Modeling the Impact of Grain Size on Corrosion Behavior of Ni-Based Alloys in Molten Chloride Salt via Cellular Automata
Abstract
1. Introduction
2. Materials and Methods
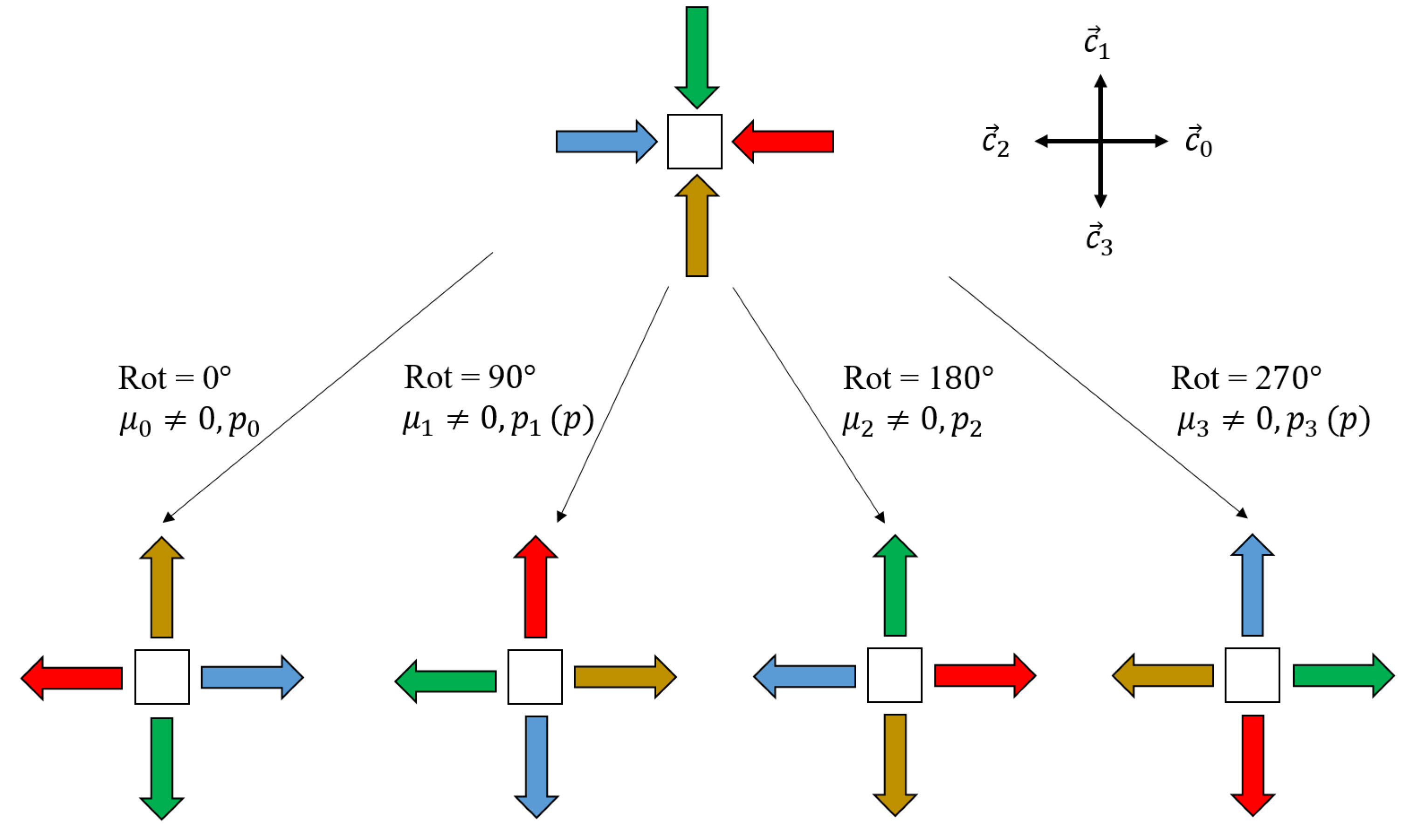
2.1. Diffusion Model
2.2. Corrosion Model
- . No reactions occur in this cell and the cell will be still filled by M (material) after the generation.
- , . In this case, a random number between 0 and 1 is generated. If , a reaction between M and Cl occurs, and M becomes the product of the interaction.
- . This case is processed similarly to the second case.
- , . Each reaction will be evaluated independently first based on its reaction rate and then put into the reaction pool if it is selected. Then, only one reaction will be picked from the pool, with probability proportional to its reaction rate.
2.3. Alloy Composition
2.4. Thickness Loss and Surface Roughness
2.5. Simulation Conditions
3. Results and Discussion
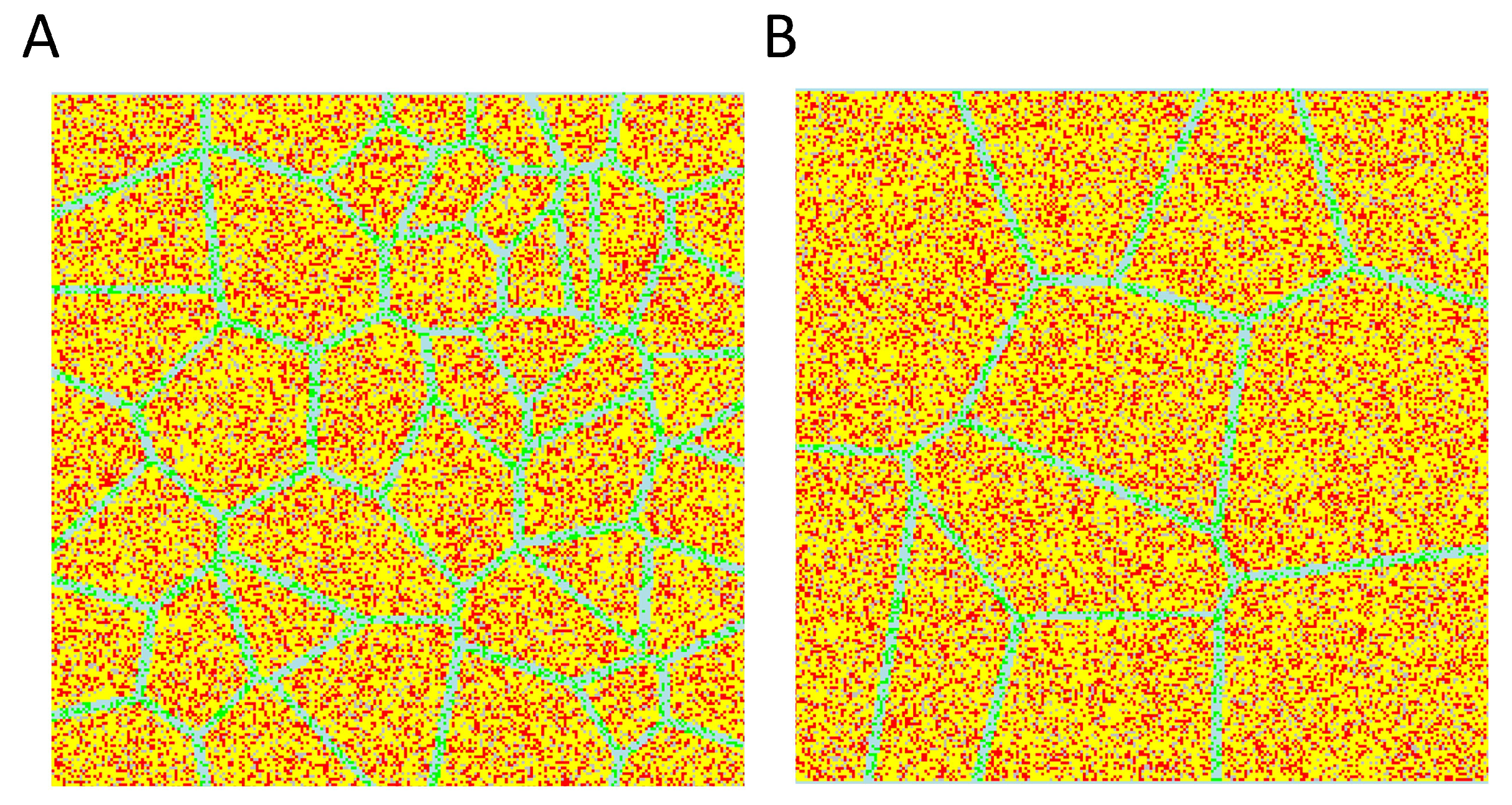
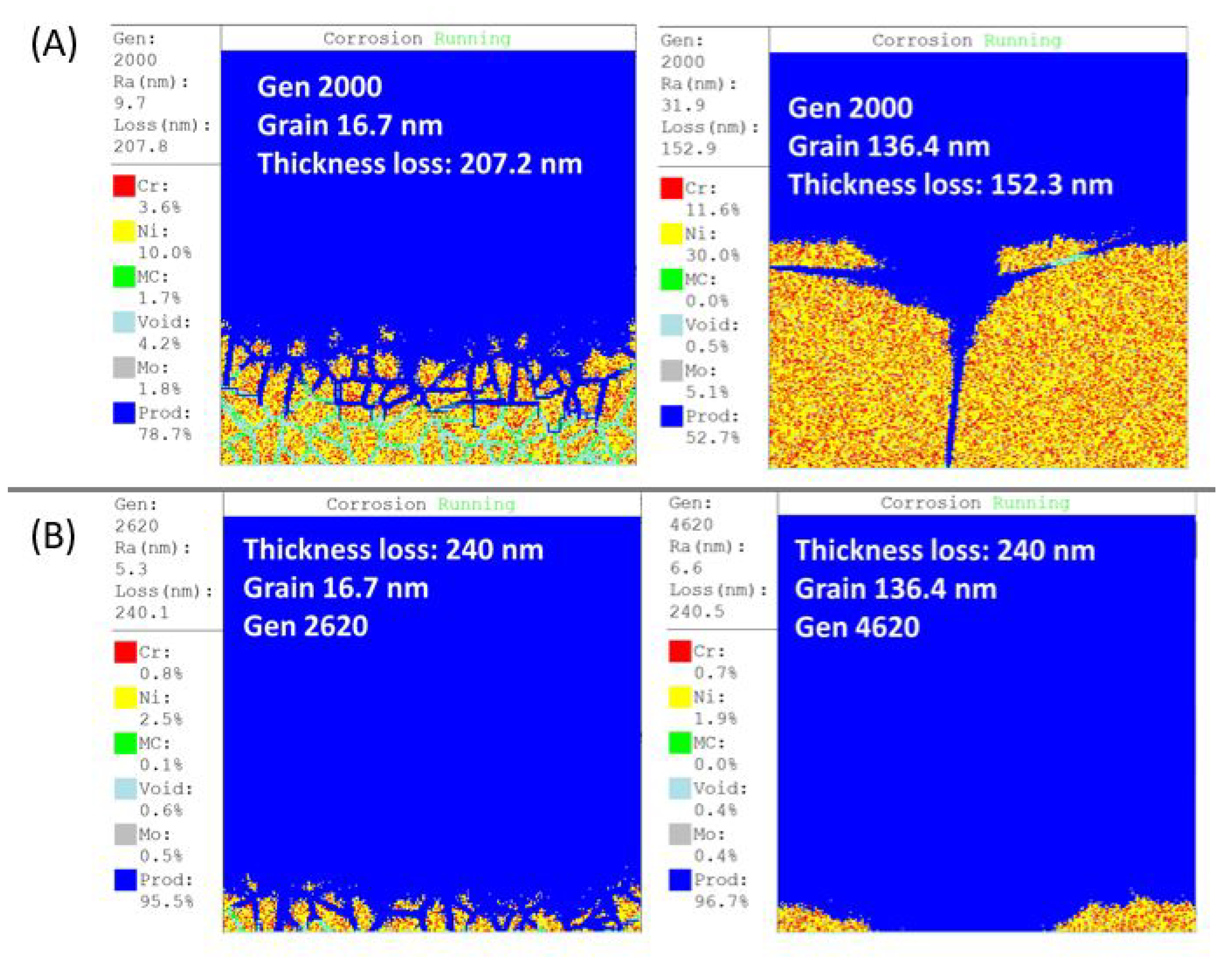
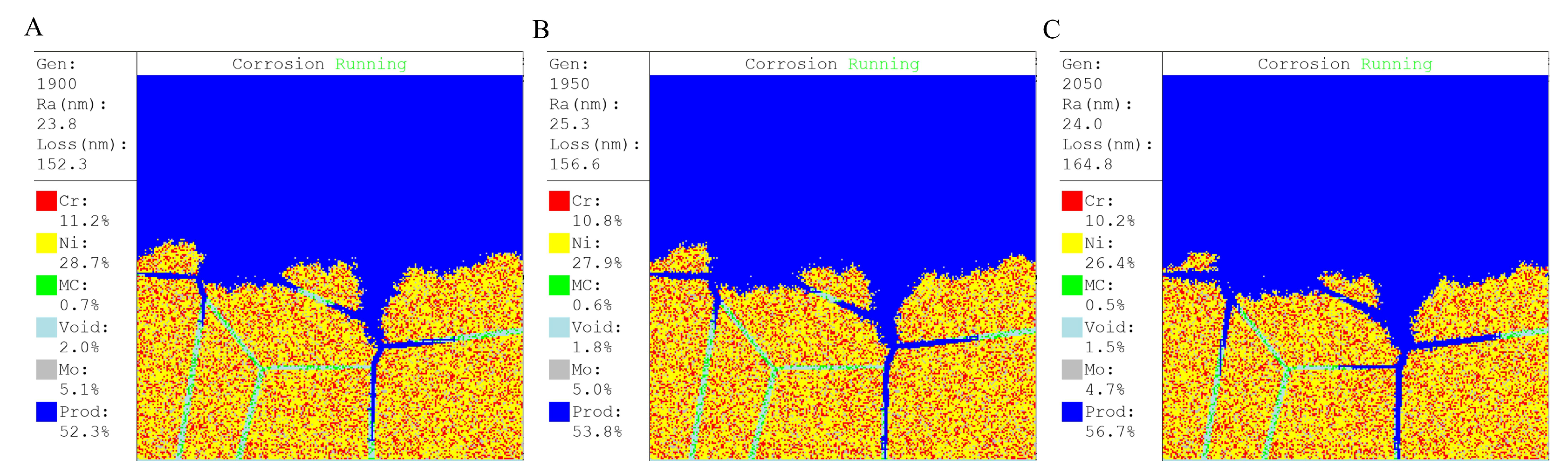
3.1. User Interface
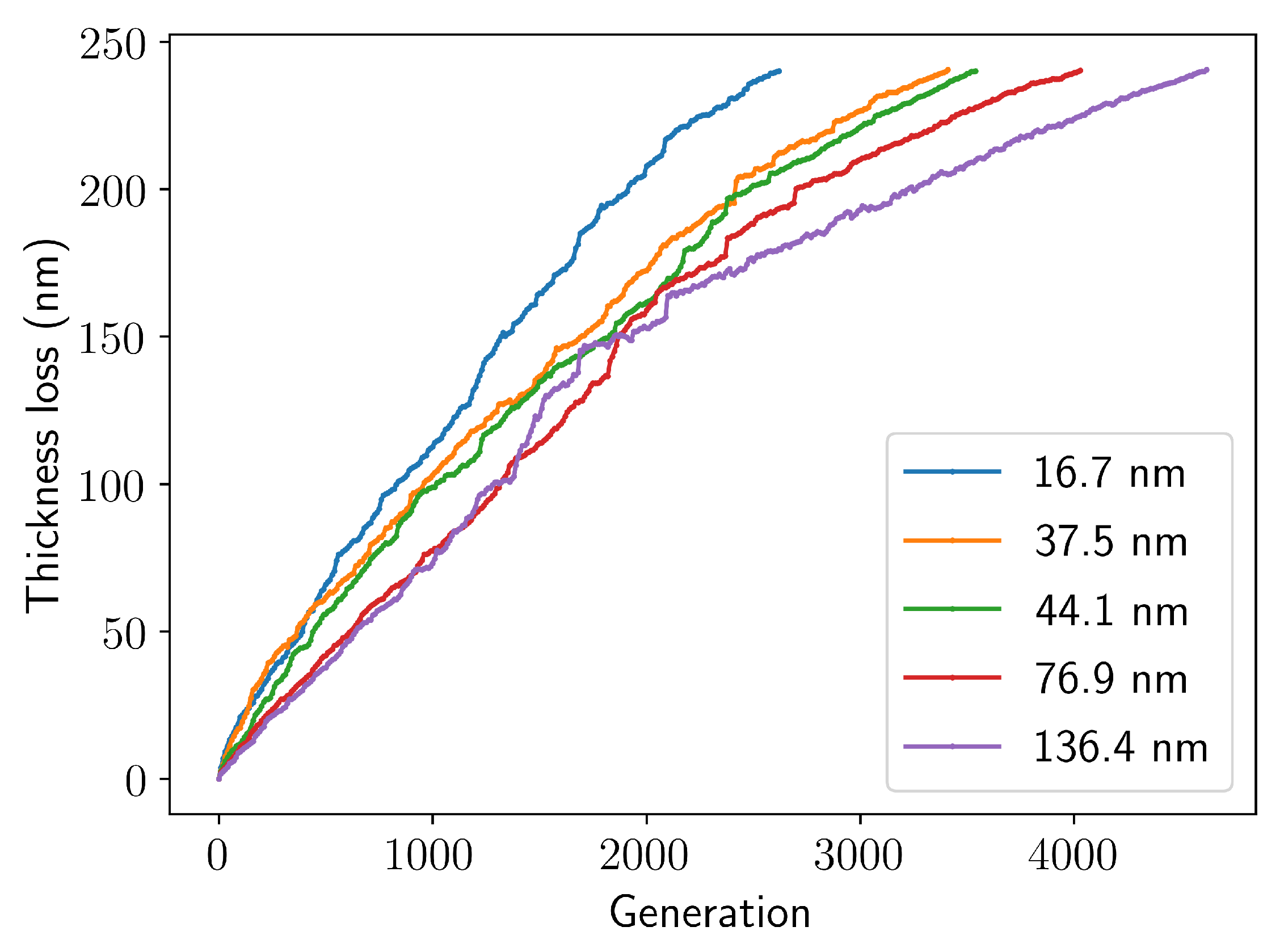
3.2. Thickness Loss
3.3. Roughness
3.4. Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSP | Concentrated Solar Power |
| CA | Cellular Automata |
| BFS | Breadth First Search |
| GUI | Graphic User Interface |
References
- Ding, W.; Bonk, A.; Bauer, T. Corrosion behavior of metallic alloys in molten chloride salts for thermal energy storage in concentrated solar power plants: A review. Front. Chem. Sci. Eng. 2018, 12, 564–576. [Google Scholar] [CrossRef]
- Williams, D.F. Assessment of Candidate Molten Salt Coolants for the NGNP/NHI Heat-Transfer Loop; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2006. [Google Scholar]
- Pragnya, P.; Gall, D.; Hull, R. In situ transmission electron microscopy of high-temperature Inconel-625 corrosion by molten chloride salts. J. Electrochem. Soc. 2021, 168, 51507. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J.; Li, Z.; Zhang, P.; Su, X. Corrosion behavior of 316SS and Ni-based alloys in a ternary NaCl-KCl-MgCl2 molten salt. Sol. Energy 2018, 171, 320–329. [Google Scholar] [CrossRef]
- Liu, B.; Wei, X.; Wang, W.; Lu, J.; Ding, J. Corrosion behavior of Ni-based alloys in molten NaCl-CaCl2-MgCl2 eutectic salt for concentrating solar power. Sol. Energy Mater. Sol. Cells 2017, 170, 77–86. [Google Scholar] [CrossRef]
- Ravi Shankar, A.; Thyagarajan, K.; Kamachi Mudali, U. Corrosion behavior of candidate materials in molten LiCl-KCl salt under argon atmosphere. Corrosion 2013, 69, 655–665. [Google Scholar] [CrossRef]
- Cho, S.H.; Oh, S.C.; Park, S.B.; Ku, K.M.; Lee, J.H.; Hur, J.M.; Lee, H.S. High temperature corrosion behavior of Ni-based alloys. Met. Mater. Int. 2012, 18, 939–949. [Google Scholar] [CrossRef]
- Kondo, M.; Nagasaka, T.; Muroga, T.; Sagara, A.; Noda, N.; Xu, Q.; Ninomiya, D.; Masaru, N.; Suzuki, A.; Terai, T. High performance corrosion resistance of nickel-based alloys in molten salt flibe. Fusion Sci. Technol. 2009, 56, 190–194. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. Corrosion 2010, 66, 075005. [Google Scholar] [CrossRef]
- Pérez-Brokate, C.F.; Di Caprio, D.; Féron, D.; De Lamare, J.; Chaussé, A. Overview of Cellular Automaton Models for Corrosion; Springer: Berlin/Heidelberg, Germany, 2014; pp. 187–196. [Google Scholar]
- Menshutina, N.V.; Kolnoochenko, A.V.; Lebedev, E.A. Cellular automata in chemistry and chemical engineering. Annu. Rev. Chem. Biomol. Eng. 2020, 11, 87–108. [Google Scholar] [CrossRef]
- Svyetlichnyy, D.S. A three-dimensional frontal cellular automaton model for simulation of microstructure evolution—Initial microstructure module. Model. Simul. Mater. Sci. Eng. 2014, 22, 1–19. [Google Scholar] [CrossRef]
- Demontis, P.; Pazzona, F.G.; Suffritti, G.B. A lattice-gas cellular automaton to model diffusion in restricted geometries. J. Phys. Chem. B 2006, 110, 13554–13559. [Google Scholar] [CrossRef]
- Bandman, O.L. A cellular automata convection-diffusion model of flows through porous media. Optoelectron. Instrum. Data Process. 2007, 43, 524–529. [Google Scholar] [CrossRef]
- Kireeva, A. Two-layer CA for simulation of catalytic reaction at dynamically varying surface temperature. J. Comput. Sci. 2015, 11, 317–325. [Google Scholar] [CrossRef]
- Scalise, D.; Schulman, R. Emulating cellular automata in chemical reaction-diffusion networks. Nat. Comput. 2016, 15, 197–214. [Google Scholar] [CrossRef]
- Bandman, O. A Hybrid Approach to Reaction-Diffusion Processes Simulation; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–16. [Google Scholar]
- Van der Weeën, P. Meso-Scale Modeling of Reaction-Diffusion Processes using Cellular Automata. Ph.D. Thesis, University of Ghent, Ghent, Belgium, 2014. [Google Scholar]
- Mai, J.; Kuzovkov, V.N.; von Niessen, W. Stochastic model for the A+B2 surface reaction: Island formation and complete segregation. J. Chem. Phys. 1994, 100, 6073–6081. [Google Scholar] [CrossRef]
- Chopard, B.; Droz, M. Cellular Automata; Springer: Berlin/Heidelberg, Germany, 1998; Volume 1, pp. 139–150. [Google Scholar]
- Feng, J.; Yuan, G.; Mao, L.; Leão, J.B.; Bedell, R.; Ramic, K.; de Stefanis, E.; Zhao, Y.; Vidal, J.; Liu, L. Probing layered structure of Inconel 625 coatings prepared by magnetron sputtering. Surf. Coat. Technol. 2021, 405, 126545. [Google Scholar] [CrossRef] [PubMed]
- Smithells, C.J.; Ransley, C.E. The diffusion of gases through metals-IV—The diffusion of oxygen and of hydrogen through nickel at very high pressures. Proc. R. Soc. Lond. Ser. A-Math. Phys. Sci. 1936, 157, 292–302. [Google Scholar]
- Cussler, E.L.; Cussler, E.L. Diffusion: Mass Transfer in Fluid Systems; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Askill, J. Tracer Diffusion Data for Metals, Alloys, and Simple Oxides; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Feng, J.; Mao, L.; Yuan, G.; Zhao, Y.; Vidal, J.; Liu, L.E. Grain size effect on corrosion behavior of Inconel 625 film against molten MgCl2-NaCl-KCl salt. Corros. Sci. 2022, 197, 110097. [Google Scholar] [CrossRef]
- Sugimoto, K.; Seto, M.; Tanaka, S.; Hara, N. Corrosion resistance of artificial passivation films of Fe2O3-Cr2O3-NiO formed by metalorganic chemical vapor deposition. J. Electrochem. Soc. 1993, 140, 1586. [Google Scholar] [CrossRef]
- Ferguson, J.B.; Lopez, H.F. Oxidation products of Inconel alloys 600 and 690 in pressurized water reactor environments and their role in intergranular stress corrosion cracking. Metall. Mater. Trans. A 2006, 37, 2471–2479. [Google Scholar] [CrossRef]
- Kumar, L.; Venkataramani, R.; Sundararaman, M.; Mukhopadhyay, P.; Garg, S.P. Studies on the oxidation behavior of Inconel 625 between 873 and 1523 K. Oxid. Met. 1996, 45, 221–244. [Google Scholar] [CrossRef]
- Feng, J. Study on the Corrosion Behavior of Inconel 625 in Molten Chloride Salt. Ph.D. Thesis, Rensselaer Polytechnic Institute, Troy, NY, USA, 2021. Available online: https://www.proquest.com/docview/2729021274?pq-origsite=gscholar&fromopenview=true&sourcetype=Dissertations%20&%20Theses (accessed on 24 July 2024).






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Gao, J.; Mao, L.; Bedell, R.; Liu, E. Modeling the Impact of Grain Size on Corrosion Behavior of Ni-Based Alloys in Molten Chloride Salt via Cellular Automata. Metals 2024, 14, 931. https://doi.org/10.3390/met14080931
Feng J, Gao J, Mao L, Bedell R, Liu E. Modeling the Impact of Grain Size on Corrosion Behavior of Ni-Based Alloys in Molten Chloride Salt via Cellular Automata. Metals. 2024; 14(8):931. https://doi.org/10.3390/met14080931
Chicago/Turabian StyleFeng, Jinghua, Jianxi Gao, Li Mao, Ryan Bedell, and Emily Liu. 2024. "Modeling the Impact of Grain Size on Corrosion Behavior of Ni-Based Alloys in Molten Chloride Salt via Cellular Automata" Metals 14, no. 8: 931. https://doi.org/10.3390/met14080931
APA StyleFeng, J., Gao, J., Mao, L., Bedell, R., & Liu, E. (2024). Modeling the Impact of Grain Size on Corrosion Behavior of Ni-Based Alloys in Molten Chloride Salt via Cellular Automata. Metals, 14(8), 931. https://doi.org/10.3390/met14080931






