Metallic Degenerately Doped Free-Electron-Confined Plasmonic Nanocrystal and Infrared Extinction Response
Abstract
:1. Introduction
2. Materials and Methods
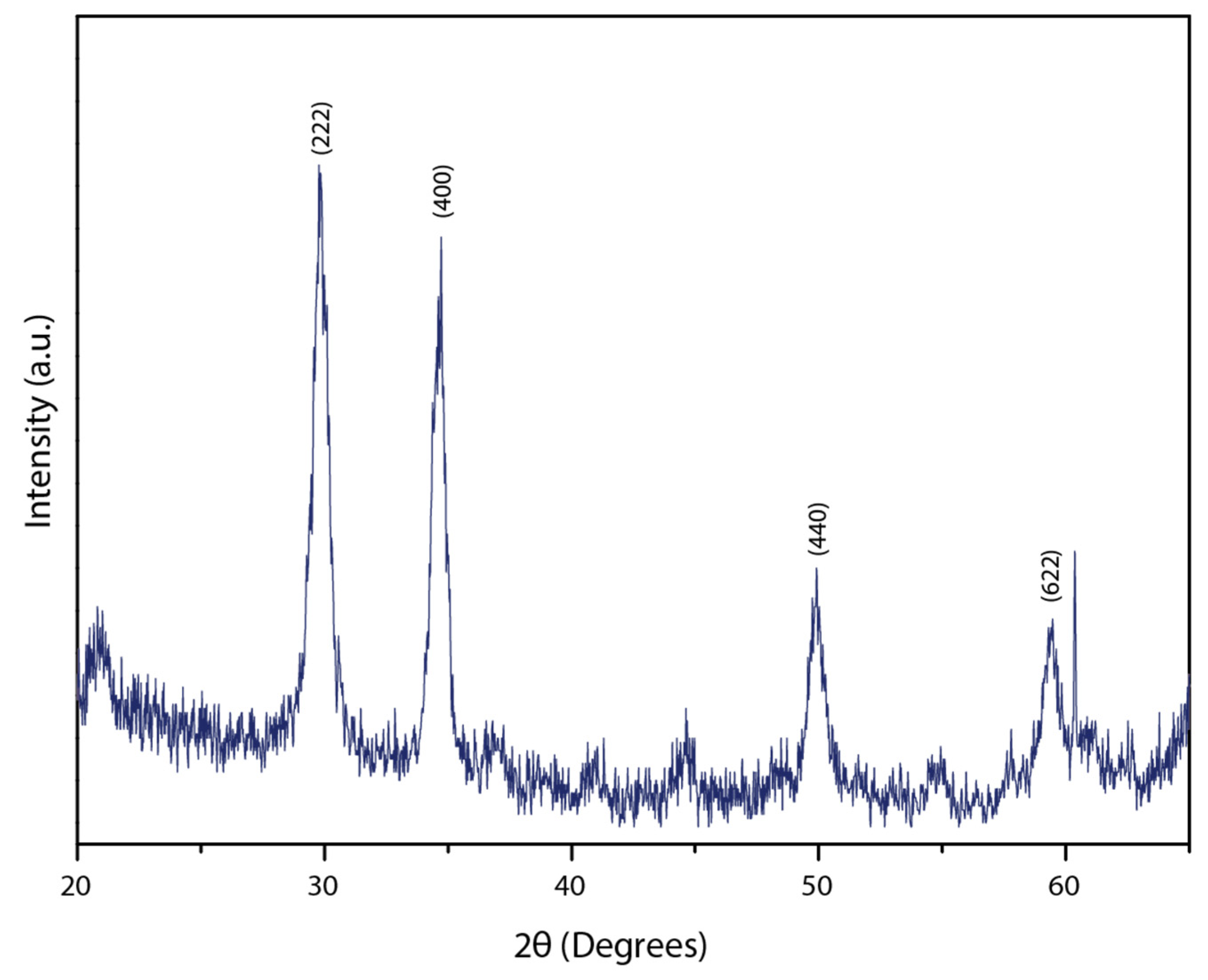
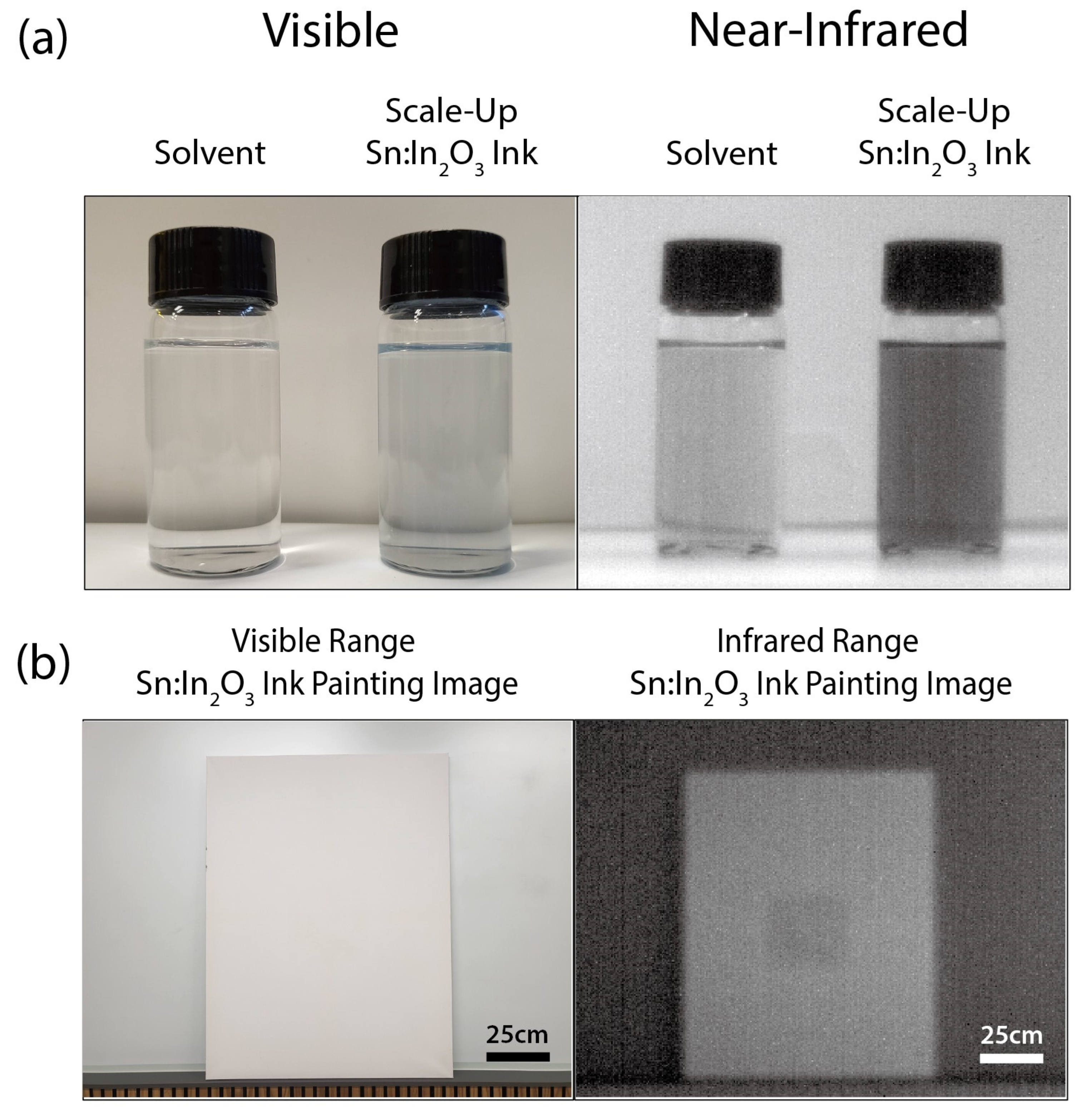
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agrawal, A.; Cho, S.H.; Zandi, O.; Ghosh, S.; Johns, R.W.; Milliron, D.J. Localized Surface Plasmon Resonance in Semiconductor Nanocrystals. Chem. Rev. 2018, 118, 3121–3207. [Google Scholar] [CrossRef]
- Kanehara, M.; Koike, H.; Yoshinaga, T.; Teranishi, T. Indium Tin Oxide Nanoparticles with Compositionally Tunable Surface Plasmon Resonance Frequencies in the Near-IR Region. J. Am. Chem. Soc. 2009, 131, 17736–17737. [Google Scholar] [CrossRef] [PubMed]
- König, T.A.F.; Ledin, P.A.; Kerszulis, J.; Mahmoud, M.A.; El-Sayed, M.A.; Reynolds, J.R.; Tsukruk, V.V. Electrically Tunable Plasmonic Behavior of Nanocube–Polymer Nanomaterials Induced by a Redox-Active Electrochromic Polymer. ACS Nano 2014, 8, 6182–6192. [Google Scholar] [CrossRef]
- Cortés, E.; Besteiro, L.V.; Alabastri, A.; Baldi, A.; Tagliabue, G.; Demetriadou, A.; Narang, P. Challenges in Plasmonic Catalysis. ACS Nano 2020, 14, 16202–16219. [Google Scholar] [CrossRef]
- Hansen, H.E.; Berge, T.B.; Seland, F.; Sunde, S.; Burheim, O.S.; Pollet, B.G. Towards Scaling up the Sonochemical Synthesis of Pt-Nanocatalysts. Ultrason. Sonochem. 2024, 103, 106794. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Chon, M.-W.; Choa, S.-H. Technology of Flexible Transparent Conductive Electrode for Flexible Electronic Devices. J. Microelectron. Packag. Soc. 2014, 21, 1–11. [Google Scholar] [CrossRef]
- Llordés, A.; Garcia, G.; Gazquez, J.; Milliron, D.J. Tunable Near-Infrared and Visible-Light Transmittance in Nanocrystal-in-Glass Composites. Nature 2013, 500, 323–326. [Google Scholar] [CrossRef]
- Wang, K.; Meng, Q.; Wang, Q.; Zhang, W.; Guo, J.; Cao, S.; Elezzabi, A.Y.; Yu, W.W.; Liu, L.; Li, H. Advances in Energy-Efficient Plasmonic Electrochromic Smart Windows Based on Metal Oxide Nanocrystals. Adv. Energy Sustain. Res. 2021, 2, 2100117. [Google Scholar] [CrossRef]
- Leppäniemi, J.; Eiroma, K.; Majumdar, H.; Alastalo, A. Far-UV Annealed Inkjet-Printed In2O3 Semiconductor Layers for Thin-Film Transistors on a Flexible Polyethylene Naphthalate Substrate. ACS Appl. Mater. Interfaces 2017, 9, 8774–8782. [Google Scholar] [CrossRef] [PubMed]
- Cummins, G.; Desmulliez, M.P.Y. Inkjet Printing of Conductive Materials: A Review. Circuit World 2012, 38, 193–213. [Google Scholar] [CrossRef]
- Sasaki, T.; Endo, Y.; Nakaya, M.; Kanie, K.; Nagatomi, A.; Tanoue, K.; Nakamura, R.; Muramatsu, A. One-Step Solvothermal Synthesis of Cubic-Shaped ITO Nanoparticles Precisely Controlled in Size and Shape and Their Electrical Resistivity. J. Mater. Chem. 2010, 20, 8153–8157. [Google Scholar] [CrossRef]
- Hong, S.-J.; Kim, Y.-H.; Han, J.-I. Development of Ultrafine Indium Tin Oxide (ITO) Nanoparticle for Ink Jet Printing by Low Temperature Synthetic Method. In Proceedings of the 2006 IEEE Nanotechnology Materials and Devices Conference, Gyeongju, Republic of Korea, 22–25 October 2006; Volume 1, pp. 464–465. [Google Scholar] [CrossRef]
- Hong, S.-J.; Kim, J.-W.; Lim, J.-W.; Choi, G.-S.; Isshiki, M. Characteristics of Printed Thin Films Using Indium Tin Oxide (ITO) Ink. Mater. Trans. 2010, 51, 1905–1908. [Google Scholar] [CrossRef]
- Runnerstrom, E.L.; Bergerud, A.; Agrawal, A.; Johns, R.W.; Dahlman, C.J.; Singh, A.; Selbach, S.M.; Milliron, D.J. Defect Engineering in Plasmonic Metal Oxide Nanocrystals. Nano Lett. 2016, 16, 3390–3398. [Google Scholar] [CrossRef]
- Yin, P.; Hegde, M.; Tan, Y.; Chen, S.; Garnet, N.; Radovanovic, P.V. Controlling the Mechanism of Excitonic Splitting in In2O3 Nanocrystals by Carrier Delocalization. ACS Nano 2018, 12, 11211–11218. [Google Scholar] [CrossRef] [PubMed]
- Saez Cabezas, C.A.; Sherman, Z.M.; Howard, M.P.; Dominguez, M.N.; Cho, S.H.; Ong, G.K.; Green, A.M.; Truskett, T.M.; Milliron, D.J. Universal Gelation of Metal Oxide Nanocrystals via Depletion Attractions. Nano Lett. 2020, 20, 4007–4013. [Google Scholar] [CrossRef]
- Benny, S.; Vidyanagar, A.V.; Bhat, S.V. Progress and Prospects with Cationic and Anionic Doping of Solution-Processed Kesterite Solar Absorbers: A Mini-Review. Energy Fuels 2024, 38, 61–72. [Google Scholar] [CrossRef]
- Kim, C.; Park, J.-W.; Kim, J.; Hong, S.-J.; Lee, M.J. A Highly Efficient Indium Tin Oxide Nanoparticles (ITO-NPs) Transparent Heater Based on Solution-Process Optimized with Oxygen Vacancy Control. J. Alloys Compd. 2017, 726, 712–719. [Google Scholar] [CrossRef]
- Maho, A.; Saez Cabezas, C.A.; Meyertons, K.A.; Reimnitz, L.C.; Sahu, S.; Helms, B.A.; Milliron, D.J. Aqueous Processing and Spray Deposition of Polymer-Wrapped Tin-Doped Indium Oxide Nanocrystals as Electrochromic Thin Films. Chem. Mater. 2020, 32, 8401–8411. [Google Scholar] [CrossRef]
- Jansons, A.W.; Hutchison, J.E. Continuous Growth of Metal Oxide Nanocrystals: Enhanced Control of Nanocrystal Size and Radial Dopant Distribution. ACS Nano 2016, 10, 6942–6951. [Google Scholar] [CrossRef]
- Cho, S.H.; Roccapriore, K.M.; Dass, C.K.; Ghosh, S.; Choi, J.; Noh, J.; Reimnitz, L.C.; Heo, S.; Kim, K.; Xie, K.; et al. Spectrally Tunable Infrared Plasmonic F,Sn:In2O3 Nanocrystal Cubes. J. Chem. Phys. 2020, 152, 014709. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, P.; Xiao, X.; Zhou, C.; Wei, H.; Yu, C. Recent Advances in Nanostructured Substrates for Surface-Enhanced Infrared Absorption Spectroscopy. Nanotechnology 2023, 34, 382002. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, W.; Liu, H.; Li, H.; Yu, H.; Hao, B. Optically Compatible Infrared Camouflage Performance of ITO Ink. RSC Adv. 2024, 14, 17355–17363. [Google Scholar] [CrossRef]
- Gibbs, S.L.; Dean, C.; Saad, J.; Tandon, B.; Staller, C.M.; Agrawal, A.; Milliron, D.J. Dual-Mode Infrared Absorption by Segregating Dopants within Plasmonic Semiconductor Nanocrystals. Nano Lett. 2020, 20, 7498–7505. [Google Scholar] [CrossRef]
- Conti III, C.R.; Quiroz-Delfi, G.; Schwarck, J.S.; Chen, B.; Strouse, G.F. Carrier Density, Effective Mass, and Nuclear Relaxation Pathways in Plasmonic Sn:In2O3 Nanocrystals. J. Phys. Chem. C 2020, 124, 28220–28229. [Google Scholar] [CrossRef]
- Mendelsberg, R.J.; Garcia, G.; Li, H.; Manna, L.; Milliron, D.J. Understanding the Plasmon Resonance in Ensembles of Degenerately Doped Semiconductor Nanocrystals. J. Phys. Chem. C 2012, 116, 12226–12231. [Google Scholar] [CrossRef]
- Mendelsberg, R.J.; Garcia, G.; Milliron, D.J. Extracting Reliable Electronic Properties from Transmission Spectra of Indium Tin Oxide Thin Films and Nanocrystal Films by Careful Application of the Drude Theory. J. Appl. Phys. 2012, 111, 063515. [Google Scholar] [CrossRef]
- Mergel, D.; Qiao, Z. Dielectric Modelling of Optical Spectra of Thin In2O3 : Sn Films. J. Phys. D Appl. Phys. 2002, 35, 794. [Google Scholar] [CrossRef]
- Araujo, J.J.; Brozek, C.K.; Liu, H.; Merkulova, A.; Li, X.; Gamelin, D.R. Tunable Band-Edge Potentials and Charge Storage in Colloidal Tin-Doped Indium Oxide (ITO) Nanocrystals. ACS Nano 2021, 15, 14116–14124. [Google Scholar] [CrossRef]
- Buonsanti, R.; Llordes, A.; Aloni, S.; Helms, B.A.; Milliron, D.J. Tunable Infrared Absorption and Visible Transparency of Colloidal Aluminum-Doped Zinc Oxide Nanocrystals. Nano Lett. 2011, 11, 4706–4710. [Google Scholar] [CrossRef]
- Cho, S.H.; Ghosh, S.; Berkson, Z.J.; Hachtel, J.A.; Shi, J.; Zhao, X.; Reimnitz, L.C.; Dahlman, C.J.; Ho, Y.; Yang, A.; et al. Syntheses of Colloidal F:In2O3 Cubes: Fluorine-Induced Faceting and Infrared Plasmonic Response. Chem. Mater. 2019, 31, 2661–2676. [Google Scholar] [CrossRef]
- Suzuki, R.; Nishi, Y.; Matsubara, M.; Muramatsu, A.; Kanie, K. Single-Crystalline Protrusion-Rich Indium Tin Oxide Nanoparticles with Colloidal Stability in Water for Use in Sustainable Coatings. ACS Appl. Nano Mater. 2020, 3, 4870–4879. [Google Scholar] [CrossRef]
- Sun, X.W.; Huang, H.C.; Kwok, H.S. On the Initial Growth of Indium Tin Oxide on Glass. Appl. Phys. Lett. 1996, 68, 2663–2665. [Google Scholar] [CrossRef]
- Kalinin, S.V.; Roccapriore, K.M.; Cho, S.H.; Milliron, D.J.; Vasudevan, R.; Ziatdinov, M.; Hachtel, J.A. Separating Physically Distinct Mechanisms in Complex Infrared Plasmonic Nanostructures via Machine Learning Enhanced Electron Energy Loss Spectroscopy. Adv. Opt. Mater. 2021, 9, 2001808. [Google Scholar] [CrossRef]
- Lozovoy, K.A.; Korotaev, A.G.; Kokhanenko, A.P.; Dirko, V.V.; Voitsekhovskii, A.V. Kinetics of Epitaxial Formation of Nanostructures by Frank–van Der Merwe, Volmer–Weber and Stranski–Krastanow Growth Modes. Surf. Coat. Technol. 2020, 384, 125289. [Google Scholar] [CrossRef]


| Properties | Scale Up Parameter | Lab Scale Parameter |
|---|---|---|
| LSPR Peak [cm−1] | 2537 | 1962 |
| Free Carrier Density [cm−3] | 2.53 × 1020 | 1.44 × 1020 |
| Bulk Plasma Frequency [cm−1] | 7525 | 5670 |
| Low-Frequency Damping Constant [cm−1] | 1569 | 1582 |
| High-Frequency Damping Constant [cm−1] | 360 | 86 |
| Crossover Frequency [cm−1] | 4447 | 3507 |
| Crossover Width [cm−1] | 277 | 311 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.-Y.; Cho, S.-H. Metallic Degenerately Doped Free-Electron-Confined Plasmonic Nanocrystal and Infrared Extinction Response. Metals 2024, 14, 843. https://doi.org/10.3390/met14080843
Park D-Y, Cho S-H. Metallic Degenerately Doped Free-Electron-Confined Plasmonic Nanocrystal and Infrared Extinction Response. Metals. 2024; 14(8):843. https://doi.org/10.3390/met14080843
Chicago/Turabian StylePark, Do-Yoon, and Shin-Hum Cho. 2024. "Metallic Degenerately Doped Free-Electron-Confined Plasmonic Nanocrystal and Infrared Extinction Response" Metals 14, no. 8: 843. https://doi.org/10.3390/met14080843






