Stabilization Effect of Interfacial Solute Segregation on θ′ Precipitates in Al-Cu Alloys
Abstract
:1. Introduction
2. Material and Methods
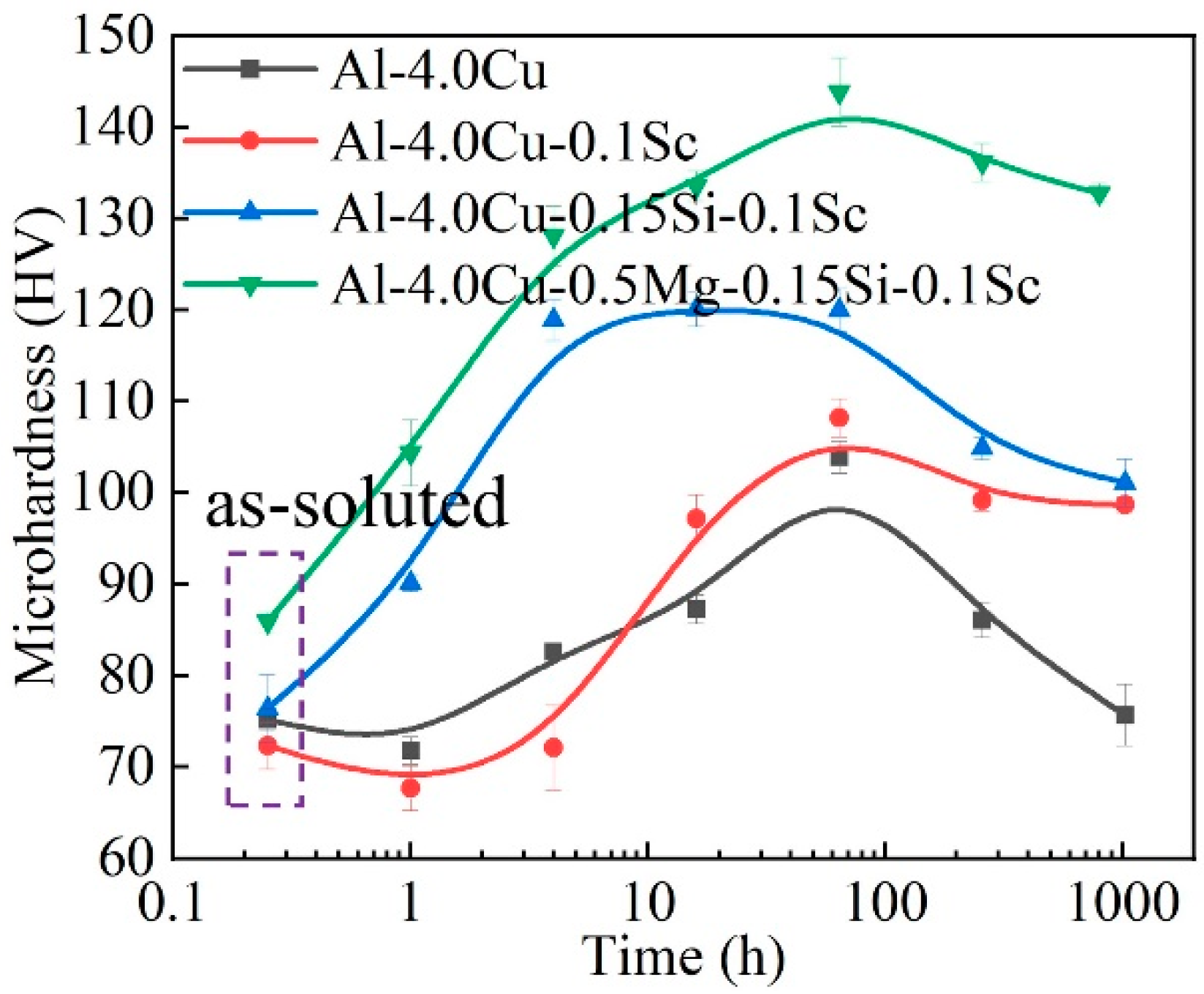
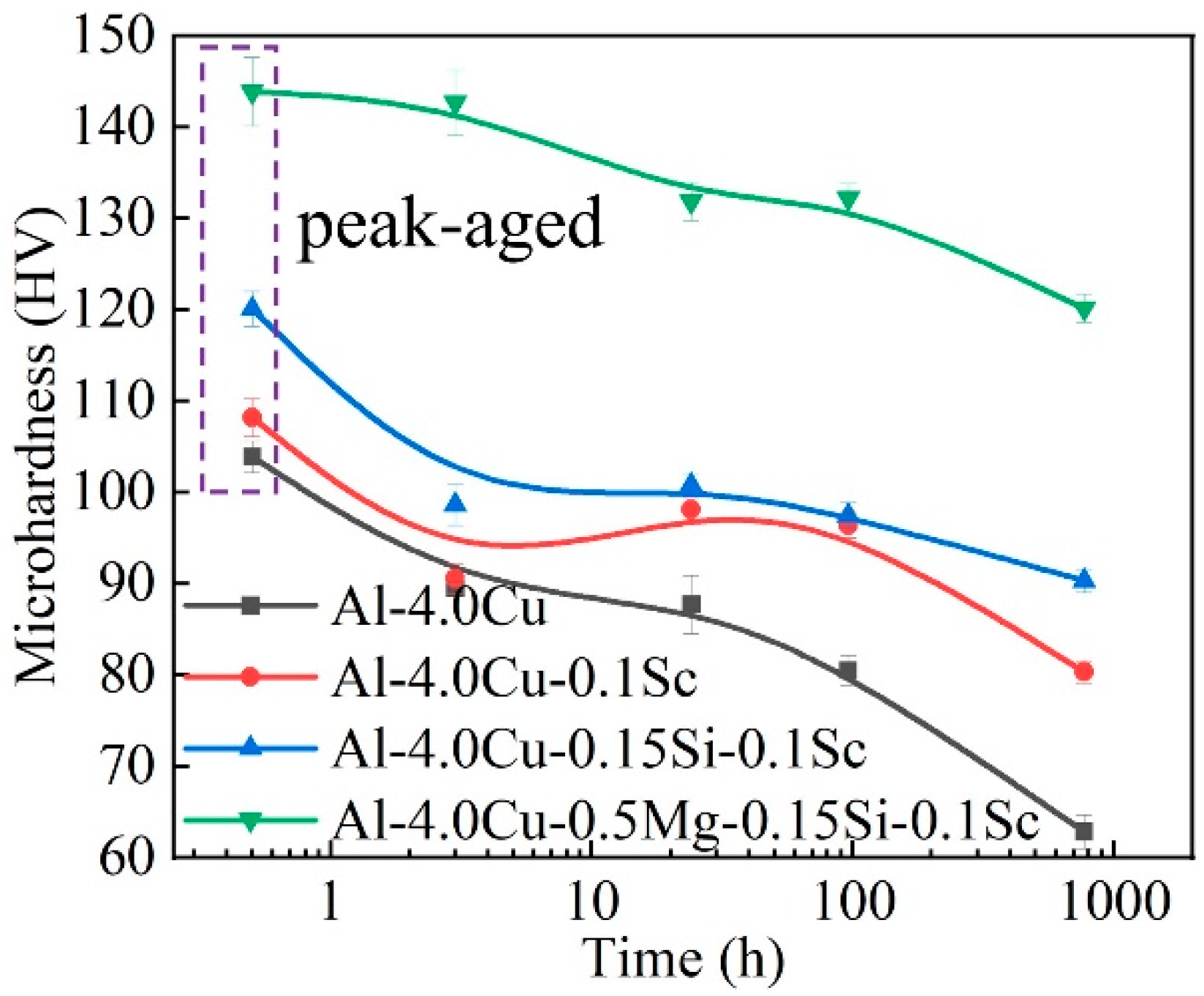
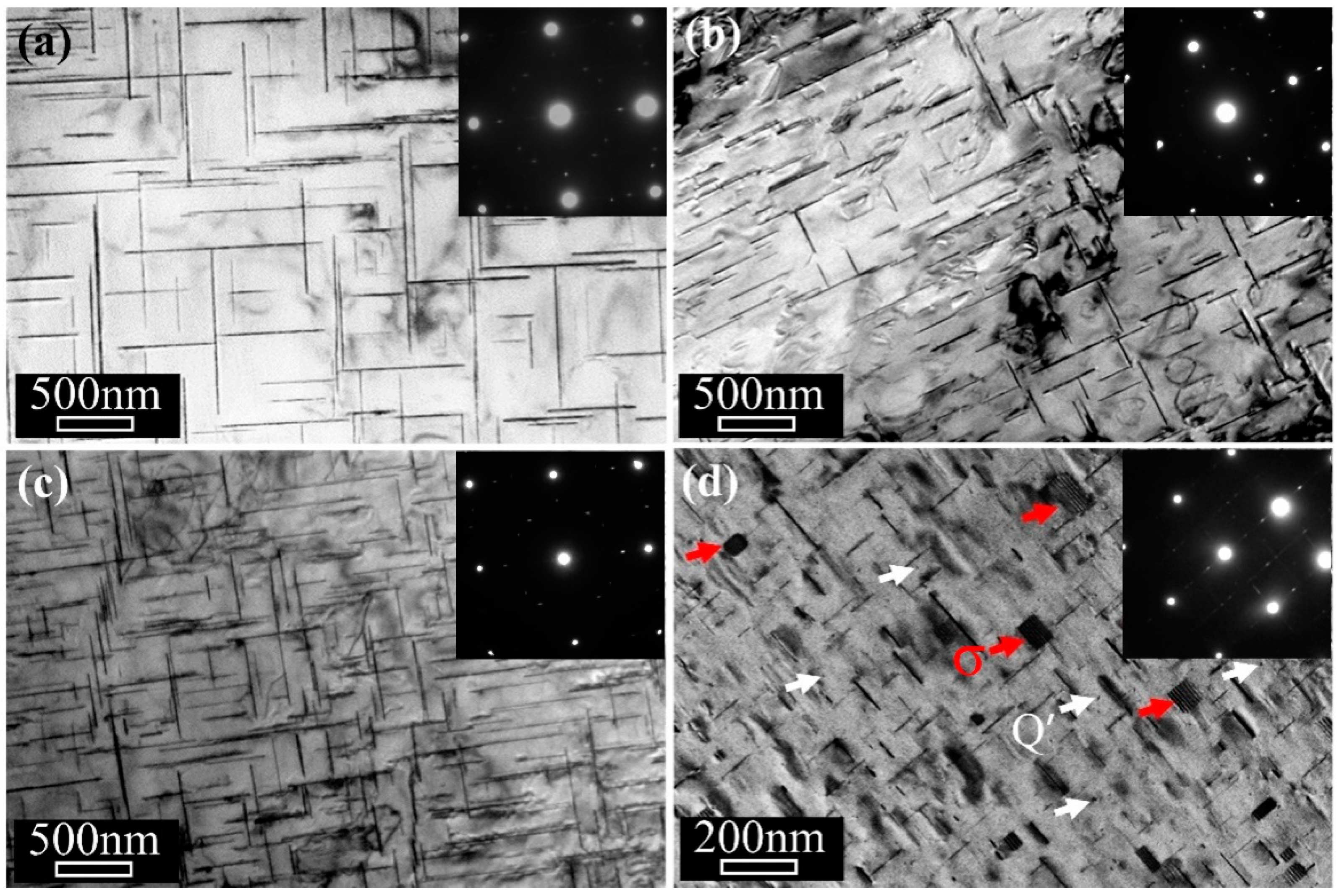
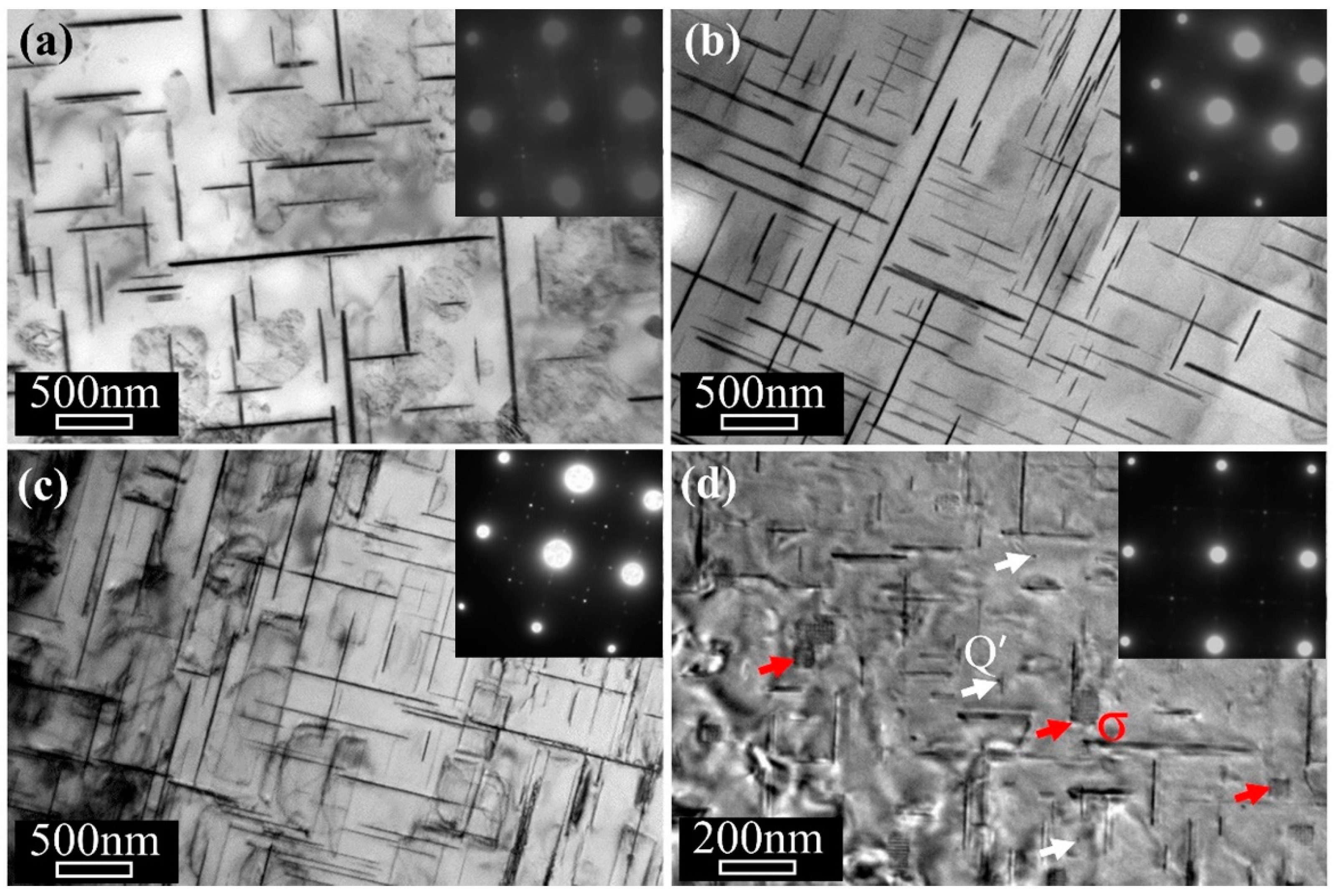
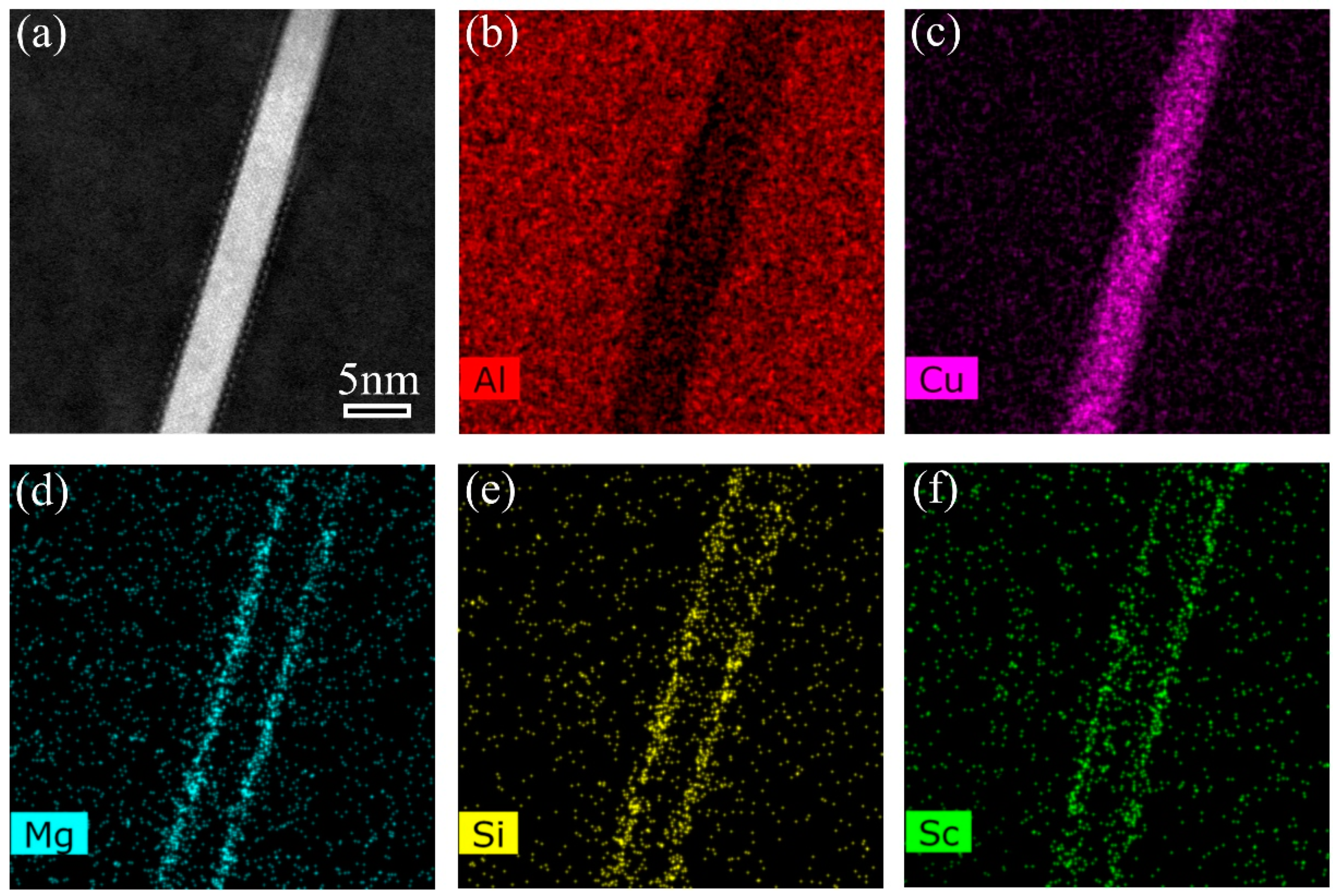
3. Results
4. Discussion
4.1. Stabilization Effect of Solute Segregation
4.2. Effect of Precipitate Evolution
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.S.; Wang, J.S.; Wang, B.; Xue, C.P.; Liu, X.G. Quantifying the effects of Sc and Ag on the microstructure and mechanical properties of Al-Cu alloys. Mater. Sci. Eng. A 2022, 831, 142355. [Google Scholar] [CrossRef]
- Xie, Y.K.; Liu, S.D.; Guo, X.B.; Liang, C.J.; Deng, Y.L. Enhancing the strength of Sc-containing Al-Cu-Li alloys by modifying the precipitation behavior through plastic deformation and heat treatment. Mater. Charact. 2024, 207, 113558. [Google Scholar] [CrossRef]
- Bansal, U.; Singh, M.P.; Sinha, S.K.; Sahu, D.K.; Mondol, S.; Makineni, S.K.; Paul, A.; Chattopadhyay, K. Strength and stability through variable micro segregation behaviour of Ta and Zr solutes at intermetallic interfaces in Al-Cu alloys. Acta Mater. 2023, 259, 119254. [Google Scholar] [CrossRef]
- Liao, G.Z.; Wei, B.; Pan, S.; Wang, S.B. Phase structure modification-based property improvement of Al-4.3Cu-1.6Mg-0.2Sc (wt%) alloy. Mater. Charact. 2023, 196, 112664. [Google Scholar] [CrossRef]
- Makineni, S.K.; Sugathan, S.; Meher, S.; Banerjee, R.; Bhattacharya, S.; Kumar, S. Enhancing elevated temperature strength of copper containing aluminium alloys by forming L12 Al3Zr precipitates and nucleating θ′′ precipitates on them. Sci. Rep. 2017, 7, 11154. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.H.; Guan, P.F.; Su, R.; Chen, H.W.; Ma, E. Segregation-sandwiched stable interface suffocates nanoprecipitate coarsening to elevate creep resistance. Mater. Res. Lett. 2020, 8, 446–453. [Google Scholar] [CrossRef]
- Yan, J.; Xiong, X.K.; Wu, C.L.; Ming, W.Q.; Xie, P.; Chen, J.H. A secondary high-temperature precursor of the θ′-phase in Al-Cu-(Sc) alloys. J. Mater. Sci. Technol. 2024, 212, 55–66. [Google Scholar] [CrossRef]
- Bansal, U.; Singh, M.P.; Mondol, S.; Sinha, S.K.; Makineni, S.K.; Paul, A.; Chattopadhyay, K. The interplay of precipitation of ordered compounds and interfacial segregation in Al-Cu-Hf-Si alloys for high-temperature strength. Acta Mater. 2022, 240, 118355. [Google Scholar] [CrossRef]
- Xue, H.; Yang, C.; Geuser, F.D.; Zhang, P.; Zhang, J.Y.; Chen, B.; Liu, F.Z.; Peng, Y.; Bian, J.J.; Liu, G.; et al. Highly stable coherent nanoprecipitates via diffusion-dominated solute uptake and interstitial ordering. Nat. Mater. 2023, 22, 434–441. [Google Scholar] [CrossRef]
- Gao, Y.H.; Cao, L.F.; Kuang, J.; Zhang, J.Y.; Sun, J. Assembling dual precipitates to improve high-temperature resistance of multi-microalloyed Al-Cu alloys. J. Alloys Compd. 2020, 822, 153629. [Google Scholar] [CrossRef]
- Polmear, I.J.; Pons, G.; Barbaux, Y.; Sanchez, C.; Morton, A.J.; Borbodge, W.E.; Octor, H.; Rogers, S. After concorde: Evaluation of creep resistant Al-Cu-Mg-Ag alloys. Mater. Sci. Technol. 1999, 15, 861–868. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Liu, K.; Pan, L.; Breton, F.; Chen, X.G. Enhanced mechanical properties of high-temperature-resistant Al-Cu cast alloy by microalloying with Mg. J. Alloys Compd. 2020, 827, 154305. [Google Scholar] [CrossRef]
- Gable, B.M.; Shiflet, G.J.; Starke, E.A. The effect of Si additions on Ω precipitation in Al-Cu-Mg-(Ag) alloys. Scripta Mater. 2004, 50, 149–153. [Google Scholar] [CrossRef]
- Luca, A.D.; Dunand, D.C.; Seidman, D.N. Scandium-enriched nanoprecipitates in aluminum providing enhanced coarsening and creep resistance. In Light Metals; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 1589–1594. [Google Scholar]
- Barlow, I.C.; Rainforth, W.M.; Jones, H. Role of silicon in the formation of the (Al5Cu6Mg2) σ phase in Al-Cu-Mg alloys. J. Mater. Sci. 2000, 35, 1413–1418. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.H.; Wang, S.B.; Liu, C.H.; Yang, S.S.; Wu, C.L. The effect of Si on precipitation in Al-Cu-Mg alloy with a high Cu/Mg ratio. Mater. Sci. Eng. A 2014, 606, 187–195. [Google Scholar] [CrossRef]
- Shower, P.; Poplawsky, J.; Bahl, S.; Shyam, A. The role of Si in determining the stability of the θ′ precipitate in Al-Cu-Mn-Zr alloys. J. Alloys Compd. 2020, 862, 158152. [Google Scholar] [CrossRef]
- Gao, Y.H.; Yang, C.; Zhang, J.Y.; Cao, L.F.; Liu, G.; Sun, J.; Ma, E. Stabilizing nanoprecipitates in Al-Cu alloys for creep resistance at 300 °C. Mater. Res. Lett. 2019, 7, 18–25. [Google Scholar] [CrossRef]
- Shyam, A.; Bahl, S. Heat-resistant aluminium alloys. Nat. Mater. 2023, 22, 425–426. [Google Scholar] [CrossRef]
- Wang, Z.J.; Liu, X.; Zhu, C.W.; Xue, J.L.; Guo, Z.C.; Zhang, Y.N.; Li, X. Influence of the interaction between Si and Sc on the microstructure and tensile properties of as casted Al-Si-Sc alloys. J. Alloys Compd. 2023, 932, 167650. [Google Scholar] [CrossRef]
- Gao, Y.S.; Jiao, J.X.; Meng, Y.; Liu, Q.M.; Cheng, L.J. Structural growth, stability and electronic characteristics of Al-Sc clusters. Comput. Theor. Chem. 2022, 1218, 113942. [Google Scholar] [CrossRef]
- Liang, S.S.; Wen, S.P.; Wu, X.L.; Huang, H.; Nie, Z.R. The synergetic effect of Si and Sc on the thermal stability of the precipitates in AlCuMg alloy. Mater. Sci. Eng. A 2020, 783, 139319. [Google Scholar] [CrossRef]
- Schueller, R.D.; Wawner, F.E.; Sachdev, A.K. Strengthening potential of the cubic σ precipitate in Al-Cu-Mg-Si alloys. J. Mater. Sci. 1994, 29, 239–249. [Google Scholar] [CrossRef]
- Chen, B.A.; Liu, G.; Wang, R.H.; Zhang, J.Y.; Jiang, L.; Song, J.J.; Sun, J. Effect of interfacial solute segregation on ductile fracture of Al-Cu-Sc alloys. Acta Mater. 2013, 61, 1676–1690. [Google Scholar] [CrossRef]
- Davydov, V.G.; Rostova, T.D.; Zakharov, V.V.; Filato, Y.A.; Yelagin, V.I. Scientific principles of making an alloying addition of scandium to aluminium alloys. Mater. Sci. Eng. A 2000, 280, 30–36. [Google Scholar] [CrossRef]
- Vo, N.Q.; Dunand, D.C.; Seidman, D.N. Improving aging and creep resistance in a dilute Al-Sc alloy by microalloying with Si, Zr and Er. Acta Mater. 2014, 63, 73–85. [Google Scholar] [CrossRef]
- Huang, L.P.; Huang, J.; Liu, W.S.; Cao, L.F.; Lia, S. Effect of minor Sc additions on precipitation and mechanical properties of a new Al-Cu-Li alloy under T8 temper. J. Alloys Compd. 2022, 927, 166860. [Google Scholar] [CrossRef]
- Jiang, L.; Rouxel, B.; Langan, T.; Dorin, T. Coupled segregation mechanisms of Sc, Zr and Mn at θ′ interfaces enhances the strength and thermal stability of Al-Cu alloys. Acta Mater. 2021, 206, 116634. [Google Scholar] [CrossRef]
- Mondol, S.; Kashyap, S.; Kumar, S.; Chattopadhyay, K. Improvement of high temperature strength of 2219 alloy by Sc and Zr addition through a novel three-stage heat treatment route. Mater. Sci. Eng. A 2018, 732, 157–166. [Google Scholar] [CrossRef]
- Knipling, K.E.; Seidman, D.N.; Dunand, D.C. Ambient- and high-temperature mechanical properties of isochronally aged Al-0.06Sc, Al-0.06Zr and Al-0.06Sc-0.06Zr (at. %) alloys. Acta Mater. 2011, 59, 943–954. [Google Scholar] [CrossRef]
- Li, B.; Liang, S.S.; Wen, S.P.; Zhao, Z.H.; Wu, X.L.; Wang, W.; Gao, K.Y.; Huang, H.; Nie, Z.R. Competition between precipitation and segregation of Sc and its effects on thermal stability of Al-Cu-Mg-Ag alloys. Mater. Lett. 2021, 297, 129927. [Google Scholar] [CrossRef]


| Alloys | Actual Composition (wt.%) | ||||
|---|---|---|---|---|---|
| Cu | Mg | Si | Sc | Al | |
| 1#—Al-4.0Cu | 3.93 | / | / | / | Bal. |
| 2#—Al-4.0Cu-0.1Sc | 3.90 | / | / | 0.10 | Bal. |
| 3#—Al-4.0Cu-0.15Si-0.1Sc | 4.21 | / | 0.18 | 0.10 | Bal. |
| 4#—Al-4.0Cu-0.5Mg-0.15Si-0.1Sc | 4.01 | 0.51 | 0.17 | 0.11 | Bal. |
| Alloys | 175 °C/~1000 h | 225 °C/~800 h |
|---|---|---|
| 1#—Al-4.0Cu | 32 | 28 |
| 2#—Al-4.0Cu-0.1Sc | 34 | 34 |
| 3#—Al-4.0Cu-0.15Si-0.1Sc | 42 | 35 |
| 4#—Al-4.0Cu-0.5Mg-0.15Si-0.1Sc | 18 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Wen, S.; Liu, B.; Hu, Y.; Wei, W.; Wu, X.; Huang, H.; Gao, K.; Xiong, X.; Nie, Z. Stabilization Effect of Interfacial Solute Segregation on θ′ Precipitates in Al-Cu Alloys. Metals 2024, 14, 848. https://doi.org/10.3390/met14080848
Liang S, Wen S, Liu B, Hu Y, Wei W, Wu X, Huang H, Gao K, Xiong X, Nie Z. Stabilization Effect of Interfacial Solute Segregation on θ′ Precipitates in Al-Cu Alloys. Metals. 2024; 14(8):848. https://doi.org/10.3390/met14080848
Chicago/Turabian StyleLiang, Shangshang, Shengping Wen, Baosheng Liu, Yong Hu, Wu Wei, Xiaolan Wu, Hui Huang, Kunyuan Gao, Xiangyuan Xiong, and Zuoren Nie. 2024. "Stabilization Effect of Interfacial Solute Segregation on θ′ Precipitates in Al-Cu Alloys" Metals 14, no. 8: 848. https://doi.org/10.3390/met14080848







