High–Strength Porous TiNbZrTaFe Alloys Fabricated by Sintering of Nanocomposite Powder Precursor with Space Holder Technique
Abstract
1. Introduction
2. Experimental Methods
3. Results and Discussion
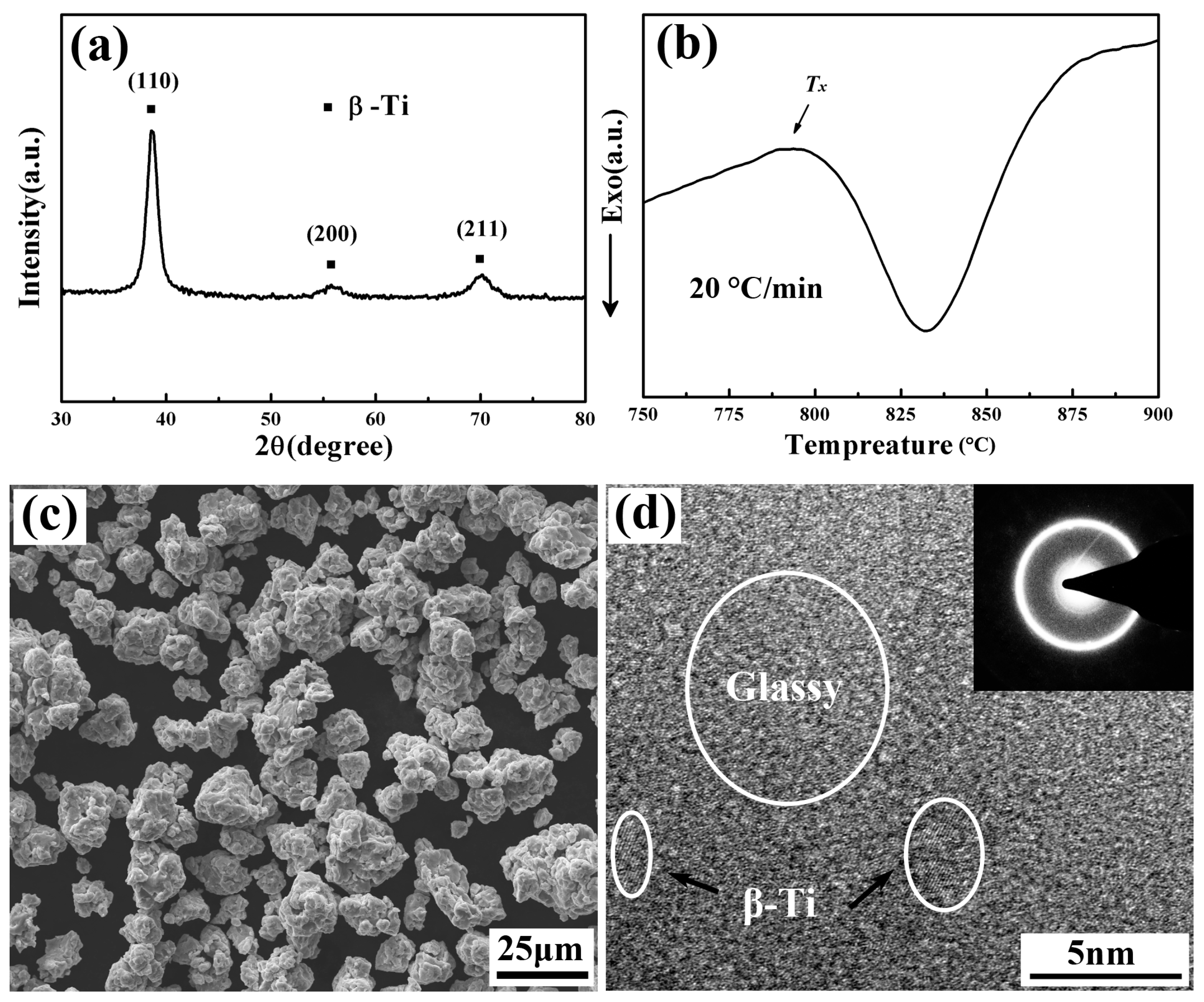
3.1. Characterization of the as–Milled TNZTF Powder
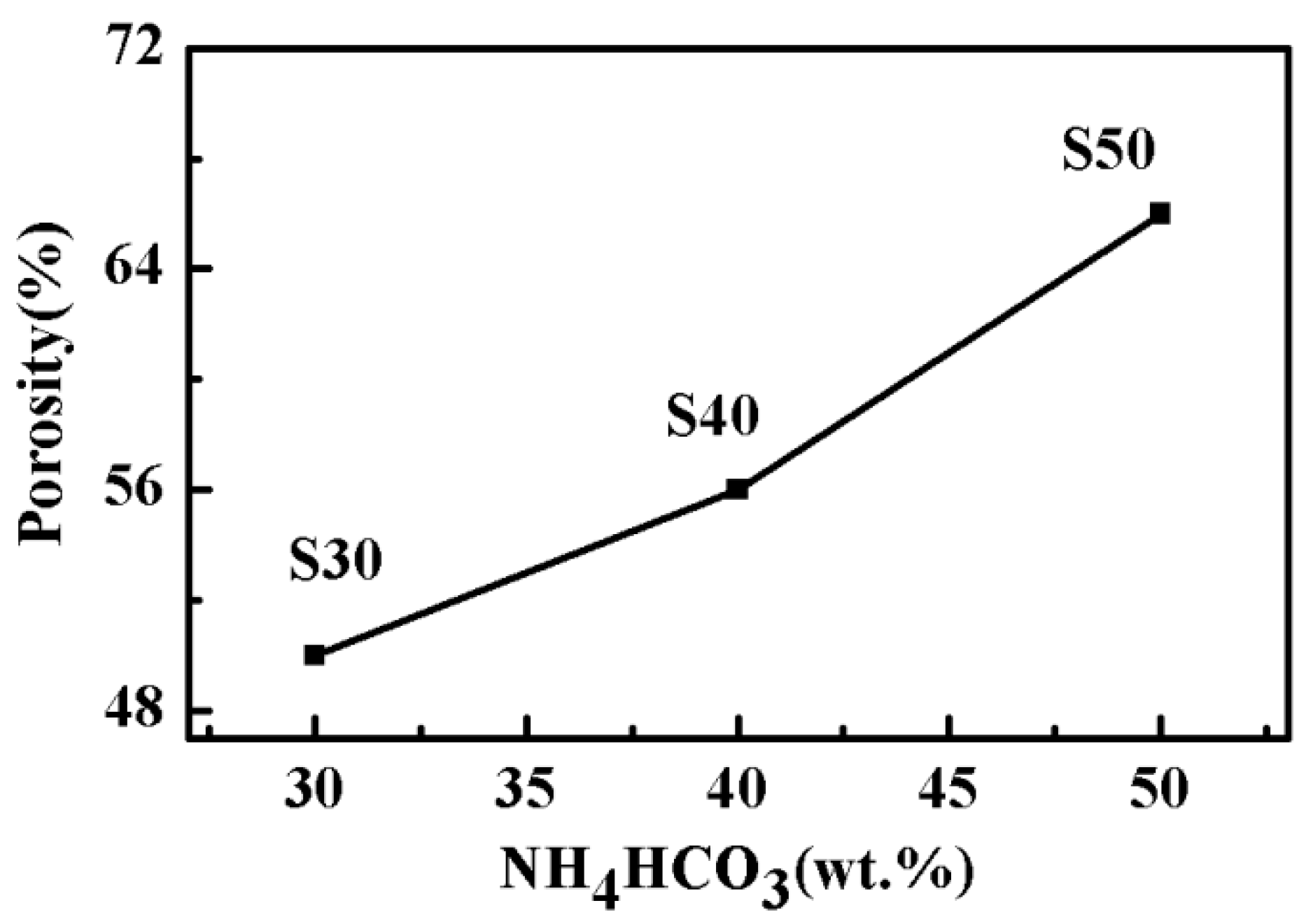
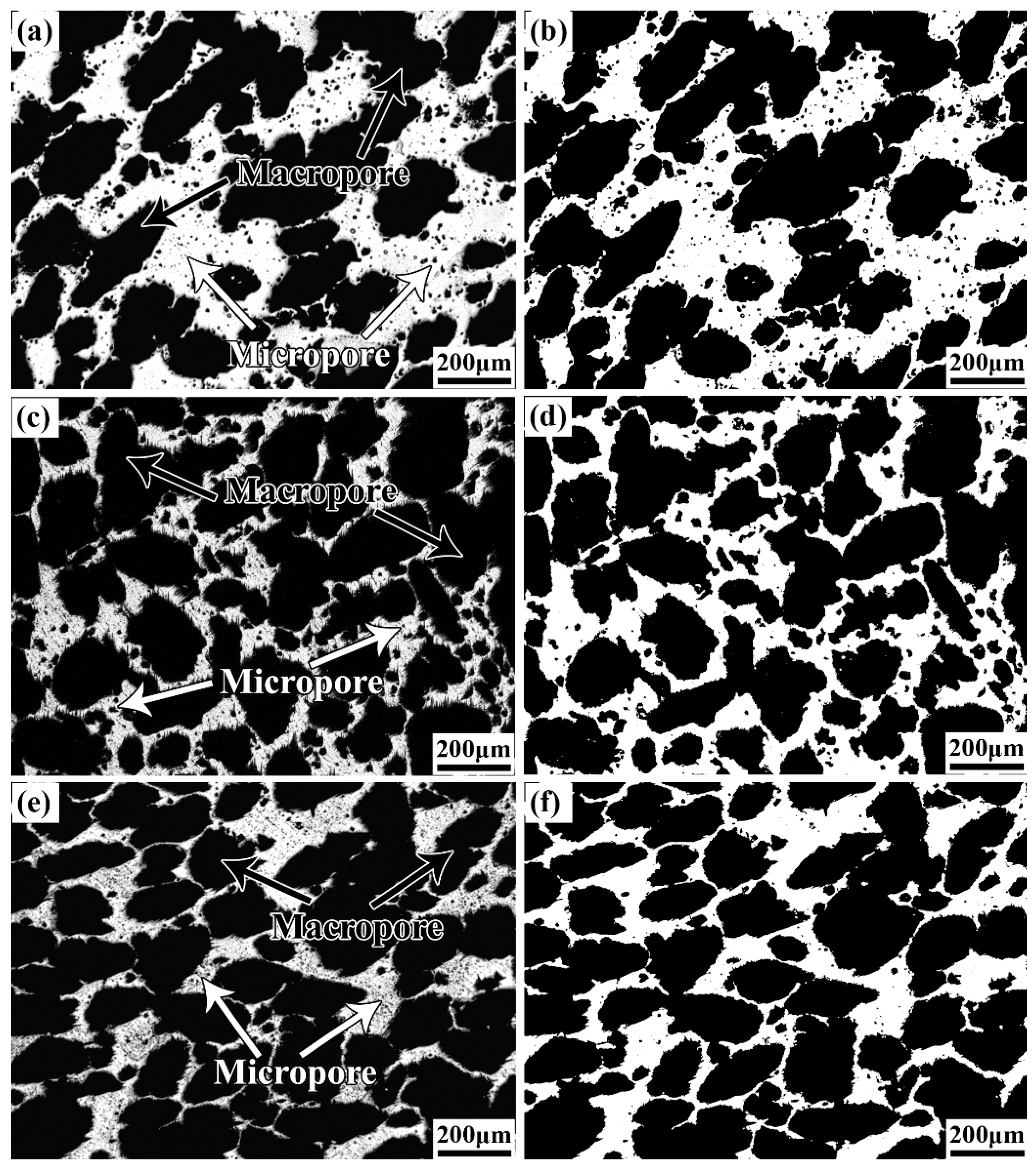
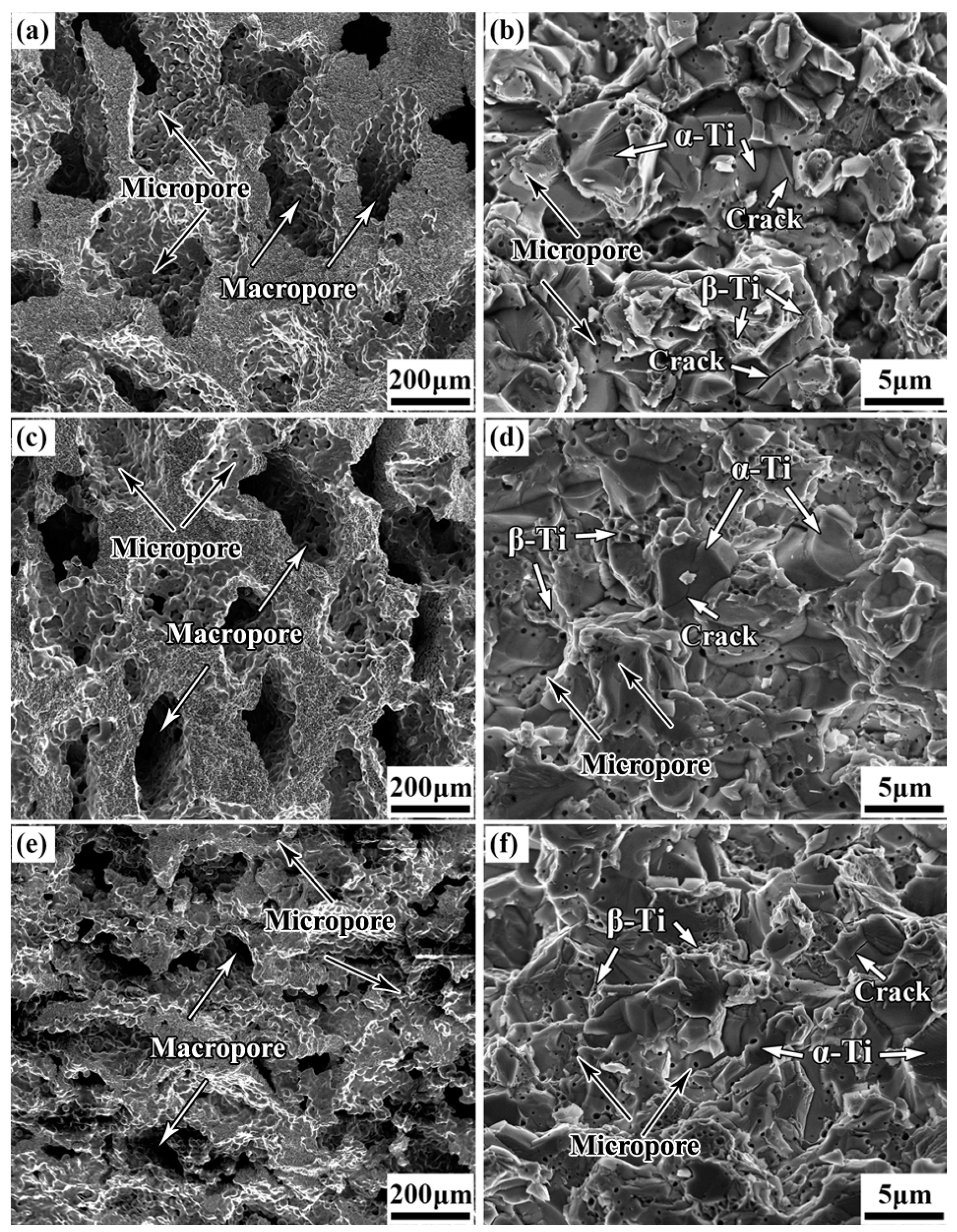
3.2. Pore Structure Characterization of the as–Prepared Porous TNZTF Alloys
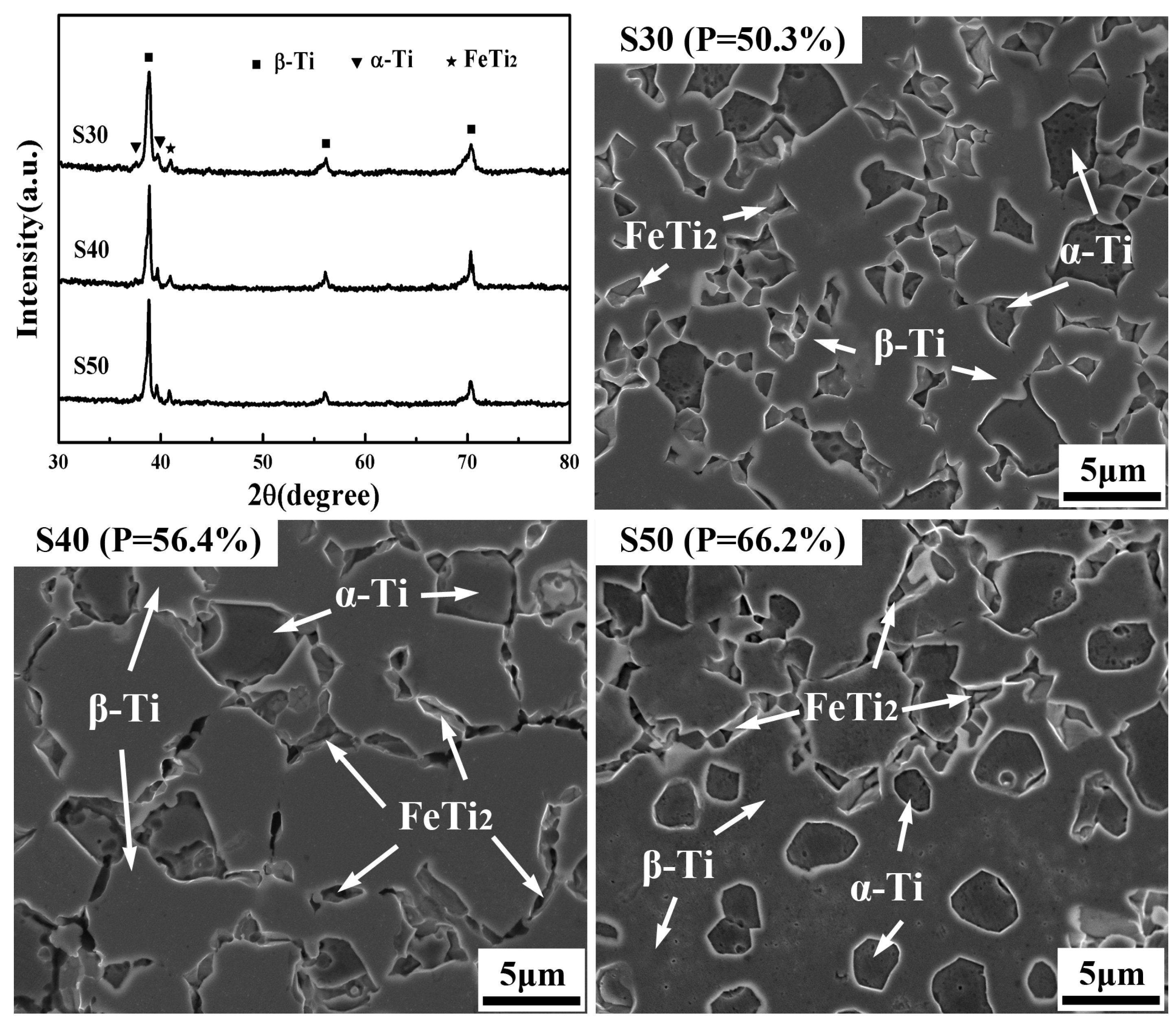
3.3. Phase Constitution and Microstructure of the as–Fabricated, Porous TNZTF Alloys
3.4. Mechanical Property of the as–Prepared TNZTF Porous Alloys
4. Conclusions
- The porous samples possess a homogeneous and fine–grained microstructure composed of equiaxed α–Ti and a small amount of FeTi2 dispersed in the β–Ti matrix. While being capable of adjusting pore structure characteristics and mechanical properties by a large margin, the addition of NH4HCO3 from 30 to 50 wt.% has little effect on their phase constitutions. The average grain size first increases and then decreases slightly with the increase in NH4HCO3 addition. The fracture mechanism indicates ductile–brittle mixed fracture characteristics in which β–Ti phase regions show a dimple structure while α–Ti phase regions display a rock candy structure together with trans–granular cracks.
- The porous alloy with a porosity of 50.3 ± 0.2% shows an excellent combination of mechanical properties with a compressive strength of 327.3 ± 2.1 MPa, and an elastic modulus of 12.2 ± 0.3 GPa, respectively. Compared with other porous titanium biomaterials, it exhibits a higher compressive strength and a low elastic modulus matched to that of the human bones. The strength enhancement is mainly attributed to the unique fine–grained microstructure obtained by the crystallization of the amorphous phase during sintering and the ductile–brittle mixed fracture mechanism.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sebbag, E.; Felten, R.; Sagez, F.; Sibilia, J.; Devilliers, H.; Arnaud, L. The world–wide burden of musculoskeletal diseases: A systematic analysis of the world health organization burden of diseases database. Ann. Rheum. Dis. 2019, 78, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.W.; Wang, L.Q.; Zhang, L.C. Towards load–bearing biomedical titanium–based alloys: From essential requirements to future developments. Prog. Mater. Sci. 2024, 144, 101277. [Google Scholar] [CrossRef]
- Li, Y.H.; Yang, C.; Zhao, H.D.; Qu, S.G.; Li, X.Q.; Li, Y.Y. New developments of Ti–based alloys for biomedical applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [PubMed]
- Pitchi, C.S.; Priyadarshini, A.; Sana, G.; Narala, S.K.R. A review on alloy composition and synthesis of β–Titanium alloys for biomedical applications. Mater. Today Proc. 2020, 26, 3297–3304. [Google Scholar] [CrossRef]
- Ma, H.Y.; Wang, J.C.; Qin, P.; Liu, Y.J.; Chen, L.Y.; Wang, L.Q.; Zhang, L.C. Advances in additively manufactured titanium alloys by powder bed fusion and directed energy deposition: Microstructure, defects, and mechanical behavior. J. Mater. Sci. Technol. 2024, 183, 32–62. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, Q.; He, Y.X.; Zhao, R.; Chu, J.H.; Niu, L.B.; Qu, J.X. Sliding and fretting wear behavior of biomedical ultrafine–grained TiNbZrTaFe/Si alloys in simulated physiological solution. Materials 2024, 17, 787. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Q.; Xie, R.Z.; Lu, X. Microstructure, mechanical property and corrosion behavior of porous Ti–Ta–Nb–Zr. Bioact. Mater. 2020, 5, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Song, C.H.; Liu, L.S.; Deng, Z.T.; Lei, H.Y.; Yuan, F.Z.; Yang, Y.Q.; Li, Y.Y.; Yu, J.K. Research progress on the design and performance of porous titanium alloy bone implants. J. Mater. Res. Technol. 2023, 23, 2626–2641. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, L.Y.; Zhou, S.F.; Luo, Z. Powder bed fusion manufacturing of beta–type titanium alloys for biomedical implant applications: A review. J. Alloys Compd. 2023, 936, 168099. [Google Scholar] [CrossRef]
- Xu, W.; Lu, X.; Tian, J.J.; Huang, C.; Chen, M.; Yan, Y.; Wang, L.N.; Qu, X.H.; Wen, C. Microstructure, wear resistance, and corrosion performance of Ti35Zr28Nb alloy fabricated by powder metallurgy for orthopedic applications. J. Mater. Sci. Technol. 2020, 41, 191–198. [Google Scholar] [CrossRef]
- El–Sayed Seleman, M.M.; Ataya, S.; Aly, H.A.; Haldar, B.; Alsaleh, N.A.; Ahmed, M.M.Z.; Bakkar, A.; Ibrahim, K.M. Effect of the Si content on the dry and wet sliding wear behavior of the developed Ti–15Mo–(0–2) Si alloys for biomedical applications. Metals 2023, 13, 1861. [Google Scholar] [CrossRef]
- Wang, J.C.; Liu, Y.J.; Rabadia, C.D.; Liang, S.X.; Sercombe, T.B.; Zhang, L.C. Microstructural homogeneity and mechanical behavior of a selective laser melted Ti–35Nb alloy produced from an elemental powder mixture. J. Mater. Sci. Technol. 2021, 61, 221–233. [Google Scholar] [CrossRef]
- Wang, W.J.; Yang, K.H.; Wang, Q.T.; Dai, P.Q.; Fang, H.; Wu, F.J.; Guo, Q.H.; Liaw, P.K.; Hua, N.B. Novel Ti–Zr–Hf–Nb–Fe refractory high–entropy alloys for potential biomedical applications. J. Alloys Compd. 2022, 906, 164383. [Google Scholar] [CrossRef]
- Tong, X.; Sun, Q.X.; Zhang, D.C.; Wang, K.; Dai, Y.L.; Shi, Z.M.; Li, Y.C.; Dargusch, M.; Huang, S.B.; Ma, J.F.; et al. Impact of scandium on mechanical properties, corrosion behavior, friction and wear performance, and cytotoxicity of a β–type Ti–24Nb–38Zr–2Mo alloy for orthopedic applications. Acta Biomater. 2021, 134, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Du, P.; Cai, Z.Y.; Li, K.; Bao, W.Z.; Yang, X.X.; Xie, G.Q. Phase–tunable equiatomic and non–equiatomic Ti–Zr–Nb–Ta high–entropy alloys with ultrahigh strength for metallic biomaterials. J. Mater. Sci. Technol. 2022, 117, 196–206. [Google Scholar] [CrossRef]
- Lee, T.; Lee, S.; Kim, I.S.; Moon, Y.H.; Kim, H.S.; Park, C.H. Breaking the limit of Young’s modulus in low–cost Ti–Nb–Zr alloy for biomedical implant applications. J. Alloys Compd. 2020, 828, 154401. [Google Scholar] [CrossRef]
- Yilmaz, E.; Gökçe, A.; Findik, F.; Gulsoy, H. Metallurgical properties and biomimetic HA deposition performance of Ti–Nb PIM alloys. J. Alloys Compd. 2018, 746, 301–313. [Google Scholar] [CrossRef]
- Li, F.P.; Jia, T.; Dang, W.; Xu, Z.L.; Zhao, K.; Tang, Y.F. Porous Ti6Al4V alloys with high strength–to–modulus ratio fabricated by unidirectional freeze casting of SiC fiber–containing slurry. Mater. Sci. Eng. A 2021, 820, 141584. [Google Scholar] [CrossRef]
- Wang, S.; Liu, L.; Li, K.; Zhu, L.; Chen, J.; Hao, Y. Pore functionally graded Ti6Al4V scaffolds for bone tissue engineering application. Mater. Des. 2019, 168, 107643. [Google Scholar] [CrossRef]
- Hafeez, N.; Liu, J.; Wang, L.Q.; Wei, D.X.; Tang, Y.J.; Lu, W.J.; Zhang, L.C. Superelastic response of low–modulus porous beta–type Ti–35Nb–2Ta–3Zr alloy fabricated by laser powder bed fusion. Addit. Manuf. 2020, 34, 101264. [Google Scholar] [CrossRef]
- Lascano, S.; Arévalo, C.; Montealegre–Melendez, I.; Muñoz, S.; Rodriguez–Ortiz, J.A.; Trueba, P.; Torres, Y. Porous Titanium for biomedical applications: Evaluation of the conventional powder metallurgy frontier and space–holder technique. Appl. Sci. 2019, 9, 982. [Google Scholar] [CrossRef]
- Rodriguez–Contreras, A.; Punset, M.; Calero, J.A.; Gil, F.J.; Ruperez, E.; Manero, J.M. Powder metallurgy with space holder for porous titanium implants: A review. J. Mater. Sci. Technol. 2021, 76, 129–149. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Attar, H.; Ehtemam–Haghighi, S.; Soro, N.; Kent, D.; Dargusch, M.S. Additive manufacturing of low–cost porous titanium–based composites for biomedical applications: Advantages, challenges and opinion for future development. J. Alloys Compd. 2020, 827, 154263. [Google Scholar] [CrossRef]
- Yang, X.L.; Du, X.F.; Xu, Z.L.; Liang, Z.S.; Xiong, L.L. Progress in processing of porous titanium: A review. Rare Met. 2024, 43, 1932–1955. [Google Scholar] [CrossRef]
- Dudina, D.V.; Bokhonov, B.B.; Olevsky, E.A. Fabrication of porous materials by spark plasma sintering: A review. Materials 2019, 12, 541. [Google Scholar] [CrossRef]
- Fujii, T.; Murakami, R.; Kobayashi, N.; Tohgo, K.; Shimamura, Y. Uniform porous and functionally graded porous titanium fabricated via space holder technique with spark plasma sintering for biomedical applications. Adv. Powder Technol. 2022, 33, 103598. [Google Scholar] [CrossRef]
- Sauceda, S.; Lascano, S.; Béjar, L.; Neves, G.O.; Chicardi, E.; Salvo, C.; Aguilar, C. Study of the effect of the floating die compaction on mechanical properties of titanium foams. Metals 2020, 10, 1621. [Google Scholar] [CrossRef]
- Yamanoglu, R.; Bahador, A.; Kondoh, K. Fabrication methods of porous titanium implants by powder metallurgy. Trans. Indian Inst. Met. 2021, 74, 2555–2567. [Google Scholar] [CrossRef]
- Kumar, R.M.; Golla, B.R. Effect of space holder on porosity, structure and mechanical properties of Al processed via power metallurgy. Trans. Indian Inst. Met. 2021, 74, 2379–2386. [Google Scholar] [CrossRef]
- Lascano, S.; Chávez–Vásconez, R.; Muñoz–Rojas, D.; Aristizabal, J.; Arce, B.; Parra, C.; Acevedo, C.; Orellana, N.; Reyes–Valenzuela, M.; Gotor, F.J.; et al. Graphene–coated Ti–Nb–Ta–Mn foams: A promising approach towards a suitable biomaterial for bone replacement. Suf. Coat. Technol. 2020, 401, 126250. [Google Scholar] [CrossRef]
- Civantos, A.; Giner, M.; Trueba, P.; Lascano, S.; Montoya–García, M.J.; Arévalo, C.; Vázquez, M.A.; Allain, J.P.; Torres, Y. In vitro bone cell behavior on porous titanium samples: Influence of porosity by loose sintering and space holder techniques. Metals 2020, 10, 696. [Google Scholar] [CrossRef]
- Gupta, J.; Ghosh, S.; Aravindan, S. Effect of Mo and space holder content on microstructure, mechanical and corrosion properties in Ti6AlxMo based alloy for bone implant. Mater. Sci. Eng. C 2021, 123, 111962. [Google Scholar] [CrossRef]
- Lantang, Y.S.F.; Kobayashi, E.; Sato, T. Fabrication of spark plasma sintered porous Ti–6Al–4V using PCA–added space holder method for bone implant materials. Mater. Today Proc. 2022, 66, 2693–2701. [Google Scholar] [CrossRef]
- Lai, T.; Xu, J.L.; Xiao, Q.F.; Tong, Y.X.; Huang, J.; Zhang, J.P.; Luo, J.M.; Liu, Y. Preparation and characterization of porous NiTi alloys synthesized by microwave sintering using Mg space holder. Trans. Nonferrous Met. Soc. China 2021, 31, 485–498. [Google Scholar] [CrossRef]
- Luo, H.J.; Zhao, J.H.; Du, H.; Yin, W.; Qu, Y. Effect of Mg powder’s particle size on structure and mechanical properties of Ti foam synthesized by space holder technique. Materials 2022, 15, 8863. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Frith, J.E.; Dehghan–Manshadi, A.; Kent, D.; Bermingham, M.; Dargusch, M. Biocompatible porous titanium scaffolds produced using a novel space holder technique. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2796–2806. [Google Scholar] [CrossRef] [PubMed]
- Arifvianto, B.; Leeflang, M.A.; Zhou, J. The compression behaviors of titanium/carbamide powder mixtures in the preparation of biomedical titanium scaffolds with the space holder method. Powder Technol. 2015, 284, 112–121. [Google Scholar] [CrossRef]
- Chávez–Vásconez, R.; Lascano, S.; Sauceda, S.; Reyes–Valenzuela, M.; Salvo, C.; Mangalaraja, R.V.; Gotor, F.J.; Arévalo, C.; Torres, Y. Effect of the processing parameters on the porosity and mechanical behavior of titanium samples with bimodal microstructure produced via hot pressing. Materials 2022, 15, 136. [Google Scholar] [CrossRef]
- Ehtemam–Haghighi, S.; Attar, H.; Okulov, I.V.; Dargusch, M.S.; Kent, D. Microstructural evolution and mechanical properties of bulk and porous low–cost Ti–Mo–Fe alloys produced by powder metallurgy. J. Alloys Compd. 2021, 853, 156768. [Google Scholar] [CrossRef]
- Xie, G.Q.; Kanetaka, H.; Kato, H.; Qin, F.X.; Wang, W. Porous Ti–based bulk metallic glass with excellent mechanical properties and good biocompatibility. Intermetallics 2019, 105, 153–162. [Google Scholar] [CrossRef]
- Xiang, T.; Chen, J.; Bao, W.Z.; Zhong, S.Y.; Du, P.; Xie, G.Q. Fabrication of porous TiZrNbTa high–entropy alloys/Ti composite with high strength and low Young’s modulus using a novel MgO space holder. J. Mater. Sci. Technol. 2023, 167, 56–73. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Z.; Lu, X.; Tian, J.J.; Chen, G.; Liu, B.W.; Li, Z.; Qu, X.H.; Wen, C. Porous Ti–10Mo alloy fabricated by powder metallurgy for promoting bone regeneration. Sci. China Mater. 2019, 62, 1053–1064. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Bermingham, M.J.; Otte, J.; Liu, Y.G.; Hou, Z.Y.; Yang, N.; Yin, Y.; Bayat, M.; Lin, W.K.; Huang, X.X.; et al. Ultrauniform, strong, and ductile 3D–printed titanium alloy through bifunctional alloy design. Science 2024, 383, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Yang, C.; Wang, F.; Zhao, H.D.; Qu, S.G.; Li, X.Q.; Zhang, W.W.; Li, Y.Y. Biomedical TiNbZrTaSi alloys designed by d–electron alloy design theory. Mater. Design 2015, 85, 7–13. [Google Scholar] [CrossRef]
- Li, Y.H.; Yang, C.; Kang, L.M.; Zhao, H.D.; Qu, S.G.; Li, X.Q.; Zhang, W.W.; Li, Y.Y. Non–isothermal and isothermal crystallization kinetics and their effect on microstructure of sintered and crystallized TiNbZrTaSi bulk alloys. J. Non–Cryst. Solids 2016, 432, 440–452. [Google Scholar] [CrossRef]
- Li, Y.H.; Yang, C.; Kang, L.M.; Zhao, H.D.; Zhang, W.W.; Li, Y.Y. Biomedical porous TiNbZrFe alloys fabricated by using NH4HCO3 as pore–forming agent through powder metallurgy route. Powder Metall. 2015, 58, 228–234. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Sun, N.; Zhu, M.R.; Qiu, Q.R.; Zhao, P.J.; Zheng, C.Y.; Bai, Q.; Zeng, Q.Y.; Lu, T.L. The contribution of pore size and porosity of 3D printed porous titanium scaffolds to osteogenesis. Biomater. Adv. 2022, 133, 112651. [Google Scholar] [CrossRef] [PubMed]
- Kamyabi, M.; Sotudeh–Gharebagh, R.; Zarghami, R.; Saleh, K. Principles of viscous sintering in amorphous powders: A critical review. Chem. Eng. Res. Des. 2017, 125, 328–347. [Google Scholar] [CrossRef]
- Paul, T.; Harimkar, S.P. Viscous flow activation energy adaptation by isochronal spark plasma sintering. Scr. Mater. 2017, 126, 37–40. [Google Scholar] [CrossRef]
- Cai, W.S.; Lu, H.Z.; Li, H.Z.; Liu, Z.; Ke, H.B.; Wang, W.H.; Yang, C. Microstructural evolution and superelastic properties of ultrafine–grained NiTi–based shape memory alloy via sintering of amorphous ribbon precursor. J. Mater. Sci. Technol. 2023, 138, 80–92. [Google Scholar] [CrossRef]
- Awad, A.H.; Aly, H.A.; Saood, M. Physical, mechanical, and corrosion properties of Ti–12Mo and Ti–15Mo alloys fabricated by elemental blend and mechanical alloying techniques. Mater. Chem. Phys. 2024, 312, 128661. [Google Scholar] [CrossRef]


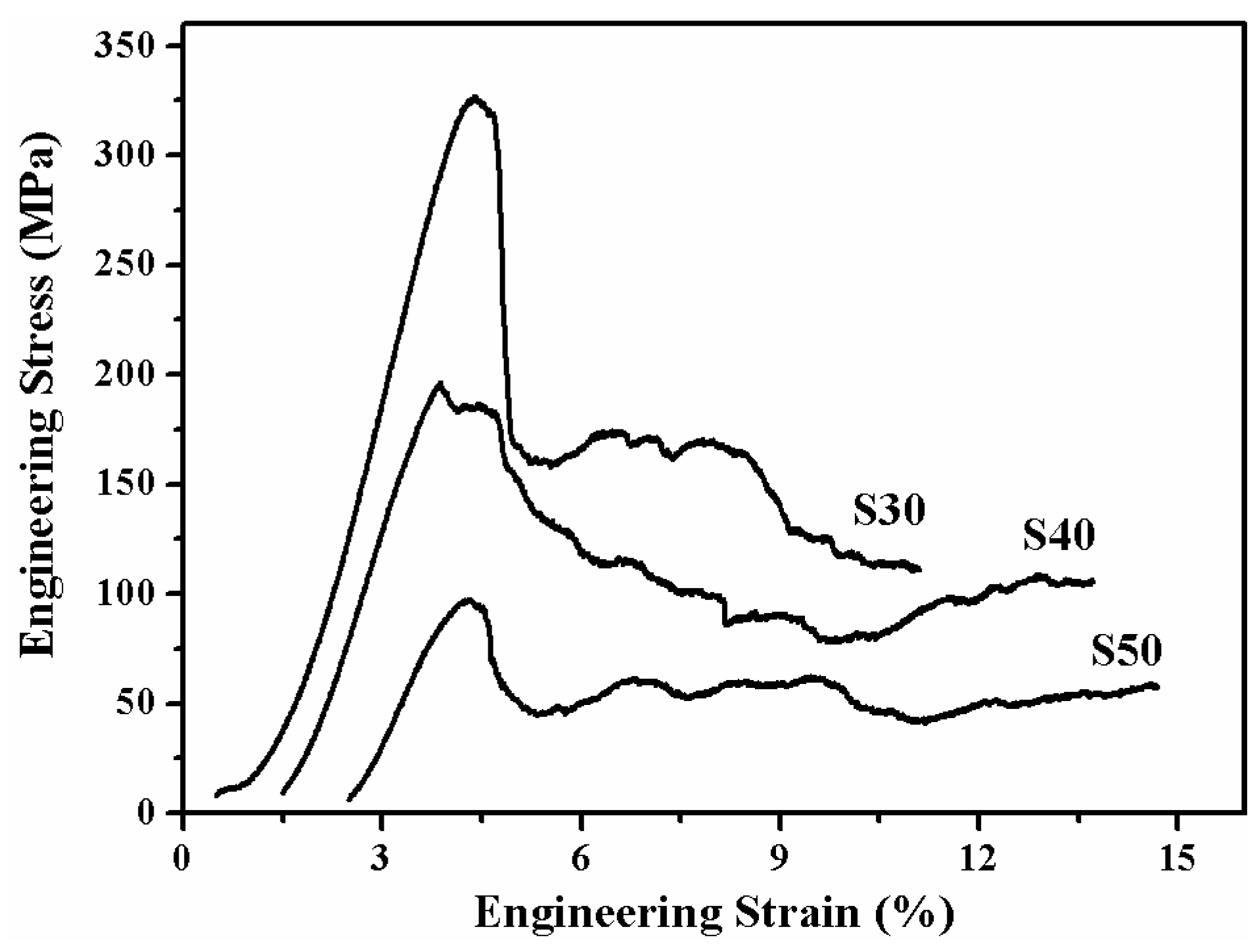
| Samples | NH4HCO3 (wt.%) | Porosity (%) | Elastic Modulus (GPa) | Compressive Strength (MPa) |
|---|---|---|---|---|
| S30 | 30 | 50.3 ± 0.2 | 12.2 ± 0.3 | 327.3 ± 2.1 |
| S40 | 40 | 56.4 ± 0.4 | 9.7 ± 0.2 | 197.2 ± 1.2 |
| S50 | 50 | 66.2 ± 0.6 | 6.4 ± 0.1 | 98.4 ± 0.6 |
| Ti59.38Nb26.6Zr8.7Fe5.32 [47] | 30 | 48 | 11 | 165 |
| Ti59.38Nb26.6Zr8.7Fe5.32 [47] | 40 | 53 | 7 | 117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; He, Y.; Zhao, R.; Niu, L.; Qu, J.; Zhang, L.-C. High–Strength Porous TiNbZrTaFe Alloys Fabricated by Sintering of Nanocomposite Powder Precursor with Space Holder Technique. Metals 2024, 14, 824. https://doi.org/10.3390/met14070824
Li Y, He Y, Zhao R, Niu L, Qu J, Zhang L-C. High–Strength Porous TiNbZrTaFe Alloys Fabricated by Sintering of Nanocomposite Powder Precursor with Space Holder Technique. Metals. 2024; 14(7):824. https://doi.org/10.3390/met14070824
Chicago/Turabian StyleLi, Yuhua, Yuxin He, Rong Zhao, Libin Niu, Juxin Qu, and Lai-Chang Zhang. 2024. "High–Strength Porous TiNbZrTaFe Alloys Fabricated by Sintering of Nanocomposite Powder Precursor with Space Holder Technique" Metals 14, no. 7: 824. https://doi.org/10.3390/met14070824
APA StyleLi, Y., He, Y., Zhao, R., Niu, L., Qu, J., & Zhang, L.-C. (2024). High–Strength Porous TiNbZrTaFe Alloys Fabricated by Sintering of Nanocomposite Powder Precursor with Space Holder Technique. Metals, 14(7), 824. https://doi.org/10.3390/met14070824








