Abstract
The technosphere consists of material stocks accumulated by human activities, which can include processing residue, such as slag. Various smelting processes generate slag, and some valuable elements are concentrated in this by-product. In this review, the extraction of critical and strategic metals from non-ferrous slags is discussed. Critical and strategic metals are materials that are vital for the nation’s economy and defence, as well as its industries, and have common features, such as expected shortfalls, increasing demand, and few substitutions. There are several definitions, methods, and classifications of critical and strategic elements by different organisations. In this study, reports from seven institutions around the world are summarised, and a list of recommended critical and strategic metals is presented. Non-ferrous slags contain a considerable amount of critical and strategic elements, and research on technology and process development using both pyro- and hydrometallurgical methods is very attractive. When it comes to the extraction of values from slag and the development of technology, it is not only important to consider the economic aspect but also to ensure the processes are low in emissions and energy consumption but high in efficiency and recycling.
1. Introduction
The extraction of metals from ores using high-temperature processing, or pyrometallurgy, produces slag as a by-product. Slag is a fusible or molten mass formed when flux, on being subjected to heat, reacts with impurities in the ore. Slag floats on top of matte and can be collected separately [1]. It can normally be divided into two groups: ferrous slags and non-ferrous slags [2]. Other slags, such as those generated from municipal waste, are excluded from this study to avoid presenting an overwhelming amount of information. The focus of this review is non-ferrous slag from metallurgical operations.
Slag is regarded as waste and has applications in brick, cement, and construction [1]; however, these is much attention due to the valuable metals it contains. Slag may cause environmental concerns for its release of potentially toxic elements; however, utilising slags as a secondary source will reduce the possibility of environmental exposure [2,3,4,5,6,7].
While there is abundant information available on copper slag, the characteristics of other slags such as nickel, tin, and vanadium slags are relatively less known, and their considerations as a potential secondary source of valuable metal warrant detailed characterisation and analysis of physical and chemical properties both for metal extraction and environmental purposes [2,7]. Subsequently, the recovery of valuable metals from ferrous and non-ferrous slag sources has been reviewed from the perspective of repurposing and reprocessing the material [6]. Ferrous slags have been effectively utilised for various applications such as cement production, road construction, and others [6]. Non-ferrous slags also have the potential to be utilised in those applications; however, they contain critical and strategic metals, which makes them more valuable as secondary sources for these metals. This review will focus on the contents of critical and strategic elements in non-ferrous slags and the extraction methods to recover them from the slag. Projected shortfalls, increasing demand, and limited substitutions make metals critical and strategic, and it is hard to secure the supply even though these metals are essential.
As mentioned, there are a few general review articles on slag, and these include the following: characterisation [2,7,8], recovery of values from slag [3,6,9], the environmental aspect [2], bioleaching potential [10,11], each commodity such as copper [12], vanadium [13], titanium [14], tungsten [15], and cobalt [4]. There has been no literature published that focuses specifically on the critical and strategic metals, particularly those contained in non-ferrous slags, and on the potential of critical and strategic metal extraction from these materials. In this review paper, the concept of technospheric mining and its application to the extraction of critical and strategic metals from non-ferrous slags are discussed. This paper also details the different critical and strategic elements found in non-ferrous slags and the processes studied and applied to extract them.
2. Research Methodology
This review paper is devoted to exploring the technospheric mining of critical and strategic metals from non-ferrous slags by introducing the terms, reviewing the literature database, and exploring relevant technologies in hydro- and pyrometallurgy.
The references in this paper were searched through ‘Google Scholar’ using keywords and were carefully chosen to reflect relevance to the topic. Publications on technospheric mining and the technosphere were used to provide a summary of the concept, except for dissertations. Most reports were chosen to introduce the classifications and methodologies of critical and strategic metals from each institute. There are also other countries or organisations available, for example India and Korea, that provide reports on critical and strategic metals. However, only seven geologically representative institutions were chosen for this review.
An extensive literature review was conducted on the recovery of critical and strategic metals from each slag using various methods. In terms of extraction methods, hydrometallurgical and pyrometallurgical methods such as leaching or roasting were the key technologies to focus on over mineral processing and bio-treatment methods unless specifically relevant and useful to restrict the scope. Some slags have limited literature available. On the other hand, copper slag had extensive literature; thus, strict and careful criteria were used to select the relevant literature; for example, the extraction of critical and strategic metals in metallic or semi-metallic forms was the main focus of choosing the literature.
3. Technosphere and Technospheric Mining
The technosphere contains materials, known as technospheric stocks, which are generated and accumulated by human and technological intervention. These stocks may include waste repositories and by-products, such as urban and industrial waste, process residue, mine waste, process effluents, slag, and tailings [16,17]. Researchers from the 1990s introduced, defined, and classified technospheric stocks and their status based on several factors relating to material flow and composition [17,18,19,20,21]. They can also be categorised based on location, type of stock, management, and state of the stocks. The subgroups of technospheric stocks are shown in Table 1 [17,22]. The concept of mining the technosphere refers to technospheric mining.

Table 1.
Sub-classification of technospheric stocks.
Technospheric mining is a term and concept referring to recovering minerals or metals from material stockpiles established by human activity and anthropogenic processes [17]. It is an umbrella concept that incorporates various terminologies common in a circular economy, such as resource recycling and urban mining. Johansson et al. [17] suggested a taxonomy in technospheric mining to address a better understanding of this newly developing field, providing a more holistic approach to reprocessing or repurposing unwanted, end-of-life, or waste materials. For example, the taxonomy also described and reviewed landfill mining [23] and urban mining [24]. There are a number of publications now highlighting the application and importance of technospheric mining and the development of technologies to recover valuable metals from technospheric stocks [25,26,27,28].
Some of the technospheric stocks, such as tailing, slag, and metallurgical by-products, have drawn attention since they contain a significant amount of critical and strategic metals [28,29]. However, accumulated tailings or slags potentially contain materials that are hazardous for humans and the environment and can cause tragic accidents when managed inappropriately. Thus, employing the technosphere as a secondary source of resources can contribute to mitigating those environmental problems. Therefore, mining the technosphere is a desirable way to reach sustainable development in social and environmental sections, along with obtaining critical and strategic metals from secondary sources.
Due to projected shortfalls, increasing demand, and limited substitutions of critical and strategic metals, the mining industry needs to pay attention to the exploitation of secondary resources that are already extracted and stored as waste to secure critical and strategic metals. Advanced technology, economic and political advocacy, and the enormous amount of technospheric stock support technospheric mining in becoming a new business model. Technospheric mining not only provides secondary sources for critical and strategic metals but also assists in achieving a circular economy that promotes sustainable development, especially in the environment. Overall, technospheric mining can provide economic, environmental, and industrial advantages.
4. Critical and Strategic Metals
Critical metals are essential for the economy and trade, and strategic metals are needed for a nation’s defence and its economy [30]. There are multiple definitions, methodologies, and classifications of critical and strategic metals from various institutes [31]. Although several publications on critical and strategic materials or metals have significantly escalated since the 2010s [32], the concept of strategic and critical raw materials publicly appeared in 1939 with a concern for securing those metals that are required in case of war but are unobtainable within the country and thus need to be imported from other countries. The list of critical and strategic materials in 1939 from the United States included antimony, asbestos, bauxite, camphor, cobalt, cryolite, ferrous alloys, iodine, mercury, opium, quinine, platinum, potash, rubber, and tin [33,34], of which some are still critical and strategic in society and others are not anymore.
It is worthwhile to note that criticality is a degree of how critical each element or metal is; for example, from the measure of 0 to 100, rather than the binary state of ‘critical’ or ‘non-critical’. Particularly, critical metals refer to those that have increasing demand with scarce supply and, therefore, become more important in the industry [35]. Similar to critical metals, some metals that are essential for a nation’s defence are classified as strategic, and they share distinct features: (1) Strategic metals are concentrated in a few countries, causing resource nationalism: (2) They are highly essential in the high-technology industry, for example, solar panels, wind turbines, and superalloys: (3) They are irreplaceable, which means few replacements for the strategic metals are available [35,36]. Several articles [37,38] mentioned the terms ‘strategic’ and ‘strategic metals’ but did not clarify what the terms mean or the methodology.
Critical and strategic metals are getting more attention with the increasing number of publications and reports, yet there is no comprehensive definition that is agreed on by the institutes or governments around the globe since the geological, political, infrastructural, and environmental situations and factors are divergent [31,32]. A conundrum in this situation is whether it is possible to have a globally defined, long-term classification and methodology for critical and strategic metals. As technology and industry evolve, the factors in the methodology change rapidly, which inevitably results in the alteration of critical and strategic metals. Jim et al. [31] also argued that having a comprehensive and concrete methodology would be ideal, although the amount of work for assessment can be incredibly large; thus, simplifying the assessment can reduce the work and, at the same time, the precision of the result as well.
Representatively, national organisations and governments, in alphabetical order, the Australian Government (AG), the British Geological Survey (BGS), the Chinese Government (CG), the European Commission (EC, referred to as EU), the Ministry of Mines from Government of India (GI), the Organisation for Economic Co-operation and Development (OECD), and the United States Department of the Interior from the United States of America (US DoI, referred to as the USA), released publications on critical and strategic materials and their strategies to secure those valuable metals.
Table 2 shows the critical and strategic metals selected by the institutions listed above. X in Table 2 means the institute chose the element as critical and/or strategic. Among metals described by several organisations, those shared in the list in Table 2 are classified as critical and strategic. This table, however, excludes non-metals such as borate (BO33− or B4O72−), helium (He), fluorine (F), phosphorus (P), and natural rubber [39,40,41], while acknowledging that the non-metals are vital for many industries. The purpose of Table 2 is to compare the common critical and strategic metals that were chosen from the seven institutes, regardless of the geological factors. For instance, all institutes categorised REEs as critical and strategic, whereas only two institutes consider cadmium (Cd) as critical and strategic. Metals classified as critical and strategic by two institutes out of seven can be considered low priority globally, for example, rhenium (Re), silver (Ag), thorium (Th), uranium (U), as well as Cd. Some metals that were chosen by only one institute were eliminated from Table 2, considering their criticality not to be as globally significant as others. The metals that were chosen only once were the following: caesium (Cs), rubidium (Rb), tellurium (Te) by the USA [42], and mercury (Hg) by BGS [43].

Table 2.
Classification of critical and strategic metals.
The Australian government initiated the Critical Minerals Strategy, which entails the vision of critical minerals by 2023, and became the global supplier of those minerals. The Critical Minerals List provides not only the critical minerals that are crucial to the technologies necessary for achieving net zero emissions as well as energy security, but also the strategic materials that are regarded as a ‘watchlist’ to ensure there is no disruption on the supply chain [40]. Among the metals and materials listed in Table 2, the strategic materials classified by Australia are Al, Cu, Ni, P, Sn, and Zn.
The British Geological Survey published a report on the supply risk of chemical elements and its methodology. The supply risk index rates each element from 4.5 to 9.5. The element with the highest index is Rare Earth Element (REE) with a rate of 9.5, and the lowest-rated metal was gold with 4.5, indicating incredibly insignificant risk. The critical metals chosen by the BGS alone are shown in Table 2. A few metals, for example, mercury (Hg) and uranium (U), were excluded from the list because they were chosen by only one institute [43].
The Chinese government released National Mineral Resources Planning in 2016 and classified 24 minerals as critical and strategic within three categories (energy, metal, and non-metal). However, this government report was only available in Chinese; thus, the alternative publication in English was used to obtain the list of critical minerals from the Chinese government [41].
The European Commission has published a report on critical raw materials every three years since 2011, and the latest report in 2023 has been mostly referenced. Based on market fluctuations, progress of technology, and production status, the list changes every three years in the reports. The critical raw metals (CRMs) in Table 2 are notably vital in Europe because of their high demand in various industries and the advancement of green and high technology. The two main parameters used by the EU in the methodology to assess the criticality of metals since 2011 are economic importance (EI) and supply risk (SR), which comprise several factors. The EU Commission is trying to ensure the consistency of the assessment throughout the years [39].
The Ministry of Mines of the Government of India published Critical Minerals for India [44]. The report included a global overview, five factors of the value chain of critical minerals, the methodology of classification, and so on. An interesting and meaningful exercise by the Government of India that was included in this report was a comparison of critical minerals chosen by different ministries in India through inter-ministerial consultation. For example, the Department of Science and Technology and the Department of Pharmaceuticals notably chose different minerals as critical; hence, a broad spectrum of critical minerals was reviewed and chosen [44].
The Organisation for Economic Co-operation and Development has 38 countries from around the globe as members, building international standards and policies and solving various issues together [45]. Hence, the OECD’s view on critical and strategic materials is significant since the organisation represents a broad perspective regardless of geological factors. In terms of critical metals, the OECD is mentioned in the case study of critical metals and mobile devices for sustainable material management. The organisation acknowledges that the definition of critical metals is subject to change over time due to factors such as geography and technology [46].
In 2020, the Energy Act of 2020 was passed by the United States Congress to boost clean energy and climate policy in the US. Following the Energy Act of 2020, the Secretary of the DoI, who is also the director of the US Geological Survey (USGS), published the draft list of critical minerals in the Federal Register. This 2021 list was consulted by the Department of Agriculture, Commerce, Defence, and Energy, and the United States Trade Representative [42]. In 2022, the Final List of Critical Minerals was published [47]. The recent reports on the critical materials from the Department of Defence (DoD) and Energy (DoE) referred to the list from the DoI [48,49]. Even if a commodity has no supply risk or trade exposure and then a single producer in the US becomes unable to produce the commodity, there is a chance to be exposed to the supply risk, which was categorised as Single Point of Failure (SPOF). With the SPOF method, Be, Ni, and Zr are included in the draft list of critical minerals. In cases where there was insufficient data for a quantitative assessment of a certain commodity, a qualitative evaluation of supply and demand was conducted. As a result, caesium (Cs), rubidium (Rb), Sc, and the REEs remained on the list [50].
5. Recovery of Critical and Strategic Metals from Non-Ferrous Slags
As demand for critical and strategic metals keeps increasing from various industries, securing primary and secondary sources of critical and strategic materials and having feasible extraction technologies are crucial for nations’ economies and defence. Hence, in the following chapter of this paper, we would like to introduce the potential of extracting critical and strategic metals from the by-product of the smelting process, non-ferrous slags. Indeed, because the recovery of critical and strategic metals from slag materials is still in its infancy, the available literature is limited. Thus, tin, copper, nickel, vanadium, and titanium slags were studied. Also, it is worth noting that the emphasis on base metals will be less than that on non-base metals since the production and supply of base metals from the primary source are more stable than the other critical and strategic metals.
5.1. Tin Slag
Tin slag is a by-product of the tin smelting process. Tin slag has drawn broad attention since it contains a significant amount of critical and strategic metals, representatively Nb and Ta [51]. It can be considered a secondary source of Nb and Ta, as there are a limited number of primary ores of those metals [52]. Several studies [52,53,54,55,56,57] have attempted to recover valuable metals from tin slag using pyro- and hydrometallurgical methods. The chemical composition of tin slag, including the critical and strategic elements, is shown in Table 3. Tin slag became a feasible candidate for secondary sources of Ta when the market price increased [58], and it can happen any time soon for other valuable metals or commodities as well.
As shown in Table 3, tin slags contain not only Sn, Nb, and Ta but also critical and strategic metals such as Ti, W, V, Cr, Ni, and REEs. The figures in Table 3 are mostly average concentrations of samples to avoid including all the samples from the same countries and regions in each paper [52,59]. The concentrations of Nb and Tb vary significantly depending on the origin of the sample: Nb 70.89 mg/kg to 14.1% and Ta 1.75 mg/kg to 20.8% as oxides. Thus, many sources actually have a high grade of Nb and Ta: Nb + Ta > 8% high, 5% < Nb + Ta < 8% low, and Nb + Ta < 5% extremely low [58]. Also, the concentrations of tin slags from Smelterskop and Elandsberg, South Africa are ancient samples from 1650 to 1850 CE. The geologists estimated that 180,000 tonnes of tin were mined in these regions before 1905 [59]. Thus, considering the grade of Sn in these regions is 11.10% SnO to 25.28 SnO2, it might be feasible to extract Sn from the historical tin slags in South Africa.

Table 3.
Compositions (%) of critical and strategic elements in tin slags.
Table 3.
Compositions (%) of critical and strategic elements in tin slags.
| Origin (Region) | Nb2O5 [Nb] | Ta2O5 [Ta] | (Ta, Nb)2O5 | TiO2 [Ti] | SnO2 [Sn] | MgO [Mg] | Al2O3 [Al] | MnO [Mn] | WO3 [W] | V2O5 [V] | Cr2O3 [Cr] | Ni2O3 [Ni] | Ce + La | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N/A | 9.35 | 2.6 | 11.95 | 5.9 | - | 5.3 | 6.56 | - | - | - | - | - | - | [52] |
| N/A | 5.2 | 7.5 | 12.7 | 1.3 | 0.7 | - | 11.2 | 3.7 | - | - | - | - | - | [53] |
| Nigeria | 14.1 | 20.8 | 34.9 | - | 0.37 | 1.2 | 5.1 | 1.7 | - | - | - | - | - | [55] |
| Malaysia | 3.43 | 3.05 | 6.48 | 7.97 | 1.33 | - | 10.42 | 0.7 | 1.4 | - | - | - | - | [56,60] |
| 4 | 4 | 8 | 11 | 0.5 | 0.5 | 9 | 0.5 | 8 | 0.5 | - | - | - | [61] | |
| Indonesia | - | - | 2.7 | 13.3 | 0.8 | - | 8.0 | 0.4 | 0.0 | - | - | - | - | [57,60] |
| Indonesia | 0.33 | 0.64 | 0.97 | 11.92 | - | - | 11.7 | - | - | - | - | - | - | [62,63] |
| (Bangka) | - | - | - | 2.38 | 1.78 | - | 0.52 | - | - | - | - | - | 1.7 | [63] |
| Spain | - | - | 18.7 | 7.3 | 0.1 | - | 6.9 | 1.7 | 0.1 | - | - | - | - | [57,60] |
| South Korea | - | - | 10.8 | 17.8 | 0.8 | - | 4.7 | 0.7 | 0.1 | - | - | - | - | [57,60] |
| Thailand | - | - | 24.9 | 15.5 | 0.7 | - | 7.0 | 2.1 | 1.0 | 0.14 | 0.65 | - | - | [57,60] |
| DR Congo | - | - | 12.7 | 1.3 | 0.7 | - | 11.2 | 3.7 | 0.2 | - | - | - | - | [57,60] |
| England | - | - | - | - | 16.44 | 2.21 | 10.60 | 0.68 | 7.31 | - | - | - | - | [64] |
| Zimbabwe | [29.9] | [27.3] | - | [0.16] | - | - | [0.17] | [2.95] | [0.41] | [0.06] | [0.03] | [0.13] | - | [65] |
| Australia | 5.5 | 10 | 15.5 | 9 | - | - | - | - | - | - | - | - | - | [62] |
| South Africa | 7 | 5 | 12 | 2 | - | - | - | - | - | - | - | - | - | [62] |
| (Smelterskop) | 70.89 * | 1.75 * | 72.64 * | 1.4 | 25.28 | 3.09 | 11.86 | 0.11 | 0.34 | 0.11 | 0.045 | 20.41 * | 456.9 * | A, [59] |
| (Elandsberg) | 0.1 | - | 0.1 | 8.61 | 11.19 ᵃ | 2.37 | 7.34 | 0.19 | - | 0.13 | 0.13 | 0.02 ᵇ | - | A, [59] |
| Brazil | - | - | - | 0.8 | - | 6.2 | 11.8 | 0.7 | - | - | - | - | - | [66] |
| Brazil | [0.3] | [0.5] | - | [1.0] | [2.0] | [4.0] | [2.0] | [0.6] | - | - | - | - | - | [51] |
* is mg/kg, not %. A is the ancient sample. ᵃ and ᵇ represent different forms of oxide; in this case, ᵃ is SnO and ᵇ is NiO.
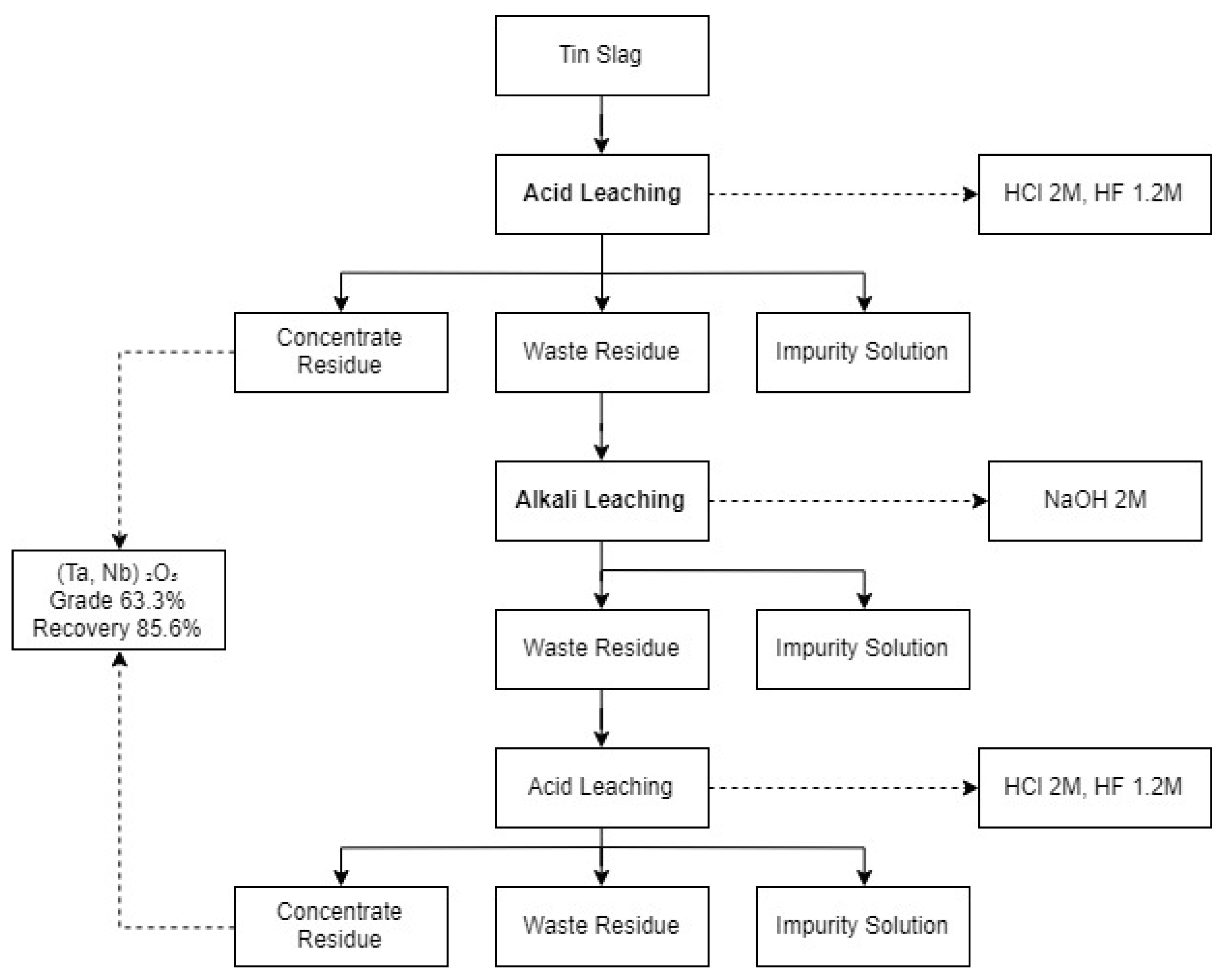
Nb and Ta are the main products that can be extracted from tin slags. As shown in Table 4, several studies [52,53,54] showed that pyrometallurgical methods, such as chlorination and carbochlorination, were mostly adapted for the recovery of Nb and Ta. For the hydrometallurgical methods, successive acid and alkali leaching were studied [55,56,57,67]. Conventional methods of extracting Nb and Ta from tin slags are hydrofluoric acid leaching or successive leaching of hydrofluoric and sulphuric acids, followed by carbochlorination. So, the most suitable process in the past would have been a combination of pyro- and hydrometallurgical methods. However, they are less utilised due to economic and environmental issues these days [53]. In June 2019, the latest study by [57], showing the best outcomes of grade and recovery of Nb and Ta, developed the acid–alkali–acid leaching process presented in Figure 1. Although a greater recovery of Nb and Ta is desirable, the grade of the product is outstanding compared to pyrometallurgical methods such as carbochlorination and roasting.

Table 4.
Extraction methods of critical and strategic metals from tin slags (Condition and recovery given for bolded method).
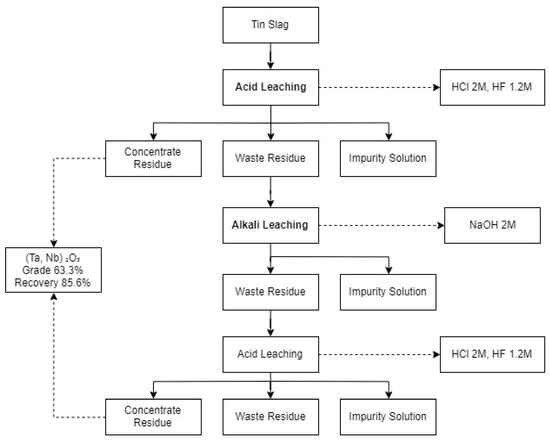
Figure 1.
The optimum process for tin slag to recover niobium and tantalum adopted from [57].
Tin slags also contain other critical and strategic metals, such as Ti and REEs, in addition to Sn. Since current studies mostly focus on recovering Nb and Ta from tin slags, a knowledge gap exists in the recovery of other critical and strategic metals. Hence, exploring a suitable process to extract other critical and strategic metals from tin slags is a beneficial and desirable provision for the mineral industry. Other research gaps and current drawbacks of utilising tin slags include the following: (1) low grade and recovery of critical and strategic metals; (2) separation of valuable metals that have chemical similarities; and (3) silica gelation. Since the latest study [57] was able to produce a commercial grade product (63.3%), the research is progressing with technology. It will be favourable to examine green reagents, for instance, organic acids.
5.2. Copper Slag
Copper slag is a by-product of copper production. As much as copper mines have been active around the world, an enormous amount of copper slag has been accumulated in dumps. Copper slag is known as a suitable material for construction, geotechnical applications, road pavements, and so on due to its physical and chemical properties [68,69]. However, this review attempts to highlight the potential economic benefits of copper slag as a great secondary source of valuable metals since copper slag contains a significant amount of critical and strategic metals, as shown in Table 5 [70].

Table 5.
Compositions (%) of critical and strategic elements in copper slags (F: Furnace slag, C: Converter slag, A: Ancient slag).
There are two forms of copper slag, which vary based on their cooling processes. Air-cooled slag and water-quenched slag show different properties. Water-quenched slag has a more granular form, like sand. Thus, it can be suitable for use in the construction industry. Air-cooled slag is slow-cooled and can be suitable for extracting valuable metals since it may contain various forms of minerals due to crystallisation with enough time [6,68,69]. However, slow-cooled slags tend to have more dense structures than fast-cooled slags, so it is sometimes harder to liberate valuable metals from slow-cooled slags.
Every ton of copper production generates 2.2 tonnes of copper slag [8]. Total reserves of copper in the world are estimated at 1 billion tonnes in 2024, and the global mine production of copper in 2023 was 22 million tonnes [102], which would have generated 44 million tonnes of slag. The largest production occurs in Chile, which was 5 million tonnes in 2023. Chile is followed by Peru, Congo, China, the USA, Russia, Indonesia, Australia, Zambia, Mexico, Kazakhstan, Canada, Poland, and others in terms of copper mine production [102]. As is shown in the active production of copper, a significant amount of copper slag keeps being dumped worldwide. It would be beneficial to recover valuable metals from copper slag for those countries that have significant amounts of dumped slag and will constantly generate slag in the future.
The amount of copper slag that is dumped in known and unknown places would be immeasurable. For example, around 2.5 million tonnes of ancient Turkish copper slag from the 13th century have been stored in the northern part of Turkey. The ancient slag also was adopted to develop the process by researchers. Based on the chemical analysis, this slag has 0.5% of cobalt, which can produce around 12,500 tonnes of cobalt [74,75], which is equivalent to 337 million US dollars (US $27,000 per tonne in June 2024) [103]. There is a literature [104] that reports a pilot-test work performed on a copper reverberatory furnace slag in Zambia and the production of cobalt from the copper slag. Around 20 million tonnes of dumped slag that contained 0.66% cobalt were processed through the furnace, which commenced in 2001.
Methods to recover critical and strategic metals from copper slags are presented in Table 6. The main valuable metals are Cu, Co, and Ni. The extraction of base metals from copper slag was excluded since base metals’ criticality is lower than that of critical and strategic metals [43,105]. To recover Co from copper slags, roasting and leaching were adopted, along with sulphurisation, flotation, precipitation, and so on. More details on the generation, characteristics, utilisation, environmental impacts, and recycling technology of copper slag, regardless of the critical and strategic elements, can be referred to in [8,9,12].

Table 6.
Extraction method for critical and strategic metals from copper slags.
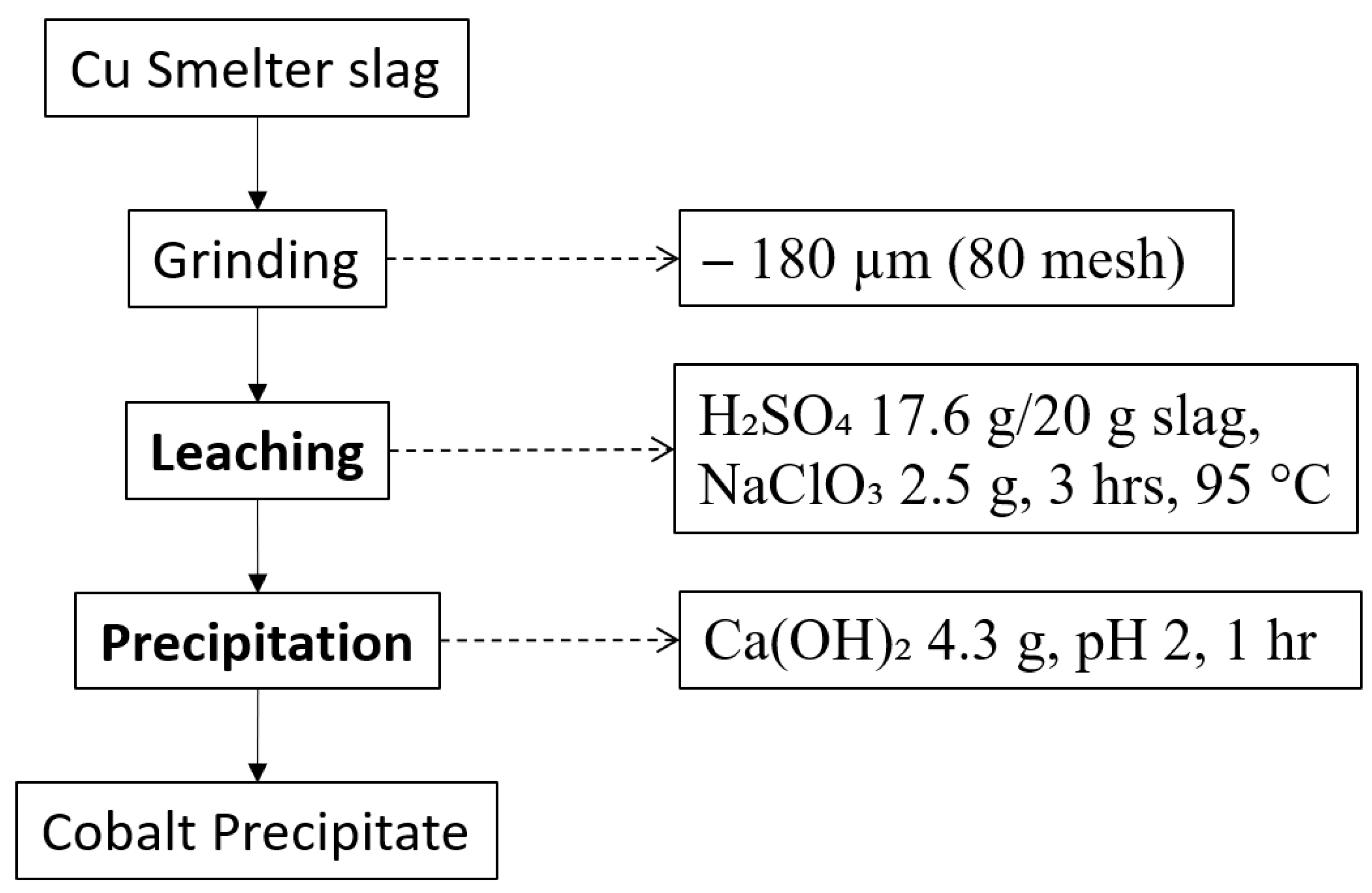
Several attempts have been made to recover Co from copper slag, mainly by hydrometallurgy approaches. They are showing remarkable results, considering that traditional methods mostly include roasting or other pyrometallurgical methods. After leaching, researchers also adapted separation and purification methods, such as solvent extraction and precipitation. Figure 2 is the process flowsheet to recover Co selectively using leaching with sulphuric acid and sodium chlorate as an oxidant and precipitation with calcium hydroxide, and it produced 98% recovery of Co, which is an outstanding result considering it was conducted under atmospheric pressure [86].
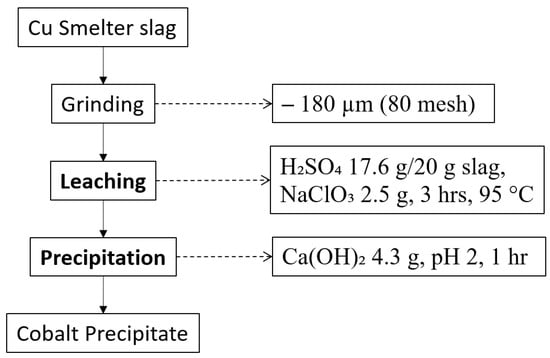
Figure 2.
Process flowsheet of copper slag adopted from [86].
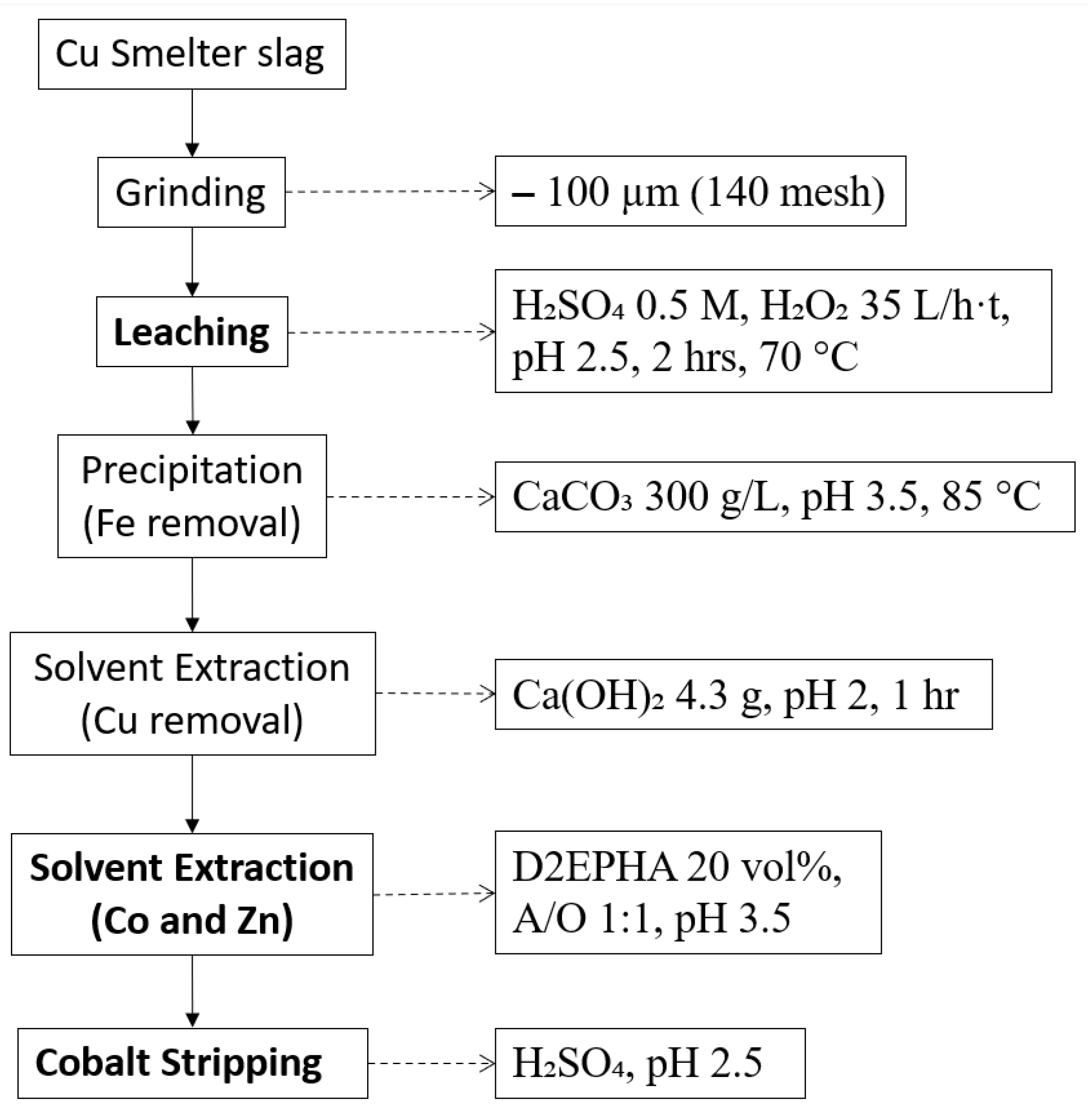
Another feasible process is shown in Figure 3. Banza and colleagues investigated a combined process of hydrometallurgical methods to extract Co, Cu, and Zn. Leaching was conducted in a sulphuric acid medium with hydrogen peroxide as an oxidant. Then, Fe was separated through precipitation, and Cu was extracted by solvent extraction. Finally, Co and Zn were recovered from the solution by solvent extraction with D2EPHA, and they were stripped separately. The overall recovery of Co was 90%, which indicates it is promising to take into consideration for adapting hydrometallurgical methods to upgrade the copper slag for the extraction of critical and strategic metals [87].
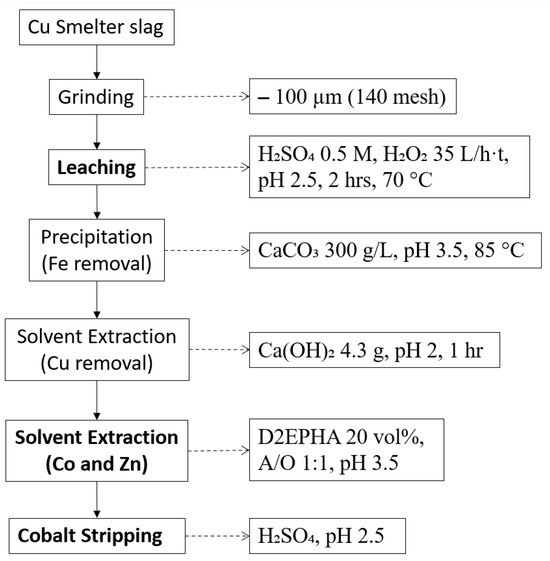
Figure 3.
Process flowsheet of copper slag adopted from [87].
A study was also conducted to recover Sn from copper slag. As shown in Table 2, Sn is one of the critical and strategic metals in copper slag. The process includes size reduction and classification, flotation, and leaching. In flotation, Cu was recovered as concentrate and Sn remained in tailing. In the leaching test, the recovery of Sn was not as efficient as that of Cu since the process was focused on Cu extraction. Consequently, there is still a research gap in the recovery of Sn from copper slag [109]. Including the processes that were mentioned above, hydrometallurgical methods are listed in Table 6 to recover critical and strategic metals from copper slag [97,98,101,113].
As one of the hydrometallurgical methods for extracting critical and strategic elements from copper slag, bioleaching has been studied for the past decades. Bioleaching is considered an environmentally friendly and sustainable process and is a gradually evolving technology [114]. In bioleaching, microorganisms play a key role as extractants [115]. A recent study by Mikoda et al. (2019) studied the feasibility of bioleaching assisted by Acidithiobacillus thiooxidans. The optimal conditions for this study were a particle size of 0.25–0.5 mm and a pulp density of 1% for 28 days. With these conditions, high recoveries of Co, Mo, REEs, and V were achieved: shaft furnace slag—Co 100%, Mo 44%, REE 70%, and V 70%; and granulated slag—Co 95%, Mo 70%, REE 99%, and V 93% [80]. Another study from the same research group suspected that the bioleaching of historical copper slag could create up to $135 worth of metals from a ton of slag. Hence, extracting values from historical copper slag can be an economically and environmentally positive option [116]. A broader perspective has been implemented by Lee and Pandey (2012), who reviewed the bioprocessing of solid wastes, including copper slag, for metal extraction [10]. Using a mixed culture that contains Acidithiobacillus and Leptospirillum with 10 g/L sulphur (S) and 4.4 g/L ferrous sulphate (Fe2⁺) at a pulp density of 10 g/L and a pH of 1.5, in 42 days, 100% recovery of Ni was achieved [117]. Mehta and Pandey [118] recovered 64% of cobalt and 50% of nickel from copper converter slag in 80 days with the optimum conditions of pH 2, pulp density 1/20, and particle size −75 µm using Thiobacillus ferrooxidans. Despite significant research efforts aimed at developing bioleaching processes, most of the experiments have been limited to the laboratory scale [10]. Since the new extraction processes should minimise the impacts on the environment and pursue economic profit, bioleaching is considered a green technology for the mining industry.
Pyrometallurgical methods tend to be used frequently owing to effective sulphurisation or reduction. Thus, there have been various implementations of pyrometallurgical methods on copper slag. A recent study by Yuksel and Teğin [73] reported a huge improvement in pyrometallurgy by reducing the temperature for roasting, which saves energy as well as the processing budget. A total of 98.4% of Co from copper slag was recovered by roasting at 600 °C for 5 h with a 3:6:6 ratio of slag: pyrite: copper concentrate and iron powder [73], whereas other studies [84,85] required 1350 °C to extract around 95% Co. Depending on the methods, Co remains in the depleted slag after roasting [84], or a cobalt-bearing alloy can be produced after reduction [85]. In these cases, recoveries of Co were 95% and 94.02%, respectively. There is also a study to identify the most suitable modifier to selectively recover Co from copper slag when the reductant is graphite. It turned out to be more titanium dioxide than calcium oxide or calcium fluoride [112].
One of the greatest achievements of exploiting slag as a secondary source is the pilot test work that was later conducted at a plant in Zambia in 2001. A 40 MW DC arc furnace was built to extract cobalt from the 20 Mt reverberatory furnace slag dump. The feed was specifically blended with slag, lime, and coal, and the tapping temperature on average was 1500 °C [104]. This example indicates that it is economical and feasible to extract valuable metals from slags, along with providing a chance to clean slag if required.
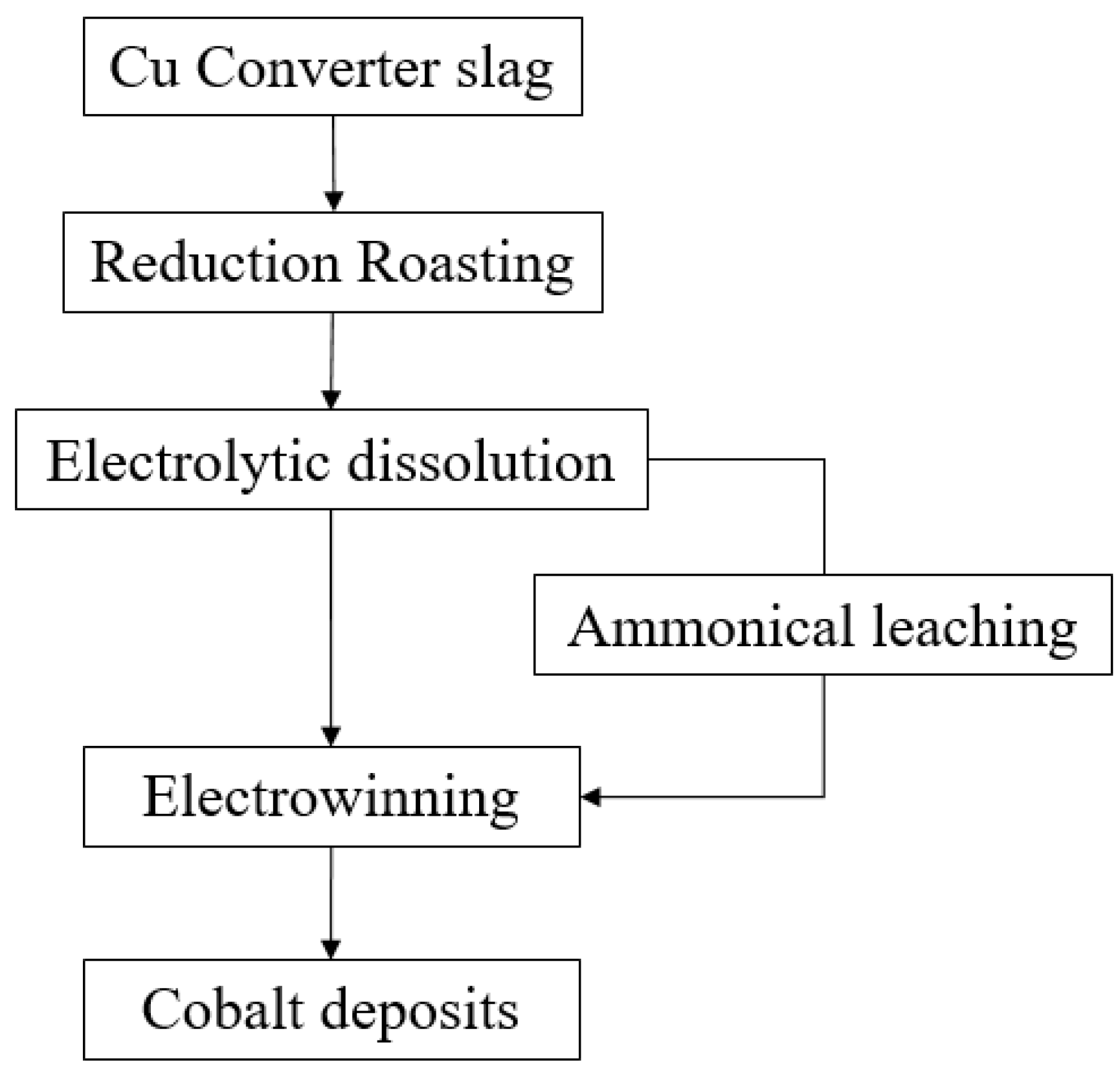
Various flowsheets combining the pyrometallurgical and hydrometallurgical methods have been widely explored to extract critical and strategic metals from copper slag. A few cases of studies in pyrometallurgy required water leaching after roasting, so they were categorised as combined methods [5,106,107,108]. A novel process was developed by Yin, Xing [111] that includes smelting, magnetic separation, and leaching with sulphuric acid. Through reductive-sulphurising smelting, a Cu–Co–Fe matte was produced and then processed under a magnetic separator. Magnetic concentrate possessed cobalt-bearing phases, which include Co–Fe alloy, and non-magnetic substances had copper-bearing phases. Subsequently, sulphuric acid leaching at 80 °C for 1 h was conducted for magnetic substances. The overall recovery of Co was 95.57% after this series of processes [111]. A research group in India investigated various methods to extract Co and Ni from copper slag: roasting with acid, water leaching and precipitation [96], pressure leaching [97], reductive-roasting, and leaching with ferric chloride [110]. The conditions of these methods are listed in Table 6. These methods showed remarkable results, with recoveries exceeding 95% of Co and Ni. Several attempts have also been made to recover Mo from copper slag by a sequence of selective reduction, roasting, and leaching with sulphuric acid [93] and by oxidative roasting followed by sulphuric acid leaching [119]. The former study achieved enrichment of Mo by 8.9 (final/initial grade: 2.67/0.3), and the latter recovered 80% of Mo. One of the most comprehensive flowsheets for processing copper slag is presented in Figure 4. It encompassed from reduction roasting to electrowinning and produced a 92% purity of Co [83]. It indicates that a combined flowsheet of hydro- and pyrometallurgical methods for extracting critical and strategic metals such as Co can produce high-purity concentrates. However, the feasibility, profitability, and viability of the process should still be assessed before applying to industry.
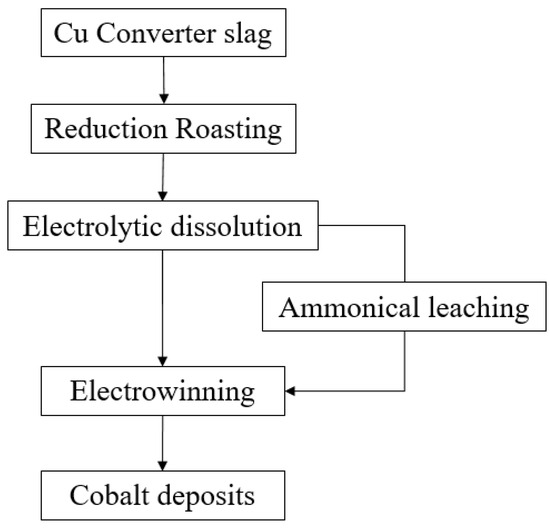
Figure 4.
Flowsheet of a selective process for cobalt from copper slag adopted from [83].
5.3. Nickel Slag
Nickel slag is a smelting waste from nickel production [120]. Slag is usually stored in dumps since they are considered as waste materials [121]. Furthermore, nickel slag contains critical and strategic metals, for example, Ni and Co, as shown in Table 7. One of the potential problems with dumped slags is that they may contain potentially toxic elements (PTEs). In case of rain, PTEs can be leached out from the slag and exposed to the environment. Therefore, the extraction of valuable metals from nickel slags is not only a great secondary source of critical and strategic metals but is also able to make the slag cleaner for the environment.
Nickel slag can be categorised based on its production stages and cooling methods: (1) furnace or converter slag and (2) slow-cooled or fast-cooled slag. They behave differently in a chemical reaction and have dissimilar crystal structures. In the pyrometallurgical process, the slag from the smelting phase is called furnace or smelter slag, and the other slag from the converting phase is distinguished as converter slag. Usually, converter slag contains a higher concentration of valuable metals than furnace slag since converting deals with a higher grade of product. For example, Ni in converter slag is 2.87–4.80%; however, the primary ores contain 1.05–2.3% of Ni [122]. For this reason, converter slag is normally going back to the circuit for processing.
Due to cooling time and methods, slow-cooled and fast-cooled slags show different crystallisations. Slow-cooled slag contains well-developed crystalline, fayalite, and minor spinel. On the other hand, fast-cooled slags are more homogeneous in terms of metal distribution and contain amorphous iron silicate glass [7].
Nickel slag contains critical and strategic metals such as Ni, Co, Ti, Al, Mg, Mn, V, and Cr, as shown in Table 7. Nickel slag also has base metals, for example, Fe, Cu, Si, etc.; however, they were excluded from Table 7 because they are regarded as less critical due to their stable supply. In recent years, there has been an increasing interest and attempt to recover Ni and Co from nickel slag in the industry. Hence, this review mostly focuses on the extraction of Ni and Co from nickel slag based on the publications.

Table 7.
Compositions (%) of critical and strategic elements in nickel slags (SC: Slow-cooled slag, FC: Fast-cooled slag, C: Converter slag, F: Furnace slag).
Table 7.
Compositions (%) of critical and strategic elements in nickel slags (SC: Slow-cooled slag, FC: Fast-cooled slag, C: Converter slag, F: Furnace slag).
| Origin (Region) | Ni | Co | Ti | Al | Mg | Mn | V | Cr | References |
|---|---|---|---|---|---|---|---|---|---|
| Brazil | 0.08 | 73 ppm | 0.04 | 1.99 | 17.2 | 0.29 | 85 ppm | 0.76 | [120] |
| 0.38 | 0.02 | - | 0.68 | 5.76 | - | - | 1.11 | [123] | |
| 1.05 | 0.243 | - | 1.66 | 0.8 | 0.05 | - | - | SC, [122,124] | |
| China | 1.48 | 0.6 | - | - | - | - | - | - | C, [125] |
| 0.9 | 0.12 | - | 1.4 | 5.83 | - | - | - | F, [126] | |
| Canada | 0.28 | 0.13 | 0.14 | 3.63 | 1.87 | 0.04 | - | - | F, [127] |
| 0.66 | 0.21 | 0.16 | 2.62 | 1.54 | 0.04 | - | - | SC, [7] | |
| 0.31 | 0.14 | 0.1 | 3.73 | 2.0 | 0.03 | - | - | FC, [7] | |
| 0.19 | 0.11 | 0.21 | 3.97 | 2.1 | 0.05 | - | - | SC, [7] | |
| 0.05 | 0.1 | 0.02 | 0.78 | 0.49 | 0.02 | - | - | FC, [7] | |
| Poland | 0.55 | 0.05 | 0.64 (TiO2) | 11.47 (Al2O3) | 2.76 (MgO) | 0.26 (MnO) | - | - | [128] |
| 0.02 | 77 ppm | 0.53 (TiO2) | 16.4 (Al2O3) | 5.52 (MgO) | 1.16 (MnO) | - | - | [128] | |
| 82 ppm | 5 ppm | 0.09 (TiO2) | 2.79 (Al2O3) | 10.06 (MgO) | 0.22 (MnO) | - | - | [128] | |
| N/A | 3.64 | 1.05 | - | 1.63 | 0.29 | - | - | - | C, [129] |
| 0.27 | 0.11 | - | 3.84 | 3.81 | - | - | - | F, [129] | |
| 0.32 | 0.16 | 0.12 | 2.86 | 2.57 | 0.05 | 0.01 | 0.05 | [121] | |
| 0.16 | 0.05 | 0.13 | 3.64 | 1.22 | 0.03 | 0.01 | 0.05 | [121] | |
| 0.12 | 0.02 | - | 6.92 (Al2O3) | 8.49 (MgO) | 0.34 (MnO) | - | - | F, [130] |
The concentrations of Ni and Co in nickel slag are from 82 ppm to 3.64% and from 5 ppm to 1.05%, respectively. The chemical composition of critical and strategic metals in nickel slag from various references is shown in Table 7. The maximum Ni grade is 3.64%, from converter slag, which is higher than the primary ore’s grade. The highest grade of Co in nickel slag is 1.05%, from converter slag [129]. Nickel slag also contains a reasonable amount of Ti and Cr which implies that nickel slag can be a secondary source of these other valuable metals.
There have been several studies in the literature on nickel slag reporting leaching behaviour [120,121,127,129], selective recovery of valuable metals [122,125,126], characterisation, and mineralogy [7,124,128], and environmental effects [130]. There has been plenty of research on the characteristics of nickel slag in the past, and recently, research has been focusing on the extraction of valuable metals from nickel slag as well. However, there is a limited number of publications on nickel slag.
Ettler, Kvapil [120] investigated the leaching behaviour of metals in nickel slag. The optimum pH was 3 in the range between pH 3 and 12. In this range, chromium shows a U-shaped leaching pattern. Nickel, cobalt, and magnesium have a decreasing trend in concentration from pH 3 to 12, with a low concentration at around pH 12. However, this study focused on the exposure of potentially toxic elements (PTEs) to the environment rather than an extraction method of valuable metals from nickel slag [120]. It is seldom appropriate to apply the result for the recovery of valuable metals to this study since the leaching test was to evaluate the environmental implications when these metals were dissolved. Thus, further studies of leaching behaviour in the extraction process for production purposes are required for a better understanding of the leaching mechanism.
Extraction processes, including the pyrometallurgical methods, were also studied. Huang et al. [125] achieved more than 97% recovery for nickel and cobalt using leaching with 0.3 M sulphuric acid and 600 kPa oxygen partial pressure at 200 °C for 80 min. Pan et al. [126] conducted selective reduction and magnetic separation to recover nickel and copper and demonstrated when the basicity increases, the enrichment of nickel improves as well. As a result, the author recovered 82.2% of nickel with a 3.25% grade.
The optimum method for the recovery of critical and strategic metals from nickel slag is high-pressure and -temperature leaching. There have been attempts to dissolve the target metals at atmospheric pressure, but it was unsuccessful due to several drawbacks, such as slow oxidation of iron, slow filtration, and high acid consumption [121]. Thus, current studies are focusing on high-pressure oxidative leaching, which provides selectivity towards Ni and Co with more than 90% of the recovery.
The common parameters of high-pressure temperature oxidative leaching from various research groups are a choice of acid and temperature. Weak sulphuric acid is a by-product of nickel production; as a result, nickel production companies have a significant amount of acid stored. It is desirable to utilise sulphuric acid to process the slag. One of the reasons that weak acid should be applied is that under atmospheric pressure and temperature, above 0.05 M of sulphuric acid reduces selectivity towards Ni and Co in nickel slag [121]. Thus, employing high-pressure and -temperature leaching with a slightly increased acid concentration is consistent with the literature as shown in Table 8. The temperature of the leaching experiment is mostly 250 °C as shown in Table 8. A high temperature, especially 250 °C, ensures that iron and aluminium do not dissolve to leach the solution at this temperature [7]. With high-pressure and -temperature leaching, an over 90% recovery of Ni and Co could be extracted in several publications which shows a promising result of the hydrometallurgical method.

Table 8.
Extraction methods of critical and strategic metals from nickel slags.
For further development of a feasible process, removing impurities such as Fe, Cu, Si, etc., in other words, improving the grade of Ni and Co, will be one of the major difficulties since target metals in starting materials are low grade. Based on the results of magnetic separation, the combination of high-pressure oxidative leaching and magnetic separation may be an option; however, there is no literature on this combination. Another way to enhance the selectivity of valuable metals can be complexation leaching, which employs a complexing agent in leaching.
Another challenge for the recovery of Ni and Co in nickel slag will be the separation of those metals since they exist together in the leach solution. So far, solvent extraction seems the optimum way to separate Ni and Co using Cyanex as an extractant [131,132,133]. Since Cyanex 272 is effective in extracting cobalt from nickel, Mubarok and Hanif (2016) [131] used an artificial solution containing only Co and Ni without impurities to separate them. As a result, the authors extracted 99.08% of Co with 10.16% of Ni at pH 5, Cyanex 272 concentration 20% (v/v), and at room temperature. However, from [132,133], it was confirmed again that Cyanex 272 is the optimal extractant and the most commercially used in the industry for the separation of Co and Ni. Further studies on separation focusing on the leach solution from nickel slag are required.
5.4. Vanadium Slag
This chapter discusses the recovery of critical and strategic metals from vanadium slag, including the chemical compositions of vanadium slags, extraction methods, and so on. Notably, most of the literature and sources of vanadium slag are from China. Several studies on the extraction of critical and strategic metals from vanadium slag have increased since the 2010s, which shows the potential of vanadium slag as a secondary source.
Vanadium is widely exploited in ferrous and non-ferrous alloys and is mostly consumed in the iron and steel industry due to its outstanding physical properties, such as fatigue resistance, hardness, and so on [13,134]. Vanadium can be found in vanadium–titanium magnetite, vanadium slag, vanadium stone coal, steel slag, spent catalyst, and fly ashes [135,136,137]. The main source of vanadium comes from vanadium titano-magnetite as a form of slag. Smelting the vanadium titano-magnetite ore enriches vanadium into solid slag, which is called vanadium slag [138]. Vanadium is mostly associated with ferrovanadium spinel (FeO·V2O3) as a main phase for both vanadium–titanium magnetite and vanadium slag [137]. Vanadium slag contains a significant amount of chromium, titanium, and vanadium. Table 9 shows the chemical compositions of vanadium slags from various origins in China.

Table 9.
Compositions (%) of critical and strategic elements in vanadium slags.
Countries that possess vanadium in the world are Australia, Russia, China, South Africa, the USA, and others [13]. In 2023, China mined 68,000 tonnes, while Russia, South Africa, and Brazil produced 35,500 tonnes all together: 20,000 tonnes, 9100 tonnes and 6400 tonnes, respectively. These countries combined have produced approximately 103,500 tonnes of vanadium in 2023, which makes up the world’s total production [102,153].
A traditional method to extract V from vanadium slag is sodium salt roasting and water leaching. However, this process can recover only 65 to 85% of the V during leaching due to its poor transfer efficiency [146]. Other well-known methods are calcification roasting, acid leaching, and direct alkaline leaching. Calcification roasting was introduced due to limited access to the sodium slag in Russia, while direct alkaline leaching was suggested to overcome the disadvantages of pyrometallurgical processes [137]. Thus, as can be seen in Table 10, several attempts from predominantly China have been made to overcome the drawbacks and to eventually achieve a higher recovery of V as well as Cr with various methods, such as electrochemical decomposition [146], microwave roasting—leaching [140], and electro-oxidation leaching [135].

Table 10.
Extraction methods of critical and strategic metals from vanadium slags.
All the roasting methods above showed more than 90% recovery of V, and some of them included a minimum of 80% of Cr. The mixture of sodium chloride and sodium carbonate achieved especially 96% of V and 91% of Cr [145]. In the case of NaOH–NaNO3 binary molten salt medium, the roasting temperature was dropped from 850 °C to 375~400 °C by the traditional method [156]. With potassium hydroxide, Li et al. (2012) tested three reaction media, such as sub-molten KOH, sub-molten NaOH, and NaOH–NaNO3 binary molten salt medium. They also dropped the roasting temperature to 200~450 °C [152]. Yan et al. (2015) put no additives for roasting at 950 °C and subsequently conducted soda roasting, which still presented a 94.13% recovery of V [150]. These attempts indicate that it is promising to establish a novel roasting method without toxic emissions.
Even without sodium salt roasting, Wang et al. (2018) established a clean metallurgical process with combined methods of pyro- and hydrometallurgy and produced V with 99.1% purity and Cr with 98.9% purity [138]. Furthermore, there have been efforts to adapt modern technology to vanadium processing. For example, electrochemistry, such as electro-oxidation, has been introduced in leaching. This method can break the silicon layer and expose more V. Hence, there is a possibility to develop further and apply this method to the industry [135,144,146].
It is interesting to note the attempts to extract valuable metals from the residue of slag processing [157,158,159]. The residue of slag processing is called ‘V–Cr-bearing reduced (reducing) residue’, and is from wastewater after roasting and neutralisation of vanadium slag [157]. V–Cr-bearing reduced slag contains 5.1% V and 13.4% Cr [158]. The methods for extracting V from V–Cr-bearing reduced residue include selective oxidation and alkaline leaching [157] and a comprehensive approach: leaching with sulphuric acid, selective oxidation, and precipitation of vanadium and chromium [158]. These methods cover separation and purification as well, showing great recoveries of V and Cr of 98.7% and 99.4%, respectively [159]. This case shows the fast development of utilising secondary waste. Therefore, developing processes to recover critical and strategic metals from secondary and tertiary sources should continue regardless of market status or metal price to the material cycle as much as possible.
5.5. Titanium Slag
In this chapter, titanium slag will be discussed briefly. Its chemical compositions are listed in Table 11, and its extraction methods are reviewed below.

Table 11.
Compositions (%) of critical and strategic elements in titanium slag.
Titanium slag is produced by a conventional method of titanium production. Ilmenite ore is upgraded to titanium slag and then to synthetic rutile [165]. Accordingly, many publications on titanium slag deal with high grades of titanium, ranging from around 70% to more than 90% as a main product [166,167,168]. Thus, ‘titanium-bearing blasting furnace slag’ (also called titanium-bearing slag) as a by-product from vanadium–titanium magnetite ore or titanomagnetite ore is more appropriate in this study [169].
The main difference between titanium slag and titanium-bearing slag is the grade of titanium. The latter shows a lower grade, less than 30%, of TiO2 in the slags [14] and around 1% of V2O5, as shown in Table 11. Much literature focuses on the upgrade and extraction of Ti from titanium-bearing slag, such as the removal of impurities [164], the enrichment of anosovite by adding SiO2 [160], and various oxidation conditions [161,170,171], as well as novel technology such as microwave treatment, super-gravity separation, and ultrasonic processes [14].
Even though Ti is considered one of the most abundant elements in the crust [165], it is critical and strategic for many applications. Thus, studies on the characteristics, mineralogy, and processing of titanium-bearing slag would be highly beneficial for waste management and process development. Another important critical and strategic element in the titanium-bearing slag is V, and Han et al. [162] presented the flowsheet of two-stage oxidation, alkali leaching and acid leaching, to extract Fe, V, and Ti.
6. Conclusions
This review attempted to present the potential of non-ferrous slag as a viable source of critical and strategic metals needed for high-technology and clean energy applications. Slag is considered waste and is mostly dumped in heaps after smelting. Non-ferrous slag materials such as tin slag, copper slag, nickel slag, vanadium slag, and titanium slag contain significant amounts of critical and strategic elements, making them potential secondary resources for such metals. Numerous studies have been conducted to extract critical and strategic metals from slag. However, most are still at the laboratory scale and are yet to be proven at pilot plant levels. In addition, their commercial viability is yet to be assessed for economic and environmental benefits. Technology development, as applied to resource recovery from slag, involves applications of mineral processing, hydrometallurgical, or pyrometallurgical techniques. As the importance of and the demand for critical and strategic metals continues to rise, it will soon be inevitable to consider extracting valuable metals in waste and by-products, such as slag, to augment the global demand for supplies. Extracting values from waste is categorised under technospheric mining, a concept that promotes a circular economy in the mining and resource industries. Until then, developing green and sustainable technologies for metal extraction and filling the knowledge gaps in the recovery of critical and strategic elements from non-ferrous slag should be actively pursued and promoted.
Author Contributions
Conceptualisation, B.L. and R.D.A.; investigation, B.L. and R.D.A.; writing—original draft preparation, B.L.; writing—review and editing, B.L., M.A. and R.D.A.; visualisation, B.L.; supervision, M.A. and R.D.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the financial support of the Science Industry PhD Fellowship from the Department of Jobs, Tourism, Science, and Innovation (JTSI), Government of Western Australia and Curtin University.
Data Availability Statement
Not applicable.
Acknowledgments
This manuscript is a part of the first author’s PhD project.
Conflicts of Interest
Author Mark Aylmore was employed by the company Mineral Resources Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Brandt, D.A.; Warner, J.C. Metallurgy Fundamentals Ferrous and Nonferrous, 5th ed.; The Goodheart-Willcox Company, Inc.: Tinley Park, IL, USA, 2009. [Google Scholar]
- Piatak, N.M.; Parsons, M.B.; Seal, R.R. Characteristics and environmental aspects of slag: A review. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]
- Lee, J.-C.; Kurniawan; Kim, E.-Y.; Chung, K.W.; Kim, R.; Jeon, H.-S. A review on the metallurgical recycling of vanadium from slags: Towards a sustainable vanadium production. J. Mater. Res. Technol. 2021, 12, 343–364. [Google Scholar] [CrossRef]
- Dehaine, Q.; Tijsseling, L.T.; Glass, H.J.; Törmänen, T.; Butcher, A.R. Geometallurgy of cobalt ores: A review. Miner. Eng. 2021, 160, 106656. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, B.; Wang, H.; Wang, M.; Wang, X. Recovery of valuable metals from copper slag. Min. Metall. Explor. 2020, 37, 1241–1251. [Google Scholar] [CrossRef]
- Shen, H.; Forssberg, E. An overview of recovery of metals from slags. Waste Manag. 2003, 23, 933–949. [Google Scholar] [CrossRef] [PubMed]
- Gbor, P.K.; Mokri, V.; Jia, C.Q. Characterization of smelter slags. J. Environ. Sci. Health Part A 2000, 35, 147–167. [Google Scholar] [CrossRef]
- Gorai, B.; Jana, R.K.; Premchand. Characteristics and utilisation of copper slag—A review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- Tian, H.; Guo, Z.; Pan, J.; Zhu, D.; Yang, C.; Xue, Y.; Li, S.; Wang, D. Comprehensive review on metallurgical recycling and cleaning of copper slag. Resour. Conserv. Recycl. 2021, 168, 105366. [Google Scholar] [CrossRef]
- Lee, J.-C.; Pandey, B.D. Bio-processing of solid wastes and secondary resources for metal extraction—A review. Waste Manag. 2012, 32, 3–18. [Google Scholar] [CrossRef]
- Srichandan, H.; Mohapatra, R.K.; Parhi, P.K.; Mishra, S. Bioleaching approach for extraction of metal values from secondary solid wastes: A critical review. Hydrometallurgy 2019, 189, 105122. [Google Scholar] [CrossRef]
- Potysz, A.; van Hullebusch, E.D.; Kierczak, J.; Grybos, M.; Lens, P.N.L.; Guibaud, G. Copper Metallurgical Slags—Current Knowledge and Fate: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2424–2488. [Google Scholar] [CrossRef]
- Moskalyk, R.; Alfantazi, A. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Shi, J.; Qiu, Y.; Yu, B.; Xie, X.; Dong, J.; Hou, C.; Li, J.; Liu, C. Titanium Extraction from Titania-Bearing Blast Furnace Slag: A Review. J. Miner. Met. Mater. Soc. 2022, 74, 654–667. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Nie, C.; Zhang, J.; Steenari, B.-M.; Ekberg, C. Comprehensive treatments of tungsten slags in China: A critical review. J. Environ. Manag. 2020, 270, 110927. [Google Scholar] [CrossRef]
- Beylot, A.; Dewulf, J.; Greffe, T.; Muller, S.; Blengini, G.-A. Mineral resources depletion, dissipation and accessibility in LCA: A critical analysis. Int. J. Life Cycle Assess. 2024, 29, 890–908. [Google Scholar] [CrossRef]
- Johansson, N.; Krook, J.; Eklund, M.; Berglund, B. An integrated review of concepts and initiatives for mining the technosphere: Towards a new taxonomy. J. Clean. Prod. 2013, 55, 35–44. [Google Scholar] [CrossRef]
- Palm, V.; Östlund, C. Lead and Zinc Flows from Technosphere to Biosphere in a City Region. Sci. Total Environ. 1996, 192, 95–109. [Google Scholar] [CrossRef]
- Hofstetter, P. Perspectives in Life Cycle Impact Assessment: A Structured Approach to Combine Models of the Technosphere, Ecosphere, and Valuesphere; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Karlsson, S. Closing the Technospheric Flows of Toxic Metals: Modeling Lead Losses from a Lead-Acid Battery System for Sweden. J. Ind. Ecol. 1999, 3, 23–40. [Google Scholar] [CrossRef]
- Palm, V. Material Flow Analysis in Technosphere and Biosphere. Ph.D. Thesis, Royal Institute of Technology, Universitetsservice US AB, Stockholm, Sweden, 2002. [Google Scholar]
- Zalasiewicz, J.; Williams, M.; Waters, C.N.; Barnosky, A.D.; Palmesino, J.; Rönnskog, A.-S.; Edgeworth, M.; Neal, C.; Cearreta, A.; Ellis, E.C.; et al. Scale and diversity of the physical technosphere: A geological perspective. Anthr. Rev. 2017, 4, 9–22. [Google Scholar] [CrossRef]
- Krook, J.; Svensson, N.; Eklund, M. Landfill Mining: A Critical Review of Two Decades of Research. Waste Manag. 2011, 32, 513–520. [Google Scholar] [CrossRef]
- Krook, J.; Carlsson, A.; Eklund, M.; Frändegård, P.; Svensson, N. Urban mining: Hibernating copper stocks in local power grids. J. Clean. Prod. 2011, 19, 1052–1056. [Google Scholar] [CrossRef]
- Reid, S.; Tam, J.; Yang, M.; Azimi, G. Technospheric Mining of Rare Earth Elements from Bauxite Residue (Red Mud): Process Optimization, Kinetic Investigation, and Microwave Pretreatment. Sci. Rep. 2017, 7, 15252. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, D.; Cleall, P.; Harbottle, M. In Situ Resource Recovery from Waste Repositories: Exploring the Potential for Mobilization and Capture of Metals from Anthropogenic Ores. J. Sustain. Metall. 2017, 3, 375–392. [Google Scholar] [CrossRef]
- Kim, J.; Azimi, G. Technospheric mining of niobium and titanium from electric arc furnace slag. Hydrometallurgy 2020, 191, 105203. [Google Scholar] [CrossRef]
- Lim, B.; Alorro, R.D. Technospheric Mining of Mine Wastes: A Review of Applications and Challenges. Sustain. Chem. 2021, 2, 686–706. [Google Scholar] [CrossRef]
- Pramanik, S.; Kumari, A.; Sahu, S.K.; Munshi, B. Extraction of metal values from iron-rich mine tailings via chloridized roasting and water leaching. Waste Manag. Bull. 2024, 2, 113–121. [Google Scholar] [CrossRef]
- Simandl, G.J.; Akam, C.; Paradis, S. Which materials are ‘critical’ and ‘strategic’. In Symposium on Strategic and Critical Metals; British Columbia Ministry of Energy and Mines: Victoria, BC, Canada, 2015; pp. 1–4. [Google Scholar]
- Jin, Y.; Kim, J.; Guillaume, B. Review of critical material studies. Resour. Conserv. Recycl. 2016, 113, 77–87. [Google Scholar] [CrossRef]
- Hofmann, M.; Hofmann, H.; Hagelüken, C.; Hool, A. Critical raw materials: A perspective from the materials science community. Sustain. Mater. Technol. 2018, 17, e00074. [Google Scholar] [CrossRef]
- Ailshie, J.F. Minerals in National Defense. ABA Sec. Miner. Nat. Res. L. Proc. 1939, 70. Available online: https://heinonline.org/HOL/LandingPage?handle=hein.journals/pabminn1&div=6&id=&page= (accessed on 3 July 2024).
- Antwerpen, F.V. Strategic Raw Materials. Ind. Eng. Chem. 1939, 31, 520–530. [Google Scholar] [CrossRef]
- Gunn, G. Critical Metals Handbook; Wiley: Hoboken, NJ, USA, 2013; pp. 1–439. [Google Scholar]
- Korinek, J.; Kim, J. Export restrictions on strategic raw materials and their impact on trade and global supply. J. World Trade 2011, 45, 255–281. [Google Scholar]
- Cote, G. Hydrometallurgy of Strategic Metals. Solvent Extr. Ion Exch. 2000, 18, 703–727. [Google Scholar] [CrossRef]
- Bortnikov, N.S.; Lobanov, K.V.; Volkov, A.V.; Galyamov, A.L.; Vikent’ev, I.V.; Tarasov, N.N.; Distler, V.V.; Lalomov, A.V.; Aristov, V.V.; Murashov, K.Y.; et al. Strategic metal deposits of the Arctic Zone. Geol. Ore Depos. 2015, 57, 433–453. [Google Scholar] [CrossRef]
- European Commission. Study on the Critical Raw Materials for the EU 2023—Final Report; Publications Office ofthe European Union: Luxembourg, 2023. [Google Scholar]
- Australian Government. Critical Minerals List 2023 Update; Critical Minerals Office: Canberra, ACT, Australia, 2023. [Google Scholar]
- Su, Y.; Hu, D. Global Dynamics and Reflections on Critical Minerals. E3S Web Conf. 2022, 352, 03045. [Google Scholar] [CrossRef]
- Applegate, J.D. 2021 Draft List of Critical Minerals; U.S. Geological Survey, Department of the Interior, Ed.; Federal Publisher: Alexandria, NSW, Australia, 2021; pp. 62199–62203. [Google Scholar]
- BGS. Risk List; British Geological Survey: Nottingham, UK, 2015. [Google Scholar]
- Mines, M.O. Critical Minerals For India; Government of India: New Delhi, India, 2023. [Google Scholar]
- OECD. OECD Secretary-General’s Report to Ministers 2021; OECD Publishing: Paris, France, 2021. [Google Scholar]
- OECD. A Sustainable Materials Management Case Study Critical Metals and Mobile Devices; OECD Publishing: Paris, France, 2011. [Google Scholar]
- USGS. 2022 Final List of Critical Minerals; Department of the Interior: Washington, DC, USA, 2022.
- United States Department of Energy. Critical Minerals and Materials; United States Department of Energy: Washington, DC, USA, 2021.
- Suliva, J.; Deese, B. Building Resilient Supply Chains, Revitalizing American Manufacturing, and Fostering Broad-Based Growth; The White House: Washington, DC, USA, 2021; pp. 1–250. [Google Scholar]
- Nassar, N.T.; Fortier, S.M. Methodology and Technical Input for the 2021 Review and Revision of the US Critical Minerals List; U.S. Geological Survey: Reston, VA, USA, 2021. [Google Scholar]
- Anes, I.A.; Garjulli, F.; Siqueira de Carvalho, M.; Tenório, J.A.S.; Espinosa, D.C.R.; Coleti, J.L. Extraction of niobium in one step from tin slag by NH4F-HCl leaching process. Can. J. Chem. Eng. 2024, 102, 168–176. [Google Scholar] [CrossRef]
- Brocchi, E.A.; Moura, F.J. Chlorination methods applied to recover refractory metals from tin slags. Miner. Eng. 2008, 21, 150–156. [Google Scholar] [CrossRef]
- Gaballah, I.; Allain, E.; Djona, M. Extraction of tantalum and niobium from tin slags by chlorination and carbochlorination. Metall. Mater. Trans. B 1997, 28, 359–369. [Google Scholar] [CrossRef]
- Gaballah, I.; Allain, E. Recycling of strategic metals from industrial slag by a hydro-and pyrometallurgical process. Resour. Conserv. Recycl. 1994, 10, 75–85. [Google Scholar] [CrossRef]
- Odo, J.U.; Okafor, W.C.; Ekpe, S.O.; Nwogbu, C.C. Extraction of Niobium from Tin Slag. Int. J. Sci. Anc Res. Publ. 2014, 4. Available online: http://www.ijsrp.org/research-paper-1114.php?rp=P353322 (accessed on 3 July 2024).
- Subramanian, C.; Suri, A.K. Recovery of niobium and tantalum from low grade tin slag—A hydrometallurgical approach. Environ. Waste Manag. Non-Ferr. Metall. Ind. 1998, 831, 100–107. [Google Scholar]
- Allain, E.; Kanari, N.; Diot, F.; Yvon, J. Development of a process for the concentration of the strategic tantalum and niobium oxides from tin slags. Miner. Eng. 2019, 134, 97–103. [Google Scholar] [CrossRef]
- Sulaiman, M.Y. Simultaneous determination of thorium and uranium in tin slag. Sci. Total Environ. 1993, 130–131, 187–195. [Google Scholar] [CrossRef]
- Chirikure, S.; Heimann, R.B.; Killick, D. The technology of tin smelting in the Rooiberg Valley, Limpopo Province, South Africa, ca. 1650–1850 CE. J. Archaeol. Sci. 2010, 37, 1656–1669. [Google Scholar] [CrossRef]
- Gaballah, I.; Allain, E.; Meyer-Joly, M.C.; Malua, K. A possible method for the characterization of amorphous slags: Recovery of refractory metal oxides from tin slags. Metall. Mater. Trans. B 1992, 23, 249–259. [Google Scholar] [CrossRef]
- Gustison, R. Electric Furnace Method of Beneficiating Tantalum-And Noibium-Containing Tin Slags and The Like; Kawecki Berylco Industries, Inc.: New York, NY, USA, 1973. [Google Scholar]
- Soedarsono, J.W.; Permana, S.; Hutauruk, J.K.; Adhyputra, R.; Rustandi, A.; Maksum, A.; Widana, K.S.; Trinopiawan, K.; Anggraini, M. Upgrading tantalum and niobium oxides content in Bangka tin slag with double leaching. IOP Conf. Ser. Mater. Sci. Eng. 2018, 316, 012052. [Google Scholar] [CrossRef]
- Munir, B.; Permana, B.; Amilia, A.; Maksum, A.; Soedarsono, J.W. Initial Study for Cerium and Lanthanum Extraction from Bangka Tin Slag through NaOH and HClO4 Leaching. MATEC Web Conf. 2019, 269, 07003. [Google Scholar] [CrossRef]
- Tylecote, F.; Photos-Jones, E.; Earl, B. The composition of tin slags from the south-west of England. World Archaeol. 1989, 20, 434–445. [Google Scholar] [CrossRef]
- Mudzanapabwe, N.T.; Chinyamakobvu, O.S.; Simbi, D.J. In situ carbothermic reduction of a ferro-columbite concentrate in the recovery of Nb and Ta as metal matrix composite from tin smelting slag waste dump. Mater. Des. 2004, 25, 297–302. [Google Scholar] [CrossRef]
- Fredericci, C.; Pizani, P.S.; Morelli, M.R. Crystallization of blast furnace slag glass melted in SnO2 crucible. J. Non-Cryst. Solids 2007, 353, 4062–4065. [Google Scholar] [CrossRef]
- Permana, S.; Soedarsono, J.W.; Rustandi, A.; Maksum, A. Other Oxides Pre-removed from Bangka Tin Slag to Produce a High Grade Tantalum and Niobium Oxides Concentrate. IOP Conf. Ser. Mater. Sci. Eng. 2016, 131, 012006. [Google Scholar] [CrossRef]
- Dhir, R.K.; de Brito, J.; Silva, R.V.; Lye, C.Q. 8—Use of Glass Cullet in Road Pavement Applications, in Sustainable Construction Materials. In Sustainable Construction Materials: Glass Cullet; Obe, R.K.D., De Brito, J., Ghataora, G.S., Lye, C.Q., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 297–325. [Google Scholar]
- Dhir, R.K.; de Brito, J.; Silva, R.V.; Lye, C.Q. 9—Use of Glass Cullet in Ceramics and Other Applications, in Sustainable Construction Materials. In Sustainable Construction Materials: Glass Cullet; Obe, R.K.D., De Brito, J., Ghataora, G.S., Lye, C.Q., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 327–387. [Google Scholar]
- Wang, L.; Xu, Y.; Tian, L.; Chen, Y.; Yang, A.; Gan, G.; Ma, Y.; Du, Y. Two-stage method recovery of metals from copper slag: Realize the resource utilization of all components. Miner. Eng. 2024, 206, 108503. [Google Scholar] [CrossRef]
- Miganei, L.; Gock, E.; Achimovičová, M.; Koch, L.; Zobel, H.; Kähler, J. New residue-free processing of copper slag from smelter. J. Clean. Prod. 2017, 164, 534–542. [Google Scholar] [CrossRef]
- Teğin, İ.; Ziyadanogullari, R. Recovery of Copper and Cobalt from Converter Slag with a New Flotation Method Using H2S. J. Environ. Sci. Eng. A 2018, 7, 69–75. [Google Scholar]
- Yuksel, U.; Teğin, İ.; Ziyadanogullari, R. Recovery of Copper and Cobalt from Copper Slags as Selective. J. Environ. Sci. Eng. A 2017, 6, 388–394. [Google Scholar] [CrossRef][Green Version]
- Bulut, G.; Perek, K.T.; Gűl, A.; Arslan, F.; Őnal, G. Recovery of metal values from copper slags by flotation and roasting with pyrite. Min. Metall. Explor. 2007, 24, 13–18. [Google Scholar] [CrossRef]
- Bulut, G. Recovery of copper and cobalt from ancient slag. Waste Manag. Res. 2006, 24, 118–124. [Google Scholar] [CrossRef]
- Arslan, C.; Arslan, F. Recovery of copper, cobalt, and zinc from copper smelter and converter slags. Hydrometallurgy 2002, 67, 1–7. [Google Scholar] [CrossRef]
- Kıyak, B.; Őzer, A.; Altundoǧan, H.S.; Erdem, M.; Tűmen, F. Cr(VI) reduction in aqueous solutions by using copper smelter slag. Waste Manag. 1999, 19, 333–338. [Google Scholar] [CrossRef]
- Altundoǧan, H.S.; Tümen, F. Metal recovery from copper converter slag by roasting with ferric sulphate. Hydrometallurgy 1997, 44, 261–267. [Google Scholar] [CrossRef]
- Yucel, O.; Addemir, O.; Tekin, A.; Nizamoglu, S. Recovery of Cobalt from Copper Slags. Miner. Process. Extr. Metall. Rev. 1992, 10, 99–107. [Google Scholar] [CrossRef]
- Mikoda, B.; Potysz, A.; Kmiecik, E. Bacterial leaching of critical metal values from Polish copper metallurgical slags using Acidithiobacillus thiooxidans. J. Environ. Manag. 2019, 236, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Potysz, A.; Kierczak, J.; Fuchs, Y.; Grybos, M.; Guibaud, G.; Lens, P.N.L.; van Hullebusch, E.D. Characterization and pH-dependent leaching behaviour of historical and modern copper slags. J. Geochem. Explor. 2016, 160, 1–15. [Google Scholar] [CrossRef]
- Potysz, A.; Kierczak, J.; Pietranik, A.; Kądziołka, K. Mineralogical, geochemical, and leaching study of historical Cu-slags issued from processing of the Zechstein formation (Old Copper Basin, southwestern Poland). Appl. Geochem. 2018, 98, 22–35. [Google Scholar] [CrossRef]
- Rudnik, E.; Burzyńska, L.; Gumowska, W. Hydrometallurgical recovery of copper and cobalt from reduction-roasted copper converter slag. Miner. Eng. 2009, 22, 88–95. [Google Scholar] [CrossRef]
- Yang, W.J.; Wang, C.Y.; Ma, B.Z.; Wang, Z.; Chen, Y.Q.; Yin, F. Recovery of cobalt from copper converter slag by a selective reduction-roasting process. Adv. Mater. Res. 2012, 550, 2186–2189. [Google Scholar] [CrossRef]
- Zhai, X.-J.; Li, N.-J.; Zhang, X.; Fu, Y.; Jiang, L. Recovery of cobalt from converter slag of Chambishi Copper Smelter using reduction smelting process. Trans. Nonferrous Met. Soc. China 2011, 21, 2117–2121. [Google Scholar] [CrossRef]
- Yang, Z.; Rui-lin, M.; Wang-dong, N.; Hui, W. Selective leaching of base metals from copper smelter slag. Hydrometallurgy 2010, 103, 25–29. [Google Scholar] [CrossRef]
- Banza, A.N.; Gock, E.; Kongolo, K. Base metals recovery from copper smelter slag by oxidising leaching and solvent extraction. Hydrometallurgy 2002, 67, 63–69. [Google Scholar] [CrossRef]
- Tshiongo, N.; Mbaya, R.K.K.; Maweja, K.; Tshabalala, L.C. Effect of cooling rate on base metals recovery from copper matte smelting slags. World Acad. Sci. Eng. Technol. 2010, 46, 273–277. [Google Scholar]
- Vítková, M.; Ettler, V.; Johan, Z.; Kríbek, B.; Sebek, O.; Mihaljevic, M. Primary and secondary phases in copper-cobalt smelting slags from the Copperbelt Province, Zambia. Mineral. Mag. 2010, 74, 581–600. [Google Scholar] [CrossRef]
- Ettler, V.; Johan, Z.; Kříbek, B.; Šebek, O.; Mihaljevič, M. Mineralogy and environmental stability of slags from the Tsumeb smelter, Namibia. Appl. Geochem. 2009, 24, 1–15. [Google Scholar] [CrossRef]
- Rozendaal, A.; Horn, R. Textural, mineralogical and chemical characteristics of copper reverb furnace smelter slag of the Okiep Copper District, South Africa. Miner. Eng. 2013, 52, 184–190. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Särkijärvi, S.; Puhakka, J.A.; Peuraniemi, E.; Junnikkala, S.; Tuovinen, O.H. Solid phase changes in chemically and biologically leached copper smelter slag. Miner. Eng. 2017, 106, 97–101. [Google Scholar] [CrossRef]
- Palacios, J.; Sánchez, M. Wastes as resources: Update on recovery of valuable metals from copper slags. Miner. Process. Extr. Metall. 2011, 120, 218–223. [Google Scholar] [CrossRef]
- Parada, F.; Parra, R.; Watanabe, T.; Hino, M.; Palacios, J.; Sánchez, M. Recovery of iron-molybdenum alloy from copper slags. In Proceedings of the VIII International Conference on ‘Molten Slags, Fluxes and Salts’, Santiago, Chile, 18–21 January 2009. [Google Scholar]
- Cardona Valencia, N. The Physical Chemistry of Copper Smelting Slags and Copper Losses at the Paipote Smelter Part 2—Characterisation of industrial slags. Can. Metall. Q. 2011, 50, 330–340. [Google Scholar] [CrossRef]
- Sukla, L.B.; Panda, S.C.; Jena, P.K. Recovery of cobalt, nickel and copper from converter slag through roasting with ammonium sulphate and sulphuric acid. Hydrometallurgy 1986, 16, 153–165. [Google Scholar] [CrossRef]
- Anand, S.; Rao, K.S.; Jena, P.K. Pressure leaching of copper converter slag using dilute sulphuric acid for the extraction of cobalt, nickel and copper values. Hydrometallurgy 1983, 10, 305–312. [Google Scholar] [CrossRef]
- Anand, S.; Rao, P.K.; Jena, P.K. Recovery of metal values from copper converter and smelter slags by ferric chloride leaching. Hydrometallurgy 1980, 5, 355–365. [Google Scholar] [CrossRef]
- Manasse, A.; Mellini, M.; Viti, C. The copper slags of the Capattoli Valley, Campiglia Marittima, Italy. Eur. J. Mineral. Eur. J. Miner. 2001, 13, 949–960. [Google Scholar] [CrossRef]
- Michailova, I.; Mehandjiev, D. Characterization of Fayalite from Copper Slags. J. Univ. Chem. Technol. Metall. 2010, 45, 317–326. [Google Scholar]
- Deng, T.; Ling, Y. Processing of copper converter slag for metal reclamation. Part I: Extraction and recovery of copper and cobalt. Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. ISWA 2007, 25, 440–448. [Google Scholar] [CrossRef]
- USGS. Mineral Commodity Summaries 2024; U.S. Geological Survey: Reston, VA, USA, 2024; p. 212.
- LME. Cobalt Price; LME: London, UK, 2024. [Google Scholar]
- Jones, R.; Denton, R.T.; Reynolds, Q.G.; Parker, J.A.L.; van Tonder, G.J.J. Recovery of cobalt from slag in a DC arc furnace at Chambishi, Zambia. J. South. Afr. Inst. Min. Metall. 2002, 102, 5–9. [Google Scholar]
- Skirrow, R.G.; Huston, D.L.; Mernagh, T.P.; Thorne, J.P.; Dulfer, H.; Senior, A.B. Critical Commodities for a High-Tech World: Australia’s Potential to Supply Global Demand; Geoscience Australia: Canberra, NSW, Australia, 2013. [Google Scholar]
- Ziyadanogullari, B. Recovery of Copper and Cobalt from Concentrate and Converter Slag. Sep. Sci. Technol. 2000, 35, 1963–1971. [Google Scholar] [CrossRef]
- Hamamci, C.; Ziyadanoğullari, B. Effect of Roasting with Ammonium Sulfate and Sulfuric Acid on the Extraction of Copper and Cobalt from Copper Converter Slag. Sep. Sci. Technol. 1991, 26, 1147–1154. [Google Scholar] [CrossRef]
- Tümen, F.; Bailey, N.T. Recovery of metal values from copper smelter slags by roasting with pyrite. Hydrometallurgy 1990, 25, 317–328. [Google Scholar] [CrossRef]
- Arslan, F.; Giray, K.; Őnal, G.; Gürkan, V. Development of a flowsheet for recovering copper and tin from copper refining slags. Eur. J. Miner. Process. Environ. Prot. 2002, 2, 94–102. [Google Scholar]
- Anand, S.; Das, R.P.; Jena, P.K. Reduction-roasting and ferric chloride leaching of copper converter slag for extracting copper, nickel and cobalt values. Hydrometallurgy 1981, 7, 243–252. [Google Scholar] [CrossRef]
- Yin, F.; Xing, P.; Li, Q.; Wang, C.; Wang, Z. Magnetic separation-sulphuric acid leaching of Cu–Co–Fe matte obtained from copper converter slag for recovering Cu and Co. Hydrometallurgy 2014, 149, 189–194. [Google Scholar] [CrossRef]
- Banda, W.; Morgan, N.; Eksteen, J.J. The role of slag modifiers on the selective recovery of cobalt and copper from waste smelter slag. Miner. Eng. 2002, 15, 899–907. [Google Scholar] [CrossRef]
- Altundogan, H.S.; Boyrazli, M.; Tumen, F. A study on the sulphuric acid leaching of copper converter slag in the presence of dichromate. Miner. Eng. 2004, 17, 465–467. [Google Scholar] [CrossRef]
- Erüst, C.; Akcil, A.; Gahan, C.S.; Tuncuk, A.; Deveci, H. Biohydrometallurgy of secondary metal resources: A potential alternative approach for metal recovery. J. Chem. Technol. Biotechnol. 2013, 88, 2115–2132. [Google Scholar] [CrossRef]
- Potysz, A.; van Hullebusch, E.D.; Kierczak, J. Perspectives regarding the use of metallurgical slags as secondary metal resources—A review of bioleaching approaches. J. Environ. Manag. 2018, 219, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Potysz, A.; Kierczak, J. Prospective (bio) leaching of historical copper slags as an alternative to their disposal. Minerals 2019, 9, 542. [Google Scholar] [CrossRef]
- Vestola, E.A.; Kuusenaho, M.K.; Närhi, H.M.; Tuovinen, O.H.; Puhakka, J.A.; Plumb, J.J.; Kaksonen, A.H. Acid bioleaching of solid waste materials from copper, steel and recycling industries. Hydrometallurgy 2010, 103, 74–79. [Google Scholar] [CrossRef]
- Mehta, K.; Pandey, B. Bio-assisted leaching of copper, nickel and cobalt from copper converter slag. Mater. Trans. JIM 1999, 40, 214–221. [Google Scholar] [CrossRef]
- Parada, F.; Sanchez, M.; Ulloa, A.; Carrasco, J.C.; Palacios, J.; Reghezza, A. Recovery of molybdenum from roasted copper slags. Miner. Process. Extr. Metall. 2010, 119, 171–174. [Google Scholar] [CrossRef]
- Ettler, V.; Kvapil, J.; Šebek, O.; Johan, Z.; Mihaljevič, M.; Ratié, G.; Garnier, J.; Quantin, C. Leaching behaviour of slag and fly ash from laterite nickel ore smelting (Niquelândia, Brazil). Appl. Geochem. 2016, 64, 118–127. [Google Scholar] [CrossRef]
- Jia, C.Q.; Xiao, J.Z.; Orr, R.G. Behavior of metals in discard nickel smelter slag upon reacting with sulfuric acid. J. Environ. Sci. Health Part A 1999, 34, 1013–1034. [Google Scholar] [CrossRef]
- Li, Y.; Perederiy, I.; Papangelakis, V.G. Cleaning of waste smelter slags and recovery of valuable metals by pressure oxidative leaching. J. Hazard. Mater. 2008, 152, 607–615. [Google Scholar] [CrossRef]
- Ibiapina, V.F.; Medeiros, M.E.; Afonso, J.C.; dos Santos, I.D. Oxidative Acidic Leaching of Ferronickel Slag for Sustainable Metals Recovery and Liability Reduction. J. Sustain. Metall. 2024, 10, 587–602. [Google Scholar] [CrossRef]
- Li, Y.; Papangelakis, V.G.; Perederiy, I. High pressure oxidative acid leaching of nickel smelter slag: Characterization of feed and residue. Hydrometallurgy 2009, 97, 185–193. [Google Scholar] [CrossRef]
- Huang, F.; Liao, Y.; Zhou, J.; Wang, Y.; Li, H. Selective recovery of valuable metals from nickel converter slag at elevated temperature with sulfuric acid solution. Sep. Purif. Technol. 2015, 156, 572–581. [Google Scholar] [CrossRef]
- Pan, J.; Zheng, G.-L.; Zhu, D.-Q.; Zhou, X.-L. Utilization of nickel slag using selective reduction followed by magnetic separation. Trans. Nonferrous Met. Soc. China 2013, 23, 3421–3427. [Google Scholar] [CrossRef]
- Gbor, P.K.; Ahmed, I.B.; Jia, C.Q. Behaviour of Co and Ni during aqueous sulphur dioxide leaching of nickel smelter slag. Hydrometallurgy 2000, 57, 13–22. [Google Scholar] [CrossRef]
- Kierczak, J.; Neel, C.; Puziewicz, J.; Bril, H. The mineralogy and weathering of slag produced by the smelting of lateritic Ni ores, Szklary, Southwestern Poland. Can. Mineral. 2009, 47, 557–572. [Google Scholar] [CrossRef]
- Baghalha, M.; Papangelakis, V.G.; Curlook, W. Factors affecting the leachability of Ni/Co/Cu slags at high temperature. Hydrometallurgy 2007, 85, 42–52. [Google Scholar] [CrossRef]
- Sakaroglou, M.; Anastassakis, G. Nickel recovery from electric arc furnace slag by magnetic separation. J. Min. Metall. A Min. 2017, 53, 3–15. [Google Scholar] [CrossRef]
- Mubarok, M.; Hanif, L. Cobalt and nickel separation in nitric acid solution by solvent extraction using Cyanex 272 and Versatic 10. Procedia Chem. 2016, 19, 743–750. [Google Scholar] [CrossRef]
- Koladkar, D.; Dhadke, P. Cobalt–Nickel separation: The extraction of cobalt (II) and nickel (II) with Bis (2-ethylhexyl) Phosphinic Acid (pia-8) in Toluene. Solvent Extr. Ion Exch. 2001, 19, 1059–1071. [Google Scholar] [CrossRef]
- Ayanda, O.S.; Adekola, F.A.; Baba, A.A.; Ximba, B.J.; Fatoki, O.S. Application of Cyanex extractant in cobalt/nickel separation process by solvent extraction. Int. J. Phys. Sci. 2013, 8, 89–97. [Google Scholar]
- Liu, S.; Wang, L.; Chen, J.; Ye, L.; Du, J. Research progress of vanadium extraction processes from vanadium slag: A review. Sep. Purif. Technol. 2024, 342, 127035. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Chen, M.; Nueraijemaiti, A.; Du, J.; Fan, X.; Tao, C.-Y. Enhanced leaching of vanadium slag in acidic solution by electro-oxidation. Hydrometallurgy 2016, 159, 1–5. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Zhang, L.; Gu, S. Mechanism of vanadium slag roasting with calcium oxide. Int. J. Miner. Process. 2015, 138, 20–29. [Google Scholar] [CrossRef]
- Gao, F.; Olayiwola, A.U.; Liu, B.; Wang, S.; Du, H.; Li, J.; Wang, X.; Chen, D.; Zhang, Y. Review of vanadium production part I: Primary resources. Miner. Process. Extr. Metall. Rev. 2021, 43, 466–488. [Google Scholar] [CrossRef]
- Wang, X.; Gao, D.; Chen, B.; Meng, Y.; Fu, Z.; Wang, M. A clean metallurgical process for separation and recovery of vanadium and chromium from V-Cr-bearing reducing slag. Hydrometallurgy 2018, 181, 1–6. [Google Scholar] [CrossRef]
- Wang, G.; Lin, M.-M.; Diao, J.; Li, H.-Y.; Xie, B.; Li, G. Novel strategy for green comprehensive utilization of vanadium slag with high-content chromium. ACS Sustain. Chem. Eng. 2019, 7, 18133–18141. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, T.; Lü, G.; Zhang, Y.; Liu, Y.; Zhang, W. Effects of Microwave Roasting on the Kinetics of Extracting Vanadium from Vanadium Slag. JOM 2016, 68, 577–584. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.-Y.; Yin, X.-C.; Yan, Z.-M.; Yan, X.-M.; Xie, B. Leaching kinetics of calcification roasted vanadium slag with high CaO content by sulfuric acid. Int. J. Miner. Process. 2014, 133, 105–111. [Google Scholar] [CrossRef]
- Jiang, T.; Wen, J.; Zhou, M.; Xue, X. Phase evolutions, microstructure and reaction mechanism during calcification roasting of high chromium vanadium slag. J. Alloys Compd. 2018, 742, 402–412. [Google Scholar] [CrossRef]
- Ning, P.; Lin, X.; Wang, X.; Cao, H. High-efficient extraction of vanadium and its application in the utilization of the chromium-bearing vanadium slag. Chem. Eng. J. 2016, 301, 132–138. [Google Scholar] [CrossRef]
- Liu, Z.; Nueraihemaiti, A.; Chen, M.; Du, J.; Fan, X.; Tao, C. Hydrometallurgical leaching process intensified by an electric field for converter vanadium slag. Hydrometallurgy 2015, 155, 56–60. [Google Scholar] [CrossRef]
- Wang, H.G.; Wang, M.Y.; Wang, X.W. Leaching behaviour of chromium during vanadium extraction from vanadium slag. Miner. Process. Extr. Metall. 2015, 124, 127–131. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, S.; Wang, S.; Qin, Y.; Du, H.; Zhang, Y. Electrochemical decomposition of vanadium slag in concentrated NaOH solution. Hydrometallurgy 2015, 151, 51–55. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, T.; Zhou, W.; Gao, H.; Xue, X. A cleaner and efficient process for extraction of vanadium from high chromium vanadium slag: Leaching in (NH4)2SO4-H2SO4 synergistic system and NH4+ recycle. Sep. Purif. Technol. 2019, 216, 126–135. [Google Scholar] [CrossRef]
- Ji, Y.; Shen, S.; Liu, J.; Xue, Y. Cleaner and effective process for extracting vanadium from vanadium slag by using an innovative three-phase roasting reaction. J. Clean. Prod. 2017, 149, 1068–1078. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, K.; Hua, W.-H.; Yang, Z.; Zhou, W.; Xie, B. Selective leaching of vanadium in calcification-roasted vanadium slag by ammonium carbonate. Hydrometallurgy 2016, 160, 18–25. [Google Scholar] [CrossRef]
- Yan, X.-M.; Xie, B.; Jiang, L.; Guo, H.-P.; Li, H.-Y. Leaching of Vanadium from the Roasted Vanadium Slag with High Calcium Content by Direct Roasting and Soda Leaching. In Rare Metal Technology 2015; Neelameggham, N.R., Alam, S., Oosterhof, H., Jha, A., Dreisinger, D., Wang, S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 209–216. [Google Scholar]
- Ning, P.; Lin, X.; Cao, H.; Zhang, Y. Selective extraction and deep separation of V(V) and Cr(VI) in the leaching solution of chromium-bearing vanadium slag with primary amine LK-N21. Sep. Purif. Technol. 2014, 137, 109–115. [Google Scholar] [CrossRef]
- Liu, H.B.; Liu, B.; Li, L.J.; Zheng, S.L.; Du, H.; Wang, S.N.; Chen, D.H.; Qi, J.; Zhang, Y. Novel methods to extract vanadium from vanadium slag by liquid oxidation technology. Adv. Mater. Res. 2012, 396, 1786–1793. [Google Scholar] [CrossRef]
- United States Geological Survey. Mineral Commodity Summaries 2020; United States Geological Survey: Reston, VA, USA, 2020; p. 204.
- Li, X.S.; Xie, B. Extraction of vanadium from high calcium vanadium slag using direct roasting and soda leaching. Int. J. Miner. Metall. Mater. 2012, 19, 595–601. [Google Scholar] [CrossRef]
- Liu, B.; Du, H.; Wang, S.-N.; Zhang, Y.; Zheng, S.-L.; Li, L.-J.; Chen, D.-H. A Novel Method to Extract Vanadium and Chromium from Vanadium Slag using Molten NaOH-NaNO3 Binary System. AIChE J. 2013, 59, 541–552. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Zheng, S.-L.; Wang, S.-N.; Liu, B.; Wang, D.-W.; Du, H.; Zhang, Y. Research and prospect on extraction of vanadium from vanadium slag by liquid oxidation technologies. Trans. Nonferrous Met. Soc. China 2014, 24, 1273–1288. [Google Scholar] [CrossRef]
- Chen, B.; Wang, M.; Huang, S.; Ge, Q.; Wang, X.; Sun, B. Extraction of vanadium from V-Cr bearing reduced residue by selective oxidation combined with alkaline leaching. Can. Metall. Q. 2018, 57, 434–438. [Google Scholar] [CrossRef]
- Wang, M.; Chen, B.; Huang, S.; Wang, X.; Liu, B.; Ge, Q.; Xie, S. A novel technology for vanadium and chromium recovery from V-Cr-bearing reducing slag. Hydrometallurgy 2017, 171, 116–122. [Google Scholar] [CrossRef]
- Chen, B.; Huang, S.; Liu, B.; Ge, Q.; Wang, M.; Wang, X. Separation and recovery of vanadium and chromium from acidic leach solution of V-Cr-bearing reducing slag. J. Environ. Chem. Eng. 2017, 5, 4702–4706. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Zhang, M.; Guo, M.; Wang, X. The Influence of SiO2 on the Extraction of Ti Element from Ti-bearing Blast Furnace Slag. Steel Res. Int. 2011, 82, 607–614. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.N.; Wang, M.Y.; Li, G.Q.; Sui, Z.T. Recovery of titanium compounds from molten Ti-bearing blast furnace slag under the dynamic oxidation condition. Miner. Eng. 2007, 20, 684–693. [Google Scholar] [CrossRef]
- Han, J.-Q.; Zhang, J.; Zhang, J.-H.; Chen, X.; Zhang, L.; Tu, G.-F. Recovery of Fe, V, and Ti in modified Ti-bearing blast furnace slag. Trans. Nonferrous Met. Soc. China 2022, 32, 333–344. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Mao, S.; Xiao, Y.; Li, J. Extraction of Valuable Metals from Titanium-bearing Blast Furnace Slag by Acid Leaching. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2024, 39, 376–385. [Google Scholar] [CrossRef]
- Chen, D.-S.; Zhao, L.-S.; Qi, T.; Hu, G.-P.; Zhao, H.-X.; Li, J.; Wang, L.-N. Desilication from titanium–vanadium slag by alkaline leaching. Trans. Nonferrous Met. Soc. China 2013, 23, 3076–3082. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Middlemas, S.; Guo, J.; Fan, P. A new, energy-efficient chemical pathway for extracting Ti metal from Ti minerals. J. Am. Chem. Soc. 2013, 135, 18248–18251. [Google Scholar] [CrossRef]
- Dong, H.; Jiang, T.; Guo, Y.; Chen, J.; Fan, X. Upgrading a Ti-slag by a roast-leach process. Hydrometallurgy 2012, 113, 119–121. [Google Scholar] [CrossRef]
- Chen, G.; Xiong, K.; Peng, J.; Chen, J. Optimization of combined mechanical activation-roasting parameters of titania slag using response surface methodology. Adv. Powder Technol. 2010, 21, 331–335. [Google Scholar] [CrossRef]
- Guo, C.; Chen, J.; Peng, J.-H.; Wan, R.-D. Green evaluation of microwave-assisted leaching process of high titanium slag on life cycle assessment. Trans. Nonferrous Met. Soc. China 2010, 20, s198–s204. [Google Scholar]
- Cai, Y.; Song, N.; Yang, Y.; Sun, L.; Hu, P.; Wang, J. Recent progress of efficient utilization of titanium-bearing blast furnace slag. Int. J. Miner. Metall. Mater. 2022, 29, 22–31. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Wang, M.; Li, G.; Sui, Z. Dynamic oxidation of the Ti-bearing blast furnace slag. ISIJ Int. 2006, 46, 458–465. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.; Zhang, L.; Zhang, L.; Tu, G.; Sui, Z. Effect of oxidization on enrichment behavior of TiO2 in titanium-bearing slag. Rare Met. 2006, 25, 106–110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).