Abstract
In this paper, the microstructure of an alloy was regulated by means of strengthening solution aging, and microstructure observation and composition analysis were carried out by means of an optical microscope and X-ray diffractometer. Combined with the Vickers hardness tester, electronic universal testing machine and high-temperature persistent creep testing machine, the mechanical properties and high-temperature properties of the alloy were tested, and the strengthening mechanism of the alloy was explored. The results showed that the dendritic morphology and structure of the alloy decreased with an increase in temperature during the solution process, and the γ′ phase morphology also changed with the solution temperature: oval → cross → cubic. The γ′ phase after solid solution at 1295 °C was closest to the cubic form. Therefore, it is believed that the 1295 °C solution treatment had the best effect. In the aging process, the uniform cubic degree of γ′ phase distribution was the highest at 1090 °C. On the basis of fixed aging temperature (1090 °C), it was found that the volume fraction of the γ′ phase increased significantly after 8 h. The γ′ phase, which was closest to the cubic form, had the largest proportion of precipitation, and the volume fraction increased to 70.3%. The minimum carbide volume was 1.0%. The hardness of the alloy reached 435.8 HV; the yield strength increased to 280.1 MPa; and the durability of the alloy under the conditions of 1000 °C/230 MPa and 870 °C/655 MPa was 99.7 h and 42.7 h, respectively, which achieved the purpose of alloy design.
1. Introduction
Nickel-based superalloys are widely used in aerospace engines and automotive supercharged turbines due to their excellent high-temperature mechanical properties, corrosion resistance and oxidation resistance [1,2,3]. Their excellent performance comes from the melting of many refractory metals, and Co is one of the main alloying elements of nickel-based superalloys [4]. However, excessive Co element will cause the alloy to exhibit hot cracking behavior during the casting process, thus reducing the high-temperature performance of the alloy; therefore, a low-cobalt nickel-based superalloy with Hf and Ta replacing the Co element is used to ensure the high-temperature performance of the alloy. Hf exists in the enriched form in the alloy; all Hf-rich phases are the last ones to solidify; and the melt has excellent fluidity and is easy to cast. The segregation of the Ta element in the γ′ phase can improve the high-temperature strength and stability of the γ′ phase and also increase the mismatch degree of the γ/γ′ phase [5,6,7,8].
The basic composition of nickel-based superalloys involves two main phases: one is the γ phase [9], which acts as the base, and the other is the γ′ phase [10], which provides the strengthening effect. The phase, as the matrix, has an external centrocubic (FCC) crystal structure [11]. This phase can dissolve a large number of alloying elements, significantly affecting the overall properties of the alloy. On the other hand, the γ′ phase, as a strengthened phase, has an L12 ordered structure, as well as a face-centered cubic arrangement, and is mainly composed of Ni3Al [12]. This special arrangement of atoms provides the alloy with high strength and excellent thermal stability and is a key factor in the ability of nickel-based superalloys to maintain high performance at high temperatures [13].
Solid solution treatment can improve the properties of the alloy by adjusting the size of the γ′ phase and eliminating the eutectic structure [14]. The microstructure and properties of the alloy are affected by the solution temperature, time and cooling rate. Wan [15] studied the effect of different solution temperatures on the microstructure and mechanical properties of forged U720LI superalloy using scanning electron microscopy and the tensile test. The results showed that higher solution treatment temperature would lead to grain coarsing and secondary γ′ phase precipitation, thus reducing the strength and ductility of the alloy. Li [16] studied the effect of solution time on the microstructure and distribution of Re and Ru elements in nickel-based single-crystal superalloy. When the solution time is as long as 20 h, the dendrite segregation in the alloy is much improved; the cubic degree of the γ′ phase is better; and the distribution of Ru and Re elements tends to be uniform. After aging heat treatment followed by solid solution treatment, the purpose is to uniformly precipitate the γ′ phase and regulate its size, shape and composition; therefore, aging is divided into single and multiple treatments [17]. Baldan R [18] studied and compared the microstructure of the alloy under different temperatures and regimes. When the material was aged in a single stage at 770 °C and 870 °C, the γ′ phase particles were coarser due to Al segregation. After bipolar aging at 980 °C for 300 min and 870 °C for 1200 min, the γ′ phase was moderate in size and uniform in distribution. Wei [19] studied the effects of three single-stage aging treatments on the microstructure properties of deformed superalloys with the help of experimental instruments and found that aging treatment at 760 °C compared to one at 730 °C resulted in an increased intra-crystal γ′ phase size, but there was no significant difference in the grain structure. After aging at 760 °C for 8 h, the coarsening degree of the strengthened γ′ phase was large, and the hardness value decreased after long-term aging at 750 °C.
In this experiment, different heat treatment schemes were designed to study the evolution of alloy microstructure during solution + aging. The change in alloy properties was analyzed in combination with high-temperature persistent creep and high-temperature tensile tests. Finally, the alloy strengthening mechanism of low-cobalt nickel-based superalloy was discussed.
2. Experimental Materials and Methods
The alloy in this experiment was melted via vacuum induction melting on the casting platform of Jiangyin Xinbaoli Company (Jiangyin, China). The specific chemical composition of the alloy is shown in Table 1.

Table 1.
Chemical composition of low-cobalt nickel-based superalloy (wt.%).
In this experiment, the solution temperature of the superalloy reported in the literature [20,21,22] was used as a reference, and differential scanning calorimetry (DSC) was used to assist the determination. The test sample (φ3 mm, δ2 mm, cylindrical sheet, approximately 17.2 mg) was taken from the middle part of the alloy ingot, and surface impurities were removed via ultrasonic wave and placed in a ceramic crucible.
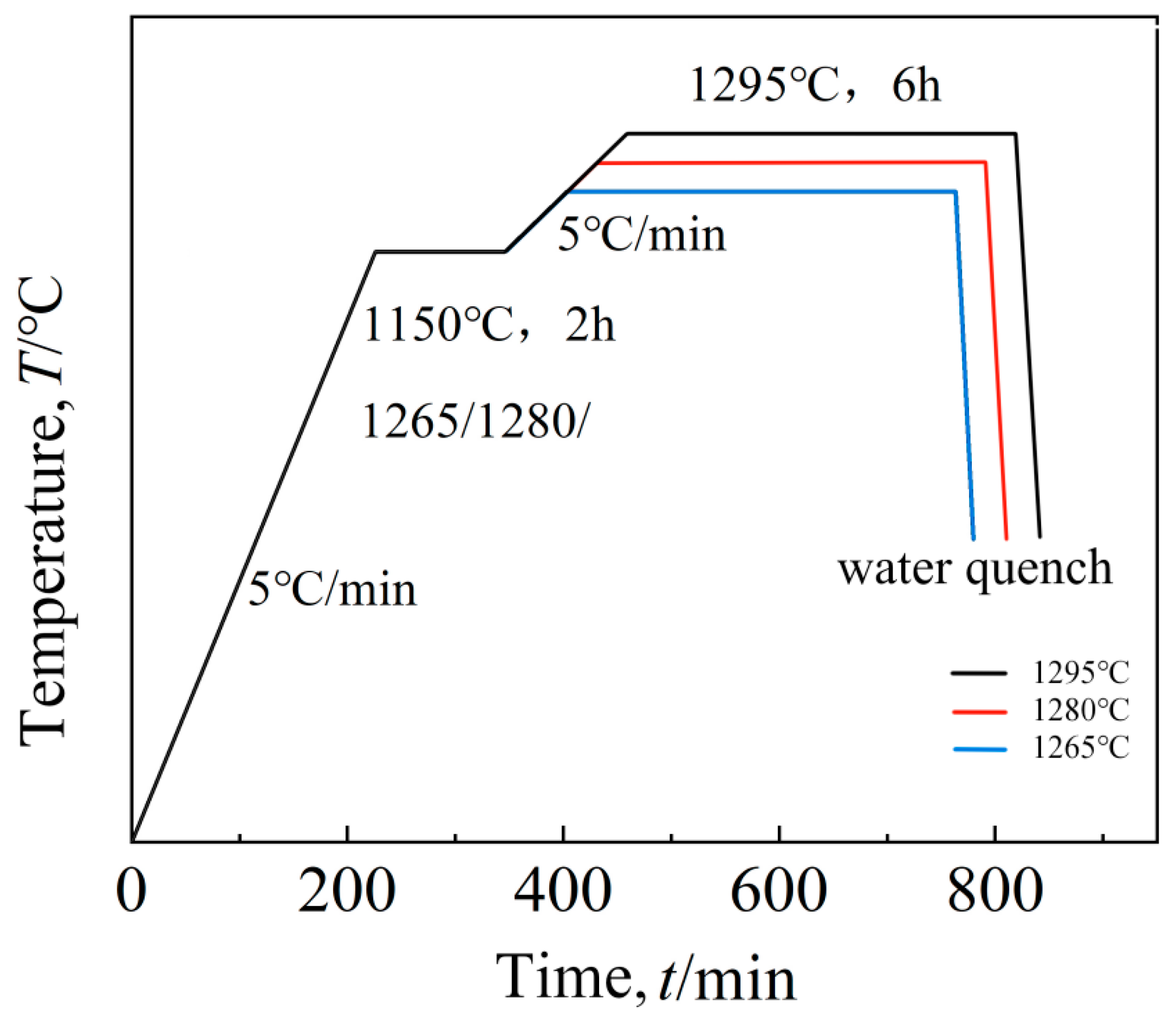
The solution treatment process flow chart of this study is shown in Figure 1, and the specific scheme is shown in Table 2.
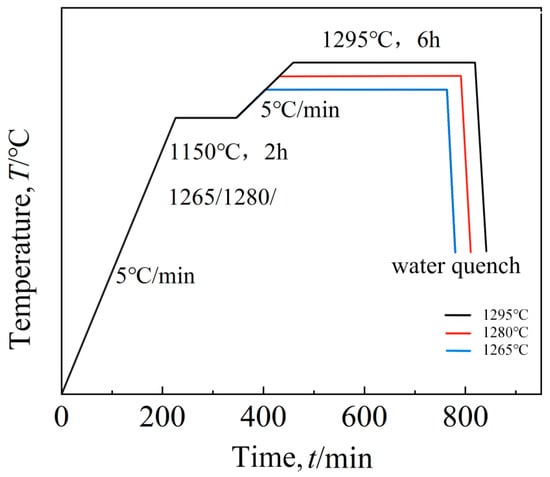
Figure 1.
Solution route.

Table 2.
Solution treatment system.
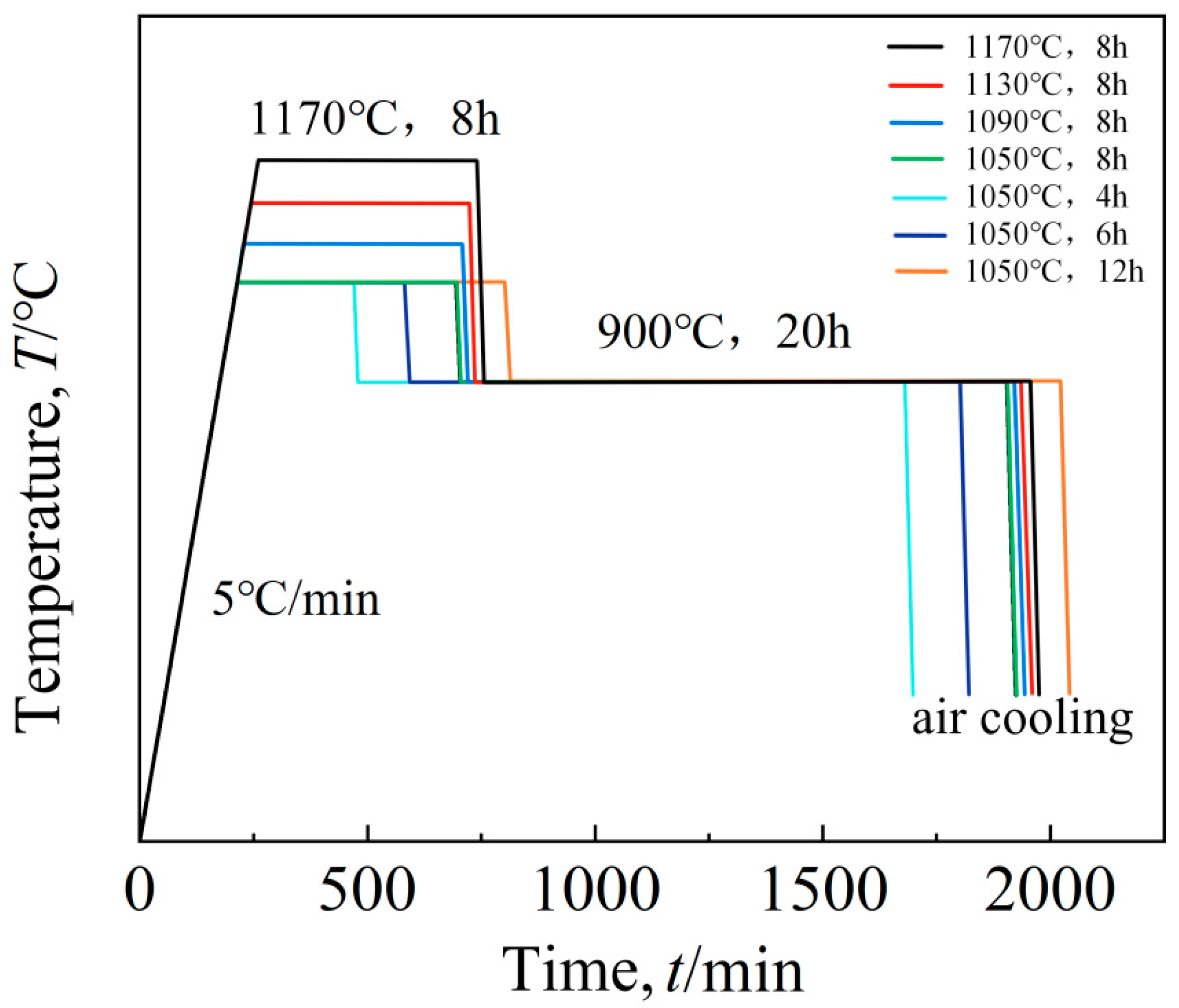
The aging process flow chart of this study is shown in Figure 2, and the specific scheme is shown in Table 3.
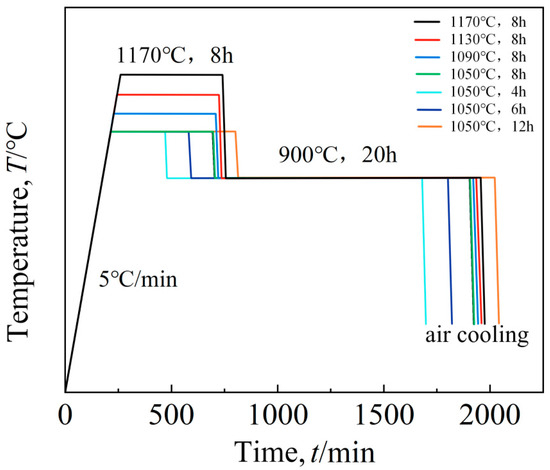
Figure 2.
Aging route.

Table 3.
Aging treatment system.
The hardness of the material was measured using an HVS-5 Vickers hardness tester (Shandong Lanhong Photoelectric Technology Co. LTD, Yantai, China). A 4.9 N load was applied in the testing process, and a conical diamond was used to press the surface of the material and maintain it for 10 s. In order to fully reflect the material properties, the hardness test point needs to run from the surface of the sample to the central area.
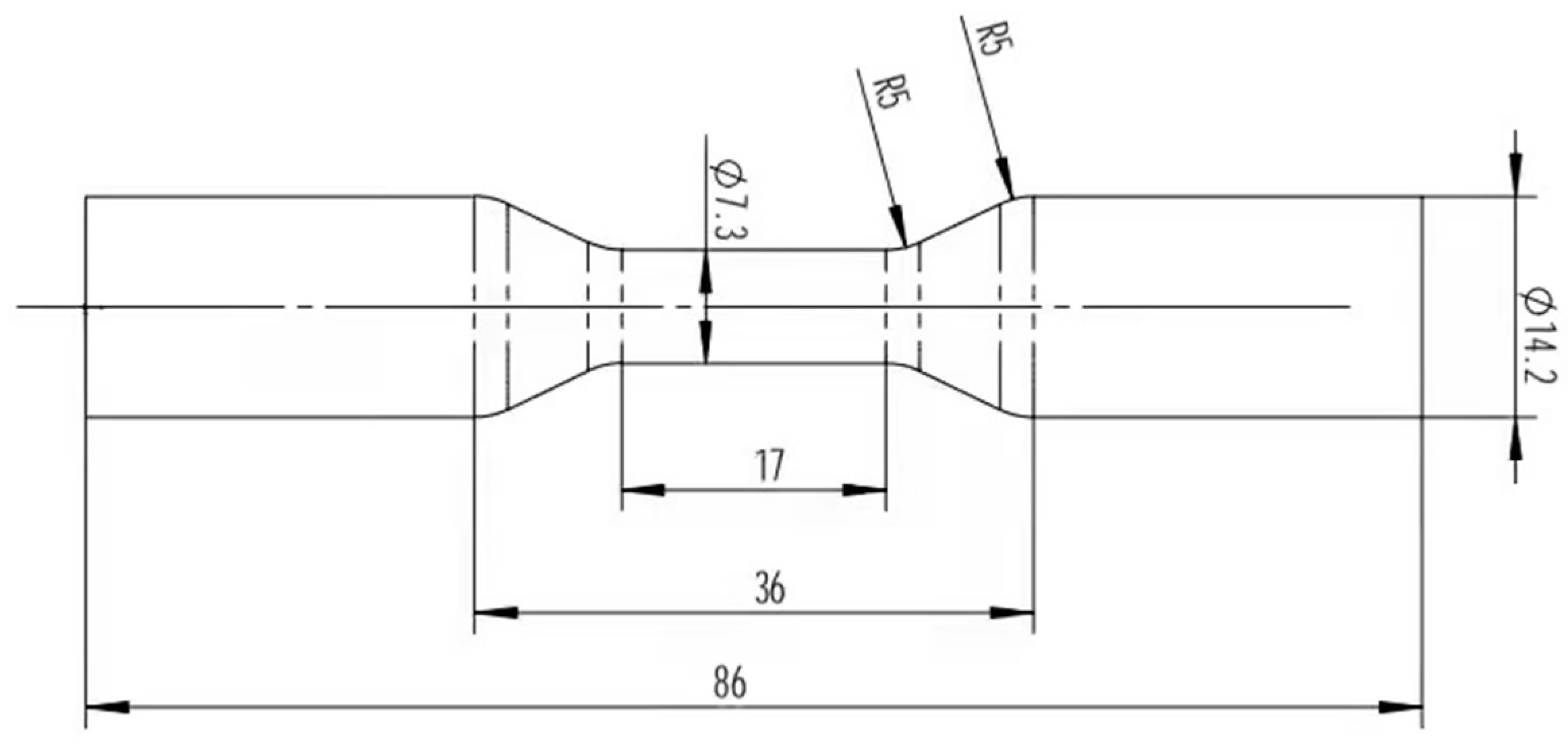
FC-20 high-temperature persistent creep testing machine (Jinan Evergrande Huifeng Test Instrument Co. LTD, Jinan, China) was selected for the high-temperature lasting performance test. The selection of experimental temperature and stress is shown in Table 4. First, the heat treatment strengthening scheme with the optimal microstructure was carried out, and after the sample was processed into standard size (Figure 3), the creep experiment was carried out under the conditions of high temperature and low stress and medium temperature and high stress.

Table 4.
Temperature and stress of high-temperature persistent creep test.
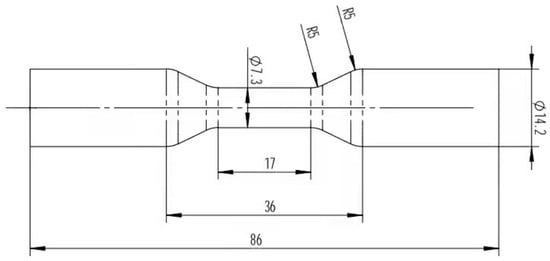
Figure 3.
Permanent creep size at high temperature (mm).
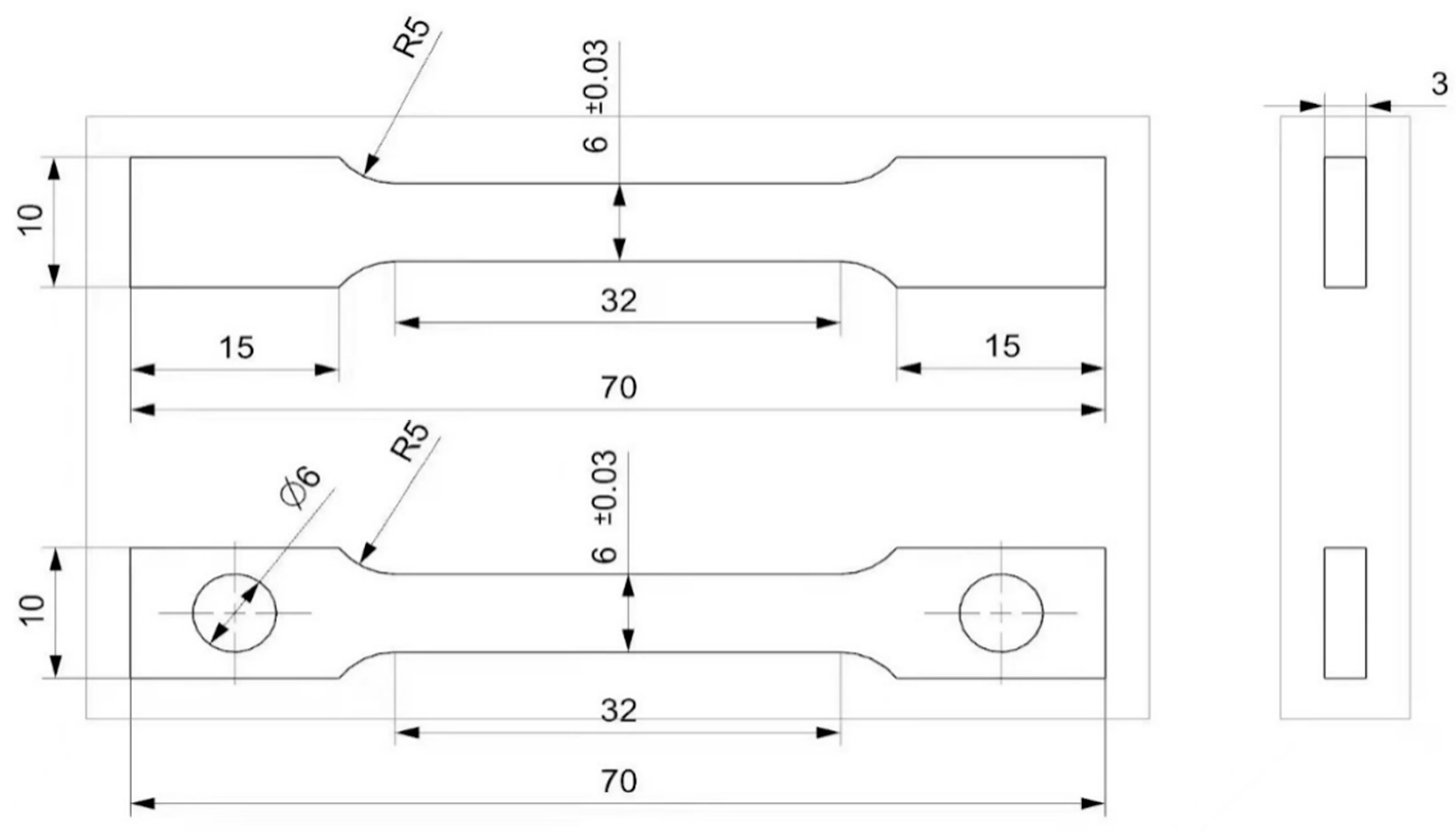
The tensile properties of the material were evaluated using an Zwick Z250 electronic universal testing machine (Shanghai Research Institute Of Materials Co. LTD, Shanghai, China ) at 1000 °C. Heat treatment of different schemes before testing (Table 5), the sample preparation strictly followed the national standard for GB/T 4338-2006 [23] high-temperature tensile testing of metals to ensure that the dimensions met the standard requirements, as shown in Figure 4.

Table 5.
Heat treatment of tensile specimens.
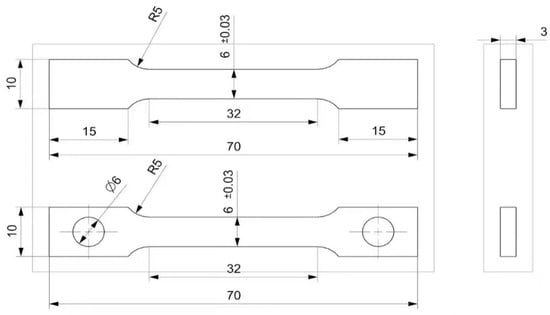
Figure 4.
Standard size of high-temperature tensile specimen (mm).
3. Results and Discussion
3.1. Effect of Solution Treatment on Microstructure
- Eutectic Structure
A high alloying degree leads to a greater tendency of segregation of alloy components. In the late solidification process, the γ′ phase forms elements such as Al and Ti, reaching the eutectic point, and precipitates large γ + γ′ eutectic structures. Due to the stress concentration during the service of the material, the eutectic structure is used as a crack channel around the material, thus destroying the material. Therefore, elimination of the eutectic structure is one of the criteria for a good or bad solution effect.
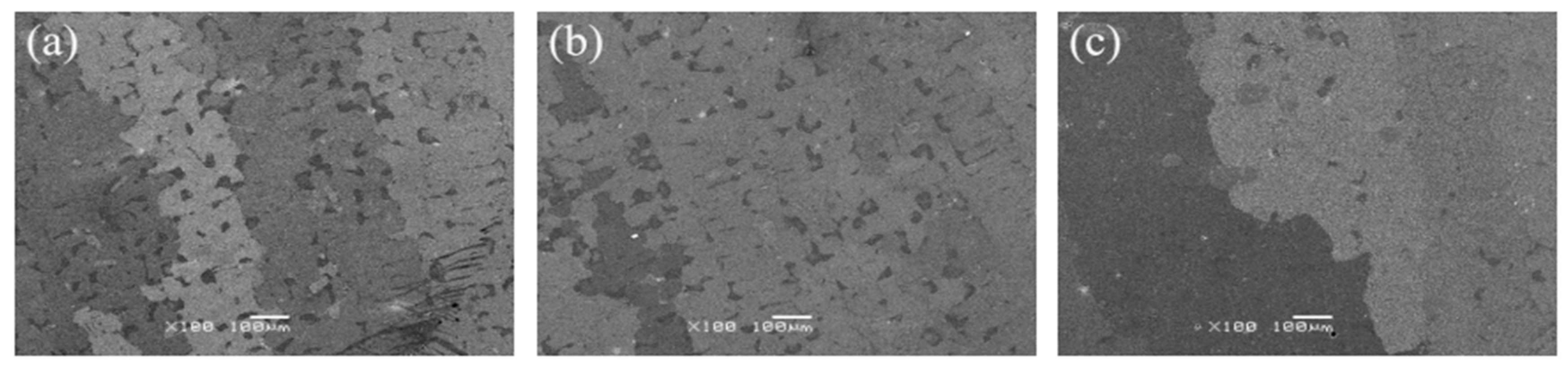
After the solution treatment of the alloy, it was observed at low magnification that after holding at 1265 °C and 1280 °C for 6 h, the alloy still showed an obvious dendrite morphology (Figure 5a,b), indicating insufficient element diffusion and poor solution effect, and the dendrites grew larger with increasing temperature. After solution treatment at 1295 °C, the dendrite morphology disappeared, and the element segregation was greatly improved, indicating that the solution effect was good.

Figure 5.
Microstructures of the alloy after heat treatment at (a) 1265 °C, (b) 1280 °C, (c) 1295 °C.
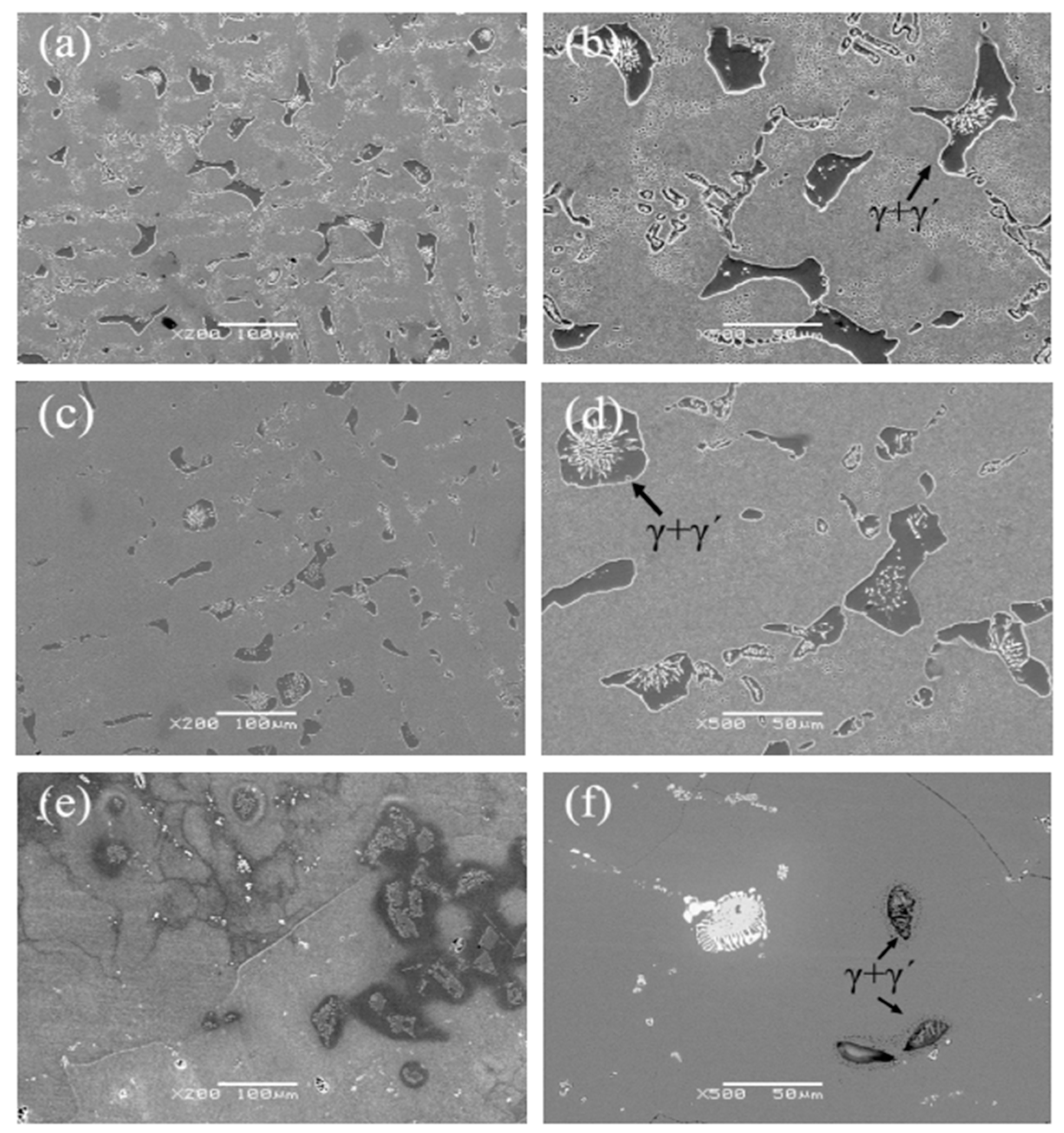
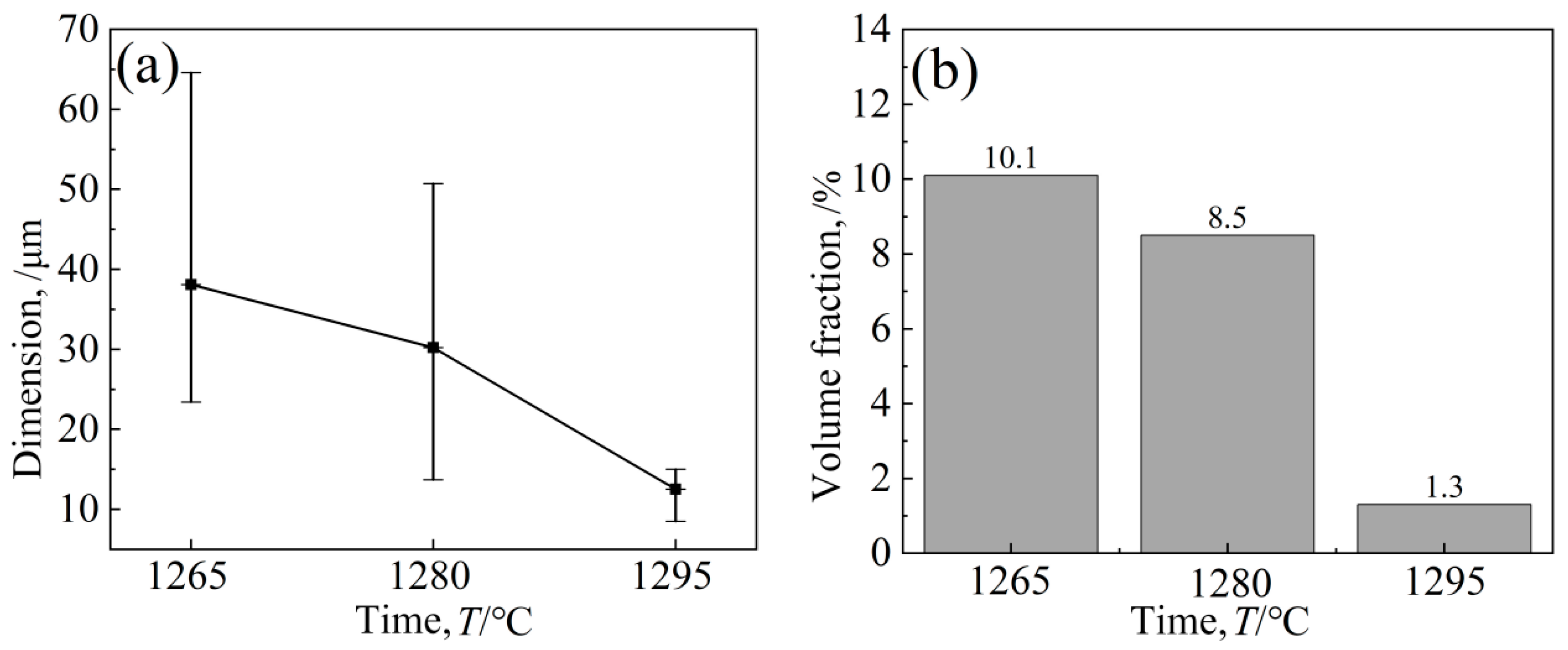
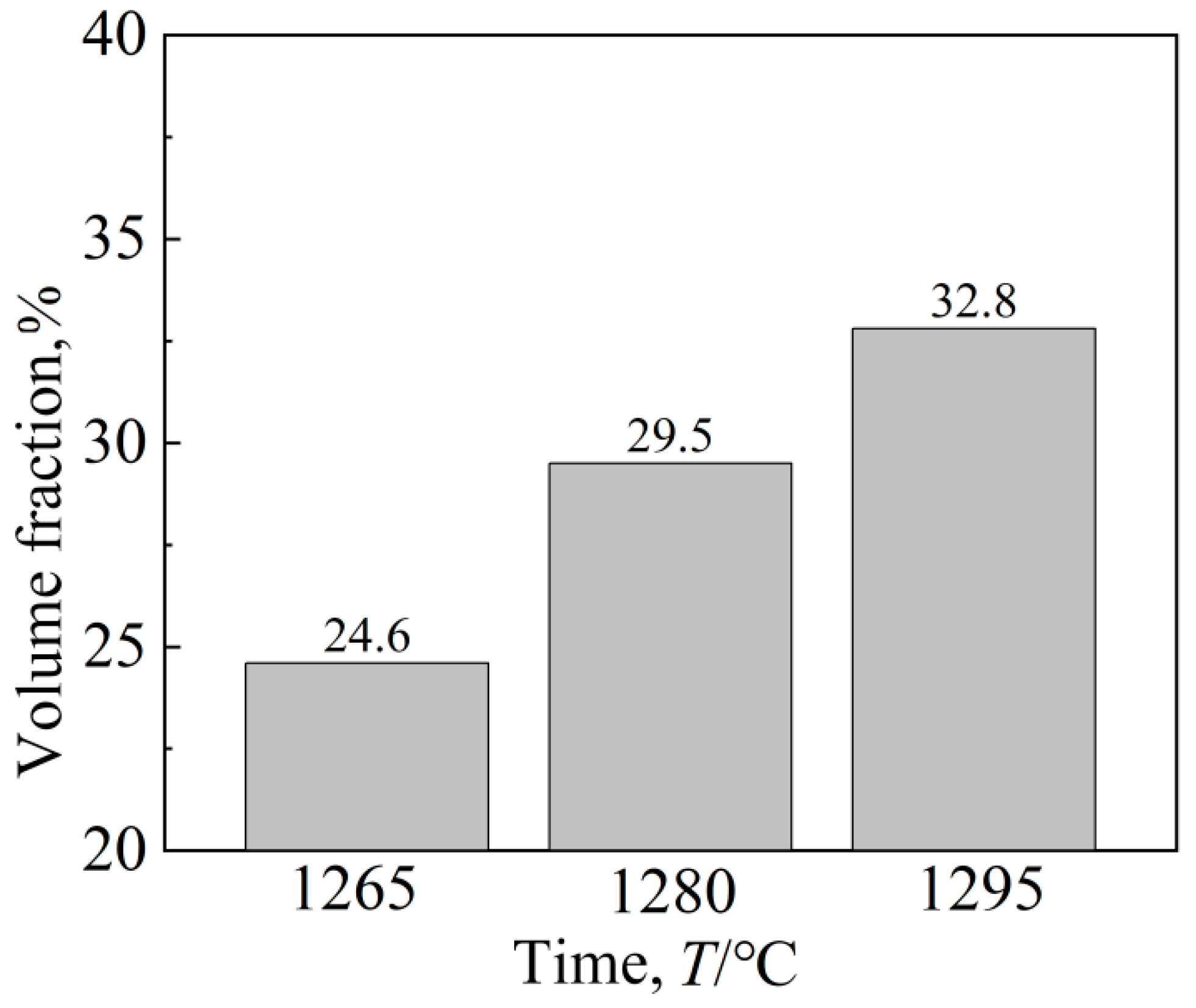
Figure 6 shows the eutectic structure morphology of a low-cobalt alloy at three solid solution heat treatment temperatures of 1265 °C, 1280 °C and 1295 °C. It was found through observation that the eutectic structure is striated or blocky on the outside and petal-like on the inside. Figure 7 shows the influence of three different solution temperatures of 1265 °C, 1280 °C and 1295 °C on the eutectic structure. The Image-Pro Plus soft (IPP 6.0)was used for quantitative calculation, and the specific data and change trend of the size and volume proportion of the eutectic structure were obtained. After heat treatment at 1265 °C, the eutectic structure size of the block ranged from 23.4 to 64.6 μm; the average size was 38.1 μm; and the volume proportion was approximately 10.1%. After solution treatment at 1280 °C, the eutectic size decreased to 13.7–50.7 μm; the average size was 30.2 μm; and the volume proportion was approximately 8.5%. After heat preservation at 1295 °C, the eutectic structure was basically eliminated; the average size was reduced to 12.5 μm; and the volume proportion was reduced to 1.3%.
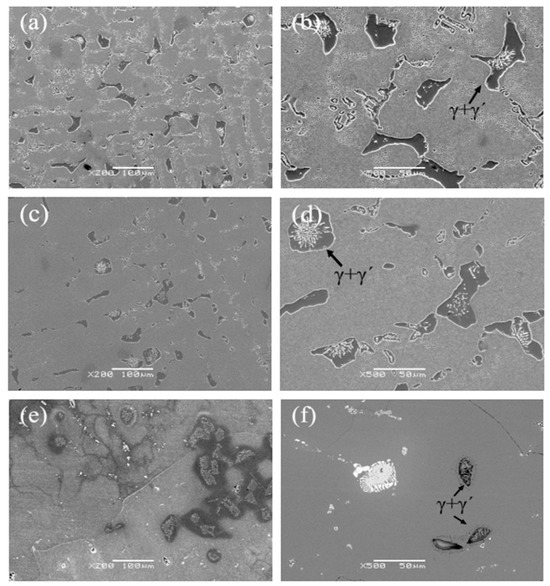
Figure 6.
Eutectic structures at different solution temperatures: (a,b) 1265 °C, (c,d) 1280 °C, (e,f) 1295 °C.
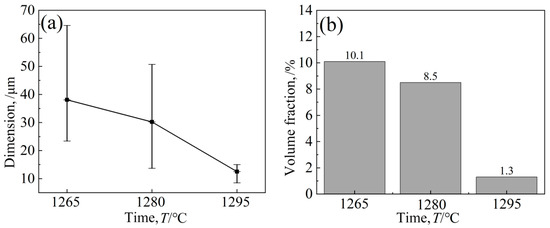
Figure 7.
Effects of different solution temperatures on eutectic structures’ (a) Dimension and (b) Volume fraction.
The results showed that the solution behavior of the γ + γ′ eutectic structure is closely related to temperature. With an increase in solution temperature, the diffusion rate of solute atoms is accelerated; the diffusion of Al, Ti and other elements is uniform; the γ + γ′ eutectic structure is gradually dissolved; and the proportion of size and volume is gradually reduced, indicating that the solution effect is better at 1295 °C.
- γ′ Phase
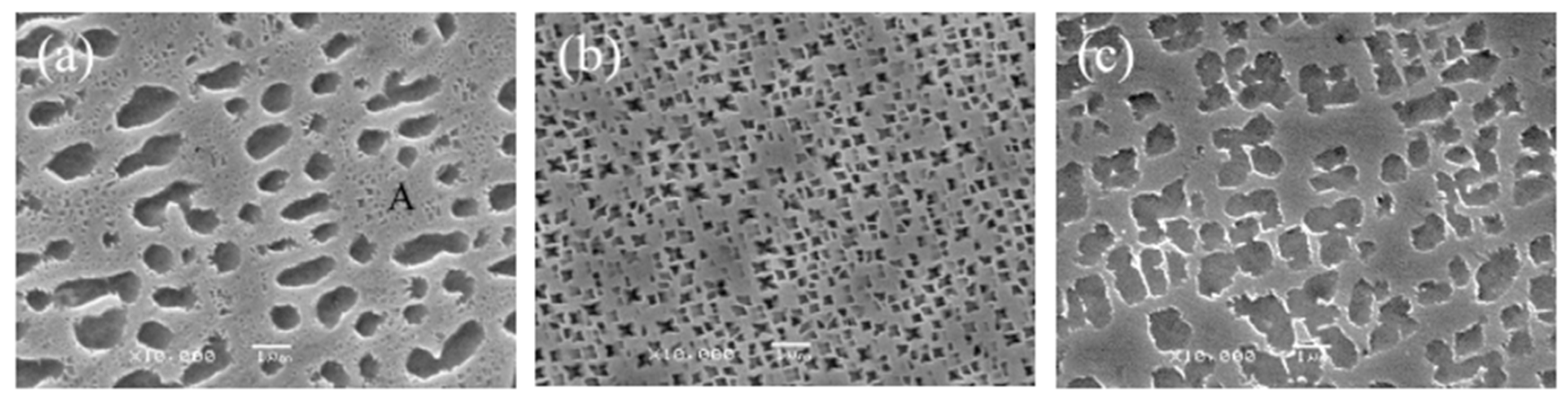
The purpose of solid solution treatment of the precipitated phase during dissolution and solidification is to prepare for subsequent aging strengthening. With the increase in solution temperature, the morphology of the γ′ phase changes to an oval cross-cube. After solid solution treatment at 1265 °C, there are two forms of the γ′ phase: one is an elliptical γ′ phase with an average size of 1.4 μm, most of which is elliptical; the other is a smaller cavity γ′ phase observed in the “A” region, the size of which is several orders of magnitude different from the elliptical γ′ phase (Figure 8a). After solid solution treatment at 1280 °C, the γ′ phase was cross-shaped and dispersed, and the particle size was approximately 0.2 μm (Figure 8b). After solid solution treatment at 1295 °C, the γ′ phase was lumpy, but some of it took the form of a small cube, and the particle size was 1.05 μm between the other two solid solution temperatures (Figure 8b).

Figure 8.
Morphology of the γ′ phase at different solution temperatures: (a) 1265 °C, (b) 1280 °C, (c) 1295 °C.
Figure 9 shows the change in the amount of γ′ phase at the three temperatures. It can be seen that the increase in solid solution temperature increases the percentage of the γ′ phase, reaching 32.8% at 1295 °C. Although the size of the γ′ phase is smallest after 1280 °C solid solution treatment, the proportion of γ′ phase precipitation is largest after 1295 °C solution treatment, and the form of γ′ is closer to cubic. It has been reported [24] that when the strengthened γ′ phase is cubic, the alloy has excellent properties.
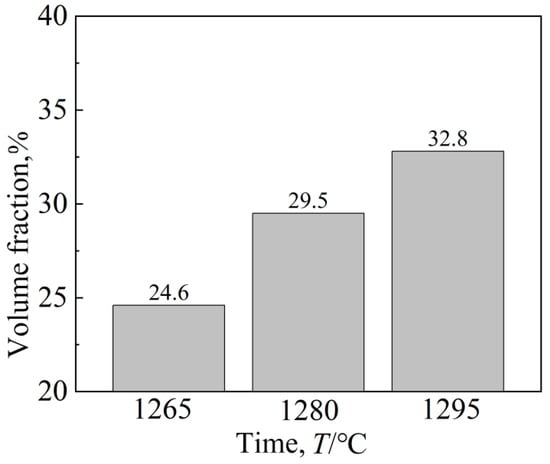
Figure 9.
Volume fraction of the γ′ phase at different solution temperatures.
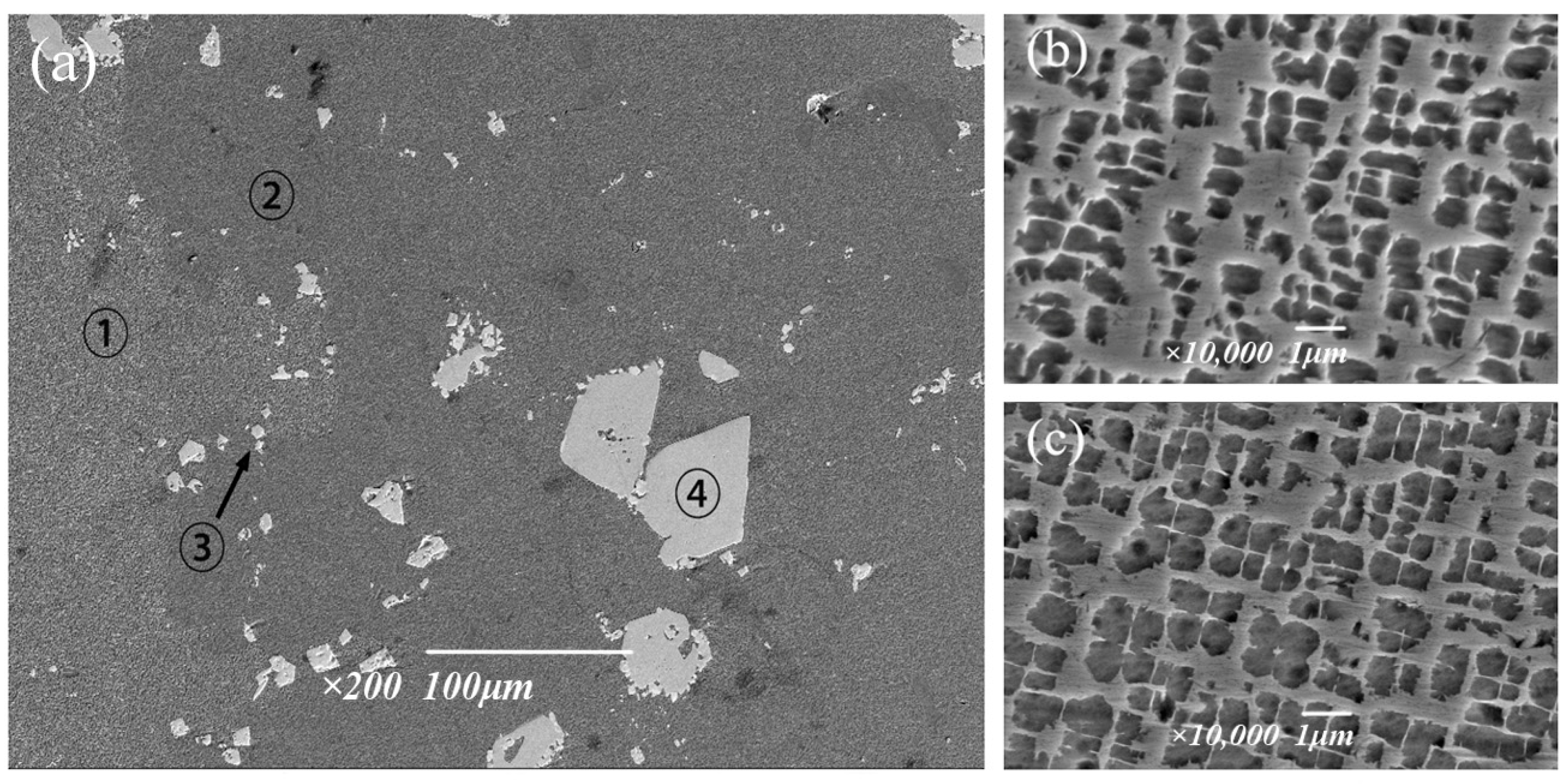
The microstructure and composition of the solution treated at 1295 °C were further analyzed using field emission scanning electron microscopy. The precipitated phase in the alloy is mainly the strengthening γ′ phase and carbide. Figure 10b,c show two gamma′ phases of similar size but in different regions. In terms of overall morphology, the edge passivation degree of region ➀ is higher, and the precipitation of the γ′ phase is lower, while the cubed degree of region ➁ is higher. The region ③ shows chain shape, which is rich Ta and Hf MC. The region ④ shows a white block, which is M6C rich in W. According to the results of energy spectrum analysis, it can be seen in Table 6 that the composition of the two γ′ phases located in different regions is relatively close, and the content difference of each element is not more than 0.4%, which also proves that the tendency of element segregation is greatly reduced after 6 h treatment at 1295 °C. This is because the high solution temperature can increase the diffusion rate of solute atoms [25], so that the main elements forming the eutectic structure, such as Al and Ti, can be uniformly diffused, and thus, the eutectic structure can be dissolved until eliminated; therefore, the segregation situation is improved, and the alloy properties are optimized. It can be determined that the solution treatment system held at 1295 °C for 6 h has the best effect, which can effectively eliminate the eutectic structure and improve the segregation situation.
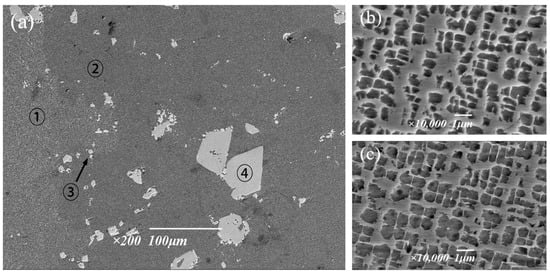
Figure 10.
Morphology of the γ′ phase after solid solution treatment at 1295 °C: (a) Entirety, (b) Region ➀, (c) Region ➁.

Table 6.
Energy spectrum analysis results (wt.%).
3.2. Effect of Aging Treatment on Microstructure
- γ′ Phase
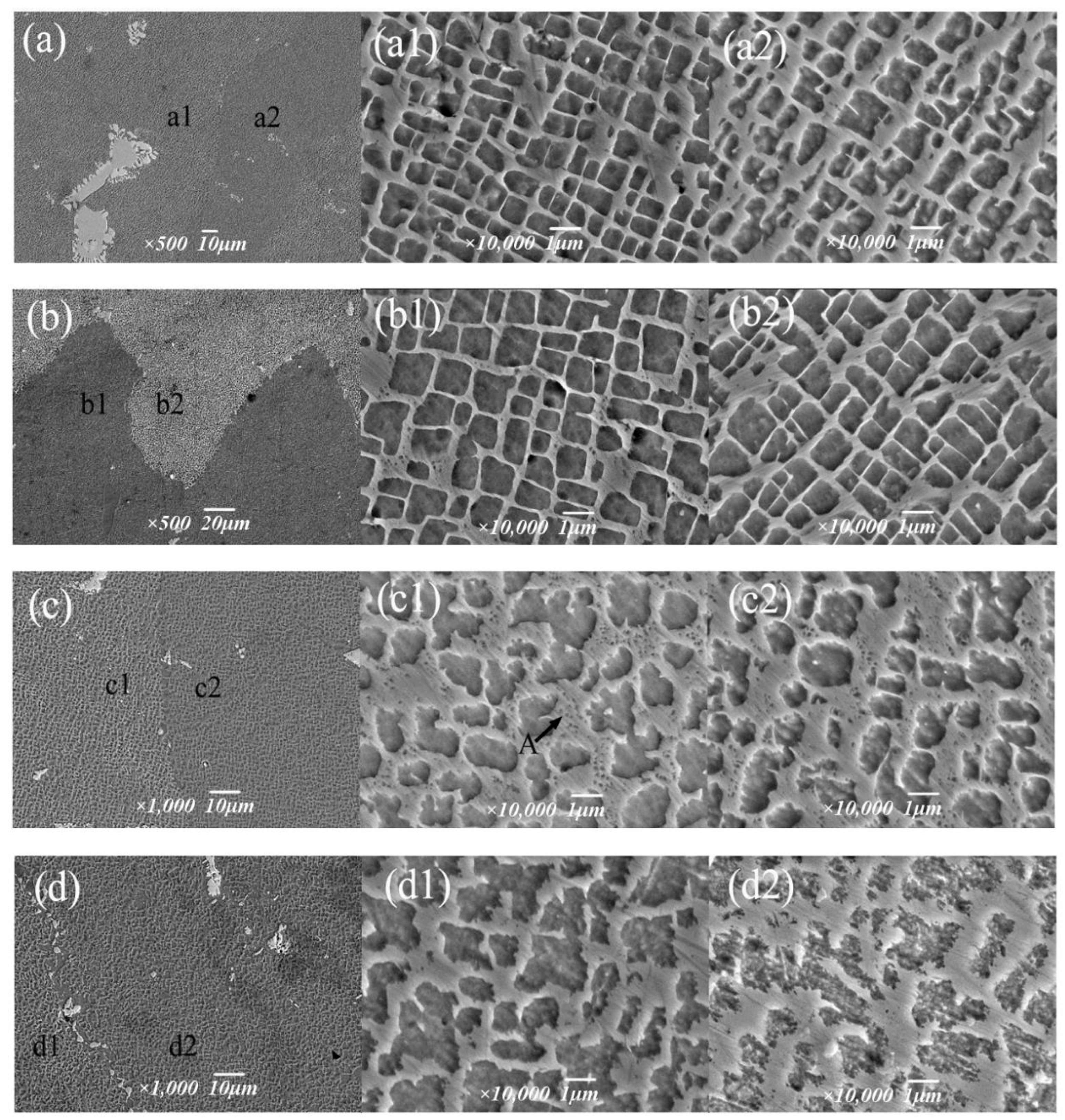
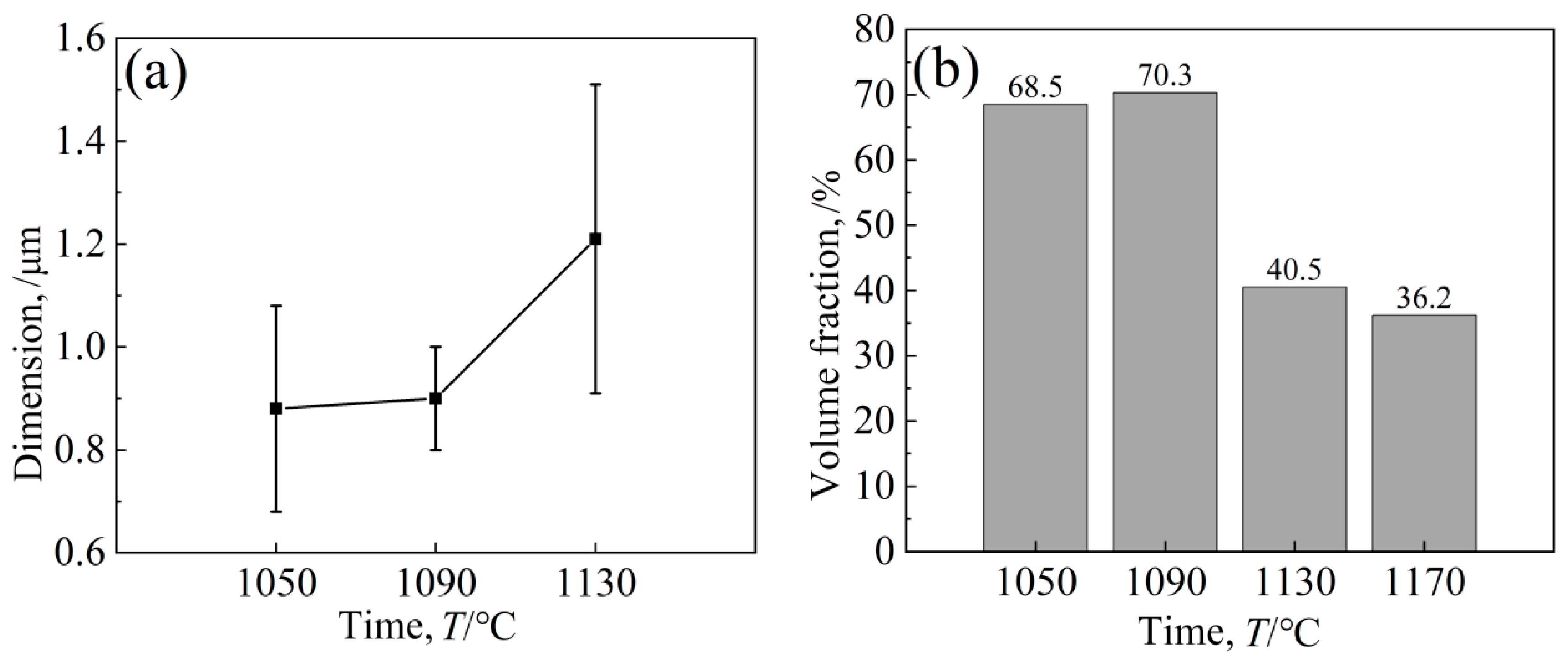
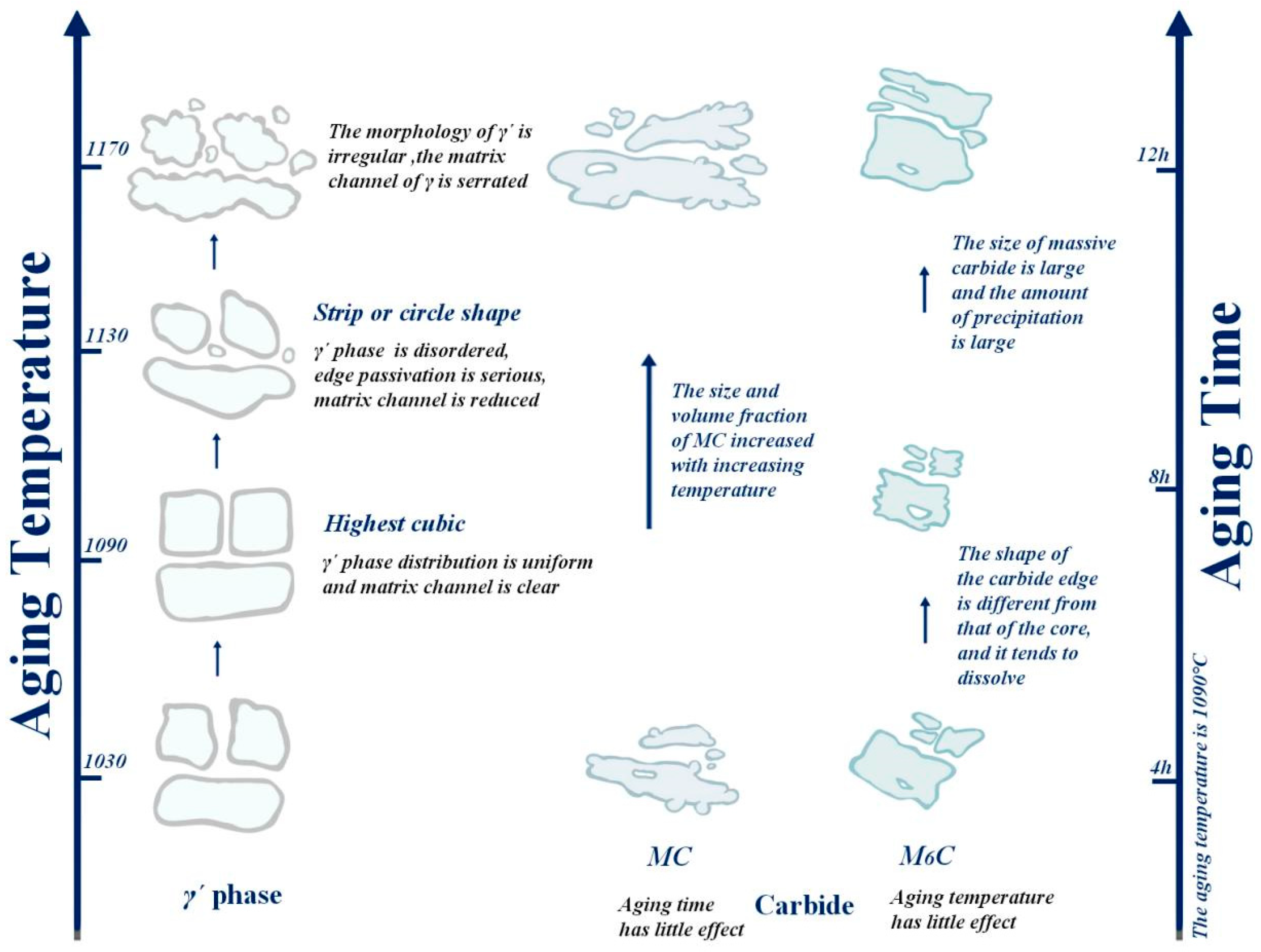
The purpose of the aging treatment is to obtain a fine gamma′ phase and enhance the high-temperature properties of the alloy via precipitation strengthening. The high-temperature properties of nickel-based alloys are largely determined by the precipitation enhancement of the γ′ phase, and further influencing factors include γ′ phase size, morphology, precipitation amount, γ/γ′ misfit degree, etc. Figure 11 shows the structure morphology of the γ′ phase in two regions at different aging temperatures. It can be intuitively seen that the aging strengthening effect is better at lower temperatures. After aging at 1090 °C, the cubic degree of the γ′ phase is highest, with a size of 0.9 μm, which is slightly increased compared to 1050 °C, but the distribution of the γ′ phase is uniform; the matrix channel is clear; and the volume fraction of γ′ phase precipitation is higher, which is 70.3%, and the morphology of the γ′ phase in different regions is not significantly different (Figure 11b). After aging at 1130 °C, the cubic degree of the γ′ phase decreased, and it took the form of a bar or round shape, with disordered arrangement, serious edge passivation and matrix channel narrowing. The average size of the γ′ phase was 1.21 μm; the volume fraction was reduced to 40.5%. In the A region of Figure 11c1, the precipitation of secondary γ´ phase particles in the matrix was also observed In Figure 11d, it was observed in the enlarged image that after aging at 1170 °C,, the matrix channels of the gamma’ phase were jagged, and some of the γ′ phases were connected together, resulting in irregular morphology and difficult measurement of the size of the γ′ phase, whose total volume fraction was 36.2%.
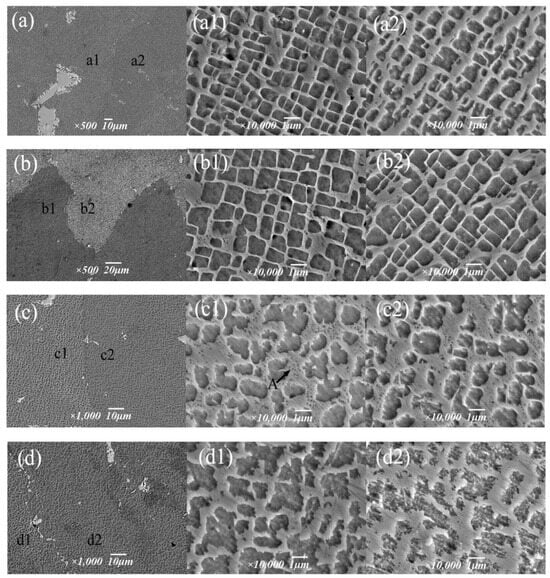
Figure 11.
Morphology of the γ′ phase at different aging temperatures: (a–a2) 1050 °C, (b–b2) 1090 °C, (c–c2) 1130 °C, (d–d2) 1170 °C.
The γ′ phases of the two regions were found via energy spectrum analysis (Table 7), and their components were relatively close, and there was no segregation problem in the as-cast structure. Nishimoto et al. [26] studied the influence of the relative creep properties of four kinds of nickel-based γ′ superalloys and found that the alloy [001] orientation had the best lasting properties when the γ′ phase morphology was cubic. The changes in the size and volume fraction of the alloy-strengthened γ′ phase are shown in Figure 12. In general, the structure of the γ′ phase after aging at 1090 °C was relatively excellent.

Table 7.
γ′ phase composition at different temperatures and regions (wt.%).
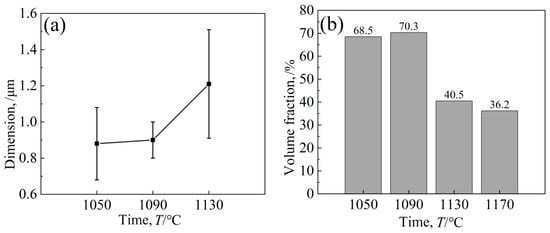
Figure 12.
Effect of different aging temperatures on γ′ phase (a) dimension and (b) volume fraction.
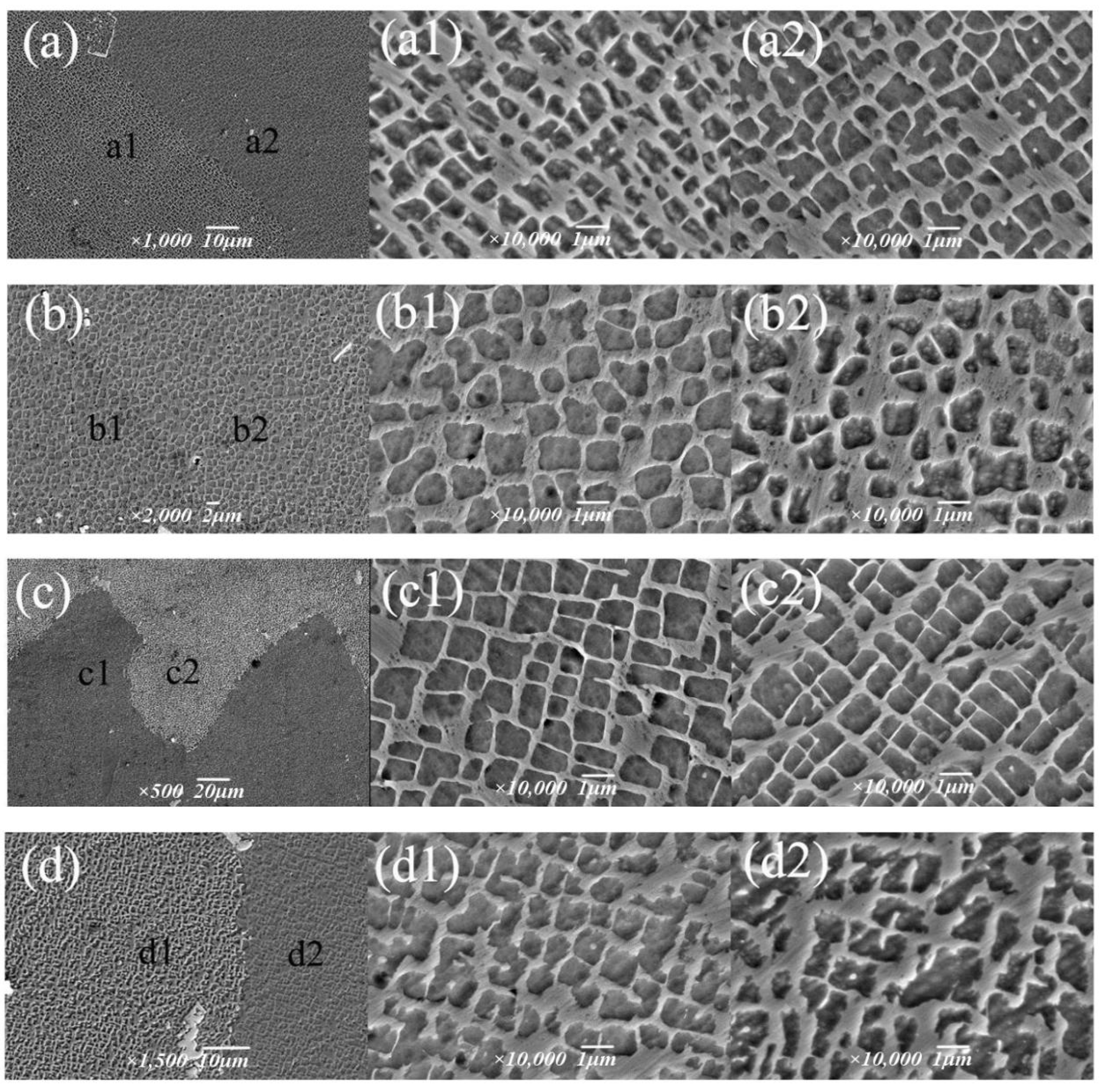
Based on an aging temperature of 1090 °C, the effects of holding time of 4 h, 6 h, 8 h and 12 h on the strengthening phase were studied (Figure 13), and it was found that the morphology transformation process of the γ′ phase was as follows: cavity shape → spherical shape → cubic shape → irregular. With the aging time, the size of the γ′ phase gradually increased from 0.72 μm to 0.9 μm, but after 12 h, γ′ phase transition became irregular, and the size decreased slightly. During the holding time of 4~8 h, the volume fraction of the γ′ phase increased from 50.9% to 70.3%, while after 12 h, the edge of the γ′ phase was passivated and partially connected; the volume fraction decreased to 56.3%; and the change trend was the same as the size. It can be seen that the alloy γ′ phase had the best morphology, size and quantity after 8 h.
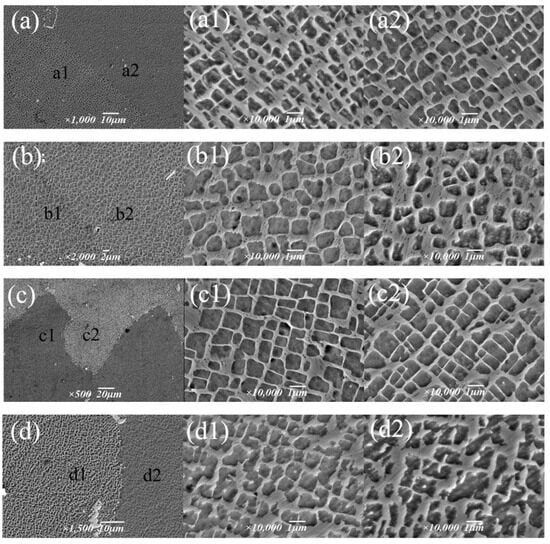
Figure 13.
Morphology of the γ′ phase under different aging times: (a–a2) 4 h, (b–b2) 6 h, (c–c2) 8 h, (d–d2) 12 h.
- Carbide
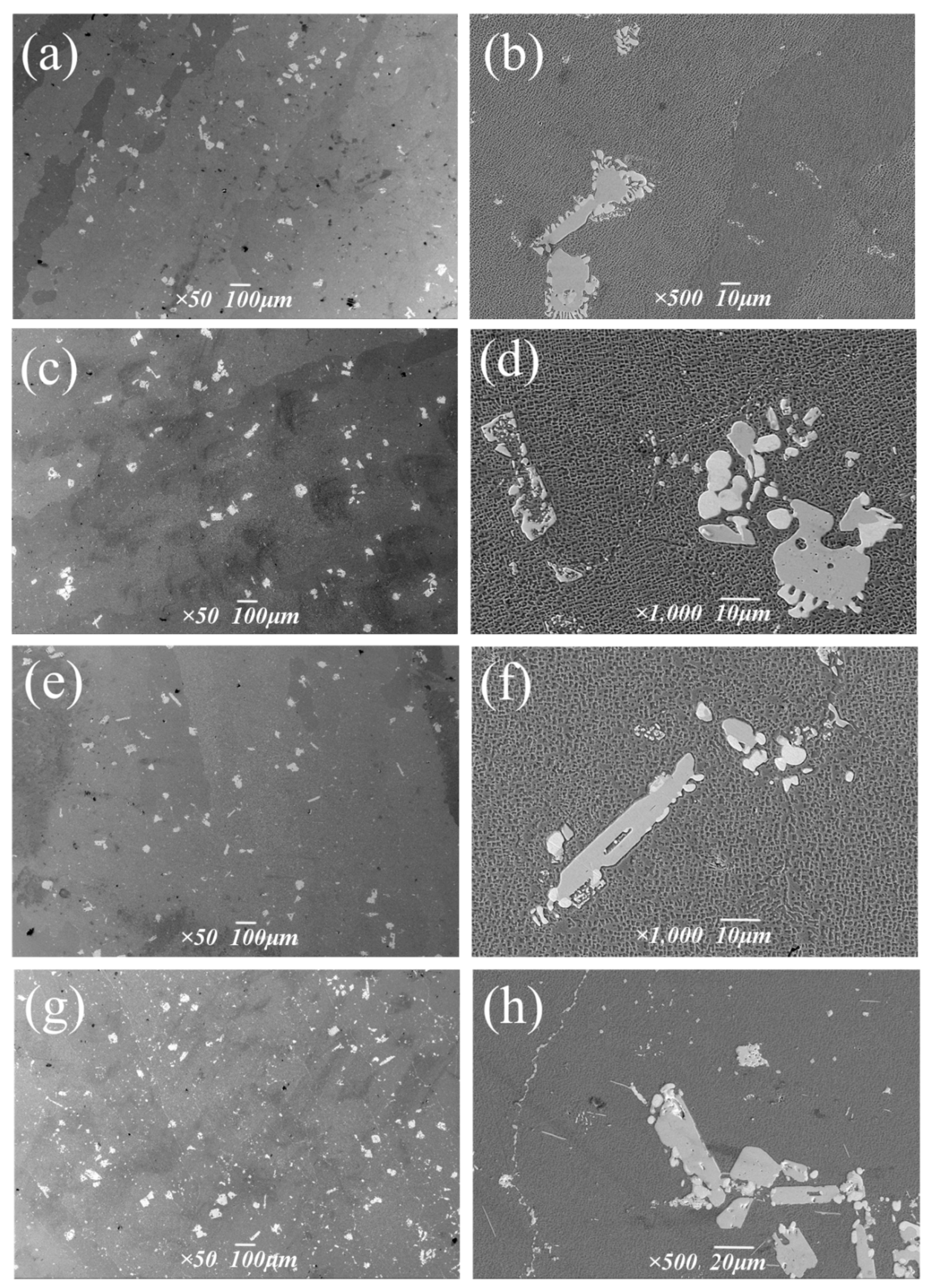
Figure 14 shows the morphology and distribution of precipitates at different aging temperatures.
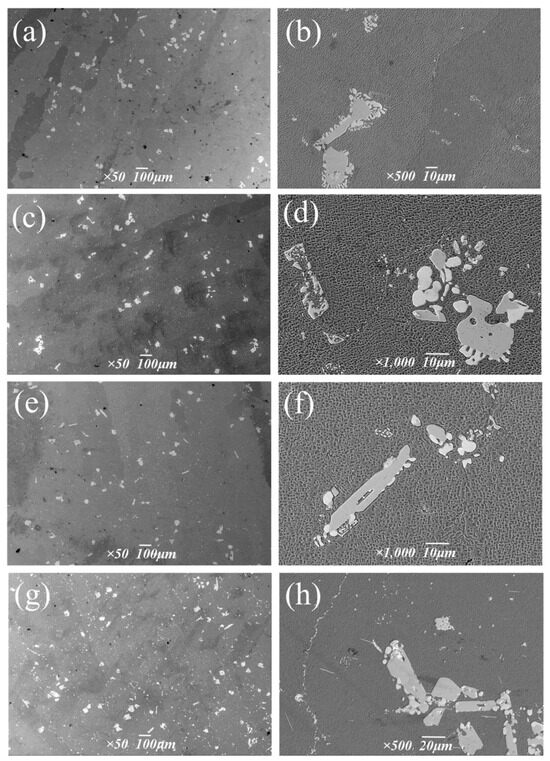
Figure 14.
Carbide morphologies at different aging temperatures: (a,b) 1050 °C, (c,d) 1090 °C, (e,f) 1130 °C, (g,h) 1170 °C.
The composition of the two forms of carbides in Figure 14h was analyzed via energy spectrum (Table 8), and its location is shown in Figure 10 of Section 3.1. The white block is M6C rich in W, and the chain form is MC rich in Ta and Hf. With the increase in aging temperature, the size and precipitation quantity of bulk M6C changed little, and the volume percentage ranged from 0.7% to 0.9%. Overall, the aging temperature had a small influence on M6C. The chain continuous MC carbides are mostly distributed at the grain boundary, which plays a role in reducing the tendency of crack initiation and expansion and strengthening the alloy [27,28,29]. It was observed that the size and volume fraction of MC carbides increased sharply at the grain boundary after treatment at 1170 °C.

Table 8.
Results of energy spectrum analysis of two kinds of carbides.
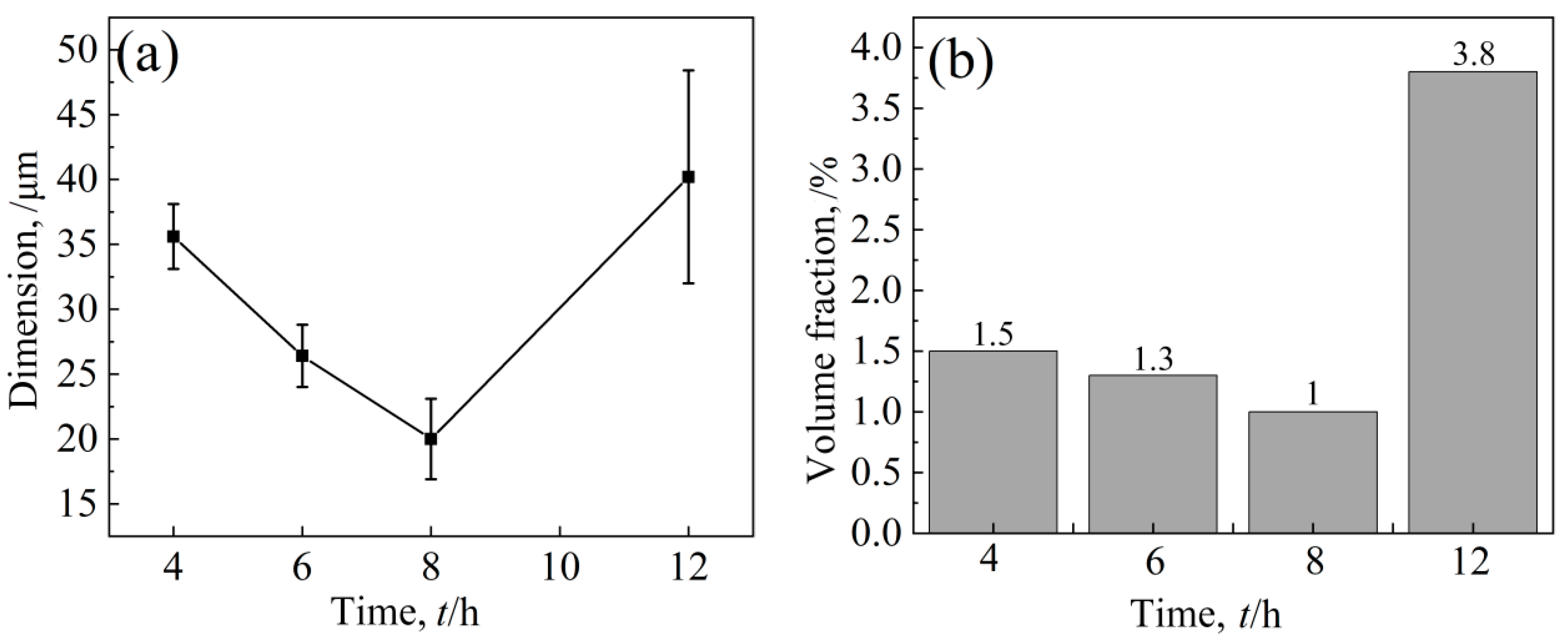
After an aging treatment at 1090 °C, the volume fraction and size of white bulk M6C decreased first and then increased with aging time (Figure 15). After an aging time of 8 h, the volume proportion of carbide was at least 1.0%, and the size was smaller by 20 μm. In general, large massive carbides are usually unfavorable to the performance of nickel-based alloys [30,31]; especially after 12 h of heat preservation, the massive carbides are larger in size, and the amount of precipitation is large. The change in temperature has little effect on the chain type MC carbides distributed at the grain boundary, and it is difficult to study the change in the size and volume fraction because of the small quantity.
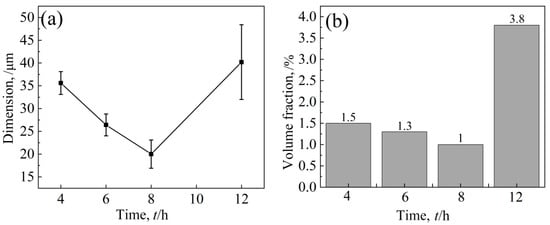
Figure 15.
Effect of holding time on carbide (a) dimension and (b) volume fraction.
Combined with the effects of aging temperature and time on the γ′ phase and carbide, it is proved that the microstructure subjected to an aging treatment at 1090 °C and 8 h best meets the requirements.
3.3. The Evolution of Low-Cobalt Alloys’ Microstructure under Heat Treatment
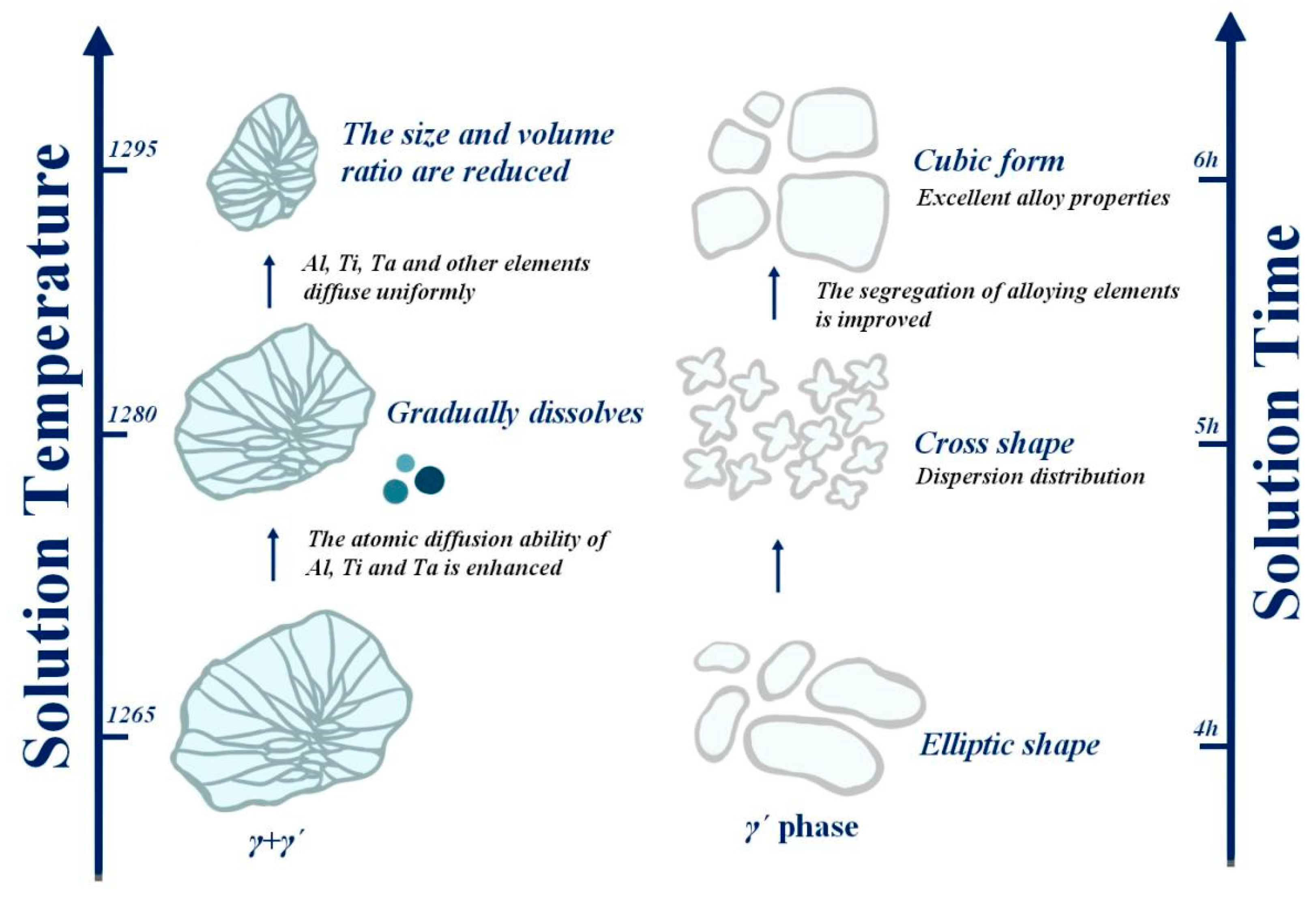
When the solution temperature reaches 1265 °C, the alloy has obvious dendrites, and the eutectic structure is formed. It was found that the outside outline of the eutectic structure is a strip or block, while the inside assumes a petal-like form. Due to the insufficient diffusion of alloying elements at this temperature, the solution effect is poor. With the increase in solution temperature, the dendrite size is positively correlated with temperature; therefore, the increase in temperature leads to an increase in dendrite size but a decrease in the eutectic structure size. The root cause of the gradual dissolution of the eutectic structure is that the temperature increase causes the acceleration of the diffusion rate of solute atoms and the improvement of the diffusion capacity of alloy elements, so that Al, Ti, Ta and other elements are uniformly diffused, and then, the segregation of alloy elements is greatly improved, and finally, the γ + γ′ eutectic structure is gradually dissolved, and the size and volume proportion are gradually reduced. When the solution temperature rises to 1295 °C, the effect of increasing the diffusion rate of solute atoms is more obvious, and most of the branched crystals disappear, and the eutectic structure is basically eliminated. This process is visually illustrated on the left side of Figure 16.
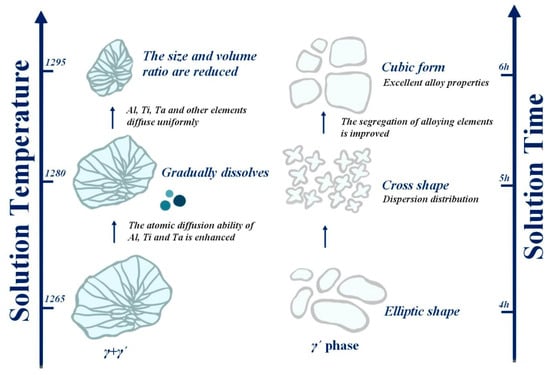
Figure 16.
Trend chart of the influence of solution temperature and time on tissue change.
During the process of raising the solution temperature, the morphology of the γ′ phase also changes—oval → cross or butterfly → cubed—as shown on the right side of Figure 16. After solid solution treatment at 1265 °C, the γ′ phase will have two forms: one is an elliptical γ′ phase, and the other is a small porous γ′ phase, the size of which is several orders of magnitude different from the elliptical γ′ (this γ′ is relatively small; only the former is considered for the time being). When the solution temperature rose to 1280 °C, the γ′ phase showed a cross shape and small dispersion. After solid solution treatment at 1295 °C, the γ′ phase was massive, but some of it was cubic. The volume fraction of the γ′ phase increases with the increase in solid solution temperature and reaches the maximum value at 1295 °C. We found that the size of the γ′ phase following the 1280 °C solid solution treatment is the smallest, but the proportion of γ′ phase precipitation after the 1295 °C solid solution treatment is the largest, and the form of γ′ is closer to a cube. At this time, the strengthened γ′ phase is cuboidal, and the alloy has excellent properties.
After the solution treatment at 1295 °C, when the solution time rises to 6 h, the main elements of the eutectic structure, such as Al and Ti, can be diffused uniformly, so that the eutectic structure can be dissolved until elimination, and the segregation situation is improved. This also proves that after 6 h of treatment at 1295 °C, the alloy properties are optimized.
In Figure 17, we can intuitively see that the aging strengthening effect is best at the aging temperature of 1090 °C. When the aging temperature is 1050 °C, part of the γ′ phase is cubic, but the cubic degree is not high. With the increase in aging temperature, when the temperature rises to 1090 °C, the cubic degree of the γ′ phase is the highest; the distribution of the γ′ phase is uniform; and the matrix channel is clear. When the temperature further increased to 1130 °C, the cubic degree of the γ′ phase decreased; the structure was rod-like or circular; the arrangement was disordered; the edge was seriously passivated; and the matrix channel became narrow. At the same time, the precipitation of secondary γ′ phase was also observed. The fundamental reason was that the residual susaturated γ′ forming elements (Al, Ti, Ta) in the matrix were limited in their diffusion path, resulting in a longer diffusion distance. These atoms could not migrate to the original γ′ phase for growth and instead precipitated on the γ matrix channel. Finally, after the aging treatment at 1170 °C, the matrix channel of the γ′ phase was zigzag, and some γ′ phases were connected together, resulting in irregular shape of the γ′ phase and difficult-to-measure size. The size of the γ′ phase decreased first and then increased with increasing aging temperature, while the volume fraction of the γ′ phase showed the opposite trend. We know that the smaller the gamma´ phase and the more dispersed it is, the better the alloy performance will be. It can be seen that the microstructure of the γ′ phase after the aging treatment at 1090 °C is the most excellent.
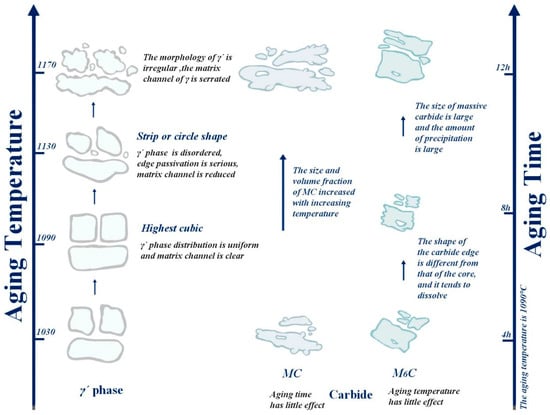
Figure 17.
Trend chart of the influence of aging temperature and time on tissue change.
Based on the aging temperature of 1090 °C, by extending the aging time, it was found that the morphology transformation process of the γ′ phase was cavity shape → spherical shape → cubed shape → irregular shape, as also visually displayed in Figure 17. It was found that the size of the γ′ phase increased with aging time within 4–8 h. This is because the increasing aging temperature promotes the diffusion rate of susaturated solute atoms in the matrix, which enhances their tendency to advance toward the two-phase interface. As a result, the γ′ phase grows convex along the existing interface in an extended way, and the volume fraction of the γ′ phase is increased. However, when the aging time is extended to 12 h, the γ′ phase begins to transform into an irregular shape, and its size decreases slightly, which is due to the re-dissolution of tiny γ′ particles. As the aging temperature further increases, the particles of the γ′ phase tend to coarsen, and the edges become blunt, resulting in a decrease in the overall integral number of the γ′ phase. It can be concluded that when the aging time is 8 h, the morphology, size and quantity of the alloy γ′ phase are the best.
Regarding carbides, the size and precipitation amount of bulk M6C do not change much with the increase in aging temperature. In general, the aging temperature has little influence on M6C. The chain continuous MC carbides are mainly distributed at the grain boundaries, which can reduce the crack initiation and propagation tendency and enhance the properties of the alloy. After the aging treatment at 1090 °C, the volume fraction and size of white block M6C first decrease and then increase with aging time. When the aging time reaches 8 h, the volume ratio and size of carbide decrease. In general, larger massive carbides are usually unfavorable to the performance of nickel-based alloys; especially after holding for 12 h, the size of massive carbides is larger, and the precipitation is larger.
Combined with the effects of aging temperature and aging time on the γ′ phase and carbide, it is proved that the microstructure subjected to an aging treatment at 1090 °C and 8 h best meets the requirements.
3.4. Variation Trend of Properties of Low-Cobalt Alloys
- (1)
- Room temperature mechanical properties
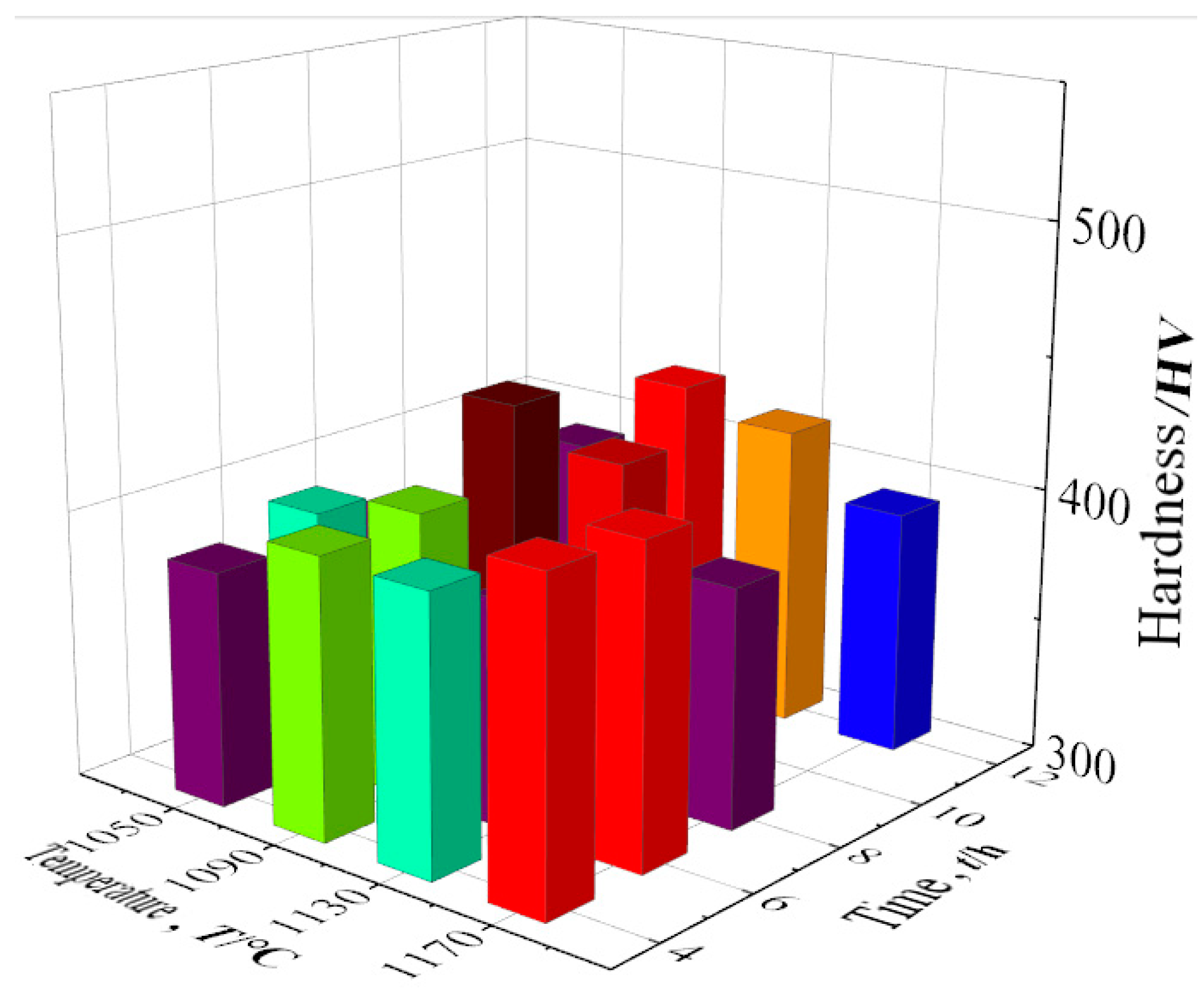
The mechanical properties and high-temperature properties of the new low-cobalt nickel-based superalloy were tested in a series of experiments, which were verified with the observation results of the heat treatment structure. Figure 18 shows the hardness values of the alloy after different aging heat treatments. For detailed data, see Table 9.
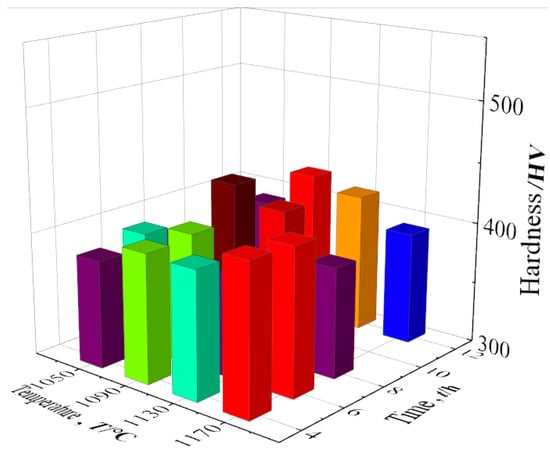
Figure 18.
Alloy hardness values in different states.

Table 9.
Alloy hardness value.
Compared with the hardness at other temperatures, the hardness at 1050 °C is generally very low, mainly due to the low aging temperature of the alloy, with the insufficient diffusion driving force of the element leading to the low diffusion rate of solute atoms in the alloy, and the precipitation amount of γ′ strengthened phase is lower.
When the aging temperature is 1090 °C, the hardness of the alloy increases gradually with the extension of holding time, and the hardness of the alloy reaches the peak value of 435.8 HV after 8 h of treatment. When the aging temperature is 1130 °C, the alloy hardness decreases first and then increases with the aging time, but the hardness value is lower than that at 1090 °C. The further increase in aging temperature leads to the continuous increase in γ′ particles, coarsening and partial strengthening phase re-dissolution, and the volume proportion decreases. When the aging temperature is 1170 °C, the high temperature leads to a large amount of γ′ strengthened phase re-dissolution, and the alloy is in an over-aging state, resulting in a decrease in the hardness of the alloy. According to the above analysis of alloy hardness, it can be concluded that in these 16 cases, the 1090 °C/8 h aging effect is the best.
- (2)
- High-temperature tensile property
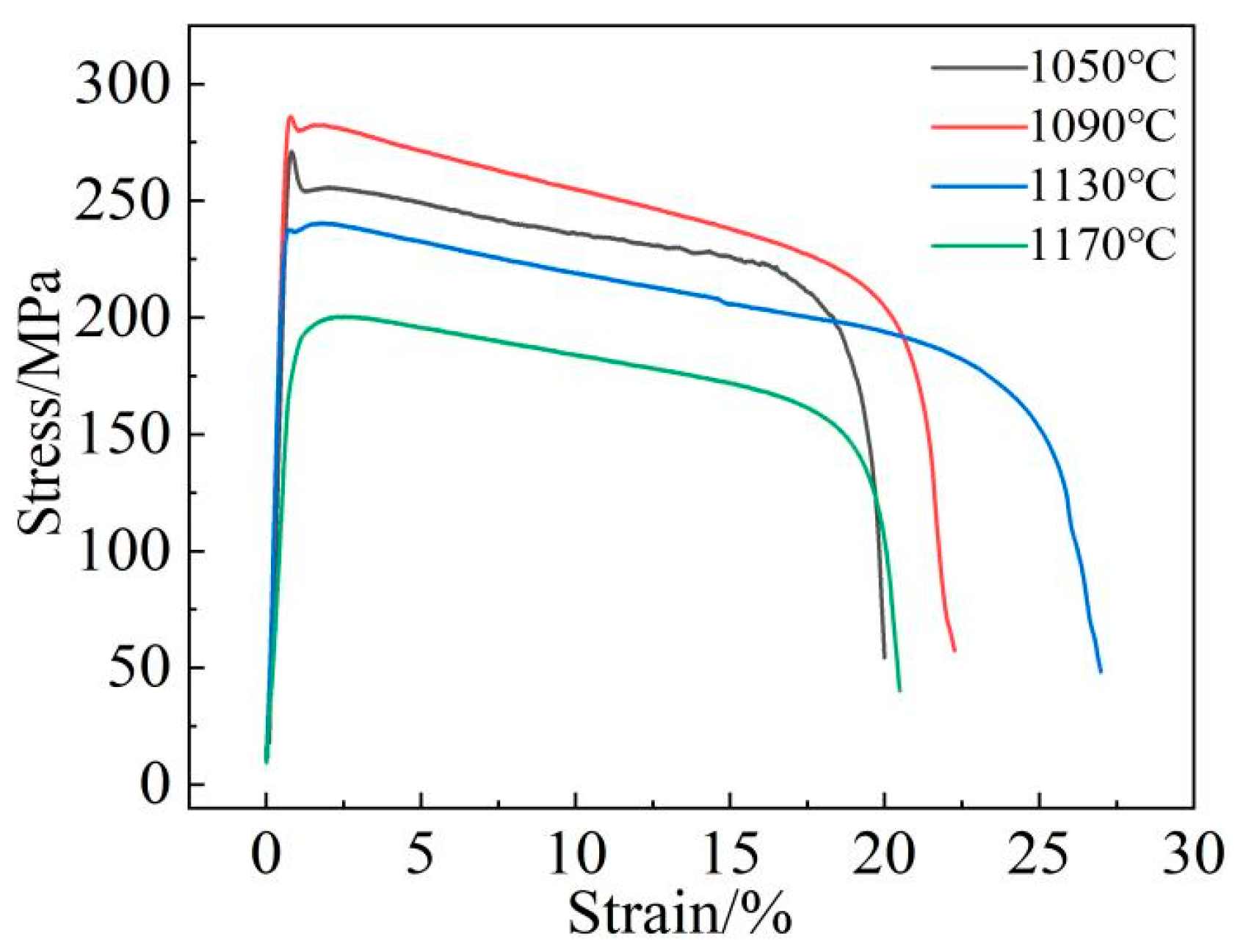
Figure 19 shows the stress–strain curve of the new low-cobalt alloy after treatment at different aging temperatures (1050/1090/1130/1170 °C, 8 h) at 1000 °C. It is clear that the alloy has the best high-temperature strength after aging at 1090 °C.
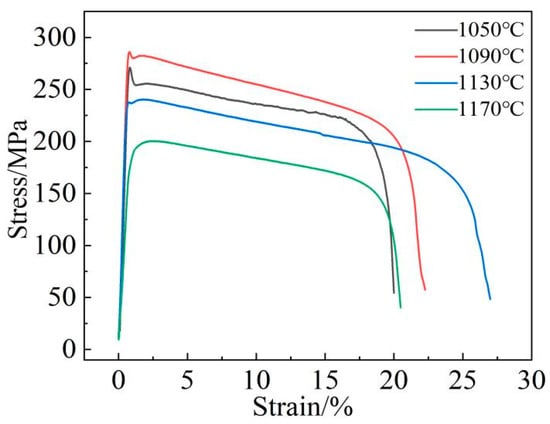
Figure 19.
Tensile curve of low-cobalt alloy at 1000 °C.
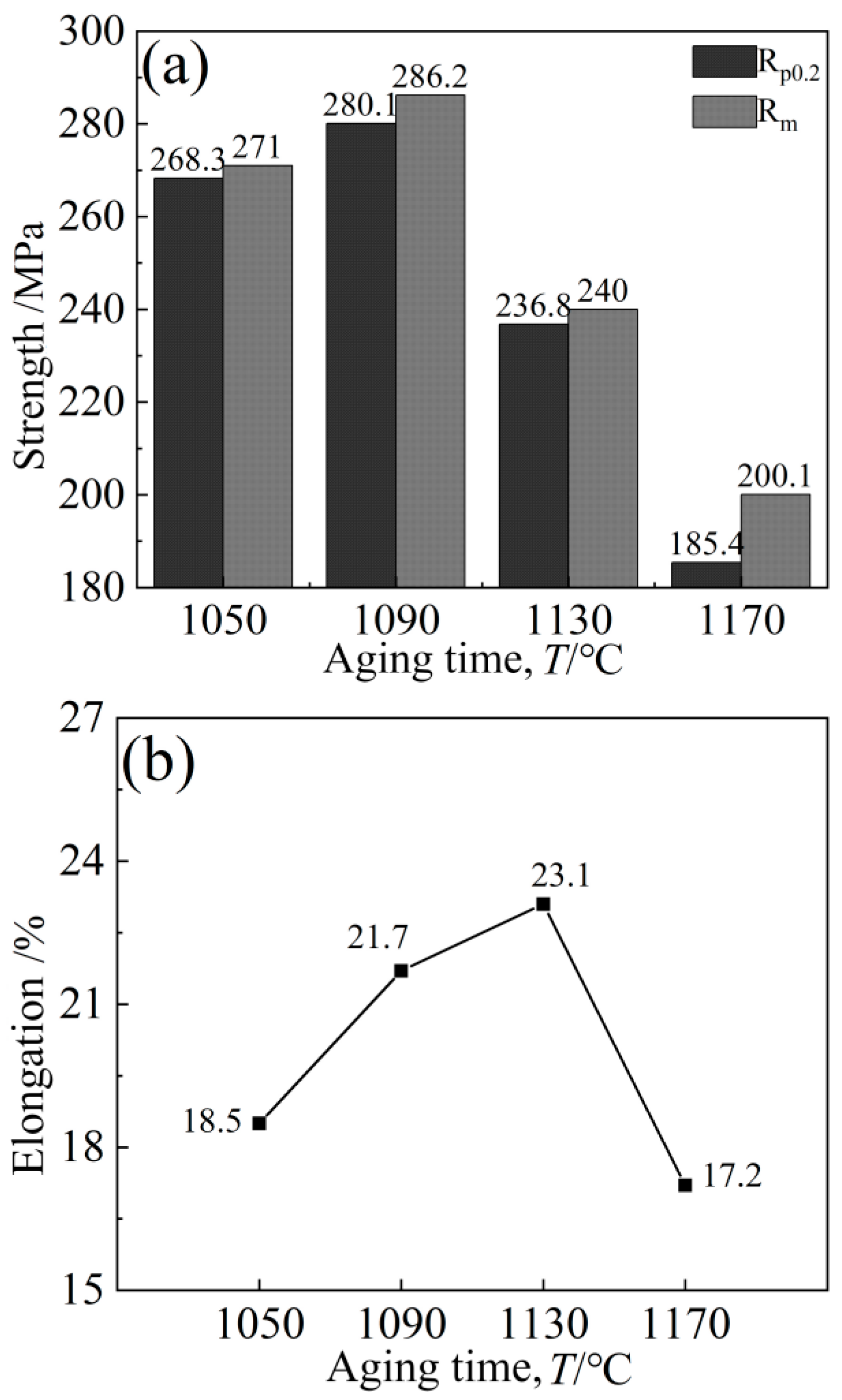
The variation characteristics of the alloy curves are basically the same, and the tensile curves are all elasto-uniform deformation. The initial deformation is elastic; the stress should become proportional; and the straight slope is large. When the applied load exceeds the yield strength of the alloy, the alloy will undergo plastic deformation until the final fracture. With the aging temperature increasing, the yield strength, tensile strength and elongation of the alloy all showed a trend of increasing first and then decreasing, but the inflection point temperatures of the strength and elongation changes were different (Figure 20), namely 1090 °C and 1130 °C, respectively.
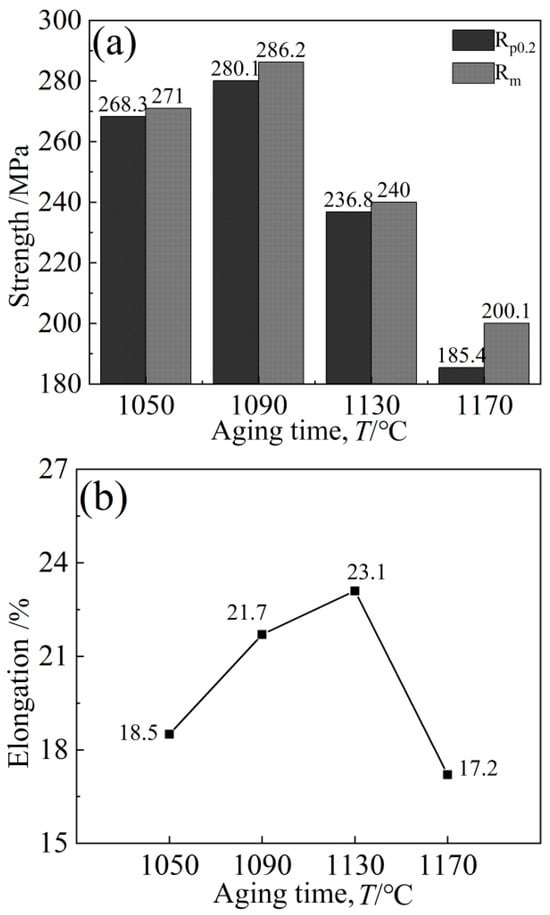
Figure 20.
Variation trend of alloy strength and elongation: (a) Strength, (b) Elongation.
- (3)
- High-temperature durability
The creep endurance properties of the sample with optimal microstructure (1090 °C, 8 h) after heat treatment were tested at high temperature and low stress (1000 °C/230 MPa) and medium temperature and high stress (870 °C/655 MPa). The test results are shown in Table 10. After the solution aging treatment, the new low-cobalt nickel-based superalloy can meet the service environment requirements of turbocharging turbine materials and achieve the initial purpose of alloy design: lasting life of more than 90 h at 1000 °C/230 MPa.

Table 10.
Creep test results.
- (4)
- Reinforcement mechanism analysis
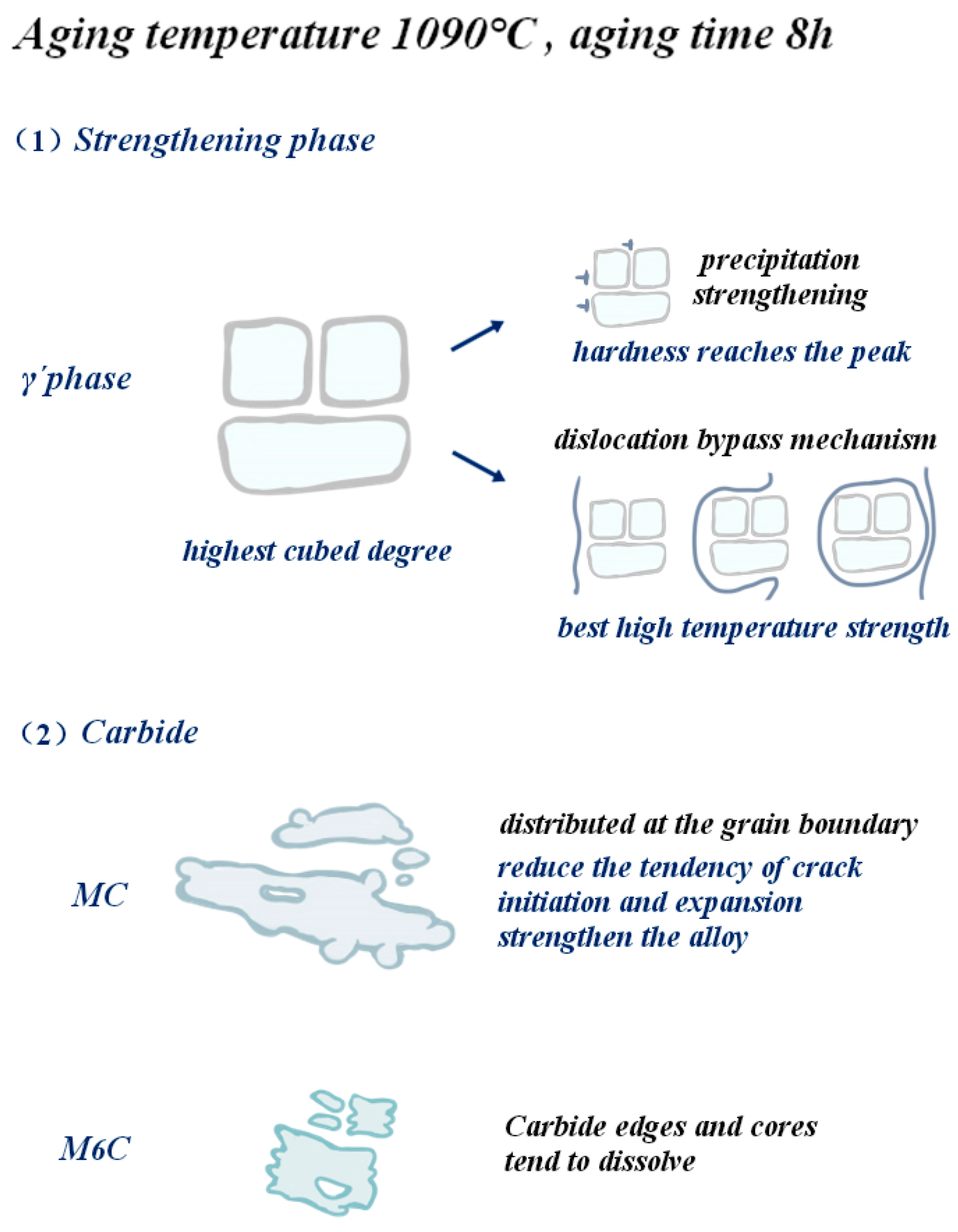
Through the above analysis, we found that when the aging temperature is 1090 °C and the holding time is 8 h, the alloy hardness reaches the peak of 435.8 HV, and the high-temperature strength is best after reaching 280.1 MPa. High-temperature durability achieves the initial purpose of alloy design: 1000 °C/230 MPa lasting life greater than 90 h. Below, we will elaborate on the mechanism of aging treatment to strengthen the properties of alloys.
The purpose of the aging process of low-cobalt nickel-based superalloy is essentially to promote the precipitation and growth of the γ′ phase through the diffusion of solute atoms. The aging temperature and time play a key role in controlling the nucleation, growth and coarsening of the precipitated phase. Figure 21 visually shows the microstructure of the precipitated phase and the enhancement of high-temperature properties when the aging temperature is 1090 °C and the holding time is 8 h. It is found that the cubic degree of the strengthened γ′ phase is the highest, and the size and volume fraction of carbide are small.
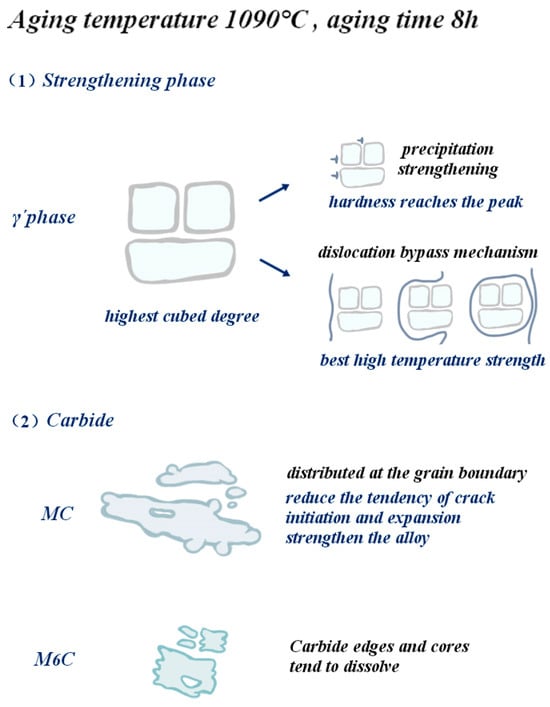
Figure 21.
Effect of optimal aging treatment on the gamma´ phase and carbide and schematic diagram of strengthening of alloy properties.
When the aging temperature is 1090 °C and the holding time is 8 h, the diffusion coefficient of susaturated solute atoms in the matrix is large, which makes them more inclined to diffuse toward the two-phase interface and enhances their tendency to advance toward the two-phase interface. As a result, the γ′ phase grows outwardly along the existing interface in an expanding manner, and the volume fraction of the γ′ phase is increased. At the same time, the form of the γ′ phase in this prescription system is cubic, and the cubic degree is the highest compared with other prescription systems. During the aging process, the morphology change in the γ′ phase is also affected by the decrease in the total energy of the alloy system. In the low-cobalt alloy, the crystal structure and lattice constant of the γ′ phase are similar to those of the matrix γ phase. Therefore, when the alloy is aged, the γ′ phase is often embedded into the [001] surface of the matrix γ phase in a coherent way, and the γ/γ′ two-phase interface energy and the elastic coherent strain energy are two key factors affecting the microstructure of the γ′ phase. With the increase in mismatch between the γ/γ′ phases, the coherent elastic strain energy becomes the primary factor affecting the total strain energy, and in order to maintain energy stability, the γ′ phase often exists in the lowest energy cubic form of the system. This is also one of the reasons why high cubic degree can be maintained after a 1090 °C, 8 h aging treatment. Regarding carbides, the chain continuous MC carbides are mainly distributed at the grain boundary, which has a tendency to reduce crack initiation and propagation and can enhance the properties of the alloy. In general, larger massive carbides are usually unfavorable to the properties of nickel-based alloys, and at this time, M6C carbides are smaller in size and have less impact on the properties of the alloy.
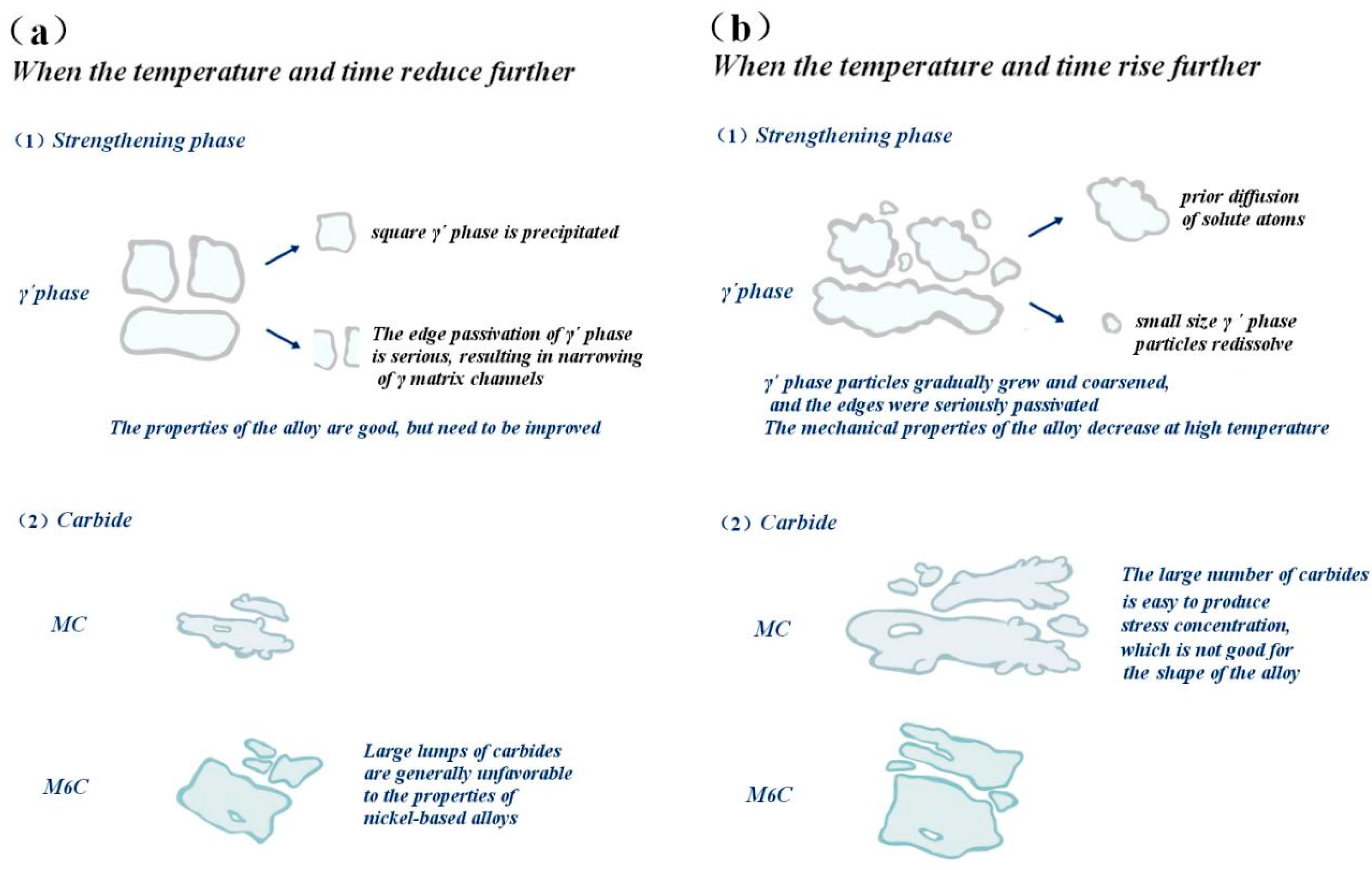
However, when the aging temperature and time deviate from the optimal conditions, the structure morphology of the γ′ phase and carbide will change, and the structure morphology change will affect the properties of the alloy; therefore, the alloy mechanics and high-temperature properties will change. This transformation process is visualized in Figure 22.
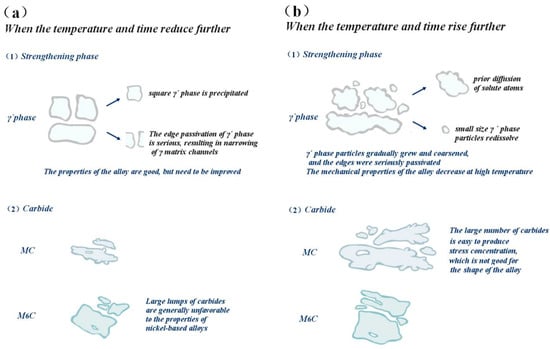
Figure 22.
The influence of deviation from the optimal aging treatment on the gamma´ phase and carbide and the trend of alloy properties: (a) Under-aging, (b) Over-aging.
When the aging temperature is lower than 1090 °C and the holding time is lower than 8 h, the cubic degree of the γ′ phase will decrease; the distribution will not be as uniform as at 1090 °C; the matrix channel will not be as clear as at a holding time of 8 h; and the volume fraction of γ′ phase precipitation will also be smaller. At this time, the alloy hardness is approximately 390 HV, which is less than the peak hardness of 435.8 HV, and the alloy hardness is generally very low, mainly due to the low aging temperature of the alloy, with the insufficient diffusion driving force of the element leading to the low diffusion rate of solute atoms in the alloy, and the precipitation amount of γ′ strengthened phase is lower. The tensile plasticity of the alloy is mainly affected by the interaction between carbide and γ′ phase change. Although both an increase in the size and a decrease in the number of strengthening phases contribute to the movement of the dislocation, thereby improving plasticity, the degree of influence varies. At this time, the carbide size increases slightly, reducing the yield strength of the alloy to 268.3 MPa.
When the aging temperature is higher than 1090 °C and the holding time is higher than 8 h, the cubic degree of the γ′ phase decreases; the structure is rod-like or circular; the arrangement is disordered; the edge is seriously passivated; and the matrix channel becomes narrow. At the same time, the precipitation of secondary γ′ phase was also observed. The fundamental reason for this is that the residual susaturated γ′ phase forming elements (Al, Ti, Ta) in the matrix are limited in the diffusion path, resulting in a longer diffusion distance, and these atoms cannot migrate to the original γ′ phase for growth but precipitate on the γ matrix channel instead. The further increase in aging temperature caused the γ′ particles to continue to increase, with coarsening occurring, and led to partial dissolution of the strengthened phase, and its volume proportion also decreased, decreasing the hardness slightly. However, the elongation increased from 18.5% to 21.7% due to the interaction between carbide and γ′ phase change.
However, when the aging temperature increased, the strengthening γ′ phase began to coarsen gradually, resulting in a reduction in the resistance of the dislocation to bypass the γ′ phase particles, and the yield strength dropped to 185.4 MPa. At the same time, the matrix channels of the γ′ phase were jagged, and some γ′ phases were connected together, which led to irregular morphology of the γ′ phase and further affected the properties of the alloy. High temperature leads to the re-dissolution of a large number of γ′ strengthened phases, leaving the alloy in an over-aged state, resulting in a decrease in hardness. The increase in large and massive M6C carbides in the alloy easily causes stress concentration, which has an adverse effect on the shaping of the alloy, decreasing the plasticity to 17.2%.
4. Conclusions
In this paper, the alloy was strengthened by means of solution and aging combined with OM and SEM to explore the microstructure evolution law in the heat treatment process of the alloy, and the optimal structure scheme was obtained, and its mechanical properties and high-temperature properties were studied. The following conclusions are drawn.
After being treated at different solution temperatures, the eutectic structure caused by element segregation gradually disappeared with the increase in temperature. After solid solution treatment at 1295 °C, the γ′ phase was closest to the cubic shape; γ′ phase precipitation accounted for the largest proportion; and the alloy had excellent properties. It is concluded that the 1295 °C solution treatment has the best effect.
The size, morphology and volume fraction of the γ′ phase in tissues treated at lower temperatures (1050 °C, 1090 °C) were significantly better than the other two temperatures, and the distribution of the γ′ phase at 1090 °C was uniform; the matrix channel was clear; and the volume fraction was higher. On the basis of fixed aging temperature (1090 °C), it was found that the volume fraction of the γ′ phase increased from 50.9% to 70.3% after 8 h and decreased after 12 h.
After an aging time of 8 h, the carbide volume proportion was at least 1.0%; the size was smaller than 20 μm; and the effect on the properties of nickel-based alloys was small. Finally, it was determined that the solution treatment at 1295 °C, 6 h+ aging at 1090 °C, 8 h was the optimal heat treatment system.
The hardness of the alloy reached the peak value of 435.8 HV after heat treatment. High-temperature tensile tests showed that the yield strength of the alloy increased from 268.3 MPa to 280.1 MPa when the aging temperature rose to 1090 °C. The results of high-temperature creep tests showed that the alloy had a long life of 99.7 h and 42.7 h, respectively, at 1000 °C/230 MPa and 870 °C/655 MPa, which achieved the purpose of alloy design.
Author Contributions
Conceptualization, L.S.; methodology, J.J.; software, L.S.; validation, Y.M. and J.J.; formal analysis, Y.M., Y.Z. and J.J.; investigation, J.C.; resources, J.C.; data curation, J.C.; writing—original draft preparation, J.J. and J.C.; writing—review and editing, L.S. and J.C.; visualization, J.C.; project administration, L.S. and Y.Z.; funding acquisition, L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (51901068), the Qinglan Project of Jiangsu Province and the High Value Patent Cultivation Project in Nanjing Institute of Technology (ZLKJ202201).
Data Availability Statement
The original contributions presented in the study are included in the article material, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Author Yue Zhang was employed by the company Helmholtz-Zentrum Hereon. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Yu, Q.; Dong, J.; Zhang, M.; Zheng, L. Thermodynamic calculation on equilibrium precipitated phases in GH720Li superalloy. Rare Met. Mater. Eng. 2010, 39, 857–861. [Google Scholar]
- Chen, J.; Xiao, J.; Lu, Z.; Wang, C.; Zhang, L. Atomic mobilities and interdiffusivities in Ni-rich fcc Ni-Co-Cr and Ni-Al-Co-Cr systems evaluated using composition profiles and HitDIC. J. Alloys Compd. 2021, 865, 158645. [Google Scholar] [CrossRef]
- Richaed, G.; Łukasz, R.; Zbigniew, G. Behaviour of Al, Co, Cr, Ni-based high entropy alloys under high-temperature thermal shock oxidising conditions. Corros. Sci. 2022, 198, 110116. [Google Scholar]
- Yang, Z.; Lu, S.; Tian, Y.; Gu, Z.; Mao, H.; Sun, J.; Vitos, L. Assessing the magnetic order dependent γ-surface of Cr-Co-Ni alloys. J. Mater. Sci. Technol. 2021, 80, 66–74. [Google Scholar] [CrossRef]
- Yu, X.; Zhou, J.; Wu, Y.; Yang, Y.; Wang, Y.; Man, Y.; Huang, A. Effect of element V on microstructure and high temperature creep properties of nickel based columnar crystal alloys. Chin. J. Mater. Res. 2016, 30, 465–472. [Google Scholar]
- Wang, Q.; Li, X.D. High temperature creep behavior analysis of nickel base alloy based on UMAT subroutine. Hot Work. Technol. 2014, 43, 112–115. [Google Scholar]
- Ban, Y.; Geng, Y.; Hou, J.; Zhang, Y.; Zhou, M.; Jia, Y.; Tian, B.; Liu, Y.; Volinsky, A.A. Properties and precipitates of the high strength and electrical conductivity Cu-Ni-Co-Si-Cr alloy. J. Mater. Sci. Technol. 2021, 93, 1–6. [Google Scholar] [CrossRef]
- Qiao, S.C.; Wang, Y.; Lv, L.X.; Tan, G.; Yang, L.; Huang, Z.Q.; Li, H. Finite element simulation and experimental study on dynamic recrystallization of Ni-Co-Cr based powder superalloy. J. Cent. South Univ. Sci. Technol. 2021, 52, 3405–3418. [Google Scholar]
- Shen, Q.K.; Kong, X.D.; Chen, X.Z. Fabrication of bulk Al-Co-Cr-Fe-Ni high-entropy alloy using combined cable wire arc additive manufacturing(CCW-AAM): Microstructure and mechanical properties. J. Mater. Sci. Technol. 2021, 74, 136–142. [Google Scholar] [CrossRef]
- Li, C.L.; Choi, S.W.; Jeong, M.O.; Hong, J.K.; Yeom, J.T.; Kang, J.H.; Mei, Q.S.; Park, C.H. Bimodal grain structures and tensile properties of a biomedical Co-20Cr-15W-10Ni alloy with different pre-strains. Rare Met. 2021, 40, 20–30. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, X.; Liu, L.; Jiang, P.; Song, D.; Subramanian, M.A. Synthesis and optical properties of Ni/Co/Cr doped BaMg6Ti6O19. Ceram. Int. 2021, 47, 34086–34091. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, W.; Wang, J.-J.; Lu, X.-G.; Zhang, L. Thermodynamic assessment of the Ni–Co–Cr system and Diffusion Study of its fcc phase. Calphad 2020, 71, 101996. [Google Scholar] [CrossRef]
- Wang, K.; Jin, H.; Xu, L.; Zhao, L.; Han, Y.; Li, H.; Song, K. Microstructure evolution of 55Ni-23Cr-13Co-based alloy during cyclic deformation at high temperature. Trans. Nonferrous Met. Soc. China 2021, 31, 3452–3468. [Google Scholar] [CrossRef]
- Li, X.; Jia, C.; Jiang, Z.; Zhang, Y.; Lv, S. Investigation of Solidification Behavior in a New High Alloy Ni-Based Superalloy. JOM 2020, 72, 4139–4147. [Google Scholar] [CrossRef]
- Wan, Z.; Hu, L.; Sun, Y.; Wang, T.; Li, Z.; Zhang, Y. Effect of solution treatment on microstructure and tensile properties of a U720LI Ni-based superalloy. Vacuum 2018, 156, 248–255. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.N.; Yu, X.X.; Wang, C.Y.; Yu, T.; Zhang, Z. Effect of solution time on the distribution and microstructure of Re and Ru elements in Nickel-based single crystal superalloys. J. Electron Microsc. 2014, 33, 318–323. [Google Scholar]
- Cowen, C.J.; Danielson, P.E.; Jablonski, P.D. The Microstructural Evolution of Inconel Alloy 740 During Solution Treatment, Aging, and Exposure at 760 °C. J. Mater. Eng. Perform. 2011, 20, 1078–1083. [Google Scholar] [CrossRef]
- Baldan, R.; da Silva, A.A.A.P.; Nunes, C.A.; Couto, A.A.; Gabriel, S.B.; Alkmin, L.B. Solution and Aging of MAR-M246 Nickel-Based Superalloy. J. Mater. Eng. Perform. 2017, 26, 465–471. [Google Scholar] [CrossRef]
- Wei, K.; Wang, T.; Li, Z.; Wan, Z.; Zhang, Y. Effect of aging regime on microstructure and properties of a new type of nickel-based deformed superalloy. J. Mater. Heat Treat. 2021, 42, 61–65. [Google Scholar]
- Shi, Z.; Dong, J.; Zhang, M.; Zheng, L. Solidification characteristics and hot tearing susceptibility of Ni-based superalloys for turbocharger turbine wheel. Trans. Nonferrous Met. Soc. China 2014, 24, 2737–2751. [Google Scholar] [CrossRef]
- Sun, M.; Wang, H.Z.; Long, H.B.; Mao, S.C.; Zhang, Z.; Han, X.D. Effect of Solid Solution Processing on the Initial Melting Temperature of Nickel-based Single-crystal Superalloy. J. Electron Microsc. 2024, 43, 146–154. [Google Scholar]
- Wang, J.; Zhou, L.; Sheng, L.; Guo, J. The microstructure evolution and its effect on the mechanical properties of a hot-corrosion resistant Ni-based superalloy during long-term thermal exposure. Mater. Des. 2012, 39, 55–62. [Google Scholar] [CrossRef]
- GB/T 4338-2006; The Standardization Administration of China. Test Method for High Temperature Tension of Metallic Materials. Standards Press of China: Beijing, China, 2006.
- Yao, Z.; Zhou, B.; Yao, K.; Wang, H.; Dong, J.; Davey, T. Influence and Sensitivity of Temperature and Microstructure on the Fluctuation of Creep Properties in Ni-Base Superalloy. Materials 2020, 13, 4758. [Google Scholar] [CrossRef] [PubMed]
- Tancret, F.; Bhadeshia, H.K.D.H.; MacKay, D.J.C. Design of a creep resistant nickel base superalloy for power plant applications. Mater. Sci. Technol. MST Publ. Inst. Met. 2003, 19, 283–290. [Google Scholar] [CrossRef]
- Nishimoto, K.; Saida, K.; Shinohara, Y. Computer aided alloy design of insert metal for transient liquid phase bonding of γ/γ′/β type high aluminium nickel base superalloy. Sci. Technol. Weld. Join. 2003, 8, 29–38. [Google Scholar] [CrossRef]
- Joseph, C.; Thuvander, M.; Persson, C.; Colliander, M.H. Precipitation of γ′ during cooling of nickel-base superalloy Haynes 282. Philos. Mag. Lett. 2021, 101, 30–39. [Google Scholar] [CrossRef]
- Ma, F.; Lu, X.Y.; Zhou, L.N.; Du, N.Y.; Lu, C.S.; Liu, H.W.; Li, D.Z. High temperature transition mechanism of M2C primary carbide in M50 Steel. Acta Met. 2024, 60, 901–914. [Google Scholar]
- Wang, L.N.; Zheng, Q.; Sun, X.F.; Guan, H.R. Oxygen and Nitrogen Effects on MC Carbide in K465 Nickel-Base Superalloy. Rare Met. Mater. Eng. 2009, 38, 13–16. [Google Scholar]
- Li, J.; Li, L.Z.; Hou, J.S.; Zhou, L.Z. Effect of Heat Treatment on microstructure evolution and mechanical Properties of a cast Nickel-based superalloy. J. Mater. Hot Tx. Technol. 2022, 43, 65–78. [Google Scholar] [CrossRef]
- Zhang, H.L.; Gong, W.; Jiang, Z.H.; Wang, P.F. Effect of Magnesium on primary carbide precipitation in GH3625 alloy. J. Iron Stl. Res. 2022, 57, 148–155. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).