Corrosion Properties and Surface Chemistry of Graphene Oxide-Coated AZ91D Magnesium Alloy in Sodium Chloride Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Characterization
3. Results
3.1. Morphological Examination
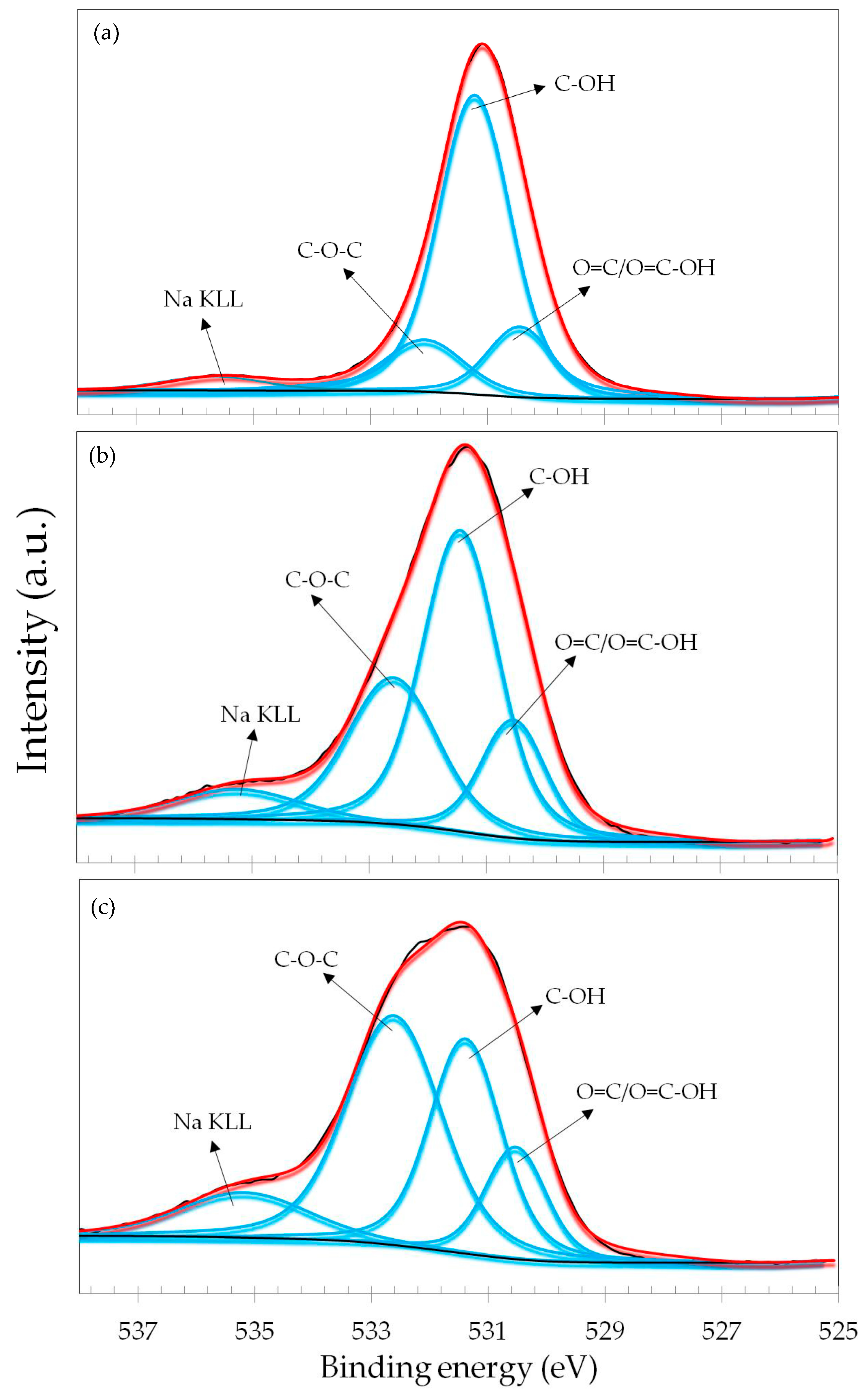
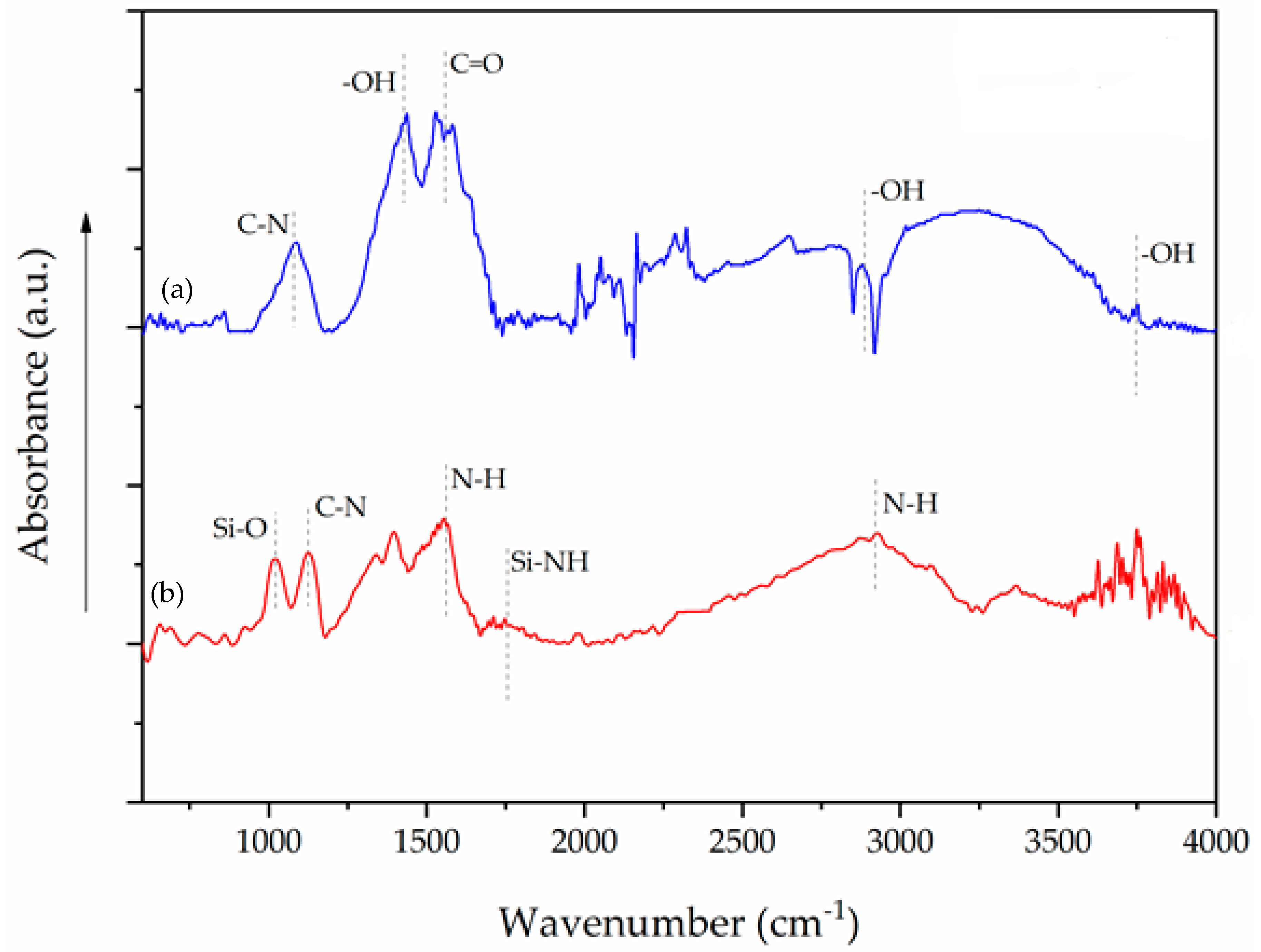
3.2. Spectroscopic Analyses
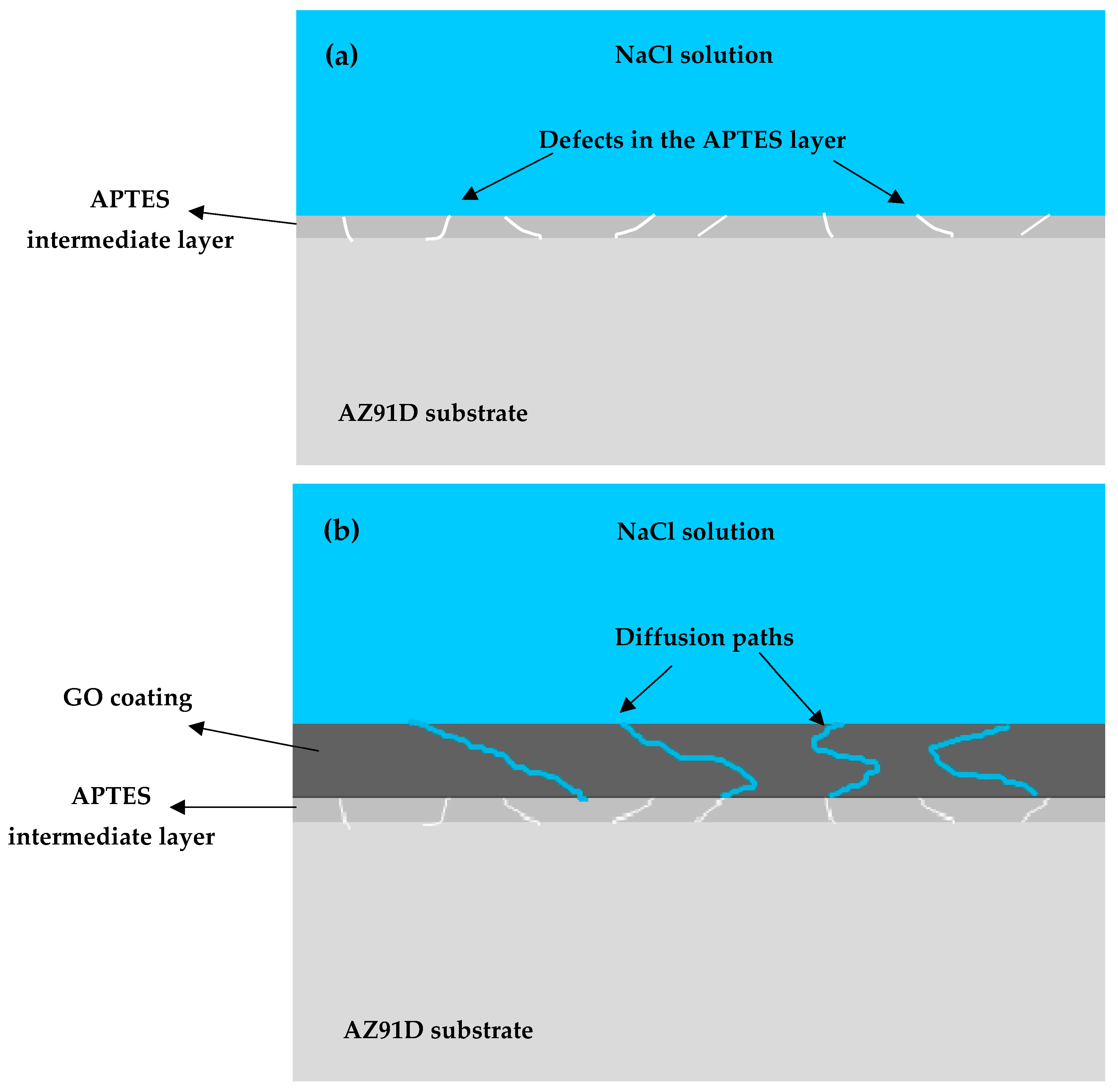
3.3. Corrosion Behavior
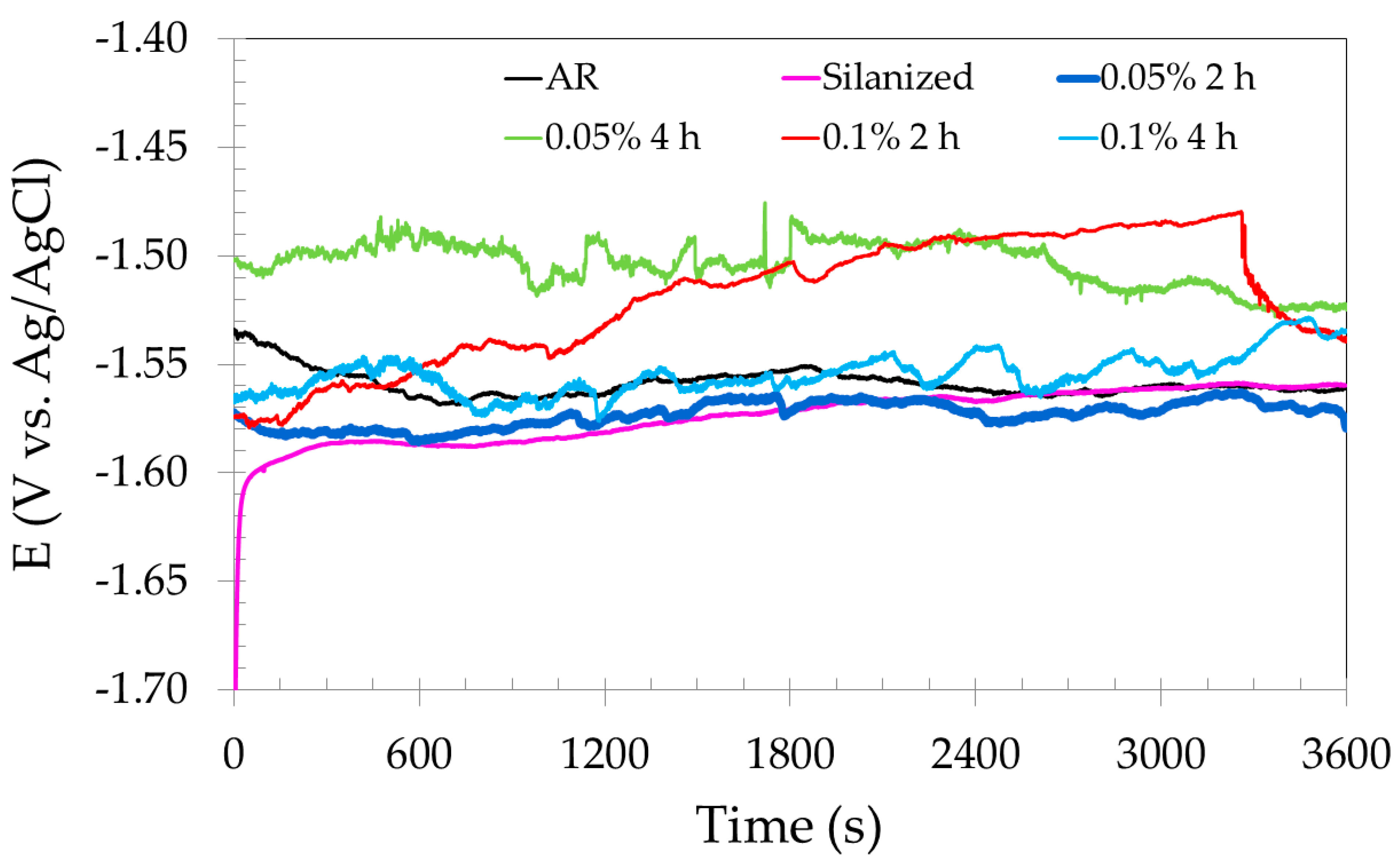
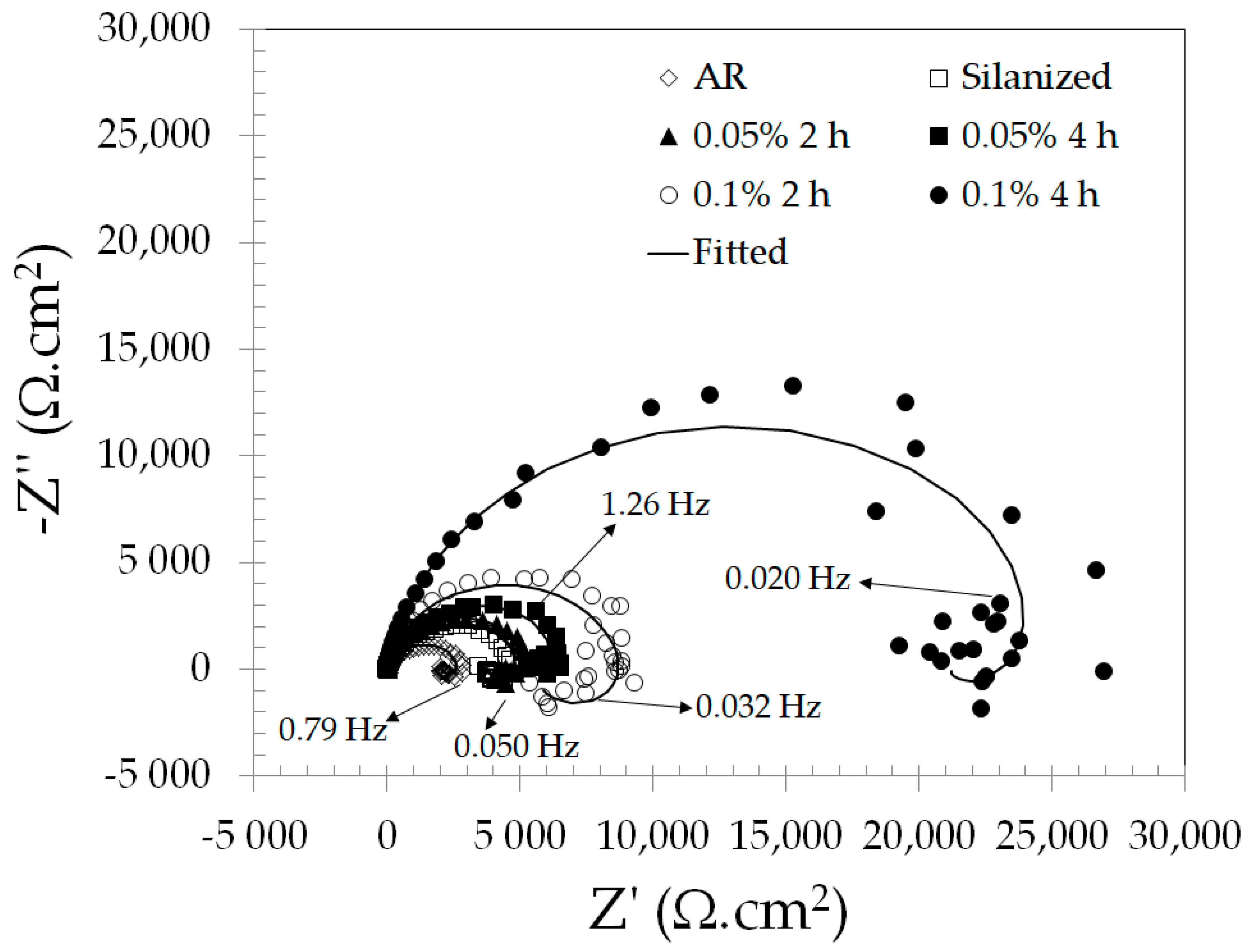
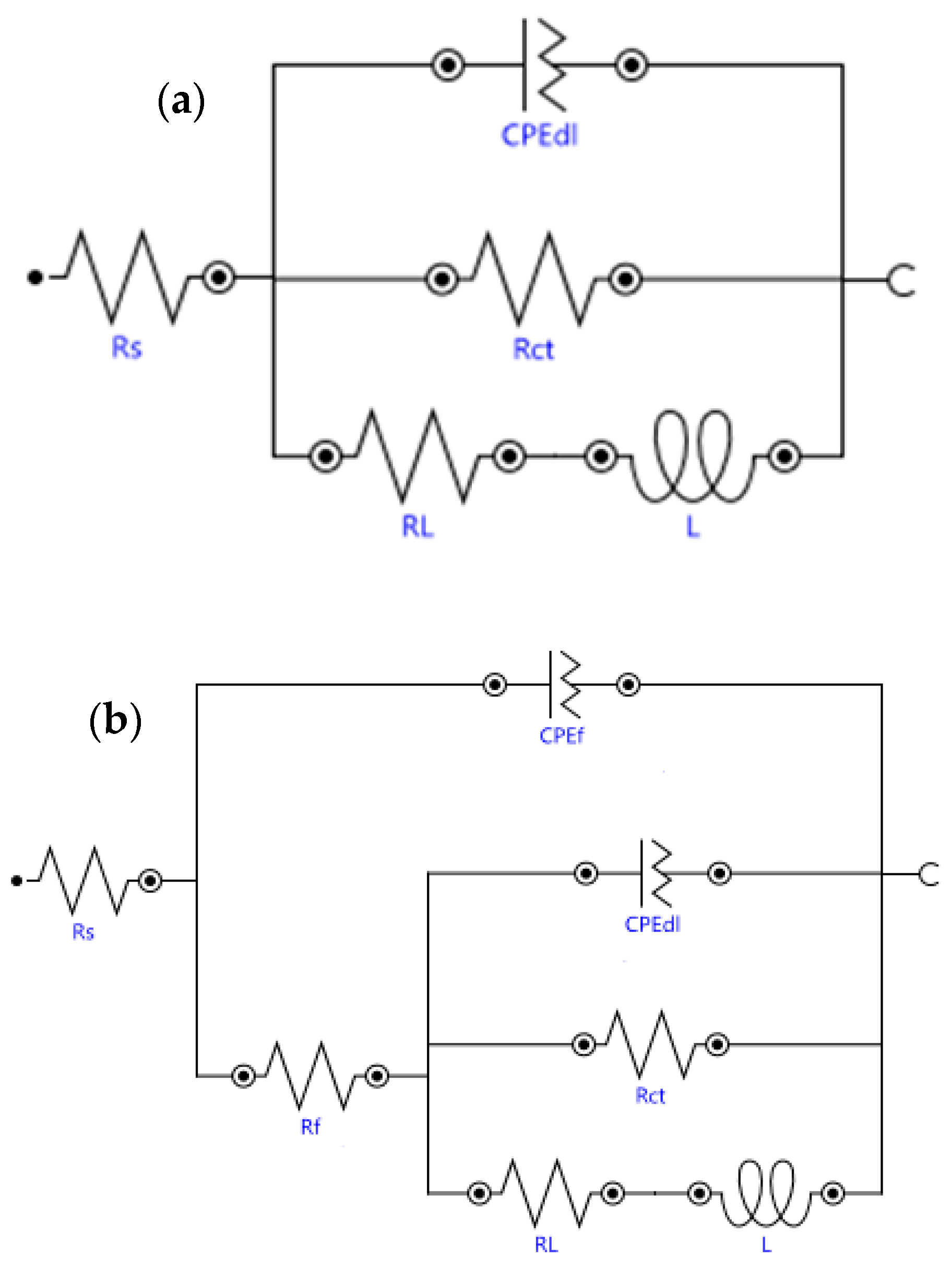
3.3.1. Open Circuit Potential (OCP) and Electrochemical Impedance Spectroscopy (EIS)
3.3.2. Potentiodynamic Polarization Curves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castellanos, A.; Altube, A.; Veja, J.M.; García-Lecina, E.; Díez, J.A.; Grande, H.J. Effect of different post-treatments on the corrosion resistance and tribological properties of AZ91D magnesium alloy coated PEO. Surf. Coat. Technol. 2015, 278, 99–107. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Zhang, X.; Yang, Q.; Zhang, J.; Li, X. Development and application of magnesium alloy partis for automotive OEMs: A review. J. Magnes. Alloys 2023, 11, 15–47. [Google Scholar] [CrossRef]
- Jouini, N.; Ruslan, M.S.M.; Ghani, J.A.; Haron, C.H.C. Sustainable high-speed milling of magnesium alloy AZ91D in dry and cryogenic conditions. Sustainability 2023, 15, 3760. [Google Scholar] [CrossRef]
- Gobara, M.; Shamekh, M.; Akid, R. Improving the corrosion resistance of AZ91D magnesium alloy through reinforcement with titanium carbides and borides. J. Magnes. Alloys 2015, 3, 112–120. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, K. Corrosion and protection of magnesium alloys: Recent advances and future perspectives. Coatings 2023, 13, 1533. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, J.; Zhu, J. One-step preparation of silicate coatings on AZ91D magnesium alloy surface for boosting its corrosion resistance. Silicon 2024, 16, 1147–1159. [Google Scholar] [CrossRef]
- Zeng, R.-C.; Zhang, F.; Lan, Z.-D.; Cui, H.-Z.; Han, E.-H. Corrosion resistance of calcium-modified zinc phosphate conversion coatings on magnesium-aluminium alloys. Corros. Sci. 2014, 88, 452–459. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Moradi-Alavian, S.; Kazempour, A. Salt-nanoparticle systems incorporated into sol-gel coatings for corrosion protection of AZ91 magnesium alloy. Prog. Org. Coat. 2019, 135, 475–482. [Google Scholar] [CrossRef]
- Grimm, M.; Lohmüller, A.; Singer, R.F.; Virtanen, S. Influence of the microstructure on the corrosion behavior of cast Mg-Al alloys. Corros. Sci. 2019, 155, 195–208. [Google Scholar] [CrossRef]
- Su, Y.; Lin, J.; Su, Y.; Zai, W.; Li, G.; Wen, C. Investigation on composition, mechanical properties, and corrosion resistance of Mg-0.5Ca-X (Sr, Zr, Sn) biological alloy. Scanning 2018, 2018, 6519310. [Google Scholar] [CrossRef]
- Pezzato, L.; Coelho, L.B.; Bertolini, R.; Settimi, A.G.; Brunelli, K.; Olivier, M.; Dabalà, M. Corrosion and mechanical properties of plasma electrolytic oxidation-coated AZ80 magnesium alloy. Mater. Corros. 2019, 70, 2103–2112. [Google Scholar] [CrossRef]
- Murakami, K.; Hino, M.; Nakai, K.; Kobayashi, S.; Saijo, A.; Kanadani, T. Mechanism of corrosion protection of anodized magnesium alloys. Mater. Trans. 2008, 49, 1057–1064. [Google Scholar] [CrossRef]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. Review of corrosion-resistant conversion coatings for magnesium and its alloys. Corrosion 2011, 67, 035005-1–035005-16. [Google Scholar] [CrossRef]
- Khiabani, A.B.; Ghanbari, A.; Yarmand, B.; Zamanian, A.; Mozafari, M. Improving corrosion behavior and in vitro bioactivity of plasma electrolytic oxidized AZ91 magnesium alloy using calcium fluoride containing electrolyte. Mater. Lett. 2018, 212, 98–102. [Google Scholar] [CrossRef]
- Hoche, H.; Groβ, S.; Oechsner, M. Development of new PVD coatings for magnesium alloys with improved corrosion properties. Surf. Coat. Technol. 2014, 259, 102–108. [Google Scholar] [CrossRef]
- Ding, R.; Li, W.; Wang, X.; Gui, T.; Li, B.; Han, P.; Tian, H.; Liu, A.; Wang, X.; Liu, X.; et al. A brief review of corrosion protective films and coatings based on graphene and graphene oxide. J. Alloys Compd. 2018, 764, 1019–1055. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Torrisi, L.; Sllipigni, L.; Cutroneo, M. Radiation effects of IR laser on graphene oxide irradiated in vacum and in air. Vacuuum 2018, 153, 122–131. [Google Scholar] [CrossRef]
- Kang, D.; Kwon, J.Y.; Cho, H.; Sim, J.; Hwang, H.S.; Kim, C.S.; Kim, Y.J.; Ruoff, R.S.; Shin, H.S. Oxidation resistance of iron and copper foils coated with reduced graphene oxide multilayers. ACS Nano 2012, 6, 7763–7769. [Google Scholar] [CrossRef]
- Medeliene, V.; Stankevic, V.; Griguceviciene, A.; Selskiene, A.; Bikulcius, G. The study of corrosion and wear resistance of copper composite coatings with inclusion of carbon nanomaterials in the copper metal matrix. Mater. Sci. 2011, 17, 132–139. [Google Scholar] [CrossRef]
- Mahato, N.; Cho, M.W. Graphene integrated polyaniline nanostructured composite coating for protecting steels from corrosion: Synthesis, characterization, and protection mechanism of the coating material in acidic environment. Constr. Build Mater. 2016, 115, 618–633. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Kasiri-Asgarani, M.; Saud, S.N.; Yaghoubidoust, F.; Akbari, E. Structure, corrosion behavior, and antibacterial properties of nano-silica/graphene oxide coating on biodegradable magnesium alloy for biomedical applications. Vacuum 2016, 131, 106–110. [Google Scholar] [CrossRef]
- Ho, C.-H.; Huang, S.-M.; Lee, S.-T.; Chang, Y.-J. Evaluation of synthesized graphene oxide as corrosion protection film coating on steel substrate by electrophoretic deposition. Appl. Surf. Sci. 2019, 477, 226–231. [Google Scholar] [CrossRef]
- Li, P.F.; Zhou, H.; Cheng, X.-H. Nano/micro tribological behaviors of a self-assembled graphene oxide nanolayer on Ti/titanium alloy substrates. Appl. Surf. Sci. 2013, 285, 937–944. [Google Scholar] [CrossRef]
- Li, P.F.; Zhou, H.; Cheng, X. Investigation of a hydrothermal reduced graphene oxide nano coating on Ti substrate and its nano-tribological behavior. Surf. Coat. Technol. 2014, 254, 298–304. [Google Scholar] [CrossRef]
- Tong, L.B.; Zhang, J.B.; Xu, C.; Wang, X.; Song, S.Y.; Jiang, Z.H.; Kamado, S.; Cheng, L.R.; Zhang, H.J. Enhanced corrosion and wear resistances by graphene oxide coating on the surface of Mg-Zn-Ca alloy. Carbon 2016, 109, 340–351. [Google Scholar] [CrossRef]
- Neupane, M.P.; Lee, S.J.; Kang, J.Y.; Park, I.S.; Bae, T.S.; Lee, M.H. Surface characterization and corrosion behavior of silanized magnesium coated with graphene for biomedical application. Mater. Chem. Phys. 2015, 163, 229–235. [Google Scholar] [CrossRef]
- Sakeye, M.; Smatt, J.-H. Comparison of different amino-functionalization procedures on a selection of metal oxide microparticles: Degree of modification and hydrolytic stability. Langmuir 2012, 28, 16491–16950. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-inhibiting coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef]
- Chu, J.H.; Tong, L.B.; Zhang, J.B.; Kamado, S.; Jiang, Z.H.; Zhang, H.J.; Sun, G.X. Bio-inspired graphene-based coatings on Mg alloy surfaces and their integrations of anti-corrosive/wearable performances. Carbon 2019, 141, 154–168. [Google Scholar] [CrossRef]
- Lin, L.; Wu, H.; Green, S.J.; Crompton, J.; Zhang, S.; Horsell, W. Formation of tunable graphene oxide coating with high adhesion. Phys. Chem. Chem. Phys. 2016, 18, 5086–5090. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yang, X.; Zhang, X.; Wang, M.; Li, P.; Zhao, Y.; Wu, G.; Chu, P.K. Effects of carbon dioxide plasma immersion ion implantation on the electrochemical properties of AZ31 magnesium alloy in physiological enviornment. Appl. Surf. Sci. 2013, 286, 257–260. [Google Scholar] [CrossRef]
- Pan, C.-J.; Pang, L.-Q.; Hou, Y.; Lin, Y.-B.; Gong, T.; Liu, T.; Ye, W.; Ding, H.-Y. Improving corrosion resistance and biocompatibility of magnesium alloy by sodium hydroxide and hydrofluoric acid treatments. Appl. Sci. 2017, 7, 33. [Google Scholar] [CrossRef]
- Man, C.; Dong, C.; Fang, Y.; Xiao, K.; Guo, C.; He, G.; Li, X. The corrosion behavior of magnesium alloy AZ31 in hot and dry atmospheric environment in Turpan, China. Int. J. Electrochem. Sci. 2015, 10, 8691–8705. [Google Scholar] [CrossRef]
- Santamaria, M.; Di Quarto, F.; Zanna, S.; Marcus, P. Initial surface film on magnesium metal: A characterization by X-ray photoelectron spectroscopy (XPS) and photocurrent spectroscopy (PCS). Electrochim. Acta 2007, 53, 1314–1324. [Google Scholar] [CrossRef]
- Jönsson, M.; Persson, D.; Thierry, D. Corrosion product formation during NaCl induced atmospheric corrosion of magnesium alloy AZ91D. Corros. Sci. 2007, 49, 1540–1558. [Google Scholar] [CrossRef]
- Feliu, S., Jr.; Galván, J.C.; Pardo, A.; Merino, M.C.; Arrabal, R. Native air-formed oxide film and its effect on magnesium alloys corrosion. Open Corros. J. 2010, 3, 80–91. [Google Scholar] [CrossRef]
- Lakshimi, R.V.; Aruna, S.T.; Anandan, C.; Bera, P.; Sampath, S. EIS and XPS studies on the self-healing properties of Ce-modified silica-alumina hybrid coatings: Evidence for Ce(III) migration. Surf. Coat. Technol. 2017, 309, 363–370. [Google Scholar] [CrossRef]
- López, A.D.F.; Lehr, I.L.; Saidman, S.B. Anodisation of AZ91D magnesium alloy in molybdate solution for corrosion protection. J. Alloys Compd. 2017, 702, 338–345. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Wang, F. Influence of cerium on passivity behavior of wrought AZ91 alloy. Electrochim. Acta 2008, 54, 706–713. [Google Scholar] [CrossRef]
- Rao, X.; Hassan, A.A.; Guyon, C.; Zhang, M.; Ognier, S.; Tatoulian, M. Plasma polymer layers with primary amino groups for immobilization of nano and microparticles. Plasma Chem. Plasma Process. 2020, 40, 589–606. [Google Scholar] [CrossRef]
- Liu, J.; Xi, T. Enhanced anti-corrosion ability and biocompatibility of PLGA coatings on MgZnYNd alloy by BTSE-APTES pretreatment for cardiovascular stent. J. Mater. Sci. Technol. 2016, 32, 845–857. [Google Scholar] [CrossRef]
- Lavorgna, M.; Romeo, V.; Martone, A.; Zarrelli, M.; Giordano, M.; Buonocore, G.G.; Qu, M.Z.; Fei, G.X.; Xia, H.S. Silanization and sílica enrichment of multiwalled carbono nanotubes: Synergistic effects on the thermal-mechanical properties of epoxy nanocomposites. Eur. Polym. J. 2013, 49, 428–438. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Zeng, R.-C.; Lin, C.-G.; Wang, L.; Chen, X.-B.; Chen, D.-C. Corrosion resistance of self-cleaning silane/polypropylene composite coatings on magnesium alloy AZ31. J. Mater. Sci. Technol. 2020, 41, 43–55. [Google Scholar] [CrossRef]
- Sánchez-López, L.; Chico, B.; Llorente, I.; Escudero, M.L.; Lozano, R.M.; García-Alonso, M.C. Covalent immobilization of graphene oxide on biomedical grande CoCr alloy by an improved multilayer system assembly via silane/GO bonding. Mater. Chem. Phys. 2022, 287, 126296. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, S.; Sun, G.; Zhong, Y.; You, B. Evaluation of scratch resistance of functionalized graphene oxide/polysiloxane nanocomposite coatings. Prog. Org. Coat. 2018, 117, 118–129. [Google Scholar] [CrossRef]
- Pei, S.; Meng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Ferrari, I.; Motta, A.; Zanoni, R.; Scaramuzzo, F.A.; Amato, F.; Dalchiele, E.A.; Marrani, A.G. Understanding the nature of graphene oxide functional groups by modulation of the electrochemical reduction: A combined experimental and theoretical approach. Carbon 2023, 203, 29–38. [Google Scholar] [CrossRef]
- Lee, Y.S.; Ji, B.C.; Seo, J.-W.; Jeon, D.I.; Kwon, S.B.; Yoo, J.H.; Kang, B.G.; Song, Y.H.; Yang, W.S.; Kang, B.K.; et al. Facile recyclable process of high-quality single layer graphene oxide via waste graphite anode scrap. Ceram. Int. 2023, 49, 34774–34779. [Google Scholar] [CrossRef]
- Gnanaseelan, N.; Marasamy, L.; Mantilla, A.; Kamaraj, S.K.; Espinosa-Faller, F.J.; Caballero-Briones, F. Exploring the impact of doping and co-doping with B and N on the properties of graphene oxide and its photocatalytic generation of hydrogen. Int. J. Hydrogen Energy 2022, 47, 40905–40919. [Google Scholar] [CrossRef]
- Oliveira, L.A.; Silva, R.M.P.; Rodas, A.C.D.; Souto, R.M.; Antunes, R.A. Surface chemistry, film morphology, local electrochemical behavior and cytotoxic response of anodized AZ31B magnesium alloy. J. Mater. Res. Technol. 2020, 9, 14754–14770. [Google Scholar] [CrossRef]
- Guo, S.; Garaj, S.; Bianco, A.; Ménard-Moyon, C. Controlling covalent chemistry on graphene oxide. Nat. Rev. Phys. 2022, 4, 247–262. [Google Scholar] [CrossRef]
- Zhong, Y.; Gu, Y.; Yu, L.; Cheng, G.; Yang, X.; Sun, M.; He, B. APTES-functionalized Fe3O4 microspheres supported Cu atom-clusters with superior catalytic activity towards 4-nitrophenol reduction. Colloids Surf. A 2018, 547, 28–36. [Google Scholar] [CrossRef]
- Li, L.; Chen, D.; Long, Y.; Wang, F.; Kang, Z. Silane modification of semi-curing epoxy surface: High interfacial adhesion for conductive coatings. Prog. Org. Coat. 2023, 174, 107228. [Google Scholar] [CrossRef]
- Iranshahi, F.; Nasiri, M.B.; Warchomicka, F.G.; Sommitsch, C. Corrosion behavior of electron beam processed AZ91 magnesium alloy. J. Magnes. Alloys 2020, 8, 1314–1327. [Google Scholar] [CrossRef]
- Liu, W.; Cao, F.; Zhong, L.; Zheng, L.; Jia, B.; Zhang, Z.; Zhang, J. Influence of rare earth element Ce and La addition on corrosion behavior of AZ91 magnesium alloy. Mater. Corros. 2009, 60, 795–803. [Google Scholar] [CrossRef]
- Osipenko, M.A.; Kasach, A.A.; Adamiec, J.; Zimowska, M.; Kurilo, I.I.; Kharytonau, D.S. Corrosion inhibition of magnesium alloy AZ31 in chloride-containing solutions by aqueous permanganate. J. Solid State Electrochem. 2023, 27, 1847–1860. [Google Scholar] [CrossRef]
- Tang, J.; Chen, L.; Li, Z.; Zhao, G.; Zhang, C. Formation of abnormal coarse grains and its effects on corrosion behaviors of solution treated ZK60 Mg alloy. Corros. Sci. 2021, 180, 109201. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R.; Han, E.-H. Corrosion characterization of Mg-8Li alloy in NaCl solution. Corros. Sci. 2009, 51, 1087–1094. [Google Scholar] [CrossRef]
- Feliu, S., Jr. Electrochemical impedance spectroscopy for the measurement of the corrosion rate of magnesium alloys: Brief review and challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Torbati-Sarraf, H.; Torbati-Sarraf, S.A.; Poursaee, A.; Langdon, T.G. Electrochemical behavior of a magnesium ZK60 alloy processed by high-pressure torsion. Corros. Sci. 2019, 154, 90–100. [Google Scholar] [CrossRef]
- Jiao, Z.-J.; Yu, C.; Wang, X.-M.; Zhou, Y.-F.; Guo, L.; Xia, Y.; Zhang, B.-C.; Zeng, R.-C. Corrosion resistance enhanced by an atomic layer deposited Al2O3/micro-arc oxidation coating on magnesium alloy AZ31. Ceram. Int. 2024, 50, 5541–5551. [Google Scholar] [CrossRef]
- Nie, L.; Xia, Y.; Zhou, Y.; Zhang, J.; Cao, F.; Zhang, J. Investigation of AZ91D magnesium alloy corrosion behavior under thin electrolyte layer using nondestructive electrochemical techniques. Int. J. Electrochem. Sci. 2016, 11, 259–276. [Google Scholar] [CrossRef]
- Guo, C.; Liu, L.; Liu, H.; Qian, F.; Zhou, Y.; Wang, L.; Li, J.; Wang, J. Effect of indium and yttrium on the corrosion behavior of AZ63 magnesium alloy. J. Alloys Compd. 2024, 985, 174068. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Wu, L.; Zhang, Y.; Deng, J.; Yao, W.; Wu, J.; Yuan, Y.; Xie, Z.; Atrens, A.; et al. Effect of solution pH value on the corrosion resistance of Co-Fe LDHs coating developed on MAO treated magnesium alloy AZ31. Colloids Surf. A 2024, 694, 134220. [Google Scholar] [CrossRef]
- Zhao, X.; Jin, Z.; Zhang, B.; Zhai, X.; Liu, S.; Sun, X.; Zhu, Q.; Hou, B. Effect of graphene oxide on anticorrosion performance of polyelectrolyte multilayer for 2A12 aluminum alloy substrates. Rsc Adv. 2017, 7, 33764. [Google Scholar] [CrossRef]
- Samadianfard, R.; Seifzadeh, D.; Dikici, B. Smart sol-gel nanocomposite containing inhibitor-stabilized g-C3N4 nanoplates for corrosion protection of magnesium alloy. Surf. Coat. Technol. 2024, 484, 130764. [Google Scholar] [CrossRef]
- Liu, S.; Huang, H.; Huang, G.; Qu, J. Influence of oxalic acid on the corrosion behavior of AZ91D magnesium alloy in deionized water. Vacuum 2023, 215, 112351. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, R.; Xu, D.; Wu, L.; Xie, Z.-H.; Yu, G. Growth mechanism and corrosion resistance of layered double hydroxide film on magnesium alloy without external addition of magnesium and aluminum salts. Colloids Surf. A 2024, 689, 133677. [Google Scholar] [CrossRef]
- Bahrampour, S.; Bordbar-Khiabani, A.; Siadati, M.H.; Gasik, M.; Mozafari, M. Improving the inflammatory-associated corrosion behavior of magnesium alloys by Mn3O4 incorporated plasma electrolytic oxidation coatings. Chem. Eng. J. 2024, 483, 149016. [Google Scholar] [CrossRef]
- Xu, X.; Qu, J.; Huang, H. Synthesis of a dibenzimidazole compound and its corrosion inhibition behavior on AZ91D Mg alloy in 3.5 wt.% NaCl solution. J. Mol. Struct. 2023, 1291, 136065. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, J.Y.; Zhao, L.; Wu, L.P. Corrosion resistance and biocompatibility of the KMgF3 coated AZ31 magnesium alloy. J. Alloys Compd. 2023, 968, 172210. [Google Scholar] [CrossRef]
- Curioni, M. The behaviour of magnesium during free corrosion and potentiodynamic polarization investigated by real-time hydrogen measurement and optical imaging. Electrochim. Acta 2014, 120, 284–292. [Google Scholar] [CrossRef]
- Chen, Y.; Ying, T.; Yang, Y.; Wang, J.; Zeng, X. Regulating corrosion resistance of Mg alloys via promoting precipitation with trace Zr alloying. Corros. Sci. 2023, 216, 111106. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Helmer, A.; Ralls, A.M.; Khan, M.U.F.; Kasar, A.K.; Gupta, R.K.; Misra, M.; Shao, S.; Menezes, P.L.; Shamsaei, N. Pitting corrosion behavior and corrosion protection performance of cold sprayed double layered noble barrier coating on magnesium-based alloy in chloride containing solutions. J. Magnes. Alloys 2023, 11, 3099–3119. [Google Scholar] [CrossRef]
- Jian, S.-Y.; Liu, Y.-C.; Chang, C.-J. Novel green and ecofriendly route for fabricating a robust corrosion protection coating on AZ91D magnesium alloy. Electrochem. Commun. 2024, 160, 107667. [Google Scholar] [CrossRef]
- Peng, Y.; Wan, S.; Liao, B.; Guo, X. Preparation and properties of multi-function composite coating on AZ91D magnesium alloy with wave-assimilation and anti-corrosion performance. Surf. Coat. Technol. 2024, 478, 130464. [Google Scholar] [CrossRef]
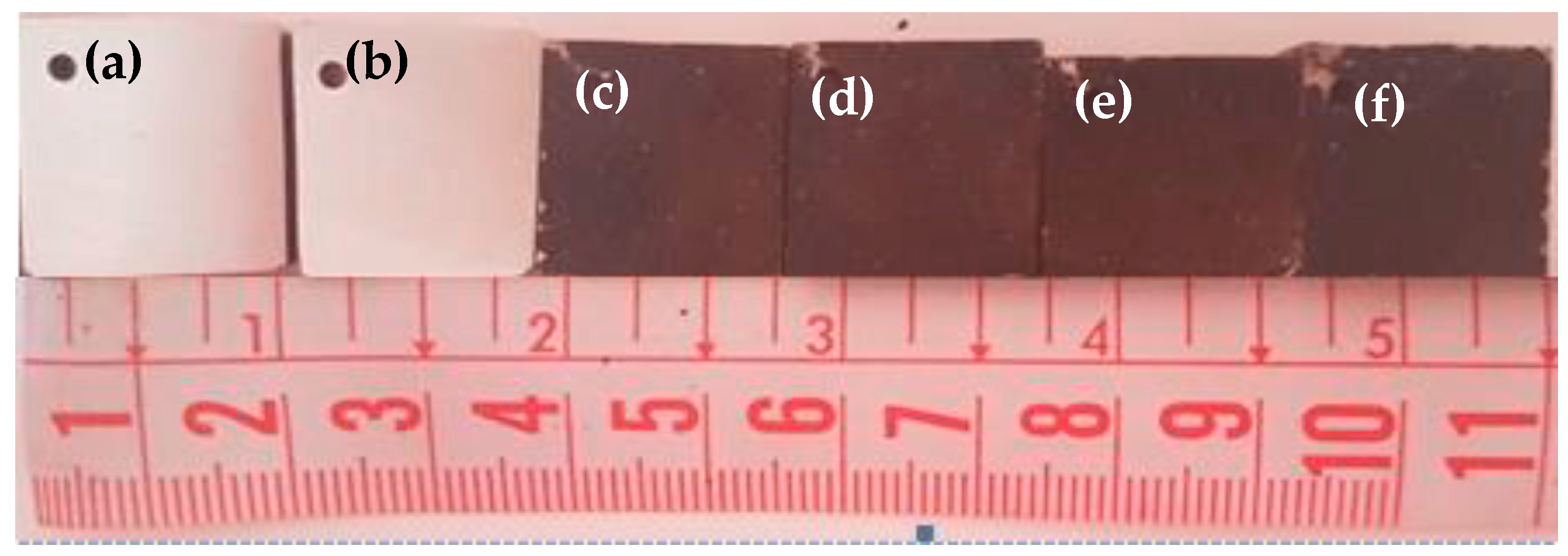
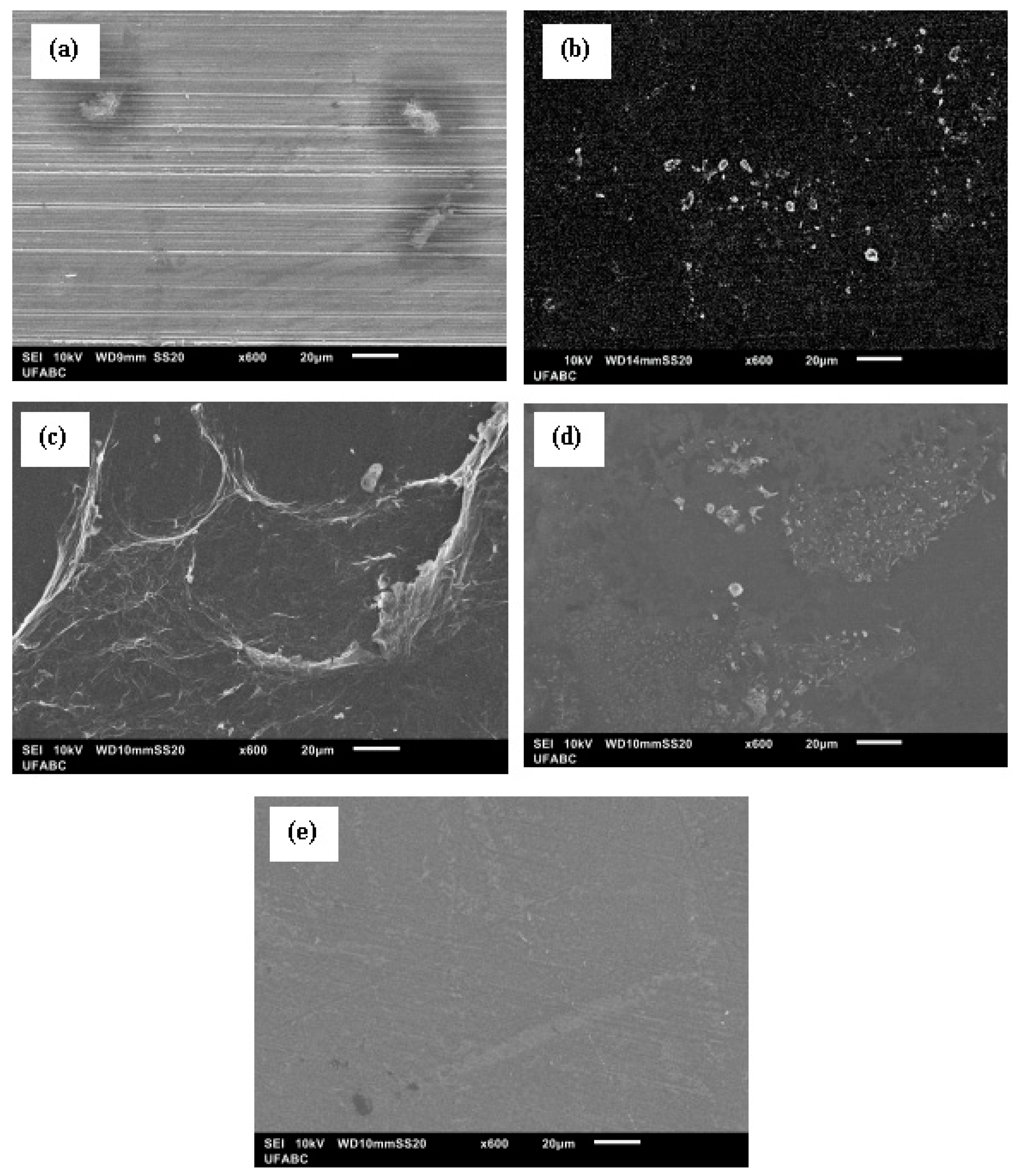

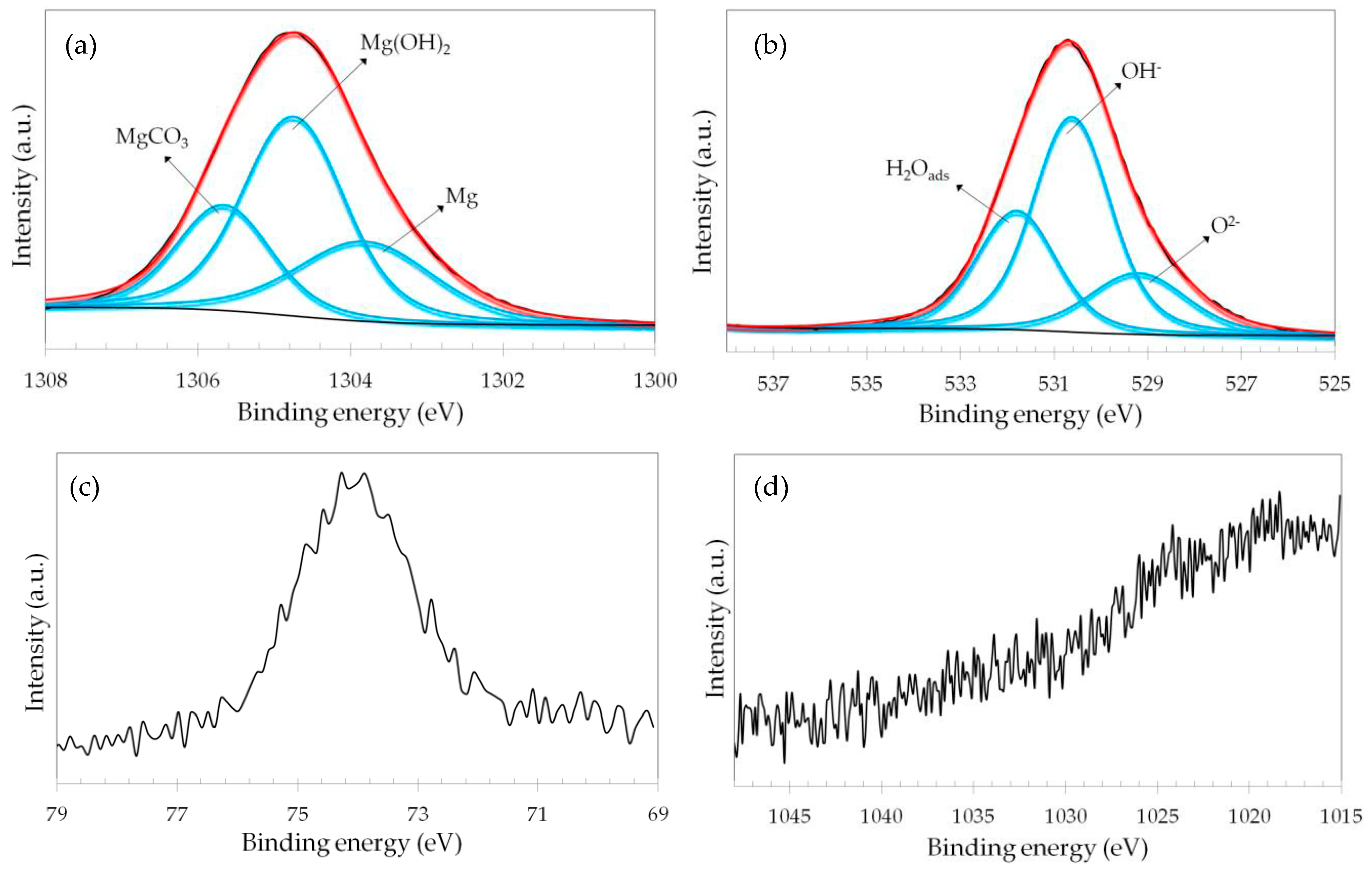
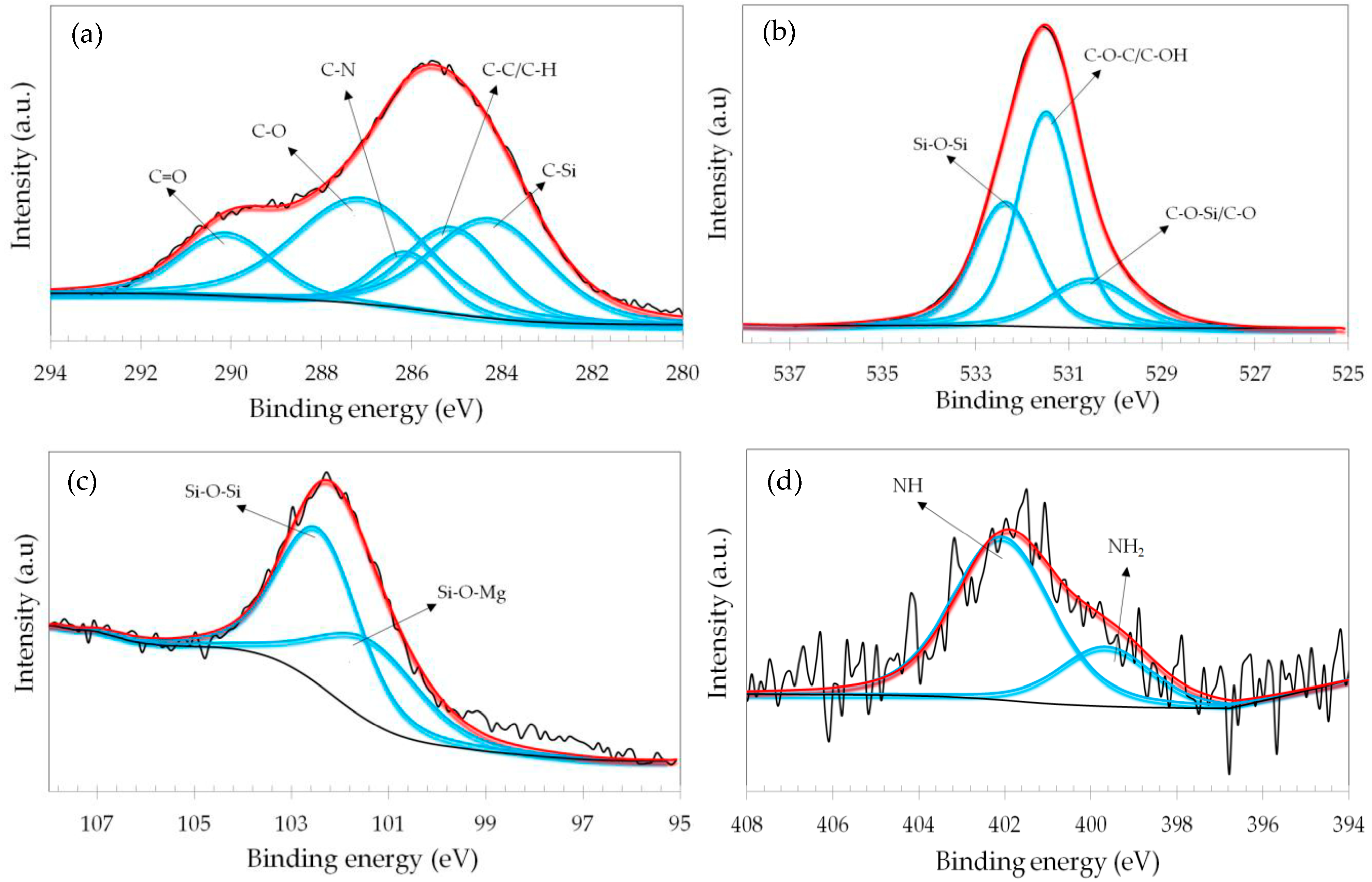




| Sample | Pretreatment | GO Concentration (wt.%) | Time (h) |
|---|---|---|---|
| AR | ---- | ---- | ---- |
| Silanized | Immersion in 3 M NaOH solution for 1 h + silanization with APTES | ---- | ---- |
| 0.05% 2 h | Immersion in 3 M NaOH solution for 1 h + silanization with APTES | 0.05 | 2 |
| 0.05% 4 h | Immersion in 3 M NaOH solution for 1 h + silanization with APTES | 0.05 | 4 |
| 0.1% 2 h | Immersion in 3 M NaOH solution for 1 h + silanization with APTES | 0.1 | 2 |
| 0.1% 4 h | Immersion in 3 M NaOH solution for 1 h + silanization with APTES | 0.1 | 4 |
| Sample | Rs (Ω·cm2) | CPEf (10−6 Ω−1·cm−2·sn) | Rf (Ω·cm2) | nf | CPEdl (10−6 Ω−1·cm−2·sn) | Rct (Ω·cm2) | ndl | RL (Ω·cm2) | L (H·cm2) | χ2 |
|---|---|---|---|---|---|---|---|---|---|---|
| AR | 24 | ---- | ---- | ---- | 8.9 | 8700 | 0.88 | 2761 | 660 | 0.34 |
| Silanized | 21 | 27.1 | 3232 | 0.78 | 5.8 | 2210 | 0.88 | 709 | 803 | 0.44 |
| 0.05% 2 h | 25 | 6.6 | 3426 | 0.92 | 0.2 | 2086 | 0.69 | 1344 | 856 | 0.14 |
| 0.05% 4 h | 52 | 10.2 | 5147 | 0.88 | 4.1 | 2356 | 0.68 | 1146 | 634 | 0.21 |
| 0.1% 2 h | 50 | 8.2 | 6323 | 0.92 | 2.1 | 3189 | 0.70 | 810 | 737 | 0.31 |
| 0.1% 4 h | 27 | 4.7 | 20,224 | 0.92 | 4.1 | 5617 | 0.87 | 1130 | 6027 | 0.56 |
| Sample | Ecorr (mV vs. Ag/AgCl) | icorr (µA·cm−2) | Epit (mV vs. Ag/AgCl) | ΔE (mV) | ipass (µA·cm−2) |
|---|---|---|---|---|---|
| AR | −1523 + 55 | 4.9 ± 0.4 | ---- | ---- | ---- |
| Silanized | −1380 ± 81 | 2.7 ± 0.3 | ---- | ---- | ---- |
| 0.05% 2 h | −1335 ± 91 | ---- | −1170 ± 65 | 211 ± 61 | 0.8 ± 0.2 |
| 0.05% 4 h | −1332 ± 52 | ---- | −980 ± 140 | 425 ± 24 | 6.2 ± 0.7 |
| 0.1% 2 h | −1408 ± 50 | ---- | −1151 ± 83 | 259 ± 63 | 1.6 ± 0.5 |
| 0.1% 4 h | −1424 ± 30 | ---- | −970 ± 101 | 538 ± 52 | 0.7 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, N.S.; Alves, A.C.; da Silva Pereira, J.A.; de Oliveira, L.A.; de Oliveira, M.C.L.; Antunes, R.A. Corrosion Properties and Surface Chemistry of Graphene Oxide-Coated AZ91D Magnesium Alloy in Sodium Chloride Solution. Metals 2024, 14, 1019. https://doi.org/10.3390/met14091019
da Silva NS, Alves AC, da Silva Pereira JA, de Oliveira LA, de Oliveira MCL, Antunes RA. Corrosion Properties and Surface Chemistry of Graphene Oxide-Coated AZ91D Magnesium Alloy in Sodium Chloride Solution. Metals. 2024; 14(9):1019. https://doi.org/10.3390/met14091019
Chicago/Turabian Styleda Silva, Nathalia Sartori, Aila Cossovan Alves, Jaine Aparecida da Silva Pereira, Leandro Antonio de Oliveira, Mara Cristina Lopes de Oliveira, and Renato Altobelli Antunes. 2024. "Corrosion Properties and Surface Chemistry of Graphene Oxide-Coated AZ91D Magnesium Alloy in Sodium Chloride Solution" Metals 14, no. 9: 1019. https://doi.org/10.3390/met14091019
APA Styleda Silva, N. S., Alves, A. C., da Silva Pereira, J. A., de Oliveira, L. A., de Oliveira, M. C. L., & Antunes, R. A. (2024). Corrosion Properties and Surface Chemistry of Graphene Oxide-Coated AZ91D Magnesium Alloy in Sodium Chloride Solution. Metals, 14(9), 1019. https://doi.org/10.3390/met14091019







