Impact of Corrosion on Tensile Properties of a Mg Alloy in a Finite Element Model of a Coronary Artery Stent Coupled with Geometry Optimization
Abstract
:1. Introduction
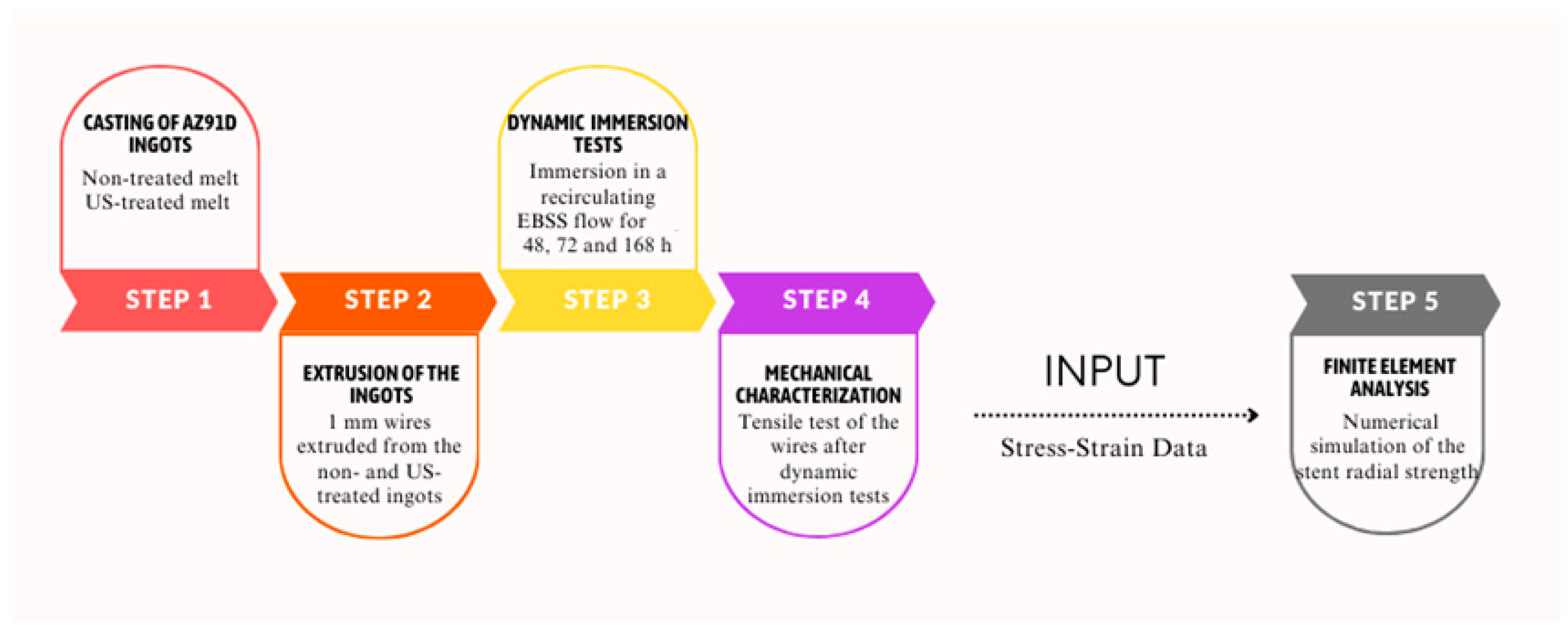
2. Materials and Methods
2.1. Dynamic Degradation Tests and Mechanical Characterisation
2.2. Numerical Simulation Methodology
3. Results and Discussion
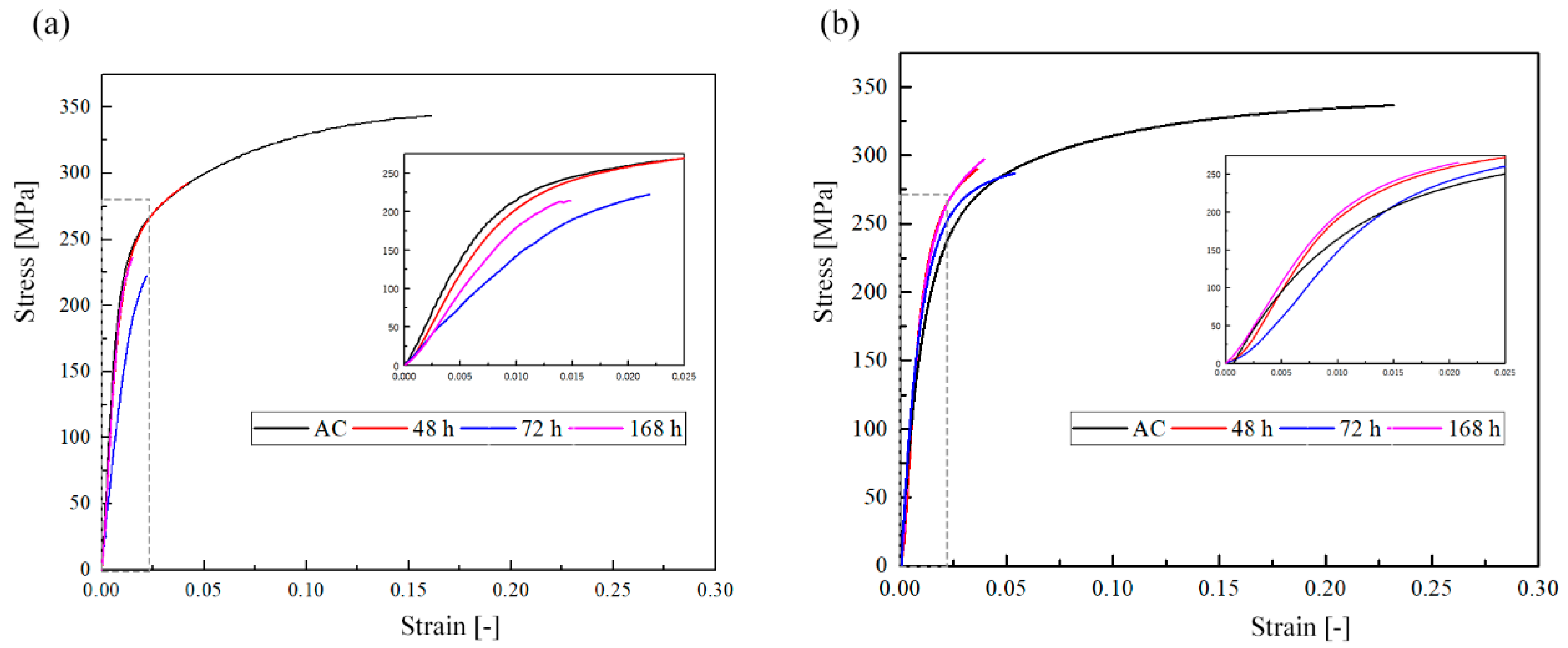
3.1. Wires Characterisation before and after Immersion
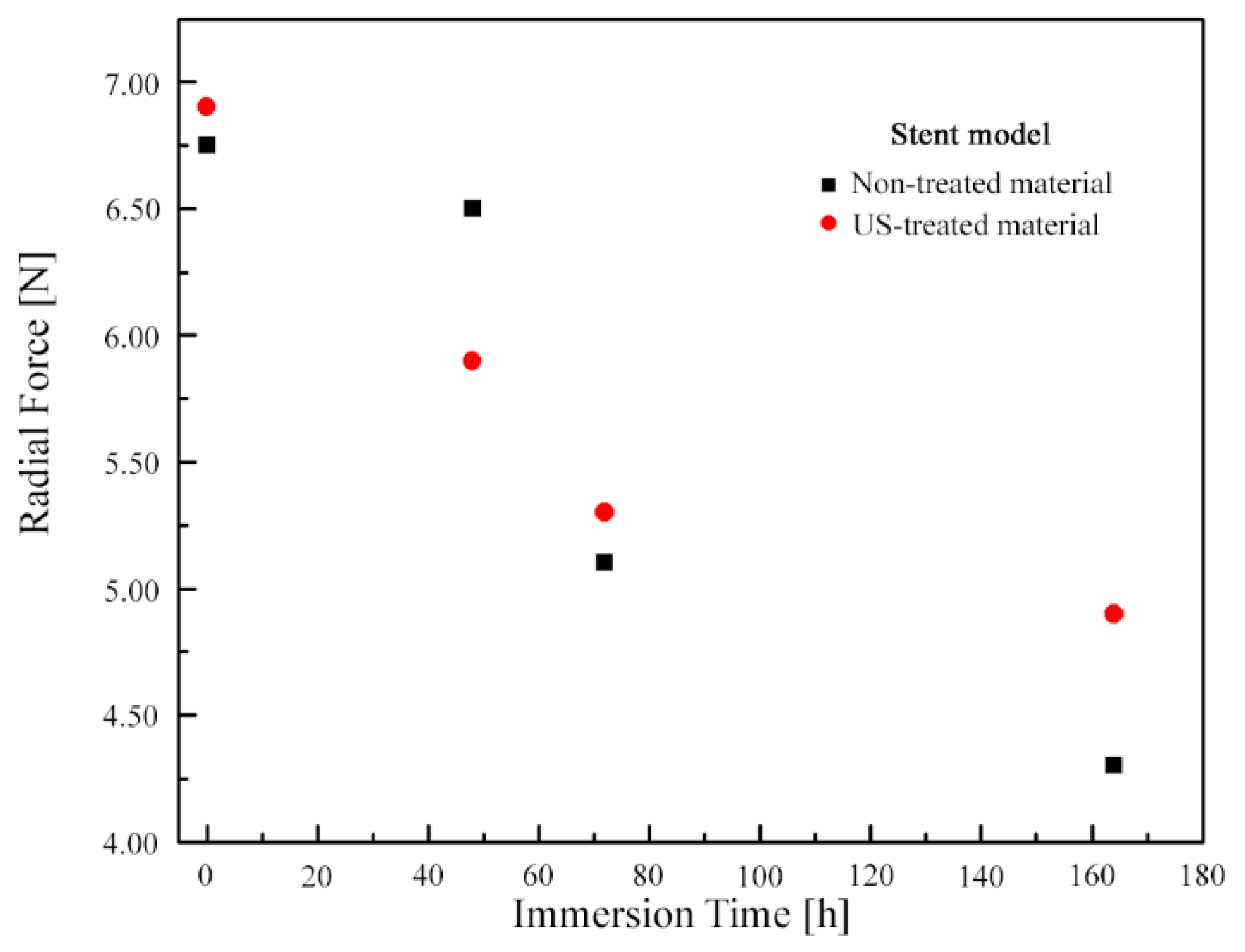
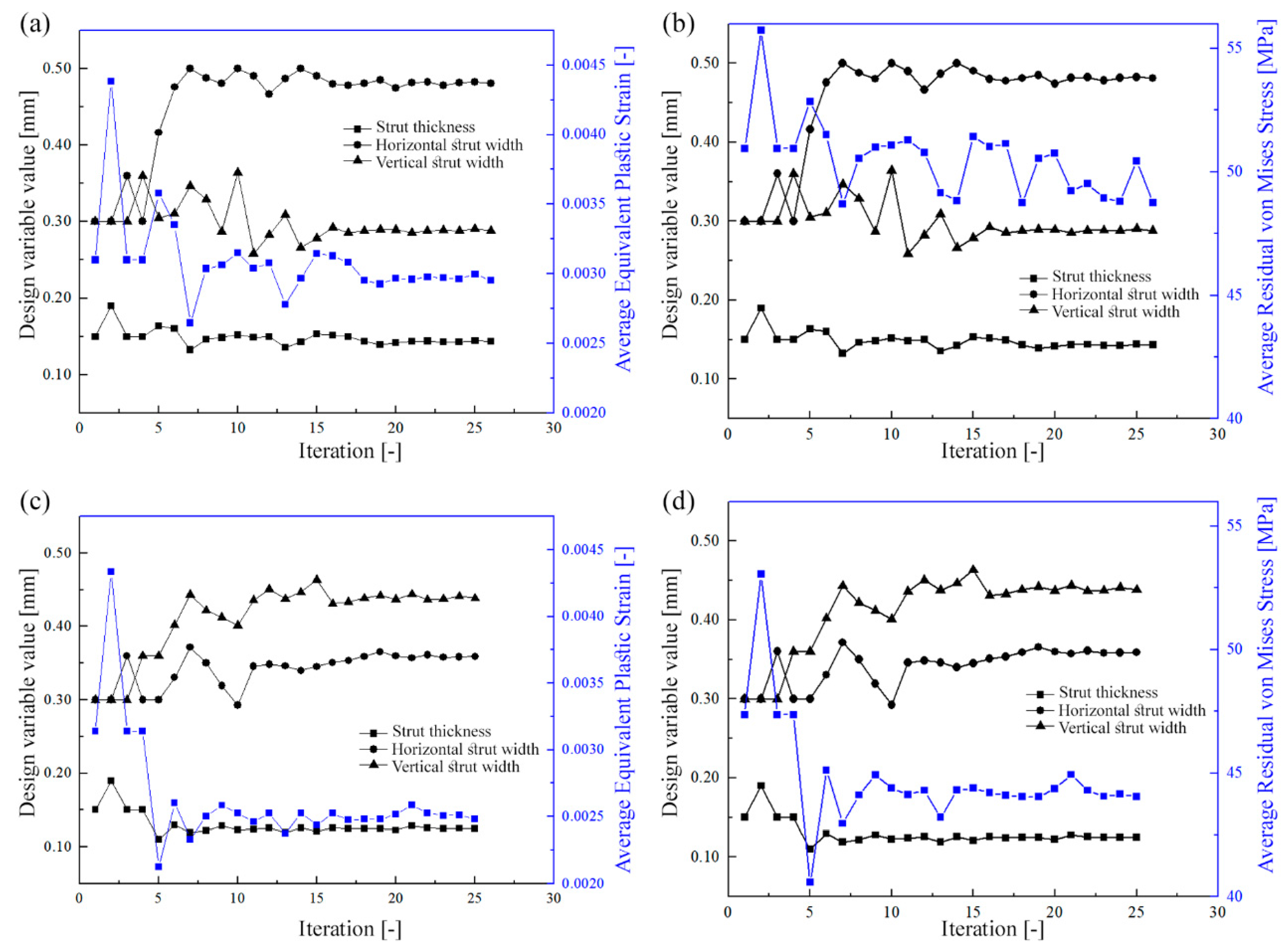
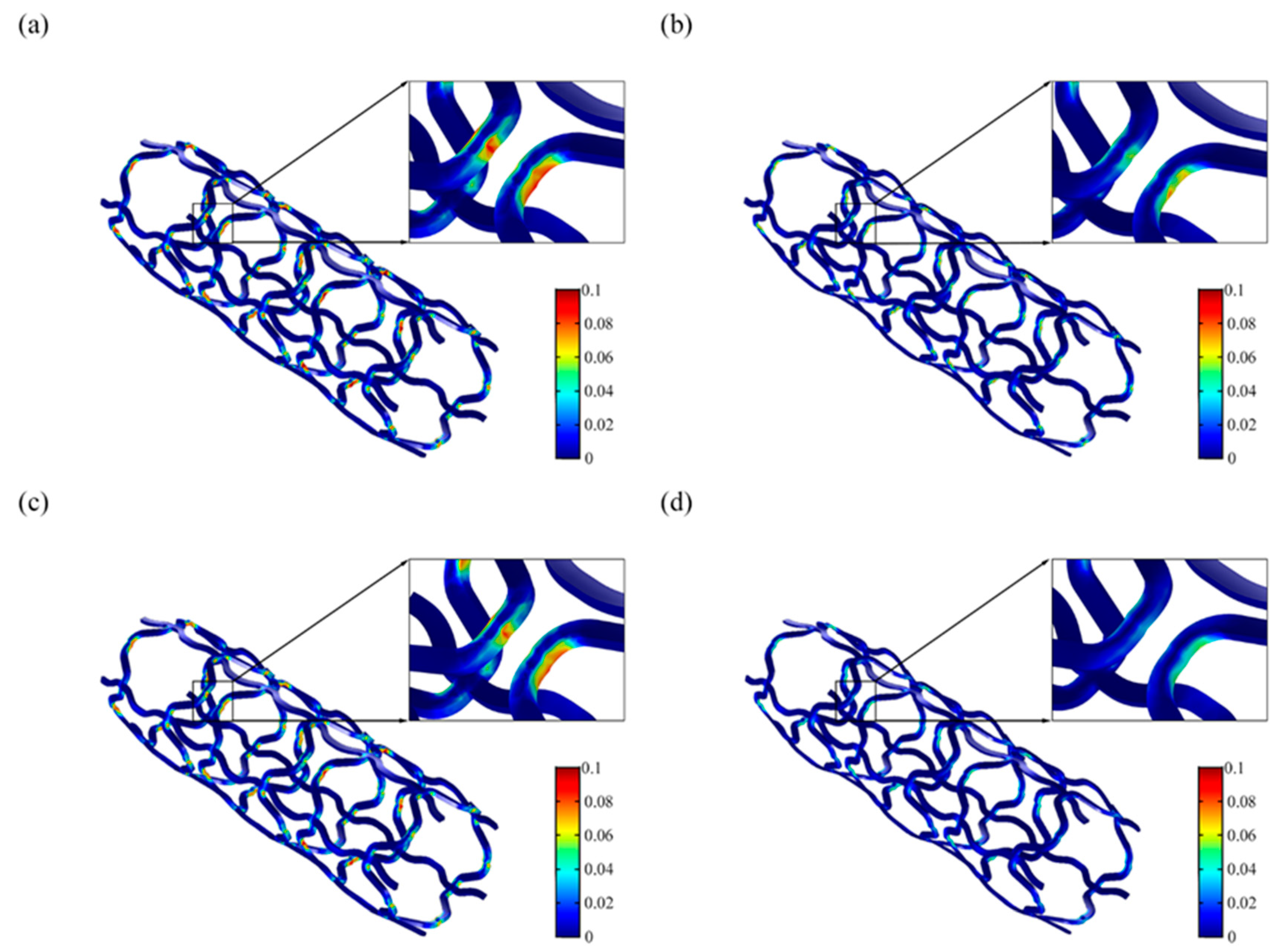
3.2. Numerical Simulation Results
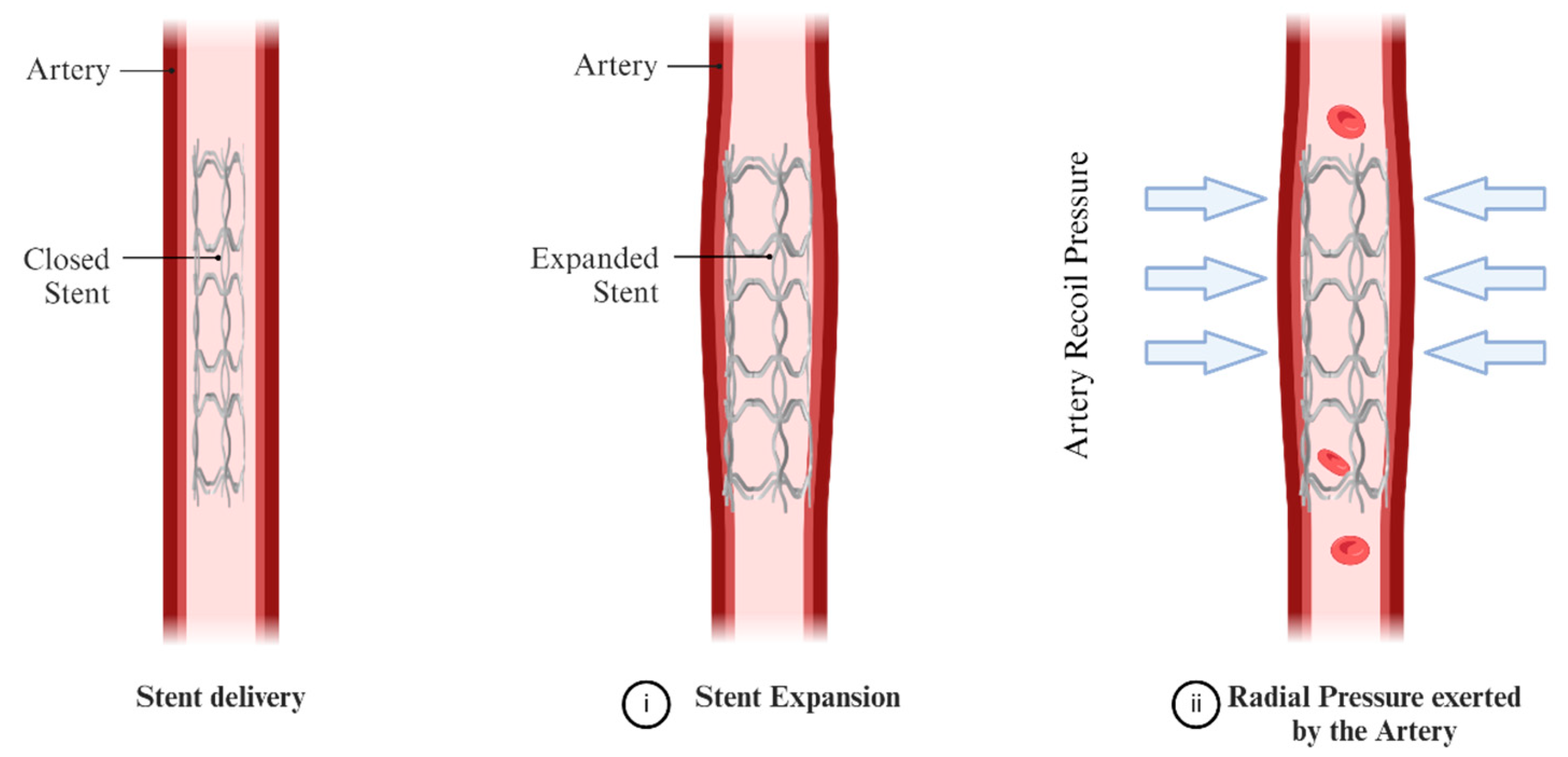
3.3. Study Clinical Relevance and Limitations
4. Conclusions
- When ti = 168 h, the decrease in radial force in the model when US-treated wire was used was less than when non-treated wire was used (~29% vs. ~36%).
- When ti = 168 h, the decrease in radial thickness, increase in horizontal strut width, increase in vertical strut width, decrease in mean residual stress, decrease in maximum residual stress, decrease in mean equivalent plastic strain, and decrease in maximum equivalent plastic strain when US-treated wire was used were each markedly greater than when the non-treated wire was used (difference ranging from ~6% to ~56%).
- These results suggest that the use of US-treated AZ91D wire may be promising for fabricating biodegradable coronary artery stents. Further studies in support of this suggestion are needed, among which is the determination of the ability of the expanded stent to withstand recoil when subjected to radial pressure exerted by the artery and fatigue performance.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hensey, M.; Sathananthan, J.; Teguh, W.P.; Mulvihill, N. (Eds.) Primary Angioplasty: A Practical Guide [Internet]; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Moses, J.W.; Leon, M.B.; Popma, J.J.; Fitzgerald, P.J.; Holmes, D.R.; O’Shaughnessy, C.; Caputo, R.P.; Kereiakes, D.J.; Williams, D.O.; Teirstein, P.S.; et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 2003, 349, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, S.; Kawashima, H.; Hara, H.; Ono, M.; Gao, C.; Wang, R.; Lunardi, M.; Sharif, F.; Wijns, W.; et al. Current perspectives on bioresorbable scaffolds in coronary intervention and other fields. Expert Rev. Med. Devices 2021, 18, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Jepson, N.; Bressloff, N.W.; Loh, P.H.; Ray, T.; Beier, S. The road to the ideal stent: A review of stent design optimisation methods, findings, and opportunities. Mater. Des. 2024, 237, 112556. [Google Scholar] [CrossRef]
- Reddy, S.R.V.; Welch, T.R.; Nugent, A.W. Biodegradable stent use for congenital heart disease. Prog. Pediatr. Cardiol. 2021, 61, 101349. [Google Scholar] [CrossRef]
- Gallinoro, E.; Almendarez, M.; Alvarez-Velasco, R.; Barbato, E.; Avanzas, P. Bioresorbable stents: Is the game over? Int. J. Cardiol. 2022, 361, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, S.; Gül, C.; Emin, N.; Gökmen, U.; Karakoç, H.; Uzun, A.; Çinici, H. Electrochemical, mechanical, and antibacterial properties of the AZ91 Mg alloy by hybrid and layered hydroxyapatite and tantalum oxide sol–gel coating. Mater. Test. 2023, 65, 1628–1644. [Google Scholar] [CrossRef]
- Brooks, E.K.; Der, S.; Ehrensberger, M.T. Corrosion and mechanical performance of AZ91 exposed to simulated inflammatory conditions. Mater. Sci. Eng.. C Mater. Biol. Appl. 2016, 60, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chen, Z.; Yang, W.; Fang, S.; Xu, B. Effect of yttrium, calcium and zirconium on ignition-proof principle and mechanical properties of magnesium alloys. J. Rare Earths 2012, 30, 74–78. [Google Scholar] [CrossRef]
- Harandi, S.E.; Mirshahi, M.; Koleini, S.; Idris, M.H.; Jafari, H.; Kadir, M.R.A. Effect of calcium content on the microstructure, hardness and in-vitro corrosion behavior of biodegradable Mg-Ca binary alloy. Mat. Res. 2013, 16, 11–18. [Google Scholar] [CrossRef]
- Ang, H.Y.; Ng, J.; Bulluck, H.; Wong, P.; Venkatraman, S.; Huang, Y.; Foin, N. Fundamentals of bioresorbable stents. In Functionalised Cardiovascular Stents; Elsevier: Amsterdam, The Netherlands, 2018; pp. 75–97. [Google Scholar]
- Jafari, S.; Harandi, S.E.; Raman, R.K.S. A Review of Stress-Corrosion Cracking and Corrosion Fatigue of Magnesium Alloys for Biodegradable Implant Applications. JOM 2015, 67, 1143–1153. [Google Scholar] [CrossRef]
- Gomes, I.V.; Pacheco, M.; Nienaber, M.; Neves, S.C.; Mei, D.; Barros, A.; Reis, R.L.; Alves, J.L.; Puga, H. Shielding AZ91D-1%Ca from corrosion through ultrasound melt treatment: A study for stent design. J. Magnes. Alloys 2023, 11, 2901–2915. [Google Scholar] [CrossRef]
- Berglund, J.; Guo, Y.; Wilcox, J. Challenges related to development of bioabsorbable vascular stents. EuroIntervention 2009, 5 (Suppl. F), F72–F79. [Google Scholar] [CrossRef] [PubMed]
- Foin, N.; Lee, R.D.; Torii, R.; Guitierrez-Chico, J.L.; Mattesini, A.; Nijjer, S.; Sen, S.; Petraco, R.; Davies, J.E.; Di Mario, C.; et al. Impact of stent strut design in metallic stents and biodegradable scaffolds. Int. J. Cardiol. 2014, 177, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, N.S.; Folgado, J.; Rodrigues, H.C. Surrogate-based multi-objective design optimization of a coronary stent: Altering geometry toward improved biomechanical performance. Int. J. Numer. Methods Biomed. Eng. 2021, 37, e3453. [Google Scholar] [CrossRef] [PubMed]
- Russ, J.B.; Li, R.L.; Herschman, A.R.; Waisman, H.; Vedula, V.; Kysar, J.W.; Kalfa, D. Design optimization of a cardiovascular stent with application to a balloon expandable prosthetic heart valve. Mater. Des. 2021, 209, 109977. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Bi, Y.; Zhao, H.; Wu, T.; Xu, F.; Zhao, G. Structural optimization and finite element analysis of poly-l-lactide acid coronary stent with improved radial strength and acute recoil rate. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2754–2764. [Google Scholar] [CrossRef] [PubMed]
- Gharleghi, R.; Wright, H.; Luvio, V.; Jepson, N.; Luo, Z.; Senthurnathan, A.; Babaei, B.; Prusty, B.G.; Ray, T.; Beier, S. A multi-objective optimization of stent geometries. J. Biomech. 2021, 125, 110575. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, J.; Wu, W.; Shi, Y.; Jin, L.; Petrini, L.; Shen, L.; Yuan, G.; Ding, W.; Ge, J.; et al. In vivo and in vitro evaluation of a biodegradable magnesium vascular stent designed by shape optimization strategy. Biomaterials 2019, 221, 119414. [Google Scholar] [CrossRef]
- Qiu, T.; He, R.; Abunassar, C.; Hossainy, S.; Zhao, L.G. Effect of two-year degradation on mechanical interaction between a bioresorbable scaffold and blood vessel. J. Mech. Behav. Biomed. Mater. 2018, 78, 254–265. [Google Scholar] [CrossRef]
- Polak-Kraśna, K.; Abaei, A.R.; Shirazi, R.N.; Parle, E.; Carroll, O.; Ronan, W.; Vaughan, T.J. Physical and mechanical degradation behaviour of semi-crystalline PLLA for bioresorbable stent applications. J. Mech. Behav. Biomed. Mater. 2021, 118, 104409. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.W.; Dunne, N.J.; Lennon, A.B.; Menary, G.H. Multi-objective optimisation of material properties and strut geometry for poly(L-lactic acid) coronary stents using response surface methodology. PLoS ONE 2019, 14, e0218768. [Google Scholar] [CrossRef] [PubMed]
- Amnieh, S.K.; Mashayekhi, M.; Shahnooshi, E.; Tavafoghi, M.; Mosaddegh, P. Biodegradable performance of PLA stents affected by geometrical parameters: The risk of fracture and fragment separation. J. Biomech. 2021, 122, 110489. [Google Scholar] [CrossRef]
- Atrens, A.; Johnston, S.; Shi, Z.; Dargusch, M.S. Viewpoint-Understanding Mg corrosion in the body for biodegradable medical implants. Scr. Mater. 2018, 154, 92–100. [Google Scholar] [CrossRef]
- Johnston, S.; Shi, Z.; Venezuela, J.; Wen, C.; Dargusch, M.S.; Atrens, A. Investigating Mg Biocorrosion In Vitro: Lessons Learned and Recommendations. JOM 2019, 71, 1406–1413. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Torii, S.; Sakamoto, A.; Kolodgie, F.D.; Virmani, R.; Finn, A.V. Fully bioresorbable vascular scaffolds: Lessons learned and future directions. Nat. Rev. Cardiol. 2019, 16, 286–304. [Google Scholar] [CrossRef] [PubMed]
- Peron, M.; Torgersen, J.; Berto, F. Mg and Its Alloys for Biomedical Applications: Exploring Corrosion and Its Interplay with Mechanical Failure. Metals 2017, 7, 252. [Google Scholar] [CrossRef]
- Gomes, I.V.; Puga, H.; Alves, J.L. Shape and functional optimization of biodegradable magnesium stents for manufacturing by ultrasonic-microcasting technique. Int. J. Interact. Des. Manuf. 2018, 12, 1059–1069. [Google Scholar] [CrossRef]
- Rieu, R.; Barragan, P.; Masson, C.; Fuseri, J.; Garitey, V.; Silvestri, M.; Roquebert, P. Radial force of coronary stents: A comparative analysis. Cathet. Cardiovasc. Intervent. 1999, 46, 380–391. [Google Scholar] [CrossRef]
- Powell, M.J.D. (Ed.) A Direct Search Optimization Method That Models the Objective and Constraint Functions by Linear Interpolation. 1994. Available online: https://link.springer.com/chapter/10.1007/978-94-015-8330-5_4 (accessed on 27 June 2024).
- Resor, C.D.; Bhatt, D.L. Thin to Ultrathin. Circ. Cardiovasc. Interv. 2018, 11, e007407. [Google Scholar] [CrossRef]
- Lupi, A.; Rognoni, A.; Secco, G.G.; Lazzero, M.; Nardi, F.; Fattori, R.; Bongo, A.S.; Agostoni, P.; Sheiban, I. Biodegradable versus durable polymer drug eluting stents in coronary artery disease: Insights from a meta-analysis of 5834 patients. Eur. J. Prev. Cardiol. 2014, 21, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Vorpahl, M.; Nakano, M.; Foerst, J.; Newell, J.B.; Sakakura, K.; Kutys, R.; Ladich, E.; Finn, A.V.; Kolodgie, F.D.; et al. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation 2014, 129, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Sheng, K.; Miao, F.; Wang, Y.; Zhang, Y.; Hou, R.; Mei, D.; Sun, Y.; Zheng, Y.; et al. Optimizing structural design on biodegradable magnesium alloy vascular stent for reducing strut thickness and raising radial strength. Mater. Des. 2022, 220, 110843. [Google Scholar] [CrossRef]
- Chevalier, B. Stent Strut Thickness: Have We Reached the Minimum? Card. Interv. Todays 2018, 12, 66–67. [Google Scholar]




| Non-Treated | US-Treated | |||
|---|---|---|---|---|
| Before Immersion | After Immersion—168 h | Before Immersion | After Immersion—168 h | |
| Tensile Strength [MPa] | 354.4 ± 2.1 | 252.7 ± 2.8 | 347.3 ± 2.3 | 293.1 ± 0.9 |
| Elongation [%] | 16.7 ± 2.6 | 2.3 ± 1.3 | 20.7 ± 1.9 | 2.46 ± 2.1 |
| Non-Treated | % Change | US-Treated | % Change | |||
|---|---|---|---|---|---|---|
| Ref. | Opt. | Ref. | Opt. | |||
| Strut Thickness [mm] | 0.200 | 0.160 | 20.0 | 0.200 | 0.125 | 37.5 |
| Horizontal Strut Width [mm] | 0.300 | 0.476 | 58.7 | 0.300 | 0.359 | 19.7 |
| Vertical Strut Width [mm] | 0.300 | 0.311 | 3.7 | 0.300 | 0.438 | 46.0 |
| Average Residual Stress [MPa] | 56 | 51 | 8.9 | 54 | 43 | 20.4 |
| Maximum Residual Stress [MPa] | 339 | 335 | 1.2 | 314 | 295 | 6.1 |
| Average Equivalent Plastic Strain [-] | 0.0048 | 0.0031 | 35.4 | 0.0049 | 0.0028 | 42.9 |
| Maximum Equivalent Plastic Strain [-] | 0.1357 | 0.1050 | 22.6 | 0.1248 | 0.0551 | 55.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, I.V.; Alves, J.L.; Puga, H. Impact of Corrosion on Tensile Properties of a Mg Alloy in a Finite Element Model of a Coronary Artery Stent Coupled with Geometry Optimization. Metals 2024, 14, 885. https://doi.org/10.3390/met14080885
Gomes IV, Alves JL, Puga H. Impact of Corrosion on Tensile Properties of a Mg Alloy in a Finite Element Model of a Coronary Artery Stent Coupled with Geometry Optimization. Metals. 2024; 14(8):885. https://doi.org/10.3390/met14080885
Chicago/Turabian StyleGomes, Inês V., José L. Alves, and Hélder Puga. 2024. "Impact of Corrosion on Tensile Properties of a Mg Alloy in a Finite Element Model of a Coronary Artery Stent Coupled with Geometry Optimization" Metals 14, no. 8: 885. https://doi.org/10.3390/met14080885
APA StyleGomes, I. V., Alves, J. L., & Puga, H. (2024). Impact of Corrosion on Tensile Properties of a Mg Alloy in a Finite Element Model of a Coronary Artery Stent Coupled with Geometry Optimization. Metals, 14(8), 885. https://doi.org/10.3390/met14080885







