Morphological and Doping Effects on Electrical Conductivity of Aluminum Metal Substrate through Pulsed Electrodeposition Coating of Cu-MWCNT
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation, Cleaning and Conditioning
2.2. Anodization Process
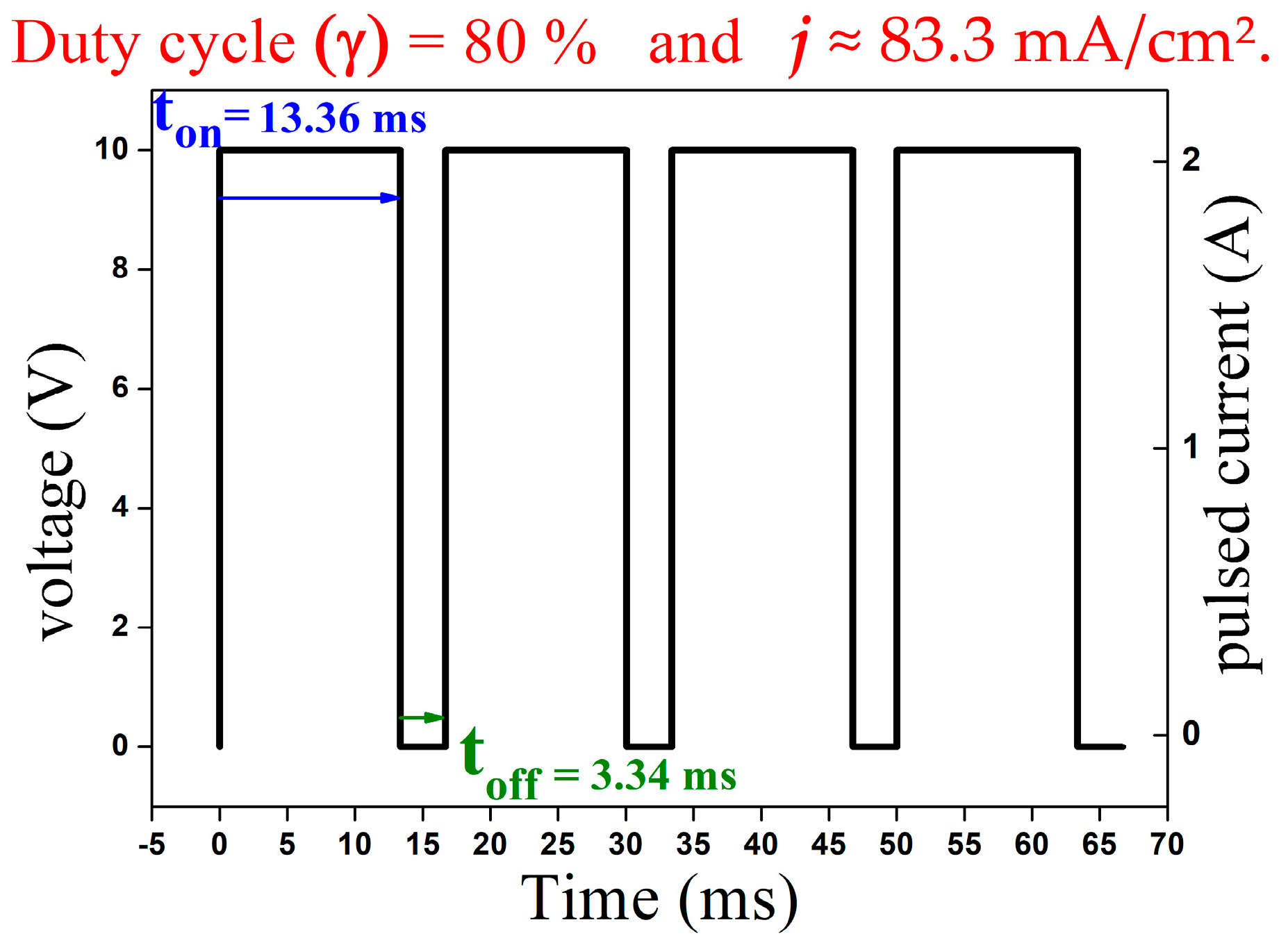
2.3. Coating of Cu-MWCNTs Using the Pulsed Electrodeposition Process
2.4. Parameterization Data from the Experiments
2.5. Morphological Characterization Using FEG-SEM
2.6. Vibrational Characterization Utilizing the Raman Spectroscopy Technique
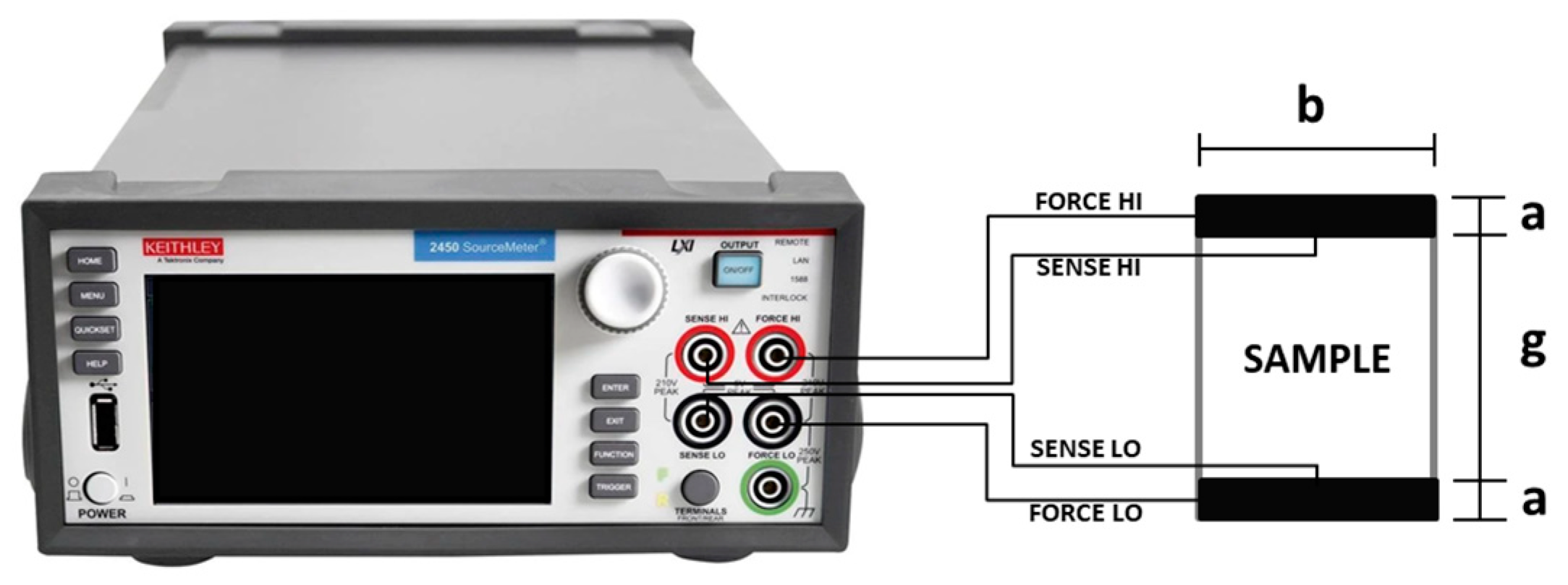
2.7. Surface Electrical Characterization Using the Four-Tip Kelvin Probe Technique
3. Results
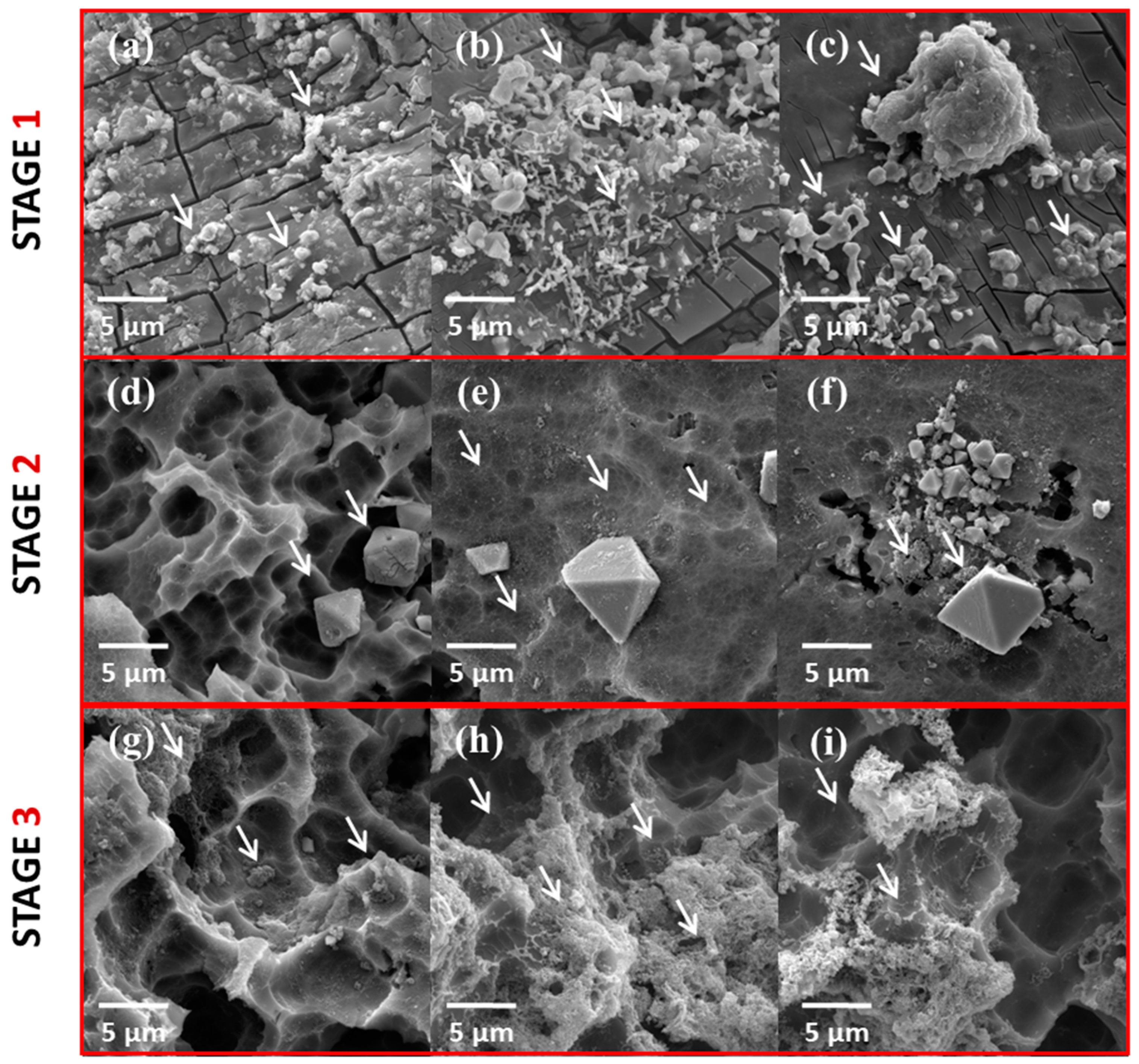
3.1. Morphological Analysis
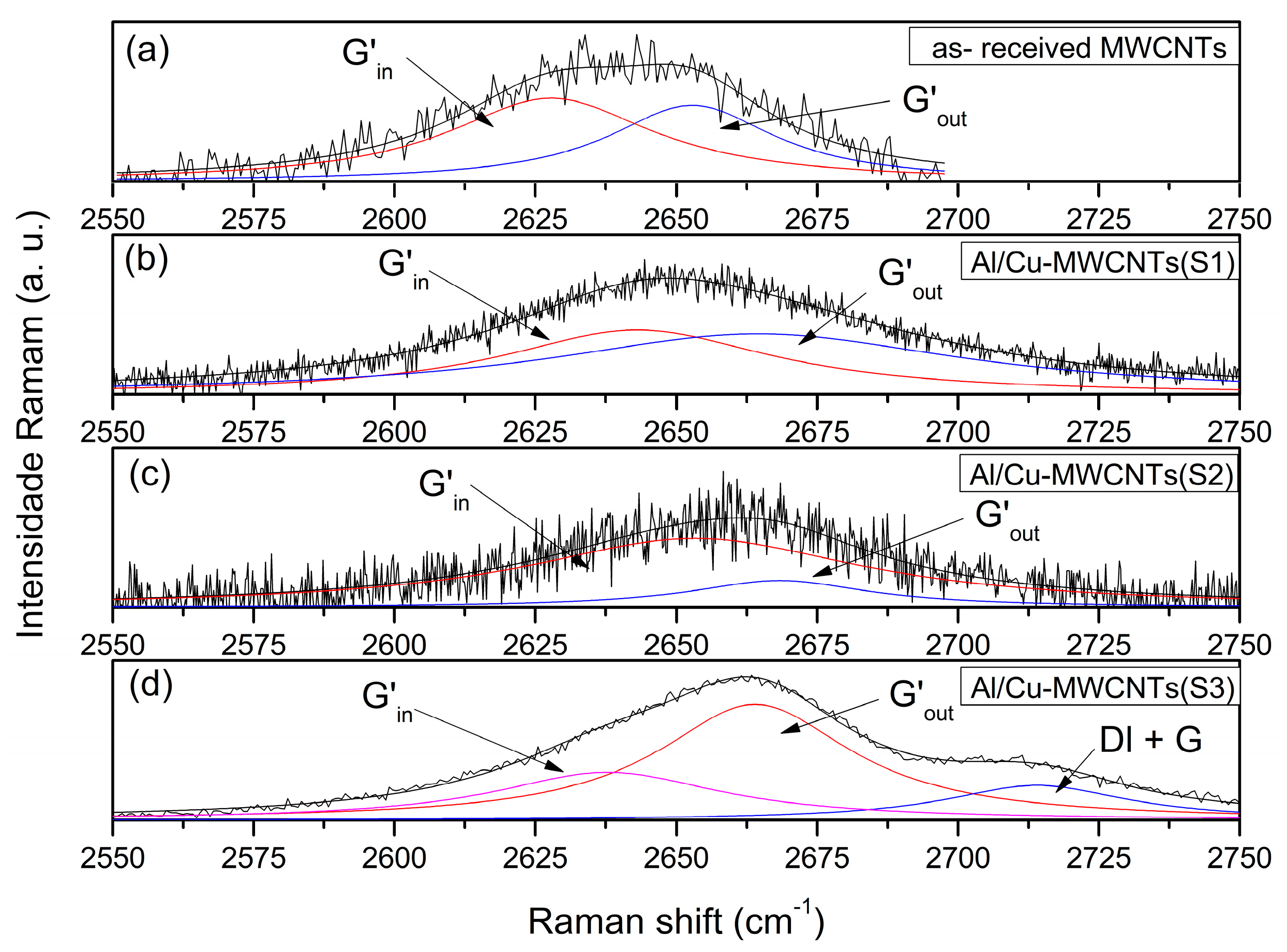
3.2. Analysis of Raman Spectra
3.3. Characterization of Electrical Properties
4. Discussion
4.1. Influence of Cu-MWCNT Morphology on Surface Electrical Conductivity Enhancement
4.2. Correlation between Vibrational Property Changes and Coating Enhancement: A Blueshift Study in Raman Spectrum
4.3. Analysis of Defect Density and Amorphous Carbon Content
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Sample | Control | Al/Cu- MWCNTs (S1) | Al/Cu- MWCNTs (S2) | Al/Cu- MWCNTs (S3) |
|---|---|---|---|---|
| (Ω) | 0.00296 ± 9.02 × 10−5 | 0.00261 ± 1.48 × 10−4 | 0.00231 ± 4.90 × 10−5 | 0.00195 ± 3.69 × 10−5 |


| Sample | Gout | Gin | G’in | G’out |
|---|---|---|---|---|
| as-received MWCNTs | 27 ± 3.0 | 25 ± 3.0 | 45 ± 2.5 | 36 ± 3 |
| Al/Cu-MWCNTs(S1) | 33 ± 3.0 | 33 ± 3.0 | 81 ± 3.0 | 57 ± 2.5 |
| Al/Cu-MWCNTs(S2) | 25 ± 3.2 | 28 ± 4.0 | 72 ± 3.0 | 38 ± 3.0 |
| Al/Cu-MWCNTs(S3) | 29 ± 4.0 | 18 ± 4.0 | 40 ± 2.2 | 34 ± 2.1 |
References
- Chen, S.; Fu, S.; Liang, D.; Chen, X.; Mi, X.; Liu, P.; Zhang, Y.; Hui, D. Preparation and properties of 3D interconnected CNTs/Cu composites. Nanotechnol. Rev. 2020, 9, 146–154. [Google Scholar] [CrossRef]
- Geng, H.; Chen, B.; Wan, J.; Shen, J.; Kondoh, K.; Li, J.S. Matrix effect on strengthening behavior of carbon nanotubes in aluminum matrix composites. Mater. Charact. 2023, 195, 112484. [Google Scholar] [CrossRef]
- Zhang, S.L.; Nguyen, N.; Leonhardt, B.; Jolowsky, C.; Hao, A.; Park, J.G.; Liang, R. Carbon-Nanotube-Based Electrical Conductors: Fabrication, Optimization, and Applications. Adv. Mater. Technol. 2019, 5, 1800811. [Google Scholar] [CrossRef]
- Gonçalves, I.L.; Vinhosa, R.A.; de Freitas, D.S.; Brasil, S.L. Pulsed electrophoretic deposition of hybrid coatings from aqueous suspensions as surface functionalization and sealing technique of anodized AA2024. Part I: Morphological characterization, analysis of the interfacial interactions, and evaluation of pore impregnation of the anodic layer. Prog. Org. Coat. 2023, 178, 107474. [Google Scholar]
- Zhang, J.; Cheng, F.; Wang, L.; Xu, Y.; Zhou, Z.; Liu, X.; Hu, Y.; Hu, X. Reinforcement study of anodizing treatment with various temperatures on aluminum substrates for stronger adhesive bonding with carbon fiber composites. Surf. Coat. Technol. 2023, 462, 129473. [Google Scholar] [CrossRef]
- Zulkifli, F.W.A.; Yazid, H.; Jani, A.M.M. Immobilization of carbon nanotubes decorated gold nanoparticles on anodized aluminium oxide (Au-CNTs-AAO) membrane for enhanced catalytic performance. Mater. Chem. Phys. 2021, 264, 12444. [Google Scholar] [CrossRef]
- Daryadel, S.; Behroozfar, A.; Morsali, S.R.; Moreno, S.; Baniasadi, M.; Bykova, J.; Bernal, R.A.; Minary-Jolandan, M. Localized pulsed electrodeposition process for three-dimensional printing of nanotwinned metallic nanostructures. Nano Lett. 2018, 18, 208–214. [Google Scholar] [CrossRef]
- Magalhães, R.; Proenca, M.P.; Araújo, J.P.; Nunes, C.; Pereira, A.M.; Sousa, C.T. Fabrication of FePt nanowires through pulsed electrodeposition into nanoporous alumina templates. Appl. Nanosci. 2022, 12, 3573–3584. [Google Scholar] [CrossRef]
- Fan, H.; Zhao, Y.; Wang, S.; Ma, R. Improvement of microstructures and properties of copper-aluminium oxide coating by pulse jet electrodeposition. Mater. Res. Express 2019, 6, 115090. [Google Scholar] [CrossRef]
- Xu, M.; Yue, G.; Bizzotto, D.; Asselin, E. Electrodeposition of aluminum onto copper-coated printed circuit boards. J. Electrochemic. Soc. 2017, 164, D729. [Google Scholar] [CrossRef]
- Rodrigues, F.; Pinheiro, P.; Sousa, M.; Angélica, R.; Paz, S.; Reis, M. Electrical Properties of Iodine-Doped Cu/f-CNT Coated Aluminum Wires by Electrophoresis with Copper Sulfate Solution. Metals 2022, 12, 787. [Google Scholar] [CrossRef]
- Mandal, P.; Mondal, S.C. Investigation of Electro-Thermal property for Cu-MWCNT composite coating on anodized 6061 aluminium alloy. Appl. Surf. Sci. 2018, 454, 138–147. [Google Scholar] [CrossRef]
- Bazbouz, M.B.; Aziz, A.; Copic, D.; De Volder, M.; Welland, M.E. Fabrication of high specific electrical conductivity and high ampacity carbon Nanotube/Copper composite wires. Adv. Electron. Mater. 2021, 7, 2001213. [Google Scholar] [CrossRef]
- ASTM B3-13; Standard Specification for Soft or Annealed Copper Wire. American Society for Testing and Materials: West Conshohocken, PA, USA, 2018.
- ASTM B193 20; Standard Test Method for Resistivity of Electrical Conductor Materials. American Society for Testing and Materials: West Conshohocken, PA, USA, 2020.
- ASTM D257-07; Standard Test Methods for DC Resistance or Conductance of Insulating Materials. American Society for Testing and Materials: West Conshohocken, PA, USA, 2012.
- Brown, S.D.M.; Jorio, A.; Corio, A.P.; Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Kneipp, K. Origin of the Breit-Wigner-Fano lineshape of the tangential G-band feature of metallic carbon nanotubes. Phys. Rev. B 2001, 63, 155414. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Reis, M.A.L.; De Sousa, F.F.; Sousa, M.E.S. Doping charge transfer in Pt/CNT systems induced by laser power heating. Chem. Phys. 2020, 530, 110591. [Google Scholar] [CrossRef]
- Alencar, R.S.; Aguiar, A.L.; Paschoal, A.R.; Freire, P.T.C.; Kim, Y.A.; Muramatsu, H.; Endo, M.; Terrones, H.; Terrones, M.; San-Miguel, A.; et al. Pressure-induced selectivity for probing inner tubes in double-and triple-walled carbon nanotubes: A resonance raman study. J. Phys. Chem. C 2014, 118, 8153–8158. [Google Scholar] [CrossRef]
- Uchida, T.; Tachibana, M.; Kurita, S.; Kojima, K. Temperature dependence of the Breit–Wigner–Fano Raman line in single-wall carbon nanotube bundles. Chem. Phys. Lett. 2004, 400, 341–346. [Google Scholar] [CrossRef]
- Eklund, P.C.; Dresselhaus, G.; Dresselhaus, M.S.; Fischer, J.E. Raman scattering from in-plane lattice modes in low-stage graphite-alkali-metal compounds. Phys. Rev. B 1977, 16, 3330. [Google Scholar] [CrossRef]
- Lazzeri, M.; Mauri, F. Nonadiabatic Kohn Anomaly in a Doped Graphene Monolayer. Phys. Rev. Lett. 2006, 97, 266407. [Google Scholar] [CrossRef]
- Brito, P.R.; Loayza, C.R.; Sousa, M.E.; Braga, E.M.; Angélica, R.S.; da Paz, S.P.; Reis, M.A. Cast aluminum surface reinforced with carbon nanotube via solubilization treatment. Met. Mater. Int. 2022, 28, 802–810. [Google Scholar] [CrossRef]
- Yan, J.; Henriksen, E.A.; Kim, P.; Pinczuk, A. Observation of anomalous phonon softening in bilayer graphene. Phys. Rev. Lett. 2008, 101, 136804. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Chakraborty, B.; Piscanec, S.; Pisana, S.; Sood, A.K.; Ferrari, A.C. Phonon renormalization in doped bilayer graphene. Phys. Rev. B 2009, 79, 155417. [Google Scholar] [CrossRef]
- Dos Reis, M.A.L.; Barbosa Neto, N.M.; De Sousa, M.E.S.; Araujo, P.T.; Simões, S.; Vieira, M.F.; Viana, F.; Loayza, C.R.L.; Borges, D.J.A.; Cardoso, D.C.S.; et al. Raman spectroscopy fingerprint of stainless steel-MWCNTs nanocomposite processed by ball-milling. AIP Adv. 2018, 8, 015323. [Google Scholar] [CrossRef]
- Ikonopisov, S. Theory of electrical breakdown during formation of barrier anodic films. Electrochim. Acta 1977, 22, 1077–1082. [Google Scholar] [CrossRef]
- Montero, I.; Albella, J.M.; Martinez-Duart, J.M. Influence of Electrolyte Concentration on the Anodization and Breakdown Characteristics of Ta2O5 Films. J. Electrochem. Soc. 1985, 132, 814. [Google Scholar] [CrossRef]
- Parkhutik, V.P. The initial stages of aluminium porous anodization studied by Auger electron spectroscopy. Corros. Sci. 1986, 26, 295–310. [Google Scholar] [CrossRef]

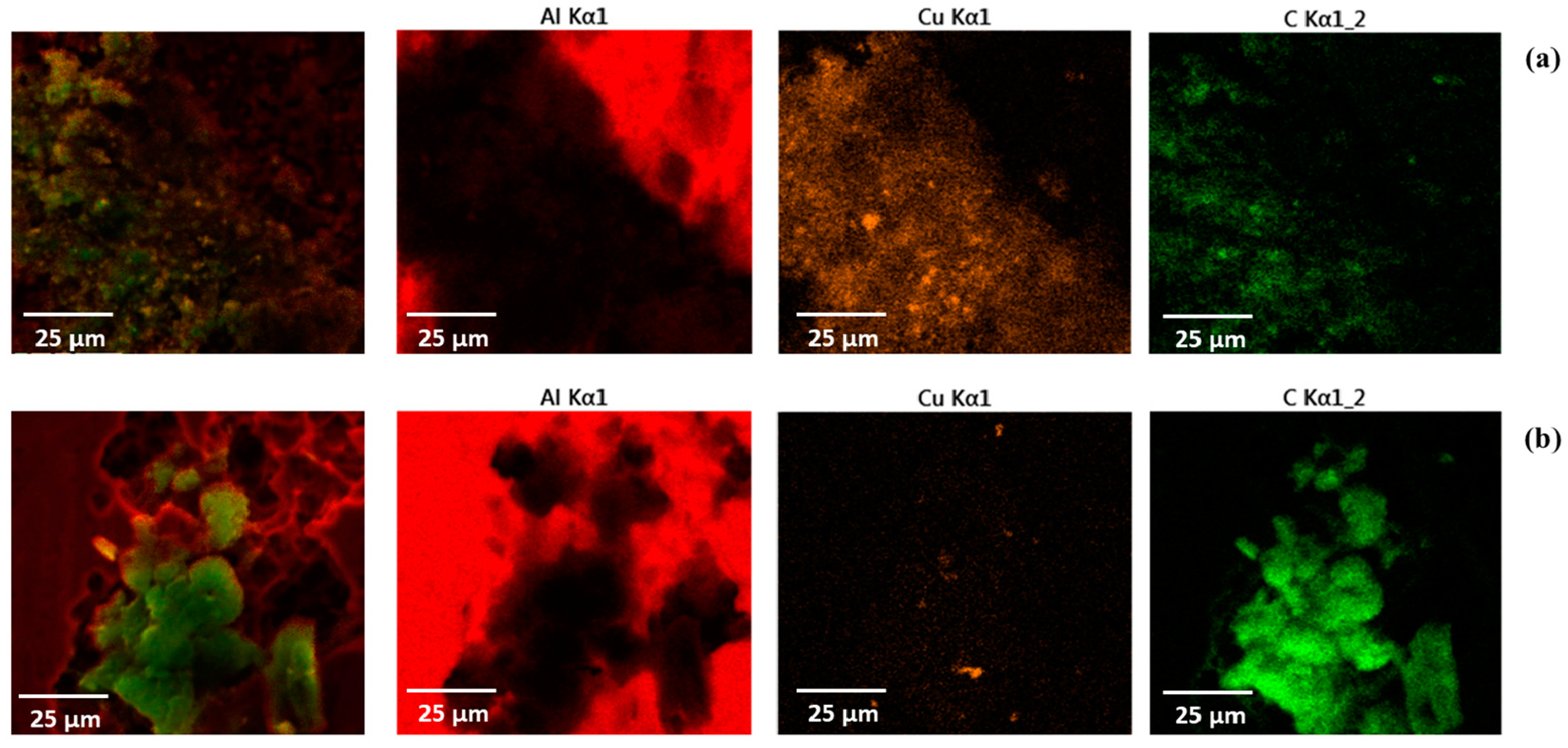
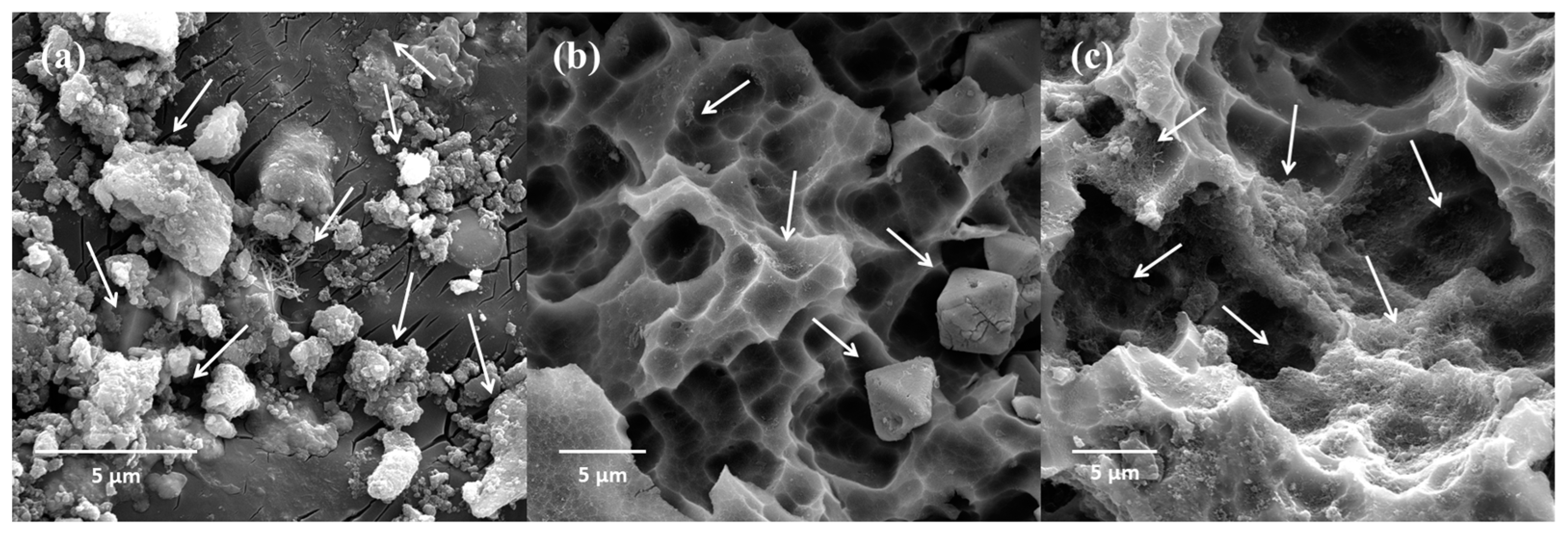




| Component | Si | Fe | Cu | Mn | Zi | Others | Al |
|---|---|---|---|---|---|---|---|
| Chemical composition limits (%) | 0.6 | 0.7 | 0.05 a 0.2 | 1 a 1.5 | 0.1 | 0.15 | remainder |
| Sample | Dl | Dr | DLO | Dmiddle | GBWF-like | Gout | Gin | D’ | G’in | G’out |
|---|---|---|---|---|---|---|---|---|---|---|
| as-received MWCNTs | 1315 | 1328 | 1353 | 1486 | - | 1567 | 1583 | 1602 | 2628 | 2653 |
| Al/Cu-MWCNTs(S1) | 1315 | 1329 | 1346 | 1484 | 1542 | 1573 | 1588 | 1608 | 2635 | 2668 |
| Al/Cu-MWCNTs(S2) | 1320 | 1337 | 1399 | 1512 | 1557 | 1576 | 1596 | 1617 | 2653 | 2668 |
| Al/Cu-MWCNTs(S3) | 1330 | 1347 | 1378 | 1491 | 1563 | 1583 | 1598 | 1617 | 2664 | 2714 |
| Sample | Control | Al/Cu- MWCNTs (S1) | Al/Cu- MWCNTs (S2) | Al/Cu- MWCNTs (S3) |
|---|---|---|---|---|
| (S) | 337.62 ± 10.1 | 383.83 ± 22.3 | 432.20 ± 9.2 | 514.30 ± 9.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.S.; Sousa, M.E.S.; Braga, E.M.; Reis, M.A.L. Morphological and Doping Effects on Electrical Conductivity of Aluminum Metal Substrate through Pulsed Electrodeposition Coating of Cu-MWCNT. Metals 2024, 14, 1060. https://doi.org/10.3390/met14091060
Silva AS, Sousa MES, Braga EM, Reis MAL. Morphological and Doping Effects on Electrical Conductivity of Aluminum Metal Substrate through Pulsed Electrodeposition Coating of Cu-MWCNT. Metals. 2024; 14(9):1060. https://doi.org/10.3390/met14091060
Chicago/Turabian StyleSilva, Alberto S., Mário E. S. Sousa, Eduardo M. Braga, and Marcos A. L. Reis. 2024. "Morphological and Doping Effects on Electrical Conductivity of Aluminum Metal Substrate through Pulsed Electrodeposition Coating of Cu-MWCNT" Metals 14, no. 9: 1060. https://doi.org/10.3390/met14091060
APA StyleSilva, A. S., Sousa, M. E. S., Braga, E. M., & Reis, M. A. L. (2024). Morphological and Doping Effects on Electrical Conductivity of Aluminum Metal Substrate through Pulsed Electrodeposition Coating of Cu-MWCNT. Metals, 14(9), 1060. https://doi.org/10.3390/met14091060







