Influence of High-Temperature Aggressive Environments on the Durability of Composites Reinforced with Refractory Particles
Abstract
1. Introduction
2. Materials, Equipment, and Research Methods
3. Results and Discussion
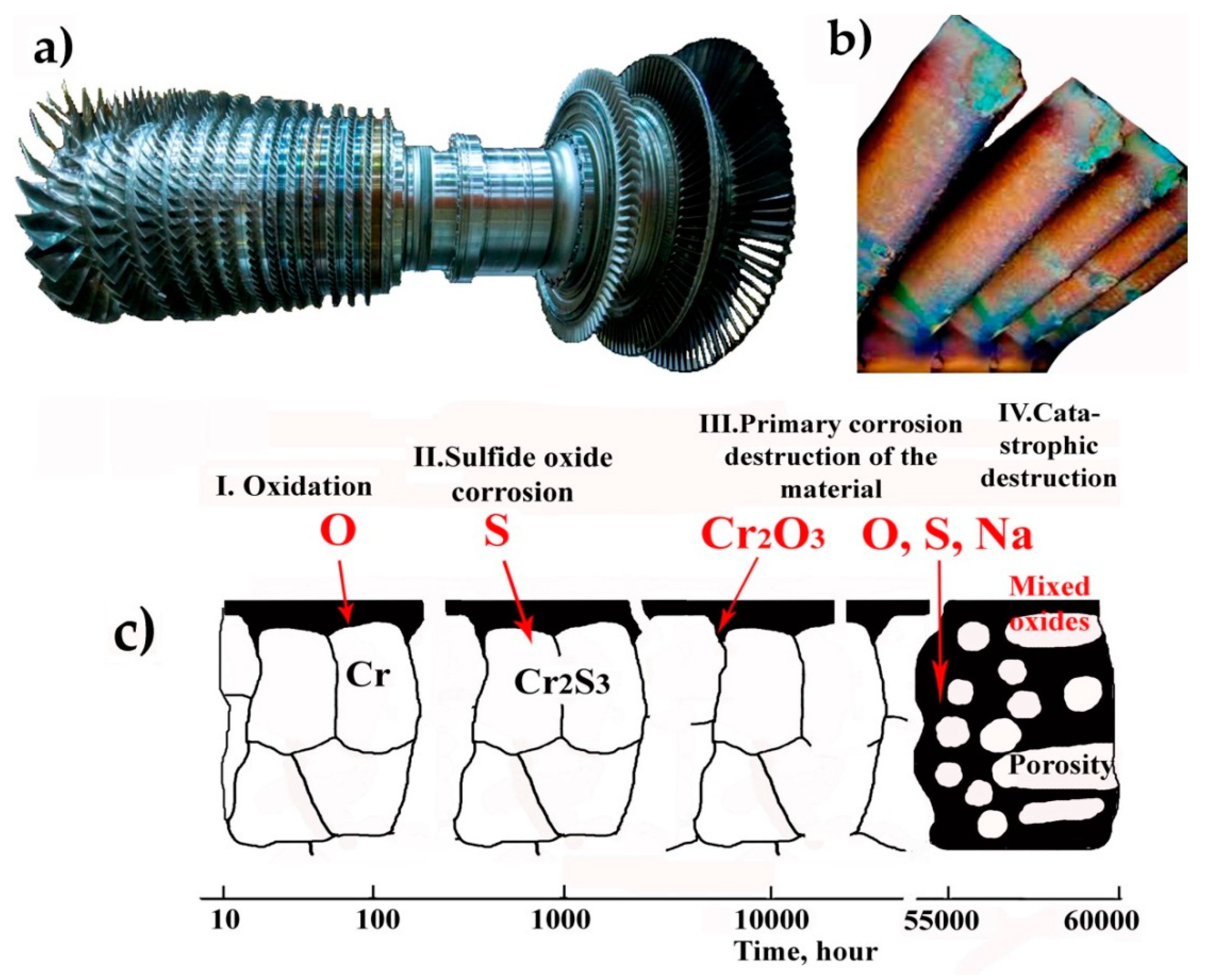
3.1. High-Temperature Corrosion of Industrial Gas Turbine Units
- -
- An increase in gas temperature, leading to higher corrosion rates;
- -
- The use of low-grade fuel, unrefined from corrosive components;
- -
- The need to maintain high surface quality under high gas flow.
3.2. Analysis of Causes and Development of a Destruction Mechanism for Gas Turbine Blades from High-Temperature Corrosion
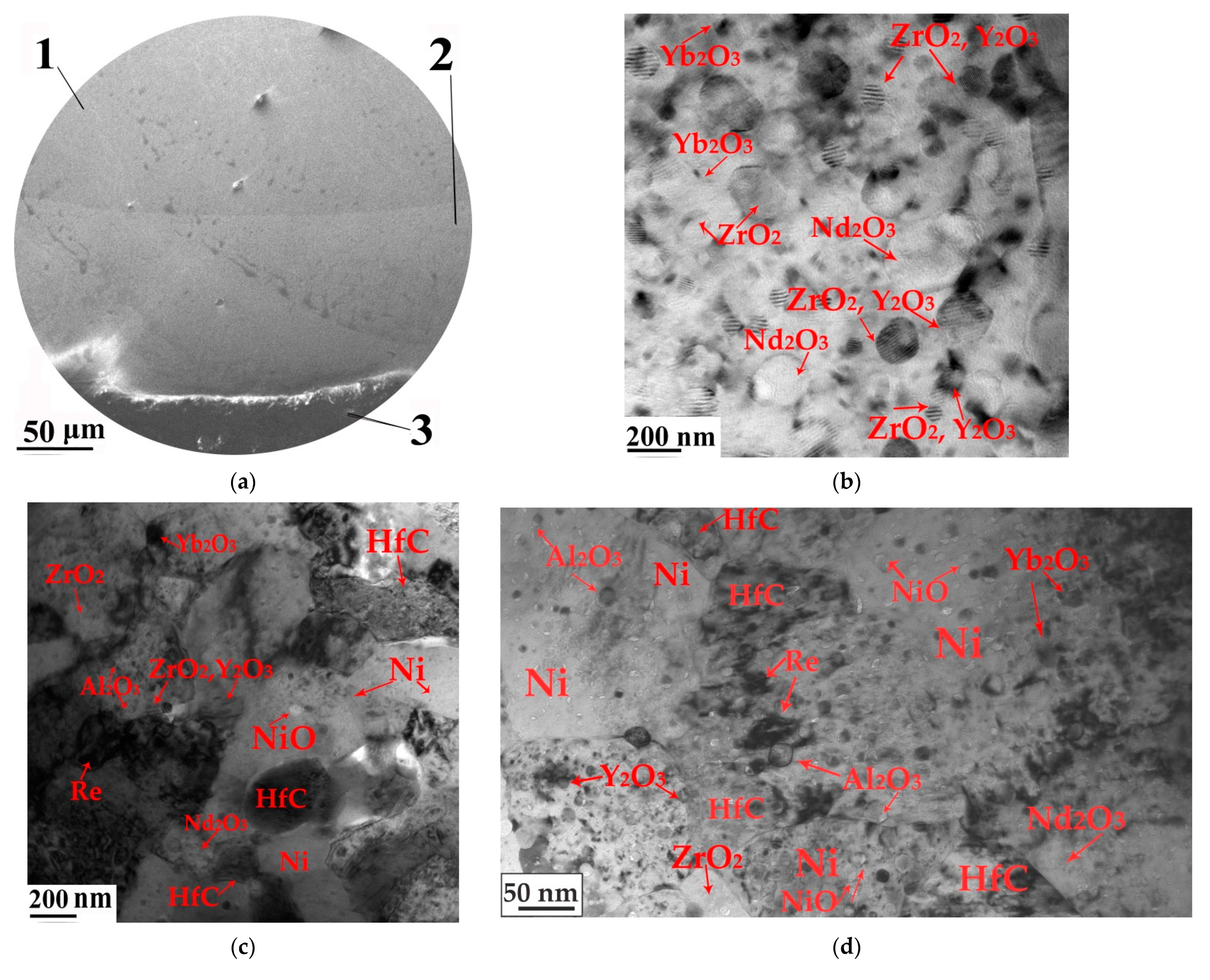
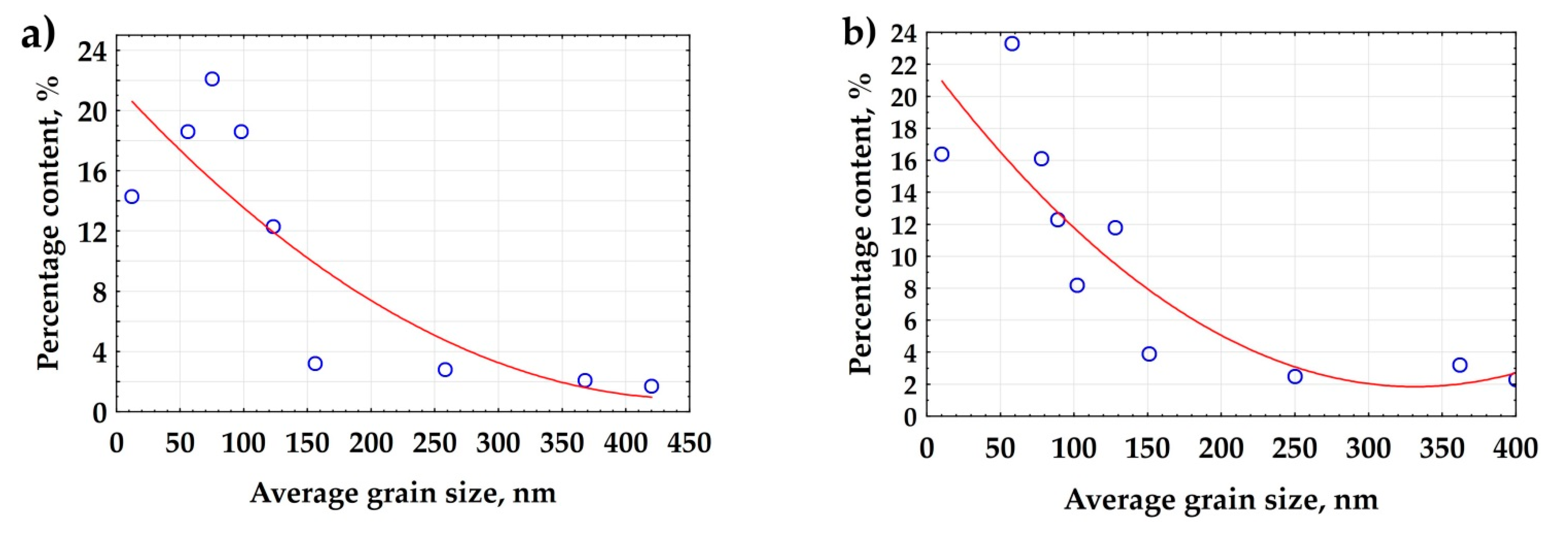
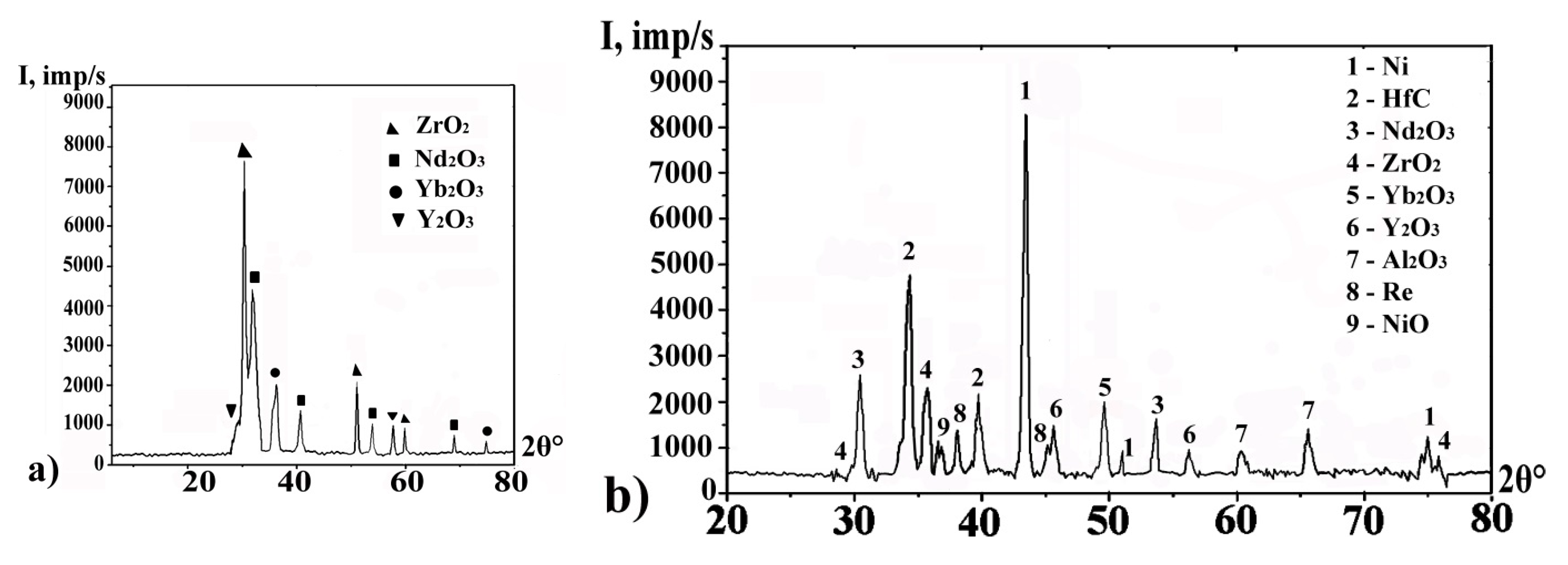
3.3. Surface Modification of Gas Turbine Units
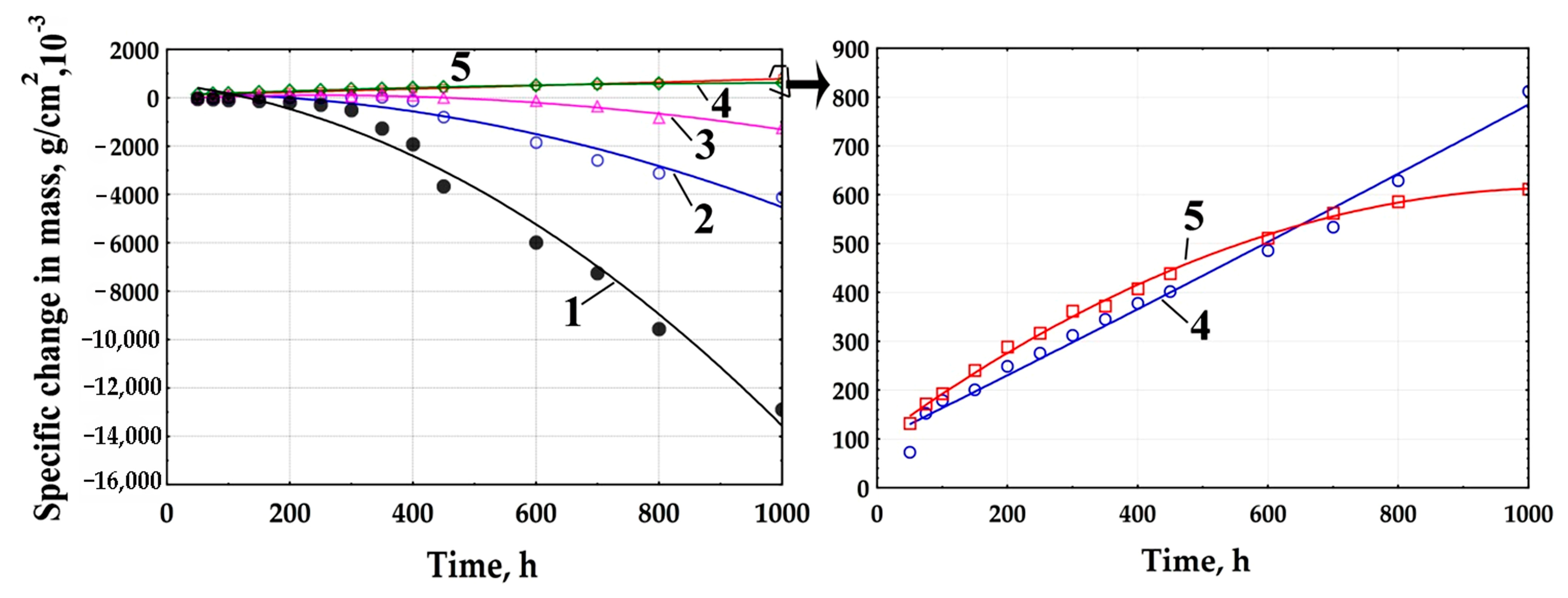
3.4. High-Temperature Corrosion Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fathyunes, L.; Mohtadi-Bonab, M.A. A Review on the Corrosion and Fatigue Failure of Gas Turbines. Metals 2023, 13, 701. [Google Scholar] [CrossRef]
- Yonezawa, K.; Kado, K.; Sugiyama, K.; Ohmori, S.; Umezawa, S. Characteristics of flow and heat transfer in first stage nozzle of a gas turbine with various flow rates of cooling gas. Mech. Eng. J. 2022, 9, 22-00053. [Google Scholar] [CrossRef]
- Yonezawa, K.; Nakai, G.; Takayasu, M.; Sugiyama, K.; Sugita, K.; Umezawa, S.; Ohmori, S. Influence of blade corrosion on aerodynamic characteristics of a gas turbine. Energy 2021, 230, 120665. [Google Scholar] [CrossRef]
- Mouna, A.; Boukortt, H.; Meliani, M.H.; Muthanna, B.G.; Suleiman, R.K.; Sorour, A.A.; Pluvinage, G.; Azari, Z. Corrosion effect, constraint and path orientation estimated in cracked gas turbine blade. Eng. Fail. Anal. 2020, 110, 104345. [Google Scholar] [CrossRef]
- Puspitasari, P.; Andoko, A.; Kurniawan, P. Failure analysis of a gas turbine blade: A review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1034, 012156. [Google Scholar] [CrossRef]
- Alnaeli, M.; Alnajideen, M.; Navaratne, R.; Shi, H.; Czyzewski, P.; Wang, P.; Eckart, S.; Alsaegh, A.; Alnasif, A.; Mashruk, S.; et al. High-Temperature Materials for Complex Components in Ammonia/Hydrogen Gas Turbines: A Critical Review. Energies 2023, 16, 6973. [Google Scholar] [CrossRef]
- Chen, W.R.; Zhao, L. Damage of Gas Turbine Engine Hot Section Components in Harsh Environments and Their Protection—A Review. In Proceedings of the Prepared for Conference of Metallurgists 2017, Vancouver, BC, Canada, 27–30 August 2017. [Google Scholar]
- Ziaei-Asl, A.; Ramezanlou, M.T. Thermo-mechanical behavior of gas turbine blade equipped with cooling ducts and protective coating with different thicknesses. Int. J. Mech. Sci. 2019, 150, 656–664. [Google Scholar] [CrossRef]
- Alqallaf, J.; Ali, N.; Teixeira, J.A.; Addali, A. Solid Particle Erosion Behaviour and Protective Coatings for Gas Turbine Compressor Blades—A Review. Processes 2020, 8, 984. [Google Scholar] [CrossRef]
- Orhon, D.; Hiner, S.D.; Kurz, R.; Benson, J. Gas Turbine Air Filtration Systems for Offshore Applications. 2015 by Turbomachinery Laboratory, Texas A&M Engineering Experiment Station. In Proceedings of the 44th Turbomachinery (31st Pump) Symposia, George R. Brown Convention Center Houston, Houston, TX, USA, 14–17 September 2015. [Google Scholar]
- Wasistho, B. Calcia–magnesia–alumina-silica particle deposition prediction in gas turbines using a Eulerian-Lagrangian approach in computational fluid dynamics. J. Mater. Res. 2020, 35, 2288–2299. [Google Scholar] [CrossRef]
- Chowdhury, T.S.; Mohsin, F.T.; Tonni, M.M.; Mita, M.N.H.; Ehsan, M.M. A critical review on gas turbine cooling performance and failure analysis of turbine blades. Int. J. Thermofluids 2023, 18, 100329. [Google Scholar] [CrossRef]
- Čerňan, J.; Janovec, M.; Hocko, M.; Cúttová, M. Damages of RD-33 Engine Gas Turbine and their Causes. Transp. Res. Procedia 2018, 35, 200–208. [Google Scholar] [CrossRef]
- Kilicarslan, A.; Kiris, M.; Administration, A.R.O.T.-R. 14Exergy Destruction Analysis of a Gas Turbine Power Plant. Hittite J. Sci. Eng. 2018, 5, 339–346. [Google Scholar] [CrossRef]
- Zhou, D.; Wei, T.; Zhang, H.; Ma, S.; Weng, S. A Damage Evaluation Model of Turbine Blade for Gas Turbine. J. Eng. Gas Turbines Power 2017, 139, 092602. [Google Scholar] [CrossRef]
- Guo, T.; Wu, H.; Shao, H.; Zhao, Q.; Liang, Z. Revealing the role of SO2 in the high-temperature corrosion diffusion of two superalloys in CO2 through molecular dynamics and thermal stability. J. Alloys Compd. 2023, 948, 169746. [Google Scholar] [CrossRef]
- Holländer, C.; Kiliani, S.; Stamm, W.; Lüsebrink, O.; Harders, H.; Wessel, E.; Müller, M.; Singheiser, L. Hot corrosion of TBC-coated components upon combustion of low-sulfur fuels. Mater. Corros. 2021, 72, 1643–1655. [Google Scholar] [CrossRef]
- Hassan, K.S.; Razooqi, A.I.; Al-Nadawi, A.K. The Effect of Coating with Nano Oxide on Pitting Corrosion of Gas Turbines Blade. AIP Conf. Proc. 2019, 2144, 040013. [Google Scholar] [CrossRef]
- Ijiri, M.; Ogi, T.; Yoshimura, T. High-temperature corrosion behavior of high-temperature and high-pressure cavitation processed Cr–Mo steel surface. Heliyon 2020, 6, e04698. [Google Scholar] [CrossRef]
- Ghosh, S.; Saha, M.; Bakshi, S.; Mondal, S. Effect of Thermal Barrier Coating in Sustainable Power production of Gas Turbines. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1187, 012034. [Google Scholar] [CrossRef]
- Salwan, G.K.; Subbarao, R.; Mondal, S. Comparison and selection of suitable materials applicable for gas turbine blades. Mater. Today Proc. 2021, 46, 8864–8870. [Google Scholar] [CrossRef]
- Li, B.; Fan, X.; Li, D.; Jiang, P. Design of Thermal Barrier Coatings Thickness for Gas Turbine Blade Based on Finite Element Analysis. Math. Probl. Eng. 2017, 2017, 2147830. [Google Scholar] [CrossRef]
- Bogdan, M.; Błachnio, J.; Kułaszka, A.; Zasada, D. Investigation of the Relationship between Degradation of the Coating of Gas Turbine Blades and Its Surface Color. Materials 2021, 14, 7843. [Google Scholar] [CrossRef] [PubMed]
- Sahith, M.S.; Giridhara, G.; Kumar, R.S. Development and analysis of thermal barrier coatings on gas turbine blades—A Review. Mater. Today Proc. 2018, 5, 2746–2751. [Google Scholar] [CrossRef]
- Kadir, A. High-temperature corrosion protection of gas turbine blades with micro-coatings and nano-coatings: Simulation and experiments. Ph.D. Thesis, University of Salford, Salford, UK, 2021. [Google Scholar]
- Wu, K.; Zhang, W.; Zheng, Z.; Debliquy, M.; Zhang, C. Room-temperature gas sensors based on titanium dioxide quantum dots for highly sensitive and selective H2S detection. Appl. Surf. Sci. 2022, 585, 152744. [Google Scholar] [CrossRef]
- Wen, J.; Song, Z.; Ding, J.; Wang, F.; Li, H.; Xu, J.; Zhang, C. MXene-derived TiO2 nanosheets decorated with Ag nanoparticles for highly sensitive detection of ammonia at room temperature. J. Mater. Sci. Technol. 2022, 114, 233–239. [Google Scholar] [CrossRef]
- Rusinov, P.; Blednova, Z.; Rusinova, A.; Kurapov, G.; Semadeni, M. Development and Research of New Hybrid Composites in Order to Increase Reliability and Durability of Structural Elements. Metals 2023, 13, 1177. [Google Scholar] [CrossRef]
- Rusinov, P.; Blednova, Z.M. Study of the structure and properties of a high-entropy ceramic composite material. Surf. Innov. 2022, 10, 217–226. [Google Scholar] [CrossRef]
- Rusinov, P.; Blednova, Z.M.; Kurapov, G.V. Functionally oriented composite layered materials with martensitic transformations. Surf. Innov. 2023, 11, 26–37. [Google Scholar] [CrossRef]
- Rusinov, P.; Blednova, Z. Physicomechanical and design characteristics of surface high-entropy alloys. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2021, 235, 1635–1644. [Google Scholar] [CrossRef]
- Ułanowicz, L.; Dudziński, A. Two-Layer Heat-Resistant Protective Coatings for Turbine Engine Blades. Coatings 2023, 13, 588. [Google Scholar] [CrossRef]
- Ugla, A.A.; Hasan, M.I.; Ibrahim, Z.A.; Kamil, D.J. Enhancing Thermal Properties of Steam Turbine Blades by Coating with Nanomaterials. Mater. Sci. Forum 2021, 1039, 281–296. [Google Scholar] [CrossRef]
- Kvasnytskyi, V.; Korzhyk, V.; Kvasnytskyi, V.; Mialnitsa, H.; Dong, C.; Pryadko, T.; Matviienko, M.; Buturlia, Y. Designing Brazing Filler Metal for Heat-Resistant Alloys Based on NI3AL Intermetallide. Eastern-Eur. J. Enterp. Technol. 2020, 6, 6–19. [Google Scholar] [CrossRef]
- Pauzi, A.A.; Ghazali, M.J.; Zamri, W.F.H.W.; Rajabi, A. Wear Characteristics of Superalloy and Hardface Coatings in Gas Turbine Applications—A Review. Metals 2020, 10, 1171. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, Y.; Li, W.; Liu, W.; Wu, Y.; Liu, F. Research Progresses on Ceramic Materials of Thermal Barrier Coatings on Gas Turbine. Coatings 2021, 11, 79. [Google Scholar] [CrossRef]
- Feng, G.; Li, H.; Yao, X.; Zhou, H.; Yu, Y.; Lu, J. Ablation resistance of HfC-TaC/HfC-SiC alternate coating for SiC-coated carbon/carbon composites under cyclic ablation. J. Eur. Ceram. Soc. 2021, 41, 3207–3218. [Google Scholar] [CrossRef]
- Li, J.; Miao, Q.; Liang, W.; Liu, R.; Zhao, H.; Sun, J.; Zhang, J.; Zang, K.; Xu, J.; Yao, W.; et al. Functionally Gradient Coatings from HfC/ HfTaC2 to Ti: Growth Process, Basic Mechanical Properties and Wear Behavior. Coatings 2022, 12, 1941. [Google Scholar] [CrossRef]
- Yoo, Y.W.; Nam, U.H.; Kim, Y.; Lee, H.I.; Park, J.K.; Byon, E. Hafnium Carbide Coatings Deposited by Suspension Vacuum Plasma Spraying for Ultra-High-Temperature Oxidation Barrier on Carbon Composites. Appl. Sci. Converg. Technol. 2021, 30, 21–24. [Google Scholar] [CrossRef]
- Dudnik, O.; Lakiza, S.; Grechanyuk, I.; Red’ko, V.; Glabay, M.; Shmibelsky, V.; Marek, I.; Ruban, A.; Grechanyuk, M. High-Entropy Ceramics for Thermal Barrier Coatings Produced from ZrO2 Doped with Rare-Earth Metal Oxides. Sov. Powder Met. Met. Ceram. 2021, 59, 556–563. [Google Scholar] [CrossRef]
- Lima, R.S. Perspectives on Thermal Gradients in Porous ZrO2-7–8 wt.% Y2O3 (YSZ) Thermal Barrier Coatings (TBCs) Manufactured by Air Plasma Spray (APS). Coatings 2020, 10, 812. [Google Scholar] [CrossRef]
- Abdulah, A.J.; Khalifa, M.Z.; Owaid, A.J. Comparative Analysis of Two Alloys (GTD-111 and IN-738) used in Blade of Gas Turbine Model MS9001E at South Baghdad Station. J. Phys. Conf. Ser. 2021, 1973, 012035. [Google Scholar] [CrossRef]
- Toocharoen, S.; Kaewkuekool, S.; Peasura, P. The Rejuvenation Heat Treatment of Nickel Base Superalloy Grade GTD111 after Long-Term Service via the Taguchi Method for Optimization. Int. J. Eng. 2021, 34, 956–965. [Google Scholar] [CrossRef]
- Tan, Z.; Wang, X.; Du, Y.; Duan, T.; Yang, Y.; Liu, J.; Liu, J.; Yang, L.; Li, J.; Zhou, Y.; et al. Temperature dependence on tensile deformation mechanisms in a novel Nickel-based single crystal superalloy. Mater. Sci. Eng. A 2020, 776, 138997. [Google Scholar] [CrossRef]
- Alsultani, K.F.; Majidi, H.S.; Abdulameer, S.; Karkush, M. High-temperature oxidation and hot corrosion behavior of the Inconel 738LC coating with and without Al2O3-CNTs. J. Mech. Behav. Mater. 2023, 32, 20220294. [Google Scholar] [CrossRef]
- Yoon, K.E.; Isheim, D.; Noebe, R.D.; Seidman, D.N. Nanoscale Studies of the Chemistry of a René N6 Superalloy. Interface Sci. 2001, 9, 249–255. [Google Scholar] [CrossRef]
- Zhilkashinova, A.; Abilev, M.; Pavlov, A.; Prokhorenkova, N.; Skakov, M.; Gradoboev, A.; Zhilkashinova, A. Ion-Plasma Spraying and Electron-Beam Treatment of Composite Cr-Al-Co-ZrO2-Y2O3 Coating on the Surface of Ni-Cr Alloy. Coatings 2021, 11, 321. [Google Scholar] [CrossRef]
- Chen, T.; Dong, S.; Deng, C.; Zhang, X.; Jiang, J.; Deng, L.; Cai, Z.; Cao, X. Proposition of a robot-based free diamond abrasive grinding process and its influence on the characteristics of plasma-sprayed 8YSZ coatings. J. Mater. Res. Technol. 2023, 26, 4423–4435. [Google Scholar] [CrossRef]
- Sudarshan, R.; Venkatesh, S.; Balasubramanian, K. Thermal Barrier Coatings for Gas-Turbine Engine Applications—A Review. PalArch’s J. Archaeol. Egypt Egyptol. 2020, 17, 15487–15500. Available online: https://archives.palarch.nl/index.php/jae/article/view/6332 (accessed on 15 May 2024).
- Poerschke, D.L.; Jackson, R.W.; Levi, C.G. Silicate Deposit Degradation of Engineered Coatings in Gas Turbines: Progress Toward Models and Materials Solutions. Annu. Rev. Mater. Res. 2017, 47, 297–330. [Google Scholar] [CrossRef]
- Murugan, M.; Ghoshal, A.; Walock, M.J.; Barnett, B.B.; Pepi, M.S.; Kerner, K.A. Sand particle-Induced deterioration of thermal barrier coatings on gas turbine blades. Adv. Aircr. Spacecr. Sci. 2017, 4, 37–52. [Google Scholar] [CrossRef]
- Padture, N.P. Environmental degradation of high-temperature protective coatings for ceramic-matrix composites in gas-turbine engines. npj Mater. Degrad. 2019, 3, 11. [Google Scholar] [CrossRef]




| Material | Ni | Cr | Co | W | Mo | Ti | Al | Ta | Hf | Yb2O3 | HfC | TaC | Re | ZrO2 | Y2O3 | Al2O3 | Nd2O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GTD111 | 60.4 | 14 | 9.5 | 3.9 | 1.5 | 4.9 | 3 | 2.8 | - | - | - | - | - | - | - | - | - |
| Inconel 738LC | 62 | 16 | 8.8 | 2.8 | 1.9 | 3.4 | 3.4 | 1.7 | - | - | - | - | - | - | - | - | - |
| Rene N6 | 57.4 | 4.2 | 12.5 | 6 | 1.4 | - | 5.75 | 7.2 | 0.15 | - | - | - | 5.4 | - | - | - | - |
| Thermal barrier layer (TBL) | - | - | - | - | - | - | - | - | - | 10 | - | - | - | 45 | 7 | - | 38 |
| Alloy 1 | 35 | - | - | - | - | - | - | - | - | 7 | 22 | - | 4 | 10 | 5 | 5 | 12 |
| Alloy 2 | 35 | - | - | - | - | - | - | - | - | 6 | 15 | 10 | 3 | 8 | 4 | 7 | 12 |
| Material | Microhardness, GPa | Grain Size, µm | Young’s Modulus, GPa | Yield Strength, MPa |
|---|---|---|---|---|
| GTD 111 | 3.45–3.65 | 0.8–0.95 | 130 | 565 |
| Inconel 738LC | 3.24–3.31 | 0.85–0.97 | 149 | 793 |
| Rene N6 | 3.7–3.8 | 0.57–0.61 | 135 | 942 |
| Thermal barrier layer (TBL) | 11.6–11.7 | 0.07–0.23 | - | - |
| Alloy 1 | 12.6–12.7 | 0.007–0.42 | - | - |
| Alloy 2 | 13.3–13.4 | 0.007–0.4 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusinov, P.; Kurapov, G.; Rusinova, A.; Semadeni, M.; Sereda, P. Influence of High-Temperature Aggressive Environments on the Durability of Composites Reinforced with Refractory Particles. Metals 2024, 14, 939. https://doi.org/10.3390/met14080939
Rusinov P, Kurapov G, Rusinova A, Semadeni M, Sereda P. Influence of High-Temperature Aggressive Environments on the Durability of Composites Reinforced with Refractory Particles. Metals. 2024; 14(8):939. https://doi.org/10.3390/met14080939
Chicago/Turabian StyleRusinov, Peter, George Kurapov, Anastasia Rusinova, Maxim Semadeni, and Polina Sereda. 2024. "Influence of High-Temperature Aggressive Environments on the Durability of Composites Reinforced with Refractory Particles" Metals 14, no. 8: 939. https://doi.org/10.3390/met14080939
APA StyleRusinov, P., Kurapov, G., Rusinova, A., Semadeni, M., & Sereda, P. (2024). Influence of High-Temperature Aggressive Environments on the Durability of Composites Reinforced with Refractory Particles. Metals, 14(8), 939. https://doi.org/10.3390/met14080939





