Development of a Dezincification-Free Alloy System for the Manufacturing of Brass Instruments
Abstract
1. Introduction
2. Materials and Methods
2.1. Simulations
2.2. Alloy Production
2.3. Investigation
2.4. Corrosion Analysis
3. Results and Discussion
3.1. Simulations Using Thermo-Calc
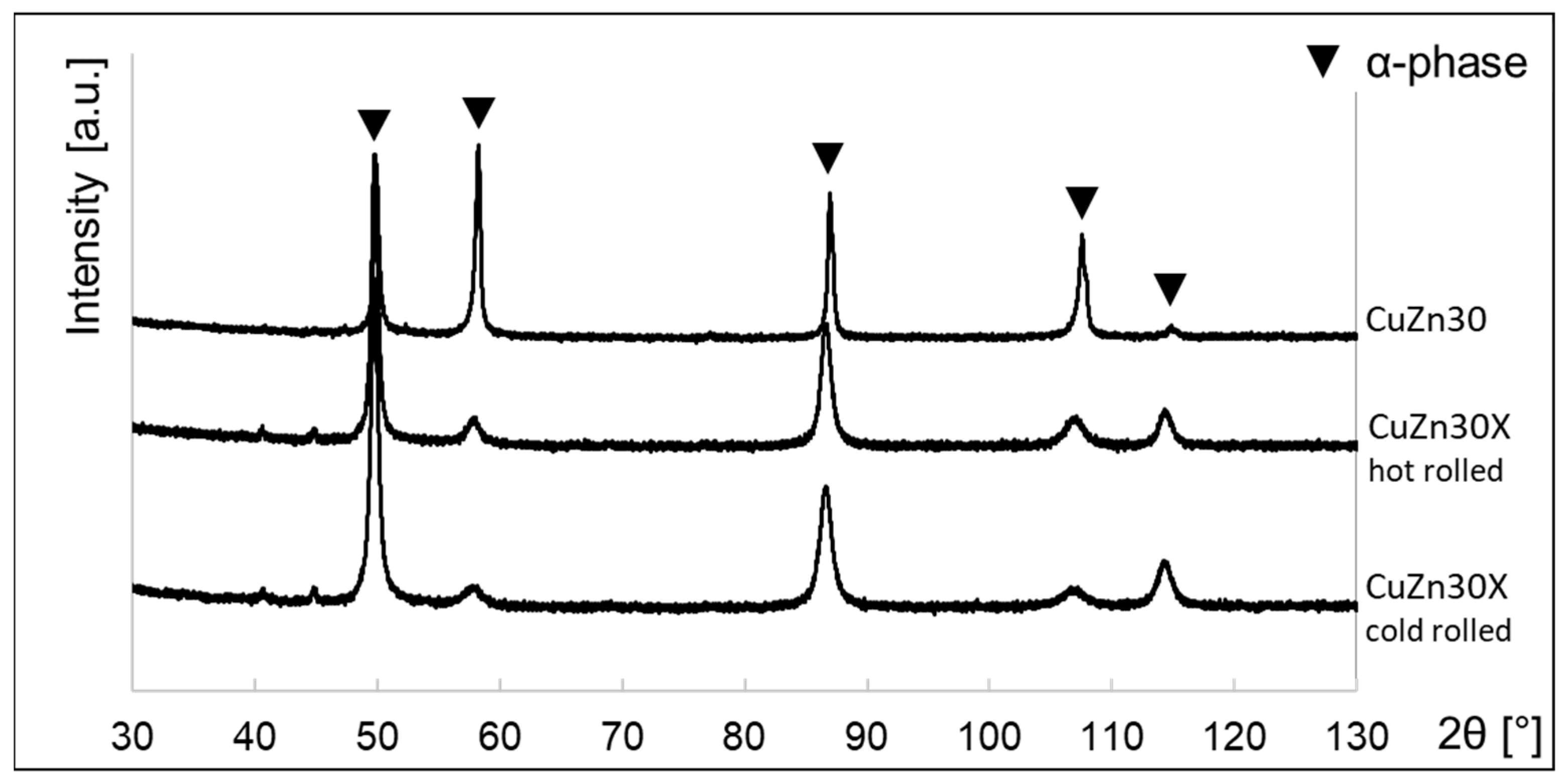
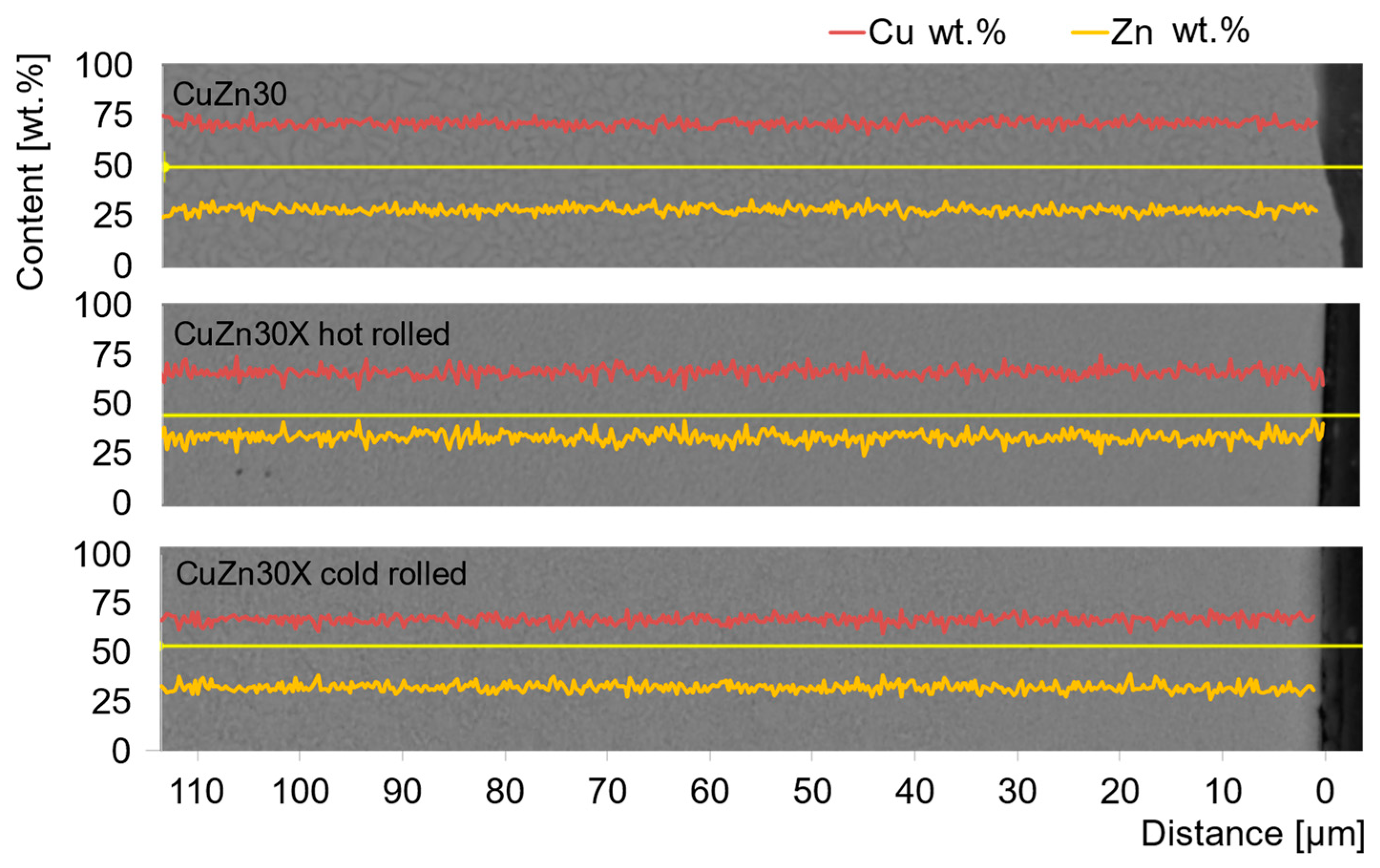
3.2. Properties of the Alloy in Cold and Hot Rolled Conditions
3.3. Corrosion Properties
3.3.1. Stress Corrosion Cracking Test
3.3.2. Dezincification Test
3.3.3. Polarization Curves
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Predel, B.; Madelung, O. Cu-Zn (Copper-Zinc); Springer Nature: Cham, Switzerland, 1994; p. 11. [Google Scholar]
- Basori, I.; Ponco, M.S.; Sari, Y. Effect of Thermomechanical Processing on the Microstructures and Mechanical Properties of Cu-28Zn-2Al Alloys. In Broad Exposure to Science and Technology II; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2022; pp. 155–160. [Google Scholar]
- Basori, I.; Nabila, Z.J.; Sari, Y. Influence of Thermomechanical Treatment on the Microstructure and Mechanical Properties of Cu-28Zn-4Mn Alloys. MSF 2022, 1057, 161–166. [Google Scholar] [CrossRef]
- Messing: Eigenschaften, Verarbeitung, Verwendung; Erw. Neuausg. von: Was muß der Ingenieur vom Messing wissen? Deutsches Kupfer-Institut e.V: Berlin, Germany, 1953.
- Kenworthy, L.; O’Driscoll, W.G. Dezincification of Brasses in Marine Environments. Corr. Tech. 1955, 2, 247. [Google Scholar]
- Stillwell, C.W.; Turnipseed, E.S. Mechanism of Dezincification—Corrosion of Brass. Ind. Eng. Chem. 1934, 26, 740–743. [Google Scholar] [CrossRef]
- Weisser, T.S. The dealloying of copper alloys. Stud. Conserv. 1975, 20, 207–214. [Google Scholar] [CrossRef]
- Heidersbach, R.H.; Verink, E.D. The Dezincification of Alpha and Beta Brasses. Corrosion 1972, 28, 397–418. [Google Scholar] [CrossRef]
- Zhou, P.; Ogle, K. The Corrosion of Copper and Copper Alloys. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2018; pp. 478–489. ISBN 978-0-12-809894-3. [Google Scholar]
- Xie, C.; Crespo Artiaga, D.; Renner, F. Corrosion Studies on Cu-Based Alloys. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2021. [Google Scholar]
- Horton, R.M. New Metallographic Evidence for Dezincification of Brass by Redisposition of Copper. Corrosion 1970, 26, 260–264. [Google Scholar] [CrossRef]
- Zhang, Y. Dezincification and Brass Lead Leaching in Premise Plumbing Systems: Effects of Alloy, Physical Conditions and Water Chemistry: Dezincification and Brass Lead Leaching in Premise Plumbing Systems: Effects of Alloy, Physical Conditions and Water Chemistry. Master’s Thesis, Virginia Tech, Blacksburg, VA, USA, 2009. [Google Scholar]
- Fink, F.W. The Dezincification of Alpha Brass with Special Reference to Arsenic. Trans. Electrochem. Soc. 1939, 75, 441. [Google Scholar] [CrossRef]
- Langenegger, E.E.; Callaghan, B.G. Use of an Empirical Potential Shift Technique for Predicting Dezincification Rates of αβ-Brasses in Chloride Media. Corrosion 1972, 28, 245–254. [Google Scholar] [CrossRef]
- Hoshi, Y.; Otake, Y.; Uchisawa, Y.; Watanabe, H.; Shitanda, I.; Itagaki, M. Real-Time Imaging of Brass Cross-Section with Dezincification Corrosion by Electrochemical Measurement Combined with Video Observation. Mater. Trans. 2023, 64, 885–888. [Google Scholar] [CrossRef]
- Karpagavalli, R.; Balasubramaniam, R. Development of novel brasses to resist dezincification. Corros. Sci. 2007, 49, 963–979. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Yang, H.; Su, Y.; Qiao, L. Study of the relationship between intergranular stress corrosion cracking and grain boundary characteristics in brass. Electrochem. Commun. 2021, 131, 107124. [Google Scholar] [CrossRef]
- Choucri, J.; Balbo, A.; Zanotto, F.; Grassi, V.; Touhami, M.E.; Mansouri, I.; Monticelli, C. Corrosion Behavior and Susceptibility to Stress Corrosion Cracking of Leaded and Lead-Free Brasses in Simulated Drinking Water. Materials 2021, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Pantazopoulos, G. A review of defects and failures in brass rods and related components. Pract. Fail. Anal. 2003, 3, 14–22. [Google Scholar] [CrossRef]
- Ledergerber, M.; Alter, M.; Cornet, E.; Hildbrand, E. Humidity in brass instruments and the prevention of corrosion. In Proceedings of the Third Vienna Talk on Music Acoustics; Mayer, A., Chatziioannou, V., Goebl, W., Eds.; Institute of Music Acoustics (Wiener Klangstil): Vienna, Austria, 2015; p. 103. [Google Scholar]
- von Steiger, A.; Allenbach, D.; Skamletz, M. (Eds.) To Play or Not to Play: Corrosion of Historic Brass Instruments. Romantic Brass Symposium 4; Erste Auflage; Edition Argus: Schliengen, Germany, 2023; ISBN 9783931264956. [Google Scholar]
- Da Louzada, M.L.C.; Costa, C.D.S.; Souza, T.N.; Da Cruz, G.L.; Levy, R.B.; Monteiro, C.A. Impact of the consumption of ultra-processed foods on children, adolescents and adults’ health: Scope review. Cad. Saude Publica 2022, 37, e00323020. [Google Scholar] [CrossRef] [PubMed]
- Straßburg, A.; Eisinger-Watzl, M.; Krems, C.; Roth, A.; Hoffmann, I. Comparison of food consumption and nutrient intake assessed with three dietary assessment methods: Results of the German National Nutrition Survey II. Eur. J. Nutr. 2019, 58, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.-J.; Tsai, M.-H.; Yu, R.-P.; Hua, L.-C.; Hu, C.-C.; Huang, C. Dezincification of brass water meters in a long-term study: Effects of anions, alkalinity, and residual chlorine. Environ. Sci. Water Res. Technol. 2021, 7, 1666–1676. [Google Scholar] [CrossRef]
- Bacon, A.L. A technical study of alloy compositions of” brass” wind musical instruments (1651–1867) utilizing non-destructive X-ray fluorescence. Ph.D. Thesis, University of London, London, UK, 2003. [Google Scholar]
- Senn, M.; Leber, H.J.; Tuchschmid, M.; Rizvic, N. Blechblasinstrumentenbau in Frankreich im 19. Jahrhundert. Analysen von Legierung und Struktur des Messings zugunsten eines historisch informierten Instrumentenbaus 2016.
- Hachberg, K. Brass in Central European instrument-making from the 16th through the 18th Centuries. Hist. Brass Soc. J. 1992, 229–252. [Google Scholar]
- Albano, M.; Fiocco, G.; Comelli, D.; Licchelli, M.; Canevari, C.; Tasso, F.; Ricetti, V.; Cofrancesco, P.; Malagodi, M. X-rays investigations for the characterization of two 17th century brass instruments from Nuremberg. Acta IMEKO 2022, 11, 1. [Google Scholar] [CrossRef]
- Davies, D.D. A note on the Dezincification of Brass and the Inhibiting Effect of Elemental Additions; Copper Development Association Inc.: New York, NY, USA, 1993. [Google Scholar]
- Lachhab, R.; Galai, M.; Ech-chebab, A.; Belakhmima, R.; Ebn Touhami, M.; Mansouri, I. Comparative study of the corrosion behavior of three alpha brass alloys used in potable water distribution equipment in aggressive soil using electrochemical measurements. Ceram. Int. 2024, 50, 4282–4295. [Google Scholar] [CrossRef]
- Herbert, T.; Wallace, J.; Cross, J. The Cambridge Companion to Brass Instruments; Cambridge University Press: Cambridge, UK, 1997; ISBN 9780521565226. [Google Scholar]
- Biezma, M.V.; Strobl, S.; Linhardt, P.; Ball, G.; Haubner, R. Dezincification in cast and heat-treated alpha-beta brass samples. Pract. Metallogr. 2023, 60, 632–642. [Google Scholar] [CrossRef]
- Lucey, V.F. The mechanism of dezincification and the effect of arsenic. II. Br. Corros. J. 1965, 1, 53–59. [Google Scholar] [CrossRef]
- Sohn, S.; Kang, T. The effects of tin and nickel on the corrosion behavior of 60Cu–40Zn alloys. J. Alloys Compd. 2002, 335, 281–289. [Google Scholar] [CrossRef]
- Galai, M.; Ouassir, J.; Ebn Touhami, M.; Nassali, H.; Benqlilou, H.; Belhaj, T.; Berrami, K.; Mansouri, I.; Oauki, B. α-Brass and (α + β) Brass Degradation Processes in Azrou Soil Medium Used in Plumbing Devices. J. Bio-Tribo-Corroson 2017, 3, 30. [Google Scholar] [CrossRef]
- Galai, M.; Benqlilou, H.; EbnTouhami, M.; Nassali, H.; Belhaj, T.; Berrami, K.; Mansouri, I.; Ouaki, B. Effect of phosphorus content of α-brass on its corrosion resistance in aggressive soil: Experimental and characterization studies. Euro-Mediterr J Environ Integr 2021, 6, 41. [Google Scholar] [CrossRef]
- Selvaraj, S.; Ponmariappan, S.; Natesan, M.; Palaniswamy, N. Dezincification of Brass and its Control—An Overview. Corros. Rev. 2003, 21, 41–74. [Google Scholar] [CrossRef]
- Yohai, L.; Schreiner, W.H.; Vázquez, M.; Valcarce, M.B. Surface characterization of copper, zinc and brass in contact with tap water inhibited with phosphate ions. Appl. Surf. Sci. 2011, 257, 10089–10095. [Google Scholar] [CrossRef]
- Seuss, F.; Gaag, N.; Virtanen, S. Corrosion mechanism of CuZn21Si3P in aggressive tap water. Mater. Corros. 2017, 68, 42–49. [Google Scholar] [CrossRef]
- Gao, Y.; Jie, J.; Zhang, P.; Wang, T.; Li, T. Corrosion Behavior of New Tin-Brass Alloys with Slightly Different Zn Content in Salt Spray Environment. Corrosion 2015, 71, 961–976. [Google Scholar] [CrossRef]
- Avramovic, Z.; Antonijevic, M. Corrosion of cold-deformed brass in acid sulphate solution. Corros. Sci. 2004, 46, 2793–2802. [Google Scholar] [CrossRef]
- Zhou, Y.; Mahmood, S.; Engelberg, D.L. Brass dezincification with a bipolar electrochemistry technique. Surf. Interfaces 2021, 22, 100865. [Google Scholar] [CrossRef]
- Liang, Z.; Jiang, K.; Zhang, T.-A.; Lin, S. Corrosion behavior of brass from the Western Zhou Dynasty in an archeological-corrosive medium. J. Alloys Compd. 2021, 865, 158579. [Google Scholar] [CrossRef]
- Sharifi, E.; Ranjbar, K. Dezincification assisted cracking of yellow brass tubes in a heat exchanger. Eng. Fail. Anal. 2022, 136, 106200. [Google Scholar] [CrossRef]
- Yaqoob, K.; Hashmi, F.; Tanveer, W.H. Failure analysis of cartridge brass shell. Eng. Fail. Anal. 2022, 138, 106325. [Google Scholar] [CrossRef]
- Andersson, J.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science: Thermo-Calc Version 2021b. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- DIN EN ISO 6509-1; DIN Deutsches Institut für Normung e. V. Korrosion von Metallen und Legierungen: Bestimmung der Entzinkungsbeständigkeit von Kupfer-Zink-Legierungen -Teil 1: Prüfverfahren (ISO 6509-1:2014). Beuth Verlag GmbH: Berlin, Germany, 2014.
- DIN 50916-1:1976-08; DIN Deutsches Institut für Normung e. V. Prüfung von Kupferlegierungen: Spannungsrißkorrosionsversuch mit Ammoniak, Prüfung von Rohren, Stangen und Profilen. Beuth Verlag GmbH: Berlin, Germany, 2023.
- DIN 50918:2018-09; DIN Deutsches Institut für Normung e. V. Korrosion der Metalle: Elektrochemische Korrosionsuntersuchungen. Beuth Verlag GmbH: Berlin, Germany, 2018.
- Kuron, D. Korrosion und Korrosionsschutz. Beurteilung, Prüfung, Schutzmaßnahmen; Normen, Technische Regeln; DIN-Taschenbuch 219, Hrsg. von DIN, Deutsches Institut für Normung e. V., 502 Seiten, 2. Auflage; Beuth Verlag GmbH: Berlin, Germany; Wien, Austria; Zürich, Switzerland, 1995; DM 178.00; ISBN 3-410-13167-1. Available online: https://katalog.ub.tu-freiberg.de/Record/0-090004558 (accessed on 20 May 2024).
- Arslan, F.; Cansever, N.; Duby, P.F. Examination of Tarnish Films Formed During SCC of α-Brass in Ammoniacal Solutions / Untersuchung der während Spannungsrisskorrosionsprüfungen an α-Messing in ammoniakhaltigen Lösungen gebildeten Anlaufschichten. Pract. Metallogr. 2001, 38, 175–188. [Google Scholar] [CrossRef]
- Ozgowicz, W.; Kalinowska-Ozgowicz, E.; Grzegorczyk, B. The microstructure and mechanical properties of the alloy CuZn30 after recrystallizion annealing. J. Achiev. Mater. Manuf. 2010, 40, 15–24. [Google Scholar]
- DIN EN 12502-2:2005-03; DIN Deutsches Institut für Normung e. V. Korrosionsschutz Metallischer Werkstoffe_- Hinweise zur Abschätzung der Korrosionswahrscheinlichkeit in Wasserverteilungs- und Speichersystemen: Teil_2: Einflussfaktoren für Kupfer und Kupferlegierungen. Deutsche Fassung EN_12502-2:2004. Beuth Verlag GmbH: Berlin, Germany, 2005.
- Namboodhiri, T.; Chaudhary, R.; Prakash, B.; Agrawal, M. The dezincification of brasses in concentrated ammonia. Corros. Sci. 1982, 22, 1037–1047. [Google Scholar] [CrossRef]
- Zhou, P.; Hutchison, M.; Erning, J.; Scully, J.; Ogle, K. An in situ kinetic study of brass dezincification and corrosion. Electrochim. Acta 2017, 229, 141–154. [Google Scholar] [CrossRef]







| Element | Cu | Zn | Ni | Sn | P |
|---|---|---|---|---|---|
| Average | 66.97 | 32.63 | 0.15 | 0.09 | 0.17 |
| Element | β-Brass X in wt.% | Other Phases X in wt.% | Other Phases Name |
|---|---|---|---|
| Ag | 2.2 | - | - |
| Al | 1.0 | 0.4 | γ-phase |
| As | 2.2 | 0.7 | Cu3As |
| Au | - | - | - |
| B | 0.01 | 0.2 | B |
| Be | 0.01 | - | - |
| Bi | - | 0.2 | Bi |
| C | 0.01 | 0.2 | Gr. |
| Ca | 0.8 | 0.01 | CaCu5 |
| Cd | - | 0.06 | Cd8Cu5 |
| Co | - | 0.02 | γ-phase |
| Cr | 0.01 | - | - |
| Fe | 0.01 | - | - |
| Mg | 1.2 | 0.02 | Cu2Mg |
| Mn | - | 0.2 | CuMnZn |
| Mo | 0.01 | . | . |
| Nb | 0.01 | . | . |
| Ni | - | 0.2 | NiZn |
| O | 0.8 | 0.01 | Cu2O |
| P | - | 0.01 2.5 | P2Zn3 Cu3P |
| Pb | - | 0.01 | Pb |
| Pt | - | - | - |
| Se | 2.7 | 0.01 | Cu2Se |
| Si | 0.6 | 0.8 | Si |
| Sn | 2.7 | 0.02 | Cu3Sn |
| Ti | 3.1 | 0.01 | CuTi |
| Zr | 2.2 | 0.01 | Cu51Zr14 |
| Alloy | φ | Hardness [HV1] | Rp0.2 [MPa] | Rm [MPa] | A [%] |
|---|---|---|---|---|---|
| CuZn30X (cold rolled) | 1.64 | 229 ± 5 | 683 ± 9 | 736 ± 9 | 1.5 ± 0.3 |
| CuZn30X (hot rolled) | 1.53 | 233 ± 5 | 604 ± 7 | 647 ± 11 | 2.9 ± 0.4 |
| CuZn30 (cold rolled) [52] | 1.61 | 680 | 2.5 |
| Element | Cu | Zn | Ni | Sn | P | O | Cl |
|---|---|---|---|---|---|---|---|
| CuZn30 | 85.4 | 3.1 | - | - | - | 6.6 | 4.9 |
| CuZn30X cold rolled | 63.9 | 15.4 | - | 0.6 | 0.4 | 8.0 | 11.7 |
| CuZn30X hot rolled | 66.8 | 19.6 | - | 0.7 | 0.5 | 8.1 | 4.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berndorf, S.; Markelov, A.; Guk, S.; Mandel, M.; Krüger, L.; Prahl, U. Development of a Dezincification-Free Alloy System for the Manufacturing of Brass Instruments. Metals 2024, 14, 800. https://doi.org/10.3390/met14070800
Berndorf S, Markelov A, Guk S, Mandel M, Krüger L, Prahl U. Development of a Dezincification-Free Alloy System for the Manufacturing of Brass Instruments. Metals. 2024; 14(7):800. https://doi.org/10.3390/met14070800
Chicago/Turabian StyleBerndorf, Susanne, Anatol Markelov, Sergey Guk, Marcel Mandel, Lutz Krüger, and Ulrich Prahl. 2024. "Development of a Dezincification-Free Alloy System for the Manufacturing of Brass Instruments" Metals 14, no. 7: 800. https://doi.org/10.3390/met14070800
APA StyleBerndorf, S., Markelov, A., Guk, S., Mandel, M., Krüger, L., & Prahl, U. (2024). Development of a Dezincification-Free Alloy System for the Manufacturing of Brass Instruments. Metals, 14(7), 800. https://doi.org/10.3390/met14070800







