Research Progress in Corrosion Behavior and Anti-Corrosion Methods of Steel Rebar in Concrete
Abstract
:1. Introduction
2. Passivation and Corrosion Mechanisms of Steel Rebars in Concrete
2.1. Passivation of Steel Rebars in Concrete
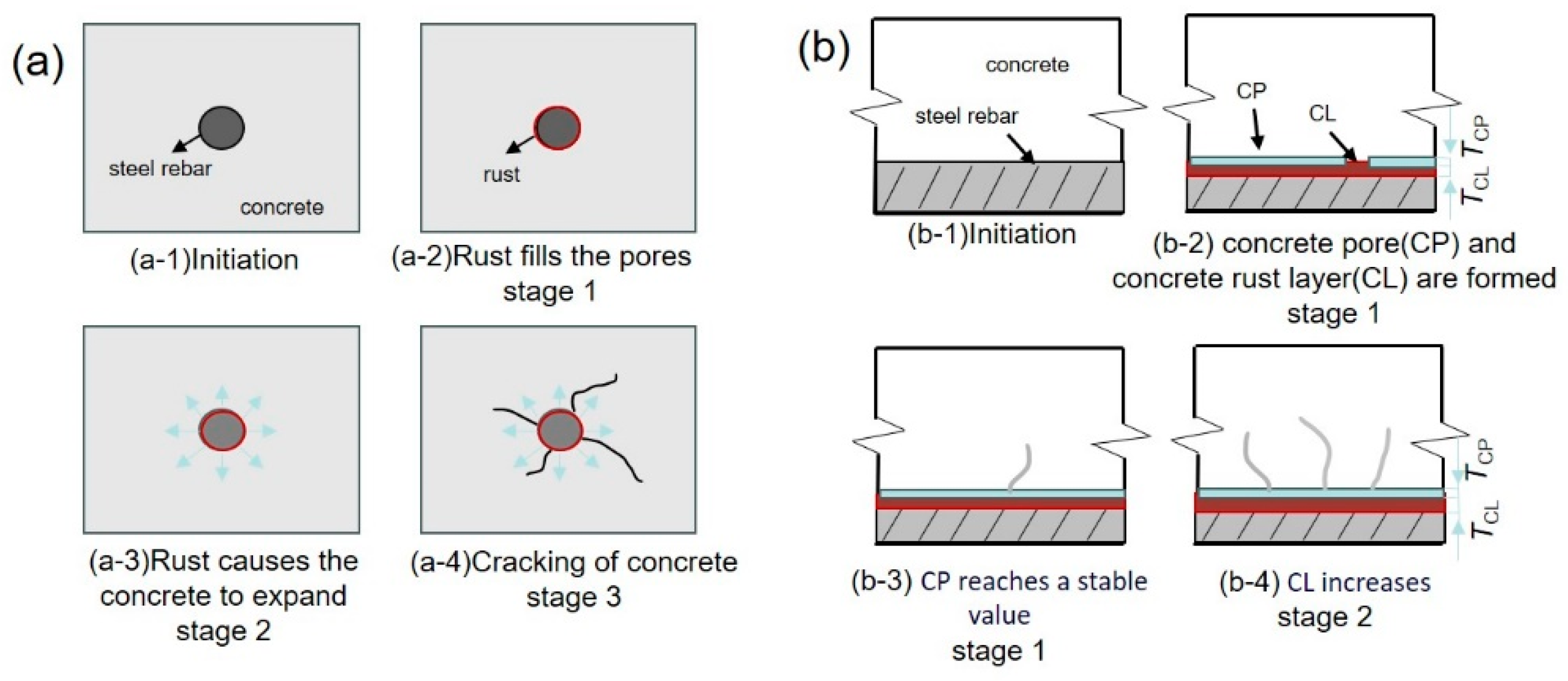
2.2. Corrosion Process of Steel Rebars in Concrete
2.3. The Main Factors Affecting the Corrosion of Steel Rebars in Concrete
2.3.1. Chloride Ion Erosion
2.3.2. Concrete Carbonization
3. Corrosion Experimental Methods for Steel Rebars in Concrete
3.1. Natural Corrosion Test Method
3.2. Artificial Climate Simulation Method
3.3. Electrification Accelerated Corrosion Method
3.4. Salt Spray Corrosion Test Method
3.5. Other Accelerated Corrosion Methods
4. Analysis of Corrosion Products and Interface Characteristics of Steel Rebars by X-CT Technology
4.1. X-CT Technology Principles
4.2. Application of X-CT Technology in Concrete Structures
4.3. Research on Steel Corrosion Products and Interface Characteristics Based on X-CT Technology
- The salutary aspect lies in the fact that as the corrosion of steel intensifies, a substantial quantity of corrosion products is generated on the steel matrix’s surface. Under such circumstances, the interface area, enriched with pores, serves as a repository for these corrosion products, significantly mitigating the expansion stress exerted by them and extending the durability of the concrete protective layer by delaying its cracking;
- The deleterious impact stems from the inadequate Ca(OH)2 protective layer present at the interfacial defects prior to the deterioration of the steel reinforcement’s passivation film. Consequently, the critical concentration of Cl− is significantly increased in these regions compared to other parts, leading to an accelerated erosion by Cl− and an exacerbation of the destructive effects on the passivation film.
5. Methods for Improving the Corrosion Resistance of Steel Rebars in Concrete Structures
5.1. Methods to Improve the Quality of the Concrete Protective Layer
5.1.1. Enhancing the Compactness of the Concrete Protective Layer
5.1.2. Apply a Protective Coating to the Concrete Surface
5.2. Methods to Improve the Corrosion Resistance of Steel Rebars
5.2.1. Apply Surface Coating of Steel Rebars
5.2.2. Alloying Design of Steel Rebars for Corrosion Resistant
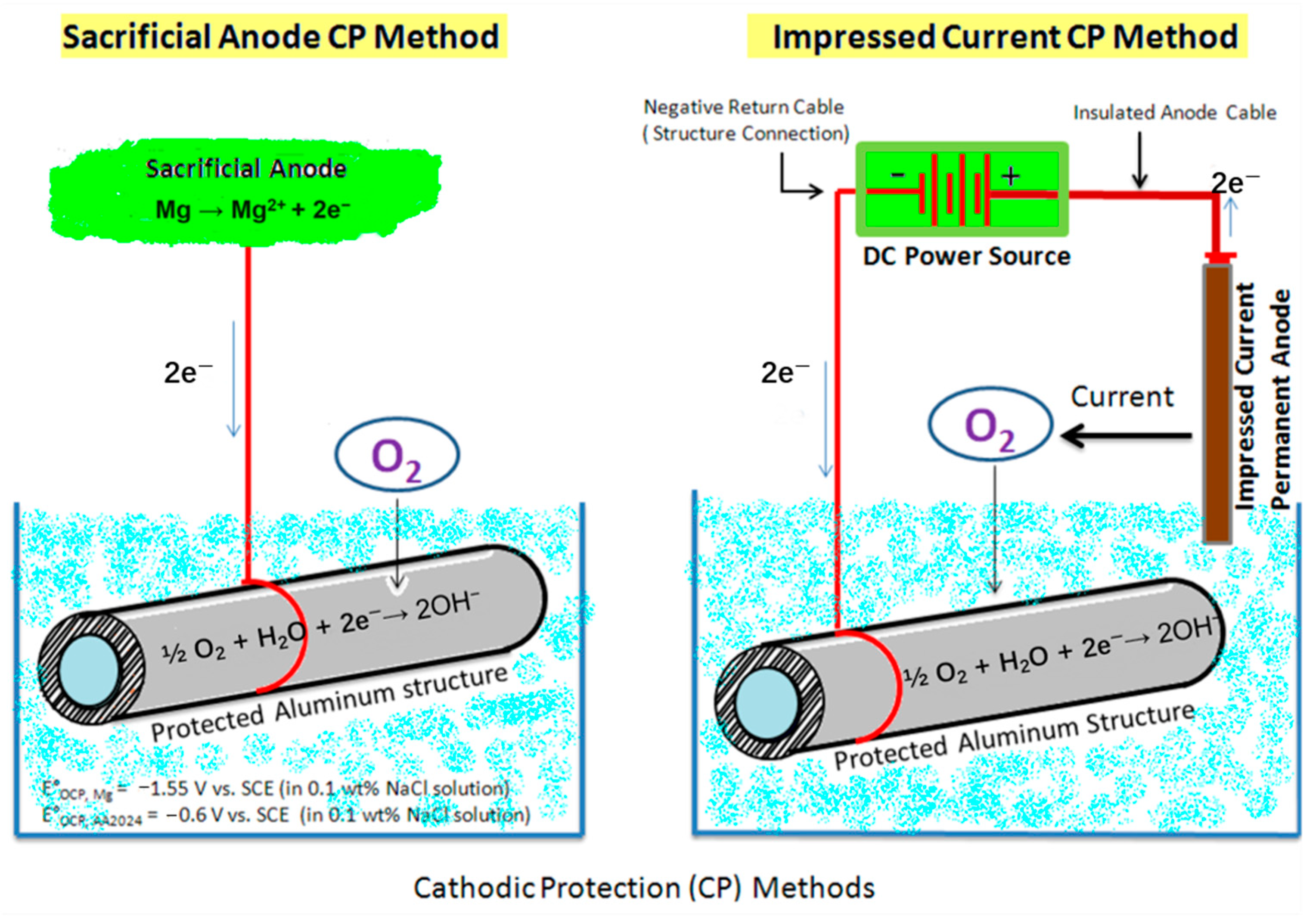
5.2.3. Electrochemical Protection of Steel Rebars
5.3. Methods to Strengthen the Bond between Steel Rebars and Concrete
6. Conclusions and Prospect
- A rigorous and meticulous analysis is undertaken to pinpoint the crucial factors that underlie the corrosion of steel bars embedded within concrete;
- It delves into the diverse methods employed for accelerated corrosion testing of steel bars within concrete, encompassing natural corrosion tests, sophisticated artificial climate simulations, electrically accelerated corrosion techniques, and rigorous salt spray corrosion tests;
- This study provides a comprehensive overview of the current research endeavors focused on steel bar corrosion products and interface characteristics, leveraging cutting-edge X-CT technology;
- This paper proposes strategies to improve reinforced concrete durability, emphasizing the importance of a high-quality concrete cover to shield rebars and the development of alloy-based, corrosion-resistant rebars.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Wang, Q.; Zhao, Y.; Li, P.; Ji, T.; Zou, G.; Qiao, Y.; Zhou, Z.; Wang, G.; Song, D. Research Progress of Macrocell Corrosion of Steel Rebar in Concrete. Coatings 2023, 13, 853. [Google Scholar] [CrossRef]
- Tang, S.W.; Yao, Y.; Andrade, C.; Li, Z.J. Recent Durability Studies on Concrete Structure. Cem. Concr. Res. 2015, 78, 143–154. [Google Scholar] [CrossRef]
- Fu, C.; Jin, N.; Ye, H.; Jin, X.; Dai, W. Corrosion Characteristics of a 4-Year Naturally Corroded Reinforced Concrete Beam with Load-Induced Transverse Cracks. Corros. Sci. 2017, 117, 11–23. [Google Scholar] [CrossRef]
- Cui, Z.; Alipour, A. Concrete Cover Cracking and Service Life Prediction of Reinforced Concrete Structures in Corrosive Environments. Constr. Build. Mater. 2018, 159, 652–671. [Google Scholar] [CrossRef]
- Greve-Dierfeld, S.v.; Bisschop, J.; Schiegg, Y. Nichtrostende Bewehrungsstähle zur Verlängerung der korrosionsfreien Lebensdauer von Stahlbetonbauwerken. Beton Stahlbetonbau 2017, 112, 601–610. [Google Scholar] [CrossRef]
- Gong, W.; Yu, H.; Ma, H.; Zhu, H. A Comprehensive Assessment of Durability and Service Life Prediction of Concrete Structures in the Simulated Tidal Zone of the South China Sea Considering Measures to Enhance Concrete Durability. J. Build. Eng. 2023, 76, 107194. [Google Scholar] [CrossRef]
- Yu, H.; Da, B.; Ma, H.; Zhu, H.; Yu, Q.; Ye, H.; Jing, X. Durability of Concrete Structures in Tropical Atoll Environment. Ocean Eng. 2017, 135, 1–10. [Google Scholar] [CrossRef]
- Da, B.; Yu, H.; Ma, H.; Zhang, Y.; Zhu, H.; Yu, Q.; Ye, H.; Jing, X. Investigation of durability of ordinary concrete structures in the South China Sea. J. Harbin Eng. Univ. 2016, 37, 1034–1040. [Google Scholar] [CrossRef]
- Chen, M.; Wei, Y.; Zheng, H.; Yu, L.; Liu, Q.; Wang, Y.; Yuan, H.; Wang, C.; Li, W. Ca-LDH-Modified Cementitious Coating to Enhance Corrosion Resistance of Steel Bars. J. Build. Eng. 2022, 51, 104301. [Google Scholar] [CrossRef]
- Shang, B.; Ma, Y.; Meng, M.; Li, Y. Feasibility of Utilizing 2304 Duplex Stainless Steel Rebar in Seawater Concrete: Passivation and Corrosion Behavior of Steel Rebar in Simulated Concrete Environments. Mater. Corros. 2019, 70, 1657–1666. [Google Scholar] [CrossRef]
- Wang, N.; Yu, H.; Bi, W.; Zhu, H.; Gong, W.; Diao, Y. Effects of Coral Sand Powder and Corrosion Inhibitors on Reinforcement Corrosion in Coral Aggregate Seawater Concrete in a Marine Environment. Struct. Concr. 2021, 22, 2650–2664. [Google Scholar] [CrossRef]
- Yang, L.; Liu, G.; Gao, D.; Zhang, C. Experimental Study on Water Absorption of Unsaturated Concrete: W/c Ratio, Coarse Aggregate and Saturation Degree. Constr. Build. Mater. 2021, 272, 121945. [Google Scholar] [CrossRef]
- Li, Y.; Aoude, H. Effects of Stainless Steel Reinforcement and Fibers on the Flexural Behaviour of High-Strength Concrete Beams Subjected to Static and Blast Loading. Eng. Struct. 2023, 291, 116398. [Google Scholar] [CrossRef]
- Rabi, M.; Shamass, R.; Cashell, K.A. Structural Performance of Stainless Steel Reinforced Concrete Members: A Review. Constr. Build. Mater. 2022, 325, 126673. [Google Scholar] [CrossRef]
- Nie, R.; Huang, Y.; Li, X.; Sun, H.; Li, D.; Ying, J. Bond of Epoxy-Coated Reinforcement to Seawater Coral Aggregate Concrete. Ocean Eng. 2020, 208, 107350. [Google Scholar] [CrossRef]
- Vijayaraghavan, J.; Jeevakkumar, R.; Venkatesan, G.; Rengasamy, M.; Thivya, J. Influence of Kaolin and Dolomite as Filler on Bond Strength of Polyurethane Coated Reinforcement Concrete. Constr. Build. Mater. 2022, 325, 126675. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.; Zhang, S.; Wu, C.L.; Zhang, C.H.; Sun, X.Y. Corrosion and Cavitation Erosion Behaviors of Laser Clad FeNiCoCr High-Entropy Alloy Coatings with Different Types of TiC Reinforcement. Surf. Coat. Technol. 2023, 471, 129910. [Google Scholar] [CrossRef]
- Wright, J.W.; Pantelides, C.P. Axial Compression Capacity of Concrete Columns Reinforced with Corrosion-Resistant Hybrid Reinforcement. Constr. Build. Mater. 2021, 302, 124209. [Google Scholar] [CrossRef]
- Zou, G.; Wang, Q.; Wang, G.; Liu, W.; Zhang, S.; Ai, Z.; Chen, H.; Ma, H.; Song, D. Revealing Excellent Passivation Performance of a Novel Cr-Alloyed Steel Rebar in Carbonized Concrete Environment. J. Mater. Res. Technol. 2023, 23, 1848–1861. [Google Scholar] [CrossRef]
- Dong, B.; Liu, W.; Zhang, T.; Chen, L.; Fan, Y.; Zhao, Y.; Yang, W.; Banthukul, W. Corrosion Failure Analysis of Low Alloy Steel and Carbon Steel Rebar in Tropical Marine Atmospheric Environment: Outdoor Exposure and Indoor Test. Eng. Fail. Anal. 2021, 129, 105720. [Google Scholar] [CrossRef]
- Zhang, Z. Influence of Threshold Chloride Concentration on Corrosion Behavior of Low-Alloy Steel Rebar in Concrete Pore Solutions. Int. J. Electrochem. Sci. 2020, 15, 9864–9873. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, X.; Li, X.; Zhou, C.; Tan, H. Effect of Carbonation on the Electrochemical Behavior of Corrosion Resistance Low Alloy Steel Rebars in Cement Extract Solution. Constr. Build. Mater. 2017, 130, 193–201. [Google Scholar] [CrossRef]
- Yoon, H.N.; Seo, J.; Kim, S.; Lee, H.K.; Park, S. Hydration of Calcium Sulfoaluminate Cement Blended with Blast-Furnace Slag. Constr. Build. Mater. 2021, 268, 121214. [Google Scholar] [CrossRef]
- He, Y.; Mao, R.; Lü, L.; Hu, S. Hydration Products of Cement-Silica Fume-Quartz Powder Mixture under Different Curing Regimes. J. Wuhan Univ. Technol. Mat. Sci. Edit. 2017, 32, 598–602. [Google Scholar] [CrossRef]
- Shang, B.; Ma, Y.; Meng, M.; Li, Y. Characterisation of Passive Film on HRB400 Steel Rebar in Curing Stage of Concrete. Chin. J. Mater. Res. 2019, 33, 659–665. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, Q.; Yuan, Y.F.; Guo, S.Y.; Pan, J. Passivation Behavior of 2507 Super Duplex Stainless Steel in Simulated Concrete Pore Solution. J. Mater. Eng Perform 2020, 29, 3141–3151. [Google Scholar] [CrossRef]
- Montemor, M.F.; Simões, A.M.P.; Ferreira, M.G.S. Chloride-Induced Corrosion on Reinforcing Steel: From the Fundamentals to the Monitoring Techniques. Cem. Concr. Compos. 2003, 25, 491–502. [Google Scholar] [CrossRef]
- Ahmad, S. Reinforcement Corrosion in Concrete Structures, Its Monitoring and Service Life Prediction—A Review. Cem. Concr. Compos. 2003, 25, 459–471. [Google Scholar] [CrossRef]
- Angst, U.M.; Geiker, M.R.; Michel, A.; Gehlen, C.; Wong, H.; Isgor, O.B.; Elsener, B.; Hansson, C.M.; François, R.; Hornbostel, K.; et al. The steel-concrete interface. Mater. Struct. 2017, 50, 143. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, X.; Yang, L.; Wang, X.; Chen, J.; Wang, Z.; Zhou, H.; Zou, J.; Wang, F. Effect of Aging Treatment on Microstructure and Corrosion Behavior of a Fe-18Cr-15Mn-0.66N Stainless Steel. J. Mater. Sci. Technol. 2022, 107, 197–206. [Google Scholar] [CrossRef]
- Liu, Y.; Weyers, R.E. Modeling the Time-to-Corrosion Cracking in Chloride Contaminated Reinforced Concrete Structures. Materials 1998, 95, 675–681. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, Y.X.; Xia, J. Experimental study and numerical simulation of corrosion-induced crack patterns of concrete structures. J. Build. Struct. 2019, 40, 138–145. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.; Gao, P.; Yuan, Y. Experimental Study on the Corrosion Characteristics of Steel Bars in Concrete Considering the Effects of Multiple Factors. Case Stud. Constr. Mater. 2024, 20, e02706. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Wang, X.; Tang, H. Effects of the Position and Chloride-Induced Corrosion of Strand on Bonding Behavior between the Steel Strand and Concrete. Structures 2023, 58, 105500. [Google Scholar] [CrossRef]
- Xiao, S.-H.; Cai, Y.-J.; Xie, Z.-H.; Guo, Y.-C.; Zheng, Y.; Lin, J.-X. Bond Durability between Steel-FRP Composite Bars and Concrete under Seawater Corrosion Environments. Constr. Build. Mater. 2024, 419, 135456. [Google Scholar] [CrossRef]
- Liu, C.; Lv, Z.; Xiao, J.; Xu, X.; Nong, X.; Liu, H. On the Mechanism of Cl− Diffusion Transport in Self-Healing Concrete Based on Recycled Coarse Aggregates as Microbial Carriers. Cem. Concr. Compos. 2021, 124, 104232. [Google Scholar] [CrossRef]
- Han, P.; Qiao, G.; Ou, J. Corrosion Kinetic Characteristics of Carbon Steel in Thermodynamically Determined Simulated Concrete Pore Solutions Driven by the Coupling Action of Cl− and CO2, as Well as Alternating Polarization. Constr. Build. Mater. 2023, 398, 132506. [Google Scholar] [CrossRef]
- Pan, D.; Niu, D.; Li, Z. Corrosion Products of Low-Alloy Steel Bars and Their Induction of Cracking in Seawater Sea-Sand Concrete Cover. Constr. Build. Mater. 2023, 389, 131800. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Wang, Y.; Wang, D.; Yuan, C.; Liu, R. Effect of Recycled Aggregate and Supplementary Cementitious Material on the Chloride Threshold for Steel Bar Corrosion in Concrete. Constr. Build. Mater. 2022, 346, 128418. [Google Scholar] [CrossRef]
- Gong, K.; Yang, M.; Liu, C.; Shen, X.; Xiao, L.; Li, M.; Mao, F. Synergistic Effect of Chloride Ions and Surface Film on Depassivation Mechanism of Q355B Steel in Simulated Concrete Pore Solution. J. Build. Eng. 2023, 78, 107742. [Google Scholar] [CrossRef]
- Ji, H.; Tian, Y.; Fu, C.; Ye, H. Transfer Learning Enables Prediction of Steel Corrosion in Concrete under Natural Environments. Cem. Concr. Compos. 2024, 148, 105488. [Google Scholar] [CrossRef]
- Long, H.; Chen, L.; Dong, B.; Sun, Y.; Yan, Y.; Chen, C. The Electronic Properties and Surface Chemistry of Passive Film on Reinforcement: Effect of Composition of Simulated Concrete Pore Solution. Constr. Build. Mater. 2022, 360, 129567. [Google Scholar] [CrossRef]
- Ogunsanya, I.G.; Hansson, C.M. Influence of Chloride and Sulphate Anions on the Electronic and Electrochemical Properties of Passive Films Formed on Steel Reinforcing Bars. Materialia 2019, 8, 100491. [Google Scholar] [CrossRef]
- Saremi, M.; Mahallati, E. A Study on Chloride-Induced Depassivation of Mild Steel in Simulated Concrete Pore Solution. Cem. Concr. Res. 2002, 32, 1915–1921. [Google Scholar] [CrossRef]
- Martin, F.J.; Olek, J. Experimental Procedure for Fundamental Studies of Reinforcing Steel Corrosion Processes. Rev. Sci. Instrum. 2003, 74, 2512–2516. [Google Scholar] [CrossRef]
- Shi, J.; Li, M.; Wu, M.; Ming, J. Role of Red Mud in Natural Passivation and Chloride-Induced Depassivation of Reinforcing Steels in Alkaline Concrete Pore Solutions. Corros. Sci. 2021, 190, 109669. [Google Scholar] [CrossRef]
- Sanjuán, M.A.; Andrade, C.; Cheyrezy, M. Concrete carbonation tests in natural and accelerated conditions. Adv. Cem. Res. 2003, 15, 171–180. [Google Scholar] [CrossRef]
- Xie, M.; Dangla, P.; Li, K. Reactive transport modelling of concurrent chloride ingress and carbonation in concrete. Mater. Struct. 2021, 54, 177. [Google Scholar] [CrossRef]
- Al-Sulaimani, G.J.; Kaleemullah, M.; Basunbul, I.A.; Rasheeduzzafar. Influence of Corrosion and Cracking on Bond Behavior and Strength of Reinforced Concrete Members. ACI Struct. J. 1990, 87, 220–231. [Google Scholar]
- Okada, K.; KobayashiI, K.; Miyagawa, T. Influence of Longitudinal Cracking Due to Reinforcement Corrosion on Characteristics of Reinforced Concrete Members. Struct. J. 1988, 85, 134–140. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Y.; Liu, L.; Pu, Q.; Jiang, L.; Chu, H.; Luo, Y.; Liu, Q.; Cai, H. Use of XPS for Quantitative Evaluation of Tensile-Stress-Induced Degradation of Passive Film on Carbon Steel in Simulated Concrete Pore Solution. Constr. Build. Mater. 2021, 274, 121779. [Google Scholar] [CrossRef]
- Jiang, J.; Yuan, Y. Relationship of Moisture Content with Temperature and Relative Humidity in Concrete. Mag. Concr. Res. 2013, 65, 685–692. [Google Scholar] [CrossRef]
- Ye, H.; Fu, C.; Jin, N.; Jin, X. Performance of reinforced concrete beams corroded under sustained service loads: A comparative study of two accelerated corrosion techniques. Constr. Build. Mater. 2018, 162, 286–297. [Google Scholar] [CrossRef]
- Feng, W.; Tarakbay, A.; Memon, S.A.; Tang, W.; Cui, H. Methods of accelerating chloride-induced corrosion in steel-reinforced concrete: A comparative review. Constr. Build. Mater. 2021, 289, 123165. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Hao, J.; Zou, G.; Zhang, P.; Wang, G.; Ai, Z.; Chen, H.; Ma, H.; Song, D. Corrosion Resistance and Corrosion Interface Characteristics of Cr-Alloyed Rebar Based on Accelerated Corrosion Testing with Impressed Current. J. Mater. Res. Technol. 2023, 22, 2996–3009. [Google Scholar] [CrossRef]
- Ramírez-Arreola, D.; de Guadalajara, U.; Aranda-García, F.; Sedano-De-La-Rosa, C.; Camacho-Vidrio, A.; Silva, R. Corrosion behavior of steel reinforcement bars embedded in concrete with sugar cane bagasse ash. Rev. Mex. De Ing. Quim. 2020, 19, 469–481. [Google Scholar] [CrossRef]
- Raupach, M. Corrosion of Steel Reinforcement in Concrete. Mater. Corros. 2009, 60, 77. [Google Scholar] [CrossRef]
- Okeniyi, J.; Loto, C.; Popoola, P. Effects of Phyllanthus muellerianus Leaf-Extract on Steel-Reinforcement Corrosion in 3.5% NaCl-Immersed Concrete. Met. Open Access Metall. J. 2016, 6, 255. [Google Scholar] [CrossRef]
- Okeniyi, J.O.; Loto, C.A.; Popoola, A.P.I. Corrosion Inhibition of Concrete Steel-Reinforcement in Saline/Marine Simulating-Environment by Rhizophora mangle L. Solid State Phenom. 2015, 227, 185–189. [Google Scholar] [CrossRef]
- Papadopoulos, M.P.; Apostolopoulos, C.A.; Zervaki, A.D.; Haidemenopoulos, G.N. Corrosion of exposed rebars, associated mechanical degradation and correlation with accelerated corrosion tests. Constr. Build. Mater. 2011, 25, 3367–3374. [Google Scholar] [CrossRef]
- Nie, S.-J.; Yi, X.-N.; Zhou, H.-L.; Zhu, H.-J.; Yang, L.-L.; Fu, F.-L.; Li, J.-Y.; Yang, H.-K.; Xu, G.-X.; Lu, S.; et al. Corrosion behavior of as-cast Al0.75CoFeCr1.25Ni high entropy alloy in 0.5 mol/L NaOH solution. Iron Steel Res. Int. 2024, 37, 175–223. [Google Scholar] [CrossRef]
- Ren, Q.; Jin, H.; Xiao, S.; Wang, F.; Chen, B. Review on long-term performance of reinforced concrete structures under simulated acid rain erosion environments. J. Traffic Transp. Eng. 2022, 22, 41–47. [Google Scholar] [CrossRef]
- Zhao, G.; Ma, Y.; Li, Y.; Liang, J.; Wang, K.; Zhou, F. An experimental study on the effect of marine corrosion on frictional properties of sliding pairs of offshore isolated bridges. In Proceedings of the 2018 7th International Conference on Energy and Environmental Protection (ICEEP 2018), Shenzhen, China, 14–15 July 2018. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; You, Z.; Yang, X. Compaction Characteristics of Asphalt Mixture with Different Gradation Type through Superpave Gyratory Compaction and X-ray CT Scanning. Constr. Build. Mater. 2016, 129, 243–255. [Google Scholar] [CrossRef]
- Chen, X.; Ren, D.; Xu, J.; Tian, G.; Jiang, S.; Huang, M.; Zhang, A.A.; Ai, C. Investigation on Mesoscopic Pore Characteristics in Asphalt Mixtures under the Coupling Effects of Segregation-Dynamic Pore Water-Salt Corrosion Based on CT Scanning Technology. Constr. Build. Mater. 2023, 404, 133041. [Google Scholar] [CrossRef]
- Morgan, I.L.; Ellinger, H.; Klinksiek, R.; Thomson, J.N. Examination of Concrete by Computerized Tomography. J. Am. Concr. Inst. 1980, 77, 23–27. [Google Scholar]
- Lawler, J.S.; Keane, D.T.; Shah, S.P. Measuring three-dimensional damage in concrete under compression. ACI Mater. J. 2001, 98, 465–475. [Google Scholar] [CrossRef]
- Houqun, C.; Shengxin, W.; Faning, D. Chapter 14—Testing Research on Large Dam Concrete Dynamic-Static Damage and Failure Based on CT Technology. In Seismic Safety of High Arch Dams; Academic Press: Oxford, UK, 2016; pp. 395–489. ISBN 978-0-12-803628-0. [Google Scholar]
- Tian, W.; Han, N. Evaluation of Meso-damage Processes in Concrete by X-ray CT Scanning Techniques under Real-Time Uniaxial Compression Testing. J. Nondestruct. Eval. 2019, 38, 44. [Google Scholar] [CrossRef]
- Glass, G.K.; Yang, R.; Dickhaus, T.; Buenfeld, N.R. Backscattered Electron Imaging of the Steel–Concrete Interface. Corros. Sci. 2001, 43, 605–610. [Google Scholar] [CrossRef]
- Page, C.L. Initiation of Chloride-induced Corrosion of Steel in Concrete: Role of the Interfacial Zone. Mater. Corros. 2009, 60, 586–592. [Google Scholar] [CrossRef]
- Mohammed, T.; Otsuki, N.; Hamada, H.; Yamaji, T. Chloride-Induced Corrosion of Steel Bars in Concrete with the Presence of Gap at the Steel-concrete Interface. ACI Mater. J. 2002, 99, 149–156. [Google Scholar]
- Zhang, R.; Castel, A.; François, R. The Corrosion Pattern of Reinforcement and Its Influence on Serviceability of Reinforced Concrete Members in Chloride Environment. Cem. Concr. Res. 2009, 39, 1077–1086. [Google Scholar] [CrossRef]
- Angst, U.M.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Chloride Induced Reinforcement Corrosion: Electrochemical Monitoring of Initiation Stage and Chloride Threshold Values. Corros. Sci. 2011, 53, 1451–1464. [Google Scholar] [CrossRef]
- Wong, H.S.; Zhao, Y.X.; Karimi, A.R.; Buenfeld, N.R.; Jin, W.L. On the Penetration of Corrosion Products from Reinforcing Steel into Concrete Due to Chloride-Induced Corrosion. Corros. Sci. 2010, 52, 2469–2480. [Google Scholar] [CrossRef]
- Chernin, L.; Val, D.V. Prediction of Corrosion-Induced Cover Cracking in Reinforced Concrete Structures. Constr. Build. Mater. 2011, 25, 1854–1869. [Google Scholar] [CrossRef]
- Beck, M.; Goebbels, J.; Burkert, A. Application of X-ray Tomography for the Verification of Corrosion Processes in Chloride Contaminated Mortar. Mater. Corros. 2007, 58, 207–210. [Google Scholar] [CrossRef]
- Itty, P.-A.; Serdar, M.; Meral, C.; Parkinson, D.; MacDowell, A.A.; Bjegović, D.; Monteiro, P.J.M. In Situ 3D Monitoring of Corrosion on Carbon Steel and Ferritic Stainless Steel Embedded in Cement Paste. Corros. Sci. 2014, 83, 409–418. [Google Scholar] [CrossRef]
- Česen, A.; Kosec, T.; Legat, A. Characterization of Steel Corrosion in Mortar by Various Electrochemical and Physical Techniques. Corros. Sci. 2013, 75, 47–57. [Google Scholar] [CrossRef]
- Šavija, B.; Luković, M.; Hosseini, S.A.S.; Pacheco, J.; Schlangen, E. Corrosion Induced Cover Cracking Studied by X-ray Computed Tomography, Nanoindentation, and Energy Dispersive X-ray Spectrometry (EDS). Mater. Struct. 2015, 48, 2043–2062. [Google Scholar] [CrossRef]
- Dong, B.; Fang, G.; Liu, Y.; Dong, P.; Zhang, J.; Xing, F.; Hong, S. Monitoring Reinforcement Corrosion and Corrosion-Induced Cracking by X-ray Microcomputed Tomography Method. Cem. Concr. Res. 2017, 100, 311–321. [Google Scholar] [CrossRef]
- Michel, A.; Pease, B.J.; Peterová, A.; Geiker, M.R.; Stang, H.; Thybo, A.E.A. Penetration of Corrosion Products and Corrosion-Induced Cracking in Reinforced Cementitious Materials: Experimental Investigations and Numerical Simulations. Cem. Concr. Compos. 2014, 47, 75–86. [Google Scholar] [CrossRef]
- Li, L.G.; Feng, J.-J.; Zhu, J.; Chu, S.-H.; Kwan, A.K.H. Pervious concrete: Effects of porosity on permeability and strength. Mag. Concr. Res. 2021, 73, 69–79. [Google Scholar] [CrossRef]
- Khoury, G.A. Effect of fire on concrete and concrete structures. Prog. Struct. Eng. Mater. 2000, 2, 429–447. [Google Scholar] [CrossRef]
- Mohammed, H.; Ahmed, H.; Kurda, R.; Alyousef, R.; Deifalla, A.F. Heat-Induced Spalling of Concrete: A Review of the Influencing Factors and Their Importance to the Phenomenon. Materials 2022, 15, 1693. [Google Scholar] [CrossRef]
- Bian, P.; Zhang, M.; Yu, Q.; Zhan, B.; Gao, P.; Guo, B.; Chen, Y. Prediction model of compressive strength of foamed concrete considering pore size distribution. Constr. Build. Mater. 2023, 409, 133705. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Bai, R.; Liu, S.; Yan, C.; Zhang, J. Evolution of the pore structure of pumice aggregate concrete and the effect on compressive strength. Rev. Adv. Mater. Sci. 2023, 62, 20230112. [Google Scholar] [CrossRef]
- Aquino, C.; Inoue, M.; Miura, H.; Mizuta, M.; Okamoto, T. The effects of limestone aggregate on concrete properties. Constr. Build. Mater. 2010, 24, 2363–2368. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, H.; Chen, X.; Li, X.; Zhou, S. Influence of aggregate variety on pore structure and microscopic interface of concrete. J. Build. Mater. 2015, 18, 133–138. [Google Scholar] [CrossRef]
- Vishalakshi, K.; Revathi, V.; Reddy, S.S. Effect of type of coarse aggregate on the strength properties and fracture energy of normal and high strength concrete. Eng. Fract. Mech. 2018, 194, 52–60. [Google Scholar] [CrossRef]
- Ichino, H.; Kuwahara, N.; Beppu, M.; Williamson, E.B.; Himi, A. Effects of the shape, size, and surface roughness of glass coarse aggregate on the mechanical properties of two-stage concrete. Constr. Build. Mater. 2024, 411, 134296. [Google Scholar] [CrossRef]
- Wang, S.; Xia, P.; Gong, F.; Zeng, Q.; Chen, K.; Zhao, Y. Multi objective optimization of recycled aggregate concrete based on explainable machine learning. J. Clean. Prod. 2024, 445, 141045. [Google Scholar] [CrossRef]
- Sarcinella, A.; Frigione, M. Sustainable and Bio-Based Coatings as Actual or Potential Treatments to Protect and Preserve Concrete. Coatings 2023, 13, 44. [Google Scholar] [CrossRef]
- Adeboye, S.; Adebowale, A.; Siyanbola, T.; Ajanaku, K. Coatings and the environment: A review of problems, progress and prospects. IOP Conf. Ser. Earth Environ. Sci. 2023, 1197, 012012. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.-C.; Li, N. A Review on Concrete Surface Treatment Part I: Types and Mechanisms. Constr. Build. Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Mao, Y.-Z.; Wei, Y.-H.; Zhao, H.-T.; Lv, C.-X.; Cao, H.-J.; Li, J. Corrosion Behavior of Epoxy-Coated Rebar with Pinhole Defect in Seawater Concrete. Acta Metall. Sin. 2018, 31, 1171–1182. [Google Scholar] [CrossRef]
- Al-Negheimish, A.; Hussain, R.R.; Alhozaimy, A.; Singh, D. Corrosion performance of hot-dip galvanized zinc-aluminum coated steel rebars in comparison to the conventional pure zinc coated rebars in concrete environment. Constr. Build. Mater. 2021, 274, 121921. [Google Scholar] [CrossRef]
- Maldonado, L.; Pech-Canul, M.; Alhassan, S. Corrosion of zinc-coated reinforcing bars in tropical humid marine environments. Anti-Corros. Methods Mater. 2006, 53, 357–361. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Ureasilicate Hybrid Coatings for Corrosion Protection of Galvanized Steel in Chloride-Contaminated Simulated Concrete Pore Solution. J. Electrochem. Soc. 2015, 162, C666. [Google Scholar] [CrossRef]
- Singh, D.; Ghosh, R. Molybdenum–phosphorus compounds based passivator to control corrosion of hot dip galvanized coated rebars exposed in simulated concrete pore solution. Surf. Coat. Technol. 2008, 202, 4687–4701. [Google Scholar] [CrossRef]
- Lau, K.; Sagüés, A.A. Corrosion of Epoxy- and Polymer/Zinc-Coated Rebar in Simulated Concrete Pore Solution. Corrosion 2009, 65, 681–694. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Q.; Tang, F.; Pan, H.; Chen, X.; Lin, Z. Mechanical Properties and Corrosion Behavior of Dual-Filler-Epoxy-Coated Steel Rebar under a Corrosive Environment. Coatings 2023, 13, 604. [Google Scholar] [CrossRef]
- Thomas, D.; Reshmy, R.; Eapen, P.; Sindhu, R.; Ulaeto, S.B.; Pugazhendhi, A.; Awasthi, M.K. Developments in smart organic coatings for anticorrosion applications: A review. Biomass Conv. Bioref. 2022, 12, 4683–4699. [Google Scholar] [CrossRef]
- Zhang, J.; Zuo, L.; Jiang, J.; Ma, H.; Song, D. Microstructure and Properties of Seawater Corrosion Resistant Rebar Steel 00Cr10MoV. J. Chin. Soc. Corros. Prot. 2016, 36, 363–369. [Google Scholar] [CrossRef]
- Araujo, L.A.A.; Arcilla, I.H.; Ramos, N.R.R.; Martín, J.E.T.M.; Chinchón-Payá, S.C.-P.; Montero, J.S.M.; Raggiotti, B.B.R.; Matres, V.M. Corrosion Initiation Period for Stainless Steel Reinforcement in Concrete Structures. In Proceedings of the XVI International Conference on Durability of Building Materials and Components, Beijing, China, 10–13 October 2023. [Google Scholar] [CrossRef]
- Hurley, M.F.; Scully, J.R. Threshold Chloride Concentrations of Selected Corrosion-Resistant Rebar Materials Compared to Carbon Steel. Corrosion 2006, 62, 892–904. [Google Scholar] [CrossRef]
- Mohamed, N.; Boulfiza, M.; Evitts, R. Corrosion of Carbon Steel and Corrosion-Resistant Rebars in Concrete Structures under Chloride Ion Attack. J. Mater. Eng. Perform. 2013, 22, 787–795. [Google Scholar] [CrossRef]
- Shi, J.; Sun, W.; Jiang, J.; Zhang, Y. Influence of Chloride Concentration and Pre-Passivation on the Pitting Corrosion Resistance of Low-Alloy Reinforcing Steel in Simulated Concrete Pore Solution. Constr. Build. Mater. 2016, 111, 805–813. [Google Scholar] [CrossRef]
- Pathak, S.S.; Mendon, S.K.; Blanton, M.D.; Rawlins, J.W. Magnesium-Based Sacrificial Anode Cathodic Protection Coatings (Mg-Rich Primers) for Aluminum Alloys. Metals 2012, 2, 353–376. [Google Scholar] [CrossRef]
- Chiriatti, L.; Mercado-Mendoza, H.; Apedo, K.L.; Fond, C.; Feugeas, F. A study of bond between steel rebar and concrete under a friction-based approach. Cem. Concr. Res. 2019, 120, 132–141. [Google Scholar] [CrossRef]
- Hachem, Y.; Ezzedine El Dandachy, M.; Khatib, J.M. Physical, Mechanical and Transfer Properties at the Steel-Concrete Interface: A Review. Buildings 2023, 13, 886. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Wang, Z.; Zhao, T.; Wang, Z. A review of the interfacial transition zones in concrete: Identification, physical characteristics, and mechanical properties. Eng. Fract. Mech. 2024, 300, 109979. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, S.; Sun, S. Determination of the Corrosion Rate of Steel Bars in Concrete Based on the Porosity of Interfacial Zone. Front. Mater. 2020, 7, 573193. [Google Scholar] [CrossRef]
- Li, N.; Ma, B.; Wang, Y.; Si, W.; Qin, F. Influence analyses of mixing approaches on properties of conventional and interlocking-dense concrete. Constr. Build. Mater. 2016, 122, 465–472. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Q.; Ding, H.; Leng, S.; Cui, H.; Xu, B.; Cui, H. Investigation of Interfacial Bonding Properties of Polyurethane Concrete and Cement Concrete/Steel Reinforcement. Adv. Mater. Sci. Eng. 2022, 2022, 5644468. [Google Scholar] [CrossRef]
- Kumar, K.; Das, S.; Garg, R.; Goyat, M.S. A Comprehensive Review on Enhancing the Strength of CFRPs through Nano-reinforcements: Applications, Characterization, and Challenges. J. Fail. Anal. Preven. 2024. [Google Scholar] [CrossRef]
- Bahmani, H.; Mostofinejad, D. Microstructure of ultra-high-performance concrete (UHPC)—A review study. J. Build. Eng. 2022, 50, 104118. [Google Scholar] [CrossRef]
- Zhang, X.; Akber, M.Z.; Zheng, W. Prediction of seven-day compressive strength of field concrete. Constr. Build. Mater. 2021, 305, 124604. [Google Scholar] [CrossRef]
- Han, X.-Y.; Wu, Z.-M.; Jia, M.-D.; Zheng, J.-J.; Yu, R.C. A new method for determining the tension-softening curve of concrete. Theor. Appl. Fract. Mech. 2023, 126, 103992. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Wang, Z.; Li, C.; Qiao, X.; Guan, H.; Zhou, Z.; Song, D. Research Progress in Corrosion Behavior and Anti-Corrosion Methods of Steel Rebar in Concrete. Metals 2024, 14, 862. https://doi.org/10.3390/met14080862
Wang Q, Wang Z, Li C, Qiao X, Guan H, Zhou Z, Song D. Research Progress in Corrosion Behavior and Anti-Corrosion Methods of Steel Rebar in Concrete. Metals. 2024; 14(8):862. https://doi.org/10.3390/met14080862
Chicago/Turabian StyleWang, Qiuyue, Zilong Wang, Chengtao Li, Xinglong Qiao, Hao Guan, Zhou Zhou, and Dan Song. 2024. "Research Progress in Corrosion Behavior and Anti-Corrosion Methods of Steel Rebar in Concrete" Metals 14, no. 8: 862. https://doi.org/10.3390/met14080862





