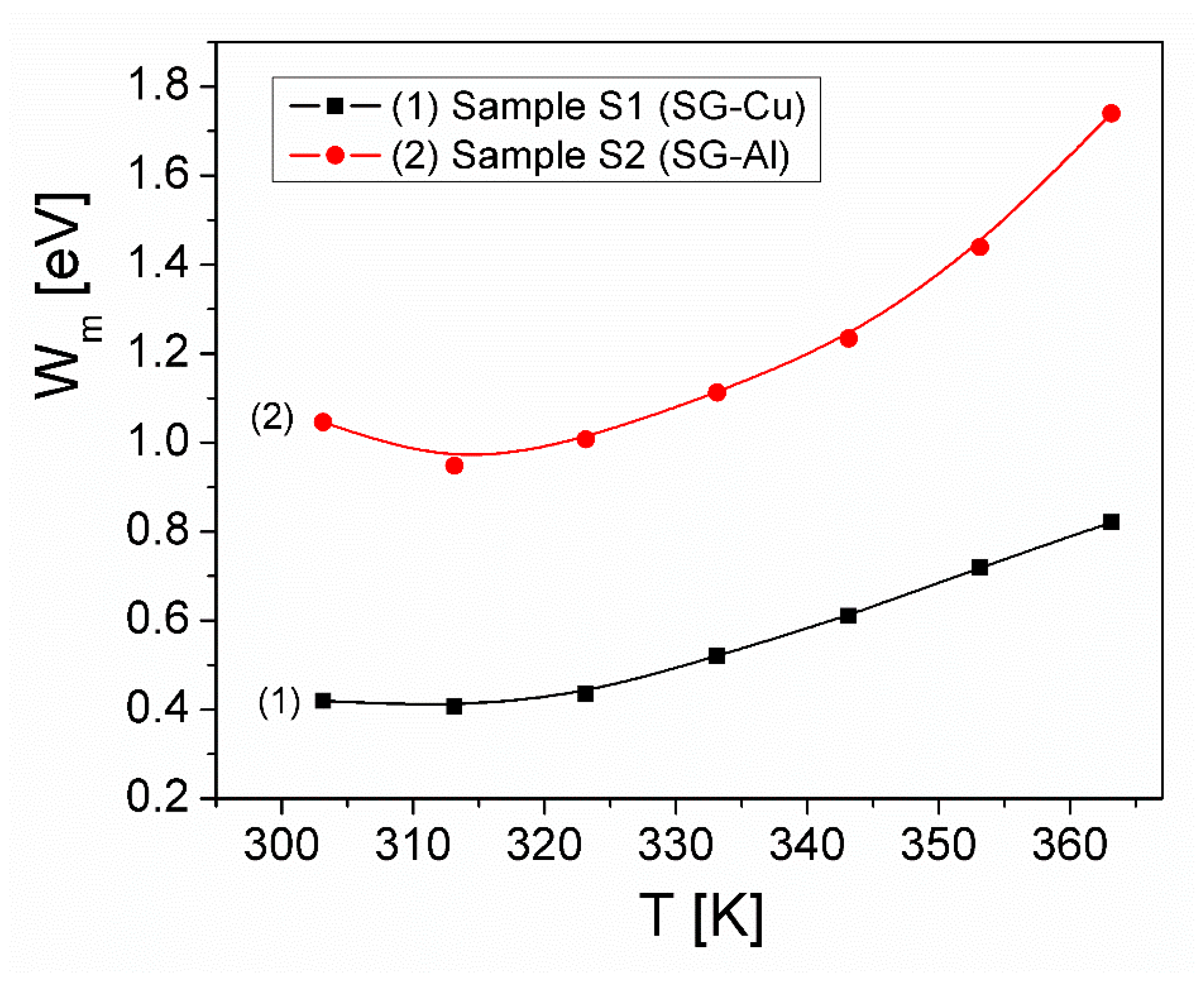
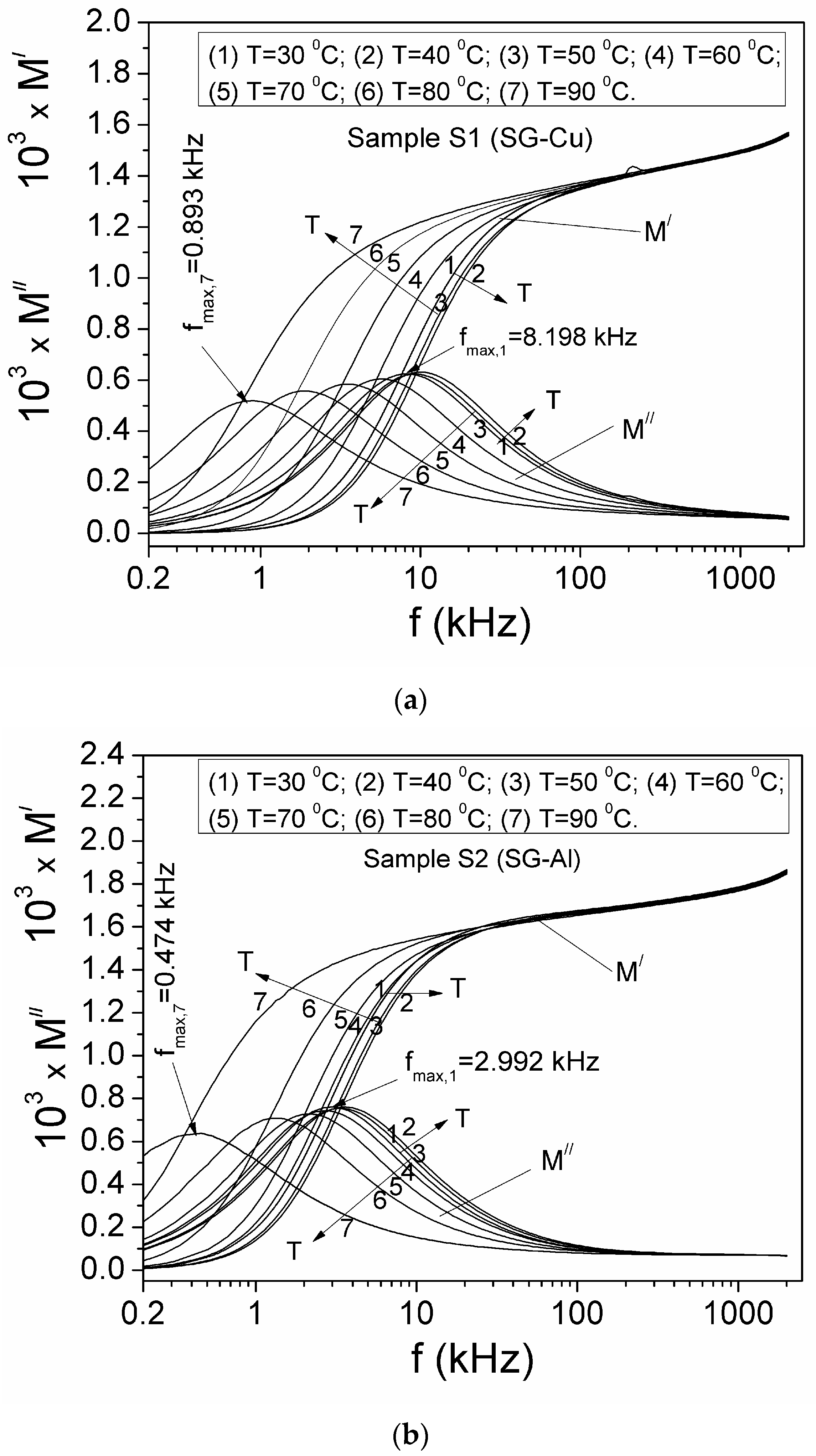
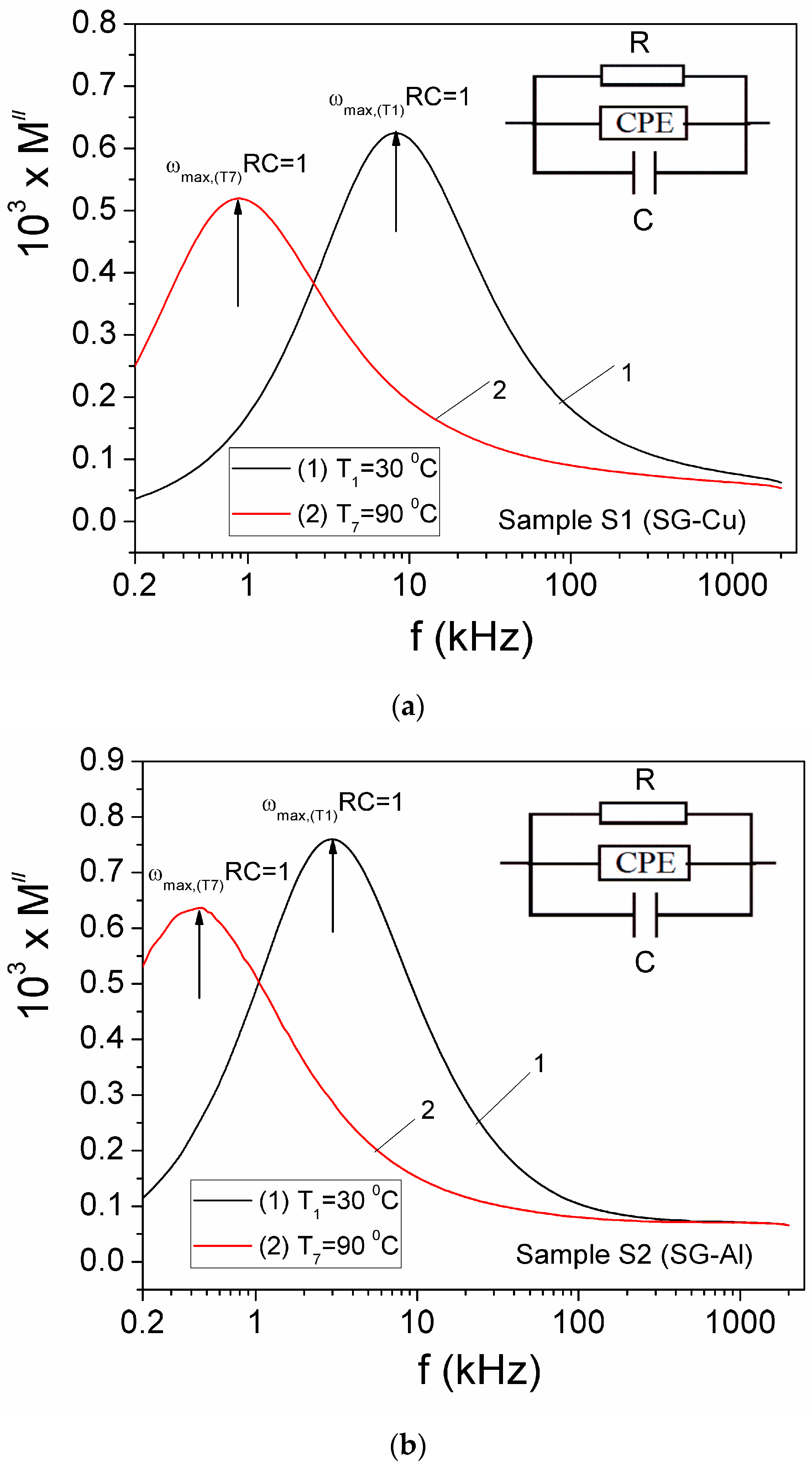
| Sample | Mean Diameter (nm) | Lattice Parameters |
|---|---|---|
| S1 (SG-Cu) | 39.4 | a = 5.5140 Å; b = 7.7510 Å; c = 5.4950 Å |
| S2 (SG-Al) | 37.7 | a = 5.5138 Å; b = 7.7508 Å; c = 5.4940 Å |
| NaTaO3 | - | a = 5.5130 Å; b = 7.7503 Å; c = 5.4941 Å |
| T [°C] | Sample S1 (SG-Cu) | Sample S2 (SG-Al) | ||
|---|---|---|---|---|
| R[nm] | W[eV] | R[nm] | W[eV] | |
| 30 | 2.86 | 0.366 | 1.90 | 0.242 |
| 40 | 2.84 | 0.375 | 1.88 | 0.248 |
| 50 | 2.82 | 0.384 | 1.87 | 0.254 |
| 54 | 2.81 | 0.388 | 1.86 | 0.256 |
| T [°C] | Sample S1 (SG-Cu) | Sample S2 (SG-Al) | ||||
|---|---|---|---|---|---|---|
| n | A0[S/msn] | ωh[s−1] | n | A0[S/msn] | ωh[s−1] | |
| 30 | 0.626 | 10.54 × 10−8 | 3.31 × 105 | 0.850 | 2.03 × 10−9 | 3.15 × 105 |
| 40 | 0.601 | 16.42 × 10−8 | 4.12 × 105 | 0.829 | 2.91 × 10−9 | 3.57 × 105 |
| 50 | 0.615 | 13.21 × 10−8 | 4.28 × 105 | 0.834 | 2.68 × 10−9 | 3.49 × 105 |
| 60 | 0.669 | 5.26 × 10−8 | 3.02 × 105 | 0.845 | 2.27 × 10−9 | 2.82 × 105 |
| 70 | 0.709 | 2.62 × 10−8 | 2.13 × 105 | 0.856 | 1.89 × 10−9 | 2.37 × 105 |
| 80 | 0.746 | 1.39 × 10−8 | 1.12 × 105 | 0.873 | 1.36 × 10−9 | 1.49 × 105 |
| 90 | 0.771 | 0.80 × 10−8 | 1.11 × 105 | 0.892 | 1.06 × 10−9 | 1.48 × 105 |
| Compounds | T[°C] | τM[μs] | Rg[MΩ] | Cg[pF] | Qg[nFsn−1] |
|---|---|---|---|---|---|
| 30 | 19.42 | 2.20 | 8.81 | 0.510 | |
| 40 | 15.88 | 1.82 | 8.71 | 0.716 | |
| 50 | 17.31 | 1.96 | 8.82 | 0.601 | |
| Sample S1 | 60 | 26.67 | 2.93 | 9.10 | 0.297 |
| 70 | 47.43 | 5.04 | 9.42 | 0.171 | |
| 80 | 79.61 | 8.06 | 9.87 | 0.109 | |
| 90 | 178.25 | 16.83 | 10.59 | 0.076 | |
| 30 | 53.21 | 6.74 | 6.75 | 0.030 | |
| 40 | 44.77 | 5.67 | 5.67 | 0.033 | |
| 50 | 47.42 | 5.97 | 5.97 | 0.033 | |
| Sample S2 | 60 | 59.70 | 7.39 | 7.39 | 0.034 |
| 70 | 70.95 | 9.12 | 9.12 | 0.035 | |
| 80 | 119.13 | 15.91 | 15.91 | 0.046 | |
| 90 | 335.80 | 35.81 | 35.80 | 0.073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaescu, I.; Sfirloaga, P.; Marin, C.N. Experimental Investigations of the AC-Conductivity in NaTaO3 Ceramic Materials Doped with Cu and Al Metal Ions. Metals 2024, 14, 793. https://doi.org/10.3390/met14070793
Malaescu I, Sfirloaga P, Marin CN. Experimental Investigations of the AC-Conductivity in NaTaO3 Ceramic Materials Doped with Cu and Al Metal Ions. Metals. 2024; 14(7):793. https://doi.org/10.3390/met14070793
Chicago/Turabian StyleMalaescu, Iosif, Paula Sfirloaga, and Catalin Nicolae Marin. 2024. "Experimental Investigations of the AC-Conductivity in NaTaO3 Ceramic Materials Doped with Cu and Al Metal Ions" Metals 14, no. 7: 793. https://doi.org/10.3390/met14070793
APA StyleMalaescu, I., Sfirloaga, P., & Marin, C. N. (2024). Experimental Investigations of the AC-Conductivity in NaTaO3 Ceramic Materials Doped with Cu and Al Metal Ions. Metals, 14(7), 793. https://doi.org/10.3390/met14070793







