Research on the Macro-Cell Corrosion Behavior of Alloyed Corrosion-Resistant Steel for a Transmission Line Steel Structure under a Chloride Corrosion Environment
Abstract
:1. Introduction
2. Materials and Methods
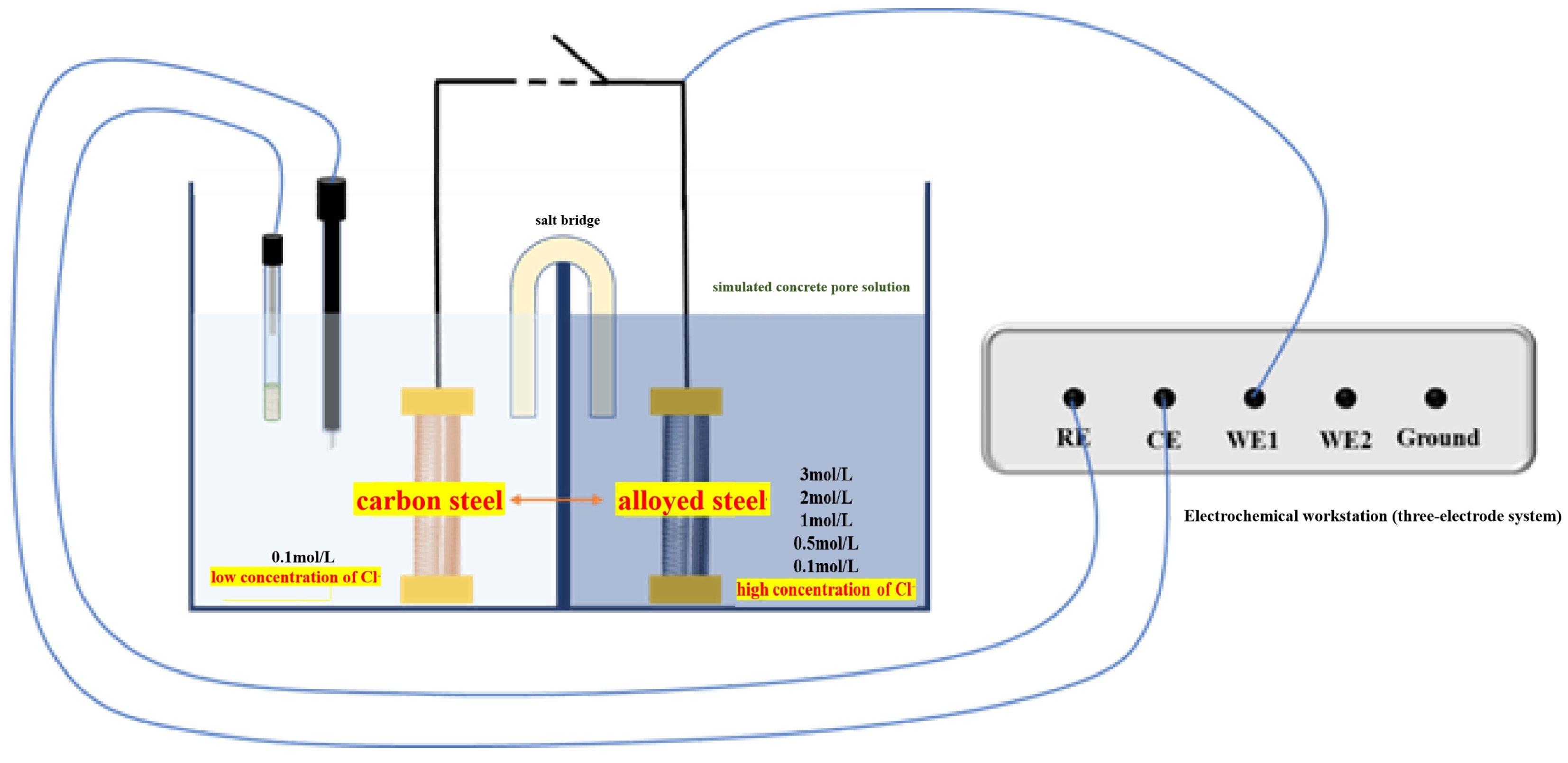
2.1. Preparation of Steel Samples and Materials
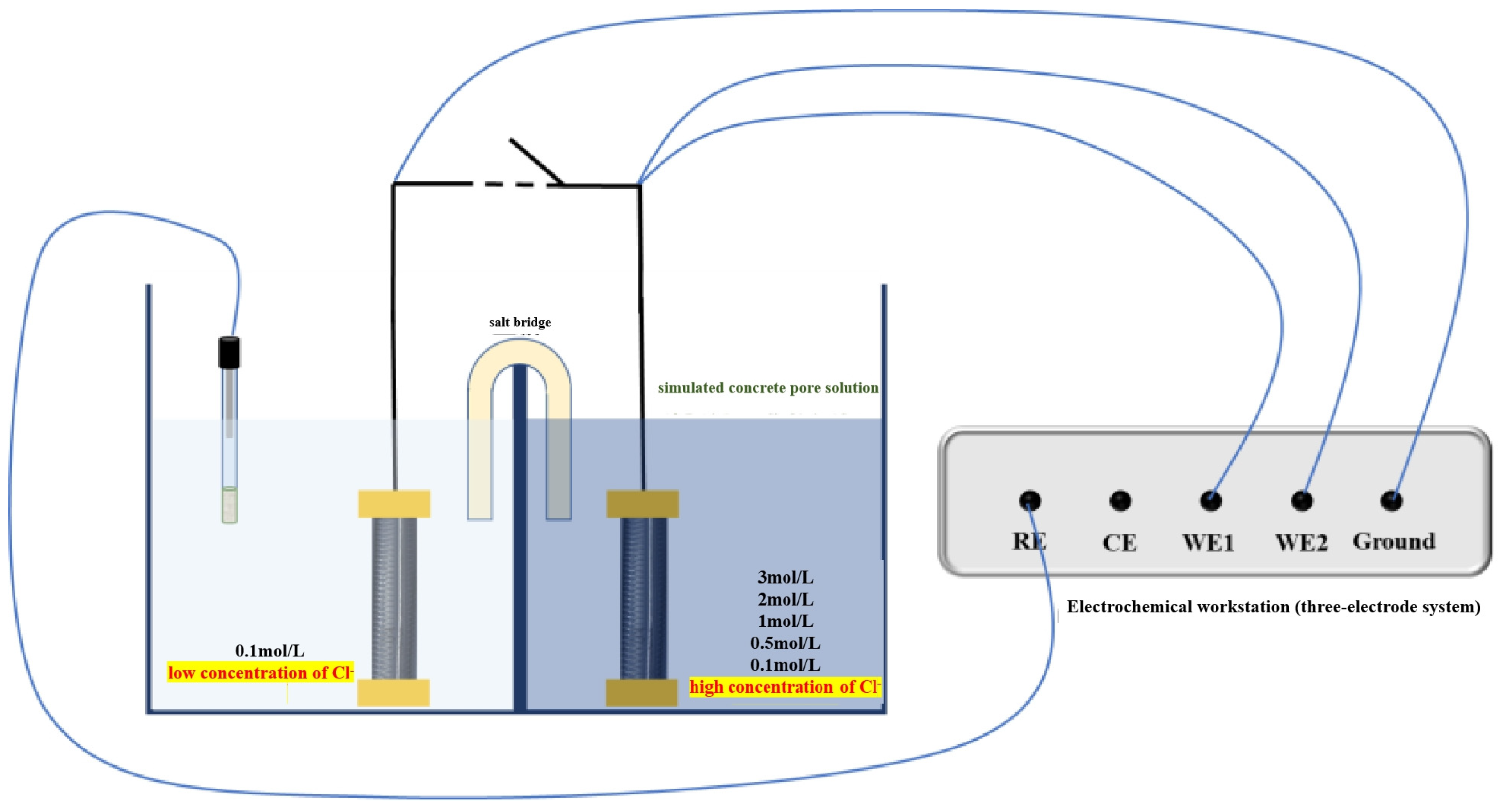
2.2. Electrochemical Testing Techniques
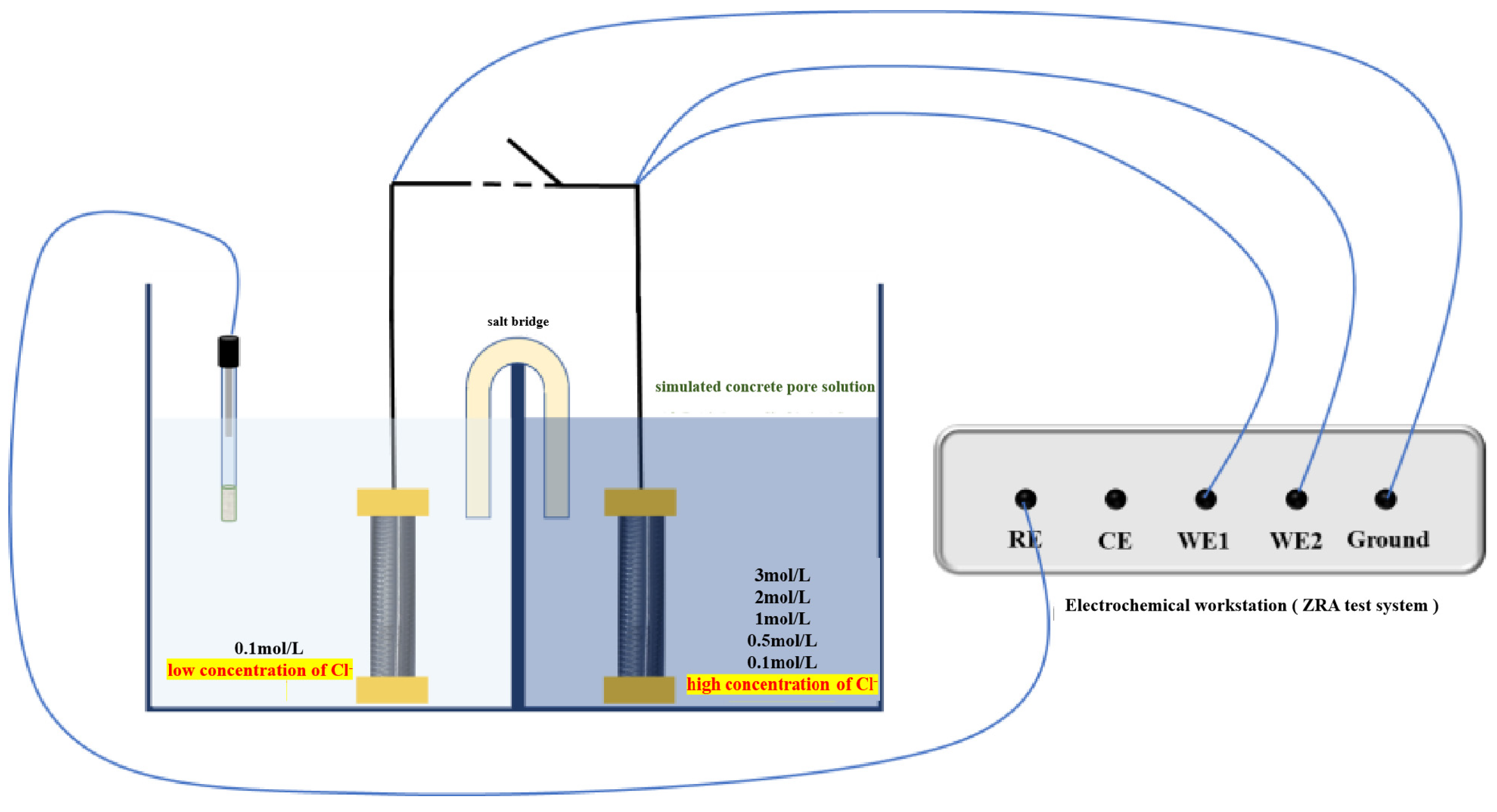
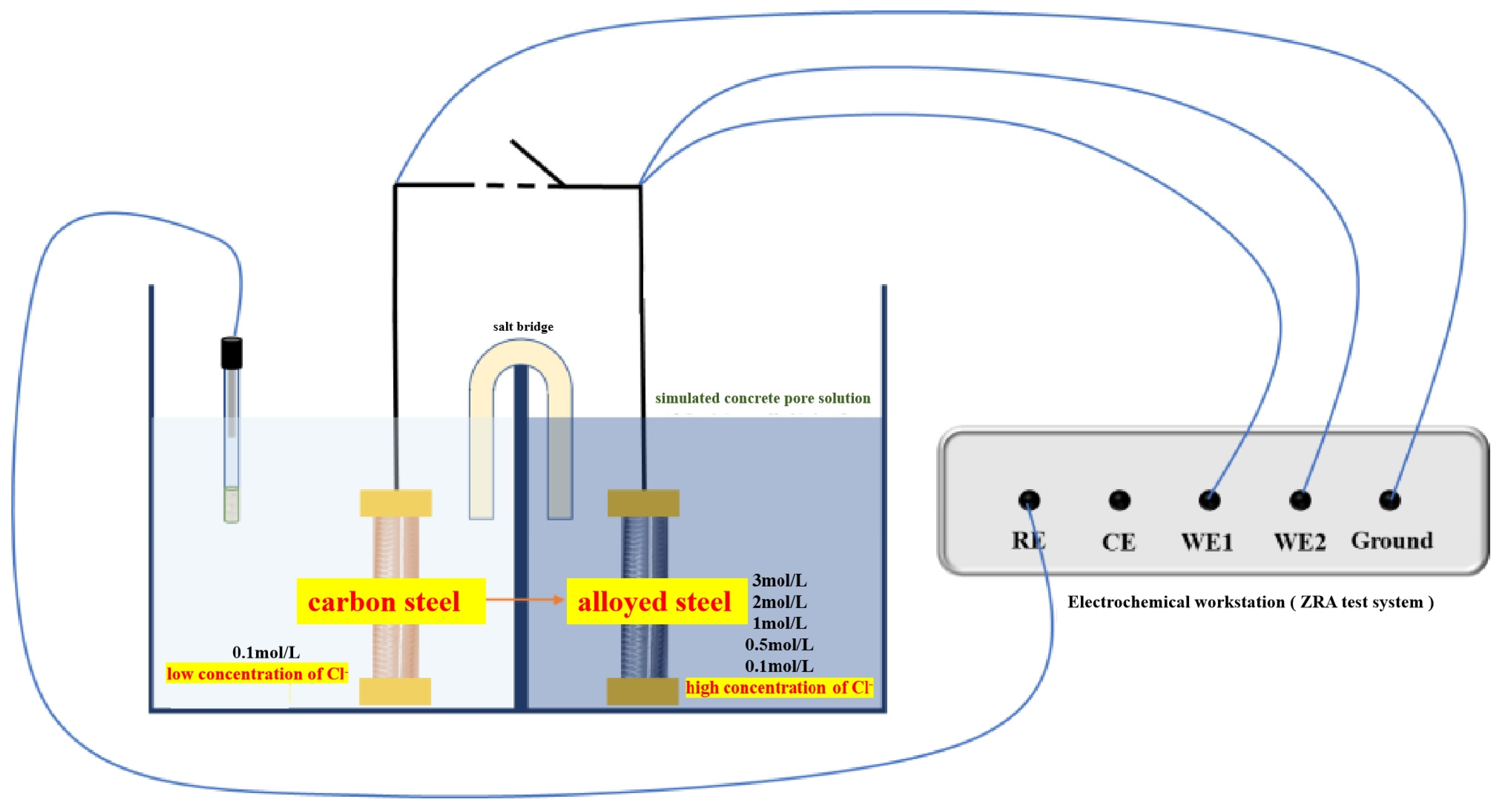
2.2.1. Macro-Cell Corrosion Current and Coupling Potential Testing
2.2.2. Electrochemical Impedance Spectroscopy (EIS) Testing
2.2.3. Potentiodynamic Polarization (PDP) Testing
3. Results and Discussion
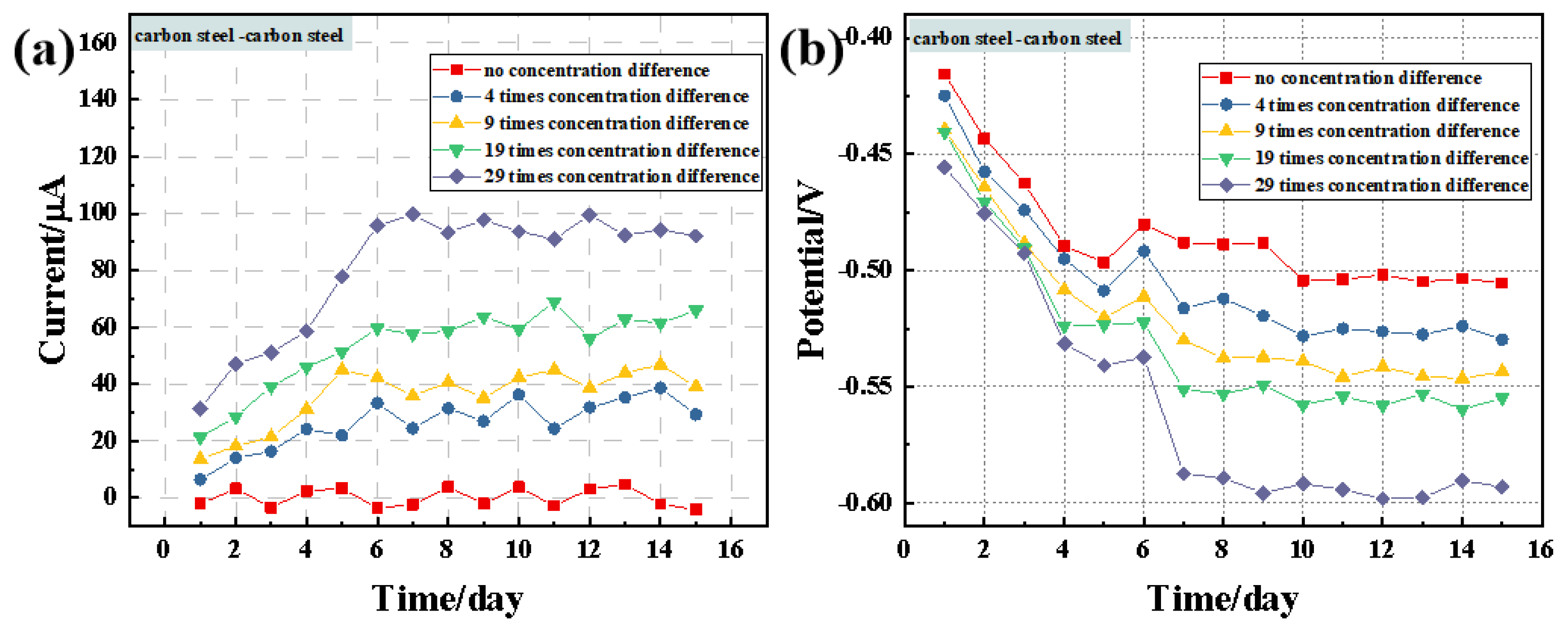
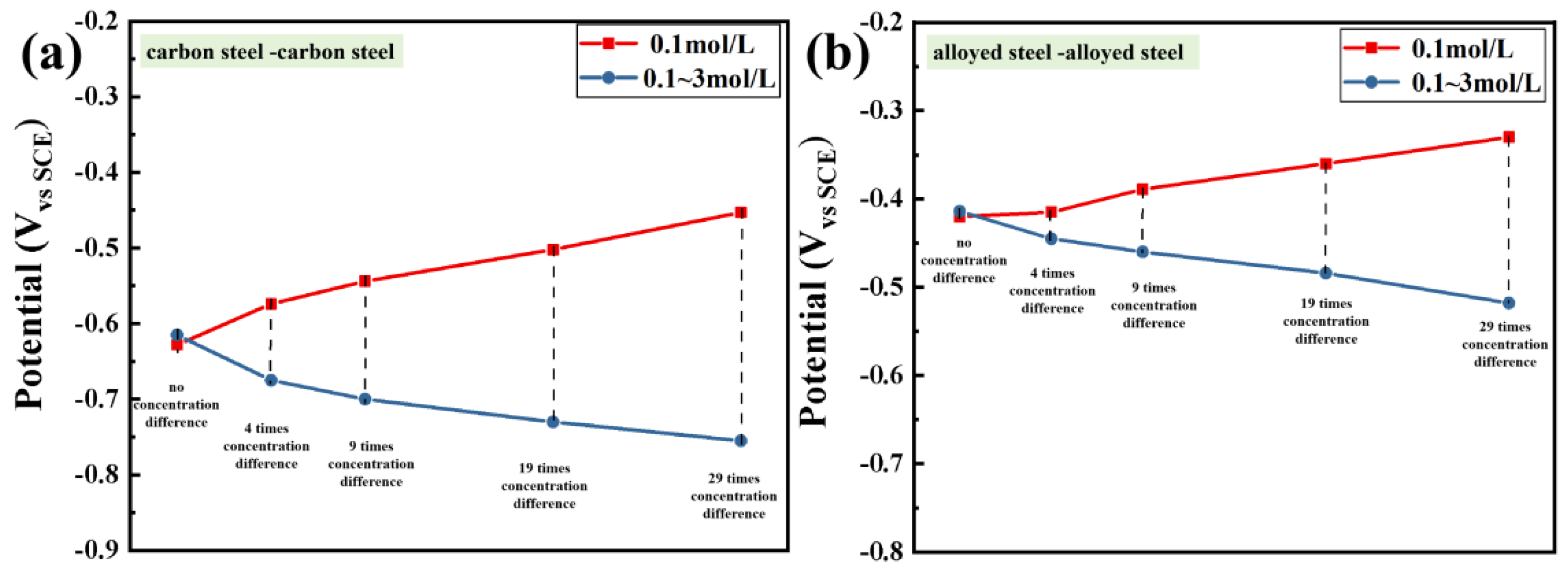
3.1. Macro-Cell Corrosion Induced by the Chloride Ion Concentration Difference for the Same Steel Based on the ZRA Testing
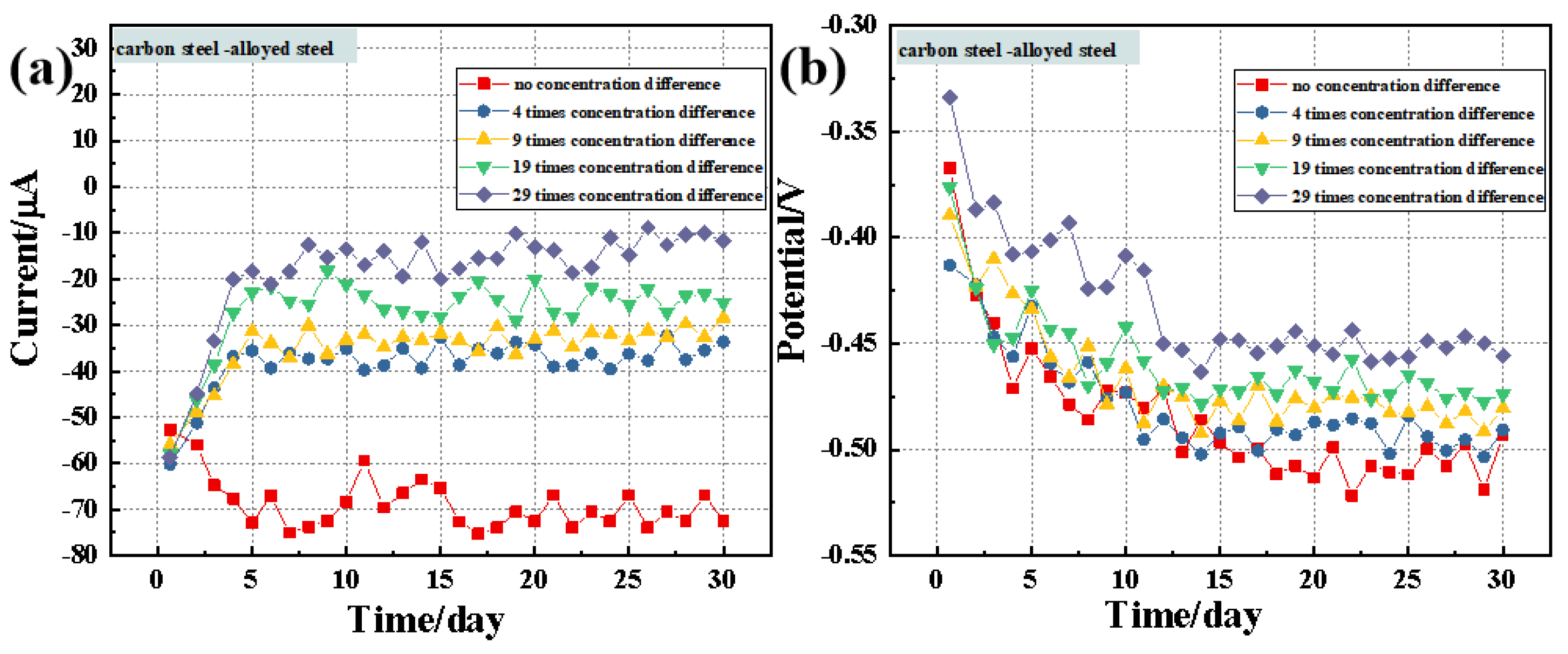
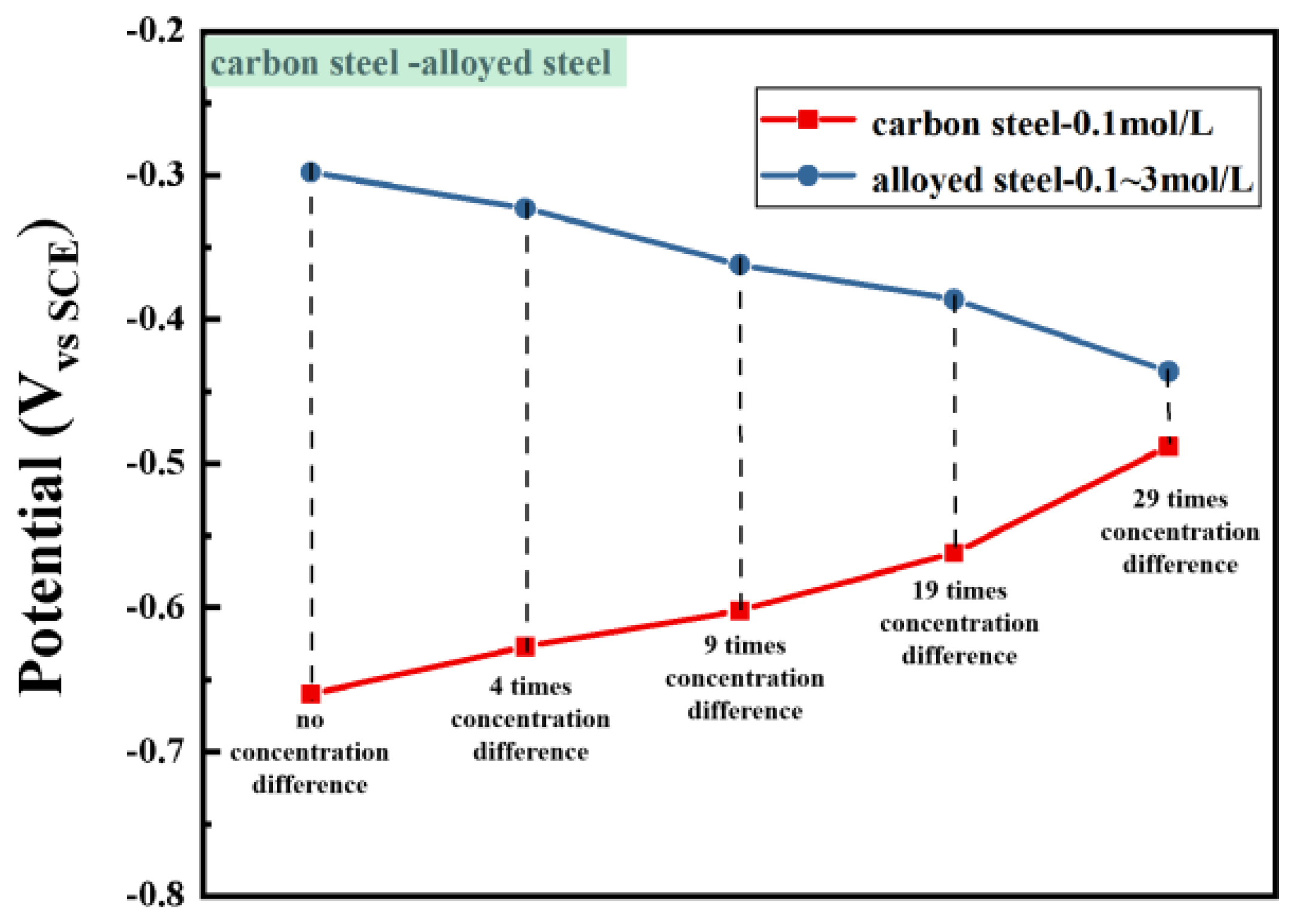
3.2. Macro-Cell Corrosion Induced by the Chloride Ion Concentration Difference in Dissimilar Steel Based on the ZRA Testing
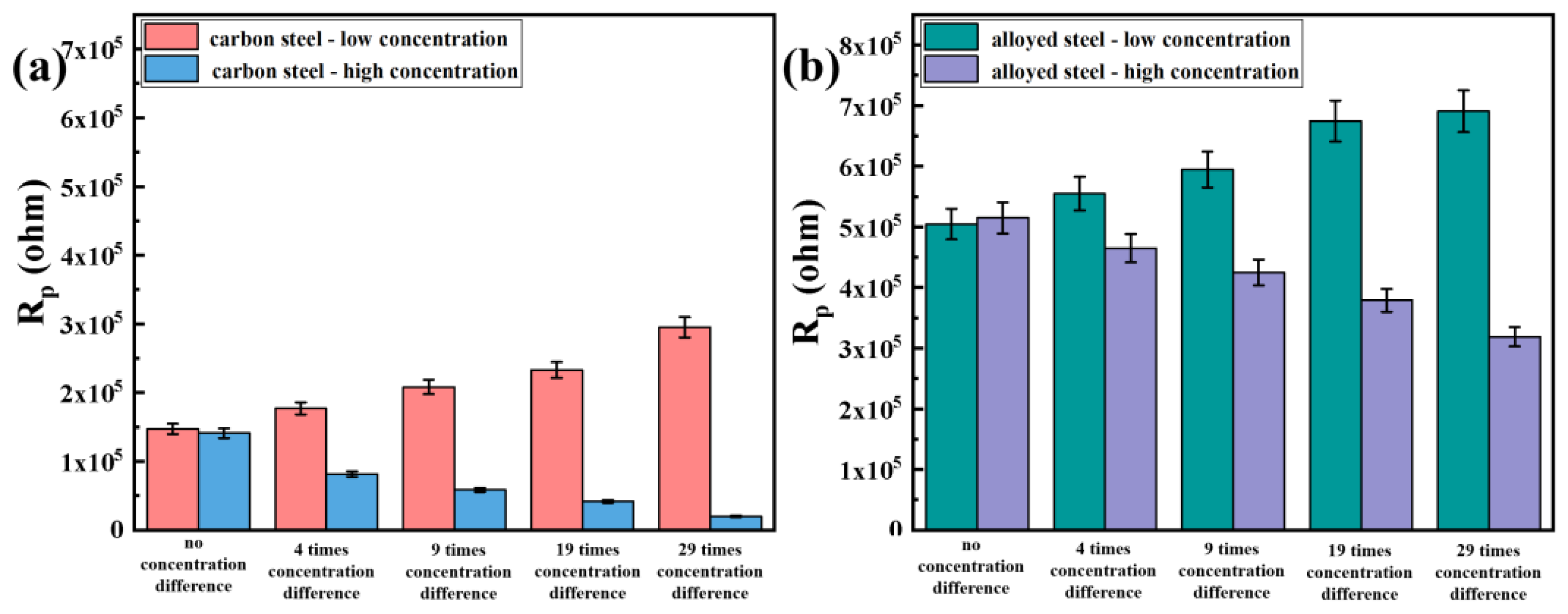
3.3. Evaluation of Corrosion Resistance after the Macro-Cell Corrosion Induced by the Chloride Ion Concentration Difference in the Same Steel
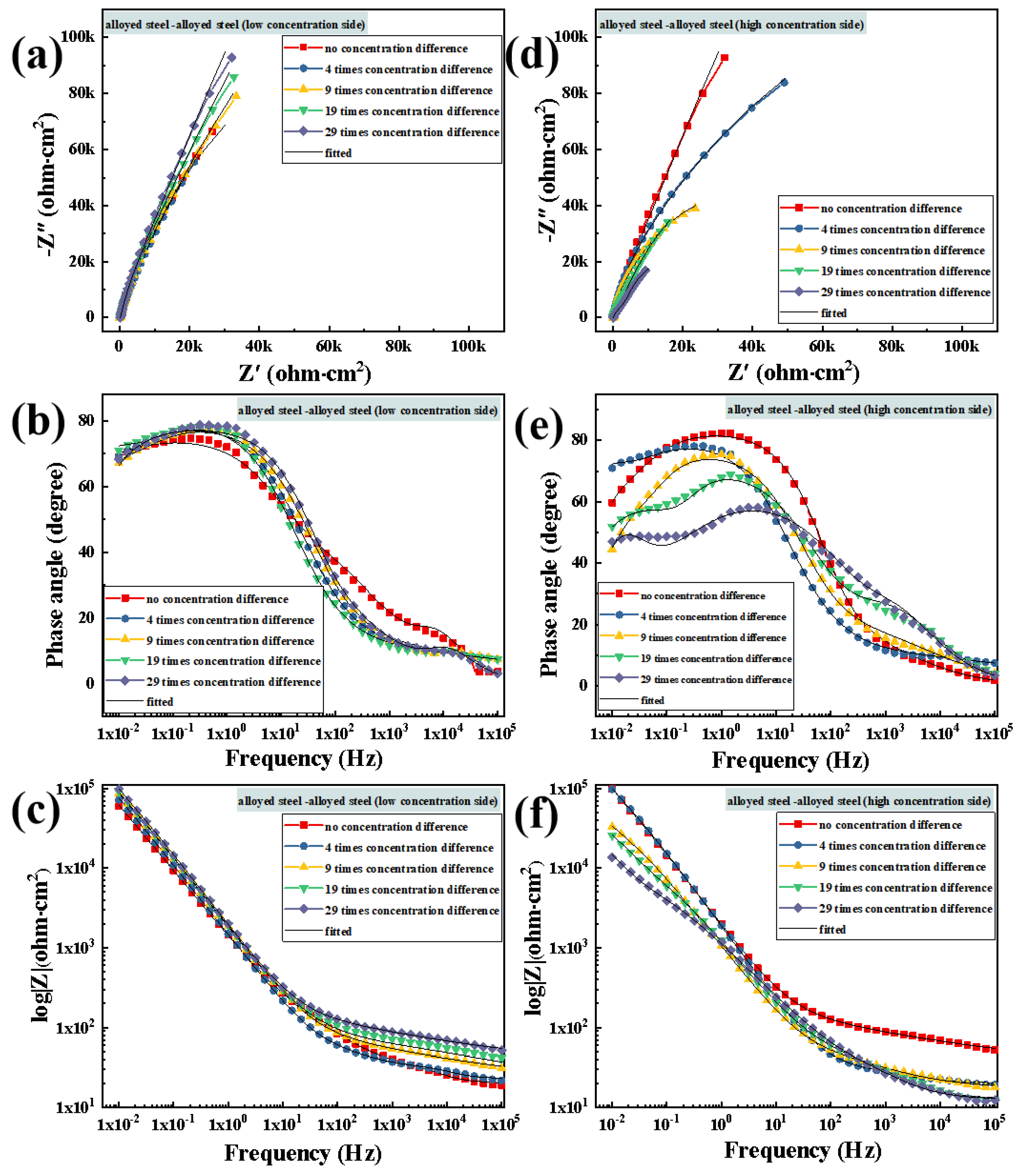
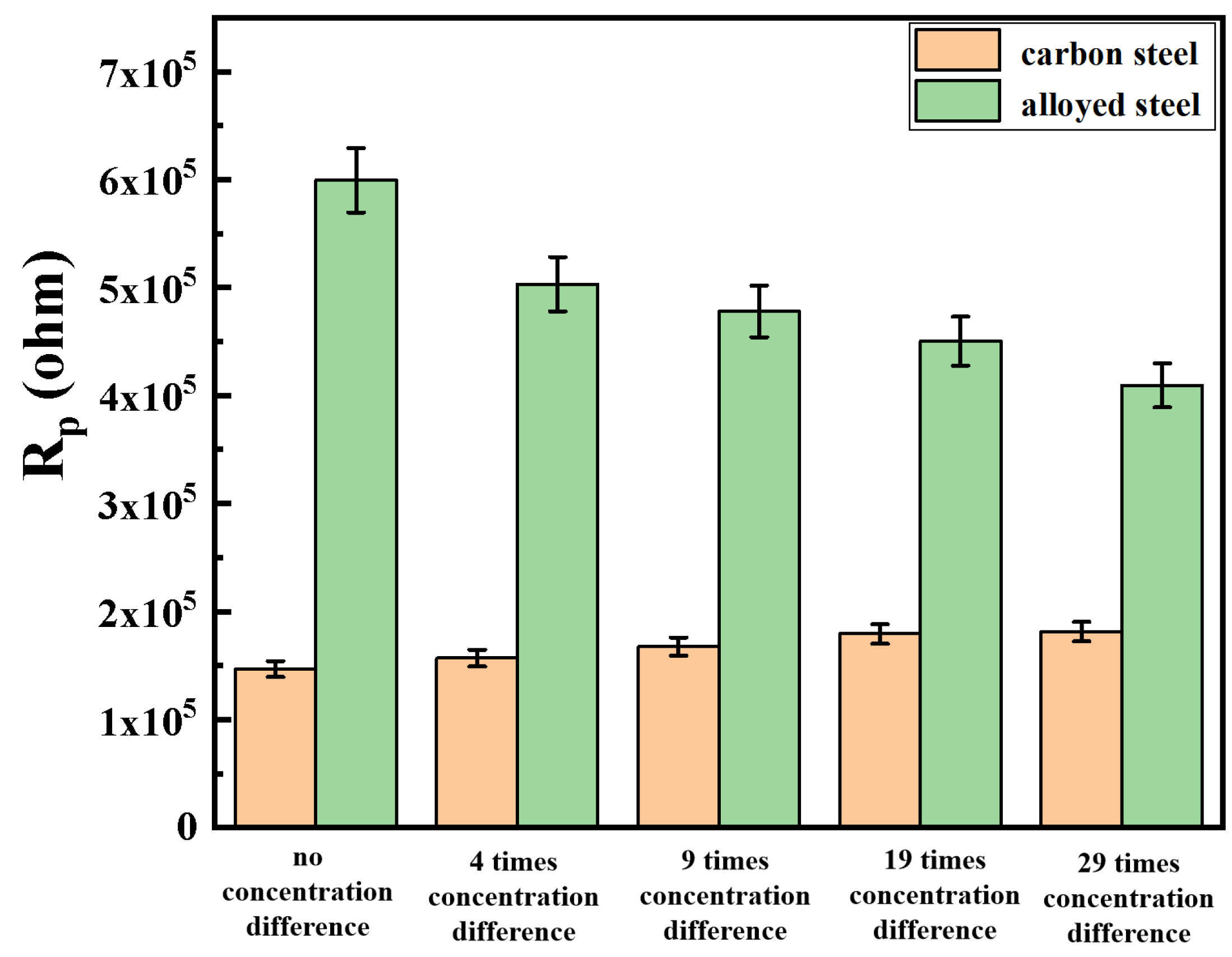
3.3.1. Electrochemical Impedance Spectroscopy Testing after Macro-Cell Corrosion
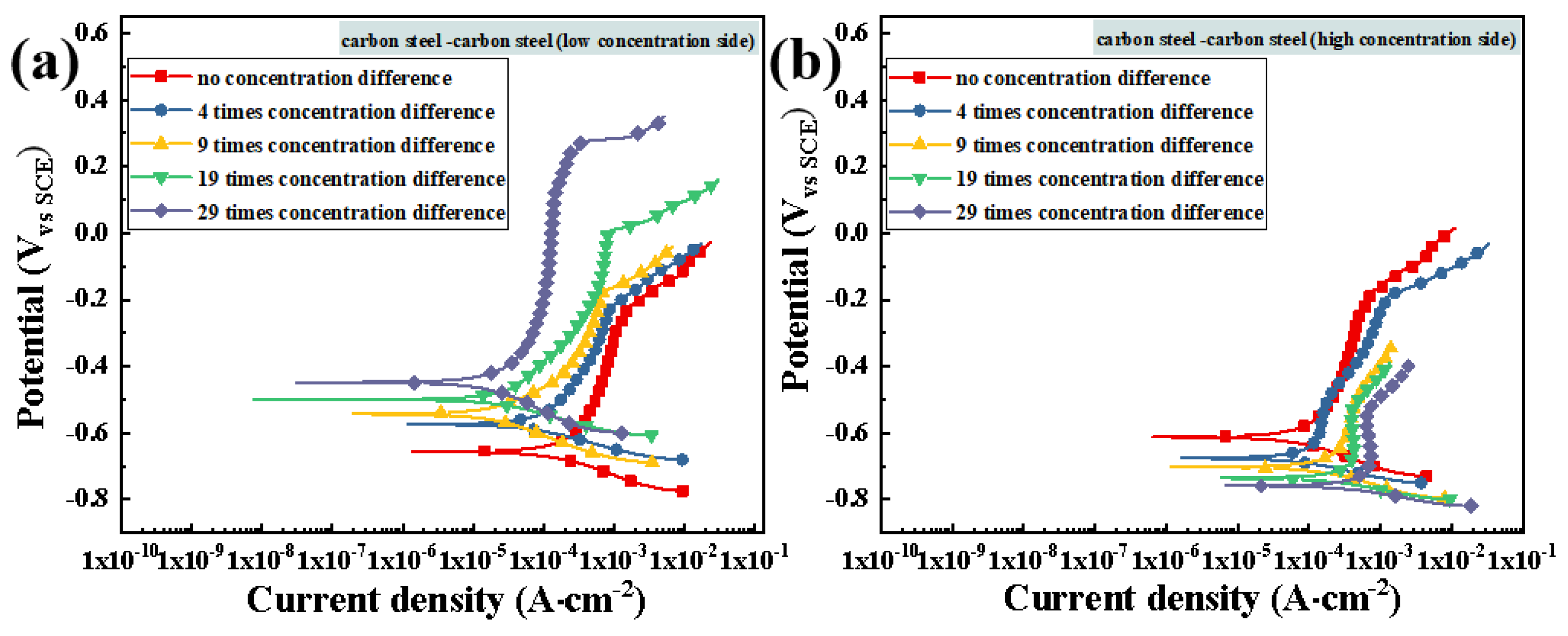
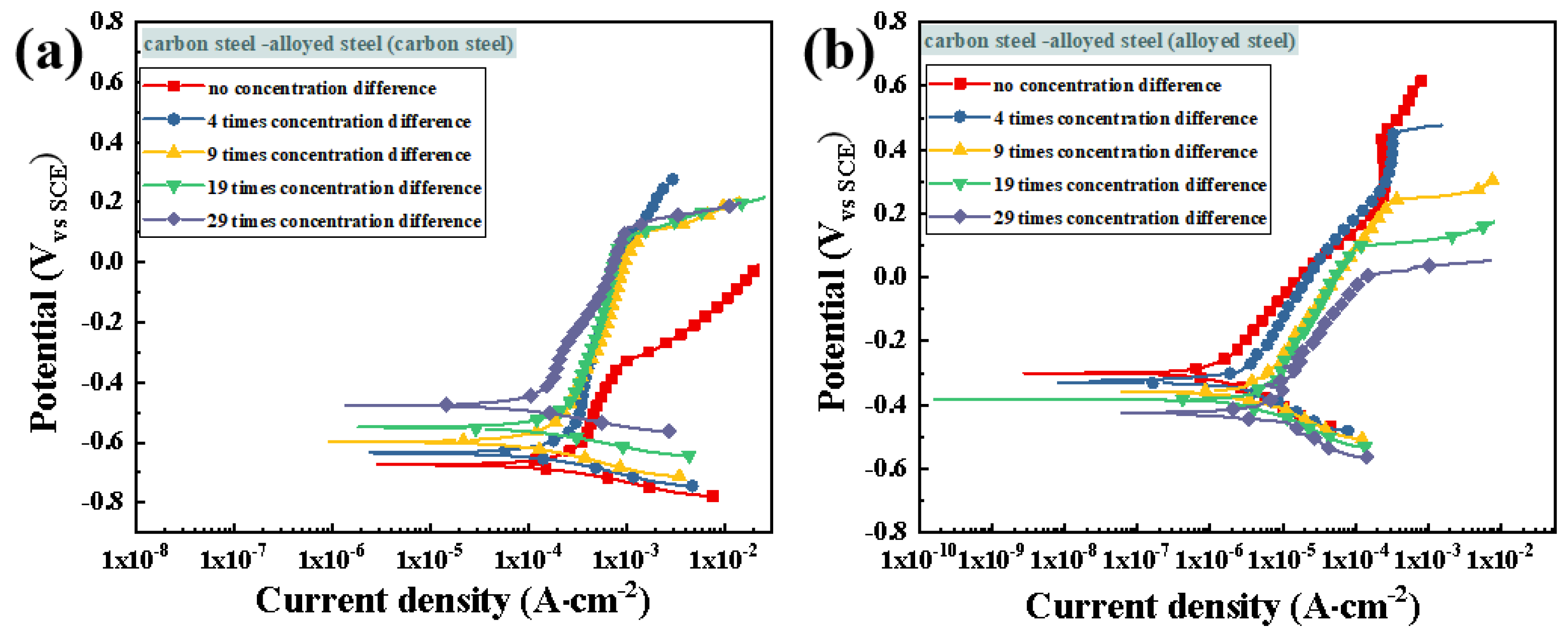
3.3.2. Potentiodynamic Polarization Testing after Macro-Cell Corrosion
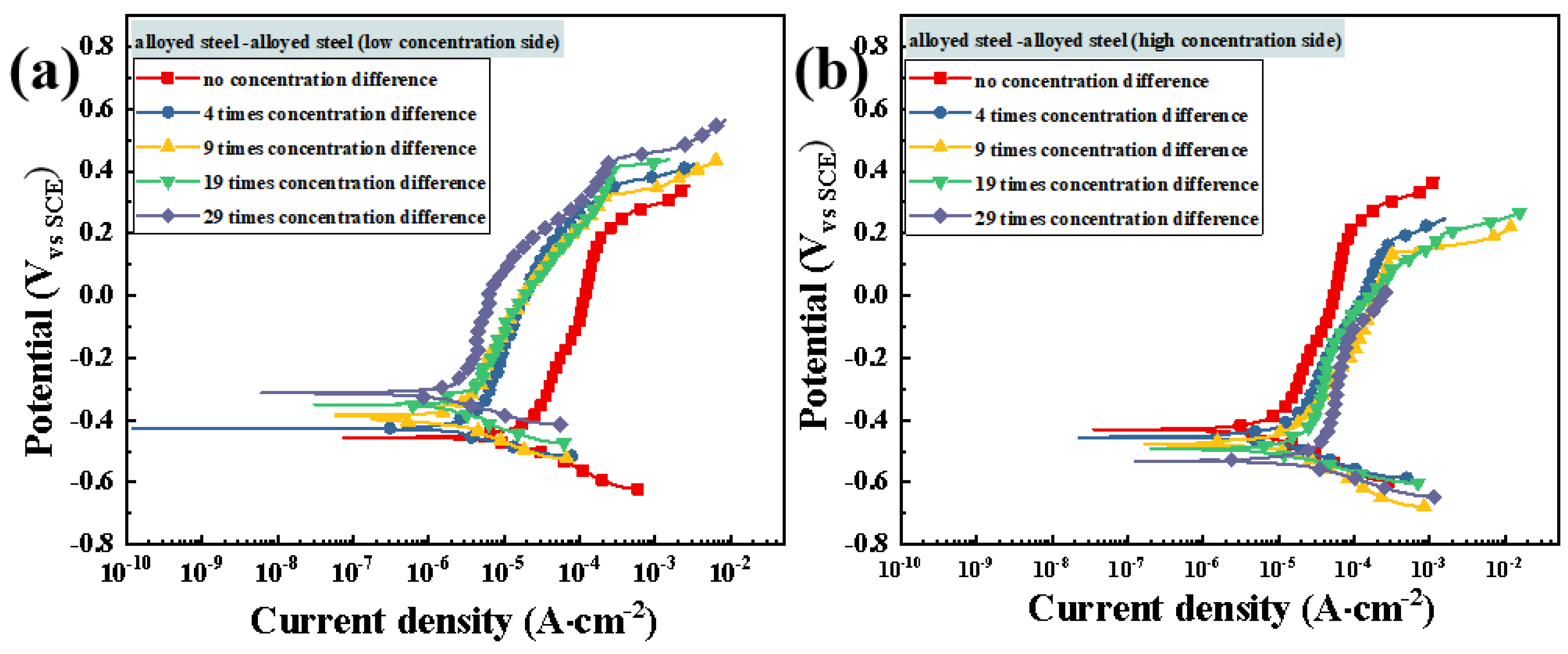
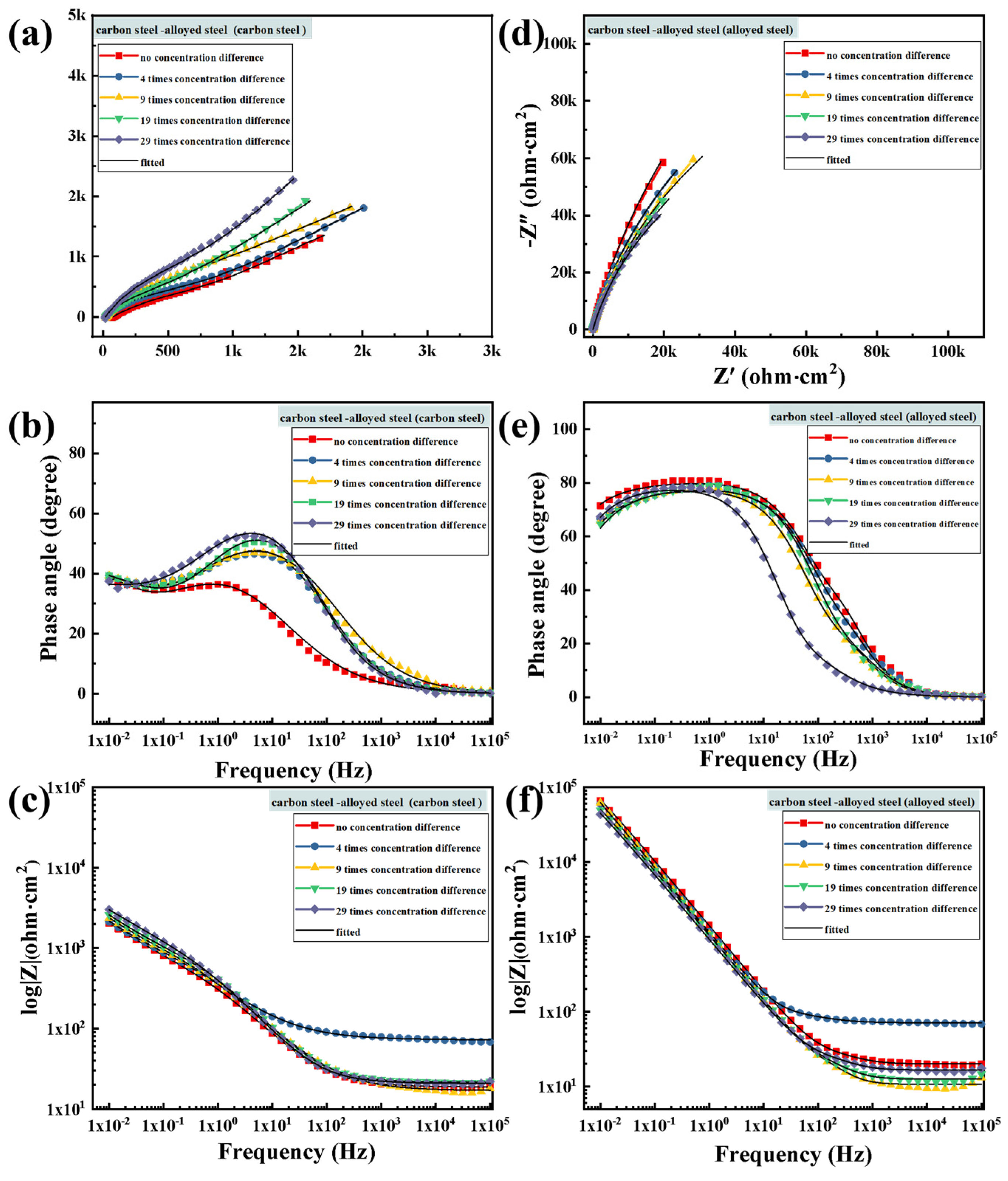
3.4. Evaluation of Corrosion Resistance after Macro-Cell Corrosion Induced by the Chloride Ion Concentration Difference in Dissimilar Steel
3.4.1. Electrochemical Impedance Spectroscopy Testing after Macro-Cell Corrosion
3.4.2. Potentiodynamic Polarization Testing after Macro-Cell Corrosion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, H.; Chen, S.; Zhou, Y.; Lin, Z.; Liang, X.; Liu, J.; Xing, F. Field test of a reinforced concrete bridge under marine environmental corrosion. Eng. Fail. Anal. 2020, 115, 104669. [Google Scholar] [CrossRef]
- Ou, Y.C.; Fan, H.D.; Nguyen, N.D. Long-term seismic performance of reinforced concrete bridges under steel reinforcement corrosion due to chloride attack. Earthq. Eng. Struct. Dyn. 2013, 42, 2113–2127. [Google Scholar] [CrossRef]
- Han, X.; Yang, D.Y.; Frangopol, D.M. Optimum maintenance of deteriorated steel bridges using corrosion resistant steel based on system reliability and life-cycle cost. Eng. Struct. 2021, 243, 112633. [Google Scholar] [CrossRef]
- Natkunarajah, K.; Masilamani, K.; Maheswaran, S.; Lothenbach, B.; Amarasinghe, D.; Attygalle, D. Analysis of the trend of pH changes of concrete pore solution during the hydration by various analytical methods. Cem. Concr. Res. 2022, 156, 106780. [Google Scholar] [CrossRef]
- Grengg, C.; Müller, B.; Staudinger, C.; Mittermayr, F.; Breininger, J.; Ungerböck, B.; Borisov, S.M.; Mayr, T.; Dietzel, M. High-resolution optical pH imaging of concrete exposed to chemically corrosive environments. Cem. Concr. Res. 2019, 116, 231–237. [Google Scholar] [CrossRef]
- Feng, Y.C.; Yang, J.B.; Zhang, P. Effects of carbonation curing regimes on alkalinity of self-compacting concretes for marine artificial reef. Constr. Build. Mater. 2023, 369, 130614. [Google Scholar] [CrossRef]
- Hansson, C.M.; Poursaee, A.; Laurent, A. Macrocell and microcell corrosion of steel in ordinary Portland cement and high performance concretes. Cem. Concr. Res. 2006, 36, 2098–2102. [Google Scholar] [CrossRef]
- Jin, Z.-H.; Jiang, C.; Gu, X.-L.; Dong, Z. Macro-cell corrosion between crossed steel bars in cracked concrete. Constr. Build. Mater. 2022, 350, 128867. [Google Scholar] [CrossRef]
- Wang, D.; Zhong, Q.; Yang, J.; Zhang, S. Effects of Cr and Ni on the microstructure and corrosion resistance of high-strength low alloy steel. J. Mater. Res. Technol. 2023, 23, 36–52. [Google Scholar] [CrossRef]
- Blikharskyy, Y.; Selejdak, J.; Kopiika, N. Corrosion Fatigue. Corrosion Fatigue Damages of Rebars under Loading in Time. Materials 2021, 14, 3416. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A. Effects of Different Parameters on Initiation and Propagation of Stress Corrosion Cracks in Pipeline Steels: A Review. Metals 2019, 9, 590. [Google Scholar] [CrossRef]
- Tsouli, S.; Lekatou, A.G.; Nikolaidis, C.; Kleftakis, S. Corrosion and tensile behavior of 316L stainless steel concrete reinforcement in harsh environments containing a corrosion inhibitor. Procedia Struct. Integr. 2019, 17, 268–275. [Google Scholar] [CrossRef]
- Martin, U.; Ress, J.; Bosch, J.; Bastidas, D. Stress corrosion cracking mechanism of AISI 316LN stainless steel rebars in chloride contaminated concrete pore solution using the slow strain rate technique. Electrochim. Acta 2020, 335, 135565. [Google Scholar] [CrossRef]
- Abreu, C.; Cristóbal, M.; Montemor, M.; Nóvoa, X.; Pena, G.; Pérez, M. Galvanic coupling between carbon steel and austenitic stainless steel in alkaline media. Electrochim. Acta 2002, 47, 2271–2279. [Google Scholar] [CrossRef]
- Karla, H.; Ueli, M.A.; Bernhard, E.; Larsen, C.K.; Geiker, M.R. Influence of mortar resistivity on the rate-limiting step of chloride-induced macro-cell corrosion of reinforcing steel. Corros. Sci. 2016, 110, 46–56. [Google Scholar]
- Li, X.; Shu, J.; Chen, L.; Bi, H. Effect of Cerium on High-Temperature Oxidation Resistance of 00Cr17NbTi Ferritic Stainless Steel. Acta Metall. Sin. (Engl. Lett.) 2014, 27, 501–507. [Google Scholar] [CrossRef]
- Rabi, M.; Cashell, K.; Shamass, R.; Desnerck, P. Bond behaviour of austenitic stainless steel reinforced concrete. Eng. Struct. 2020, 221, 111027. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.; Guo, S.; Xiao, Y. Study on galvanic corrosion behavior of 5083 aluminum alloy and 30CrMnSiA steel in different Cl- concentrations. J. Chin. Soc. Corros. Protect. 2021, 41, 883–891. [Google Scholar]
- Hao, L.; Li, N.; Li, D. Galvanic Corrosion Behavior of Hastelloy C-276 Alloy and 16MnR Steel in Hydrochloric Acid. Mater. Prot. 2011, 44, 49–51+8. [Google Scholar]
- Peng, Z.; Wu, J.; Wang, C. Effect of Temperature on Galvanic Corrosion Behavior of Industrial Pure Titanium and Low Alloy Steel. Corros. Sci. Prot. Technol. 2013, 25, 463–469. [Google Scholar]
- Wang, P.; Cai, J.; Cheng, X.; Ma, L.; Li, X. Inhibition of galvanic corrosion between crystallographic orientations in low alloy steel by grain ultra-refinement. Mater. Today Commun. 2022, 31, 103742. [Google Scholar] [CrossRef]
- Okonkwo, B.O.; Ming, H.; Wang, J.; Han, E.-H.; Rahimi, E.; Davoodi, A.; Hosseinpour, S. A new method to determine the synergistic effects of area ratio and microstructure on the galvanic corrosion of LAS A508/309 L/308 L SS dissimilar metals weld. J. Mater. Sci. Technol. 2021, 78, 38–50. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, W.; Sun, Y.; Yang, W.; Chen, L.; Xie, J.; Li, W. Corrosion behavior of the 3 wt.% Ni weathering steel with replacing 1 wt.% Cr in the simulated tropical marine atmospheric environment. J. Phys. Chem. Solids 2023, 175, 111221. [Google Scholar] [CrossRef]
- Yang, B.; Shi, C.; Teng, J.; Gong, X.; Ye, X.; Li, Y.; Lei, Q.; Nie, Y. Corrosion behaviours of low Mo Ni-(Co)-Cr-Mo alloys with various contents of Co in HF acid solution. J. Alloys Compd. 2019, 791, 2125–2224. [Google Scholar] [CrossRef]
- Vedalakshmi, R.; Kumar, K.; Raju, V.; Rengaswamy, N. Effect of prior damage on the performance of cement based coatings on rebar: Macrocell corrosion studies. Cem. Concr. Compos. 2000, 22, 417–421. [Google Scholar] [CrossRef]
- Zou, G.; Wang, Q.; Wang, G.; Liu, W.; Zhang, S.; Ai, Z.; Chen, H.; Ma, H.; Song, D. Revealing excellent passivation performance of a novel Cr-alloyed steel rebar in carbonized concrete environment. J. Mater. Res. Technol. 2023, 23, 1848–1861. [Google Scholar] [CrossRef]
- Feng, X.; Zuo, Y.; Tang, Y.; Zhao, X.; Zhao, J. The influence of strain on the passive behavior of carbon steel in cement extract. Corros. Sci. 2012, 65, 542–548. [Google Scholar] [CrossRef]
- Lu, C.-F.; Wang, W.; Li, Q.-T.; Hao, M.; Xu, Y. Effects of micro-environmental climate on the carbonation depth and the pH value in fly ash concrete. J. Clean. Prod. 2018, 181, 309–317. [Google Scholar] [CrossRef]
- Pilvara, A.; Ramezanianpour, A.A.; Rajaie, H. New method development for evaluation concrete chloride ion permeability. Constr. Build. Mater. 2015, 93, 790–797. [Google Scholar] [CrossRef]
- Jun, P.H.; Min, S.J.; Moonil, K.K.Y.A. Corrosion risk of steel fibre in concrete. Constr. Build. Mater. 2015, 101, 239–245. [Google Scholar]
- Uthaman, S.; George, R.; Vishwakarma, V.; Harilal, M.; Philip, J. Enhanced seawater corrosion resistance of reinforcement in nanophase modified fly ash concrete. Constr. Build. Mater. 2019, 221, 232–243. [Google Scholar] [CrossRef]
- Qian, S.Y.; Zhang, J.Y.; Qu, D.Y. Theoretical and experimental study of microcell and macrocell corrosion in patch repairs of concrete structures. Cem. Concr. Compos. 2006, 28, 685–695. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Guo, X.; Zhang, G. Galvanic corrosion between N80 carbon steel and 13Cr stainless steel under supercritical CO2 conditions. Corros. Sci. 2018, 147, 260–272. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, S.; Geng, W.-T.; Hu, Q.; Zhou, L. Corrosion behavior of the low alloy weathering steels coupled with stainless steel in simulated open atmosphere. Mater. Chem. Phys. 2022, 288, 126409. [Google Scholar] [CrossRef]
- Schmuki, P.; Böhni, H. Metastable pitting and semiconductive properties of passive films. J. Electrochem. Soc. 1992, 139, 1908–1913. [Google Scholar] [CrossRef]
- Searson, P.C.; Latanision, R.M.; Stimming, U. Analysis of the photoelectrochemical response of the passive film on iron in neutral solutions. J. Electrochem. Soc. 1988, 135, 1358–1363. [Google Scholar] [CrossRef]
- Kashani, F.R.; Rezaei, M. Electrochemical studies and molecular simulations on the use of molybdic acid for stabilization of AISI 304 stainless steel passive film in sulfuric acid medium. J. Mol. Liq. 2021, 344, 11773. [Google Scholar]
- Carmezim, M.; Simões, A.; Montemor, M.; Belo, M.D.C. Capacitance behaviour of passive films on ferritic and austenitic stainless steel. Corrosion. Sci. 2005, 47, 581–591. [Google Scholar] [CrossRef]
- Paola, A.D. Semiconducting properties of passive films on stainless steels. Electrochim. Acta 1989, 34, 203–210. [Google Scholar] [CrossRef]
- Hakiki, N.; Boudin, S.; Rondot, B.; Belo, M.D.C. The electronic structure of passive films formed on stainless steels. Corros. Sci. 1995, 37, 1809–1822. [Google Scholar] [CrossRef]
- Martínez, I.; Andrade, C. Application of EIS to cathodically protected steel: Tests in sodium chloride solution and in chloride contaminated concrete. Corros. Sci. 2008, 50, 2948–2958. [Google Scholar] [CrossRef]


| Fe | C | Si | Mn | P | S | V | Cr | Mo | |
|---|---|---|---|---|---|---|---|---|---|
| alloyed steel | Balance | 0.02 | 0.48 | 1.48 | 0.01 | 0.01 | 0.05 | 10.06 | 1.28 |
| carbon steel | Balance | 0.25 | 0.8 | 1.6 | 0.045 | 0.045 | - | - | - |
| Icorr (A/cm2) | 0 Times | 4 Times | 9 Times | 19 Times | 29 Times |
|---|---|---|---|---|---|
| carbon steel (0.1 mol/L) | 2.23 × 10−4 | 1.44 × 10−4 | 1.09 × 10−4 | 8.31 × 10−5 | 6.62 × 10−5 |
| carbon steel (0.1~3 mol/L) | 2.18 × 10−4 | 5.36 × 10−4 | 7.69 × 10−4 | 8.67 × 10−4 | 9.13 × 10−4 |
| Icorr (A/cm2) | 0 Times | 4 Times | 9 Times | 19 Times | 29 Times |
|---|---|---|---|---|---|
| alloyed steel (0.1 mol/L) | 2.67 × 10−6 | 2.13 × 10−6 | 2.38 × 10−6 | 2.01 × 10−6 | 1.61 × 10−6 |
| alloyed steel (0.1~3 mol/L) | 2.62 × 10−6 | 3.63 × 10−6 | 5.21 × 10−6 | 5.34 × 10−6 | 5.56 × 10−6 |
| Icorr (A/cm2) | 0 Times | 4 Times | 9 Times | 19 Times | 29 Times |
|---|---|---|---|---|---|
| carbon steel | 5.34 × 10−4 | 3.24 × 10−4 | 5.28 × 10−4 | 2.13 × 10−4 | 9.23 × 10−5 |
| alloyed steel | 8.86 × 10−7 | 9.71 × 10−7 | 1.06 × 10−6 | 1.14 × 10−6 | 1.25 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, F.; Zhou, X.; Ding, Z.; Zhang, S.; Zou, G.; Wang, G.; Wang, X.; Qiao, X.; Xu, J.; Song, D. Research on the Macro-Cell Corrosion Behavior of Alloyed Corrosion-Resistant Steel for a Transmission Line Steel Structure under a Chloride Corrosion Environment. Metals 2024, 14, 879. https://doi.org/10.3390/met14080879
Lyu F, Zhou X, Ding Z, Zhang S, Zou G, Wang G, Wang X, Qiao X, Xu J, Song D. Research on the Macro-Cell Corrosion Behavior of Alloyed Corrosion-Resistant Steel for a Transmission Line Steel Structure under a Chloride Corrosion Environment. Metals. 2024; 14(8):879. https://doi.org/10.3390/met14080879
Chicago/Turabian StyleLyu, Feng, Xinyue Zhou, Zheng Ding, Sijie Zhang, Gongnian Zou, Guowei Wang, Xing Wang, Xinglong Qiao, Jiahao Xu, and Dan Song. 2024. "Research on the Macro-Cell Corrosion Behavior of Alloyed Corrosion-Resistant Steel for a Transmission Line Steel Structure under a Chloride Corrosion Environment" Metals 14, no. 8: 879. https://doi.org/10.3390/met14080879




