Mechanical Properties and Wear Resistance of Biodegradable ZnMgY Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structural and Chemical Composition Analysis
3.2. Vickers Microhardness Determination of Hot-Rolled Zn3Mg1Y Alloy
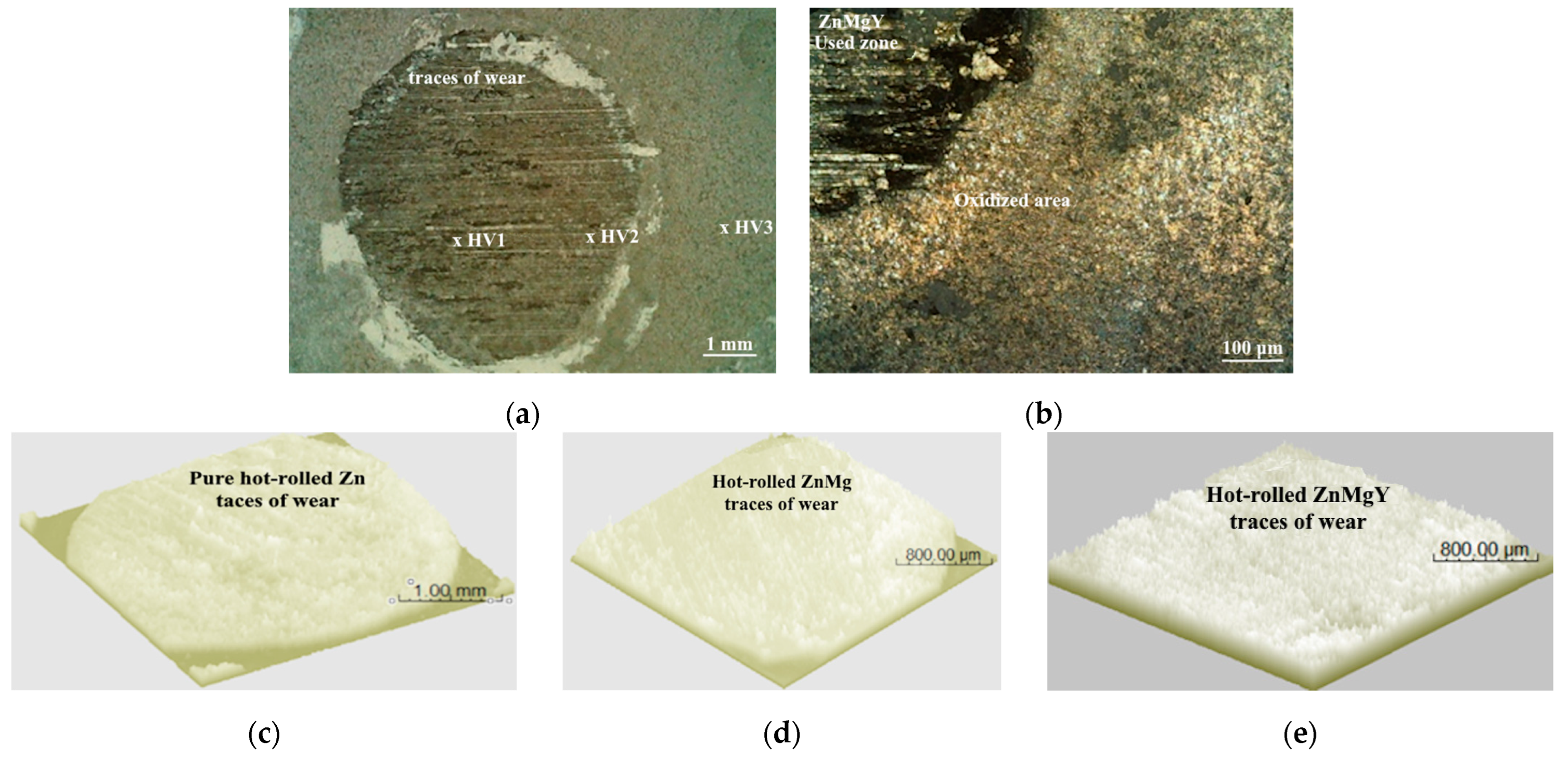
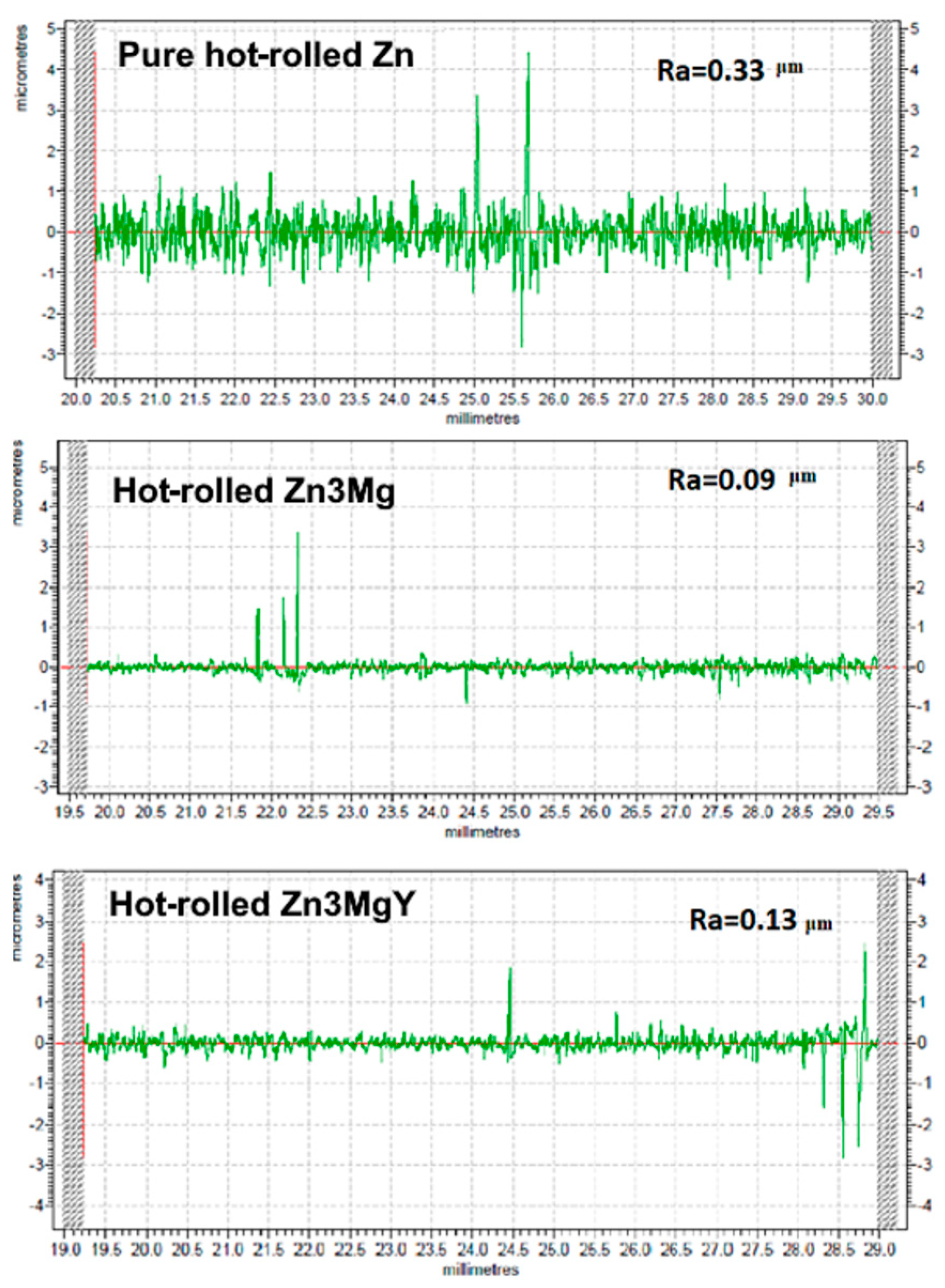
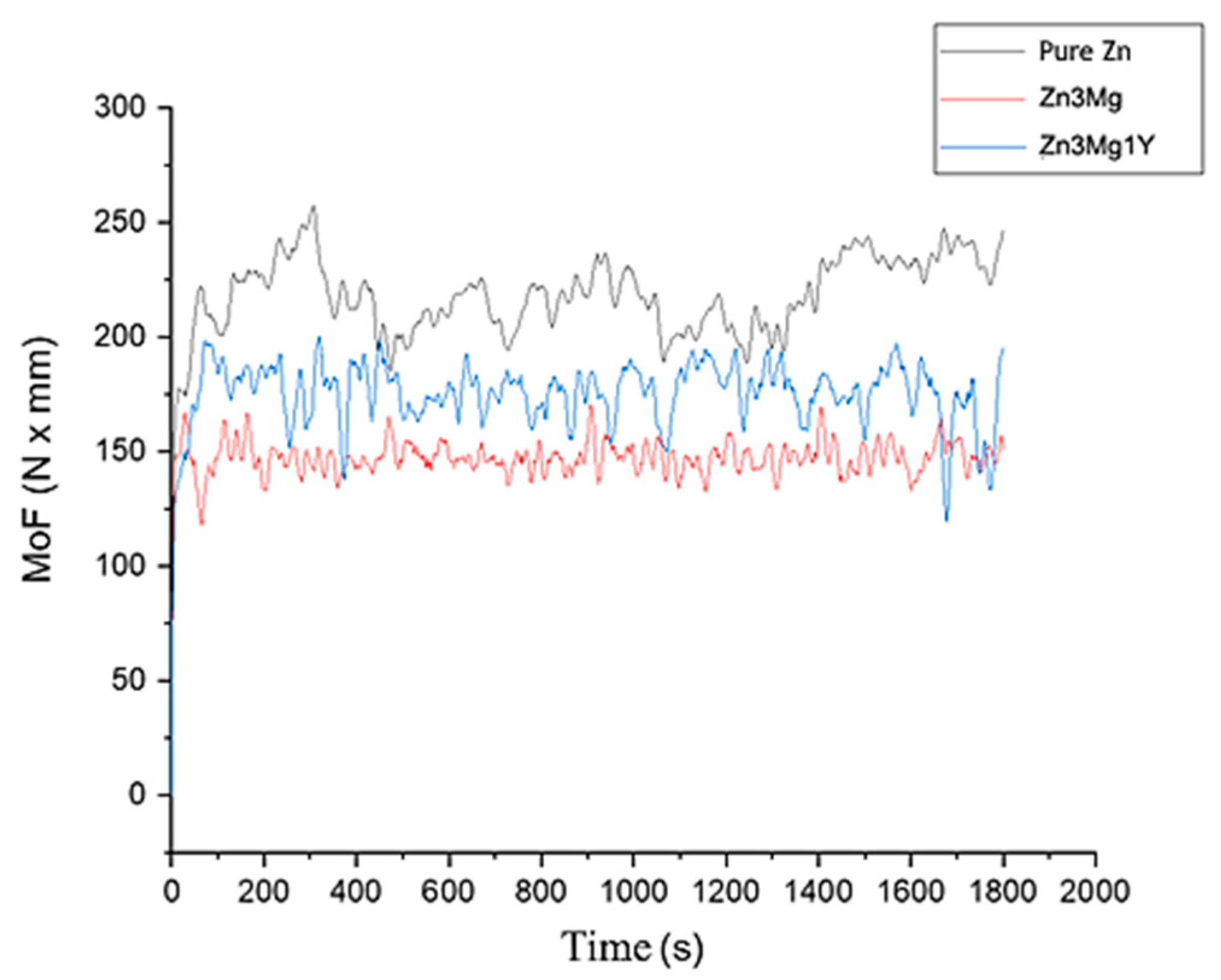
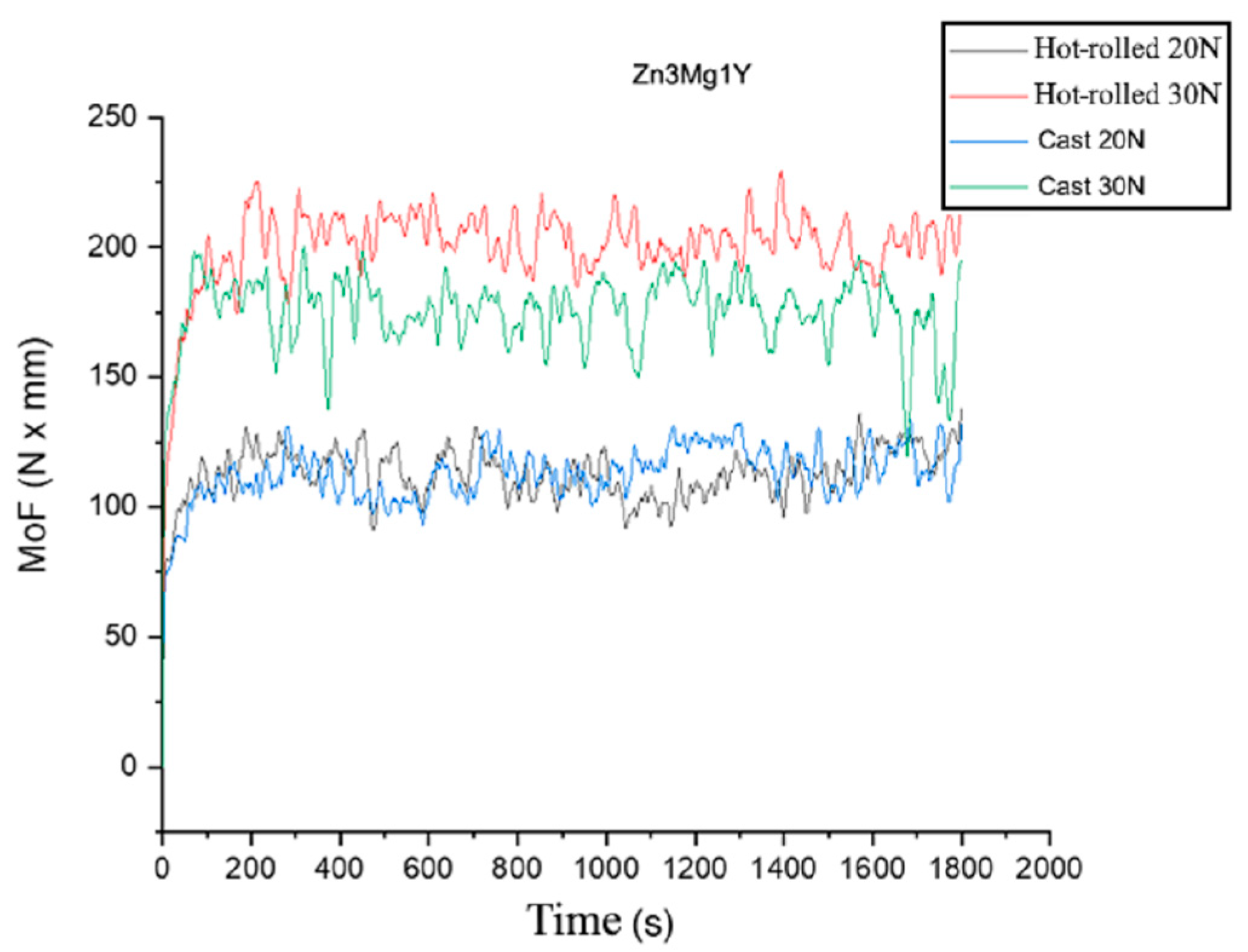
3.3. Wear Resistance Tests of Experimental Hot-Rolled Materials
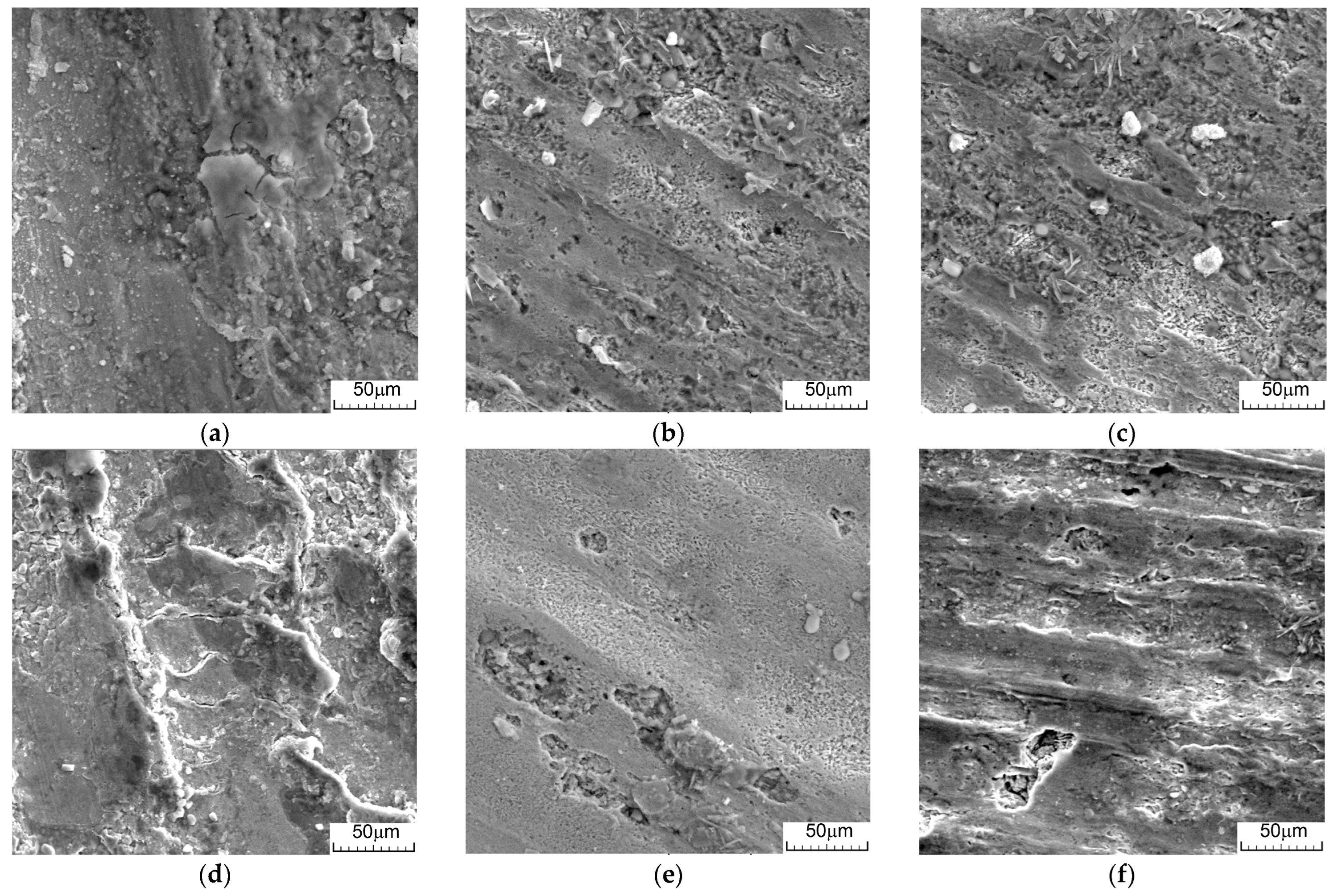
3.4. Microstructural Analysis of Hot-Rolled and Worn Alloys
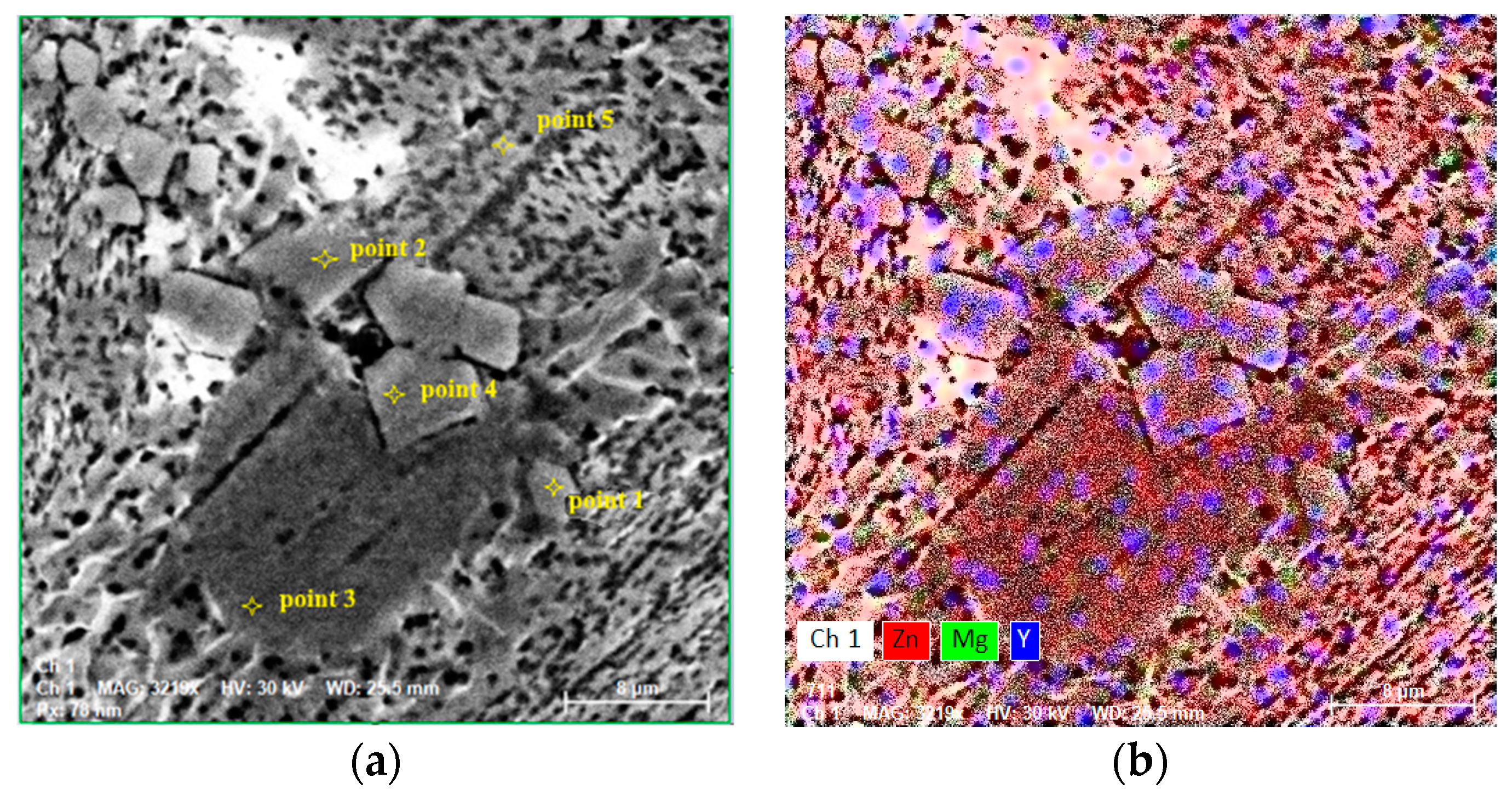
3.5. Chemical Analysis by EDS of Hot-Rolled and Worn Sample Surfaces
4. Conclusions
- -
- Mg and Y addition to pure Zn improves mechanical properties such as microhardness and wear resistance;
- -
- The hot-rolling process altered the shape and size of the compounds by reducing the YZn12 dimensions and spreading them in the eutectic;
- -
- Both the hot rolling and Y addition contributed to the improved workability of the Zn3Mg1Y alloy;
- -
- The analysis of hot-rolled test samples from pure Zn, Zn3Mg, and Zn3Mg1Y showed a significant increase in microhardness due to the Mg and Y additions, as well as an increase in microhardness in the worn zone;
- -
- The worn surface, in addition to the embrittlement phenomenon characteristic of stressed metallic materials, exhibited more pronounced oxidation compared to the unaffected surface in the electrolytic environment due to the increase in the exposed surface area and the surface stresses caused by wear.
- -
- Further investigations will include analysis regarding the effect of material wear products on cell survival, cell viability, and adhesion tests, followed by in vivo implantation tests in laboratory mice or rabbits.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cai, A.Y.; Zhu, Y.J.; Qi, C. Biodegradable inorganic nanostructured biomaterials for drug delivery. Adv. Mater. Interfaces 2020, 7, 2000819. [Google Scholar] [CrossRef]
- Khan, A.R.; Grewal, N.S.; Zhou, C.; Yuan, K.; Zhang, H.J.; Jun, Z. Recent advances in biodegradable metals for implant ap-plications: Exploring in vivo and in vitro responses. Results Eng. 2023, 20, 101526. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteu, A.; Bezina-Cimdina, L. Biodegradable Materials and Metallic Implants—A Review. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Vojtech, D.; Kubasek, J.; Serak, J.; Novak, P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef]
- Kaur, K.; Gupta, R.; Saraf, S.A.; Saraf, S.K. Zinc: The metal of life. Compr. Rev. Food Sci. Food Saf. 2014, 13, 358–376. [Google Scholar] [CrossRef]
- Li, H.F.; Shi, Z.Z.; Wang, L.N. Opportunities and challenges of biodegradable Zn-based alloys. J. Mater. Sci. Technol. 2020, 46, 136–138. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Zhou, F.; Yang, Y.; Chang, R.; Qiu, K.; Pu, Z.; Li, L.; Zheng, Y. Microalloying with Mn in Zn–Mg alloy for future biodegradable metals application. Mater. Des. 2016, 94, 95–104. [Google Scholar] [CrossRef]
- Tang, Z.; Niu, J.; Huang, H.; Zhang, H.; Pei, J.; Ou, J.; Yuan, G. Potential biodegradable Zn-Cu binary alloys developed for cardiovascular implant applications. J. Mech. Behav. Biomed. Mater. 2017, 72, 182–191. [Google Scholar] [CrossRef]
- Yue, R.; Huang, H.; Ke, G.; Zhang, H.; Pei, J.; Xue, G.; Yuan, G. Microstructure, mechanical properties and in vitro degradation behavior of novel Zn-Cu-Fe alloys. Mater. Charact. 2017, 134, 114–122. [Google Scholar] [CrossRef]
- Bowen, P.K.; Seitz, J.-M.; Guillory, R.J.; Braykovich, J.P.; Zhao, S.; Goldman, J.; Drelich, J.W. Evaluation of wrought Zn-Al alloys (1, 3, and 5 wt% Al) through mechanical and in vivo testing for stent applications. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Z.; Yu, J.; Liu, X.F.; Wang, L.N. Fabrication and characterization of novel biodegradable Zn-Mn-Cu alloys. J. Mater. Sci. Technol. 2018, 34, 1008–1015. [Google Scholar] [CrossRef]
- Liu, L.; Meng, Y.; Dong, C.; Yan, Y.; Volinsky, A.A.; Wang, L.N. Initial formation of corrosion products on pure zinc in simulated body fluid. J. Mater. Sci. Technol. 2018, 34, 2271–2282. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, F.; Liu, X. Protection of magnesium alloys: From physical barrier coating to smart self-healing coating. J. Alloys Compd. 2021, 853, 157010. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S. Surface Modification of Biodegradable Zinc Alloy for Biomedical Applications. BioNanoScience 2023, 13, 1381–1398. [Google Scholar] [CrossRef]
- Panaghie, C.; Cimpoesu, R.; Istrate, B.; Cimpoesu, N.; Bernevig, M.A.; Zegan, G.; Roman, A.M.; Chelariu, R.; Sodor, A. New Zn3Mg-xY Alloys: Characteristics, Microstructural Evolution and Corrosion Behavior. Materials 2021, 14, 2505. [Google Scholar] [CrossRef] [PubMed]
- Panaghie, C.; Cimpoeșu, N.; Benchea, M.; Roman, A.M.; Manole, V.; Alexandru, A.; Cimpoesu, R.; Cazacu, M.M.; Wnuk, I.; Zegan, G. “In-vitro” Tests on New Biodegradable Metallic Material Based on ZnMgY. Arch. Metall. Mater. 2022, 67, 587–594. [Google Scholar] [CrossRef]
- Chen, K.; Gu, X.; Zheng, Y. Feasibility, challenges and future prospects of biodegradable zinc alloys as orthopedic internal fixation implants. Smart Mater. Manuf. 2024, 2, 100042. [Google Scholar] [CrossRef]
- Xu, W.; Yu, A.; Lu, X.; Tamaddon, M.; Ng, L.; Hayat, M.D. Synergistic interactions between wear and corrosion of Ti-16Mo orthopedic alloy. J Mater Res Technol. 2020, 9, 9996–10003. [Google Scholar] [CrossRef]
- Savaskan, T.; Azaklı, Z. An investigation of lubricated friction and wear properties of Zn–40Al–2Cu–2Si alloy in comparison with SAE 65 bearing bronze. Wear 2008, 264, 920–928. [Google Scholar] [CrossRef]
- Darabont, D.-C.; Moraru, R.I.; Antonov, A.E.; Bejinariu, C. Managing new and emerging risks in the context of ISO 45001 standard. Qual.-Access Success 2017, 18, 11–14. [Google Scholar]
- Egami, M.; Ohnuma, I.; Enoki, M.; Ohtani, H.; Abe, E. Thermodynamic origin of solute-enriched stacking-fault in dilute Mg-Zn-Y alloys. Mater. Des. 2020, 188, 108452. [Google Scholar] [CrossRef]
- Cha, G.H.; Lee, S.Y.; Nash, P.; Pan, Y.Y.; Nash, A. Binary alloy phase diagrams (Ni-Sb; Ni-Sc; Ni-Se; Ni-Si; Ni-Sm; Ni-Sn). In ASM Handbook. Volume 3. Alloys Phase Diagrams; ASM International: Russell Township, OH, USA, 1991; pp. 317–318. [Google Scholar]
- Prosek, T.; Nazarov, A.; Bexell, U.; Thierry, D.; Serak, J. Corrosion mechanism of model zinc–magnesium alloys in atmospheric conditions. Corros. Sci. 2008, 50, 2216–2231. [Google Scholar] [CrossRef]
- Ji, C.; Ma, A.; Jiang, J.; Song, D.; Liu, H.; Guo, S. Research status and future prospects of biodegradable Zn-Mg alloys. J. Alloys Compd. 2024, 993, 174669. [Google Scholar] [CrossRef]
- Ma, S.-Y.; Liu, L.M.; Wang, S.Q. The microstructure, stability, and elastic properties of 14H long-period stacking-ordered phase in Mg–Zn–Y alloys: A first-principles study. J. Mater. Sci. 2014, 49, 737–748. [Google Scholar] [CrossRef]
- Vencl, A.; Bobić, I.; Bobić, B.; Jakimovska, K.; Svoboda, P.; Kandeva, M. Erosive wear properties of ZA-27 alloy-based nanocomposites: Influence of type, amount, and size of nanoparticle reinforcements. Friction 2019, 7, 340–350. [Google Scholar] [CrossRef]
- Panaghie, C.; Cimpoesu, R.; Zegan, G.; Roman, A.M.; Ivanescu, M.C.; Aelenei, A.A.; Benchea, M.; Cimpoesu, N.; Ioanid, N. In Vitro Corrosion Behavior of Zn3Mg0.7Y Biodegradable Alloy in Simulated Body Fluid (SBF). Appl. Sci. 2022, 12, 2727. [Google Scholar] [CrossRef]
- Panaghie, C.; Zegan, G.; Sodor, A.; Cimpoesu, N.; Lohan, N.-M.; Istrate, B.; Roman, A.M.; Ioanid, N. Analysis of Degradation Products of Biodegradable ZnMgY Alloy. Materials 2023, 16, 3092. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Currat, R.; De Boissieu, M.; Sato, T.J.; Takakura, H.; Tsai, A.P. Dynamics of the ZnMgY icosahedral phase. J. Condens. Matter Phys. 2002, 14, 1847. [Google Scholar] [CrossRef]
- Gong, H.; Wang, K.; Strich, R.; Zhou, J.G. In vitro biodegradation behavior, mechanical properties, and cytotoxicity of biodegradable Zn-Mg alloy. J. Biomed. Mater. Res. B. Appl. Biomater. 2015, 103, 1632–1640. [Google Scholar] [CrossRef]
- Zhuo, X.; Wu, Y.; Ju, J.; Liu, H.; Jiang, J.; Hu, Z.; Bai, J.; Xue, F. Recent progress of novel biodegradable zinc alloys: From the perspective of strengthening and toughening. J. Mater. Res. Technol. 2022, 17, 244–269. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, D.; Zhang, X.; Su, Y.; Shi, Z.; Wang, K.; Lin, J.; Li, Y.; Lin, J.; Wen, C. Microstructure, mechanical properties, biocompatibility, and in vitro corrosion and degradation behavior of a new Zn-5Ge alloy for biodegradable implant materials. Acta Biomater. 2018, 82, 197–204. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef]
- Laurian, T.; Tudor, A.; Antoniac, I.; Miculescu, F. A micro-scale abrasion test to study the influence of counterface roughness on the wear resistance of UHMWPE. J. Optoel. Adv. Mater. 2007, 9, 3383–3388. [Google Scholar]

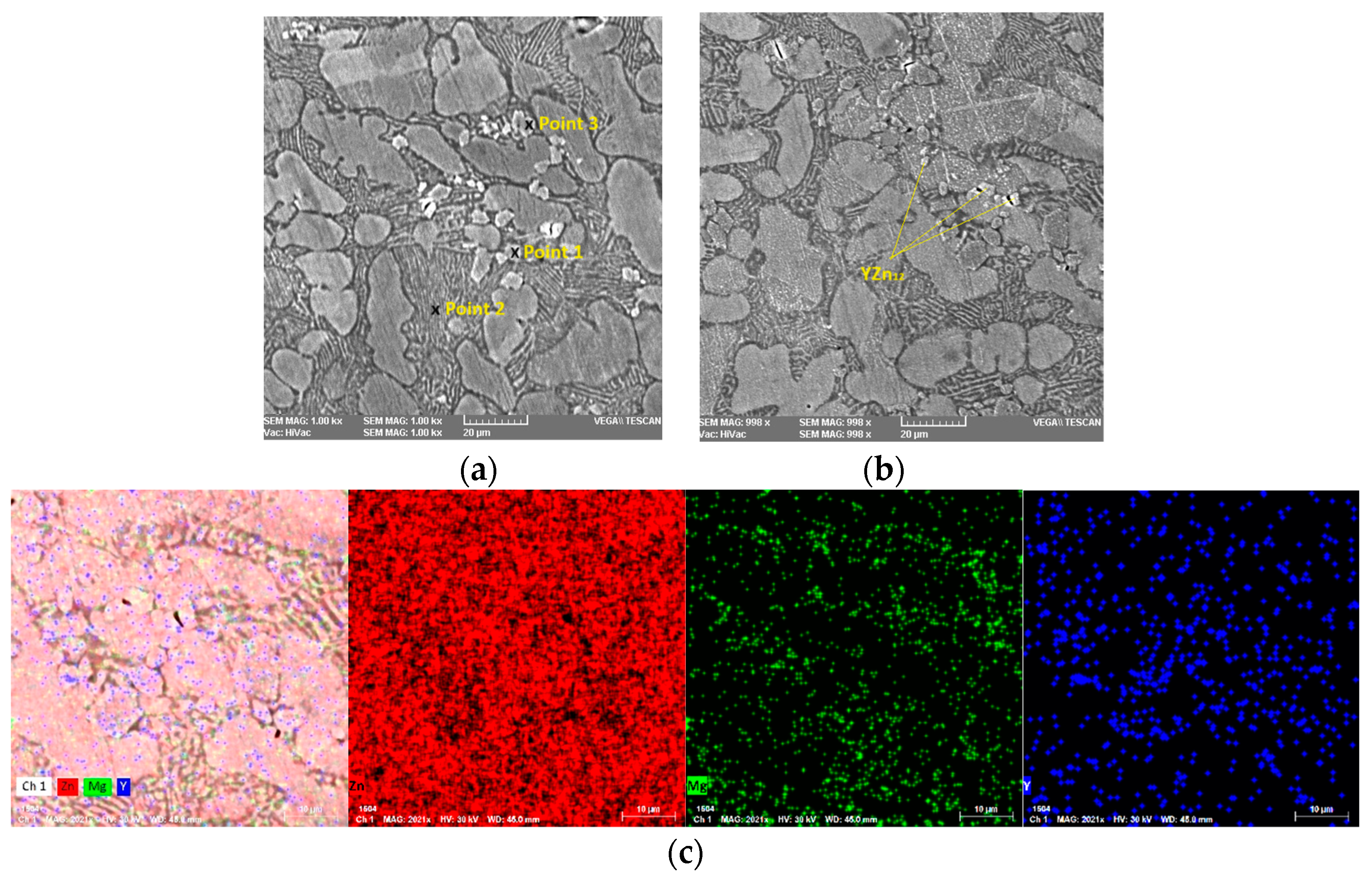



| Chemical Composition | Zn | Mg | Y | |||
|---|---|---|---|---|---|---|
| wt% | at% | wt% | at% | wt% | at% | |
| General (1 mm2) | 96.1 | 93.1 | 3.0 | 6.3 | 0.9 | 0.7 |
| Point 1 | 90.4 | 92.5 | - | - | 9.6 | 7.5 |
| Point 2 | 99.2 | 97.4 | 0.8 | 2.6 | - | - |
| Point 3 | 92.8 | 94.1 | 0.2 | 0.7 | 7 | 5.2 |
| EDS error [%] | 2.1 | 0.9 | 0.2 | |||
| Material | Zn3Mg1Y [HV] | Zn3Mg [HV] | Zn [HV] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Test Report | Min. | Max. | Mean | Min. | Max. | Mean | Min. | Max. | Mean |
| Point 1 (20 N—dry) | 84.1 | 203.2 | 157.2 | 74.2 | 129.0 | 100.3 | 104.2 | 108.7 | 107.2 |
| Point 2 (20 N—dry) | 116.9 | 138.1 | 126.9 | 102.3 | 129.0 | 115.3 | 107.6 | 160 | 130.0 |
| Point 3 (20 N—dry) | 74.2 | 97.5 | 88.2 | 90.0 | 93.4 | 92.2 | 53.2 | 61.7 | 56.2 |
| Point 1 (30 N—dry) | 206 | 222.3 | 215.3 | 224.6 | 233.3 | 229.8 | 101.2 | 99.5 | 98.4 |
| Point 2 (30 N—dry) | 116.0 | 128.0 | 120.2 | 172.2 | 189.4 | 179.4 | 106.4 | 122.2 | 115.3 |
| Point 3 (30 N—dry) | 90.8 | 99.3 | 94.7 | 55.6 | 69.7 | 64.7 | 31.2 | 33.7 | 32.8 |
| Material (Hot-Rolled State) | Mass before Wear [g] | Mass after Wear [g] | Mass Loss [g] | Average Wear Rate ×10−2 [mg/g] |
|---|---|---|---|---|
| Pure Zn (2 kg) | 7.498 | 7.493 | 0.005 | 2.01 |
| Zn3Mg (2 kg) | 11.952 | 11.951 | 0.001 | 0.3 |
| Zn3Mg1Y (2 kg) | 15.745 | 15.738 | 0.007 | 0.23 |
| Pure Zn (3 kg) | 7.520 | 7.498 | 0.022 | 1.1 |
| Zn3Mg (3 kg) | 11.964 | 11.952 | 0.012 | 0.6 |
| Zn3Mg1Y (3 kg) | 15.759 | 15.745 | 0.014 | 0.7 |
| Zn3Mg0.4Y Hot-Rolled | Zn | Mg | Y | |||
|---|---|---|---|---|---|---|
| wt% | at% | wt% | at% | wt% | at% | |
| General | 95.78 | 91.03 | 3.24 | 8.28 | 0.97 | 0.68 |
| Point 1 | 99.5 | 98.7 | 0.49 | 1.3 | - | - |
| Point 2 | 90.4 | 92.75 | - | - | 9.6 | 7.3 |
| Point 3 | 99.9 | 99.9 | - | - | - | - |
| Point 4 | 92.92 | 94.7 | - | - | 7.1 | 5.3 |
| Point 5 | 99.4 | 99.3 | 0.13 | 0.33 | 0.45 | 0.33 |
| EDS Error % | 2.3 | 0.1 | 0.3 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimpoesu, N.; Paleu, V.; Panaghie, C.; Roman, A.-M.; Cazac, A.M.; Cioca, L.-I.; Bejinariu, C.; Lupescu, S.C.; Axinte, M.; Popa, M.; et al. Mechanical Properties and Wear Resistance of Biodegradable ZnMgY Alloy. Metals 2024, 14, 836. https://doi.org/10.3390/met14070836
Cimpoesu N, Paleu V, Panaghie C, Roman A-M, Cazac AM, Cioca L-I, Bejinariu C, Lupescu SC, Axinte M, Popa M, et al. Mechanical Properties and Wear Resistance of Biodegradable ZnMgY Alloy. Metals. 2024; 14(7):836. https://doi.org/10.3390/met14070836
Chicago/Turabian StyleCimpoesu, Nicanor, Viorel Paleu, Catalin Panaghie, Ana-Maria Roman, Alin Marian Cazac, Lucian-Ionel Cioca, Costica Bejinariu, Stefan Constantin Lupescu, Mihai Axinte, Mihai Popa, and et al. 2024. "Mechanical Properties and Wear Resistance of Biodegradable ZnMgY Alloy" Metals 14, no. 7: 836. https://doi.org/10.3390/met14070836








