The Preparation and Properties of a Ni-SiO2 Superamphiphobic Coating Obtained by Electrodeposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvent Configuration and Preparation of Superamphiphobic Coating
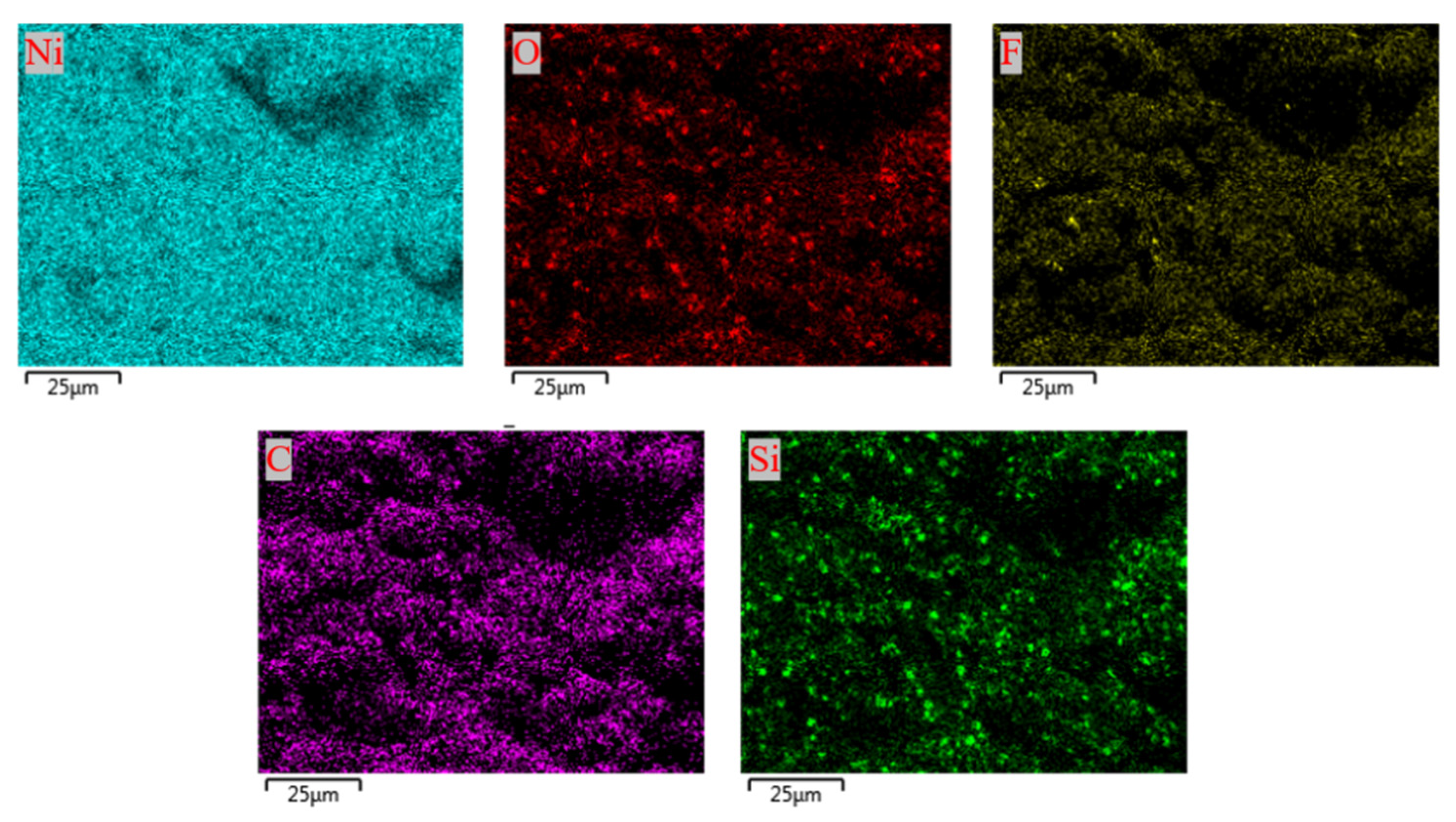
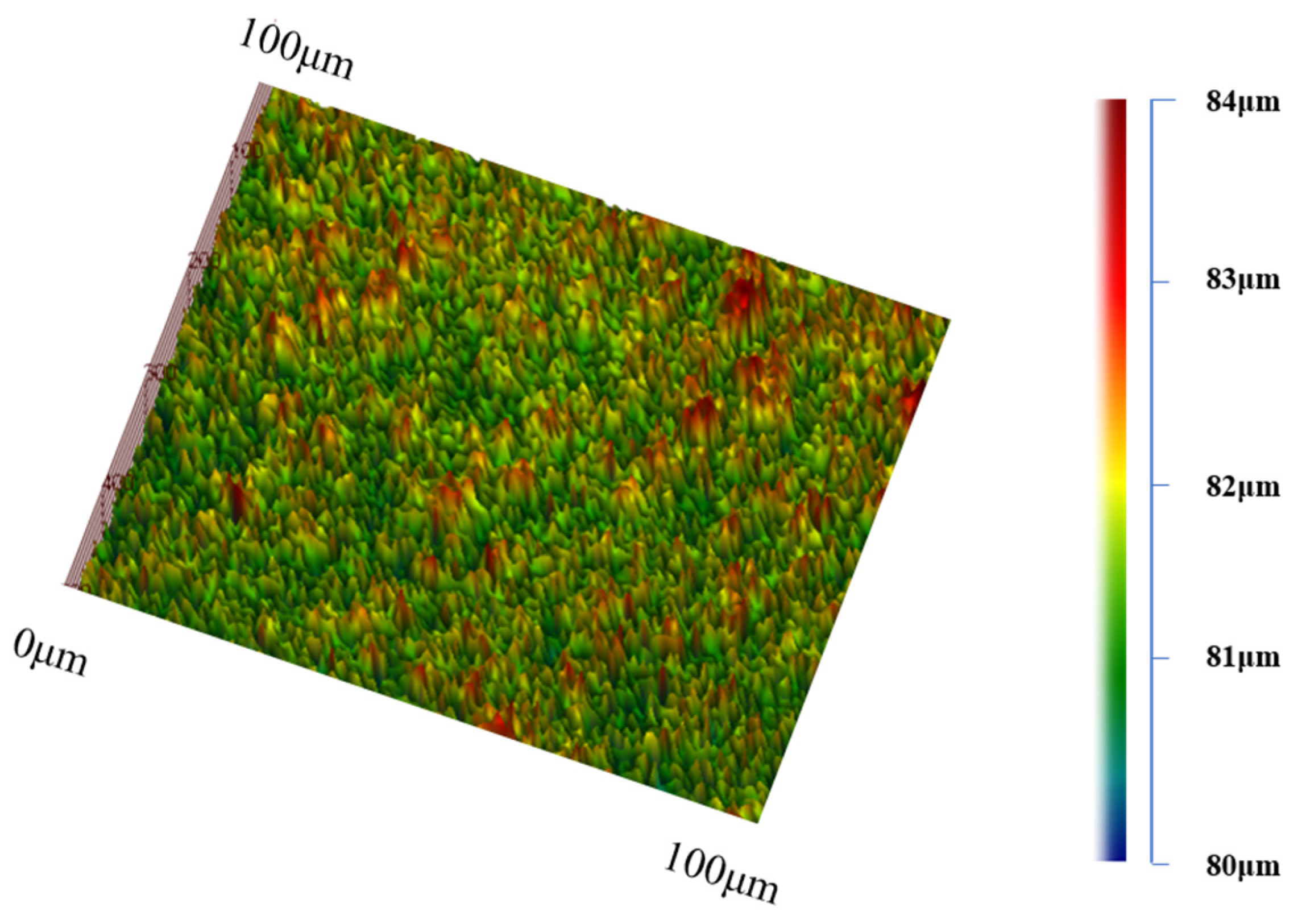
2.2. Characterization
2.3. Coating Chemical Stability Test
2.4. Coating Corrosion Resistance Test
2.5. Wear Resistance Test of the Coating
2.6. High-Temperature Resistance Test of the Coating
3. Results and Discussion
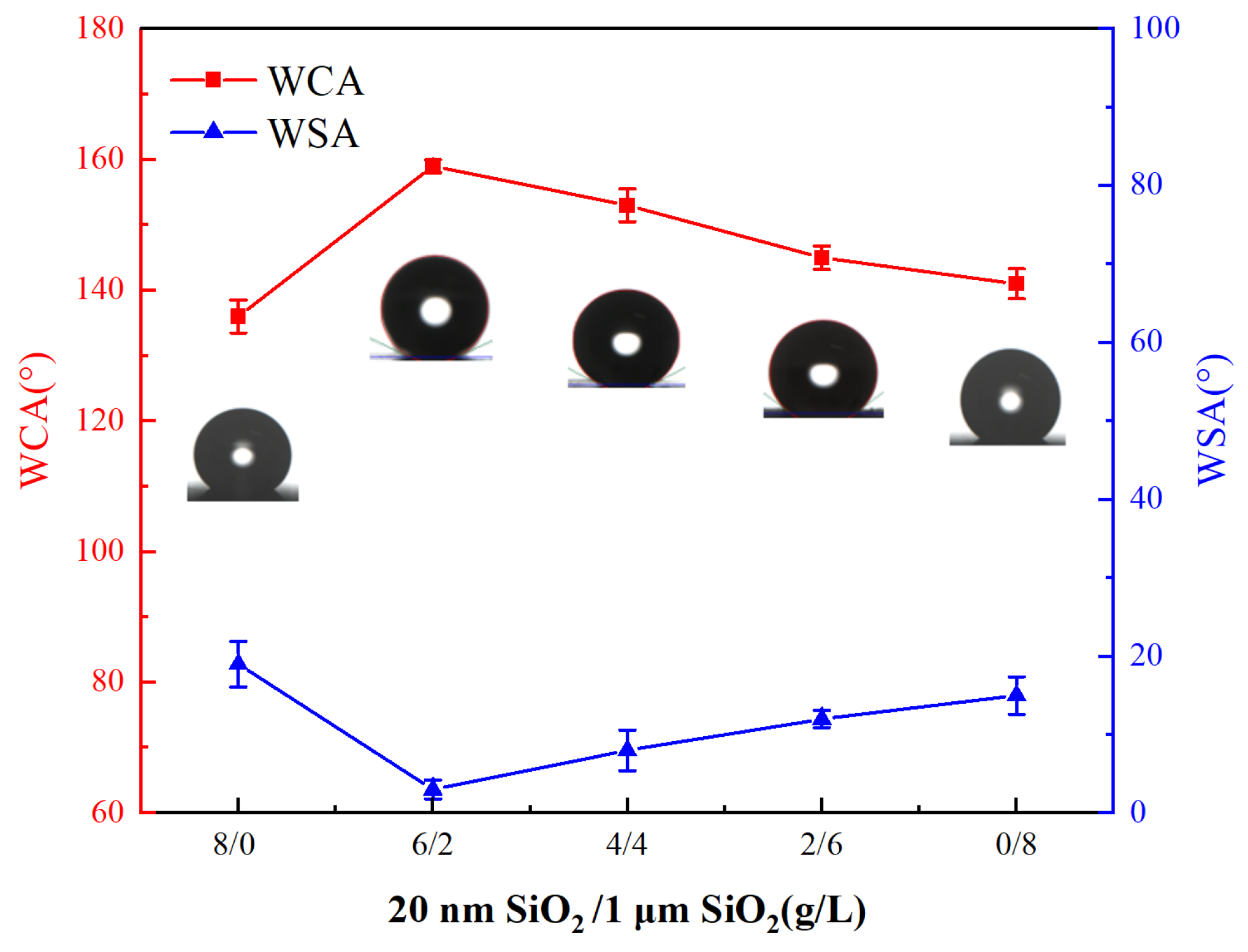
3.1. Effect of SiO2 Particle Concentration of Different Particle Sizes in the Plating Solution on the Coating
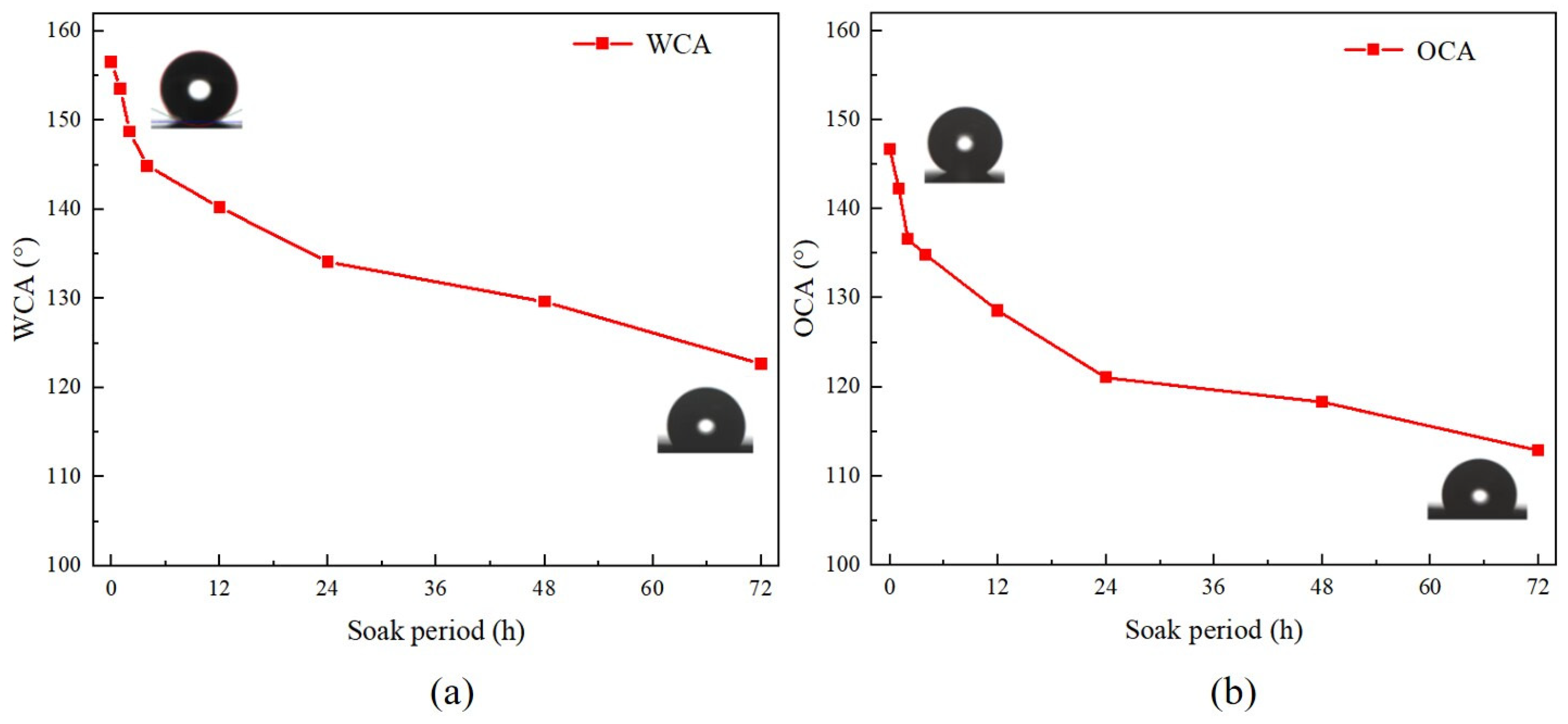
3.2. Chemical Stability of the Coating
3.3. Corrosion Resistance of the Coating
3.3.1. Polarization Curve Analysis
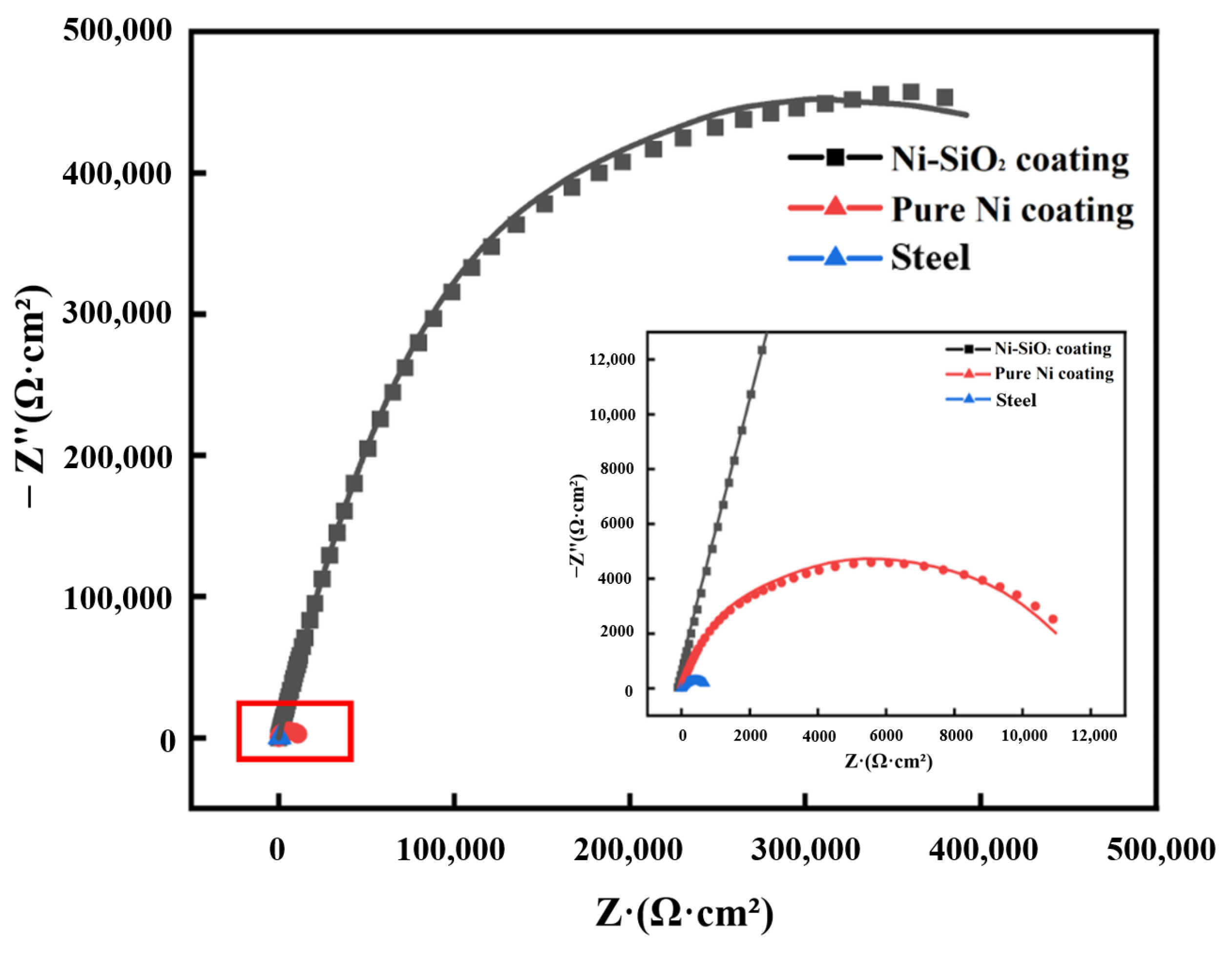
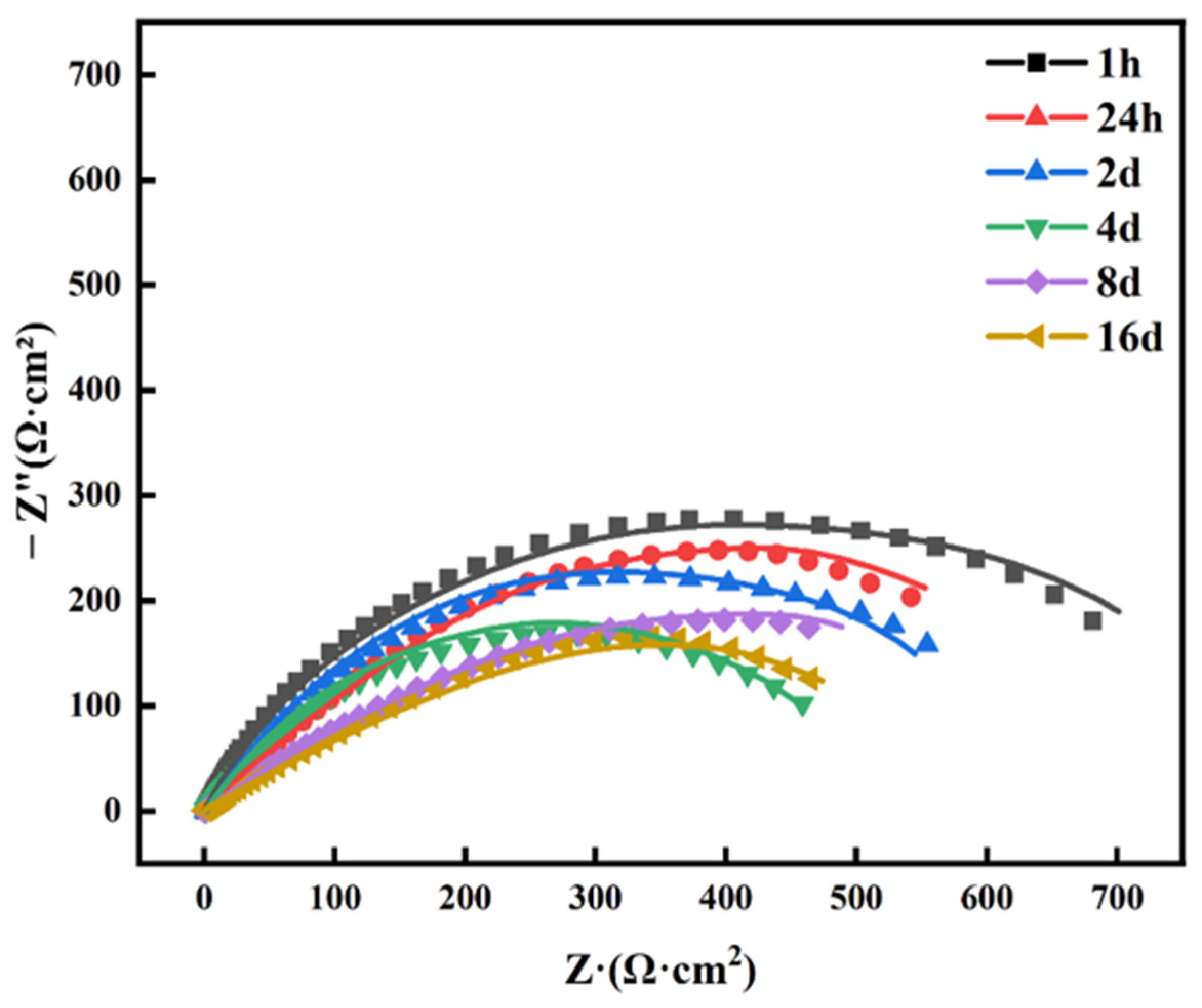
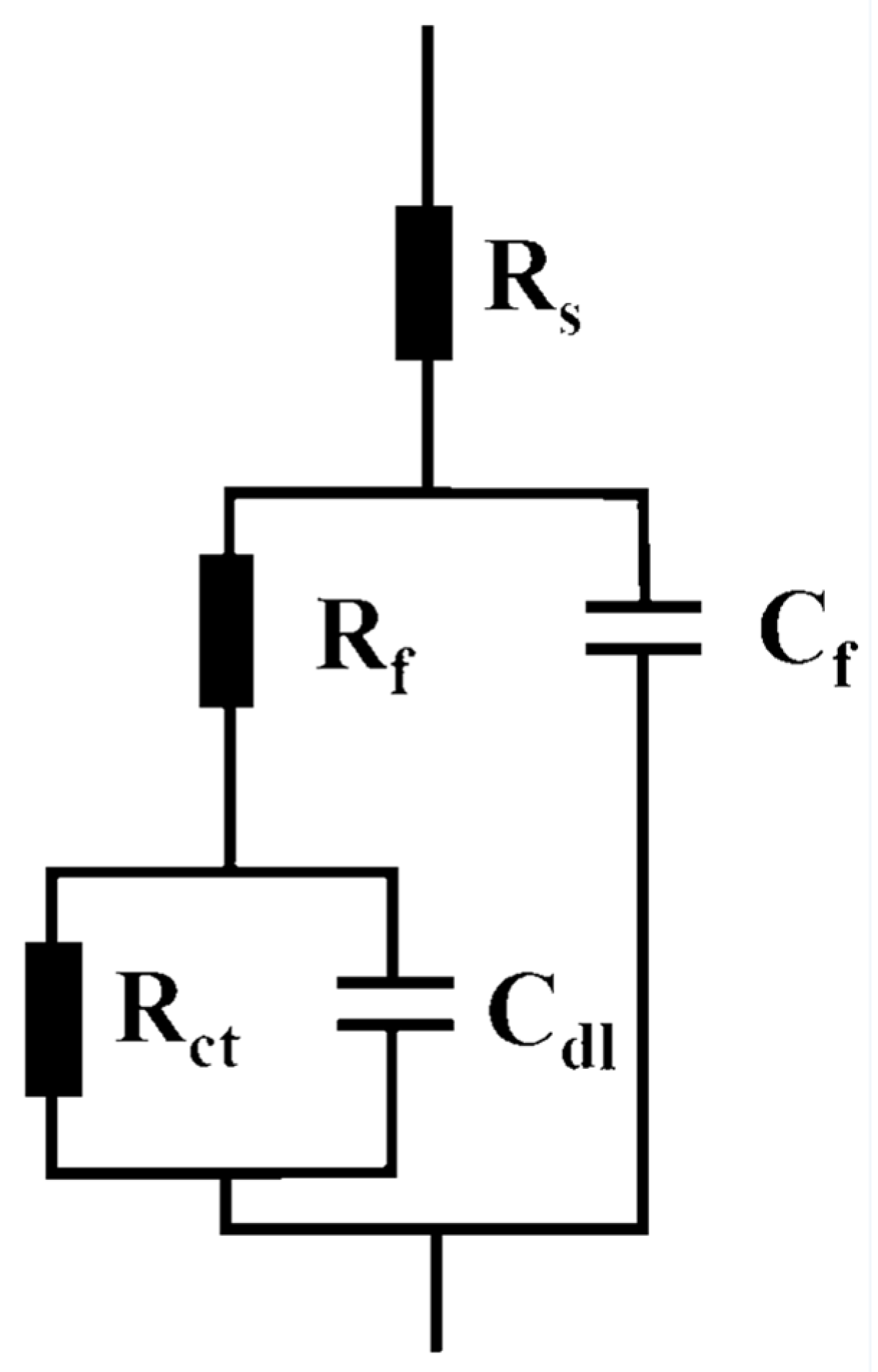
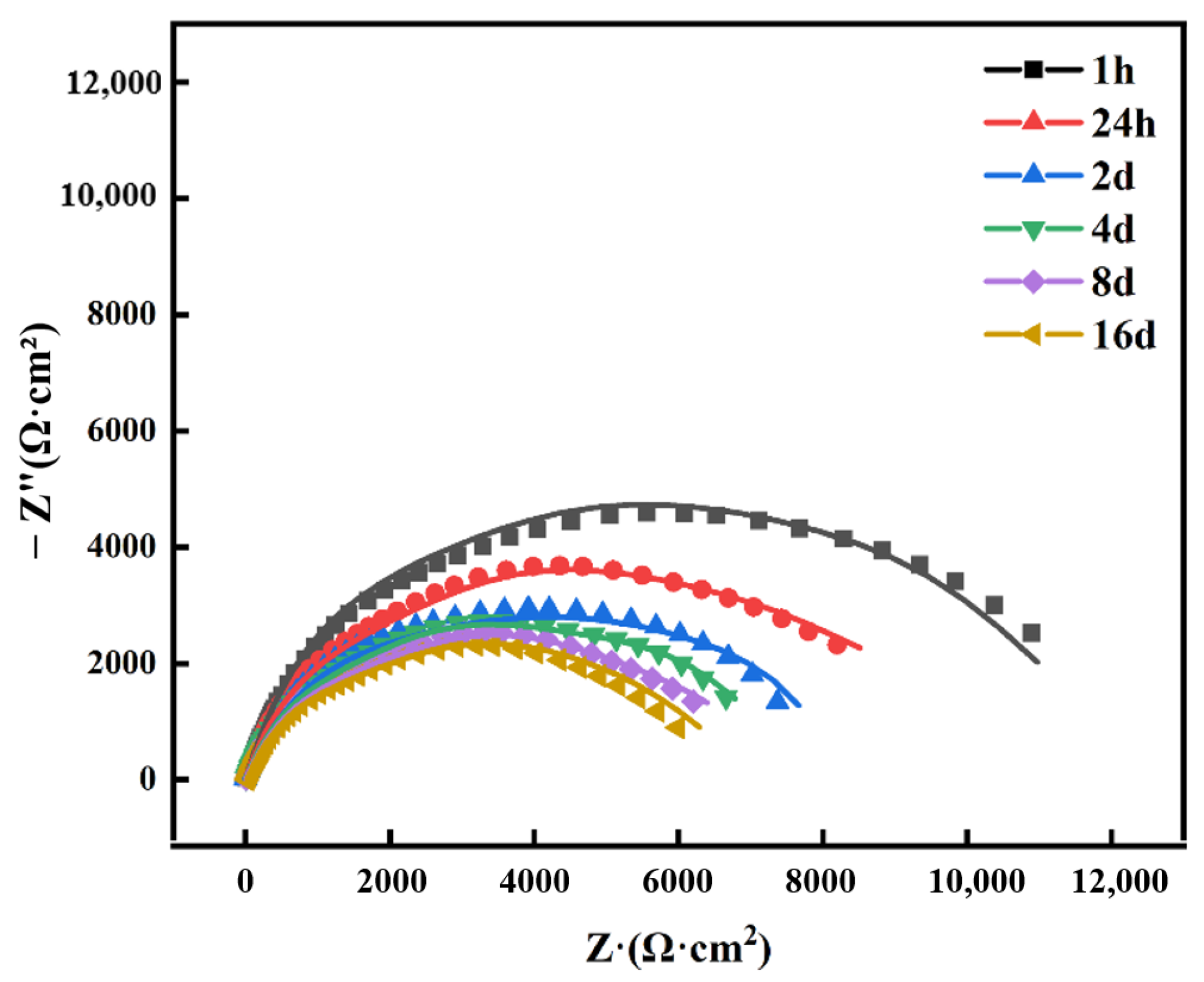
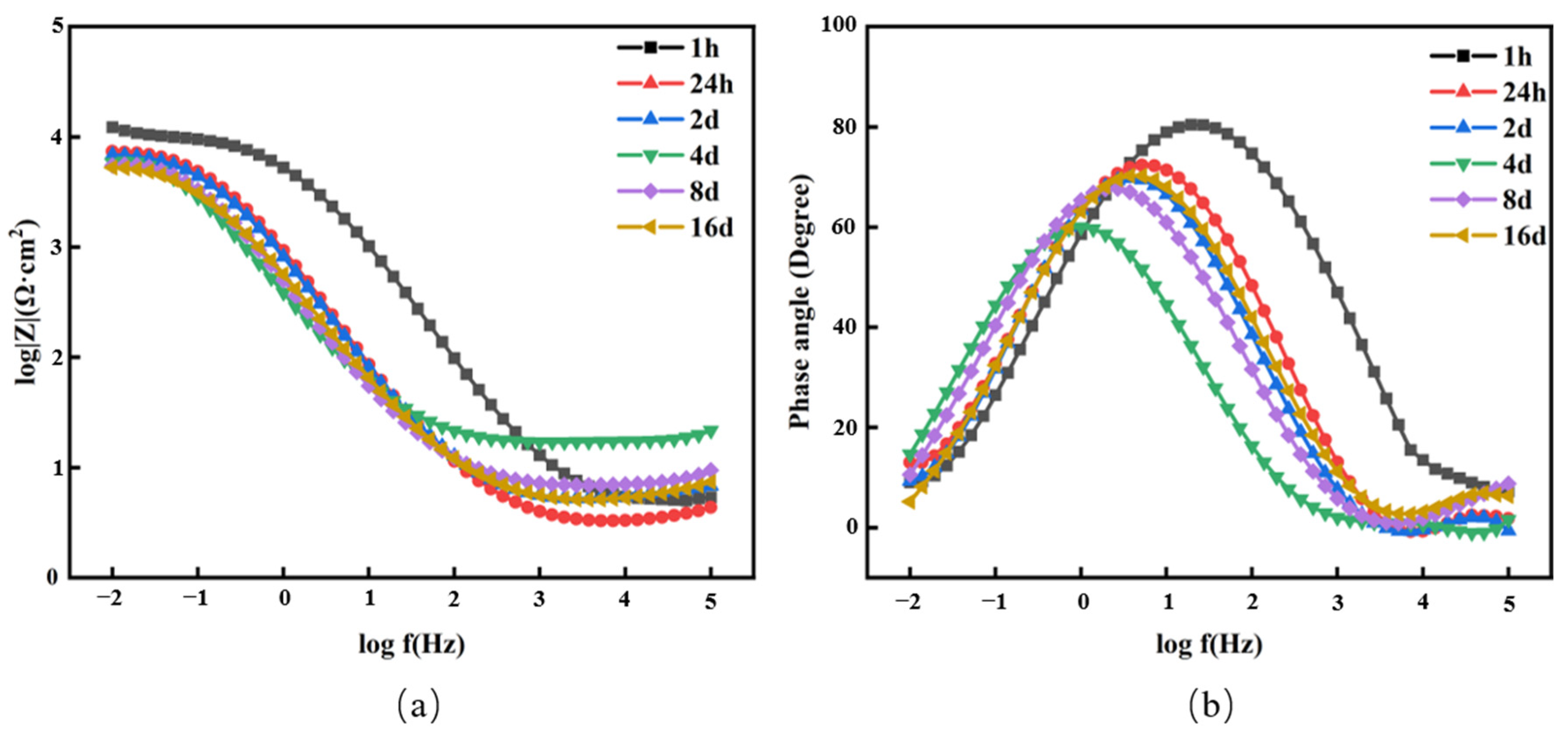
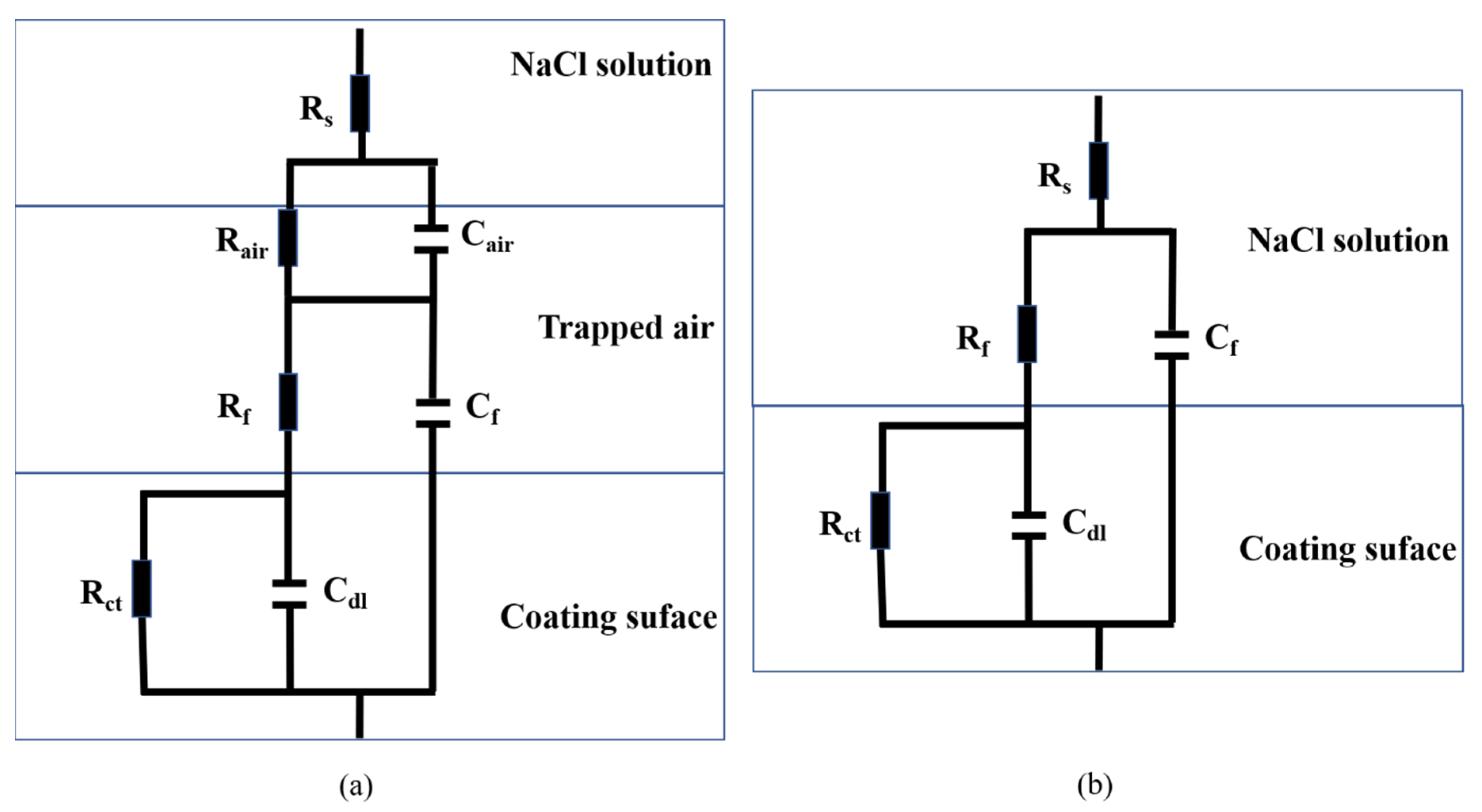
3.3.2. EIS Analysis
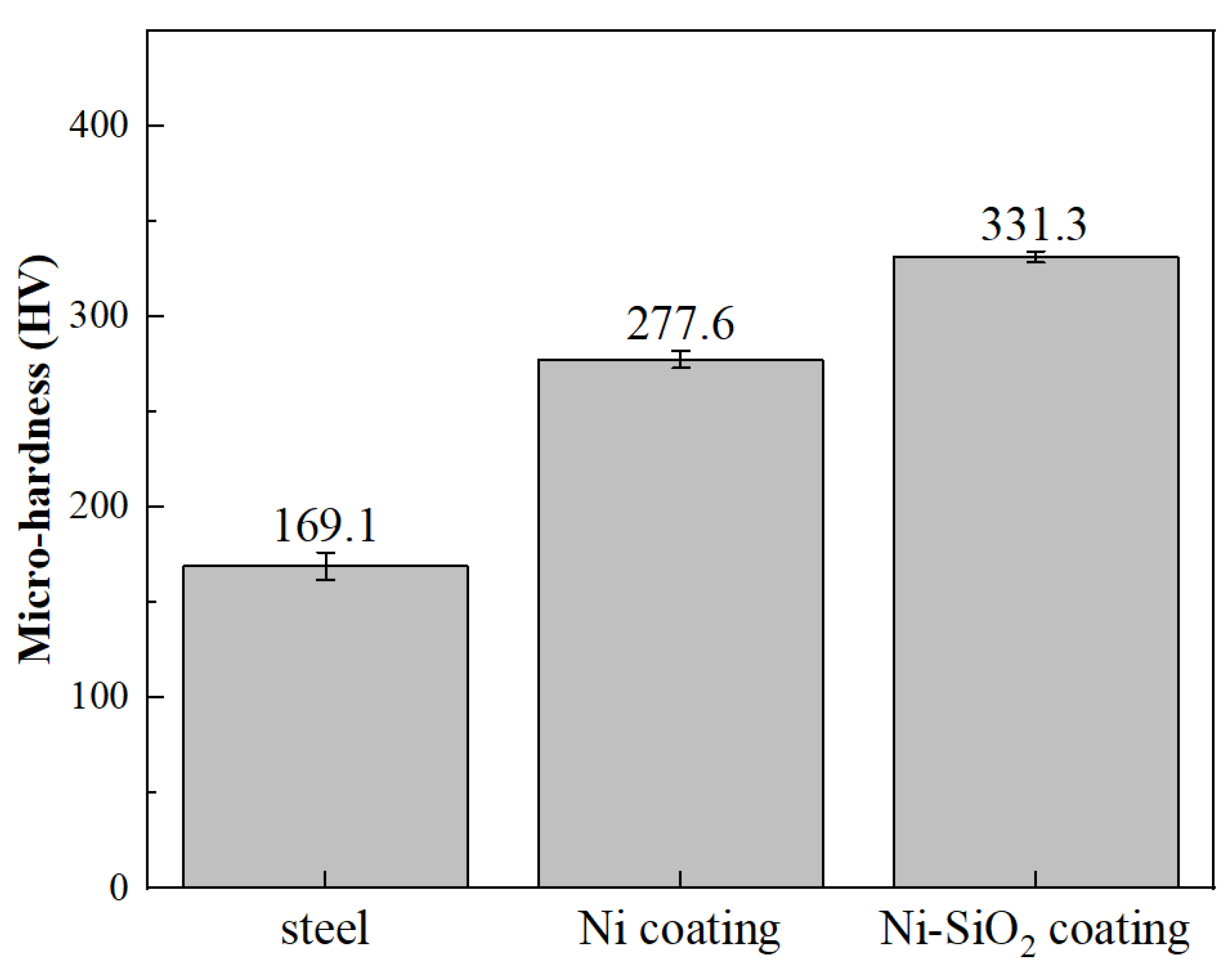
3.4. The Wear Resistance of the Coating
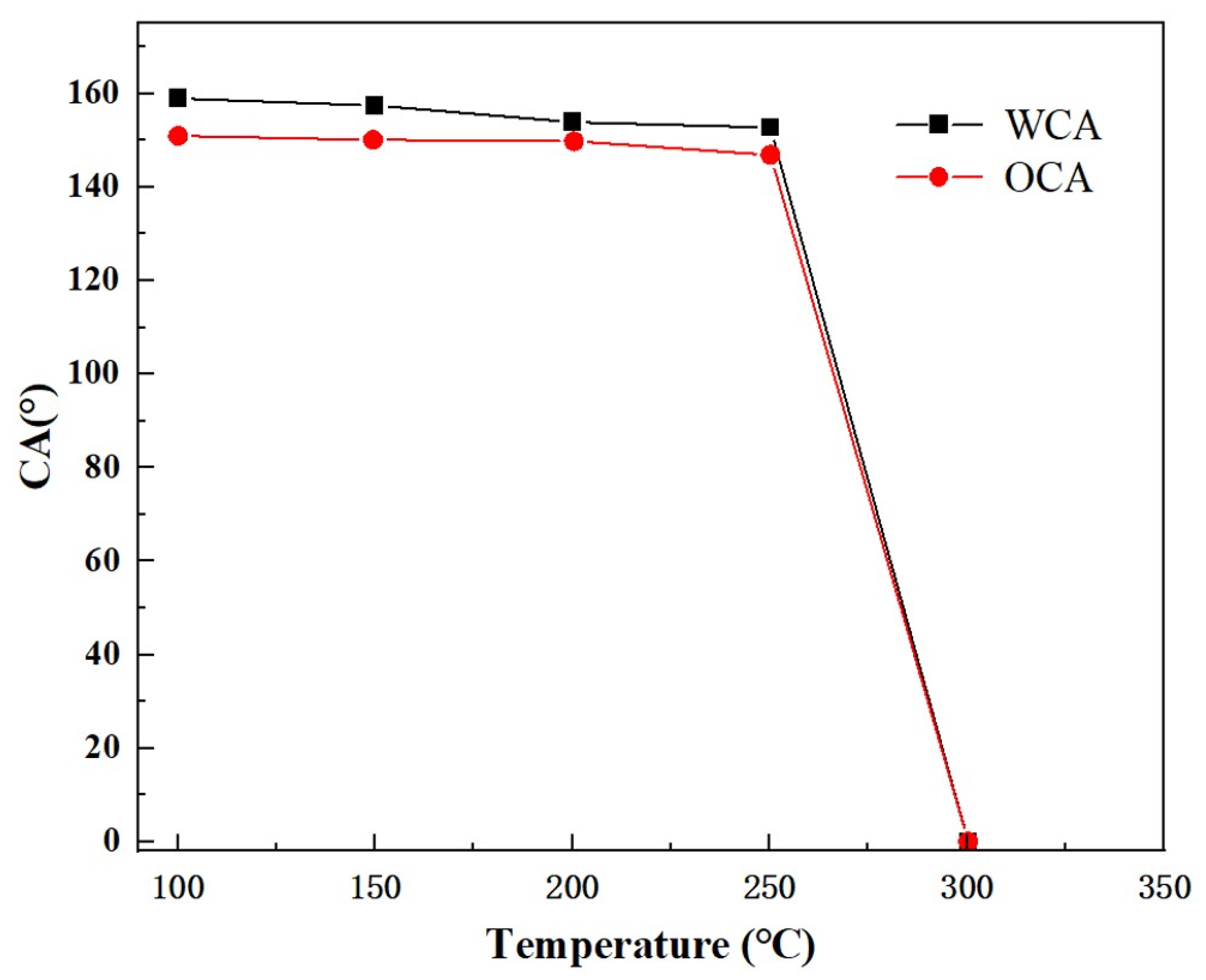
3.5. The High-Temperature Resistance of the Coating
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fihri, A.; Bovero, E.; Al-Shahrani, A.; Al-Ghamdi, A.; Alabedi, G. Recent progress in superhydrophobic coatings used for steel protection: A review. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 378–390. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Manoudis, P.N. Superhydrophobic and superamphiphobic materials for the conservation of natural stone: An overview. Constr. Build. Mater. 2022, 320, 126175. [Google Scholar] [CrossRef]
- Abu Jarad, N.; Imran, H.; Imani, S.M.; Didar, T.F.; Soleymani, L. Fabrication of Superamphiphobic Surfaces via Spray Coating; a Review. Adv. Mater. Technol. 2022, 7, 2101702. [Google Scholar] [CrossRef]
- She, Z.; Li, Q.; Wang, Z.; Li, L.; Chen, F.; Zhou, J. Novel Method for Controllable Fabrication of a Superhydrophobic CuO Surface on AZ91D Magnesium Alloy. ACS Appl. Mater. Interfaces 2012, 4, 4348–4356. [Google Scholar] [CrossRef]
- Shang, W.; Chen, B.; Shi, X.; Chen, Y.; Xiao, X. Electrochemical corrosion behavior of composite MAO/sol–gel coatings on magnesium alloy AZ91D using combined micro-arc oxidation and sol–gel technique. J. Alloys Compd. 2009, 474, 541–545. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Tian, Y.; Zhang, H.; Wang, R.; Zhang, B.; Zhang, H.; Zhang, Q. Robust Organic–Inorganic Composite Films with Multifunctional Properties of Superhydrophobicity, Self-Healing, and Drag Reduction. Ind. Eng. Chem. Res. 2019, 58, 4468–4478. [Google Scholar] [CrossRef]
- Han, Z.; Feng, X.; Guo, Z.; Niu, S.; Ren, L. Flourishing Bioinspired Antifogging Materials with Superwettability: Progresses and Challenges. Adv. Mater. 2018, 30, 1704652. [Google Scholar] [CrossRef]
- Guo, F.; Wen, Q.; Peng, Y.; Guo, Z. Multifunctional hollow superhydrophobic SiO2 microspheres with robust and self-cleaning and separation of oil/water emulsions properties. J. Colloid Interface Sci. 2017, 494, 54–63. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, M.; Guo, Z. Multifunctional superamphiphobic SiO2 coating for crude oil transportation. Chem. Eng. J. 2018, 334, 1584–1593. [Google Scholar] [CrossRef]
- Si, Y.; Yang, F.; Guo, Z. Bio-inspired one-pot route to prepare robust and repairable micro-nanoscale superhydrophobic coatings. J. Colloid Interface Sci. 2017, 498, 182–193. [Google Scholar] [CrossRef]
- Zhang, X.; Si, Y.; Mo, J.; Guo, Z. Robust micro-nanoscale flowerlike ZnO/epoxy resin superhydrophobic coating with rapid healing ability. Chem. Eng. J. 2017, 313, 1152–1159. [Google Scholar] [CrossRef]
- Li, D.; Guo, Z. Stable and self-healing superhydrophobic MnO2@fabrics: Applications in self-cleaning, oil/water separation and wear resistance. J. Colloid Interface Sci. 2017, 503, 124–130. [Google Scholar] [CrossRef]
- Wen, G.; Gao, X.; Tian, P.; Zhong, L.; Wang, Z.; Guo, Z. Modifier-free fabrication of durable and multifunctional superhydrophobic paper with thermostability and anti-microbial property. Chem. Eng. J. 2018, 346, 94–103. [Google Scholar] [CrossRef]
- Si, Y.; Guo, Z. Eco-friendly functionalized superhydrophobic recycled paper with enhanced flame-retardancy. J. Colloid Interface Sci. 2016, 477, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guo, Z. Facile fabrication of ultraviolet light cured fluorinated polymer layer for smart superhydrophobic surface with excellent durability and flame retardancy. J. Colloid Interface Sci. 2019, 547, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, D.; Guo, Z. Highly fluorinated F-APP-TiO2 particle with hierarchical core-shell structure and its application in multifunctional superamphiphobic surface: Mechanical robustness, self-recovery and flame retardancy. J. Colloid Interface Sci. 2020, 560, 777–786. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, M.; Guo, Z. Robust, heat-resistant and multifunctional superhydrophobic coating of carbon microflowers with molybdenum trioxide nanoparticles. J. Colloid Interface Sci. 2017, 506, 649–658. [Google Scholar] [CrossRef]
- Wen, G.; Guo, Z. Nonflammable superhydrophobic paper with biomimetic layered structure exhibiting boiling-water resistance and repairable properties for emulsion separation. J. Mater. Chem. A Mater. Energy Sustain. 2018, 6, 7042–7052. [Google Scholar] [CrossRef]
- Qu, M.; Yang, X.; Peng, L.; Liu, L.; Yang, C.; Zhao, Z.; Liu, X.; Zhang, T.; He, J. High reliable electromagnetic interference shielding carbon cloth with superamphiphobicity and environmental suitability. Carbon 2021, 174, 110–122. [Google Scholar] [CrossRef]
- Si, Y.; Chen, L.; Yang, F.; Guo, F.; Guo, Z. Stable Janus superhydrophilic/hydrophobic nickel foam for directional water transport. J. Colloid Interface Sci. 2018, 509, 346–352. [Google Scholar] [CrossRef]
- Liang, X.; Guo, Z. Mechano-adjusted anisotropic surface for manipulating water droplets. Chem. Eng. J. 2020, 395, 125110. [Google Scholar] [CrossRef]
- Si, Y.; Dong, Z. Bioinspired Smart Liquid Directional Transport Control. Langmuir 2020, 36, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Hu, C.; Yang, C.; An, K.; Tang, F.; Tan, J.; Liu, C. Rough Structure of Electrodeposition as a Template for an Ultrarobust Self-Cleaning Surface. ACS Appl. Mater. Interfaces 2017, 9, 16571–16580. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; Zhu, J. Fabrication of superamphiphobic surface on Cu substrate via a novel and facile dip coating method. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128379. [Google Scholar] [CrossRef]
- Geng, Z.; He, J. An effective method to significantly enhance the robustness and adhesion-to-substrate of high transmittance superamphiphobic silica thin films. J. Mater. Chem. A 2014, 2, 16601–16607. [Google Scholar] [CrossRef]
- Wang, C.F.; Chiou, S.F.; Ko, F.H.; Chou, C.T.; Lin, H.C.; Huang, C.F.; Chang, F.C. Fabrication of Biomimetic Super-Amphiphobic Surfaces Through Plasma Modification of Benzoxazine Films. Macromol. Rapid Commun. 2006, 27, 333–337. [Google Scholar] [CrossRef]
- Sheen, Y.; Huang, Y.; Liao, C.; Chou, H.; Chang, F. New approach to fabricate an extremely super-amphiphobic surface based on fluorinated silica nanoparticles. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1984–1990. [Google Scholar] [CrossRef]
- Deng, X.; Mammen, L.; Butt, H.-J.; Vollmer, D. Candle Soot as a Template for a Transparent Robust Superamphiphobic Coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef]
- Wang, H.; Chi, G.; Jia, Y.; Yu, F.; Wang, Z.; Wang, Y. A novel combination of electrical discharge machining and electrodeposition for superamphiphobic metallic surface fabrication. Appl. Surf. Sci. 2020, 504, 144285. [Google Scholar] [CrossRef]
- Li, Q.; Xia, F.; Liu, G.; Yao, L. Microstructure and Properties of Jet Pulse Electrodeposited Ni-TiN Nanocoatings. J. Mater. Eng. Perform. 2022, 31, 8823–8829. [Google Scholar] [CrossRef]
- Jin, P.; Sun, C.; Zhang, Z.; Zhou, C.; Williams, T. Fabrication of the Ni-W-SiC thin film by pulse electrodeposition. Surf. Coat. Technol. 2020, 392, 125738. [Google Scholar] [CrossRef]
- Cheng, X.; He, Y.; Song, R.; Li, H.; Liu, B.; Zhou, H.; Yan, L. Study of mechanical character and corrosion properties of La2O3 nanoparticle reinforced Ni-W composite coatings. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129799. [Google Scholar] [CrossRef]
- Yan, L.; Yan, S.; He, Y.; He, Y.; Li, H.; Song, R.; Zhou, H.; Cheng, X. Preparation, corrosion resistance and mechanical properties of electroless Ni-B/α-ZrP composite coatings. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130132. [Google Scholar] [CrossRef]
- Qin, B.; Zhou, S.; Chen, H.; Wang, M. Superior corrosion and wear resistance of AZ91D Mg alloy via electrodeposited SiO2–Ni-based composite coating. Mater. Chem. Phys. 2022, 283, 126001. [Google Scholar] [CrossRef]
- Shakoor, R.A.; Waware, U.S.; Kahraman, R.; Popelka, A.; Yusuf, M.M. Corrosion Behavior of Electrodeposited Ni-B Coatings Modified with SiO2 Particles. Int. J. Electrochem. Sci. 2017, 12, 4384–4391. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Trans. Faraday Soc. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Patankar, N.A. Transition between superhydrophobic states on rough surfaces. Langmuir 2004, 20, 7097–7102. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, J.; Liu, P.; Yu, H. Boron nitride-epoxy inverse “nacre-like” nanocomposite coatings with superior anticorrosion performance. Corros. Sci. 2021, 183, 109333. [Google Scholar] [CrossRef]
- Hu, C.; Xie, X.; Ren, K. A facile method to prepare stearic acid-TiO2/zinc composite coating with multipronged robustness, self-cleaning property, and corrosion resistance. J. Alloys Compd. 2021, 882, 160636. [Google Scholar] [CrossRef]
- Xing, S.; Wang, L.; Jiang, C.; Liu, H.; Zhu, W.; Ji, V. Influence of Y2O3 nanoparticles on microstructures and properties of electrodeposited Ni–W–Y2O3 nanocrystalline coatings. Vacuum 2020, 181, 109665. [Google Scholar] [CrossRef]
















| Reagent Name | Chemical Formula | Concentration |
|---|---|---|
| Nickel sulfate | NiSO4·6H2O (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) | 240 g/L |
| Nickel chloride | NiCl2·6H2O (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) | 40 g/L |
| Boric acid | H3BO3 (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) | 35 g/L |
| Sodium lauryl sulfate | C12H25SO4Na (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) | 0.15 g/L |
| 20 nm SiO2 | SiO2 (Lavasi (Beijing) New Material Technology Co., Ltd., Beijing, China) | 0, 2, 4, 6, 8 g/L |
| 1 μm SiO2 | SiO2 (Lavasi (Beijing) New Material Technology Co., Ltd., Beijing, China) | 0, 2, 4, 6, 8 g/L |
| Test | 20 nm SiO2 Concentration (g/L) | 1 μm SiO2 Concentration (g/L) |
|---|---|---|
| 1 | 8 | 0 |
| 2 | 6 | 2 |
| 3 | 4 | 4 |
| 4 | 2 | 6 |
| 5 | 0 | 8 |
| Experimental Parameters | Numerical Setting |
|---|---|
| Temperature (°C) | 50 |
| pH | 4.6 |
| Mixing speed (r/min) | 150 |
| Electrodeposited current density (A/dm2) | 10 |
| Electrodeposition time (min) | 20 |
| Sample Name | Ecorr (mVSCE) | icorr (A·cm−2) | βa (mV·dec−1) | βc (mV·dec−1) | η |
|---|---|---|---|---|---|
| Steel | −513.29 | 7.62 × 10−5 | 83.6 | 1011.3 | -- |
| Pure nickel coating | −466.31 | 3.06 × 10−5 | 206.9 | 132.6 | 59.8% |
| Ni-SiO2 superamphiphobic coating | −360.97 | 8.32 × 10−7 | 277.4 | 103.3 | 98.9% |
| Time | Rf (Ω·cm2) | Cf (F·cm−2) | Rct (Ω·cm2) | Cdl | χ2 | |
|---|---|---|---|---|---|---|
| Y0 (Ω−1·cm−2·sn) | n | |||||
| 1 h | 8.312 | 3.224 × 10−4 | 900.1 | 1.371 × 10−3 | 0.688 | 3.77 × 10−4 |
| 24 h | 10.561 | 3.301 × 10−4 | 877.5 | 4.009 × 10−3 | 0.720 | 3.53 × 10−3 |
| 2 days | 5.688 | 3.325 × 10−4 | 730.5 | 2.932 × 10−3 | 0.733 | 1.12 × 10−4 |
| 4 days | 6.097 | 2.432 × 10−4 | 573.9 | 2.188 × 10−3 | 0.701 | 1.45 × 10−3 |
| 8 days | 5.933 | 1.401 × 10−4 | 772.4 | 4.177 × 10−3 | 0.519 | 3.87 × 10−4 |
| 16 days | 12.032 | 2.256 × 10−4 | 583.1 | 4.202 × 10−3 | 0.628 | 3.51 × 10−4 |
| Time | Rf (Ω·cm2) | Cf (F·cm−2) | Rct (Ω·cm2) | Cdl | χ2 | |
|---|---|---|---|---|---|---|
| Y0 (Ω−1·cm−2·sn) | n | |||||
| 1 h | 25.61 | 2.451 × 10−5 | 12,911 | 4.323 × 10−5 | 0.736 | 3.31 × 10−3 |
| 24 h | 36.29 | 7.026 × 10−5 | 9017 | 8.145 × 10−5 | 0.697 | 5.15 × 10−3 |
| 2 days | 44.51 | 7.309 × 10−5 | 8216 | 1.378 × 10−4 | 0.745 | 4.01 × 10−4 |
| 4 days | 33.97 | 8.991 × 10−5 | 8133 | 2.891 × 10−4 | 0.733 | 3.23 × 10−4 |
| 8 days | 29.06 | 8.635 × 10−5 | 7514 | 1.941 × 10−4 | 0.789 | 7.92 × 10−4 |
| 16 days | 30.12 | 7.157 × 10−5 | 7056 | 1.033 × 10−4 | 0.751 | 8.73 × 10−4 |
| Time | Rair Ω·cm2 | Cair F·cm−2 | Rf Ω·cm2 | Cf F·cm−2 | Rct Ω·cm2 | Cdl | χ2 | |
|---|---|---|---|---|---|---|---|---|
| Y0 (Ω−1·cm−2·sn) | n | |||||||
| 1 h | 3.270 × 105 | 3.021 × 10−5 | 29.19 | 2.406 × 10−6 | 6.826 × 105 | 2.537 × 10−5 | 0.703 | 4.91 × 10−3 |
| 1 day | 1.532 × 105 | 4.488 × 10−5 | 28.17 | 4.153 × 10−6 | 2.121 × 105 | 1.505 × 10−5 | 0.739 | 3.22 × 10−4 |
| 2 days | 1.231 × 105 | 4.617 × 10−5 | 33.51 | 5.961 × 10−6 | 1.307 × 105 | 3.022 × 10−5 | 0.621 | 6.81 × 10−4 |
| 4 days | -- | -- | 38.12 | 1.158 × 10−5 | 12,103 | 4.165 × 10−5 | 0.608 | 4.51 × 10−4 |
| 8 days | -- | -- | 36.38 | 1.235 × 10−5 | 9871 | 2.315 × 10−5 | 0.599 | 6.44 × 10−4 |
| 16 days | -- | -- | 35.61 | 1.375 × 10−5 | 8962 | 8.188 × 10−5 | 0.608 | 8.09 × 10−4 |
| Time | Steel | Pure Nickel Coating | Ni-SiO2 Superamphiphobic Coating |
|---|---|---|---|
| 1 h | 908.41 | 1.291 × 104 | 1.100 × 107 |
| 24 h | 888.06 | 9.053 × 103 | 3.653 × 105 |
| 2 days | 736.19 | 8.216 × 103 | 2.238 × 105 |
| 4 days | 580.00 | 8.133 × 103 | 1.214 × 104 |
| 8 days | 778.33 | 7.514 × 103 | 9.907 × 103 |
| 16 days | 595.13 | 7.056 × 103 | 8.997 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Tong, S.; Wang, S.; Wan, Z.; Xing, X.; Cui, G. The Preparation and Properties of a Ni-SiO2 Superamphiphobic Coating Obtained by Electrodeposition. Metals 2024, 14, 1047. https://doi.org/10.3390/met14091047
Liu J, Tong S, Wang S, Wan Z, Xing X, Cui G. The Preparation and Properties of a Ni-SiO2 Superamphiphobic Coating Obtained by Electrodeposition. Metals. 2024; 14(9):1047. https://doi.org/10.3390/met14091047
Chicago/Turabian StyleLiu, Jianguo, Songlin Tong, Shuaihua Wang, Zhiyao Wan, Xiao Xing, and Gan Cui. 2024. "The Preparation and Properties of a Ni-SiO2 Superamphiphobic Coating Obtained by Electrodeposition" Metals 14, no. 9: 1047. https://doi.org/10.3390/met14091047
APA StyleLiu, J., Tong, S., Wang, S., Wan, Z., Xing, X., & Cui, G. (2024). The Preparation and Properties of a Ni-SiO2 Superamphiphobic Coating Obtained by Electrodeposition. Metals, 14(9), 1047. https://doi.org/10.3390/met14091047






