Experimental Characterization and First-Principles Calculations of Zn Segregation at the β″-Mg5Al2Si4/Al Interfaces in Al-Mg-Si Alloys
Abstract
1. Introduction
2. Materials and Methods
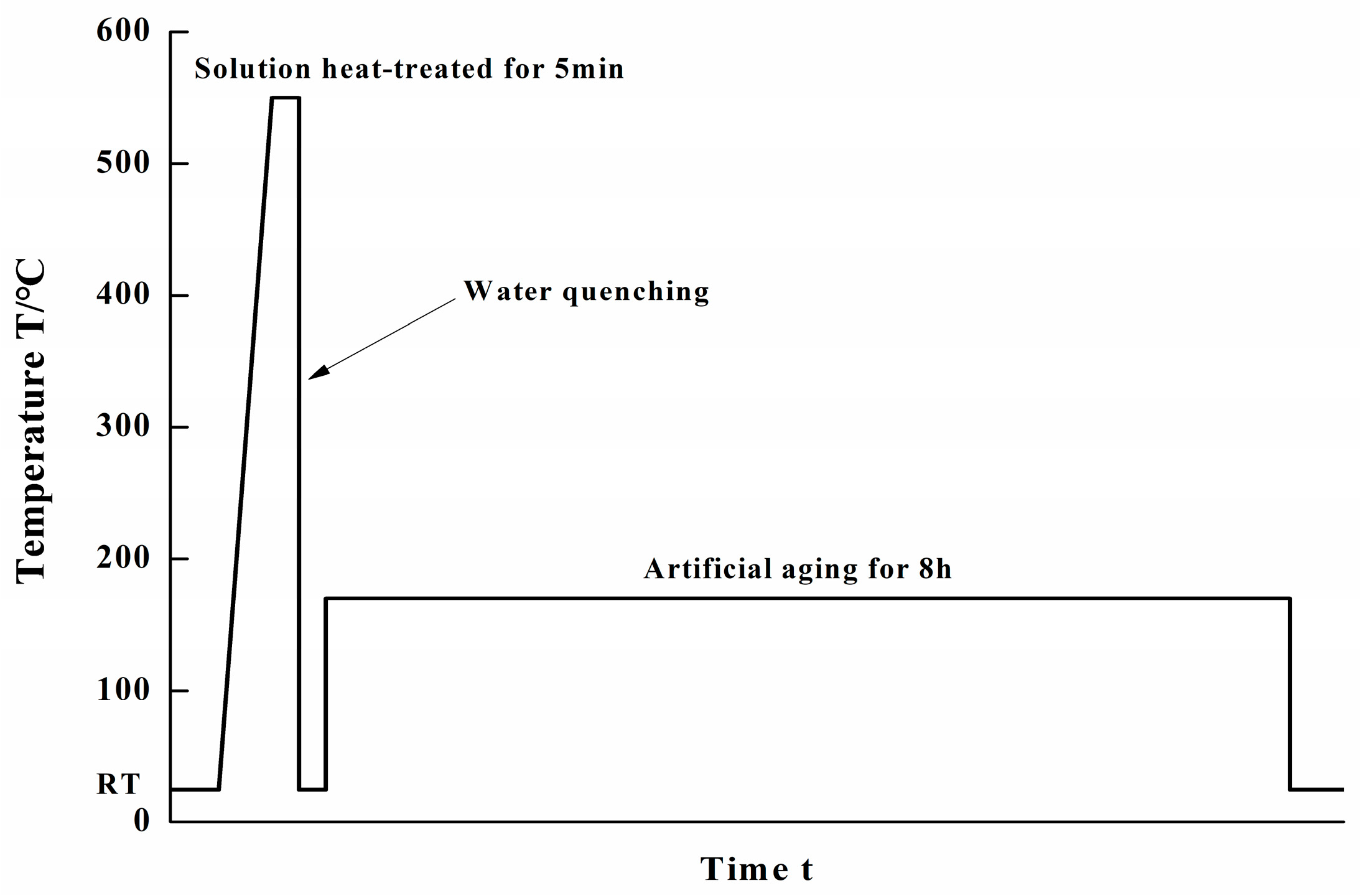
2.1. Experiments
2.2. DFT Calculations
3. Results and Discussions
3.1. Comparative Analysis of Mechanical Properties in Zn-Containing and Zn-Free Alloys
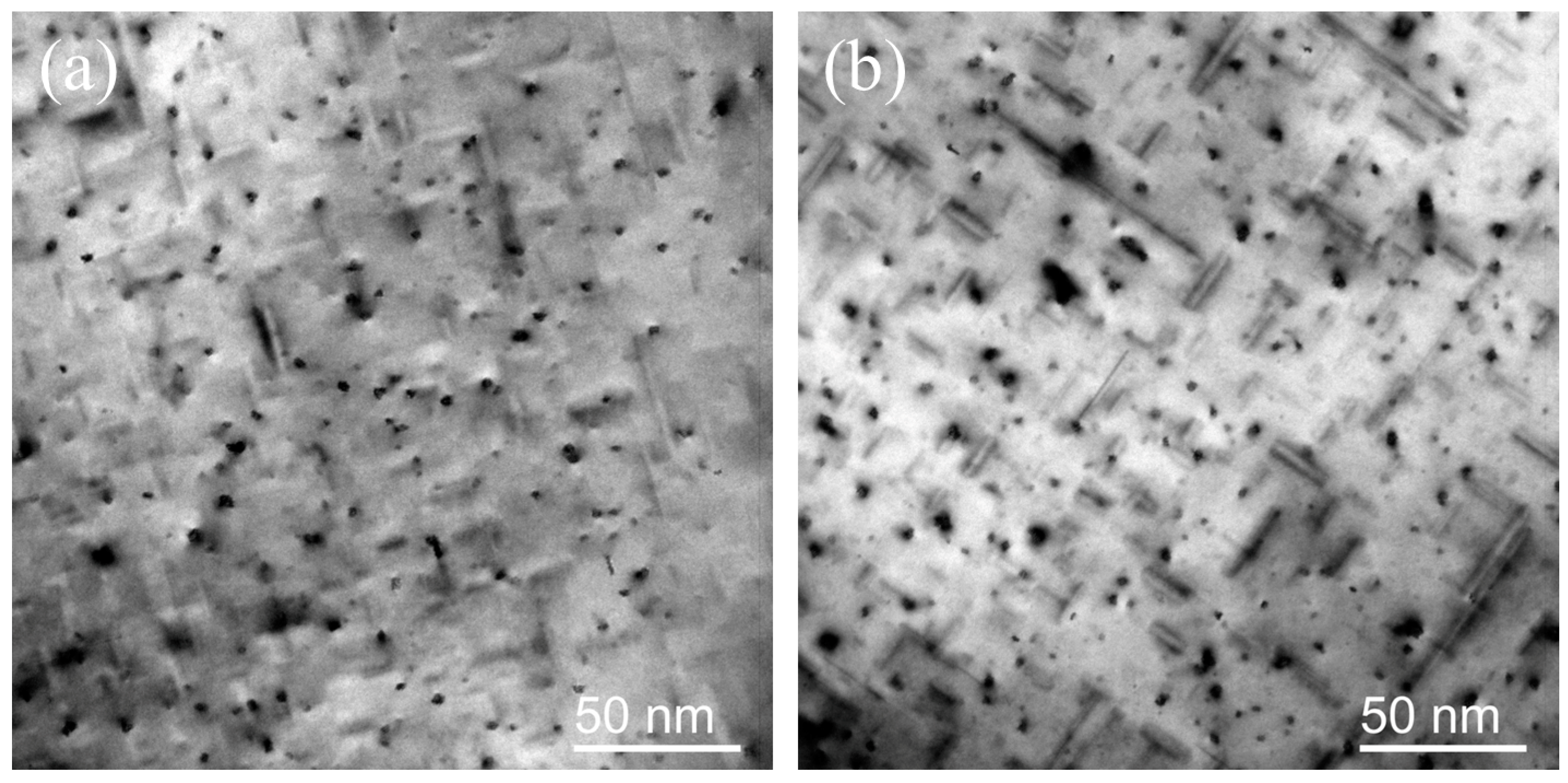
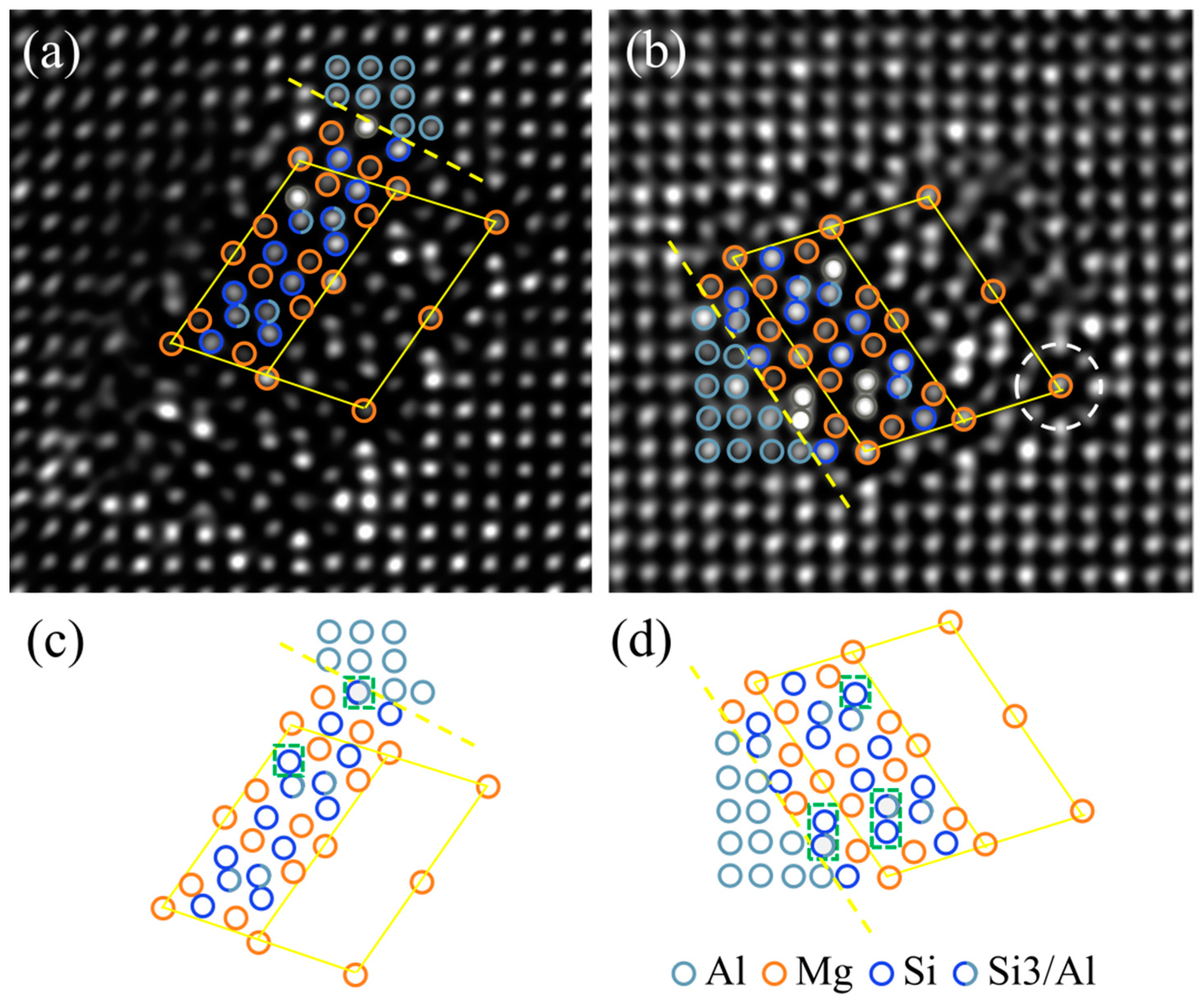
3.2. The Experimental Characterization of Zn Segregation at β″/Al Interfaces
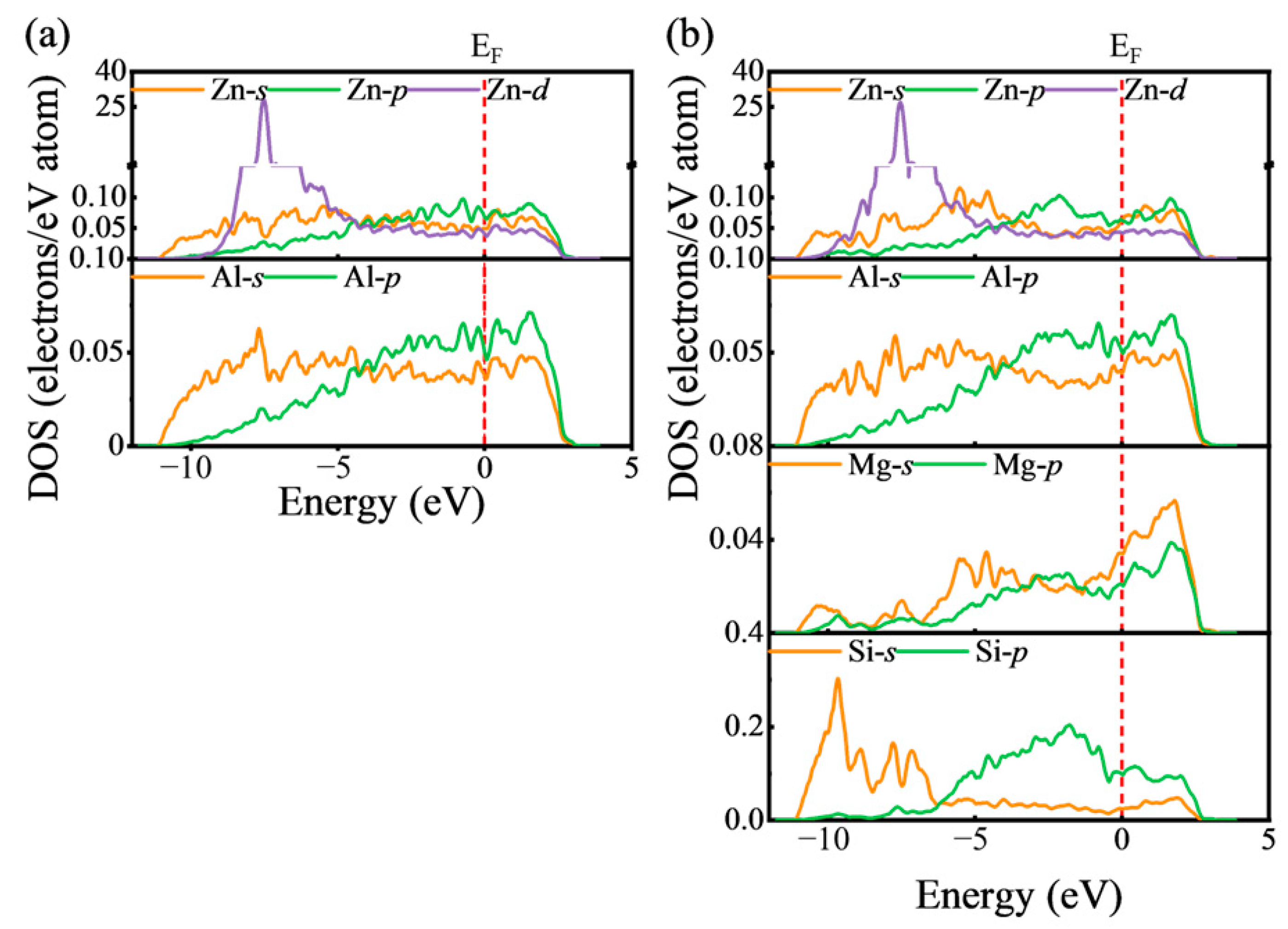
3.3. The Modelling of Zn Segregation at β″/Al Interfaces
3.3.1. The Stability of β″/Al Interfaces
3.3.2. The Occupation Sites of Zn at β″/Al Interfaces
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jin, M.; Shao, G.J. Effect of Cu addition on precipitation phases at early stage in 6082 Al-Mg-Si alloy aged. Trans. Nonferr. Met. Soc. China 2009, 19, 1–7. [Google Scholar]
- Li, H.; Wang, X.L.; Shi, Z.X.; Zheng, Z. Precipitation behaviors of Al-Mg-Si-(Cu) aluminum alloys during continuous heating. Trans. Nonferr. Met. Soc. China 2011, 21, 2028–2034. [Google Scholar]
- Deng, D.H. Effect of Heat Treatment Process on Corrosion Resistance and Microstructure of Al-Mg-Si-Cu Alloy. Master’s Thesis, Hunan University, Changsha, China, 2018. [Google Scholar]
- Zandbergen, H.W.; Andersen, S.J.; Jansen, J. Structure Determination of Mg5Si6 Particles in Al by Dynamic Electron Diffraction Studies. Science 1997, 277, 1221–1225. [Google Scholar] [CrossRef]
- Hasting, H.S.; Frøseth, A.G.; Andersen, S.J.; Vissers, R.; Walmsley, J.C.; Marioara, C.D.; Danoix, F.; Lefebvre, W.; Holmestad, R. Composition of β″ precipitates in Al-Mg-Si alloys by atom probe tomography and first principles calculations. J. Appl. Phys. 2009, 106, 691. [Google Scholar] [CrossRef]
- Saito, T.; Mortsell, E.A.; Wenner, S.; Marioara, C.D.; Andersen, S.J.; Friis, J.; Matsuda, K.; Holmestad, R. Atomic Structures of Precipitates in Al-Mg-Si Alloys with Small Additions of Other Elements. Adv. Eng. Mater. 2018, 20, 1800125. [Google Scholar] [CrossRef]
- Zhang, M.S.; Wang, J.S.; Han, J.Q.; Sui, H.M.; Huang, H.B.; Jin, K.; Qian, F. Optimization of heat treatment process of Al-Mg-Si cast alloys with Zn additions by simulation and experimental investigations. Calphad 2019, 67, 101684. [Google Scholar] [CrossRef]
- Yang, F.; Wang, J.; Wen, T.; Huang, S.; Zhang, L.; Wu, J.; Zhen, J.; Shan, L.; Dong, X.; Yang, H. Double-peak age strengthening of an Al-Mg-Si-Zn alloy processed by laser powder bed fusion. J. Mater. Sci. Technol. 2024, 192, 82–94. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.Y.; Liu, Z.T.; Wang, H.Y. Effect of Mg/Si ratio on synergistic improvement of formability and yield strength in Al-Mg-Si-Zn alloys. Mater. Charact. 2024, 214, 114095. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Z.H.; Yan, L.Z.; Li, X.W.; Huang, S.H.; Yan, H.W.; Zhang, Y.A.; Xiong, B.Q. Effects of Zn addition on the age hardening behavior and precipitation evolution of an Al-Mg-Si-Cu alloy. Mater. Charact. 2018, 145, 258–267. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Z.H.; Yan, L.Z.; Li, X.W.; Huang, S.H.; Yan, H.W.; Zhang, Y.A.; Xiong, B.Q. Natural aging behavior in pre-aged Al-Mg-Si-Cu alloys with and without Zn addition. J. Alloys Compd. 2019, 773, 496–502. [Google Scholar] [CrossRef]
- Saito, T.; Wenner, S.; Osmundsen, E.; Marioara, C.D.; Andersen, S.J.; Røyset, J.; Lefebvre, W.; Holmestad, R. The effect of Zn on precipitation in Al-Mg-Si alloys. Philos Mag. 2014, 94, 2410–2425. [Google Scholar] [CrossRef]
- Guo, M.X.; Du, J.Q.; Zheng, C.H.; Zhang, J.S.; Zhuang, L.Z. Influence of Zn contents on precipitation and corrosion of Al-Mg-Si-Cu-Zn alloys for automotive applications. J. Alloys Compd. 2019, 778, 256–270. [Google Scholar] [CrossRef]
- Bartawi, E.H.; Marioara, C.D.; Shaban, G.; Rahimi, E.; Mishin, O.V.; Sunde, J.K.; Gonzalez-Garcia, Y.; Holmestad, R.; Ambat, R. Effects of grain boundary chemistry and precipitate structure on intergranular corrosion in Al-Mg-Si alloys doped with Cu and Zn. Corros. Sci. 2024, 236, 112227. [Google Scholar] [CrossRef]
- Xu, X.H.; Zhu, W.B.; Yuan, M.F.; Liang, C.J.; Deng, Y.L. The effect of Zn content on the microstructure and mechanical properties of the Al-Mg-Si alloy. Mater. Charact. 2023, 198, 112714. [Google Scholar] [CrossRef]
- Yang, W.C.; Shen, W.W.; Zhang, R.R.; Cao, K.L.; Zhang, J.; Liu, L. Enhanced age-hardening by synergistic strengthening from MgSi and MgZn precipitates in Al-Mg-Si alloy with Zn addition. Mater. Charact. 2020, 169, 110579. [Google Scholar] [CrossRef]
- Mizuno, M.; Sugita, K.; Araki, H. Structural transition of vacancy–solute complexes in Al-Mg-Si alloys. Metals 2022, 12, 2. [Google Scholar] [CrossRef]
- Lan, J.; Chen, Z.; Liu, L.; Zhang, Q.; He, M.; Li, J.; Peng, X.; Fan, T. The thermal properties of L12 phases in aluminum enhanced by alloying elements. Metals 2021, 11, 1420. [Google Scholar] [CrossRef]
- Xue, B.; Xiao, W.; Li, X.; Gao, G.; Li, X.; Zhang, Y.; Wang, L.; Xiong, B. Comprehensive investigation on the structural, electronic and mechanical properties of T-Mg₃₂(Al, Zn)₄₉ phases in Al-Mg-Zn alloys. J. Mater. Sci. Technol. 2024, 173, 237–246. [Google Scholar] [CrossRef]
- Saito, T.; Ehlers, F.J.H.; Lefebvre, W.; Hernandez-Maldonado, D.; Bjørge, R.; Marioara, C.D.; Andersen, S.J.; Holmestad, R. HAADF-STEM and DFT investigations of the Zn-containing β″ phase in Al-Mg-Si alloys. Acta Mater. 2014, 78, 245–253. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.K.; Chen, L.Q.; Wolverton, C. First-principles calculations of β″-Mg5Si6/α-Al interfaces. Acta Mater. 2007, 55, 5934–5947. [Google Scholar] [CrossRef]
- Ehlers, F.J.H.; Dumoulin, S. Interface configuration stability and interfacial energy for the β″ phase in Al-Mg-Si as examined with a first principles based hierarchical multi-scale scheme. J. Alloys Compd. 2014, 591, 329–336. [Google Scholar] [CrossRef]
- Saito, T.; Ehlers, F.J.H.; Lefebvre, W.; Hernandez-Maldonado, D.; Bjørge, R.; Marioara, C.D.; Andersen, S.J.; Mørtsell, E.A.; Holmestad, R. Cu atoms suppress misfit dislocations at the β″/Al interface in Al-Mg-Si alloys. Scr. Mater. 2016, 110, 6–9. [Google Scholar] [CrossRef]
- Liu, C.H.; Ma, P.P.; Zhan, L.H.; Huang, M.H.; Li, J.J. Solute Sn-induced formation of composite β′/β″ precipitates in Al-Mg-Si alloy. Scr. Mater. 2018, 155, 68–72. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- van de Walle, A.; Asta, M.; Ceder, G. The alloy theoretic automated toolkit: A user guide. Calphad 2002, 26, 539–553. [Google Scholar] [CrossRef]
- Manz, T.A. Introducing DDEC6 atomic population analysis: Part 3. Comprehensive method to compute bond orders. RSC Adv. 2017, 7, 45552–45581. [Google Scholar] [CrossRef]
- Limas, N.G.; Manz, T.A. Introducing DDEC6 atomic population analysis: Part 4. Efficient parallel computation of net atomic charges, atomic spin moments, bond orders, and more. RSC Adv. 2018, 8, 2678–2707. [Google Scholar] [CrossRef] [PubMed]



| Interface | Lattice Parameter | Energy of Formation (kJ/mol) | Interfacial Energy (mJ/m2) | |||
|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | γ (°) | |||
| A | 14.92 | 6.606 | 4.054 | 105.6 | 1.06 | 162 |
| Ref. [21] | 14.94 | 6.591 | 4.046 | 106.5 | 1.06 | 124 |
| B | 15.01 | 6.651 | 4.007 | 105.4 | 1.29 | 116 |
| Ref. [21] | 14.83 | 6.651 | 4.052 | 106.8 | 1.27 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yu, M.; Xiao, W.; Li, Y.; Yan, L.; Yu, R.; Li, X.; Li, Z.; Zhang, Y.; Xiong, B. Experimental Characterization and First-Principles Calculations of Zn Segregation at the β″-Mg5Al2Si4/Al Interfaces in Al-Mg-Si Alloys. Metals 2024, 14, 933. https://doi.org/10.3390/met14080933
Li Y, Yu M, Xiao W, Li Y, Yan L, Yu R, Li X, Li Z, Zhang Y, Xiong B. Experimental Characterization and First-Principles Calculations of Zn Segregation at the β″-Mg5Al2Si4/Al Interfaces in Al-Mg-Si Alloys. Metals. 2024; 14(8):933. https://doi.org/10.3390/met14080933
Chicago/Turabian StyleLi, Ying, Mingyang Yu, Wei Xiao, Yanan Li, Lizhen Yan, Rui Yu, Xiwu Li, Zhihui Li, Yongan Zhang, and Baiqing Xiong. 2024. "Experimental Characterization and First-Principles Calculations of Zn Segregation at the β″-Mg5Al2Si4/Al Interfaces in Al-Mg-Si Alloys" Metals 14, no. 8: 933. https://doi.org/10.3390/met14080933
APA StyleLi, Y., Yu, M., Xiao, W., Li, Y., Yan, L., Yu, R., Li, X., Li, Z., Zhang, Y., & Xiong, B. (2024). Experimental Characterization and First-Principles Calculations of Zn Segregation at the β″-Mg5Al2Si4/Al Interfaces in Al-Mg-Si Alloys. Metals, 14(8), 933. https://doi.org/10.3390/met14080933






