Experimental and Mathematical Investigation of Hydrogen Absorption in LaNi5 and La0.7Ce0.1Ga0.3Ni5 Compounds
Abstract
:1. Introduction
2. Experimental Setup
3. Results
3.1. Experimental Results
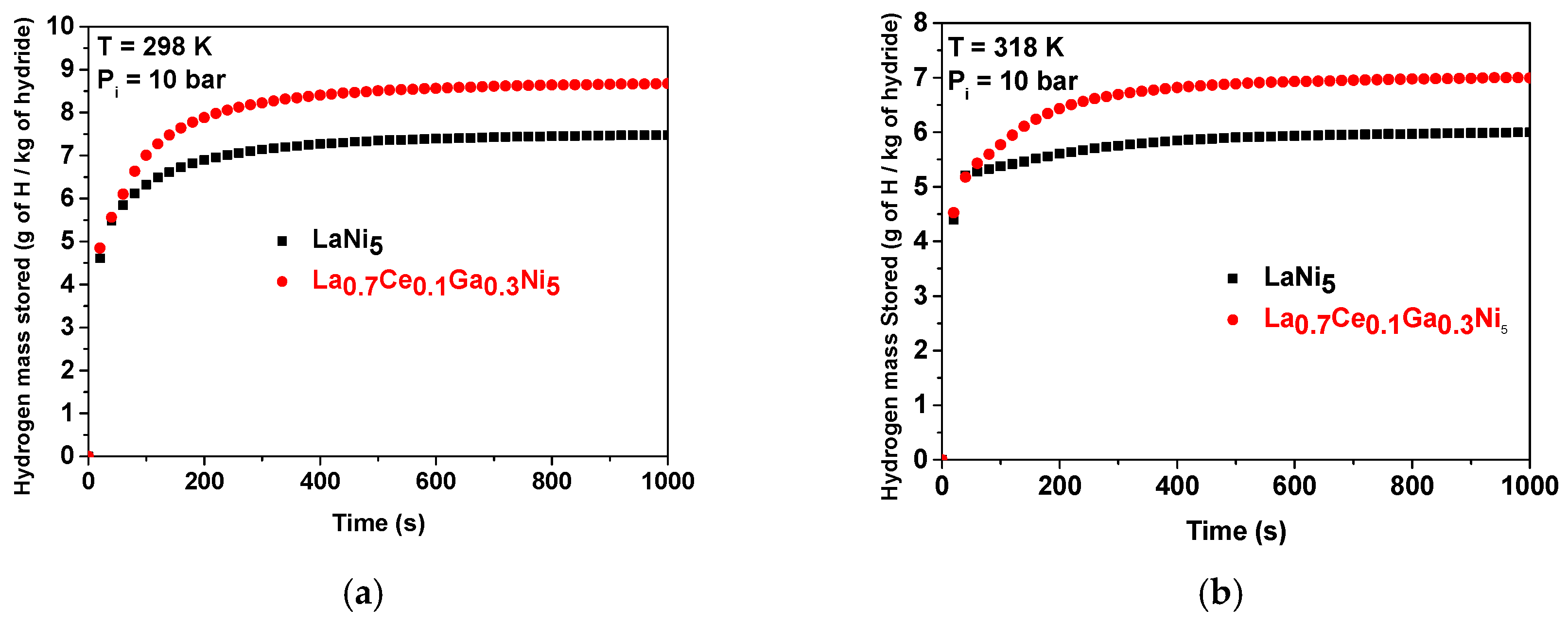
- Absorption kinetics
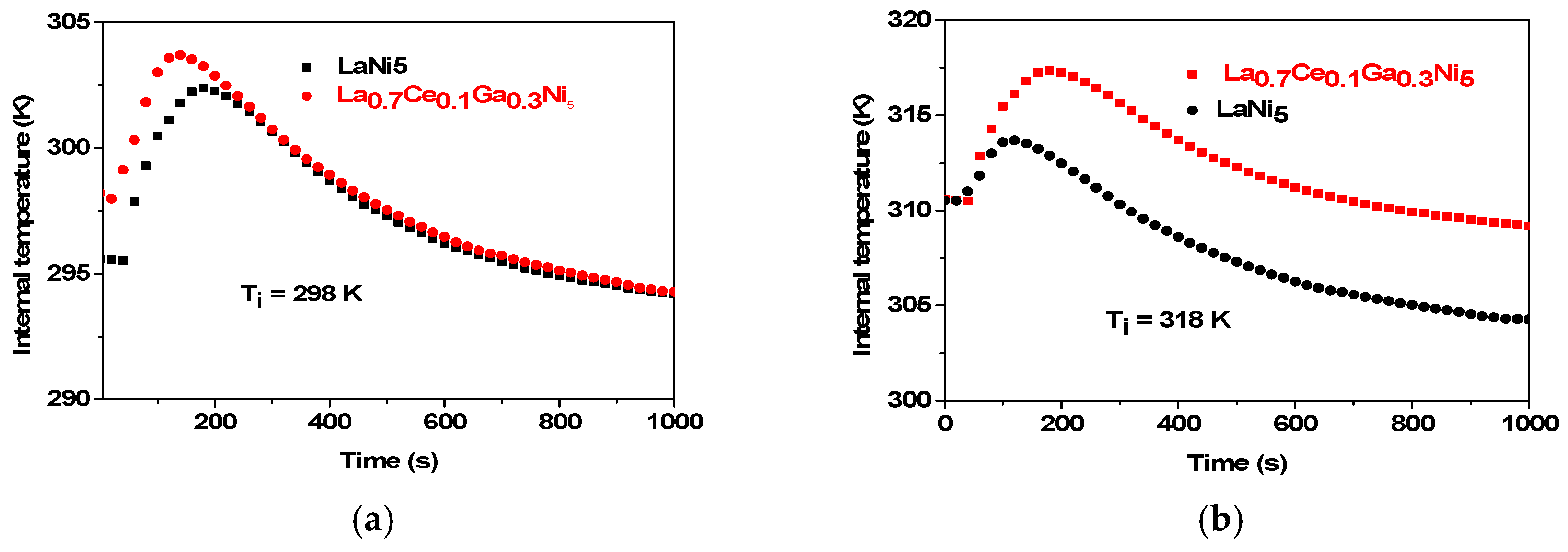
- Internal temperature
- Absorption isotherms
- Hysteresis measurement
3.2. Modeling
- Description of the model
4. Results and Discussions
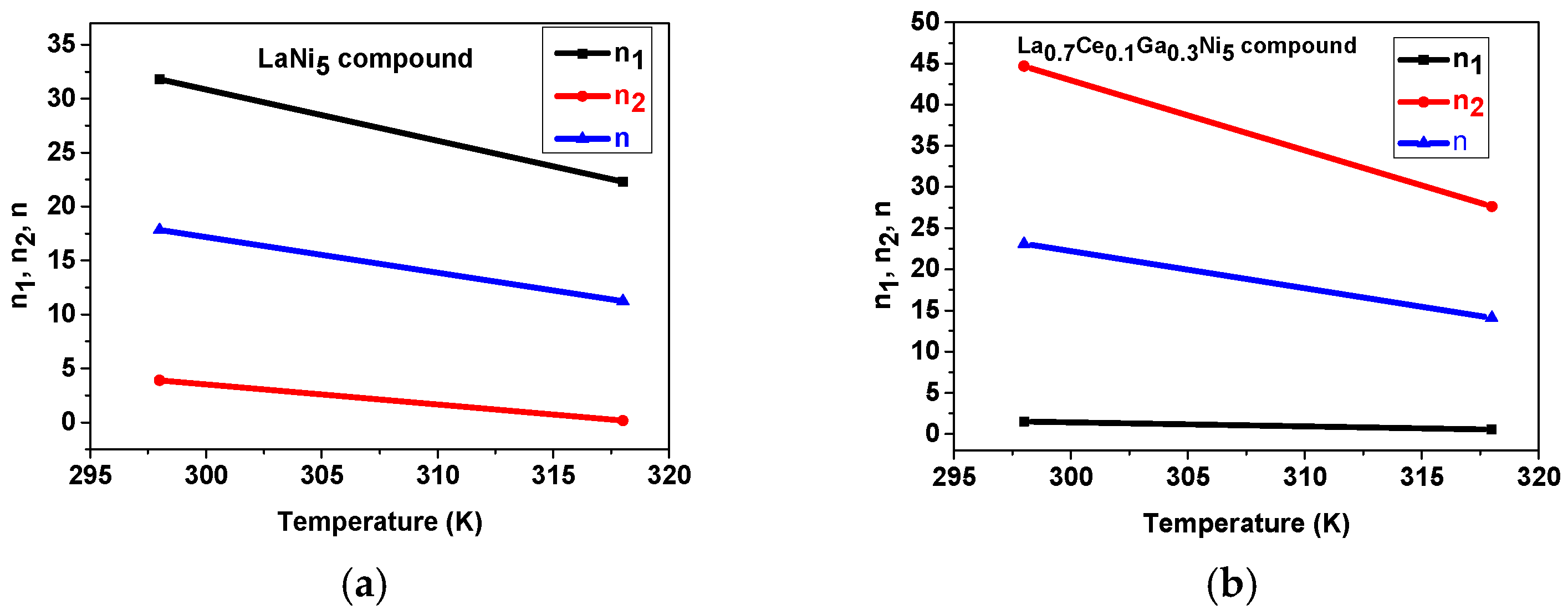
4.1. Adjustment of Experimental Isotherms
4.2. Energy Analysis: Absorption Energies
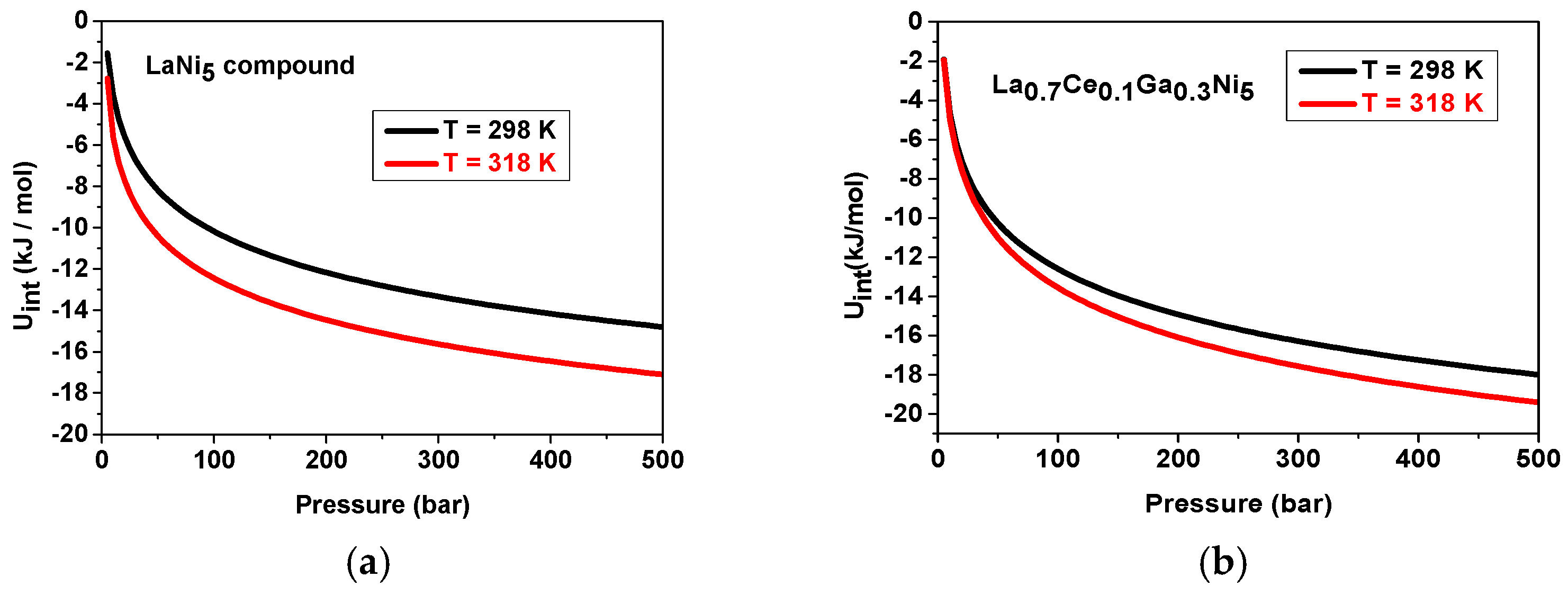
- Thermodynamic properties: Internal and Gibbs energies
- Internal energy
- Gibbs Energy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shabani, B.; Andrews, J. Standalone solar-hydrogen systems powering fire contingency networks. Int. J. Hydrogen Energy 2015, 27, 5509–5517. [Google Scholar] [CrossRef]
- Barahona, I.; Almulhim, T. Renewable energies and circular economies: A systematic literature review before the ChatGPT boom. Energy Rep. 2024, 11, 2656–2669. [Google Scholar] [CrossRef]
- Assaf, J.; Shabani, B. Economic analysis and assessment of a standalone solar-hydrogen combined heat and power system integrated with solar-thermal collectors. Int. J. Hydrogen Energy 2016, 41, 18389–18404. [Google Scholar] [CrossRef]
- Talukdar, M.; Blum, P.; Heinemann, N.; Miocic, J. Techno-economic analysis of underground hydrogen storage in Europe. iScience 2024, 27, 108771. [Google Scholar] [CrossRef]
- Yin, L.; Yang, H.; Ju, Y. Review on the key technologies and future development of insulation structure for liquid hydrogen storage tanks. Int. J. Hydrogen Energy 2024, 57, 1302–1315. [Google Scholar] [CrossRef]
- Morales-Ospino, R.; Celzard, A.; Fierro, V. Strategies to recover and minimize boil-off losses during liquid hydrogen storage. Renew. Sustain. Energy Rev. 2023, 182, 113360. [Google Scholar] [CrossRef]
- Drawer, C.; Lange, J.; Kaltschmitt, M. Metal hydrides for hydrogen storage—Identification and evaluation of stationary and transportation applications. J. Energy Storage 2024, 77, 109988. [Google Scholar] [CrossRef]
- Malleswararao, K.; Kumar, P.; Dutta, P.; Murthy, S.S. Experimental investigations on a coupled metal hydride based thermal energy storage system. Int. J. Hydrogen Energy 2024, 56, 1371–1383. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, X.; Xu, Z.; Tsui, G.; Zhou, Q.; Tang, R.; Xiao, F.; Chan, K. Development of AB2-type TiZrCrMnFeCoV intermetallic high-entropy alloy for reversible room-temperature hydrogen storage. J. Energy Storage 2024, 57, 109553. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V. Laves type intermetallic compounds as hydrogen storage materials: A review. J. Alloys Compd. 2022, 916, 165219. [Google Scholar] [CrossRef]
- Ugaddan, E.; Violi, D.; Fiume, V.; Barale, J.; Luetto, C.; Rizzi, P.; Baricco, M. AB5-based metal hydride embedded in polyethylene and polymethylmethacrylate for hydrogen storage. Int. J. Hydrogen Energy 2024, 78, 952–961. [Google Scholar] [CrossRef]
- Lys, A.; Fadonougbo, J.O.; Faisal, M.; Suh, J.-Y.; Lee, Y.-S.; Shim, J.-H.; Park, J.; Cho, Y.W. Enhancing the Hydrogen Storage Properties of AxBy Intermetallic Compounds by Partial Substitution: A Short Review. Hydrogen 2020, 1, 38–63. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Zhao, Y.; Zhang, X.; Ma, H.; He, X.; Zhou, S.; Wang, L.; Yan, H. Composition design of (LaCeCa)1(NiMnAl)5 alloys by uniform design method and their hydrogen storage performance. J. Alloy. Metall. Syst. 2023, 2, 100006. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Z.; Zheng, J.; Li, X.; Akiba, E.; Li, H.-W. Perspectives and challenges of hydrogen storage in solid-state hydrides. Chin. J. Chem. Eng. 2021, 29, 1–12. [Google Scholar] [CrossRef]
- Joubert, J.-M.; Paul-Boncour, V.; Cuevas, F.; Zhang, J.; Latroche, M. LaNi5 related AB5 compounds: Structure, properties and applications. J. Alloys Compd. 2021, 862, 158163. [Google Scholar] [CrossRef]
- Spodaryk, M.; Gasilova, N.; Züttel, A. Hydrogen storage and electrochemical properties of LaNi5-xCux hydride-forming alloys. J. Alloys Compd. 2019, 775, 175–180. [Google Scholar] [CrossRef]
- Mansouri, M.; Shtender, V.; Tunsu, C.; Yilmaz, D.; Messaoudi, O.; Ebin, B.; Sahlberg, M.; Petranikova, M. Production of AB5 materials from spent Ni-MH batteries with further tests of hydrogen storage suitability. J. Power Sources 2022, 539, 231459. [Google Scholar] [CrossRef]
- Liu, Y.; Chabane, D.; Elkedim, O. Optimization of LaNi5 hydrogen storage properties by the combination of mechanical alloying and element substitution. Int. J. Hydrogen Energy 2024, 53, 394–402. [Google Scholar] [CrossRef]
- Kumar, A.; Muthukumar, P.; Sharma, P.; Kumar, E.A. Absorption based solid state hydrogen storage system: A review. Sustain. Energy Technol. Assess. 2022, 52, 102204. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Gbadamosi, A.O.; Epelle, E.I.; Abdulrasheed, A.A.; Haq, B.; Patil, S.; Al-Shehri, D.; Kamal, M.S. Hydrogen production, transportation, utilization, and storage: Recent advances towards sustainable energy. J. Energy Storage 2023, 73, 109207. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, S.; Lu, H.; Wu, J.; Yan, K.; Cheng, H.; Liu, J. Stability of LaNi5-xCox alloys cycled in hydrogen—Part 1 evolution in gaseous hydrogen storage performance. Int. J. Hydrogen Energy 2019, 44, 15159–15172. [Google Scholar] [CrossRef]
- Liu, J.; Li, K.; Cheng, H.; Yan, K.; Wang, Y.; Liu, Y.; Jin, H.; Zheng, Z. New insights into the hydrogen storage performance degradation and Al functioning mechanism of LaNi5-xAlx alloys. Int. J. Hydrogen Energy 2017, 42, 24904–24914. [Google Scholar] [CrossRef]
- Wan, C.; Zhao, S.; Wang, H. Tuning phase structure and electrochemical hydrogen storage properties of A5B19-type La–Y–Ni–Mn-based superlattice alloys by partial Al substitution. Int. J. Hydrogen Energy 2024, 49, 51–58. [Google Scholar] [CrossRef]
- Lv, W.; Yuan, J.; Zhang, B.; Wu, Y. Influence of the substitution Ce for La on structural and electrochemical characteristics of La0.75-xCexMg0.25Ni3Co0.5 (x=0, 0.05, 0.1, 0.15, 0.2 at. %) hydrogen storage alloys. J. Alloys Compd. 2018, 730, 360–368. [Google Scholar] [CrossRef]
- Tarasov, B.; Bocharnikov, M.; Yanenko, Y.; Fursikov, P.; Lototskyy, M. Cycling stability of RNi5 (R = La, La+Ce) hydrides during the operation of metal hydride hydrogen compressor. Int. J. Hydrogen Energy 2018, 43, 4415–4427. [Google Scholar] [CrossRef]
- Wen, X.; Wang, B.; Li, C.; Liu, T. Effect of La doping on the structural stability and hydrogen adsorption behavior of Ce-La alloys. J. Alloys Compd. 2024, 982, 173553. [Google Scholar] [CrossRef]
- Silvestri, L.; Forcina, A.; Silvestri, C.; Traverso, M. Circularity potential of rare earths for sustainable mobility: Recent developments, challenges and future prospects. J. Clean. Prod. 2021, 292, 26089. [Google Scholar] [CrossRef]
- Cha, S.; Kim, J.; Yun, J.; Rhyee, J. Magnetocaloric and hydrogen storage multi-functional properties of Eu4Ga8Ge16 compounds. J. Alloys Compd. 2023, 968, 17217. [Google Scholar] [CrossRef]
- Qin, C.; Wang, H.; Jiang, W.; Liu, J.; Ouyang, L.; Zhu, M. Comparative study of Ga and Al alloying with ZrFe2 for high-pressure hydrogen storage. Int. J. Hydrogen Energy 2022, 47, 13409–13417. [Google Scholar] [CrossRef]
- Janjua, M.R.S.A. Hydrogen as an energy currency: Encapsulation of inorganic Ga12N12 with alkali metals for efficient H2 adsorption as hydrogen storage materials. J. Phys. Chem. Solids 2022, 160, 110352. [Google Scholar] [CrossRef]
- Oueslati, K.; Sakly, A.; Lima, E.C.; Ayachi, F.; Ben Lamine, A. Statistical physics modeling of the removal of Resorcinol from aqueous effluents by activated carbon from avocado seeds. J. Mol. Liq. 2022, 63, 119386. [Google Scholar] [CrossRef]
- Wjihi, S.; Sellaoui, L.; Bouzid, M.; Dhaou, H.; Knani, S.; Jemni, A.; Ben Lamine, A. Theoretical study of hydrogen sorption on LaNi5 using statistical physics treatment: Microscopic and macroscopic investigation. Int. J. Hydrogen Energy 2017, 42, 2699–2712. [Google Scholar] [CrossRef]
- Prasad, J.S.; Muthukumar, P. Performance and energy efficiency of a solid-state hydrogen storage system: An experimental study on La0.7Ce0.1Ca0.3Ni5. Appl. Therm. Eng. 2022, 216, 119030. [Google Scholar] [CrossRef]
- Ge, Y.T. Characterisation of pressure-concentration-temperature profiles for metal hydride hydrogen storage alloys with model development. Energy Storage 2024, 6, 504. [Google Scholar] [CrossRef]
- Bouaziz, N.; Briki, C.; Dhahri, E.; Jemni, A.; Ben Lamine, A. Experimental and theoretical investigation of absorption and desorption of hydrogen in the LaNi4Co0.5Mn0.5 alloy. Chem. Eng. Sci. 2022, 251, 117453. [Google Scholar] [CrossRef]
- Schwarzer, M.; Schwarzer, M.; Hertl, N.; Hertl, N.; Nitz, F.; Nitz, F.; Borodin, D.; Borodin, D.; Fingerhut, J.; Fingerhut, J.; et al. Adsorption and Absorption Energies of Hydrogen with Palladium. J. Physic Chem. 2022, 126, 14500–14508. [Google Scholar] [CrossRef] [PubMed]
- Ziemiański, P.P.; Derkowski, A. Structural and textural control of high-pressure hydrogen adsorption on expandable and non-expandable clay minerals in geologic conditions. Int. J. Hydrogen Energy 2022, 47, 28794–28805. [Google Scholar] [CrossRef]
- Lam, S.T.; Ballinger, R.; Forsberg, C. Modeling and predicting total hydrogen adsorption in nanoporous carbon materials for advanced nuclear systems. J. Nucl. Mater. 2018, 511, 328–340. [Google Scholar] [CrossRef]
- Knani, S.; Mathlouthi, M.; Ben Lamine, A. Modeling of the psychophysical responsecurves using the grand canonical ensemble in statistical physics. Food Biophys. 2007, 2, 183–192. [Google Scholar] [CrossRef]
- Belkhiria, S.; Alsawi, A.; Briki, C.; Altarifi, S.M.; Dhaou, M.H.; Jemni, A. Physical Analysis and Mathematical Modeling of the Hydrogen Storage Process in the MmNi4.2Mn0.8 Compound. Materials 2024, 17, 2237. [Google Scholar] [CrossRef]
- Mechi, N.; Khemis, I.B.; Dhaou, H.; Zghal, S.; Lamine, A.B. Morphologic, Structural, Steric, Energetic and Thermodynamic Studies of the Mechanical Alloy Mg50Ni45Ti5 for Hydrogen Storage. J. Phys. Chem. J. Biophys. 2017, 7, 2–11. [Google Scholar] [CrossRef]
- Bouaziz, N.; Ben Manaa, M.; Ben Lamine, A. Theoretical study of hydrogen absorption-desorption on LaNi3.8Al1.2-xMnx using statistical physics treatment. Phys. B 2017, 525, 46–59. [Google Scholar] [CrossRef]
- Bouaziz, N.; Ben Torkia, Y.; Aouaini, F.; Nakbi, A.; Dhaou, H.; Ben Lamine, A. Statistical physics modeling of hydrogen absorption onto LaNi4.6Al0.4: Stereographic and energetic interpretations. Sep. Sci. Technol. 2019, 54, 1520–5754. [Google Scholar] [CrossRef]
- Wjihi, S.; Dhaou, H.; Ben Yahia, M.; Knani, S.; Jemni, A.; Ben Lamine, A. Statistical physics modeling of hydrogen desorption from LaNi4.75Fe0.25: Stereographic and energetic interpretations. Phys. B 2015, 497, 112–120. [Google Scholar] [CrossRef]
- Belkhiria, S.; Briki, C.; Dhaou, M.H.; Jemni, A. Experimental and numerical study of hydrogen adsorption by the Ni0.6Mg0.4Fe2O4 compound. Int. J. Hydrogen Energy 2024, 64, 990–1000. [Google Scholar] [CrossRef]

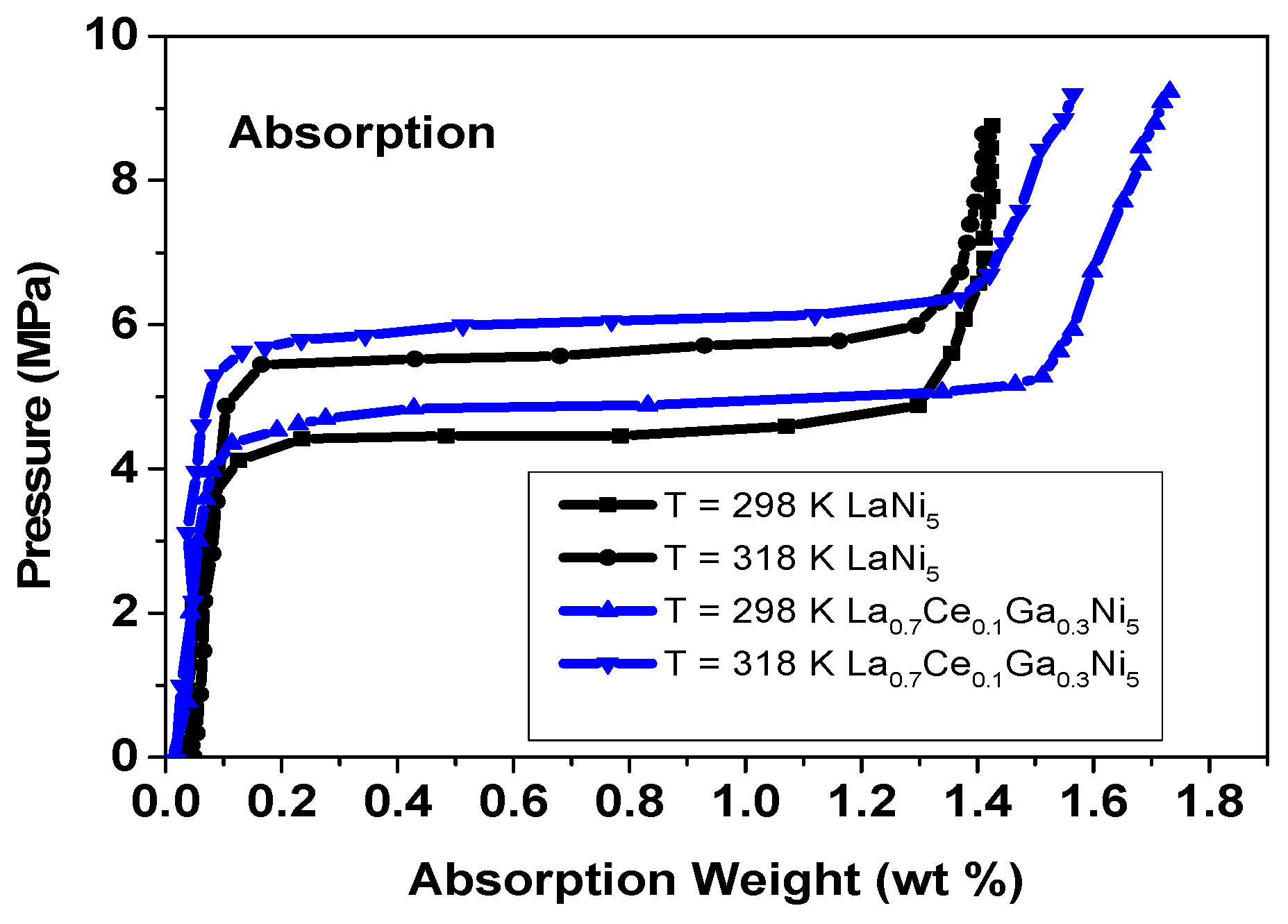




| Compound | Temperature (K) | Peq (Absorption) (bar) | Peq (Desorption) (bar) | Hys |
|---|---|---|---|---|
| LaNi5 | 298 | 4.50 | 4.00 | 0.11 |
| 318 | 5.60 | 5.20 | 0.07 | |
| La0.7Ce0.1Ga0.3Ni5 | 298 | 4.88 | 4.45 | 0.09 |
| 318 | 6.05 | 5.75 | 0.05 |
| LaNi5 Compound | ||||||||
|---|---|---|---|---|---|---|---|---|
| T (K) | n1 | n2 | n | Nm1 | Nm2 | P1 | P2 | R-Square |
| 298 | 31.81 | 3.88 | 17.84 | 0.04 | 0.022 | 4.52 | 1.80 | 0.98 |
| 318 | 22.29 | 0.17 | 11.23 | 0.06 | 0.22 | 5.62 | 0.26 | 0.98 |
| La0.7Ce0.1Ga0.3Ni5 Compound | ||||||||
| 298 | 1.50 | 44.65 | 23.08 | 0.09 | 0.03 | 2.71 | 4.92 | 0.98 |
| 318 | 0.54 | 27.62 | 14.08 | 0.12 | 0.05 | 0.87 | 6.07 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belkhiria, S.; Alsawi, A.; Hraiech, I.; Dhaou, M.H.; Jemni, A. Experimental and Mathematical Investigation of Hydrogen Absorption in LaNi5 and La0.7Ce0.1Ga0.3Ni5 Compounds. Metals 2024, 14, 967. https://doi.org/10.3390/met14090967
Belkhiria S, Alsawi A, Hraiech I, Dhaou MH, Jemni A. Experimental and Mathematical Investigation of Hydrogen Absorption in LaNi5 and La0.7Ce0.1Ga0.3Ni5 Compounds. Metals. 2024; 14(9):967. https://doi.org/10.3390/met14090967
Chicago/Turabian StyleBelkhiria, Sihem, Abdulrahman Alsawi, Ibtissem Hraiech, Mohamed Houcine Dhaou, and Abdelmajid Jemni. 2024. "Experimental and Mathematical Investigation of Hydrogen Absorption in LaNi5 and La0.7Ce0.1Ga0.3Ni5 Compounds" Metals 14, no. 9: 967. https://doi.org/10.3390/met14090967






