Enhanced Recovery of Lithium and Cobalt from Spent Lithium-Ion Batteries Using Ultrasound-Assisted Deep Eutectic Solvent Leaching
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of DES
2.3. Leaching Procedure
2.4. Analytical Techniques
3. Results and Discussion
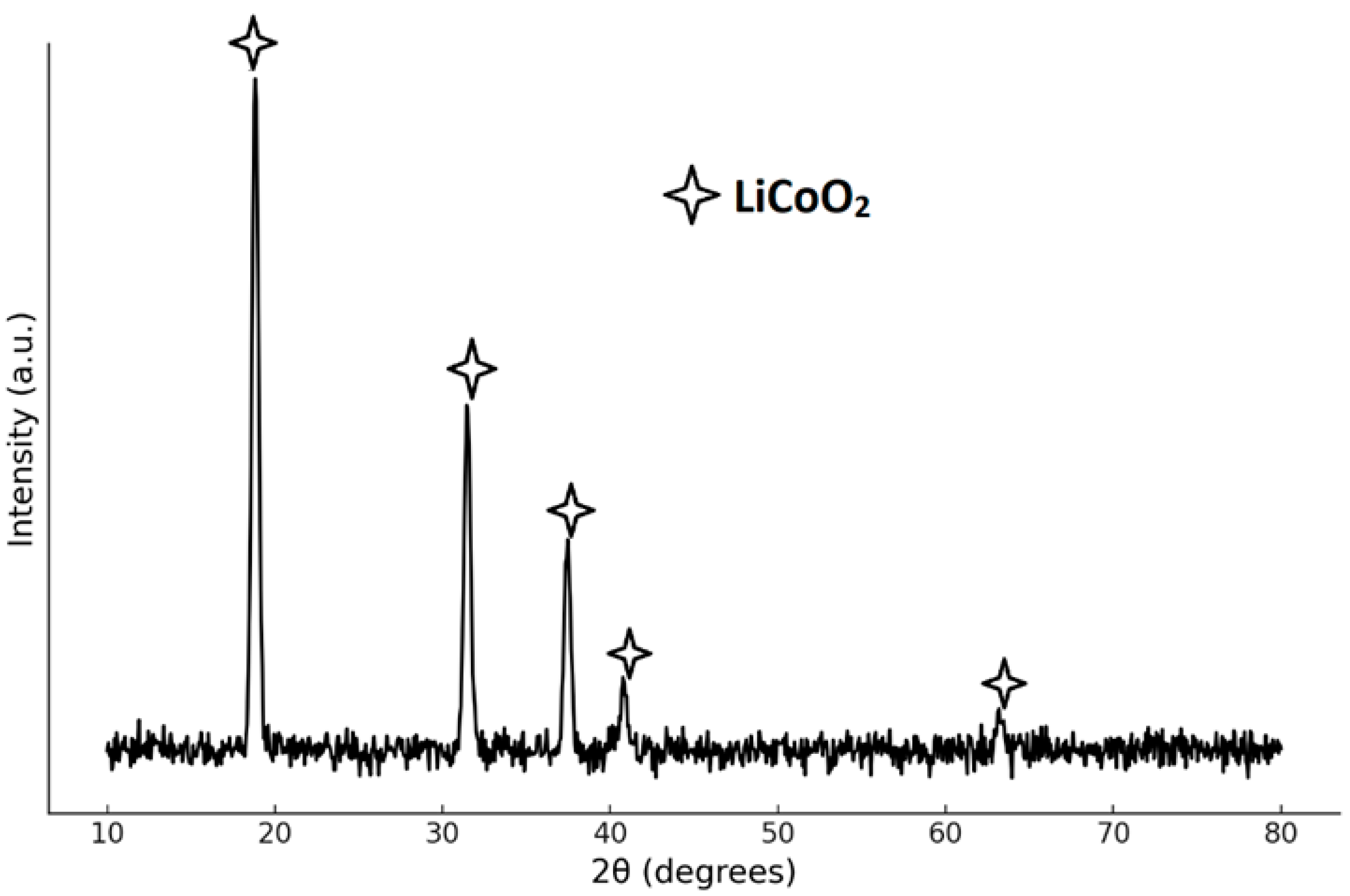
3.1. Characterization of LCO Powder Sample
3.2. Leaching Experiments
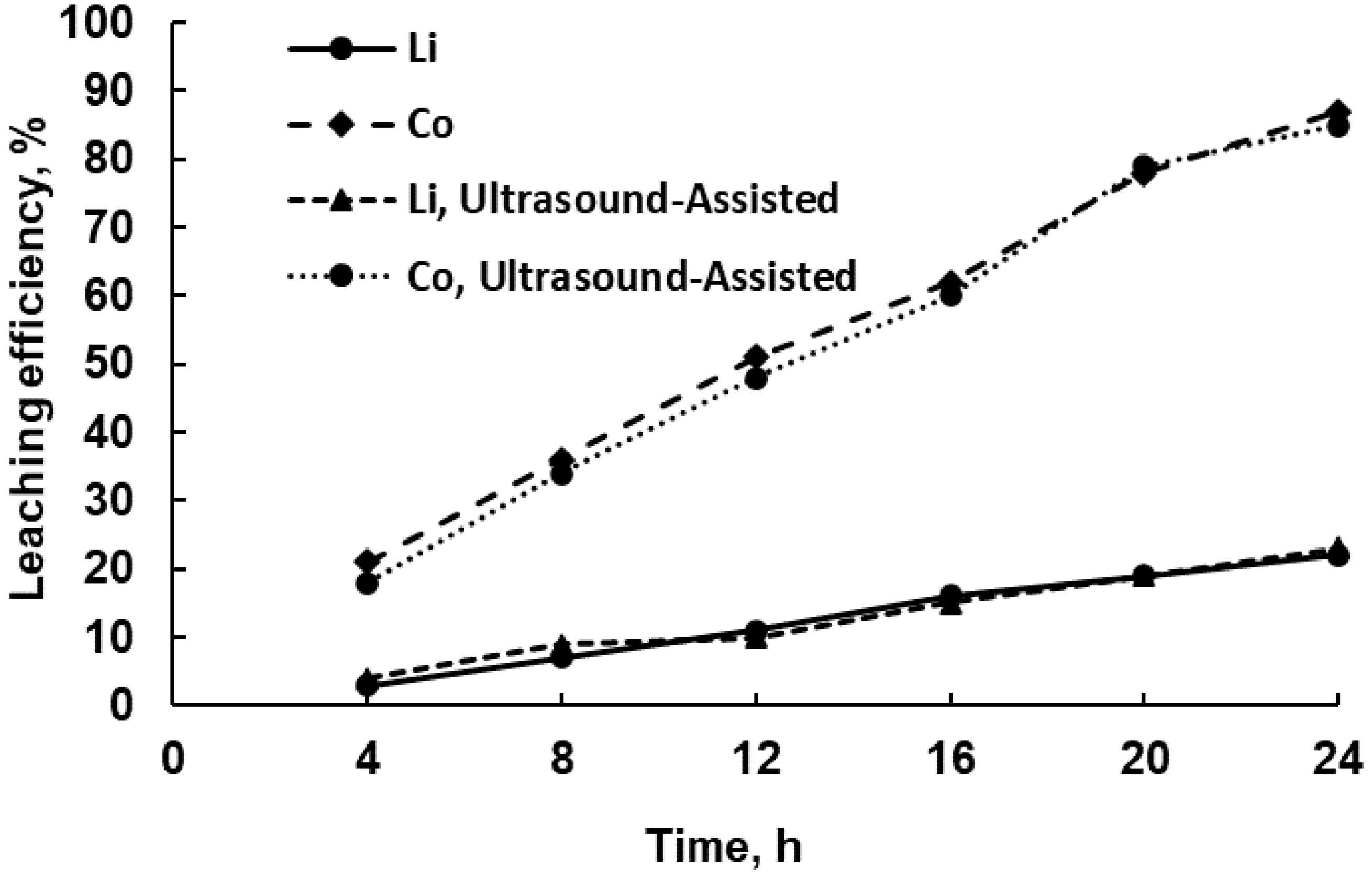
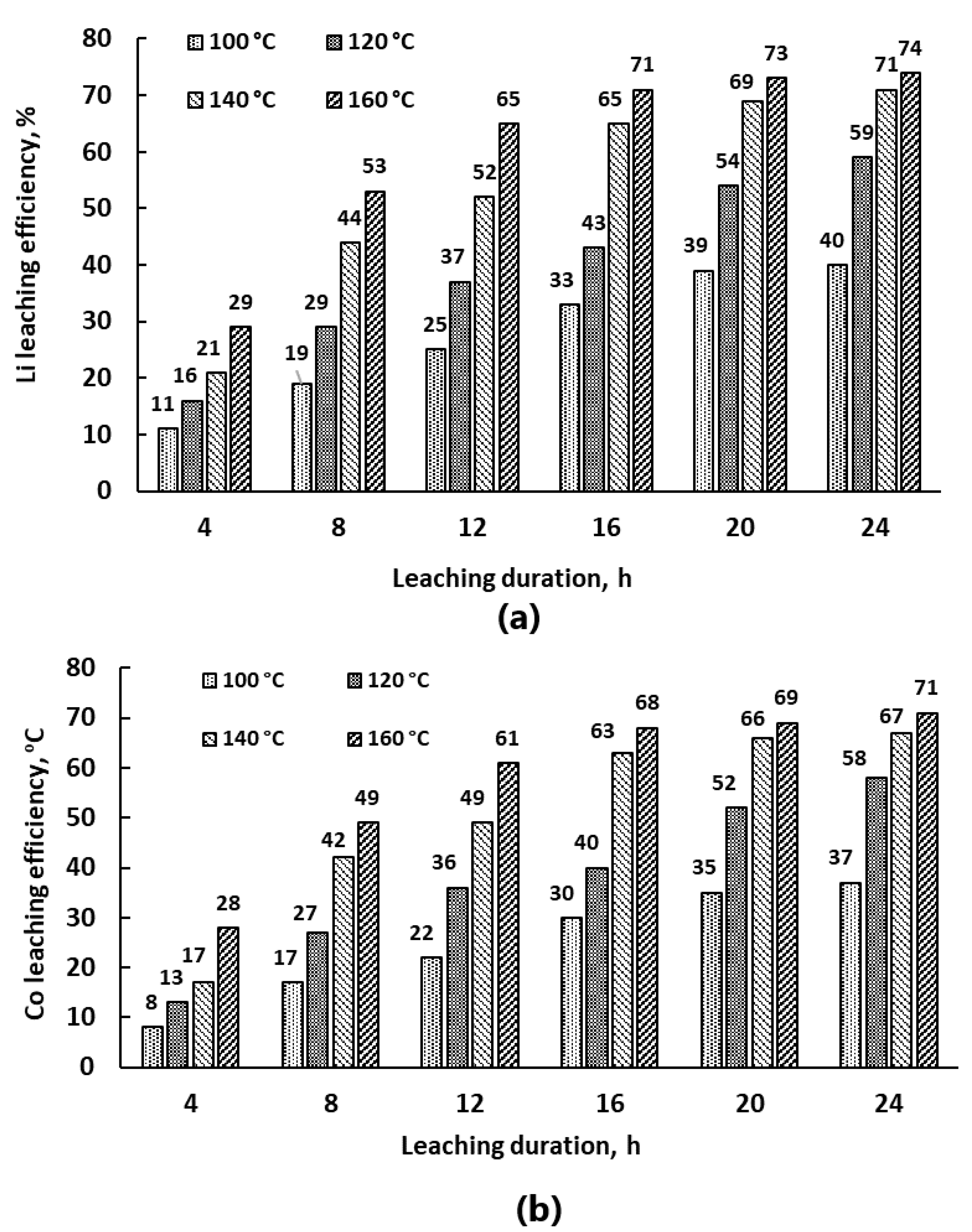
3.2.1. Effect of Leaching Duration on Leaching Efficiency
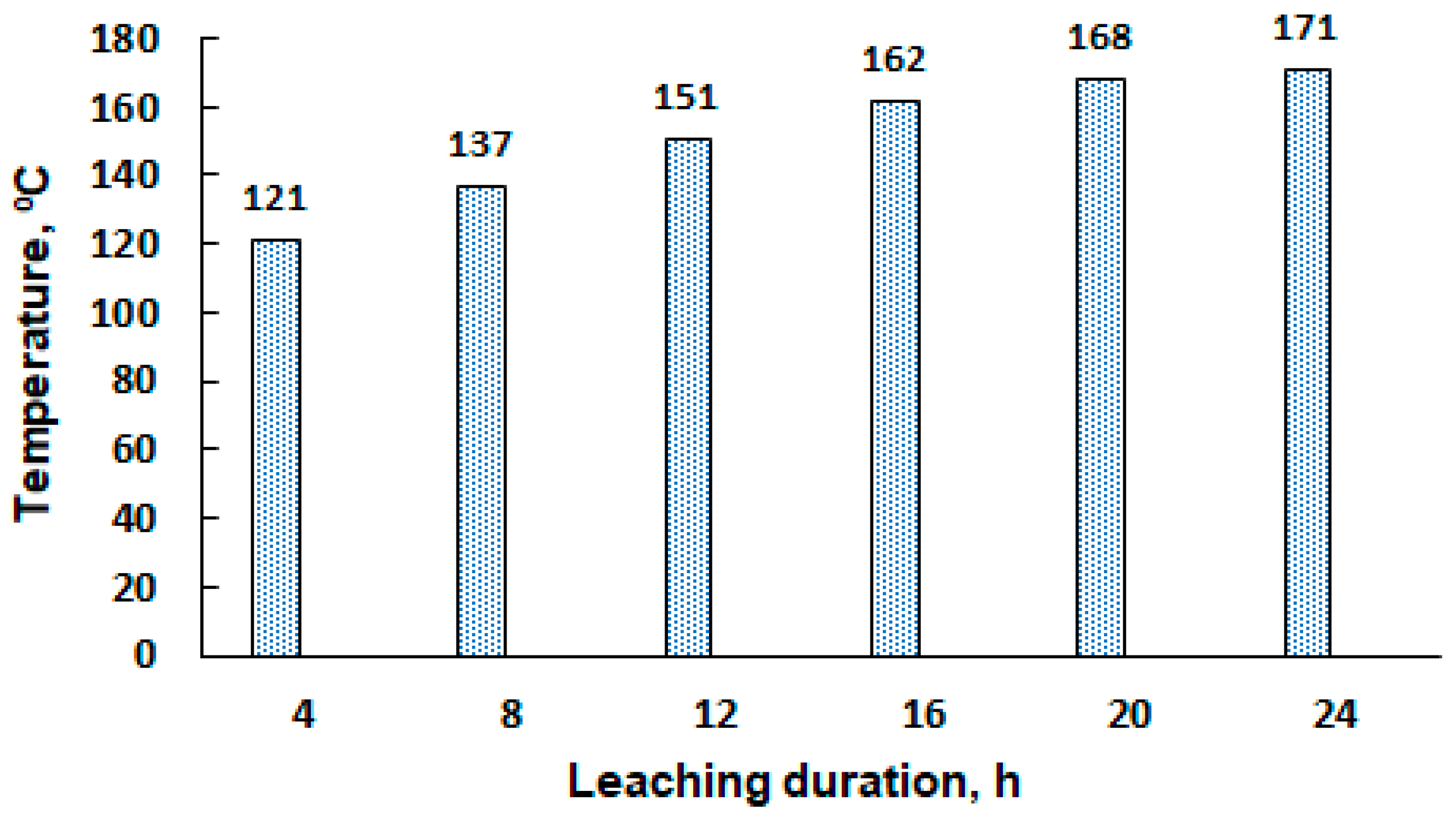
3.2.2. Leaching under Periodic Ultrasound Exposure
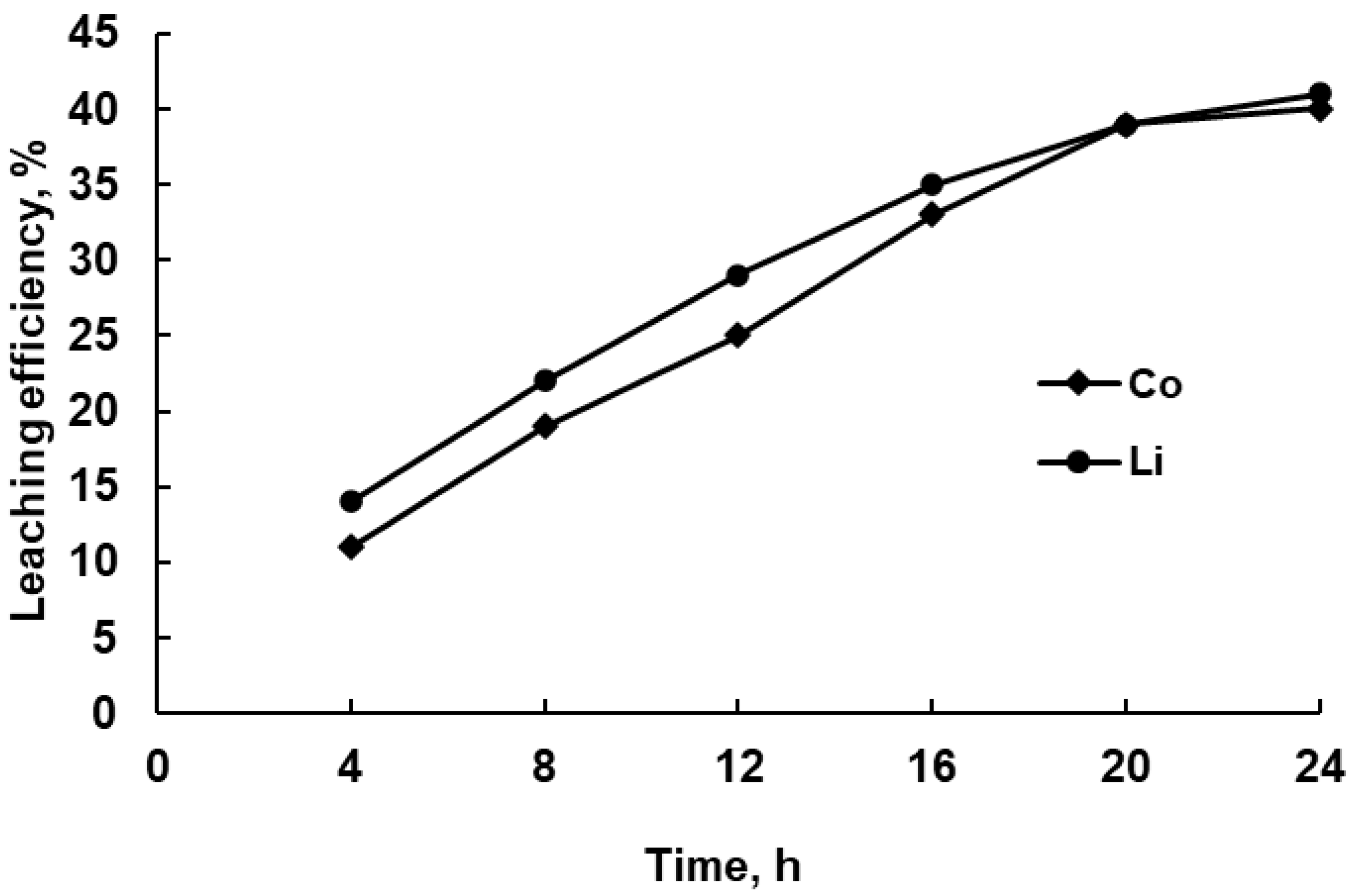
3.2.3. Effect of Temperature on Leaching Efficiency
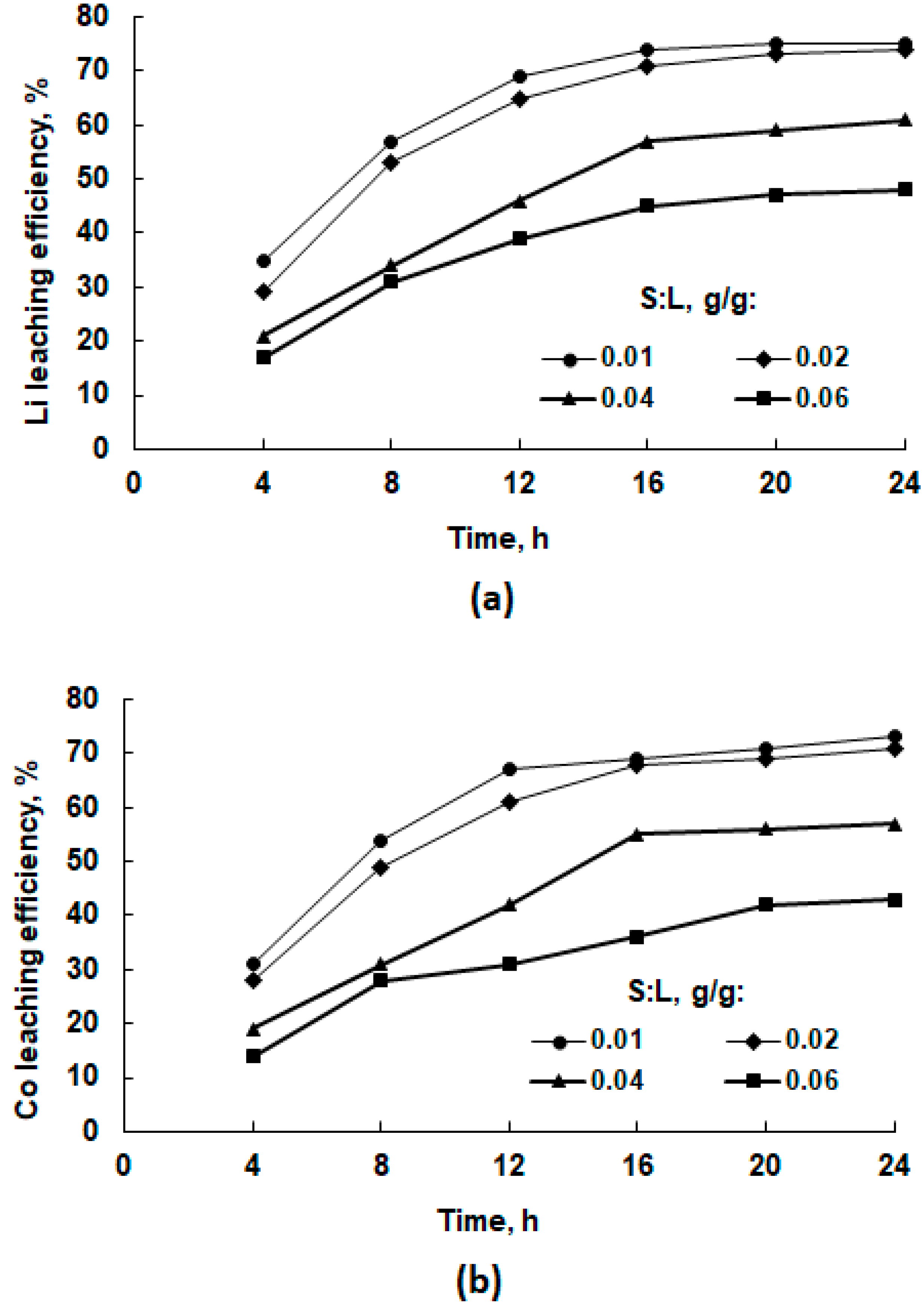
3.2.4. Effect of S:L Ratio on Leaching Efficiency
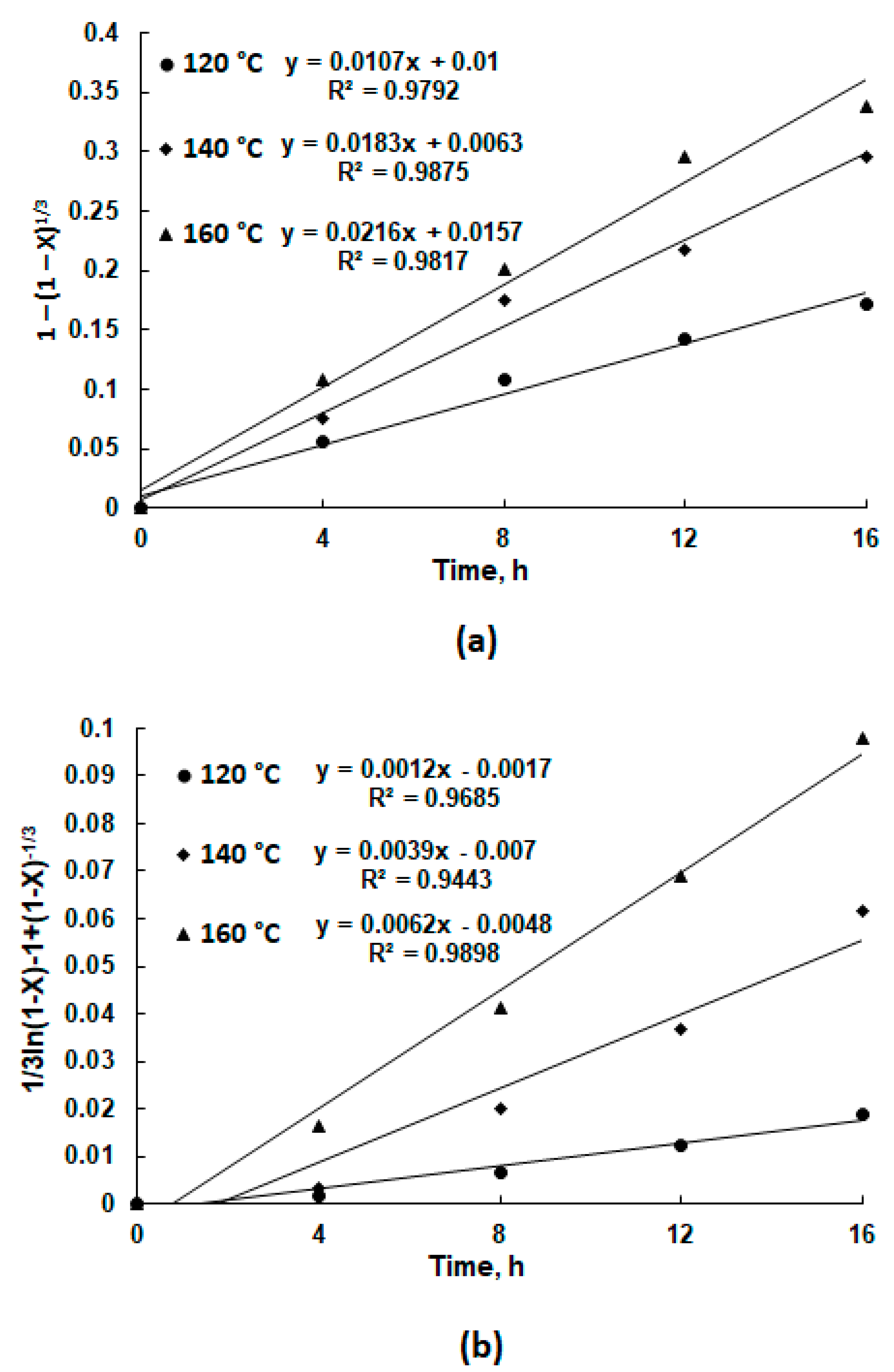
3.3. Leaching Kinetics Study
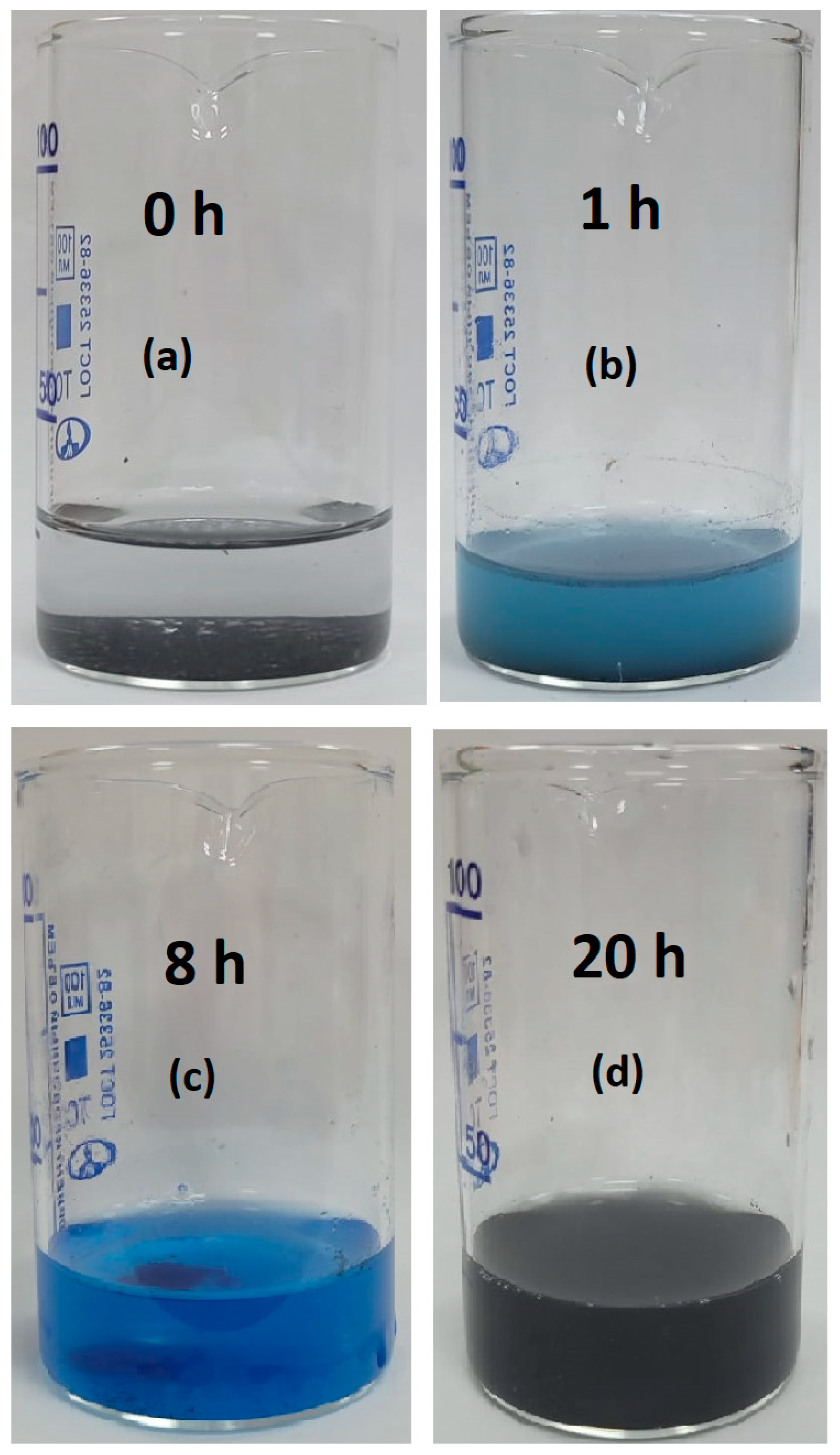
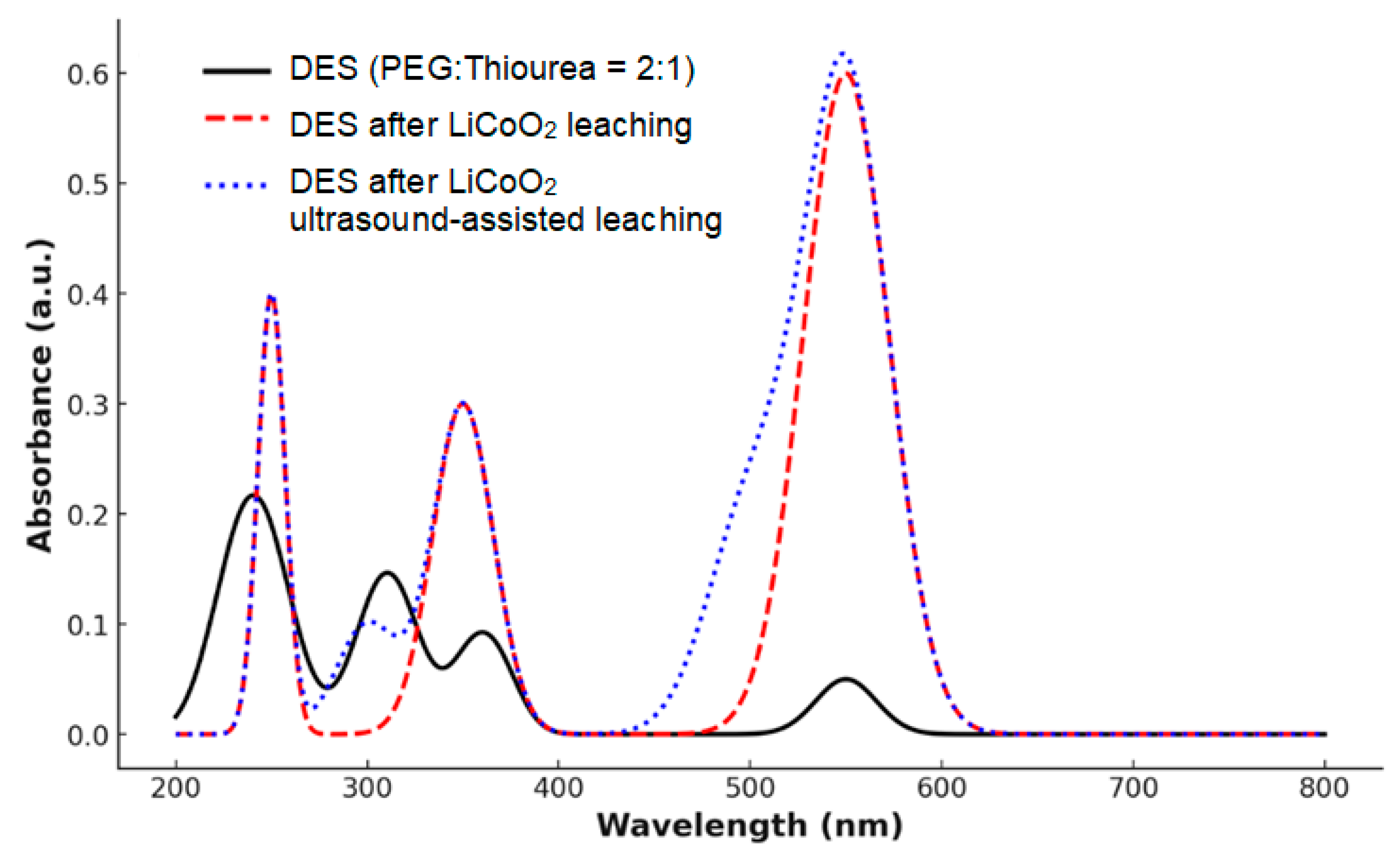
3.4. FTIR and UV–Vis Spectra Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Chen, B.; Ma, J.; Cui, G.; Chen, L. Reviving lithium cobalt oxide-based lithium secondary batteries-toward a higher energy density. Chem. Soc. Rev. 2018, 47, 6505–6602. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J. Cobalt in lithium-ion batteries. Science 2020, 367, 979–980. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wan, J.; Xiang, Y.; Zhu, J.; Leng, Q.; Wang, M.; Xu, L.; Yang, Y. Recent advances and historical developments of high voltage lithium cobalt oxide materials for rechargeable Li-ion batteries. J. Power Sources 2020, 460, 228062. [Google Scholar] [CrossRef]
- Gourley, S.W.D.; Or, T.; Chen, Z. Breaking free from cobalt reliance in lithium-ion batteries. iScience 2020, 23, 101505. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Cao, H.; Zhang, Y.; Sun, Z. Recycling of spent lithium-ion batteries in view of lithium recovery: A critical review. J. Clean. Prod. 2019, 228, 801–813. [Google Scholar] [CrossRef]
- Ordoñez, J.; Gago, E.J.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Liu, L. Solving spent lithium-ion battery problems in China: Opportunities and challenges. Renew. Sustain. Energy Rev. 2015, 52, 1759–1767. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A critical review and analysis on the recycling of spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Li, M.; Chen, R.; Wu, F.; Amine, K.; Lu, J. The recycling of spent lithium-ion batteries: A review of current processes and technologies. Electrochem. Energy Rev. 2018, 1, 461–482. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A mini-review on metal recycling from spent lithium ion batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical processes for recycling spent lithium-ion batteries: A critical review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Meshram, P.; Mishra, A.; Sahu, R. Environmental impact of spent lithium ion batteries and green recycling perspectives by organic acids–A review. Chemosphere 2020, 242, 125291. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Yuan, G.; Aleksandrov, A.; Zhang, T.; Li, Z.; Fathollahi-Fard, A.M.; Ivanov, M. Recycling of spent Lithium-ion Batteries: A comprehensive review for identification of main challenges and future research trends. Sustain. Energy Technol. Assess. 2022, 53, 102447. [Google Scholar] [CrossRef]
- Wang, M.M.; Zhang, C.C.; Zhang, F.S. An environmental benign process for cobalt and lithium recovery from spent lithium-ion batteries by mechanochemical approach. Waste Manag. 2016, 51, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tan, Q.; Li, J. Unveiling the role and mechanism of mechanochemical activation on lithium cobalt oxide powders from spent lithium-ion batteries. Environ. Sci. Technol. 2018, 52, 13136–13143. [Google Scholar] [CrossRef]
- Guan, J.; Li, Y.; Guo, Y.; Su, R.; Gao, G.; Song, H.; Yuan, H.; Liang, B.; Guo, Z. Mechanochemical process enhanced cobalt and lithium recycling from wasted lithium-ion batteries. ACS Sustain. Chem. Eng. 2017, 5, 1026–1032. [Google Scholar] [CrossRef]
- Tran, M.K.; Rodrigues, M.T.F.; Kato, K.; Babu, G.; Ajayan, P.M. Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat. Energy 2019, 4, 339–345. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; McKenzie, K.J.; Obi, S.U. Solubility of metal oxides in deep eutectic solvents based on choline chloride. J. Chem. Eng. Data 2006, 51, 1280–1282. [Google Scholar] [CrossRef]
- Zante, G.; Boltoeva, M. Review on hydrometallurgical recovery of metals with deep eutectic solvents. Sustain. Chem. 2020, 1, 238–255. [Google Scholar] [CrossRef]
- Liu, C.; Yan, Q.; Zhang, X.; Lei, L.; Xiao, C. Efficient recovery of end-of-life NdFeB permanent magnets by selective leaching with deep eutectic solvents. Environ. Sci. Technol. 2020, 54, 10370–10379. [Google Scholar] [CrossRef]
- Topcu, M.A.; Kalem, V.; Rüşen, A. Processing of anode slime with deep eutectic solvents as a green leachant. Hydrometallurgy 2021, 205, 105732. [Google Scholar] [CrossRef]
- Jung, J.C.Y.; Sui, P.C.; Zhang, J. A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments. J. Energy Storage 2021, 35, 102217. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, H.; Yong, W.F.; She, Q.; Esteban, J. Status and advances of deep eutectic solvents for metal separation and recovery. Green Chem. 2022, 24, 1895–1929. [Google Scholar]
- Tian, G.; Liu, H. Review on the mineral processing in ionic liquids and deep eutectic solvents. Miner. Process. Extr. Metall. Rev. 2024, 45, 130–153. [Google Scholar] [CrossRef]
- Suffia, S.; Dutta, D. Applications of deep eutectic solvents in metal recovery from E-wastes in a sustainable way. J. Mol. Liq. 2024, 394, 123738. [Google Scholar] [CrossRef]
- Martín, M.I.; García-Díaz, I.; López, F.A. Properties and perspective of using deep eutectic solvents for hydrometallurgy metal recovery. Miner. Eng. 2023, 203, 108306. [Google Scholar] [CrossRef]
- Lu, B.; Du, R.; Wang, G.; Wang, Y.; Dong, S.; Zhou, D.; Wang, S.; Li, C. High-efficiency leaching of valuable metals from waste Li-ion batteries using deep eutectic solvents. Environ. Res. 2022, 212, 113286. [Google Scholar] [CrossRef]
- Jafari, M.; Shafaie, S.Z.; Abdollahi, H.; Entezari-Zarandi, A. Green recycling of spent Li-ion batteries by deep eutectic solvents (DESs): Leaching mechanism and effect of ternary DES. J. Environ. Chem. Eng. 2022, 10, 109014. [Google Scholar] [CrossRef]
- Peeters, N.; Binnemans, K.; Riaño, S. Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents. Green Chem. 2020, 22, 4210–4221. [Google Scholar] [CrossRef]
- Yan, Q.; Ding, A.; Li, M.; Liu, C.; Xiao, C. Green leaching of lithium-ion battery cathodes by ascorbic acid modified guanidine-based deep eutectic solvents. Energy Fuels 2023, 37, 1216–1224. [Google Scholar] [CrossRef]
- Luo, Y.; Yin, C.; Ou, L.; Zhang, C. Highly efficient dissolution of the cathode materials of spent Ni–Co–Mn lithium batteries using deep eutectic solvents. Green Chem. 2022, 24, 6562–6570. [Google Scholar] [CrossRef]
- Chenthamara, B.; Gardas, R.L. Beyond the Conventional Leaching: Exploring Pyruvic Acid-Based Deep Eutectic Solvents for Sustainable Recycling of Spent Lithium-Ion Battery Cathode Material. ACS Sustain. Chem. Eng. 2024, 12, 12827–12836. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Liu, X.; Yin, X.; Yang, Y. Novel ternary deep eutectic solvents used for recycling lithium and cobalt from waste lithium-ion batteries. Sep. Purif. Technol. 2025, 354, 128934. [Google Scholar] [CrossRef]
- Luo, Y.; Ou, L.; Yin, C. Extraction of precious metals from used lithium-ion batteries by a natural deep eutectic solvent with synergistic effects. Waste Manag. 2023, 164, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, Y.; Liu, Z.; Zhou, L.; Li, Z.; Jiang, J.; Wei, L.; Ren, P.; Mu, T. Efficient dissolution of lithium-ion batteries cathode LiCoO2 by polyethylene glycol-based deep eutectic solvents at mild temperature. ACS Sustain. Chem. Eng. 2020, 8, 11713–11720. [Google Scholar] [CrossRef]
- Turan, M.D.; Silva, J.P.; Sarı, Z.A.; Nadirov, R.; Toro, N. Dissolution of chalcopyrite in presence of chelating agent and hydrogen peroxide. Trans. Indian Inst. Met. 2022, 75, 273–280. [Google Scholar] [CrossRef]
- Li, L.; Zhai, L.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Recovery of valuable metals from spent lithium-ion batteries by ultrasonic-assisted leaching process. J. Power Sources 2014, 262, 380–385. [Google Scholar] [CrossRef]
- Jiang, F.; Chen, Y.; Ju, S.; Zhu, Q.; Zhang, L.; Peng, J.; Wang, X.; Miller, J.D. Ultrasound-assisted leaching of cobalt and lithium from spent lithium-ion batteries. Ultrason. Sonochemistry 2018, 48, 88–95. [Google Scholar] [CrossRef]
- Yan, S.; Sun, C.; Zhou, T.; Gao, R.; Xie, H. Ultrasonic-assisted leaching of valuable metals from spent lithium-ion batteries using organic additives. Sep. Purif. Technol. 2021, 257, 117930. [Google Scholar] [CrossRef]
- Esmaeili, M.; Rastegar, S.O.; Beigzadeh, R.; Gu, T. Ultrasound-assisted leaching of spent lithium ion batteries by natural organic acids and H2O2. Chemosphere 2020, 254, 126670. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shixing, W.; Ullah, E.; Sajjad, M.; Zhang, L.; Fu, L. Enhanced metal recovery using ultrasound assisted leaching (UAL). An overview. J. Mol. Liq. 2024, 410, 125545. [Google Scholar] [CrossRef]
- Gbor, P.K.; Jia, C.Q. Critical evaluation of coupling particle size distribution with the shrinking core model. Chem. Eng. Sci. 2004, 59, 1979–1987. [Google Scholar] [CrossRef]
- Nadirov, R.; Karamyrzayev, G. Selective ozone-assisted acid leaching of copper from copper smelter slag by using isopropanol as a solvent. Minerals 2022, 12, 1047. [Google Scholar] [CrossRef]
- Nadirov, R.; Karamyrzayev, G. Enhancing Synthetic Zinc Ferrite Hydrochloric Acid Leaching by Using Isopropanol as a Solvent. Min. Metall. Explor. 2022, 39, 1743–1751. [Google Scholar] [CrossRef]

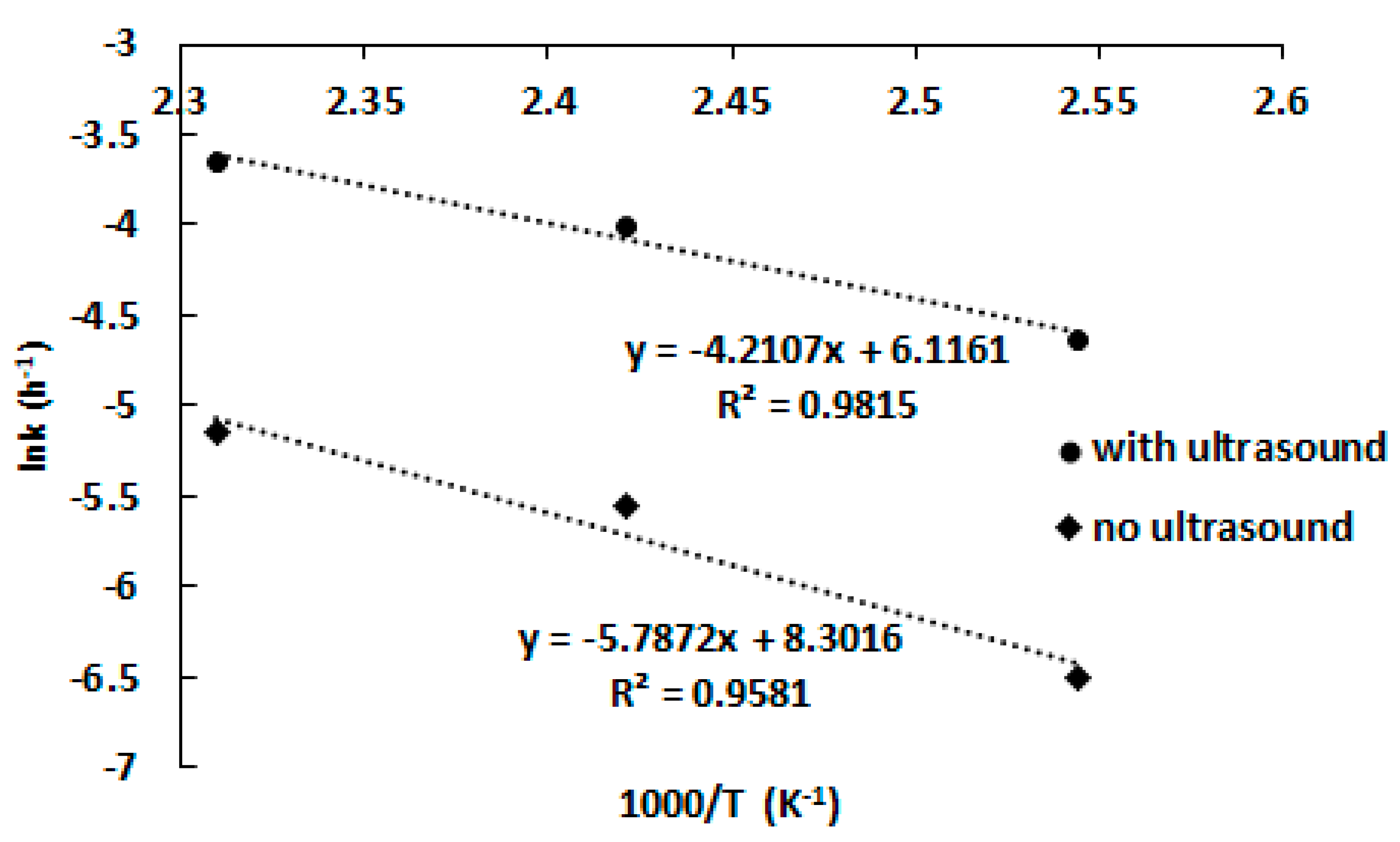

| Equation | Conditions | Temperature, °C | ||
|---|---|---|---|---|
| 120 | 140 | 160 | ||
| 2 | No ultrasound | R2 = 0.5873 | R2 = 0.6438 | R2 = 0.6271 |
| Ultrasound | R2 = 0.6942 | R2 = 0.7115 | R2 = 0.7009 | |
| 3 | No ultrasound | R2 = 0.7065 | R2 = 0.6794 | R2 = 0.4951 |
| Ultrasound | R2 = 0.9792 | R2 = 0.9875 | R2 = 0.9817 | |
| 4 | No ultrasound | R2 = 0.9898 | R2 = 0.9443 | R2 = 0.9685 |
| Ultrasound | R2 = 0.7463 | R2 = 0.7738 | R2 = 0.7916 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ketegenov, T.; Kamunur, K.; Mussapyrova, L.; Batkal, A.; Nadirov, R. Enhanced Recovery of Lithium and Cobalt from Spent Lithium-Ion Batteries Using Ultrasound-Assisted Deep Eutectic Solvent Leaching. Metals 2024, 14, 1052. https://doi.org/10.3390/met14091052
Ketegenov T, Kamunur K, Mussapyrova L, Batkal A, Nadirov R. Enhanced Recovery of Lithium and Cobalt from Spent Lithium-Ion Batteries Using Ultrasound-Assisted Deep Eutectic Solvent Leaching. Metals. 2024; 14(9):1052. https://doi.org/10.3390/met14091052
Chicago/Turabian StyleKetegenov, Tlek, Kaster Kamunur, Lyazzat Mussapyrova, Aisulu Batkal, and Rashid Nadirov. 2024. "Enhanced Recovery of Lithium and Cobalt from Spent Lithium-Ion Batteries Using Ultrasound-Assisted Deep Eutectic Solvent Leaching" Metals 14, no. 9: 1052. https://doi.org/10.3390/met14091052






