Influence of Top Slag Containing TiO2 and VOx on Hot Metal Pre-Desulfurization
Abstract
1. Introduction
2. Experimental Details
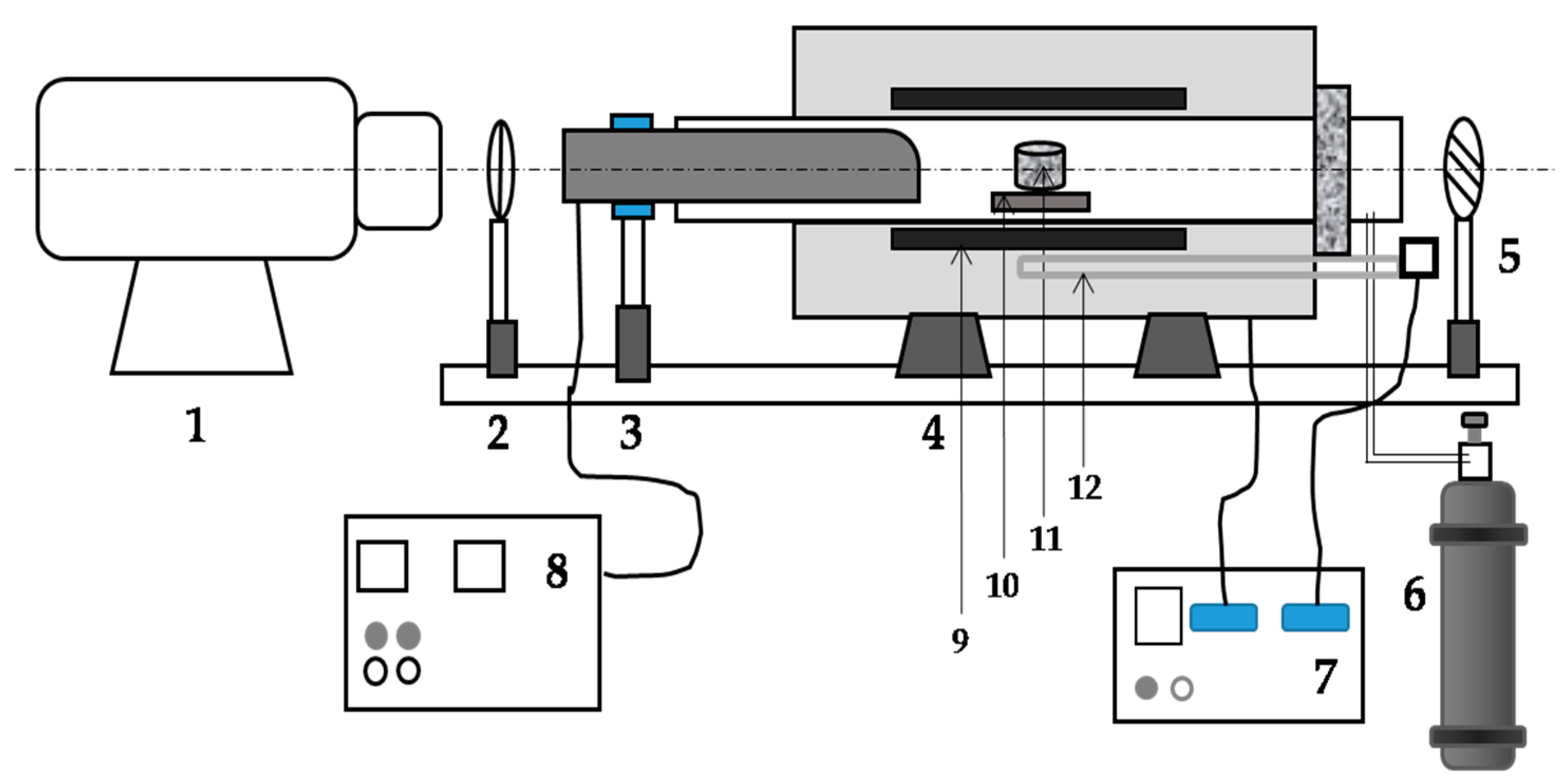
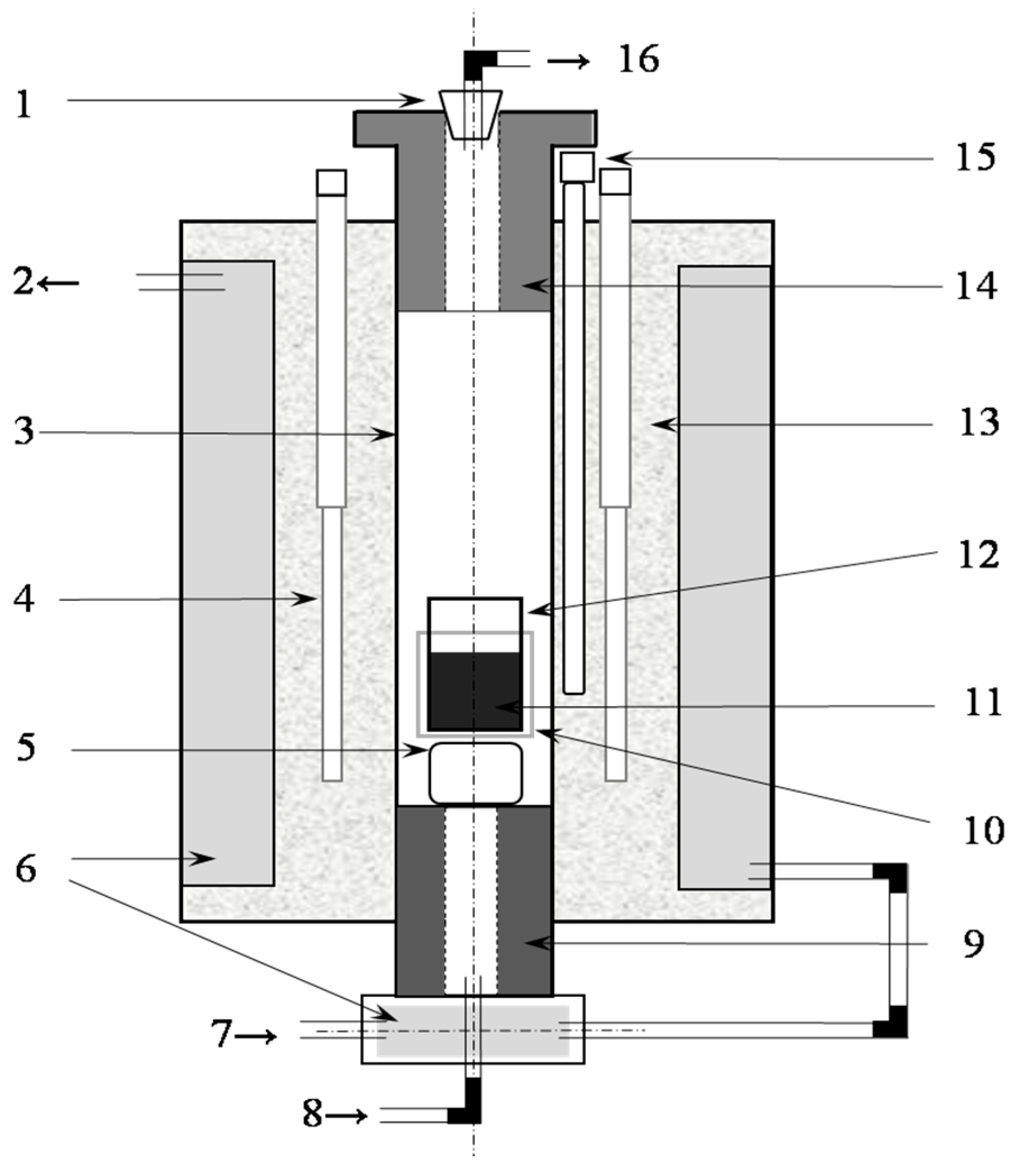
2.1. Equipment and Materials
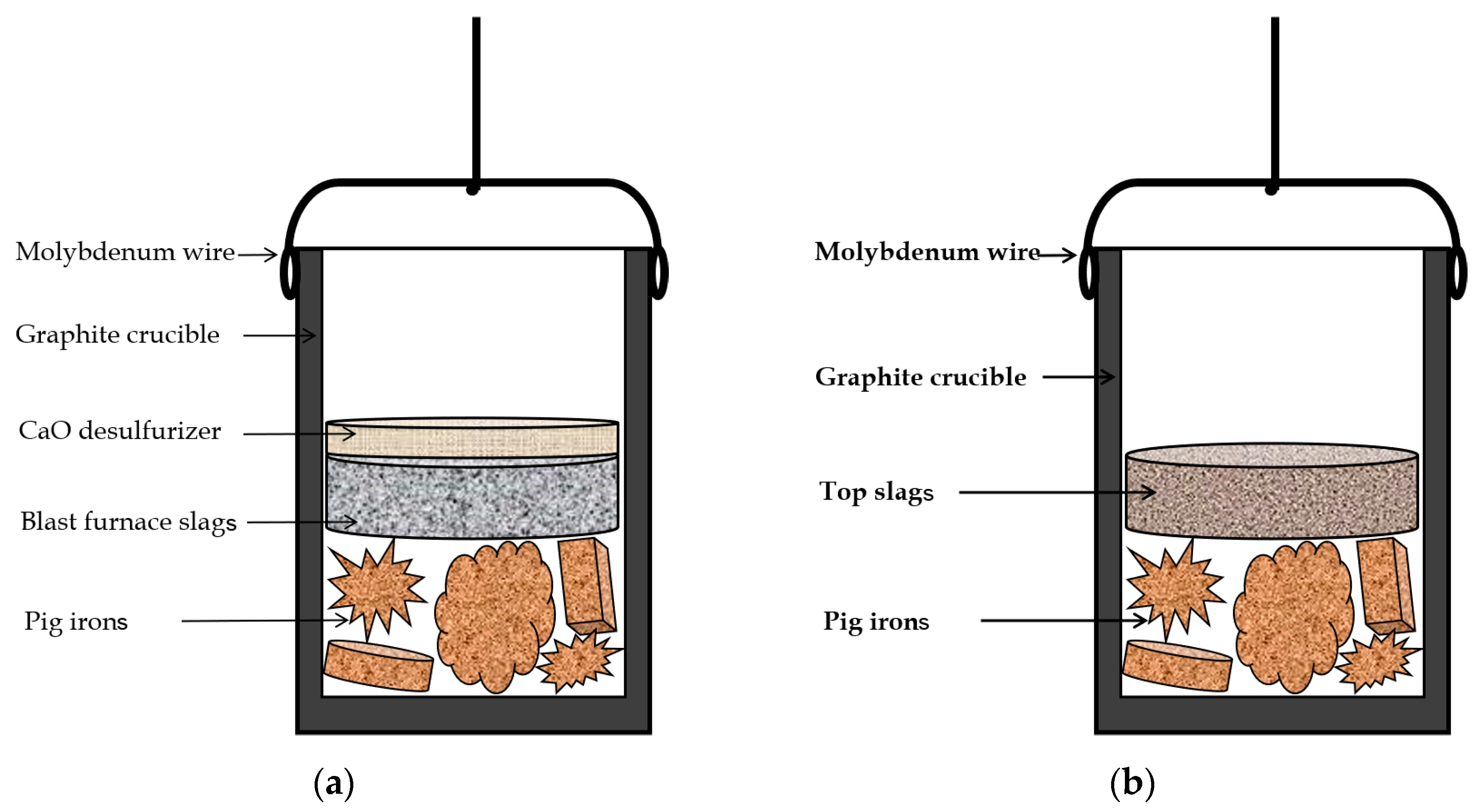
2.2. Procedures
2.3. Analysis
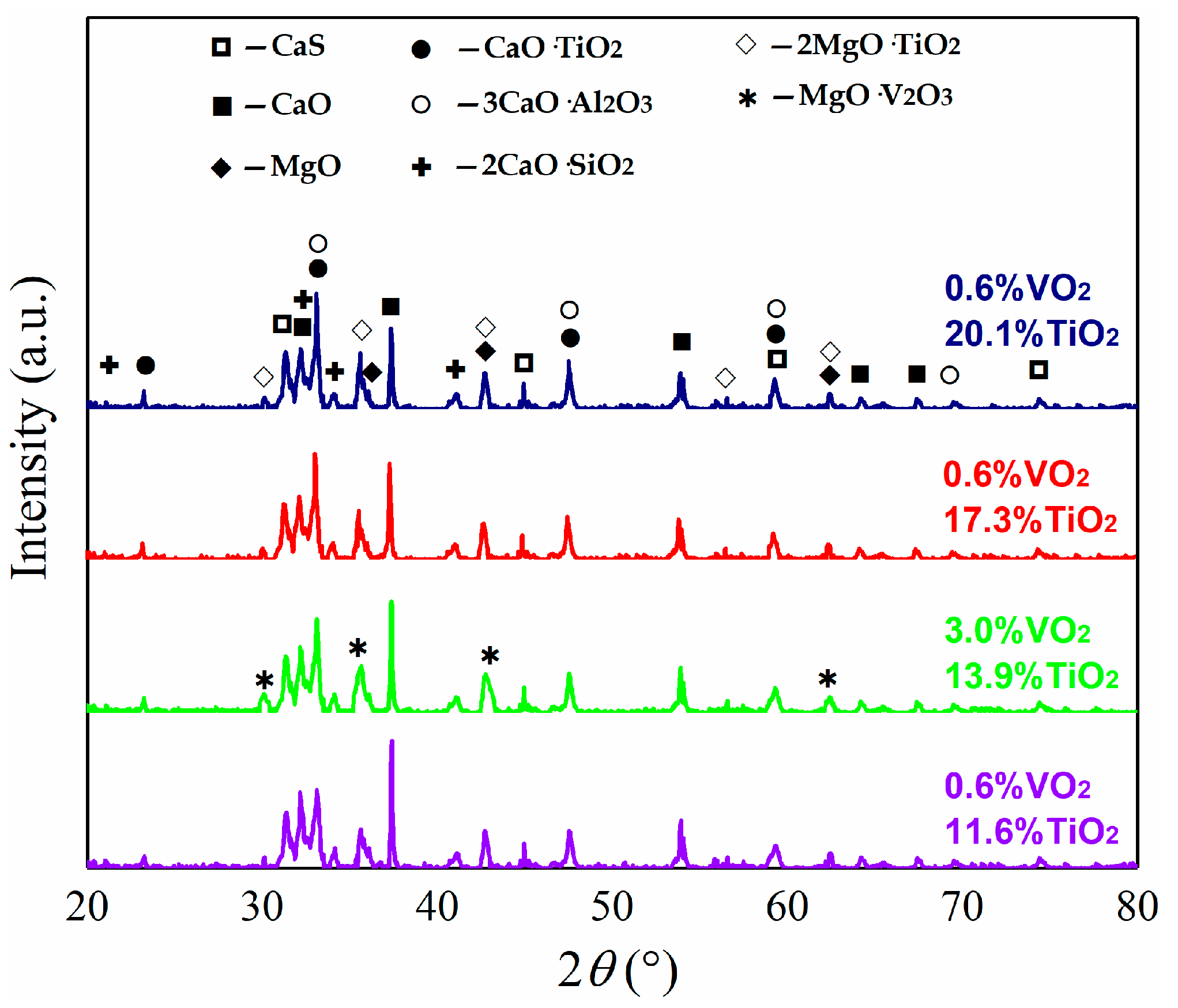
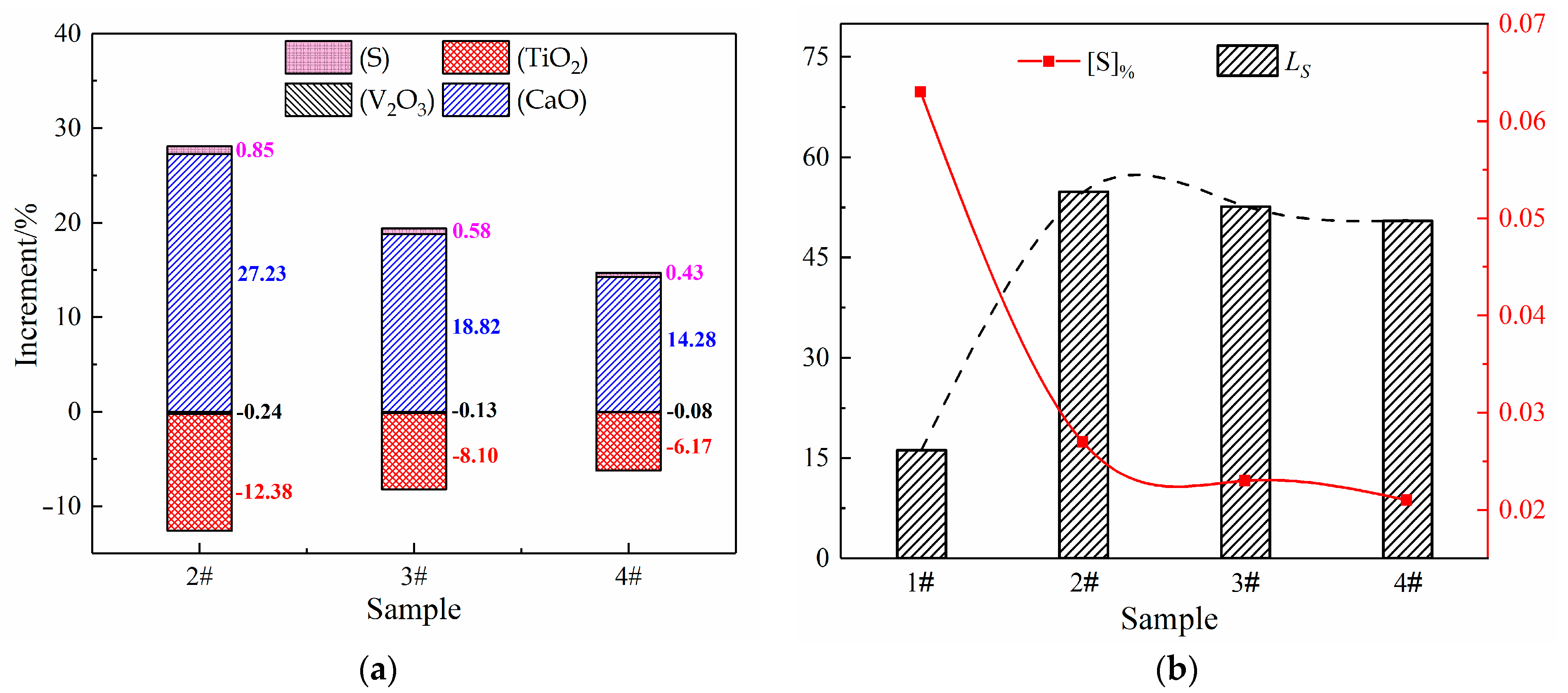
3. Results
4. Discussion
4.1. Influence of BF Slag
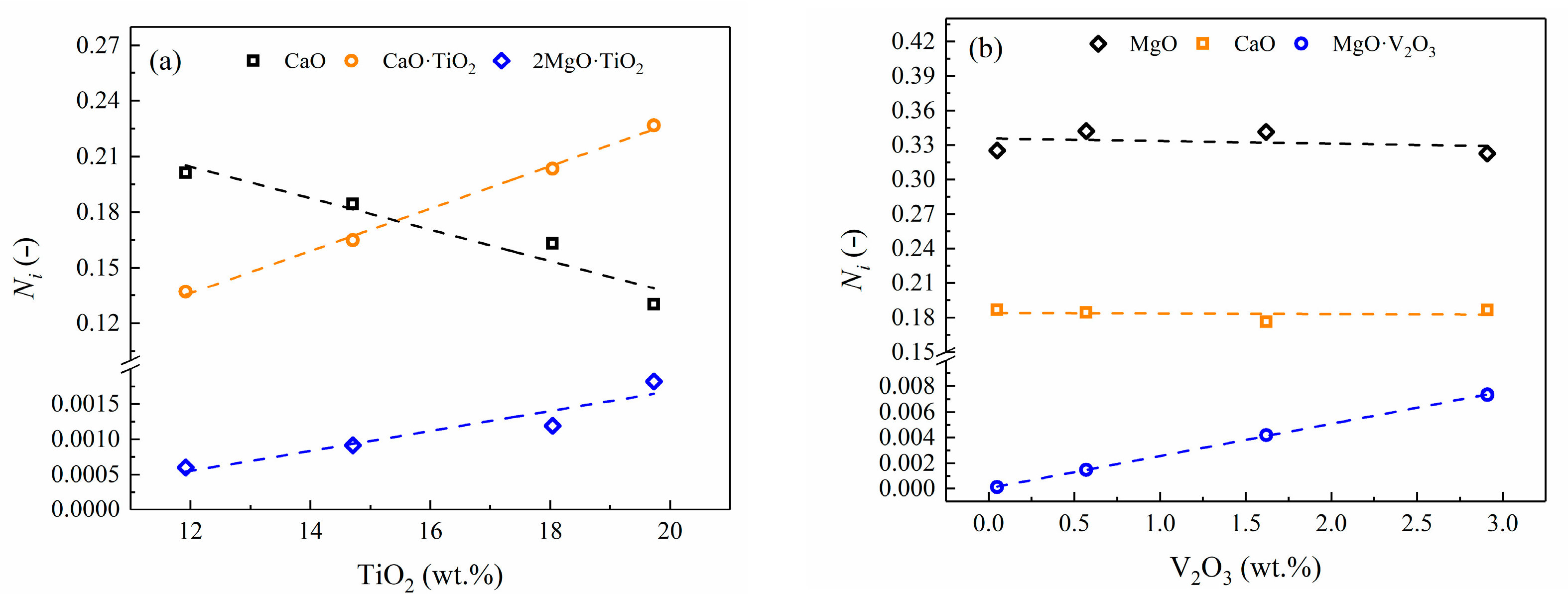
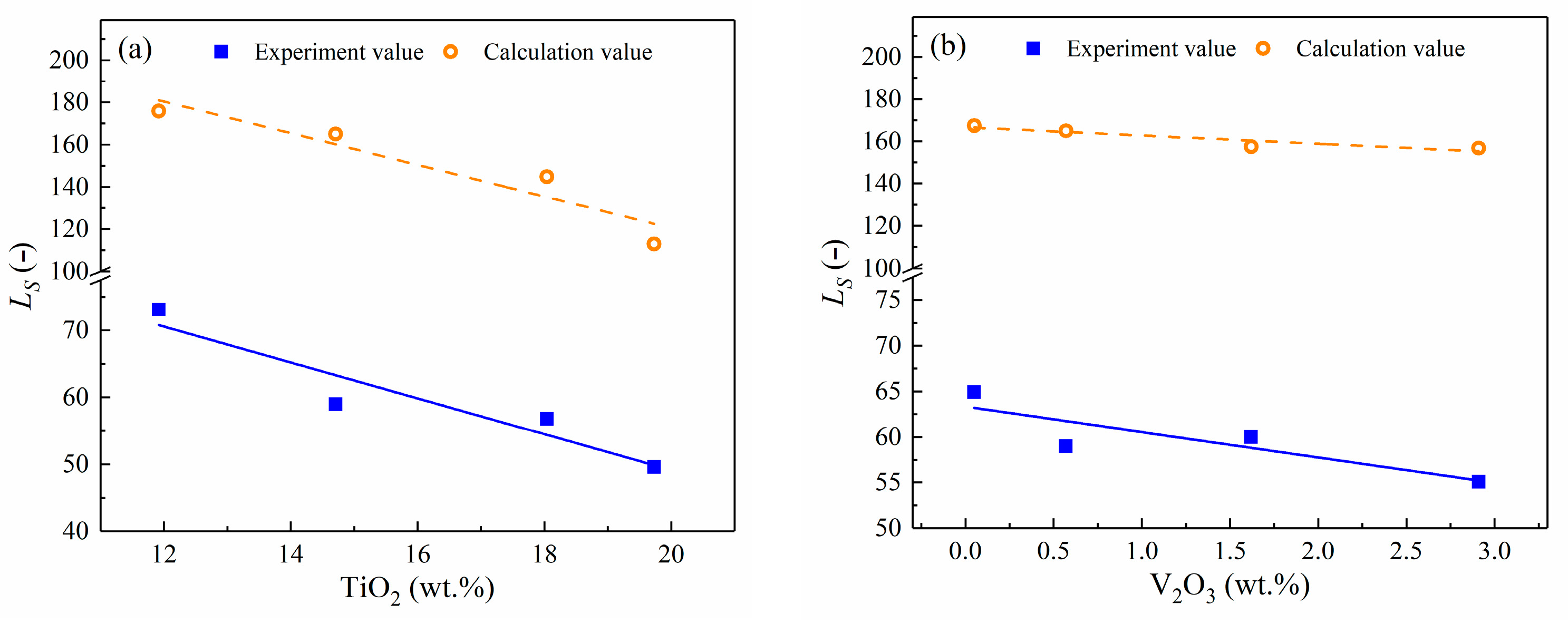
4.2. Influence of TiO2 and VOx
4.2.1. Coexistence Theory Model of the Desulfurization Slag
4.2.2. Effect of TiO2 and VOx on LS
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Zhang, B.; Liu, C.; Jiang, M. A new method of red mud recycling in the process of hot metal pretreatment. Metall. Res. Technol. 2020, 117, 115. [Google Scholar] [CrossRef]
- Matsui, A.; Uchida, Y.I.; Kikuchi, N.; Miki, Y. Effects of temperature and oxygen potential on removal of sulfur from desulfurization slag. ISIJ Int. 2017, 57, 1012–1018. [Google Scholar] [CrossRef]
- Biswas, A.K. Principles of Blast Furnace Ironmaking, Theory and Practice. Steel Times 1983, 211, 111. [Google Scholar]
- Irons, G.A.; Guthrie, R.I.L. The kinetics of molten iron desulfurization using magnesium vapor. Metall. Trans. B 1981, 12, 755–767. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, X.; Yan, Z.; Qin, Y.; Bai, C. Desulphurisation ability of blast furnace slag containing high Al2O3 and 5 mass% TiO2 at 1773 K. Ironmak. Steelmak. 2016, 43, 378–384. [Google Scholar] [CrossRef]
- Yang, B.W.; Hu, Y.H.; Liu, S.P.; Zhang, X.D.; Zheng, C.Z.; Wang, H.B. Influence of desulfurization slag containing oxides of vanadium and titanium on semi-steel pre-desulfurization. J. Iron Steel Res. Int. 2022, 29, 939–950. [Google Scholar] [CrossRef]
- Zhou, W.; Li, T.; Lan, D.; Sun, C.; Yang, S. Influence of TiO2, Al2O3, and basicity on viscosity and structure of high titanium-bearing blast furnace slag. Materials 2023, 16, 2575. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.M. Distribution behaviors of vanadium between hot metal and FeO-SiO2-MnO slag system. Iron Steel/Gangtie 2011, 46, 14–20. [Google Scholar]
- Liu, S.; Wang, L.; Chen, J.; Ye, L.; Du, J. Research progress of vanadium extraction processes from vanadium slag: A review. Sep. Purif. Technol. 2024, 342, 127035. [Google Scholar] [CrossRef]
- Park, D.; Jung, J.S.; Lee, J. Thermodynamic investigation of vanadium oxide in CaO–SiO2–VOx system at 1873 K. J. Am. Ceram. Soc. 2023, 106, 767–779. [Google Scholar] [CrossRef]
- He, Y.; Chen, C.; Yang, X. Thermodynamic analysis of vanadium distribution behavior in blast furnaces and basic oxygen furnaces. High Temp. Mater. Process. 2023, 42, 20220259. [Google Scholar] [CrossRef]
- Huang, W.; Chen, M.; Shen, X.; Yu, S.; Wang, N.; Xu, L. Phase diagrams and thermodynamic properties of the FeO–SiO2–V2O3 systems. Steel Res. Int. 2017, 88, 1600324. [Google Scholar] [CrossRef]
- Oros, T.J.; Son, K.; Hodge, A.M.; Kassner, M.E. The high temperature creep and fracture behavior of Inconel 718 produced by additive manufacturing. Scr. Mater. 2024, 251, 116208. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, D.J.; Lee, H.G. Reaction kinetics of desulfurization of molten pig iron using CaO–SiO2–Al2O3–Na2O slag systems. ISIJ Int. 2001, 41, 216–224. [Google Scholar] [CrossRef]
- He, X.F.; Wang, X.H.; Chen, S.H.; Jiang, M.; Huang, F.X.; Wang, W.J. Inclusion composition control in tyre cord steel by top slag refining. Ironmak. Steelmak. 2014, 41, 676–684. [Google Scholar] [CrossRef]
- Salina, V.A.; Sychev, A.V.; Zhuchkov, V.I.; Leont’ev, L.I.; Babenko, A.A. Thermodynamic simulation of the influence of the temperature and the basicity of a boron-containing slag on steel desulfurization. Russ. Metall. 2018, 2018, 427–431. [Google Scholar] [CrossRef]
- Lin, Y.; Yi, Y.; Fang, M.; Ma, W.; Liu, W. Prediction model for SiO2 activity in the CaO-Al2O3-SiO2-MgO quaternary slag system. Minerals 2023, 13, 509. [Google Scholar] [CrossRef]
- Sun, H.; Yang, J.; Zhang, R.H.; Yang, W.K. Effect of slag basicity on dephosphorization at lower basicity and lower temperature based on industrial experiments and ion-molecular coexistence theory. Metall. Mater. Trans. B 2021, 52, 3403–3422. [Google Scholar] [CrossRef]
- Sun, H.; Yang, J.; Yang, W.K.; Zhang, R.H. Comprehensive evaluation of phosphorus enrichment capacity for decarburization slag at different temperatures based on industrial experiments, mineral phase analysis and ion–molecule coexistence theory. Metall. Mater. Trans. B 2023, 54, 115–145. [Google Scholar] [CrossRef]
- Yang, X.M.; Jiao, J.S.; Ding, R.C.; Shi, C.B.; Guo, H.J. A thermodynamic model for calculating sulphur distribution ratio between CaO–SiO2–MgO–Al2O3 ironmaking slags and carbon saturated hot metal based on the ion and molecule coexistence theory. ISIJ Int. 2009, 49, 1828–1837. [Google Scholar] [CrossRef]
- Yang, X.M.; Shi, C.B.; Zhang, M.; Chai, G.M.; Wang, F. A thermodynamic model of sulfur distribution ratio between CaO–SiO2–MgO–FeO–MnO–Al2O3 slags and molten steel during LF refining process based on the ion and molecule coexistence theory. Metall. Mater. Trans. B 2011, 42, 1150–1180. [Google Scholar] [CrossRef]
- Sun, H.; Yang, J.; Yang, W.; Zhang, R. Evaluation of phosphorus enrichment capacity of CaO–SiO2–FeO–MgO–MnO–P2O5–Al2O3 dephosphorization slag based on ion-molecule coexistence theory. Steel Res. Int. 2023, 94, 2200662. [Google Scholar] [CrossRef]
- Yang, X.M.; Zhang, M.; Shi, C.B.; Chai, G.M.; Zhang, J. A sulfide capacity prediction model of CaO-SiO2-MgO-FeO-MnO-Al2O3 slags during the LF refining process based on the ion and molecule coexistence theory. Metall. Mater. Trans. B 2012, 43, 241–266. [Google Scholar] [CrossRef]
- Zhang, K.H.; Zhang, Y.L.; Li, F.S.; Wu, T. A thermodynamic model for calculation of sulfur distribution ratio between CaO–SiO2–Al2O3–Na2O–TiO2–(MgO) slags and carbon saturated hot metal. J. Iron Steel Res 2018, 30, 265–272. [Google Scholar]
- Oshima, H. Phase Relations in the System MgO-V2O3-VO2 at 1200 °C. J. Am. Ceram. Soc. 1980, 63, 504–508. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, W.; Zuo, H. Viscosity and desulphurisation behaviour of CaO–SiO2–MgO–Al2O3–BaO–MnO slag. Ironmak. Steelmak. 2023, 50, 1420–1426. [Google Scholar] [CrossRef]
- Hao, X.; Wang, X. A New Sulfide Capacity Model for CaO-Al2O3-SiO2-MgO Slags Based on Corrected Optical Basicity. Steel Res. Int. 2016, 87, 359–363. [Google Scholar] [CrossRef]
- Vuolio, T.; Visuri, V.V.; Paananen, T.; Fabritius, T. Identification of rate, extent, and mechanisms of Hot metal resulfurization with CaO-SiO2-Na2O slag systems. Metall. Mater. Trans. B 2019, 50, 1791–1807. [Google Scholar] [CrossRef]
- Yang, B.; Song, B.; Li, L.; Chen, L. Effect of vanadium and Titanium on desulfurization of CaO slag in liquid iron. Metals 2019, 9, 1239. [Google Scholar] [CrossRef]
- Dong, K.; Wu, L.; Liu, W.J.; Zhu, R. Desulfurization of CaO–Al2O3–SiO2–TiO2 Slag System. ISIJ Int. 2014, 54, 2248–2254. [Google Scholar] [CrossRef][Green Version]

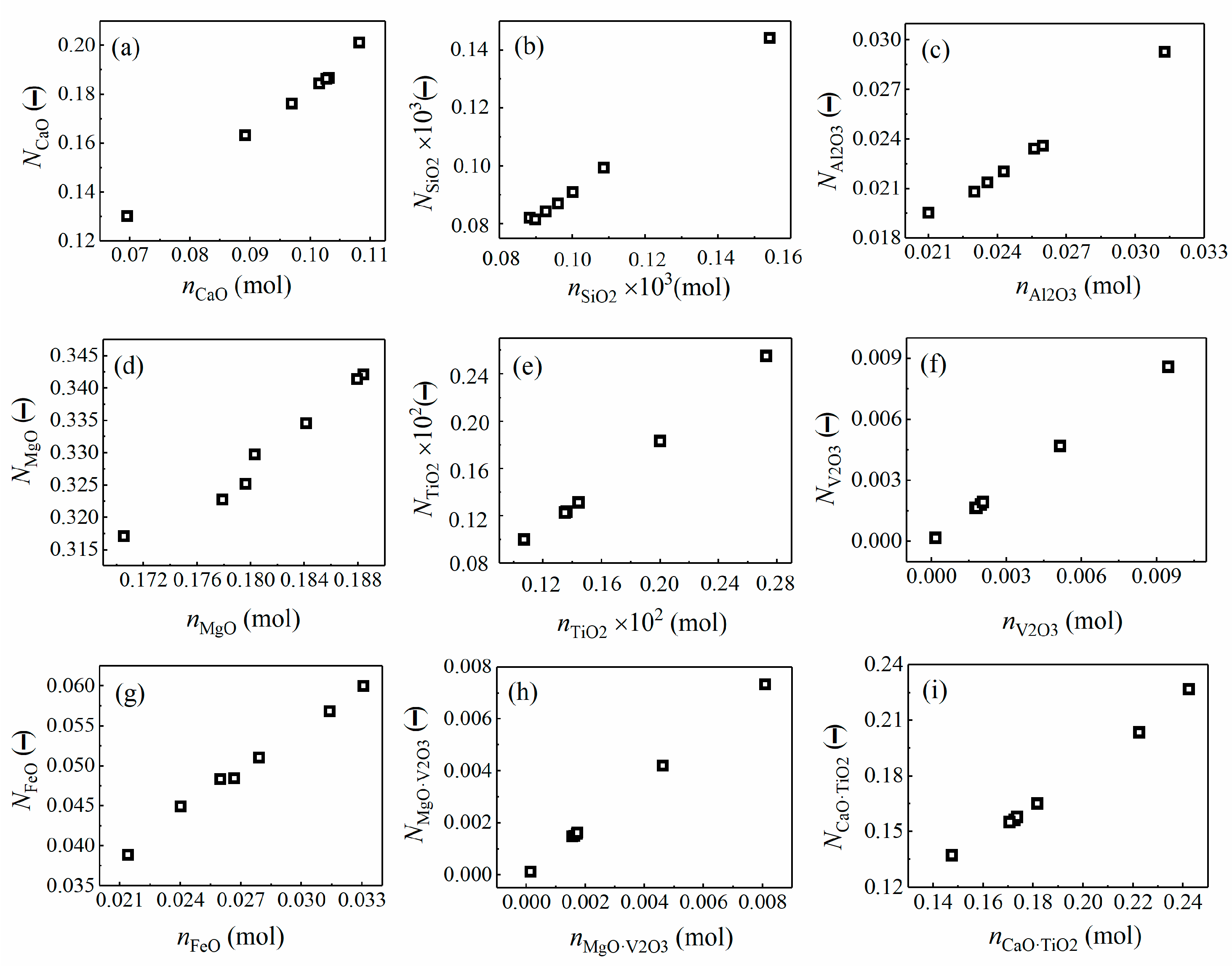
| C | Si | Mn | P | S | V | Ti |
|---|---|---|---|---|---|---|
| 4.23 | 0.08 | 0.12 | 0.07 | 0.08 | 0.28 | 0.19 |
| CaO | SiO2 | Al2O3 | MgO | TiO2 | V2O3 | FeO | S |
|---|---|---|---|---|---|---|---|
| 27.96 | 22.63 | 11.41 | 8.55 | 24.03 | 0.55 | 2.17 | 0.63 |
| Name | CaO | SiO2 | Al2O3 | MgO | TiO2 | FeC2O4·2H2O | VOC2O4·5H2O |
|---|---|---|---|---|---|---|---|
| Purity | 98.0 | 98.5 | 99.0 | 98.0 | 98.0 | 98.0 | 99.0 |
| Sample | BF Slag | CaO Desulfurization Agent |
|---|---|---|
| 1# | 10 | 0 |
| 2# | 5 | 5 |
| 3# | 10 | 5 |
| 4# | 15 | 5 |
| Sample | CaO | SiO2 | Al2O3 | MgO | TiO2 | VO2 |
|---|---|---|---|---|---|---|
| a | 44.3 | 14.2 | 12.3 | 8.5 | 20.1 | 0.6 |
| b | 46.3 | 14.8 | 12.4 | 8.6 | 17.3 | 0.6 |
| c | 48.8 | 15.6 | 12.3 | 8.5 | 14.2 | 0.6 |
| d | 50.5 | 16.2 | 12.5 | 8.6 | 11.6 | 0.6 |
| e | 49.4 | 15.8 | 12.1 | 8.5 | 14.2 | - |
| f | 47.6 | 15.3 | 12.3 | 8.4 | 14.6 | 1.8 |
| g | 47.1 | 15.1 | 12.3 | 8.6 | 13.9 | 3.0 |
| Sample | [S] | CaO | SiO2 | Al2O3 | MgO | TiO2 | V2O3 | FeO | (S) | R(CaO/SiO2) | Melting Point of Slag/K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1# | 0.063 | 27.34 | 22.81 | 11.37 | 8.16 | 23.94 | 0.51 | 2.83 | 1.02 | 1.20 | 1565 |
| 2# | 0.027 | 54.57 | 16.19 | 8.04 | 4.31 | 11.65 | 0.31 | 1.48 | 1.48 | 3.37 | 1696 |
| 3# | 0.023 | 46.16 | 18.27 | 9.16 | 5.57 | 15.93 | 0.42 | 2.14 | 1.21 | 2.53 | 1687 |
| 4# | 0.021 | 41.62 | 19.05 | 10.07 | 6.32 | 17.86 | 0.47 | 2.31 | 1.06 | 2.18 | 1611 |
| a | 0.014 | 43.73 | 13.43 | 11.68 | 8.04 | 19.73 | 0.55 | 1.73 | 0.67 | 3.26 | 1667 |
| b | 0.012 | 45.68 | 13.62 | 11.15 | 7.76 | 18.04 | 0.61 | 2.01 | 0.70 | 3.35 | 1652 |
| c | 0.012 | 47.18 | 14.54 | 12.06 | 8.07 | 14.71 | 0.57 | 1.54 | 0.68 | 3.25 | 1668 |
| d | 0.010 | 48.75 | 15.87 | 12.12 | 7.33 | 11.92 | 0.63 | 1.87 | 0.69 | 3.07 | 1683 |
| e | 0.011 | 48.02 | 15.28 | 11.87 | 7.65 | 13.97 | 0.05 | 2.26 | 0.71 | 3.14 | 1681 |
| f | 0.011 | 46.26 | 14.63 | 12.34 | 8.18 | 14.06 | 1.62 | 1.92 | 0.68 | 3.16 | 1672 |
| g | 0.013 | 46.01 | 14.17 | 11.73 | 7.86 | 13.81 | 2.91 | 2.38 | 0.69 | 3.25 | 1675 |
| Component | Mole | “Activity” |
|---|---|---|
| Ca2+ + O2– | n1 = nCaO | N1 = 2n1/Σni = NCaO |
| Mg2+ +O2– | n2 = nMgO | N2 = 2n2/Σni = NMgO |
| Fe2+ + O2– | n3 = nFeO | N3 = 2n3/Σni = NFeO |
| SiO2 | n4 = nSiO2 | N4 = n4/Σni = NSiO2 |
| Al2O3 | n5 = nAl2O3 | N5 = n5/Σni = NAl2O3 |
| TiO2 | n6 = nTiO2 | N6 = n6/Σni = NTiO2 |
| V2O3 | n7 = nV2O3 | N7 = n7/Σni = NV2O3 |
| MgO·V2O3 | n8 = nMgO·V2O3 | N8 = n8/Σni = NMgO·V2O3 |
| 2CaO·SiO2 | n9 = n2CaO·SiO2 | N9 = n9/Σni = N2CaO·SiO2 |
| CaO·SiO2 | n10 = nCaO·SiO2 | N10 = n10/Σni = NCaO·SiO2 |
| 3CaO·Al2O3 | n11 = n3CaO·Al2O3 | N11 = n11/Σni = N3CaO·Al2O3 |
| 2MgO·SiO2 | n12 = n2MgO·SiO2 | N12 = n12/Σni = N2MgO·SiO2 |
| CaO·TiO2 | n13 = nCaO·TiO2 | N13 = n13/Σni = NCaO·TiO2 |
| 2MgO·TiO2 | n14 = n2MgO·TiO2 | N14 = n14/Σni = N2MgO·TiO2 |
| 2FeO·TiO2 | n15 = n2FeO·TiO2 | N15 = n15/Σni = N2FeO·TiO2 |
| 2CaO·Al2O3·SiO2 | n16 = n2CaO·Al2O3·SiO2 | N16 = n16/Σni = N2CaO·Al2O3·SiO2 |
| 3CaO·MgO·2SiO2 | n17 = n3CaO·MgO·2SiO2 | N17 = n17/Σni = N3CaO·MgO·2SiO2 |
| Reaction | ΔG°/(J·mol−1) | Equilibrium Equation |
|---|---|---|
| (Mg2+ + O2–) + (V2O3) = (MgO·V2O3) | –21,416 + 5.54T [25] | K1 = N8/(N2·N7) |
| 2(Ca2+ + O2–) + (SiO2) = (2CaO·SiO2) | –102,090 − 24.27T [23] | K2 = N9/(N12·N4) |
| (Ca2+ + O2–) + (SiO2) = (CaO·SiO2) | –21,757 − 36.82T [23] | K3 = N10/(N1·N4) |
| 3(Ca2+ + O2–) + (Al2O3) = (3CaO·Al2O3) | –21,771 − 29.31T [20] | K4 = N11/(N13·N5) |
| 2(Mg2+ + O2–) + (SiO2) = (2MgO·SiO2) | –56,902 − 3.35T [21] | K5 = N12/(N22·N4) |
| (Ca2+ + O2–) + (TiO2) = (CaO·TiO2) | –79,900 − 3.35T [24] | K6 = N13/(N1·N6) |
| 2(Mg2+ + O2–) + (TiO2) = (2MgO·TiO2) | –25,500 + 1.26T [24] | K7 = N14/(N22·N6) |
| 2(Fe2+ + O2–) + (TiO2) = (2FeO·TiO2) | –33,913 + 5.86T [24] | K8 = N15/(N32·N6) |
| 2(Ca2+ + O2–) + (Al2O3) + (SiO2) = (2CaO·Al2O3·SiO2) | –116,315 − 38.91T [21] | K9 = N16/(N12·N5·N4) |
| 3(Ca2+ + O2–) + (Mg2+ + O2–) + 2(SiO2) = (3CaO·MgO·2SiO2) | –205,016 − 31.80T [21] | K10 = N17/(N13·N2·N42) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Song, B.; Chen, L.; Sun, H.; Northwood, D.O.; Waters, K.E.; Ma, H. Influence of Top Slag Containing TiO2 and VOx on Hot Metal Pre-Desulfurization. Metals 2024, 14, 910. https://doi.org/10.3390/met14080910
Yang B, Song B, Chen L, Sun H, Northwood DO, Waters KE, Ma H. Influence of Top Slag Containing TiO2 and VOx on Hot Metal Pre-Desulfurization. Metals. 2024; 14(8):910. https://doi.org/10.3390/met14080910
Chicago/Turabian StyleYang, Biwen, Bo Song, Liang Chen, Honghong Sun, Derek O. Northwood, Kristian E. Waters, and Hao Ma. 2024. "Influence of Top Slag Containing TiO2 and VOx on Hot Metal Pre-Desulfurization" Metals 14, no. 8: 910. https://doi.org/10.3390/met14080910
APA StyleYang, B., Song, B., Chen, L., Sun, H., Northwood, D. O., Waters, K. E., & Ma, H. (2024). Influence of Top Slag Containing TiO2 and VOx on Hot Metal Pre-Desulfurization. Metals, 14(8), 910. https://doi.org/10.3390/met14080910







