Engineering Corrosion Resistance in Magnesium Alloys for Biomedical Applications: A Synergy of Zn/Ca Atomic Ratio and Texture-Based Approach
Abstract
:1. Introduction
2. Experimental
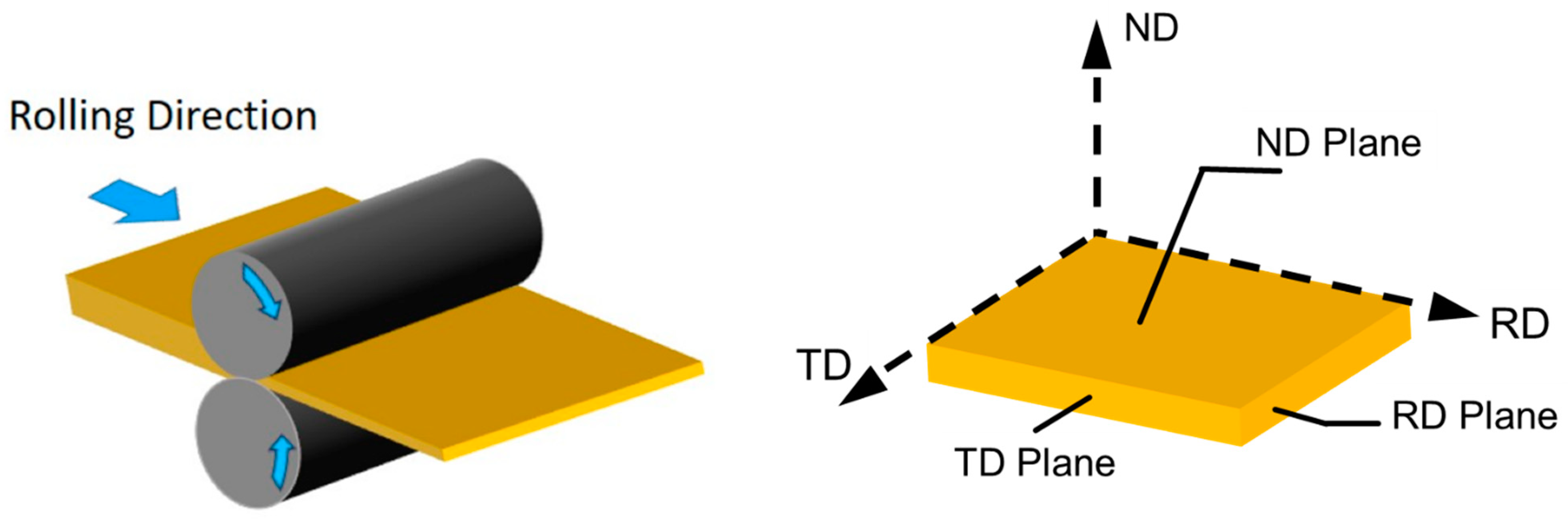
2.1. Materials
2.2. Chemical Composition and X-ray Diffraction
2.3. Microstructural Analysis
2.4. EBSD Analysis
2.5. Mechanical Properties
2.6. Electrochemical Behaviour
2.7. Immersion Test
3. Results and Discussion
3.1. Chemical Composition and XRD Phase Analysis
3.2. Microstructural Analysis
3.3. Crystallographic Texture Analysis
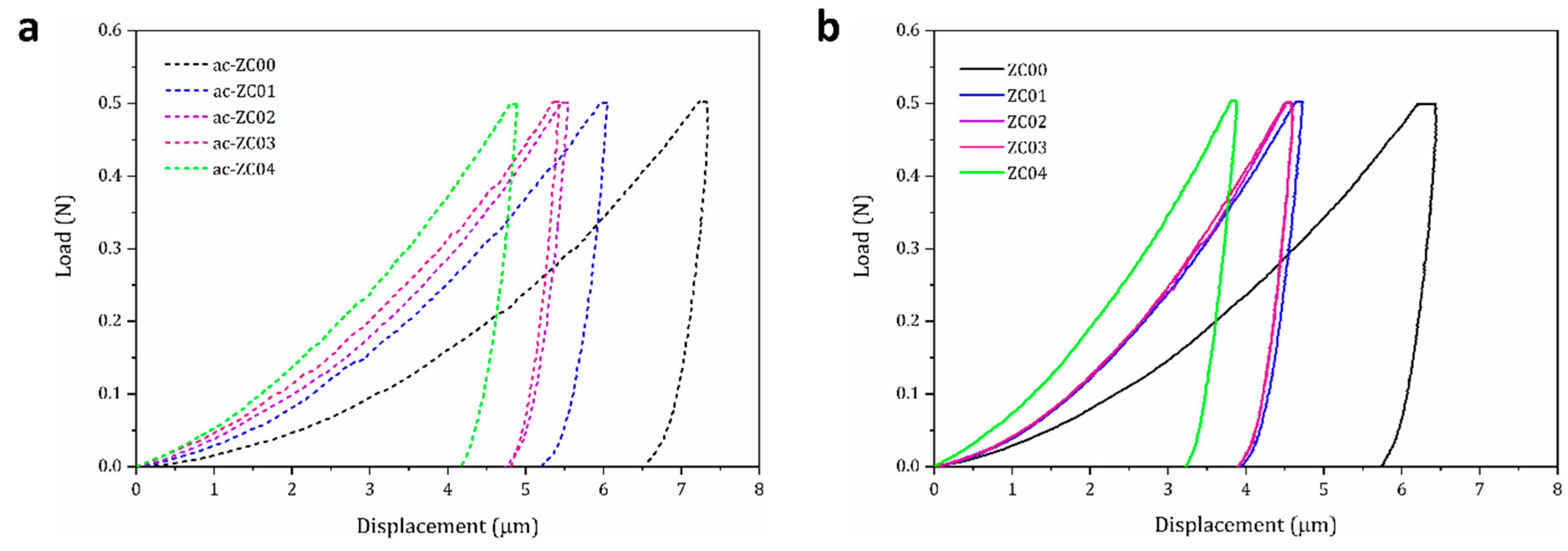
3.4. Mechanical Properties
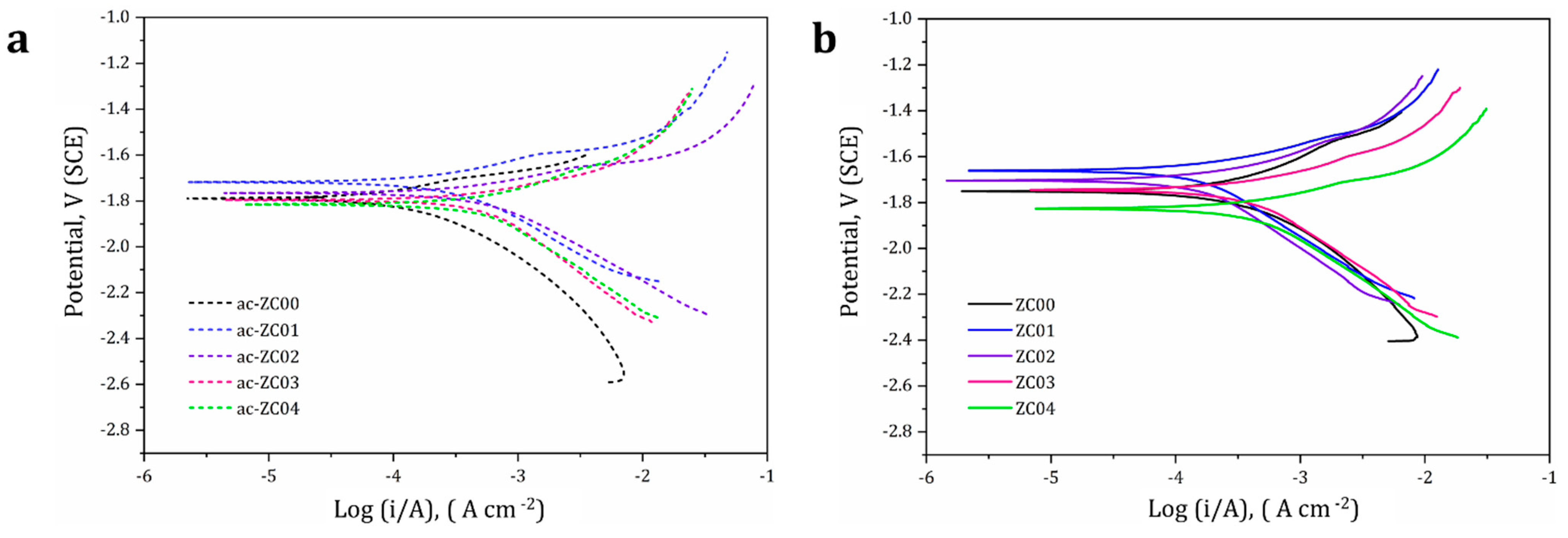
3.5. Corrosion Behaviour
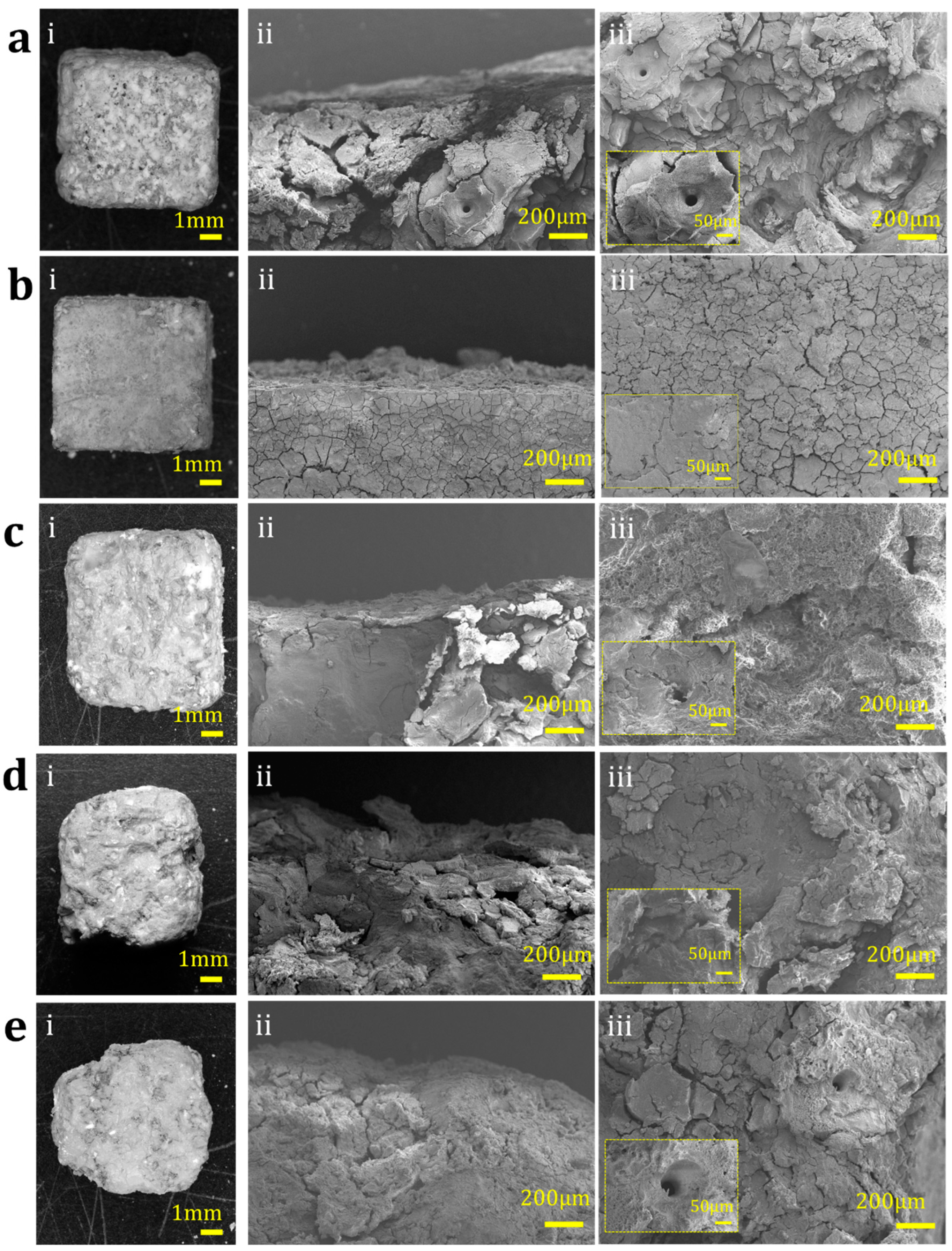
3.6. Degradation Behaviour in Simulated Body Fluid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uppal, G.; Thakur, A.; Chauhan, A.; Bala, S. Magnesium based implants for functional bone tissue regeneration—A review. J. Magnes. Alloys 2022, 10, 356–386. [Google Scholar] [CrossRef]
- Behera, M.; Panemangalore, D.B.; Shabadi, R. Additively manufactured magnesium-based bio-implants and their challenges. Trans. Indian Natl. Acad. Eng. 2021, 6, 917–932. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Emerging magnesium-based biomaterials for orthopedic implantation. Emerg. Mater. Res. 2020, 8, 305–319. [Google Scholar] [CrossRef]
- Li, C.; Huang, T.; Liu, Z. Effects of thermomechanical processing on microstructures, mechanical properties, and biodegradation behaviour of dilute Zn–Mg alloys. J. Mater. Res. Technol. 2023, 23, 2940–2955. [Google Scholar] [CrossRef]
- Tekumalla, S.; Seetharaman, S.; Bau, N.Q.; Wong, W.L.E.; Goh, C.S.; Shabadi, R.; Gupta, M. Influence of Cerium on the Deformation and Corrosion of Magnesium. J. Eng. Mater. Technol. 2016, 138, 031011. [Google Scholar] [CrossRef]
- Zerankeshi, M.M.; Alizadeh, R.; Gerashi, E.; Asadollahi, M.; Langdon, T.G. Effects of heat treatment on the corrosion behavior and mechanical properties of biodegradable Mg alloys. J. Magnes. Alloys 2022, 10, 1737–1785. [Google Scholar] [CrossRef]
- Becerra, L.H.C.; Rodríguez, M.A.L.H.; Solís, H.E.; Arroyo, R.L.; Castro, A.T. Bio-inspired biomaterial Mg-Zn-Ca: A review of the main mechanical and biological properties of Mg-based alloys. Biomed. Phys. Eng. Express 2020, 6, 042001. [Google Scholar]
- Black, J. Biological Performance of Materials: Fundamentals of Biocompatibility; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Gu, X.N.; Zheng, Y.F. A review on magnesium alloys as biodegradable materials. Front. Mater. Sci. China 2010, 4, 111–115. [Google Scholar] [CrossRef]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Moreno, L.; Matykina, E.; Yasakau, K.A.; Blawert, C.; Arrabal, R.; Mohedano, M. As-cast and extruded MgZnCa systems for biodegradable implants: Characterization and corrosion behavior. J. Magnes. Alloys 2023, 11, 1102–1120. [Google Scholar] [CrossRef]
- Zander, D.; Zumdick, N.A. Influence of Ca and Zn on the microstructure and corrosion of biodegradable Mg–Ca–Zn alloys. Corros. Sci. 2015, 93, 222–233. [Google Scholar] [CrossRef]
- Pu, Z.; Song, G.L.; Yang, S.; Outeiro, J.C.; Dillon, O.W.; Puleo, D.A.; Jawahir, I.S. Grain refined and basal textured surface produced by burnishing for improved corrosion performance of AZ31B Mg alloy. Corros. Sci. 2012, 57, 192–201. [Google Scholar] [CrossRef]
- Ahmadkhaniha, D.; Fedel, M.; Sohi, M.H.; Deflorian, F. Corrosion behavior of severely plastic deformed magnesium based alloys: A review. Surf. Eng. Appl. Electrochem. 2017, 53, 439–448. [Google Scholar] [CrossRef]
- Bahmani, A.; Arthanari, S.; Shin, K.S. Formulation of corrosion rate of magnesium alloys using microstructural parameters. J. Magnes. Alloys 2020, 8, 134–149. [Google Scholar] [CrossRef]
- Li, Q.; Huang, G.J.; Huang, X.D.; Pan, S.W.; Tan, C.L.; Liu, Q. On the texture evolution of Mg–Zn–Ca alloy with different hot rolling paths. J. Magnes. Alloys 2017, 5, 166–172. [Google Scholar] [CrossRef]
- Gu, X.N.; Xie, X.H.; Li, N.; Zheng, Y.F.; Qin, L. In vitro and in vivo studies on a Mg-Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012, 8, 2360–2374. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; Dargusch, M. Influence of microstructure on the corrosion of diecast AZ91D. Corros. Sci. 1998, 41, 249–273. [Google Scholar] [CrossRef]
- Bahmani, A.; Lotfpour, M.; Taghizadeh, M.; Kim, W.J. Corrosion behavior of severely plastically deformed Mg and Mg alloys. J. Magnes. Alloys 2022, 10, 2607–2648. [Google Scholar] [CrossRef]
- Du, H.; Wei, Z.; Liu, X.; Zhang, E. Effects of Zn on the microstructure, mechanical property and bio-corrosion property of Mg-3Ca alloys for biomedical application. Mater. Chem Phys. 2011, 125, 568–575. [Google Scholar] [CrossRef]
- Duley, P.; Sanyal, S.; Bandyopadhyay, T.K.; Mandal, S. Homogenization-induced age-hardening behavior and room temperature mechanical properties of Mg-4Zn-0.5Ca-0.16Mn (wt%) alloy. Mater. Des. 2019, 164, 107554. [Google Scholar] [CrossRef]
- Lu, Y.; Bradshaw, A.R.; Chiu, Y.L.; Jones, I.P. Effects of secondary phase and grain size on the corrosion of biodegradable Mg-Zn-Ca alloys. Mater. Sci. Eng. C 2015, 48, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Song, G.-L.; Atrens, A. Recently deepened insights regarding Mg corrosion and advanced engineering applications of Mg alloys. J. Magnes. Alloys 2023, 11, 3948–3991. [Google Scholar] [CrossRef]
- Cao, F.; Xiao, B.; Wang, Z.; Ying, T.; Zheng, D.; Atrens, A.; Song, G.-L. A Mg alloy with no hydrogen evolution during dissolution. J. Magnes. Alloys 2023, 11, 2084–2095. [Google Scholar] [CrossRef]
- Luo, J.; Yan, H.; Zheng, N.; Chen, R.S. Effects of zinc and calcium concentration on the microstructure and mechanical properties of hot-rolled Mg-Zn-Ca sheets. Acta Metall. Sin. (Engl. Lett.) 2016, 29, 205–216. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Geng, L.; Lu, C. Effects of calcium on texture and mechanical properties of hot-extruded Mg-Zn-Ca alloys. Mater. Sci. Eng. A 2012, 539, 56–60. [Google Scholar] [CrossRef]
- Mirzadeh, H. Grain refinement of magnesium alloys by dynamic recrystallization (DRX): A review. J. Mater. Res. Technol. 2023, 25, 7050–7077. [Google Scholar] [CrossRef]
- Zhang, E.; Yang, L. Microstructure, mechanical properties and bio-corrosion properties of Mg-Zn-Mn-Ca alloy for biomedical application. Mater. Sci. Eng. A 2008, 497, 111–118. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Abdul-Kadir, M.R.; Idris, M.H.; Farahany, S. Relationship between the corrosion behavior and the thermal characteristics and microstructure of Mg-0.5Ca-xZn alloys. Corros. Sci. 2012, 64, 184–197. [Google Scholar] [CrossRef]
- Gungor, A.; Incesu, A. Effects of alloying elements and thermomechanical process on the mechanical and corrosion properties of biodegradable Mg alloys. J. Magnes. Alloys 2021, 9, 241–253. [Google Scholar] [CrossRef]
- Schmid-Fetzer, R.; Kozlov, A. Thermodynamic aspects of grain growth restriction in multicomponent alloy solidification. Acta Mater. 2011, 59, 6133–6144. [Google Scholar] [CrossRef]
- Robson, J.D.; Stanford, N.; Barnett, M.R. Effect of particles in promoting twin nucleation in a Mg-5 wt.% Zn alloy. Scr. Mater. 2010, 63, 823–826. [Google Scholar] [CrossRef]
- Liu, C.M.; Zhu, X.R.; Zhou, H.T. Phase Diagrams of Magnesium Alloys; Central South University Press: Changsha, China, 2006; Volume 35, p. 63. [Google Scholar]
- Wang, Y.; Xin, Y.; Yu, H.; Lv, L.; Liu, Q. Formation and microstructure of shear bands during hot rolling of a Mg-6Zn-0.5Zr alloy plate with a basal texture. J. Alloys Compd. 2015, 644, 147–154. [Google Scholar] [CrossRef]
- Wang, M.; Xin, R.; Wang, B.; Liu, Q. Effect of initial texture on dynamic recrystallization of AZ31 Mg alloy during hot rolling. Mater. Sci. Eng. A 2011, 528, 2941–2951. [Google Scholar] [CrossRef]
- Chun, Y.B.; Davies, C.H.J. Texture effects on development of shear bands in rolled AZ31 alloy. Mater. Sci. Eng. A 2012, 556, 253–259. [Google Scholar] [CrossRef]
- Zhen, L.; Zou, D.L.; Xu, C.Y.; Shao, W.Z. Microstructure evolution of adiabatic shear bands in AM60B magnesium alloy under ballistic impact. Mater. Sci. Eng. A 2010, 527, 5728–5733. [Google Scholar] [CrossRef]
- Hadorn, J.P.; Hantzsche, K.; Yi, S.; Bohlen, J.; Letzig, D.; Wollmershauser, J.A.; Agnew, S.R. Role of solute in the texture modification during hot deformation of Mg-rare earth alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2012, 43, 1347–1362. [Google Scholar] [CrossRef]
- Bugnet, M.; Kula, A.; Niewczas, M.; Botton, G.A. Segregation and clustering of solutes at grain boundaries in Mg-rare earth solid solutions. Acta Mater. 2014, 79, 66–73. [Google Scholar] [CrossRef]
- Zeng, Z.R.; Zhu, Y.M.; Xu, S.W.; Bian, M.Z.; Davies, C.H.J.; Birbilis, N.; Nie, J.F. Texture evolution during static recrystallization of cold-rolled magnesium alloys. Acta Mater. 2016, 105, 479–494. [Google Scholar] [CrossRef]
- Trang, T.T.T.; Zhang, J.H.; Kim, J.H.; Zargaran, A.; Hwang, J.H.; Suh, B.C.; Kim, N.J. Designing a magnesium alloy with high strength and high formability. Nat. Commun. 2018, 9, 2522. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Incesu, A.; Gungor, A. Mechanical properties and biodegradability of Mg–Zn–Ca alloys: Homogenization heat treatment and hot rolling. J. Mater. Sci. Mater. Med. 2020, 31, 123. [Google Scholar] [CrossRef]
- Pulido-González, N.; Torres, B.; García-Rodríguez, S.; Rodrigo, P.; Bonache, V.; Hidalgo-Manrique, P.; Mohedano, M.; Rams, J. Mg–1Zn–1Ca alloy for biomedical applications. Influence of the secondary phases on the mechanical and corrosion behaviour. J. Alloys Compd. 2020, 831, 154735. [Google Scholar] [CrossRef]
- Rad, H.R.B.; Idris, M.H.; Kadir, M.R.A.; Farahany, S.; Fereidouni, A.; Yahya, M.Y. Characterization and corrosion behavior of biodegradable Mg-Ca and Mg-Ca-Zn implant alloys. Appl. Mech. Mater. 2012, 121–126, 568–572. [Google Scholar] [CrossRef]
- Shi, H.; Xu, C.; Hu, X.; Gan, W.; Wu, K.; Wang, X. Improving the Young’s modulus of Mg via alloying and compositing—A short review. J. Magnes. Alloys 2022, 10, 2009–2024. [Google Scholar] [CrossRef]
- Nayak, S.; Bhushan, B.; Jayaganthan, R.; Gopinath, P.; Agarwal, R.D.; Lahiri, D. Strengthening of Mg based alloy through grain refinement for orthopaedic application. J. Mech. Behav. Biomed. Mater. 2016, 59, 57–70. [Google Scholar] [CrossRef]
- Jung, Y.G.; Yang, W.; Kim, Y.J.; Kim, S.K.; Yoon, Y.O.; Lim, H.; Kim, D.H. Effect of Ca addition on the microstructure and mechanical properties of heat-treated Mg-6.0Zn-1.2Y-0.7Zr alloy. J. Magnes. Alloys 2021, 9, 1619–1631. [Google Scholar] [CrossRef]
- ASTM G59-97; Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements. ASTM International: West Conshohocken, PA, USA, 2014.
- Zhang, B.; Hou, Y.; Wang, X.; Wang, Y.; Geng, L. Mechanical properties, degradation performance and cytotoxicity of Mg-Zn-Ca biomedical alloys with different compositions. Mater. Sci. Eng. C 2011, 31, 1667–1673. [Google Scholar] [CrossRef]
- Yin, P.; Li, N.F.; Lei, T.; Liu, L.; Ouyang, C. Effects of Ca on microstructure, mechanical and corrosion properties and biocompatibility of Mg-Zn-Ca alloys. J. Mater. Sci. Mater. Med. 2013, 24, 1365–1373. [Google Scholar] [CrossRef]
- Li, H.X.; Qin, S.K.; Ma, Y.Z.; Wang, J.; Liu, Y.J.; Zhang, J.S. Effects of Zn content on the microstructure and the mechanical and corrosion properties of as-cast low-alloyed Mg–Zn–Ca alloys. Int. J. Miner. Metall. Mater. 2018, 25, 800–809. [Google Scholar] [CrossRef]
- Xin, R.; Luo, Y.; Zuo, A.; Gao, J.; Liu, Q. Texture effect on corrosion behavior of AZ31 Mg alloy in simulated physiological environment. Mater. Lett. 2012, 72, 1–4. [Google Scholar] [CrossRef]
- Jiang, B.; Xiang, Q.; Atrens, A.; Song, J.; Pan, F. Influence of crystallographic texture and grain size on the corrosion behaviour of as-extruded Mg alloy AZ31 sheets. Corros. Sci. 2017, 126, 374–380. [Google Scholar] [CrossRef]
- Gerashi, E.; Alizadeh, R.; Langdon, T.G. Effect of crystallographic texture and twinning on the corrosion behavior of Mg alloys: A review. J. Magnes. Alloys 2022, 10, 313–325. [Google Scholar] [CrossRef]
- Xin, R.; Li, B.; Li, L.; Liu, Q. Influence of texture on corrosion rate of AZ31 Mg alloy in 3.5 wt.% NaCl. Mater. Des. 2011, 32, 4548–4552. [Google Scholar] [CrossRef]
- Meng, X.; Jiang, Z.; Zhu, S.; Guan, S. Effects of Sr addition on microstructure, mechanical and corrosion properties of biodegradable Mg–Zn–Ca alloy. J. Alloys Compd. 2020, 838, 155611. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Li, J. Effects of calcium addition on phase characteristics and corrosion behaviors of Mg-2Zn-0.2Mn-xCa in simulated body fluid. J. Alloys Compd. 2017, 728, 37–46. [Google Scholar] [CrossRef]
- Abdel-Gawad, S.A.; Shoeib, M.A. Corrosion studies and microstructure of Mg−Zn−Ca alloys for biomedical applications. Surf. Interfaces 2019, 14, 108–116. [Google Scholar] [CrossRef]
- Dhanapal, A.; Boopathy, S.R.; Balasubramanian, V. Influence of pH value, chloride ion concentration and immersion time on corrosion rate of friction stir welded AZ61A magnesium alloy weldments. J. Alloys Compd. 2012, 523, 49–60. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Cao, F.; Shi, Z.; Bowen, P.K. Advances in Mg corrosion and research suggestions. J. Magnes. Alloys 2013, 1, 177–200. [Google Scholar] [CrossRef]
- Johnston, S.; Shi, Z.; Atrens, A. The influence of pH on the corrosion rate of high-purity Mg, AZ91 and ZE41 in bicarbonate buffered Hanks’ solution. Corros. Sci. 2015, 101, 182–192. [Google Scholar] [CrossRef]







| Nomenclature | Material Condition | Nominal Composition (by wt%) |
|---|---|---|
| ac-ZC00 | As-cast | Commercially pure magnesium (99.99%) |
| ac-ZC01 | As-cast | Mg 97.2%, Zn 2.5%, Ca 0.3% |
| ac-ZC02 | As-cast | Mg 97%, Zn 2.5%, Ca 0.5% |
| ac-ZC03 | As-cast | Mg 96.6%, Zn 2.5%, Ca 0.9% |
| ac-ZC04 | As-cast | Mg 96.35%, Zn 2.5%, Ca 1.15% |
| ZC00 | Hot-rolled | Commercially pure magnesium (99.99%) |
| ZC01 | Hot-rolled | Mg 97.2%, Zn 2.5%, Ca 0.3% |
| ZC02 | Hot-rolled | Mg 97%, Zn 2.5%, Ca 0.5% |
| ZC03 | Hot-rolled | Mg 96.6%, Zn 2.5%, Ca 0.9% |
| ZC04 | Hot-rolled | Mg 96.35%, Zn 2.5%, Ca 1.15% |
| Material | Elemental Composition (wt%) | Zn/Ca Atomic Ratio | ||
|---|---|---|---|---|
| Mg | Zn | Ca | ||
| ZC00 | 100 | - | - | - |
| ZC01 | Balance | 2.5 ± 0.3 | 0.3 ± 0.02 | 5 |
| ZC02 | Balance | 2.41 ± 0.2 | 0.5 ± 0.04 | 3 |
| ZC03 | Balance | 2.35 ± 0.7 | 0.9 ± 0.01 | 1.7 |
| ZC04 | Balance | 2.5 ± 0.1 | 1.15 ± 0.01 | 1.3 |
| Material | Grain Size (µm) Mean ± S.D for n = 3 | Volume Fraction of Secondary Phases (%) |
| ZC00 | 342 ± 5.7 | 0 |
| ZC01 | 173.8 ± 2.5 | 0.9 |
| ZC02 | 158.1 ± 3 | 1.5 |
| ZC03 | 136.7 ± 3.7 | 3.4 |
| ZC04 | 107.2 ± 2.8 | 13.5 |
| Material System | Ecorr (V, SCE) | Icorr (×10−4) (A cm−2) | CR (mm yr−1) |
|---|---|---|---|
| ac-ZC00 | −1.73 | 3.42 ± 0.7 | 7.6 ± 1.1 |
| ac-ZC01 | −1.7 | 2.35 ± 1.0 | 5.2 ± 0.8 |
| ac-ZC02 | −1.75 | 4.2 ± 0.3 | 9.4 ± 0.2 |
| ac-ZC03 | −1.78 | 4.7 ± 0.3 | 10.5 ± 0.2 |
| ac-ZC04 | −1.82 | 5.2 ± 0.9 | 10.8 ± 0.6 |
| ZC00 | −1.7 | 2.86 ± 0.11 | 6.4 ± 0.03 |
| ZC01 | −1.67 | 1.68 ± 0.4 | 3.7 ± 0.1 |
| ZC02 | −1.72 | 3.02 ± 0.8 | 6.7 ± 0.7 |
| ZC03 | −1.75 | 3.5 ± 0.7 | 7.8 ± 0.8 |
| ZC04 | −1.82 | 4.17 ± 0.3 | 8.7 ± 0.5 |
| Days | pH Values | ||||
|---|---|---|---|---|---|
| ZC00 | ZC01 | ZC02 | ZC03 | ZC04 | |
| Day 0 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 |
| Day 21 | 9.53 | 9.23 | 10.09 | 10.28 | 10.33 |
| Material System | Electrochemical Studies | Immersion Studies | |
|---|---|---|---|
| Icorr (× 10−4) (A cm−2) | CR (mm yr−1) | CRi (mm yr−1) | |
| ZC00 | 2.86 ± 0.11 | 6.4 ± 0.03 | 3.6 ± 0.05 |
| ZC01 | 1.68 ± 0.4 | 3.7 ± 0.1 | 1.4 ± 0.02 |
| ZC02 | 3.02 ± 0.8 | 6.7 ± 0.7 | 5.7 ± 0.1 |
| ZC03 | 3.5 ± 0.7 | 7.8 ± 0.8 | 13.1 ± 0.07 |
| ZC04 | 4.17 ± 0.3 | 8.7 ± 0.5 | 16.7 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behera, M.; Shabadi, R.; Gruescu, C. Engineering Corrosion Resistance in Magnesium Alloys for Biomedical Applications: A Synergy of Zn/Ca Atomic Ratio and Texture-Based Approach. Metals 2024, 14, 1002. https://doi.org/10.3390/met14091002
Behera M, Shabadi R, Gruescu C. Engineering Corrosion Resistance in Magnesium Alloys for Biomedical Applications: A Synergy of Zn/Ca Atomic Ratio and Texture-Based Approach. Metals. 2024; 14(9):1002. https://doi.org/10.3390/met14091002
Chicago/Turabian StyleBehera, Manisha, Rajashekhara Shabadi, and Cosmin Gruescu. 2024. "Engineering Corrosion Resistance in Magnesium Alloys for Biomedical Applications: A Synergy of Zn/Ca Atomic Ratio and Texture-Based Approach" Metals 14, no. 9: 1002. https://doi.org/10.3390/met14091002






