Effects of Different Austenitising Conditions on the Strength–Ductility Balance in a High-Strength Low-Alloy Steel
Abstract
:1. Introduction
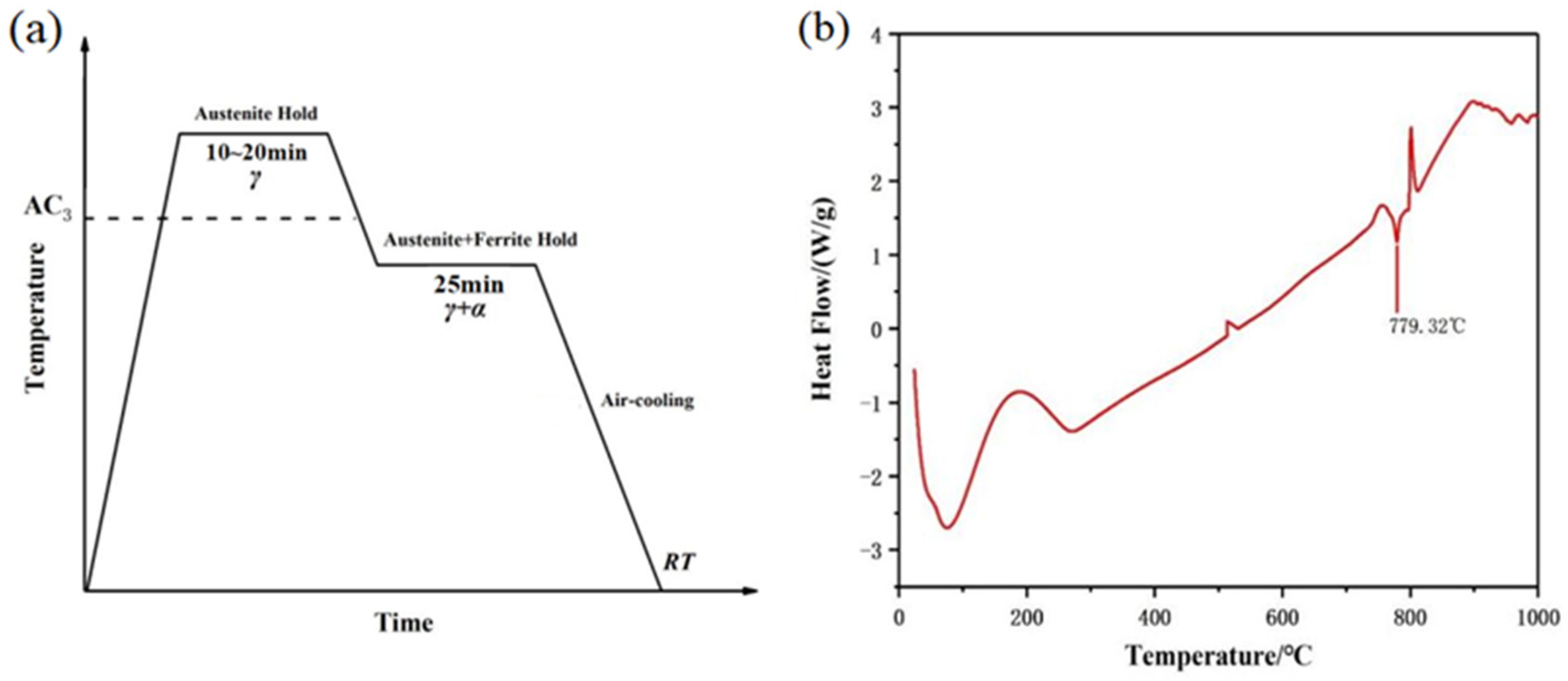
2. Materials and Experimental Procedure
3. Result and Discussion
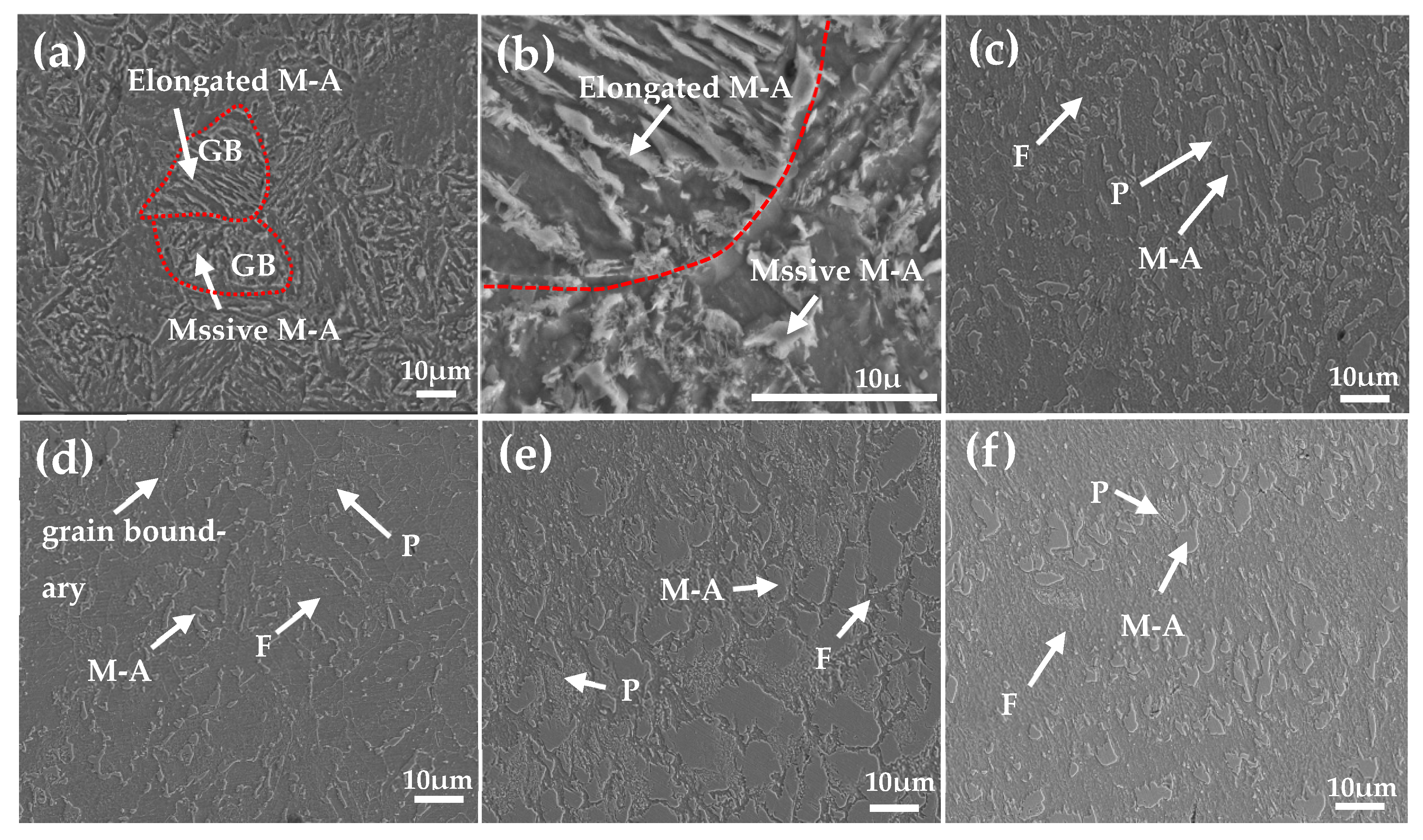
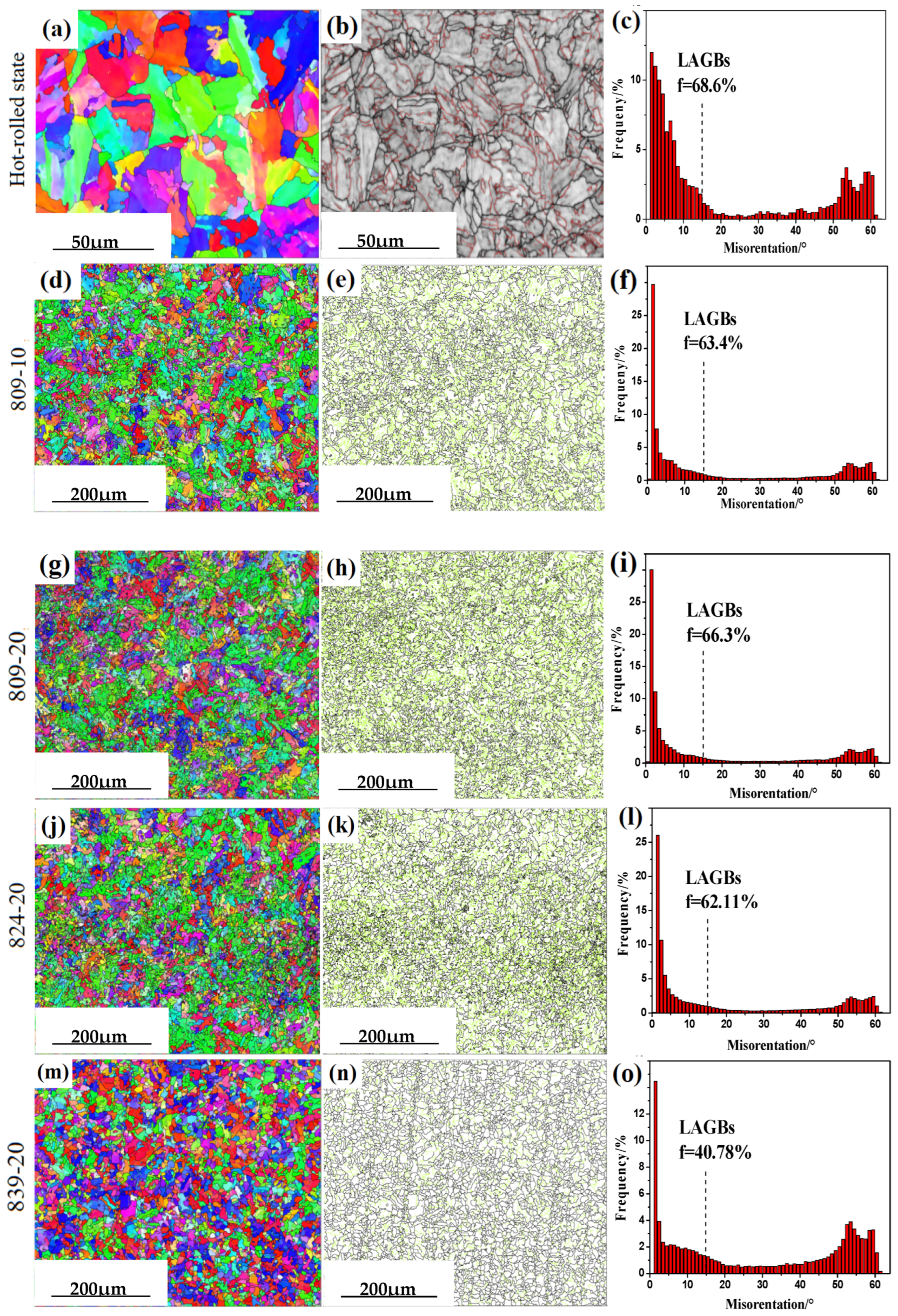
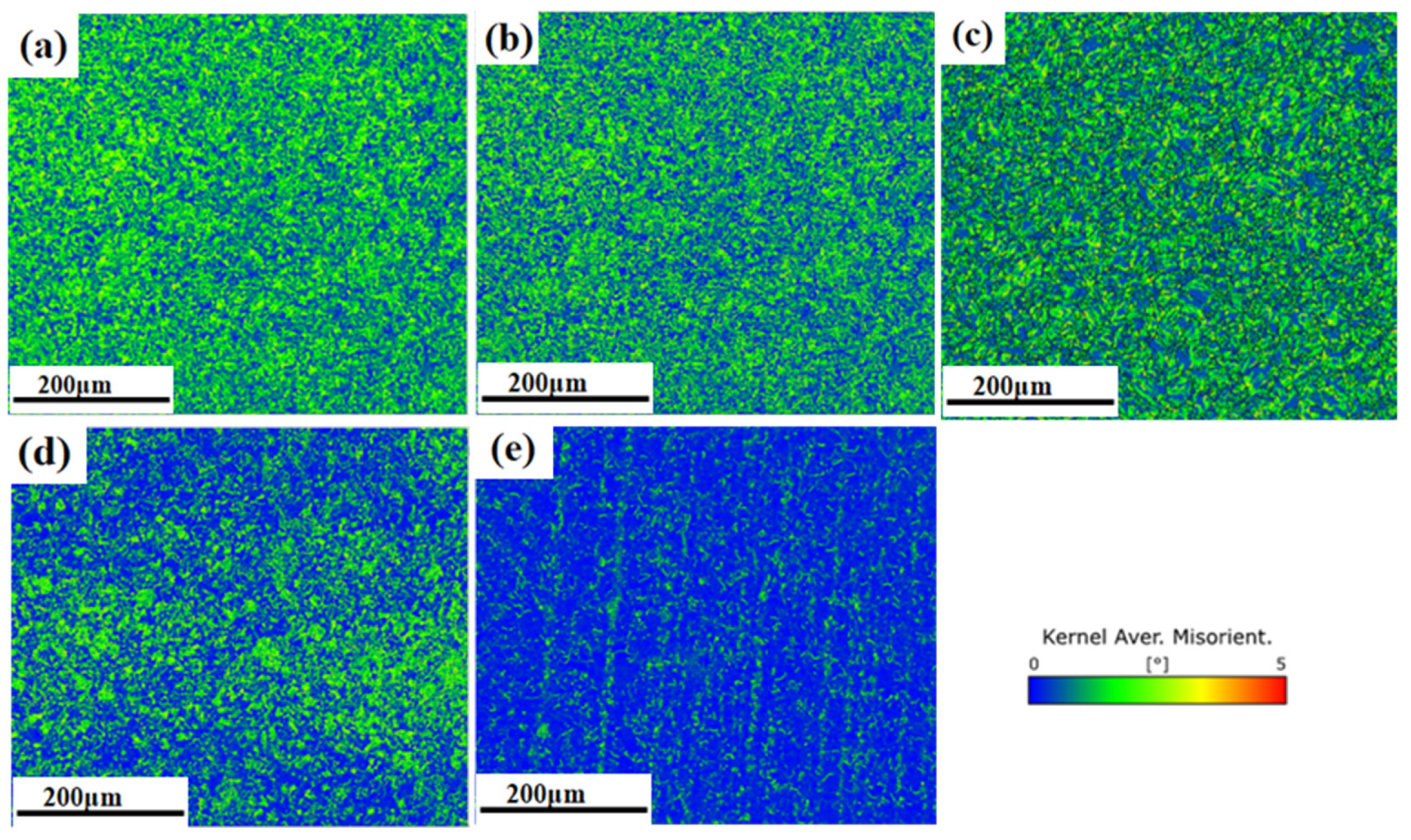
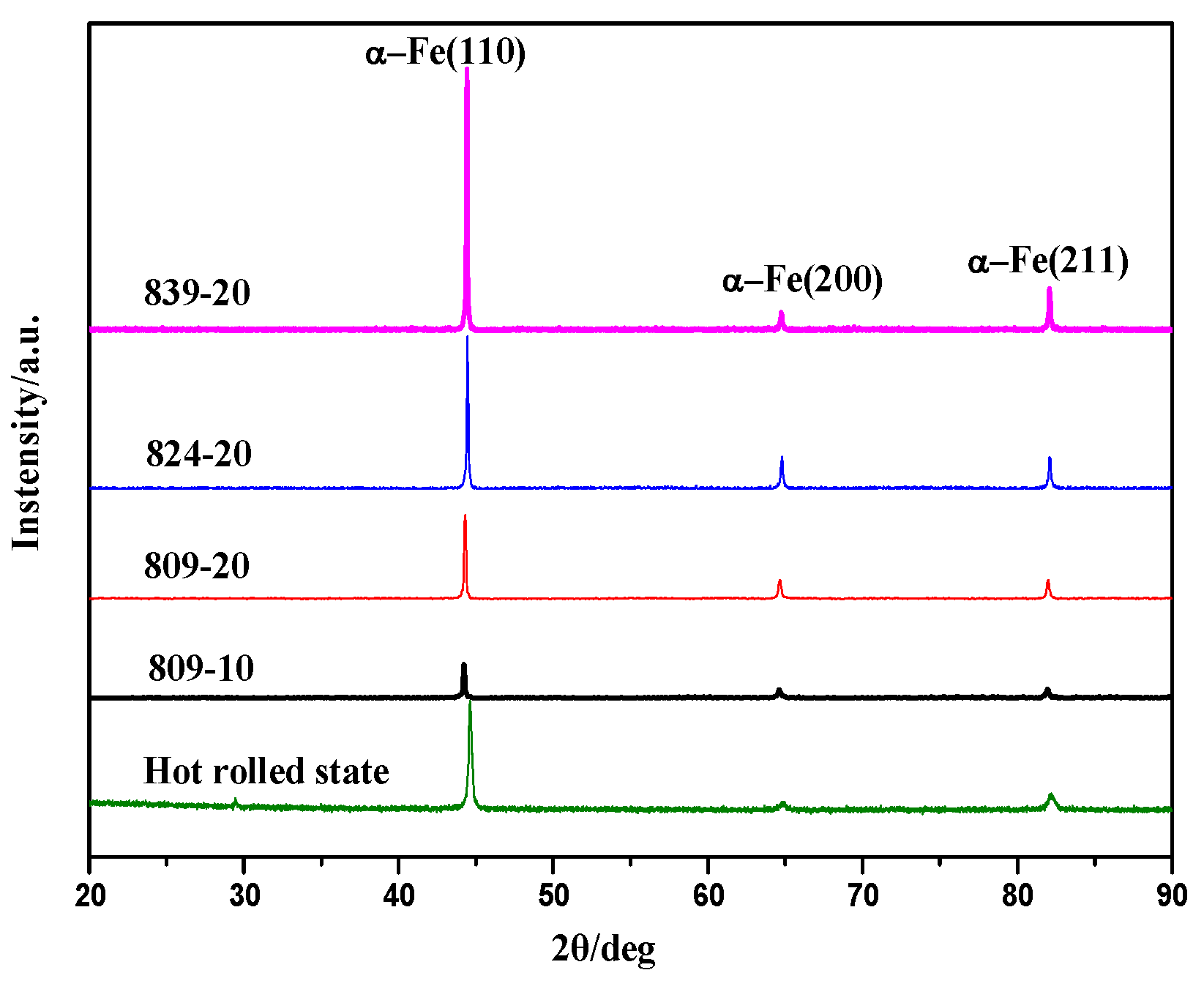
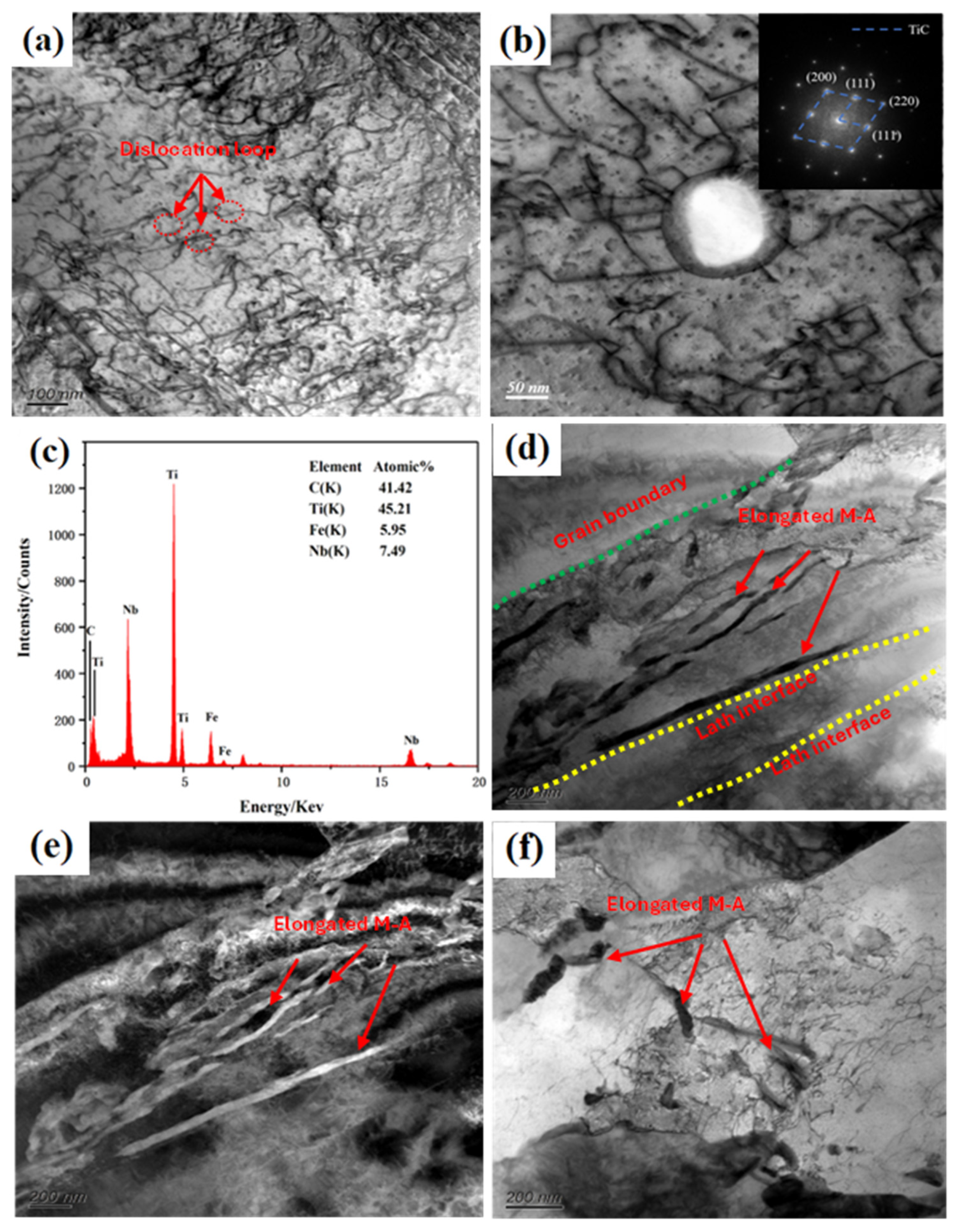
3.1. Microstructural Characteristics
3.2. Mechanical Properties
3.3. Effect of Austenitising Conditions on Microstructural Properties
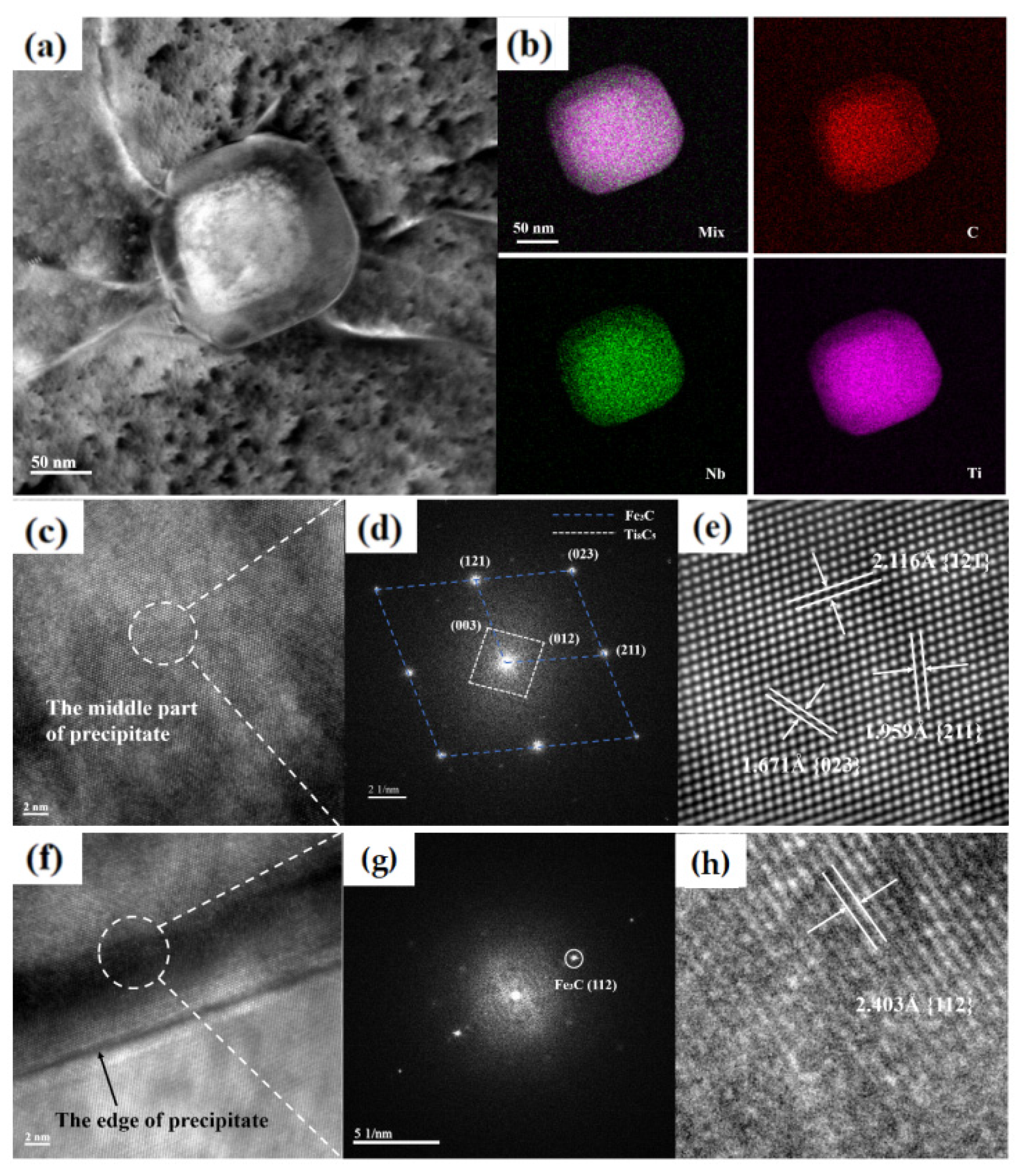
3.4. Nano-Sized Precipitate Structure
3.5. Strengthening Mechanism
4. Conclusions
- (1)
- When the hot-rolled sample was reheated to 15 °C above the austenitic transition temperature for 20 min and then cooled to 25 °C below the austenitic transition temperature for 25 min, more low-angle boundaries were formed, and the smallest effective grain size was achieved.
- (2)
- Compared with the hot-rolled sample, the tensile and yield strengths of the 810-20 sample (the sample was first heated to 15 °C above the austenite transition temperature for 20 min, then cooled to 25 °C below the austenite transition point for 25 min, and finally air-cooled to room temperature) increased by 10.2% and 3.1%, respectively, while the elongation increased by 162.5%, exhibiting a good strength–ductility balance.
- (3)
- By adopting an appropriate austenitisation process, precipitates can refine crystalline grains during austenitisation, thus enhancing the comprehensive mechanical properties of the steel.
- (4)
- Excessively high austenitisation temperatures coarsen the steel microstructure, decreasing the microstructural refinement efficiency via precipitation and consequently weakening the comprehensive mechanical properties of the steel.
- (5)
- After heat treatment, a large number of fine particles with sizes of approximately 5–12 nm are dispersed in the matrix, and a few precipitates have nearly spherical shapes with sizes of approximately 102–130 nm. The precipitate exhibits a core–shell structure, in which the Fe–C compound forms the shell, and the Ti–C compound forms the core.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sietsma, J.J.; Forum, M.S. The Formation of Multiphase Microstructures in Low-Alloy Steel. Mater. Sci. Forum 2010, 638–642, 3520–3530. [Google Scholar] [CrossRef]
- Muneer, W.; Hu, K.; Chen, S.; Liu, D.T.; Liu, T.; Zhan, X.H. Comparative study of microstructure and mechanical properties using a novel filler rod ER 4943 and autogenously butt welded joint during laser welding of AA 6061-T6 in 1G position. Mod. Phys. Lett. B 2022, 24, 256–264. [Google Scholar] [CrossRef]
- Arsenlis, A.; Cai, W.; Tang, M.; Rhee, M.; Oppelstrup, T.; Hommes, G.; Pierce, T.G.; Bulatov, V.V. Enabling strain hardening simulations with dislocation dynamics. Model. Simul. Mater. Sci. Eng. 2007, 15, 553–559. [Google Scholar] [CrossRef]
- Khorrami, M.; Hanzaki, A.Z.; Abedi, H.R.; Moallemi, M.; Mola, J.; Chen, G.H. On the effect of Mn-content on the strength-ductility balance in Ni-free high N transformation induced plasticity steels. Mater. Sci. Eng. A 2021, 814, 141260. [Google Scholar] [CrossRef]
- Kuhr, B.R.; Aifantis, K.E. Interpreting the inverse Hall-Petch relationship and capturing segregation hardening by measuring the grain boundary yield stress through MD indentation. Mater. Sci. Eng. A 2019, 745, 107–114. [Google Scholar] [CrossRef]
- Nie, W.J.; Shang, C.J.; Guan, H.L.; Zhang, X.B.; Chen, S.H. Control of microstructures of ferritebainite(F/B) dual-phase steels and analysis of their resistance to deformation behavior. Acta Metall. Sin. 2012, 3, 298–306. [Google Scholar] [CrossRef]
- Shang, C.J.; Wang, X.M.; Zhou, Z.J.; Liang, X.; Miao, C.L.; He, X.L. Evolution of intermediate transformation microstructures in Mn-Mo-Nb-B low carbon microalloyed steel. Acta Metall. Sin. 2008, 3, 287–291. [Google Scholar]
- Klueh, R.L.; Nasreldin, A.M. Microstructure and mechanical properties of a 3Cr-1.5Mo steel. Mater. Trans. A 1987, 18, 1279–1290. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, P.; Li, D.; Li, Y. Influence of the decomposition behavior of retained austenite during tempering on the mechanical properties of 2.25Cr-1Mo-0.25 V steel. Mater. Sci. Eng. A 2019, 742, 540–552. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, N.; Li, D.; Zhang, J.; Luo, Y.; Zhang, R. Predictive value of APE1, BRCA1, ERCC1 and TUBB3 expression in patients with advanced non-small cell lung cancer (NscLc) receiving first-line platinum-paclitaxel chemotherapy. Acta Metall. Sin. 2014, 50, 777–786. [Google Scholar] [CrossRef]
- Yan, G.; Han, L.; Li, C.; Luo, X.; Gu, J. Characteristic of retained austenite decomposition during tempering and its effect on impact toughness in SA508 Gr.3 steel. J. Nucl. Mater. 2017, 483, 167–175. [Google Scholar] [CrossRef]
- Lan, L.; Qiu, C.; Zhao, D.; Gao, X.; Du, L. Analysis of microstructural variation and mechanical behaviors in submerged arc welded joint of high strength low carbon bainitic steel. Mater. Sci. Eng. A 2012, 558, 592–601. [Google Scholar] [CrossRef]
- Tian, D.; Karjalainen, L.P.; Qian, B.; Chen, X. Cleavage fracture model for granular bainite in simulated coarse-grained heat-affected zones of high-strength low-alloyed steels. JSME Int. J. Ser. A 1997, 40, 179–188. [Google Scholar] [CrossRef]
- Cui, P.C.; Xia, T.; Wang, Y.Q.; Jia, C.H.; Wang, H.L.; Bai, Q.Q.; Zheng, H.B.; Lai, Z.H.; Liu, Y.; Zhu, J.C.; et al. Uncovering the isothermal transformation dynamics and elemental diffusion behavior of reversed austenite in PH13-8Mo steel. Mater. Chem. Phys. 2024, 312, 128666. [Google Scholar] [CrossRef]
- Li, H.X.; Lee, C.W.; Venezuelaa, J.; Kimc, H.; Atrens, A. Hydrogen diffusion and hydrogen embrittlement of a 1500 MPa hot-stamped steel 22MnB5 in different austenitizing condition. Mater. Sci. Eng. A 2024, 897, 146349. [Google Scholar] [CrossRef]
- Santos, S.L.; Toloczko, F.R.; Silva, D.M.; Santos, S.F. Investigating the role of the austenitizing temperature and cooling rate on the martensitic transformation kinetics in a SAE 9254 spring steel. J. Alloys Metall. Syst. 2024, 5, 100065. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Wang, P.; Li, D.Z.; Li, Y.Y. Effects of rare earth on microstructure and impact toughness of low alloy Cr-Mo-V steels for hydrogenation reactor vessels. J. Mater. Sci. Technol. 2020, 45, 1–14. [Google Scholar] [CrossRef]
- Farichild, D. A study concerning intercritical HAZ microstructure andtoughness in HSLA steels. Weld. J. 1991, 70, 321–329. [Google Scholar]
- Kim, B. Microstructure and local brittle zone phenomena in high.strength low-alloy steel welds. Metall. Trans. A 1991, 22, 139–149. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from field aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Gladman, T. On the theory of the effect of precipitate particles on grain growth in metals. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1966, 42, 298–309. [Google Scholar]
- Alexander, V.; Dmitry, S.; Semen, S.; Nikolay, K. Modeling of austenitization kinetics under continuous heating of steels with complex microstructure. Proc. Manuf. 2019, 37, 613–620. [Google Scholar] [CrossRef]
- Ding, Y.F.; Wu, M.J.; Li, Y.P.; Sun, Y.D.; Zhao, Y.L.; Ran, G. Quantitative in-situ investigation of dislocation loop motion inFe9Cr1.5W ferritic/martensitic steel under the coupling effects of constant stress and irradiation. J. Nucl. Mater. 2024, 598, 155166. [Google Scholar] [CrossRef]
- Xie, Z.J.; Han, G.; Zhou, W.H.; Zeng, C.Y.; Shang, C.J. Study of retained austenite and nano-scale precipitation and their effects on properties of a low alloyed multi-phase steel by the two-step intercritical treatment. Mater. Charact. 2016, 113, 60–66. [Google Scholar] [CrossRef]
- Okada, H.; Matsuda, F.; Lkeuchi, K.; Li, Z. Decomposition behaviour of M-A constituent and recovery of toughness deterioration of HAZ toughness in 780 and 980 MPa class HSLA steels welded with high heat inputs and its improvement. Weld. Int. 1995, 9, 211–216. [Google Scholar] [CrossRef]
- Zener, C. Theory of growth of spherical precipitates from solid solution. J. Appl. Phys. 1949, 20, 950–953. [Google Scholar] [CrossRef]
- Davenport, A.T.; Brossard, L.C.; Miner, R.E.J.J. Precipitation in microalloyed high-strength low-alloy steels. JOM 1975, 27, 21–27. [Google Scholar] [CrossRef]
- Melnyk, C.; Grant, D.; Keener, S.G.; Ganser, R.V.; Schroeder, S. Improving the properties of cryomilled light alloys using spark plasma sintering and hot isostatic pressing. JOM 2011, 63, 65–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, H. Effects of Nb on microstructure and continuous cooling transformation of coarse grain heat-affected zone in 610MPa class HSLA structural steels. Mater. Sci. Eng. A 2009, 499, 182–186. [Google Scholar] [CrossRef]
- Kim, D.W.; Kwon, D.Y.; Kang, J. Strengthening of high nitrogen austenitic stainless steel by Nb addition. Mater. Charact. 2024, 209, 113776. [Google Scholar] [CrossRef]
- Pereda, B.; Rodriguez-Ibabe, J.M.; López, B. Improved model of kinetics of strain induced precipitation and microstructure evolution of Nb microalloyed steels during multipass rolling. ISIJ Int. 2008, 48, 1457–1466. [Google Scholar] [CrossRef]
- Iza-Mendia, A.; Gutierrez, I.J.M.S. Generalization of the existing relations between microstructure and yield stress from ferrite-pearlite to high strength steels. Mater. Sci. Eng. A 2013, 561, 40–51. [Google Scholar] [CrossRef]
- Pickering, F.B. Physical metallurgy and the design of steels. Phys. Metall. Des. Steel 1978, 23, 90–100. [Google Scholar]
- Yan, Z.; Zou, K.; Cheng, M.P.; Zhou, Z.P.; Song, L.J. Revealing relationships between heterogeneous microstructure and strengthening mechanism of austenitic stainless steels fabricated by directed energy deposition. J. Mater. Res. Technol. 2021, 15, 582–594. [Google Scholar] [CrossRef]
- Kestenbach, H.J.; Campos, S.S.; Gallego, J.; Morales, E.V. Discussion of “Precipitation behavior and its effect on strengthening of an HSLA-Nb/Ti steel”. Metall. Mater. Trans A 2003, 34, 1013–1017. [Google Scholar] [CrossRef]


| C | Si | Mn | P | Ti | Cr | S | Mo | Nb | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 0.3 | 0.25 | 1.39 | 0.007 | 0.15 | 0.3 | 0.003 | 0.19 | 0.04 | Bal. |
| Sample | Rot-rolled | 809-10 | 809-20 | 824-10 | 824-20 | 839-10 | 839-20 |
|---|---|---|---|---|---|---|---|
| Yield strength/MPa | 564 | 443 | 583 | 551 | 445 | 416 | 463 |
| Tensile strength/MPa | 751 | 693 | 843 | 798 | 775 | 705 | 767 |
| Elongation/% | 8 | 25 | 21 | 16.5 | 20 | 18.5 | 19.5 |
| Sample | Average Effective Grain Size/μm | Dislocation Density/nm−2 | Average Large Precipitate Size/nm | Average Small Precipitate Size/nm |
|---|---|---|---|---|
| Rot-rolled | 6.26 | 1.26 × 10−2 | 109.58 | 8.56 |
| 809-10 | 4.35 | 0.491 × 10−2 | 113.43 | 8.83 |
| 809-20 | 3.68 | 0.906 × 10−2 | 115.68 | 9.28 |
| 824-20 | 5.35 | 0.641 × 10−2 | 121.32 | 12.21 |
| 839-20 | 7.26 | 0.437 × 10−2 | 126.74 | 13.52 |
| Sample | Austenite Transformation Fraction/% | |
|---|---|---|
| By Model in Reference [19] | By JMatPro | |
| 809-10 | 41.5 | 49.1 |
| 809-20 | 87.6 | 93 |
| 824-20 | 100 | 100 |
| 839-20 | 100 | 100 |
| Sample | Precipitation Hardening/MPa | Grain Refining Hardening/MPa | Dislocation Hardening/MPa | σy |
|---|---|---|---|---|
| Rot-rolled | 50.32 | 279.7 | 224.6 | 595.36 |
| 809-10 | 64.99 | 335.6 | 126.18 | 566.23 |
| 809-20 | 65.91 | 364.8 | 171.36 | 643.36 |
| 824-20 | 66.19 | 302.6 | 144.10 | 556.75 |
| 839-20 | 66.43 | 259.8 | 119.02 | 496.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Dong, D.; Jiang, Z.; Chen, T.; Li, Y. Effects of Different Austenitising Conditions on the Strength–Ductility Balance in a High-Strength Low-Alloy Steel. Metals 2024, 14, 850. https://doi.org/10.3390/met14080850
Luo L, Dong D, Jiang Z, Chen T, Li Y. Effects of Different Austenitising Conditions on the Strength–Ductility Balance in a High-Strength Low-Alloy Steel. Metals. 2024; 14(8):850. https://doi.org/10.3390/met14080850
Chicago/Turabian StyleLuo, Liang, Duyu Dong, Zheng Jiang, Tao Chen, and Yimin Li. 2024. "Effects of Different Austenitising Conditions on the Strength–Ductility Balance in a High-Strength Low-Alloy Steel" Metals 14, no. 8: 850. https://doi.org/10.3390/met14080850





