Passivation Behavior of Chromium Alloyed High-Strength Rebar in Simulated Concrete Pore Solution
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Material Preparation
2.2. Simulated Solution
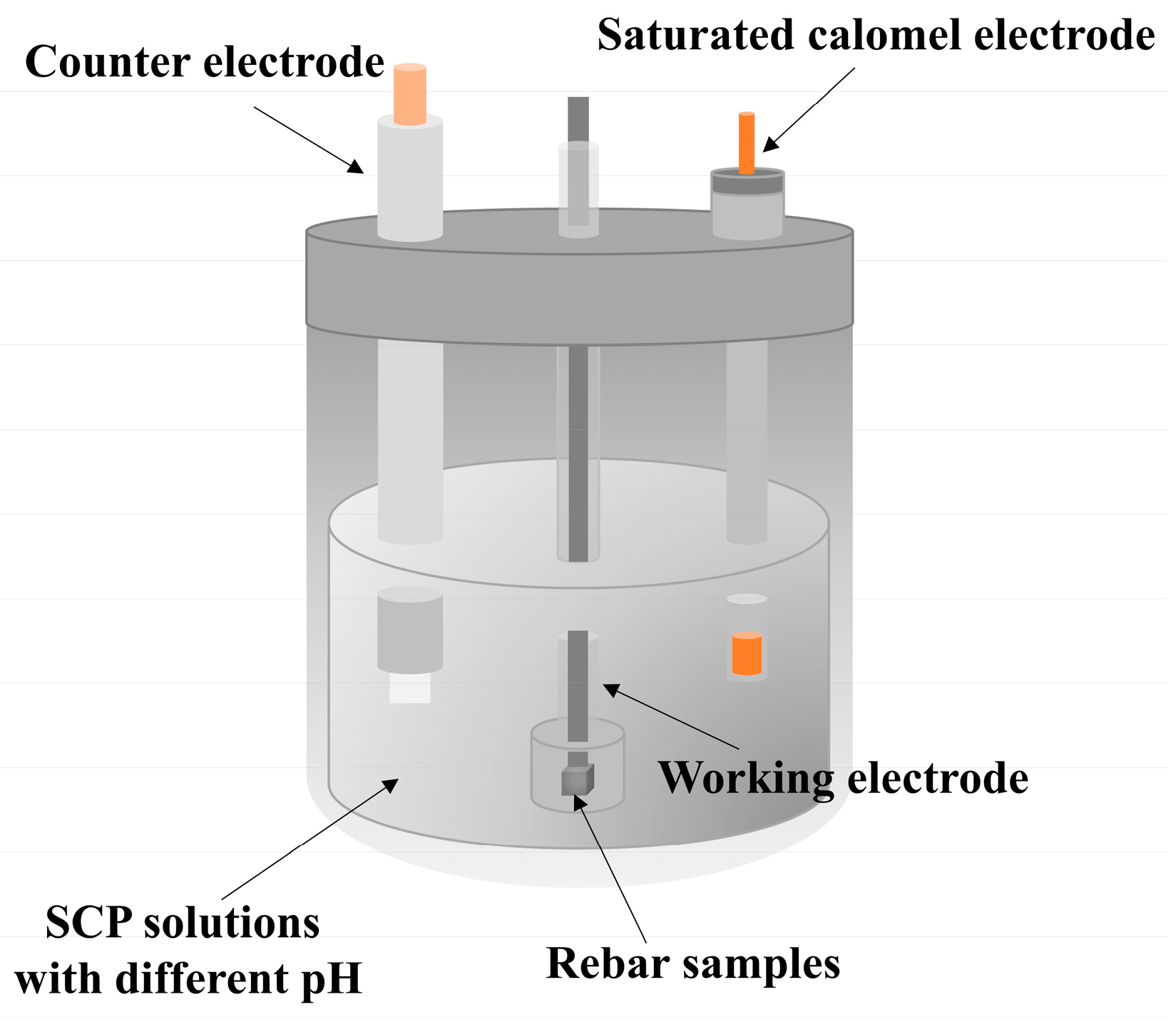
2.3. Electrochemical Test
2.4. Characterization Method
3. Results and Analysis
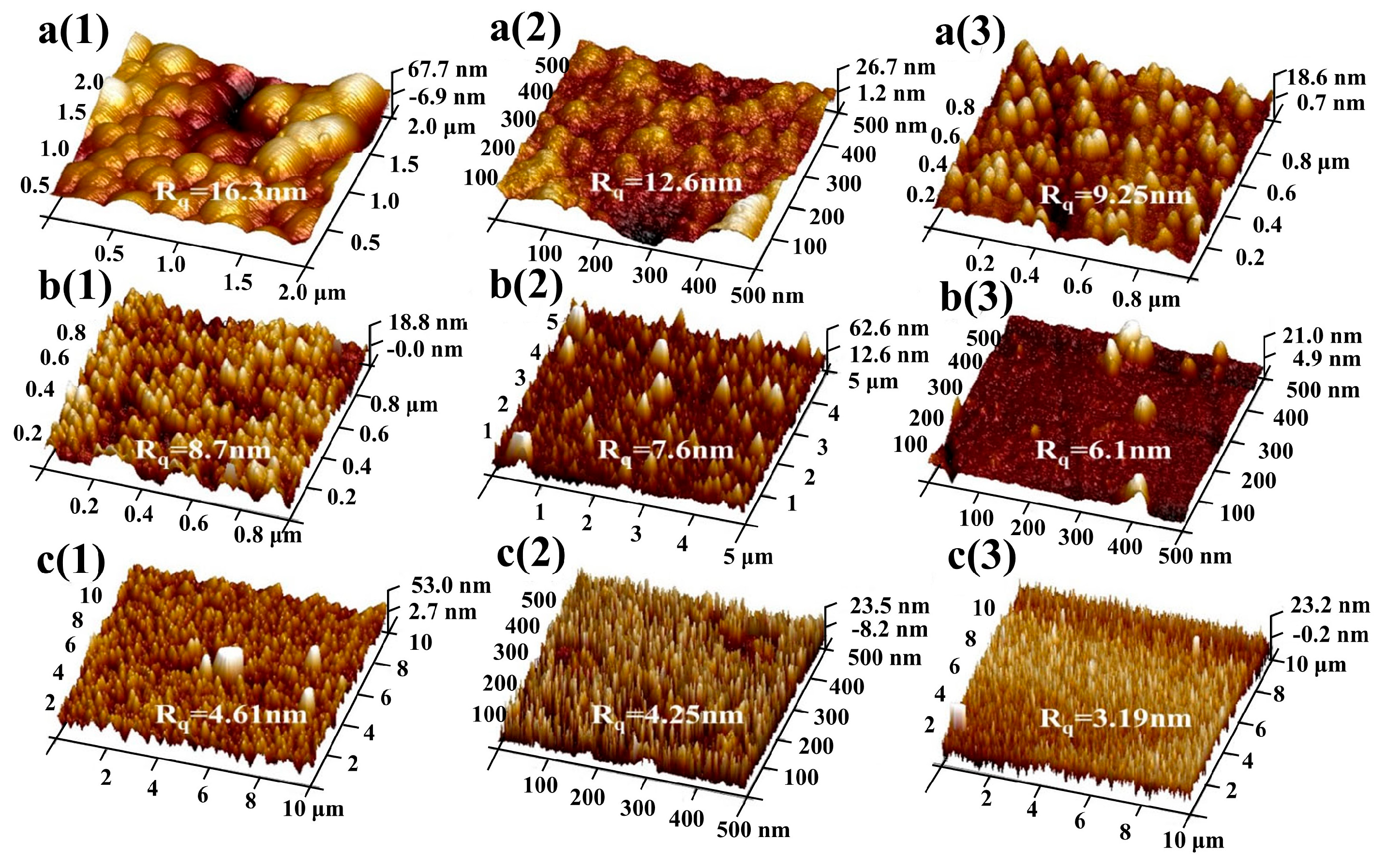
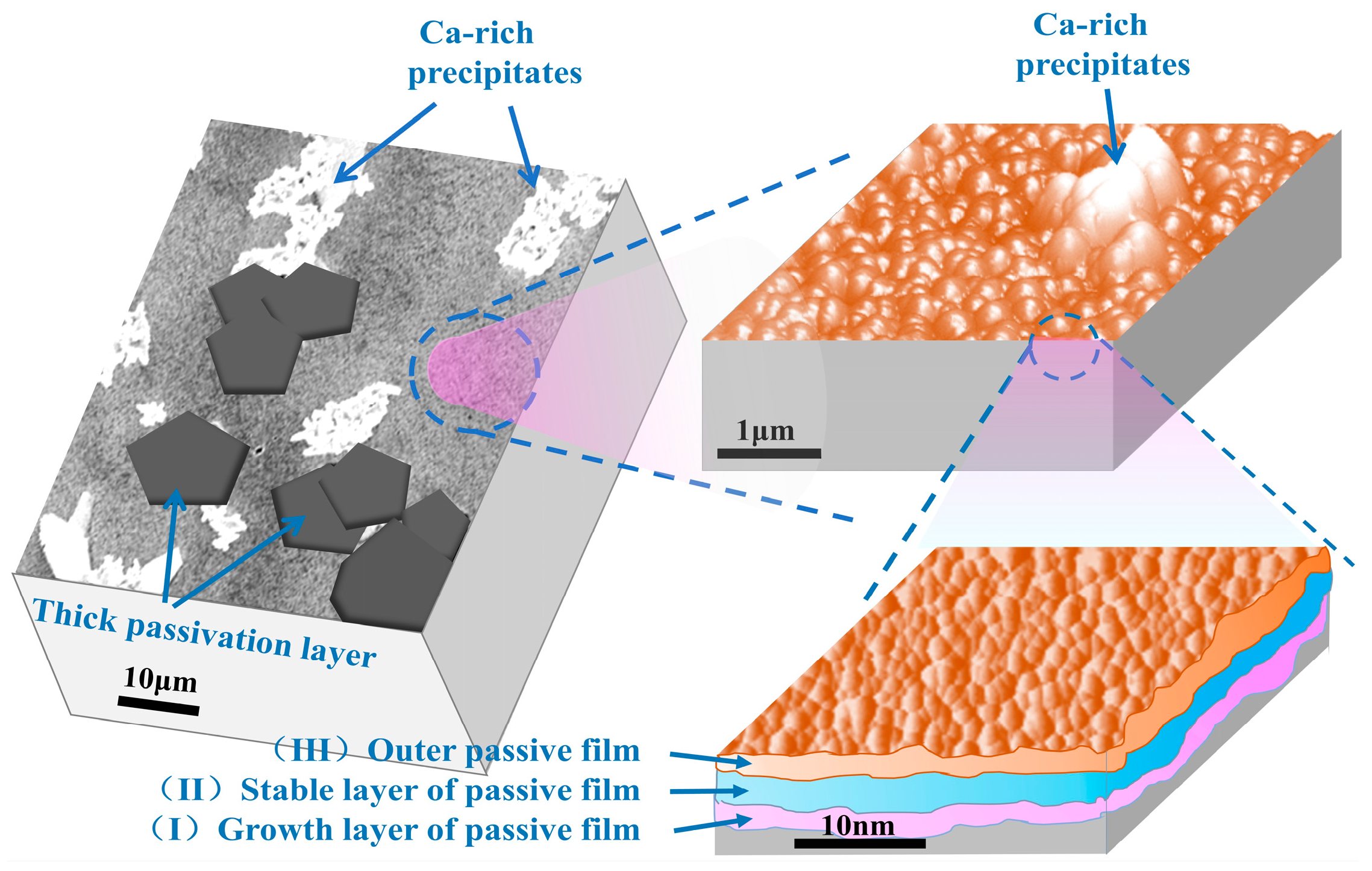
3.1. Morphology Analysis of Passive Film
3.2. Structure Analysis of Passive Film
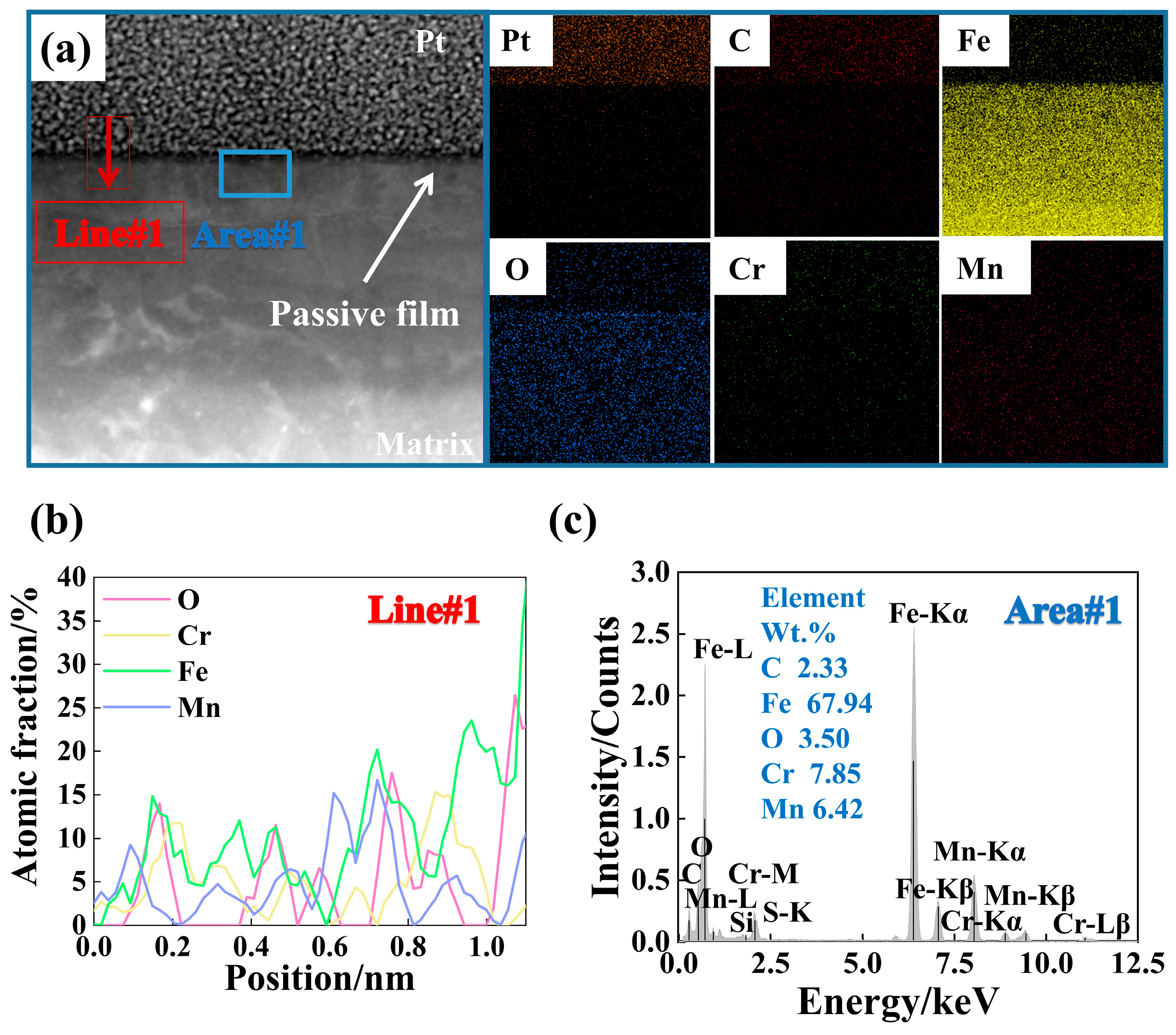
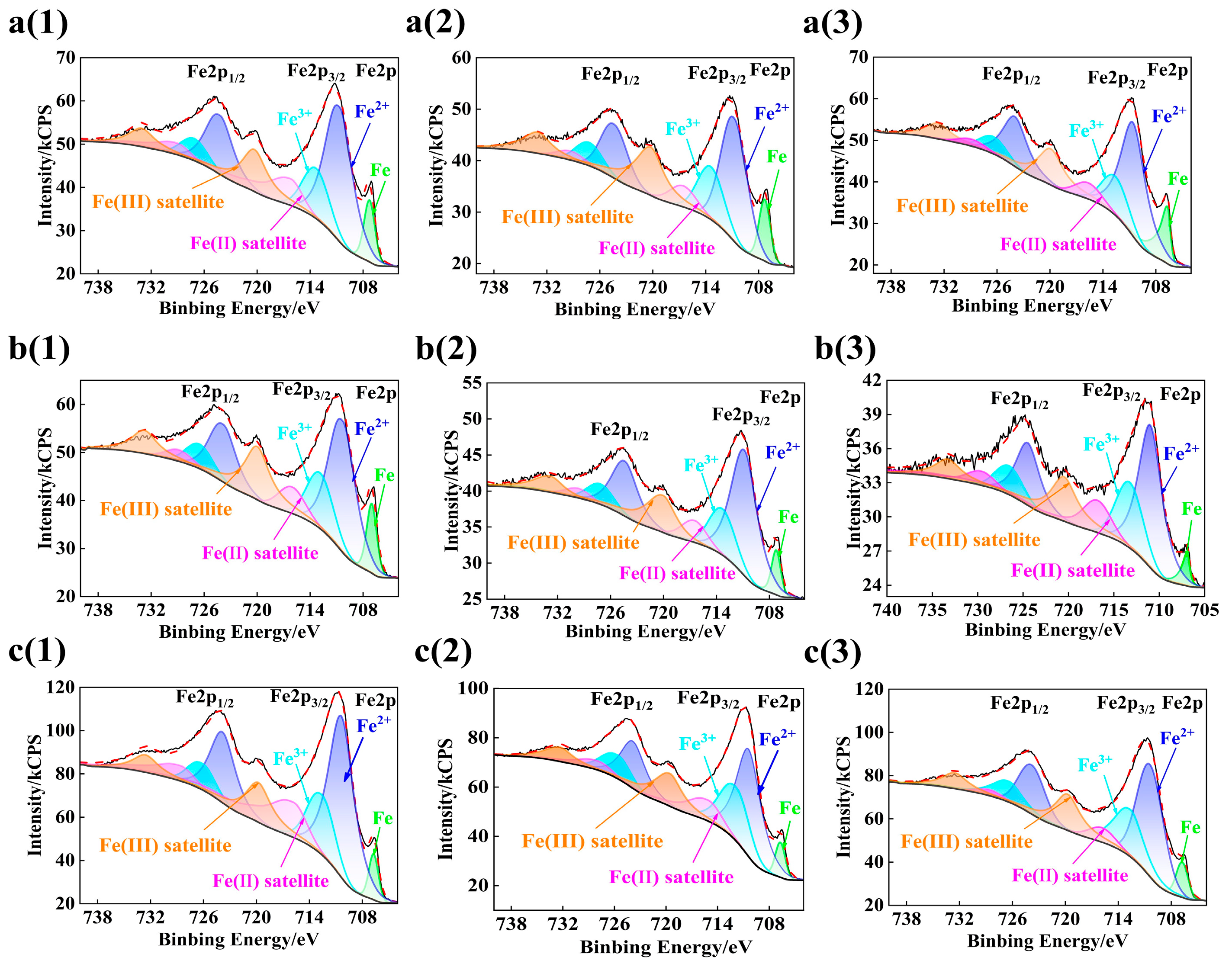
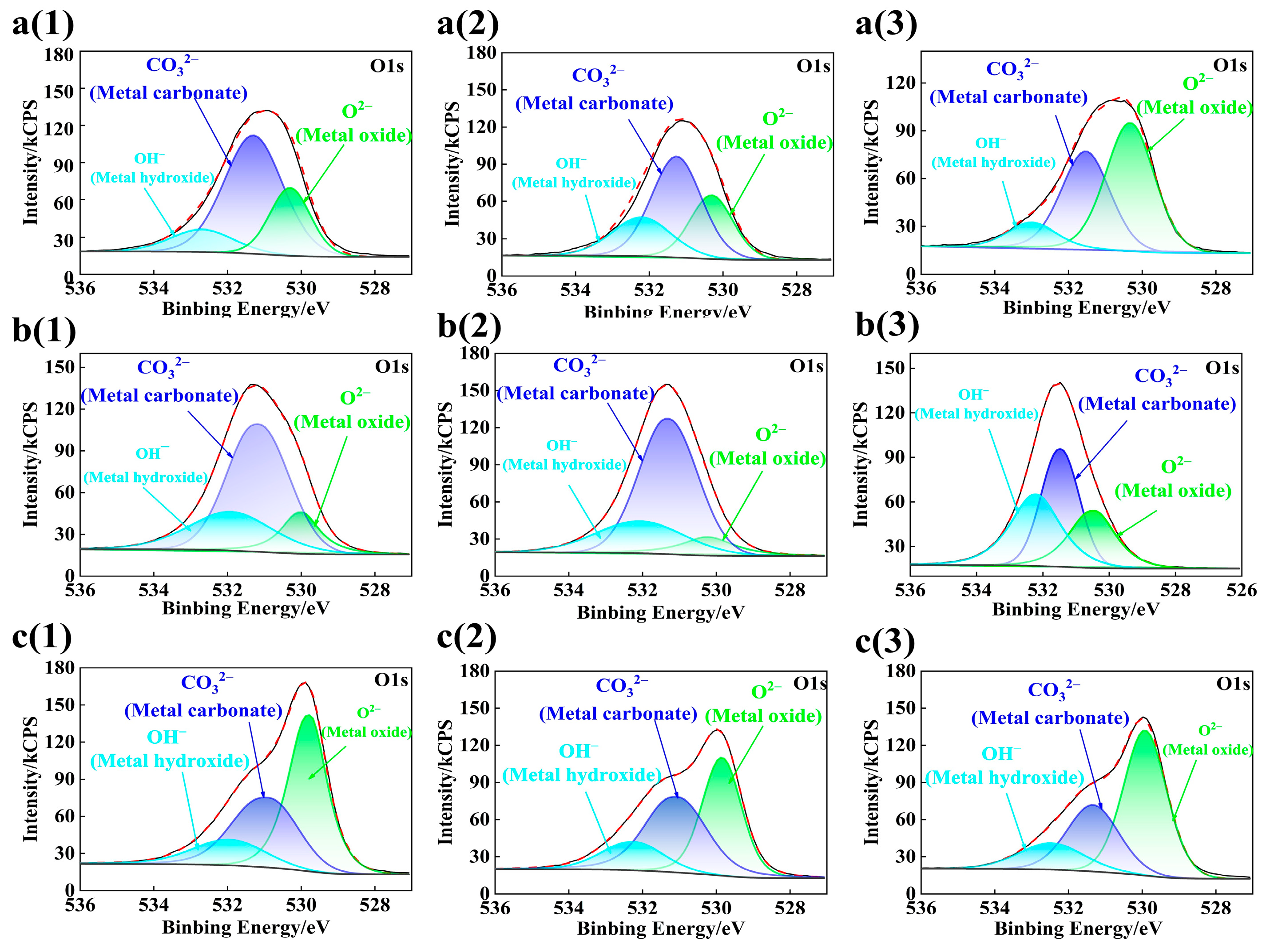
3.3. Composition Analysis of Passive Film
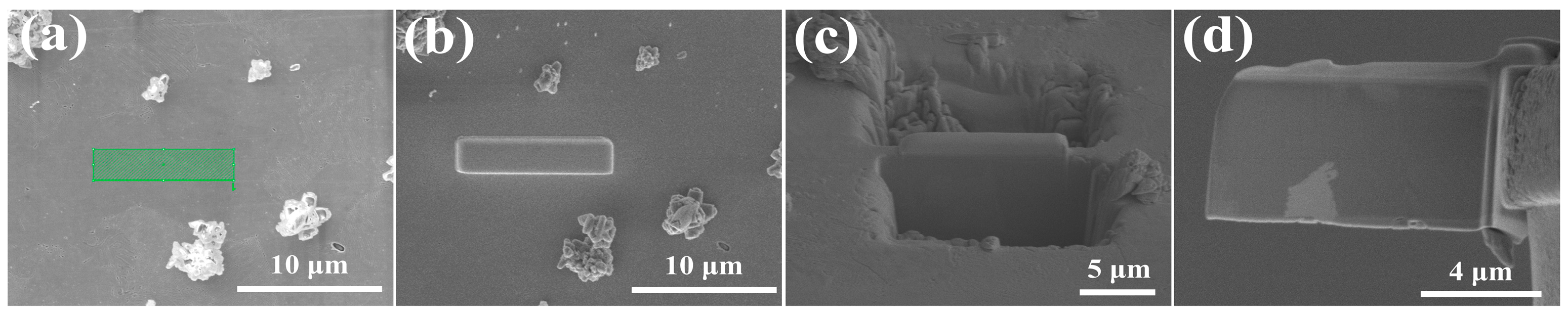
3.4. Analysis of the Passive Film Thickness
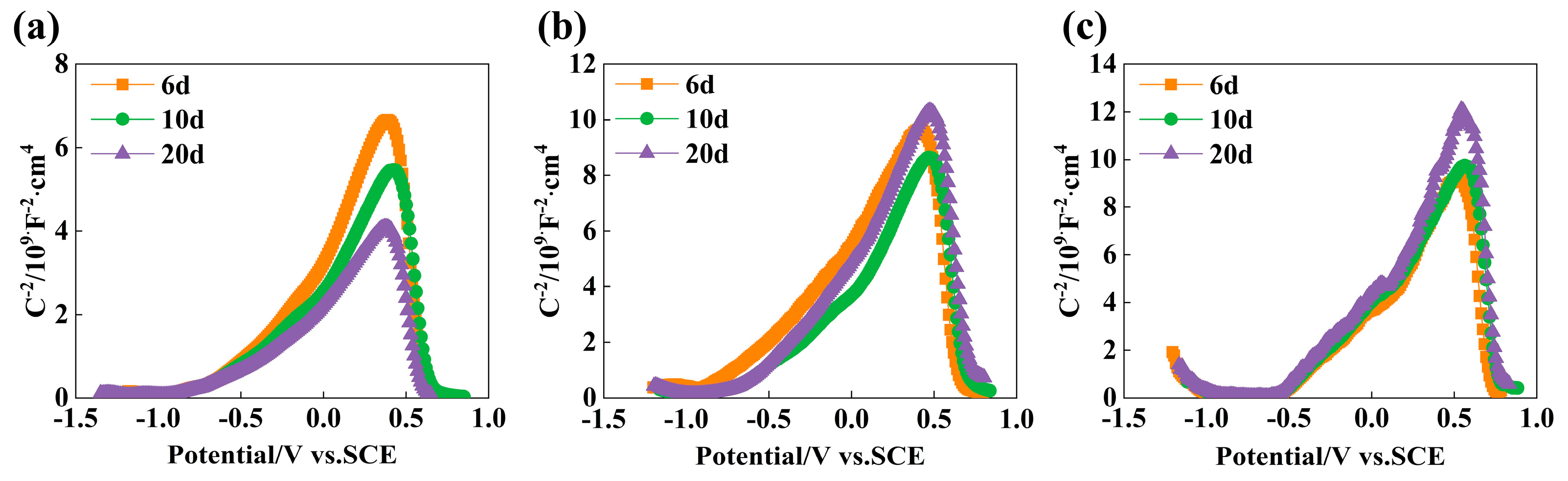
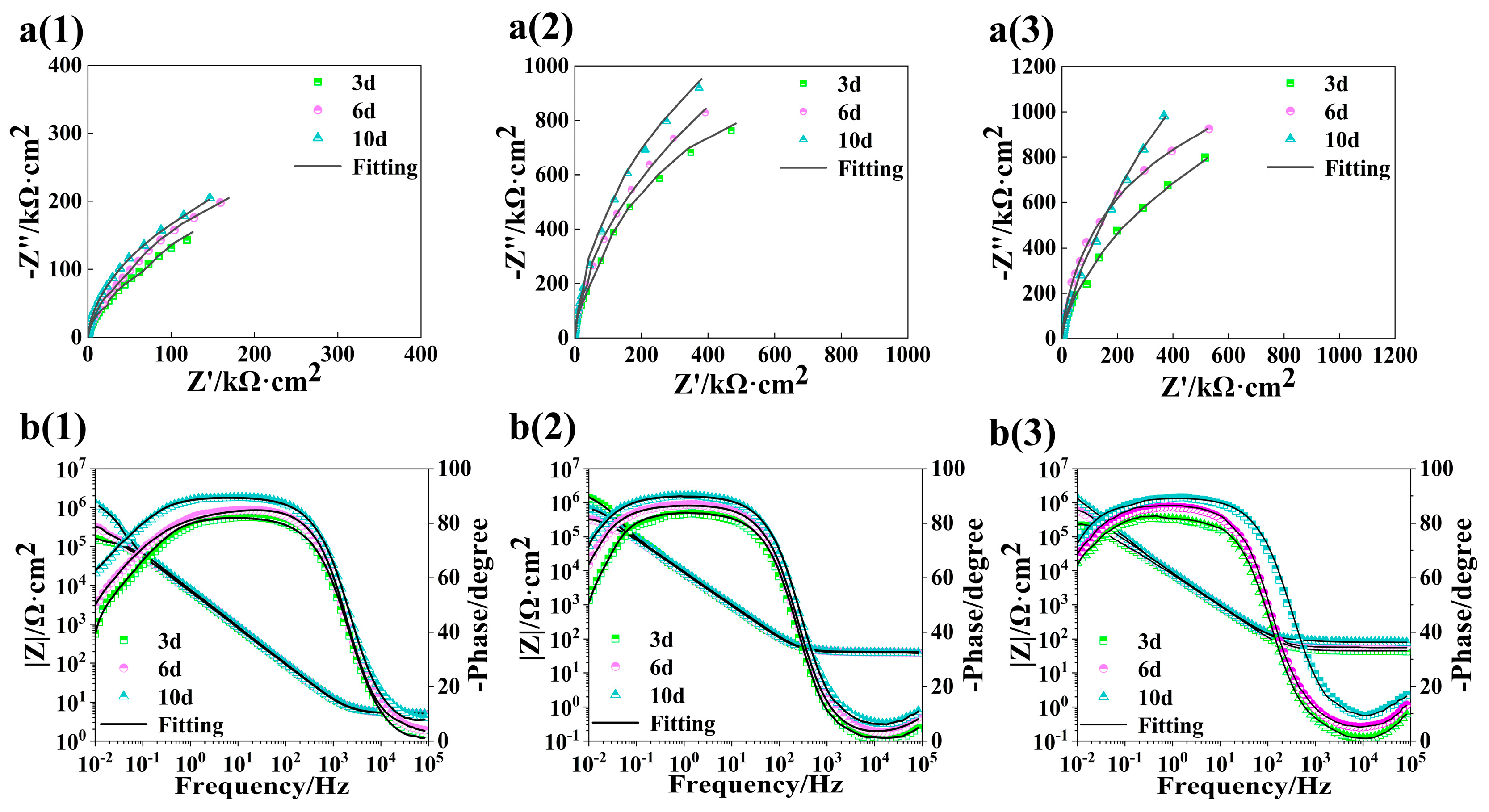
3.5. Performance Analysis of Passive Film
4. Conclusions
- In this study, SEM, AFM, TEM, XPS, and electrochemical tests are used to study the passivation behavior of chromium alloyed high-strength rebar in SCP solutions with different pH values, and the structure and composition of the passive film are analyzed. The main key conclusions are as follows:
- In SCP solution with pH 10.5, the passive film on the surface of the rebar is mainly composed of contiguous nanoparticles, and the surface is distributed with a multilateral passivation layer and calcium-rich precipitates, which enhance the compactness of the passive film.
- When the rebar is passivated in the SCP solution with pH 10.5 for 10 d, the passive film is relatively stable, and its crystal structure is a rhombohedral crystal system. The passivating film on the surface of chromium-alloyed high-strength rebar mainly presents a three-layer structure. The structural changes from the inside to the outside are the growth layer of the inner passive film, the stable layer of the passive film, and the outer passive film layer. The outer layer of the passive film is mainly composed of Fe oxide, the intermediate stable layer of the passive film is mainly composed of Fe oxide and Cr oxide, and the inner growth layer of the passive film is mainly composed of Cr oxide.
- In SCP solution with pH 13.5, pH 12.5, and pH 10.5, the thickness of the passive film increases with the increasing passivation time, and the passive film of the experimental rebar is the thickest after immersion 10 d. The passive film formed in the pH 10.5 environment is the thickest and the passive film resistance is the largest at the same immersion time. In a high alkaline solution, the passive film is an n-type semiconductor. In the passivation process of low alkaline solution, the Cr element accumulates in the passive film, and the passive film exhibits p-n type semiconductors with better corrosion resistance.
5. Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, J.; Wu, M.; Ming, J. Degradation effect of carbonation on electrochemical behavior of 2304 duplex stainless steel in simulated concrete pore solutions. Corros. Sci. 2020, 177, 109006. [Google Scholar] [CrossRef]
- Jin, Z.; Xiong, C.; Zhao, T.; Du, Y.; Zhang, X.; Li, N.; Yu, Y.; Wang, P. Passivation and depassivation properties of Cr–Mo alloyed corrosion-resistant steel in simulated concrete pore solution. Cem. Concr. Compos. 2022, 126, 104375. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, X.; Gong, N.; Zhang, Y.; Wu, H.; Mao, X.; Niu, G. Passivation behavior of Cr-modified rebar in simulated concrete pore solutions with different pH. J. Mater. Res. Technol. 2023, 26, 246–259. [Google Scholar] [CrossRef]
- Caprili, S.; Salvatore, W.; Valentini, R. Corroded TempCore® vs dual-phase steel reinforcing bars. Constr. Build. Mater. 2021, 277, 122301. [Google Scholar] [CrossRef]
- Macdonald, D.D. The history of the Point Defect Model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 564, 1761–1772. [Google Scholar] [CrossRef]
- Moser, R.D.; Singh, P.M.; Kahn, L.F.; Kurtis, K.E. Chloride-induced corrosion resistance of high-strength stainless steels in simulated alkaline and carbonated concrete pore solutions. Corros. Sci. 2012, 57, 241–253. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, X.; Cao, Y.; Yang, H. Natural passivation behavior and its influence on chloride-induced corrosion resistance of stainless steel in simulated concrete pore solution. J. Mater. Res. Technol. 2020, 96, 12378–12390. [Google Scholar] [CrossRef]
- Sun, H.; Wu, X.; Han, E.-H. Effects of temperature on the oxide film properties of 304 stainless steel in high temperature lithium borate buffer solution. Corros. Sci. 2009, 5112, 2840–2847. [Google Scholar] [CrossRef]
- Massoud, T.; Maurice, V.; Klein, L.H.; Seyeux, A.; Marcus, P. Nanostructure and local properties of oxide layers grown on stainless steel in simulated pressurized water reactor environment. Corros. Sci. 2014, 84, 198–203. [Google Scholar] [CrossRef]
- Lv, S.; Li, K. Semiconducting behaviour and corrosion resistance of passive film on corrosion-resistant steel rebars. Materials 2022, 1521, 7644. [Google Scholar] [CrossRef]
- Goyal, A.; Pouya, H.S.; Ganjian, E.; Claisse, P. A review of corrosion and protection of steel in concrete. Arab. J. Sci. Eng. 2018, 43, 5035–5055. [Google Scholar] [CrossRef]
- Xie, Y.; Qian, C.; Xu, Y.; Wei, M.; Du, W. Effect of fine aggregate type on early-age performance, cracking analysis and engineering applications of C50 concrete. Constr. Build. Mater. 2022, 323, 126633. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Liu, H.; Zhu, C.; Wu, Y.; He, C.; Wang, Z. Recycled aggregate concrete with the incorporation of rice husk ash: Mechanical properties and microstructure. Constr. Build. Mater. 2022, 351, 128934. [Google Scholar] [CrossRef]
- Tang, B.; Fan, M.; Yang, Z.; Sun, Y.; Yuan, L. A comparison study of aggregate carbonation and concrete carbonation for the enhancement of recycled aggregate pervious concrete. Constr. Build. Mater. 2023, 371, 130797. [Google Scholar] [CrossRef]
- Tam, V.W.; Soomro, M.; Evangelista, A.C.J. A review of recycled aggregate in concrete applications (2000–2017). Constr. Build. Mater. 2018, 172, 272–292. [Google Scholar] [CrossRef]
- Xu, L.; Wu, P.; Zhu, X.; Zhao, G.; Ren, X.; Wei, Q.; Xie, L. Structural characteristics and chloride intrusion mechanism of passive film. Corros. Sci. 2022, 207, 110563. [Google Scholar] [CrossRef]
- Ai, Z.; Jiang, J.; Sun, W.; Jiang, X.; Yu, B.; Wang, K.; Zhang, Z.; Song, D.; Ma, H.; Zhang, J. Enhanced passivation of alloy corrosion-resistant steel Cr10Mo1 under carbonation—Passive film formation, the kinetics and mechanism analysis. Cem. Concr. Compos. 2018, 92, 178–187. [Google Scholar] [CrossRef]
- Loh, N.D.; Sen, S.; Bosman, M.; Tan, S.F.; Zhong, J.; Nijhuis, C.A.; Král, P.; Matsudaira, P.; Mirsaidov, U. Multistep nucleation of nanocrystals in aqueous solution. Nat. Chem. 2017, 91, 77–82. [Google Scholar] [CrossRef]
- Freire, L.; Carmezim, M.; Ferreira, M.a.; Montemor, M. The passive behaviour of AISI 316 in alkaline media and the effect of pH: A combined electrochemical and analytical study. Electrochim. Acta 2010, 5521, 6174–6181. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Vafaeian, S. Passivation behavior of a ferritic stainless steel in concentrated alkaline solutions. J. Mater. Res. Technol. 2015, 44, 423–428. [Google Scholar] [CrossRef]
- Ai, Z.; Jiang, J.; Sun, W.; Song, D.; Ma, H.; Zhang, J.; Wang, D. Passive behaviour of alloy corrosion-resistant steel Cr10Mo1 in simulating concrete pore solutions with different pH. Appl. Surf. Sci. 2016, 389, 1126–1136. [Google Scholar] [CrossRef]
- GB 1499.2-2018; Steel for the Reinforcement Concrete-Part 2: Hot Rolled Ribbed Bars. China Standard Press: Beijing, China, 2018.
- Shi, J.; Wang, D.; Ming, J.; Sun, W. Long-term electrochemical behavior of low-alloy steel in simulated concrete pore solution with chlorides. J. Mater. Civ. Eng. 2018, 304, 04018042. [Google Scholar] [CrossRef]
- Maurice, V.; Marcus, P. Passive films at the nanoscale. Electrochim. Acta 2012, 84, 129–138. [Google Scholar] [CrossRef]
- Jeon, S.; Heo, T.; Hwang, S.-Y.; Ciston, J.; Bustillo, K.C.; Reed, B.W.; Ham, J.; Kang, S.; Kim, S.; Lim, J.; et al. Reversible disorder-order transitions in atomic crystal nucleation. Science 2021, 3716528, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Cheng, X.; Li, X.; Pan, Y.; Li, J. Effect of Cr on the passive film formation mechanism of steel rebar in saturated calcium hydroxide solution. Appl. Surf. Sci. 2016, 389, 1182–1191. [Google Scholar] [CrossRef]
- Koga, G.Y.; Albert, B.; Roche, V.; Nogueira, R.P. On the intrinsic passivating ability of Belite-Ye’elimite-Ferrite towards carbon steel: A straightforward comparison with ordinary Portland cement. Corros. Sci. 2019, 147, 141–151. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Y.; Liu, L.; Pu, Q.; Jiang, L.; Chu, H.; Luo, Y.; Liu, Q.; Cai, H. Use of XPS for quantitative evaluation of tensile-stress-induced degradation of passive film on carbon steel in simulated concrete pore solution. Constr. Build. Mater. 2021, 274, 121779. [Google Scholar] [CrossRef]
- Ghods, P.; Isgor, O.B.; Brown, J.; Bensebaa, F.; Kingston, D. XPS depth profiling study on the passive oxide film of carbon steel in saturated calcium hydroxide solution and the effect of chloride on the film properties. Appl. Surf. Sci. 2011, 25710, 4669–4677. [Google Scholar] [CrossRef]
- Shang, B.; Ma, Y.; Meng, M.; Li, Y. The roles carbonate and bicarbonate ions play on pre-passive HRB400 rebars in simulated pore solutions of deteriorating concrete. Mater. Corros. 2018, 6912, 1800–1810. [Google Scholar] [CrossRef]
- Barroux, A.; Duguet, T.; Ducommun, N.; Nivet, E.; Delgado, J.; Laffont, L.; Blanc, C. Combined XPS/TEM study of the chemical composition and structure of the passive film formed on additive manufactured 17-4PH stainless steel. Surf. Interfaces 2021, 22, 100874. [Google Scholar] [CrossRef]
- You, N.; Shi, J.; Zhang, Y. Electrochemical performance of low-alloy steel and low-carbon steel immersed in the simulated pore solutions of alkali-activated slag/steel slag pastes in the presence of chlorides. Corros. Sci. 2022, 205, 110438. [Google Scholar] [CrossRef]
- Miserque, F.; Huet, B.; Azou, G.; Bendjaballah, D.; L’hostis, V. X-ray photoelectron spectroscopy and electrochemical studies of mild steel FeE500 passivation in concrete simulated water. EDP Sci. 2006, 136, 89–97. [Google Scholar] [CrossRef]
- Dong, Z.H.; Shi, W.; Zhang, G.A.; Guo, X.P. The role of inhibitors on the repassivation of pitting corrosion of carbon steel in synthetic carbonated concrete pore solution. Electrochim. Acta 2011, 5617, 5890–5897. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, J.; Zhang, G.-H.; Fan, X.-H.; Zhang, L. Effect of temperature on the passive film structure and corrosion performance of CoCrFeMoNi high-entropy alloy. Corros. Sci. 2022, 208, 110661. [Google Scholar] [CrossRef]
- Feng, X.; Lu, X.; Zuo, Y.; Zhuang, N.; Chen, D. The effect of deformation on metastable pitting of 304 stainless steel in chloride contaminated concrete pore solution. Corros. Sci. 2016, 103, 223–229. [Google Scholar] [CrossRef]
- Li, L.; Dong, C.; Xiao, K.; Yao, J.; Li, X. Effect of pH on pitting corrosion of stainless steel welds in alkaline salt water. Constr. Build. Mater. 2014, 68, 709–715. [Google Scholar] [CrossRef]
- Hakiki, N.; Montemor, M.; Ferreira, M.; da Cunha Belo, M. Semiconducting properties of thermally grown oxide films on AISI 304 stainless steel. Corros. Sci. 2000, 424, 687–702. [Google Scholar] [CrossRef]
- Hakiki, N.; Boudin, S.; Rondot, B.; Belo, M.D.C. The electronic structure of passive films formed on stainless steels. Corros. Sci. 1995, 3711, 1809–1822. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, X.; Li, X.; Lu, T.J. Corrosion behavior of low-Cr steel rebars in alkaline solutions with different pH in the presence of chlorides. J. Electroanal. Chem. 2017, 803, 40–50. [Google Scholar] [CrossRef]
- Pan, Z.; Luo, H.; Zhao, Q.; Cheng, H.; Wei, Y.; Wang, X.; Zhang, B.; Li, X. Tailoring microstructure and corrosion behavior of CoNiVAlx medium entropy alloys via Al addition. Corros. Sci. 2022, 207, 110570. [Google Scholar] [CrossRef]
- Brug, G.; van den Eeden, A.L.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 5521, 6218–6227. [Google Scholar] [CrossRef]
- Han, S.; Zhang, J.; Lei, X.; Yang, R.; Wang, N. Insight into the anisotropic electrochemical corrosion behaviors of laser metal deposited Ni-based single crystal superalloy. Corros. Sci. 2023, 217, 111111. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, B.; Zhang, J.; Zhang, W.; Zhao, M.; Li, N.; Xiong, M.; Chen, J.; Yu, Y.; Song, S.Y. Influence of Ru-Ni-Nb combined cathode modification on corrosion behavior and passive film characteristics of Ti-6Al-4V Alloy used for oil country tubular goods. Corros. Sci. 2022, 207, 110569. [Google Scholar] [CrossRef]




| Brand | C | Si | Mn | P | S | O | Cr | Fe | Ceq |
|---|---|---|---|---|---|---|---|---|---|
| HRB500E | 0.20 | 0.61 | 1.42 | 0.026 | 0.021 | 0.008 | 0.84 | Bal. | 0.445 |
| Solution | Concentration/mol·L−1 | pH | Potential Values/mV | |||
|---|---|---|---|---|---|---|
| Ca(OH)2 | NaOH | KOH | NaHCO3 | |||
| 1 | saturation | 0.1 | 0.2 | - | 13.5 | −392 |
| 2 | saturation | - | - | 0.014 | 12.5 | −343 |
| 3 | saturation | - | - | 0.036 | 10.5 | −277 |
| Time | pH 13.5 | pH 12.5 | pH 10.5 |
|---|---|---|---|
| 3 d | 2.76 | 2.92 | 3.06 |
| 6 d | 2.91 | 3.18 | 3.41 |
| 10 d | 2.95 | 3.95 | 5.01 |
| Time | Nq/1020 × cm−3 | Na/1020 × cm−3 | EFB/V | ||||
|---|---|---|---|---|---|---|---|
| pH 13.5 | pH 12.5 | pH 10.5 | pH 10.5 | pH 13.5 | pH 12.5 | pH 10.5 | |
| 3 d | 14.16 | 14.65 | 27.12 | 20.01 | −0.65 | −0.79 | −0.56 |
| 6 d | 13.47 | 13.98 | 25.83 | 16.33 | −0.70 | −0.69 | −0.56 |
| 10 d | 12.84 | 13.27 | 22.18 | 12.05 | −0.67 | −0.66 | −0.58 |
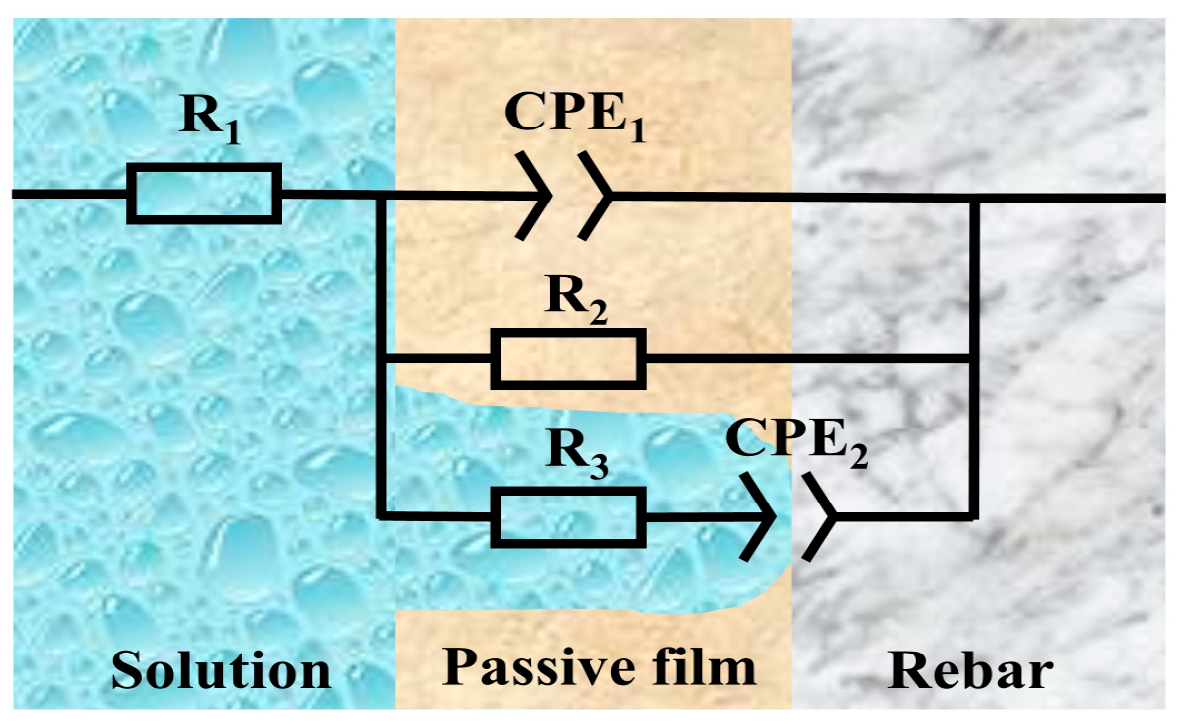
| pH Value | Time | R1 (Ω·cm2) | CPE1 | R2 (Ω·cm2) | R3 (Ω·cm2) | CPE2 | C1 (F·cm−2) | C2 (F·cm−2) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Y1 (Ω−1·cm2·sn) | n1 | Y2 (Ω−1·cm2·sn) | n2 | |||||||
| 13.5 | 3 d | 5.10 | 2.16 × 10−5 | 0.96 | 8.83 × 103 | 7.16 × 105 | 2.27 × 10−5 | 0.98 | 2.06 × 10−5 | 2.14 × 10−5 |
| 6 d | 5.20 | 1.70 × 10−5 | 0.96 | 1.39 × 104 | 1.92 × 106 | 1.76 × 10−5 | 0.96 | 1.60 × 10−5 | 1.27 × 10−5 | |
| 10 d | 5.37 | 1.44 × 10−5 | 0.95 | 2.99 × 104 | 2.16 × 106 | 1.47 × 10−5 | 0.95 | 1.38 × 10−5 | 8.94 × 10−6 | |
| 12.5 | 3 d | 30.91 | 1.36 × 10−5 | 0.94 | 1.06 × 104 | 8.59 × 105 | 6.36 × 10−6 | 0.95 | 1.20 × 10−5 | 4.46 × 10−6 |
| 6 d | 34.57 | 1.22 × 10−5 | 0.95 | 1.60 × 104 | 1.75 × 106 | 5.60 × 10−6 | 0.96 | 1.12 × 10−5 | 3.56 × 10−6 | |
| 10 d | 41.65 | 1.06 × 10−5 | 0.95 | 3.41 × 104 | 2.33 × 106 | 4.62 × 10−6 | 0.95 | 1.00 × 10−5 | 2.94 × 10−6 | |
| 10.5 | 3 d | 34.77 | 6.68 × 10−6 | 0.99 | 1.43 × 104 | 1.10 × 106 | 4.53 × 10−6 | 0.97 | 6.52 × 10−6 | 3.45 × 10−6 |
| 6 d | 79.09 | 5.43 × 10−7 | 0.98 | 2.41 × 104 | 2.09 × 106 | 4.10 × 10−6 | 0.94 | 4.97 × 10−7 | 2.46 × 10−6 | |
| 10 d | 82.63 | 2.59 × 10−7 | 0.94 | 7.25 × 104 | 2.84 × 106 | 2.73 × 10−6 | 0.96 | 2.01 × 10−8 | 1.92 × 10−6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, H.; Gu, S.; Wang, J.; Wei, F.; Xie, X.; Li, Z.; Yang, H.; Zeng, Z.; Li, C. Passivation Behavior of Chromium Alloyed High-Strength Rebar in Simulated Concrete Pore Solution. Metals 2024, 14, 859. https://doi.org/10.3390/met14080859
Bao H, Gu S, Wang J, Wei F, Xie X, Li Z, Yang H, Zeng Z, Li C. Passivation Behavior of Chromium Alloyed High-Strength Rebar in Simulated Concrete Pore Solution. Metals. 2024; 14(8):859. https://doi.org/10.3390/met14080859
Chicago/Turabian StyleBao, Hongxia, Shangjun Gu, Jie Wang, Fulong Wei, Xiang Xie, Zhiying Li, Hui Yang, Zeyun Zeng, and Changrong Li. 2024. "Passivation Behavior of Chromium Alloyed High-Strength Rebar in Simulated Concrete Pore Solution" Metals 14, no. 8: 859. https://doi.org/10.3390/met14080859






