Realization of Bio-Coal Injection into the Blast Furnace
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. BF Operational Trials
2.2.2. Dust Sampling and Characterization
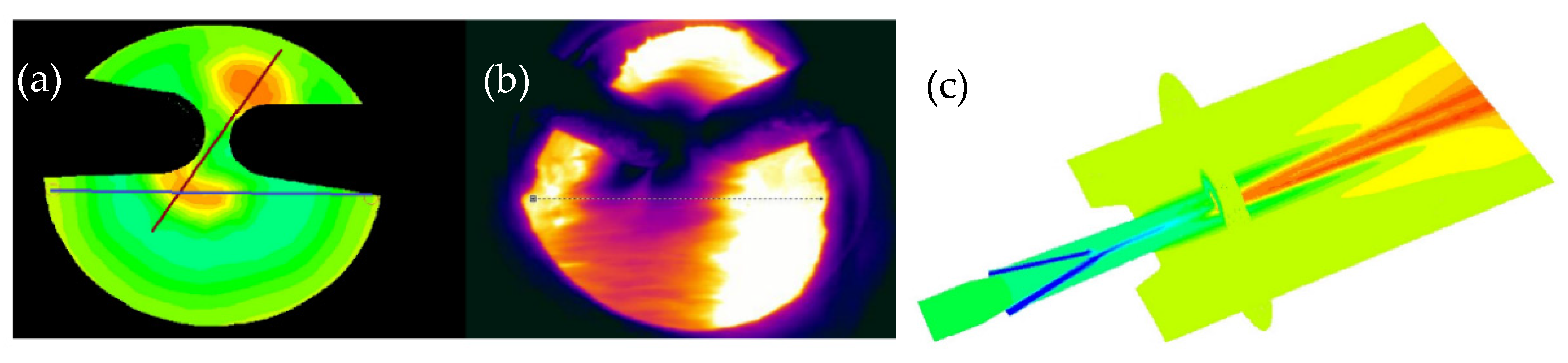
2.2.3. Raceway Monitoring
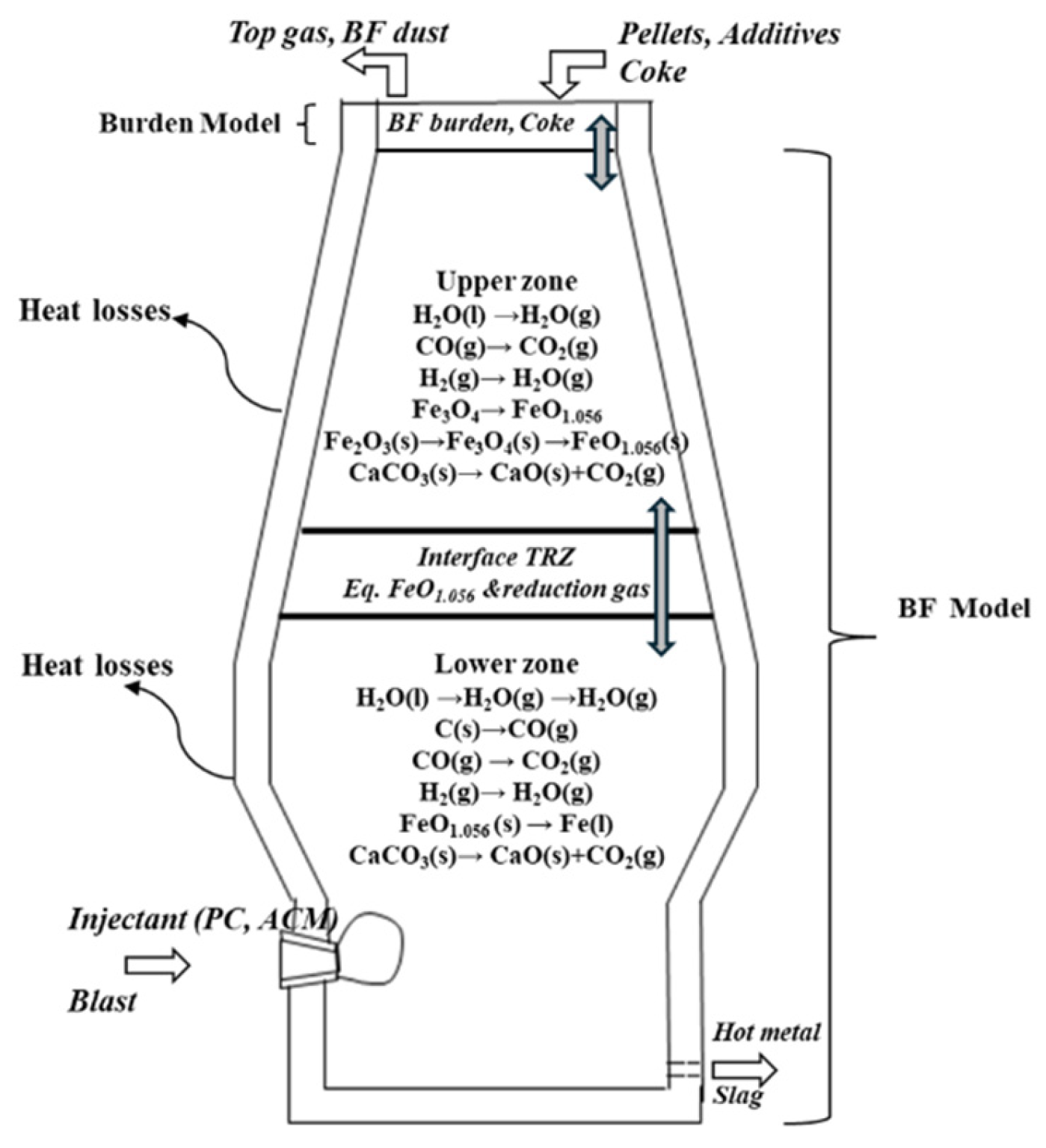
2.2.4. BF Trial Evaluation and Theoretical Prediction of the Effects of Bio-Coal Injection
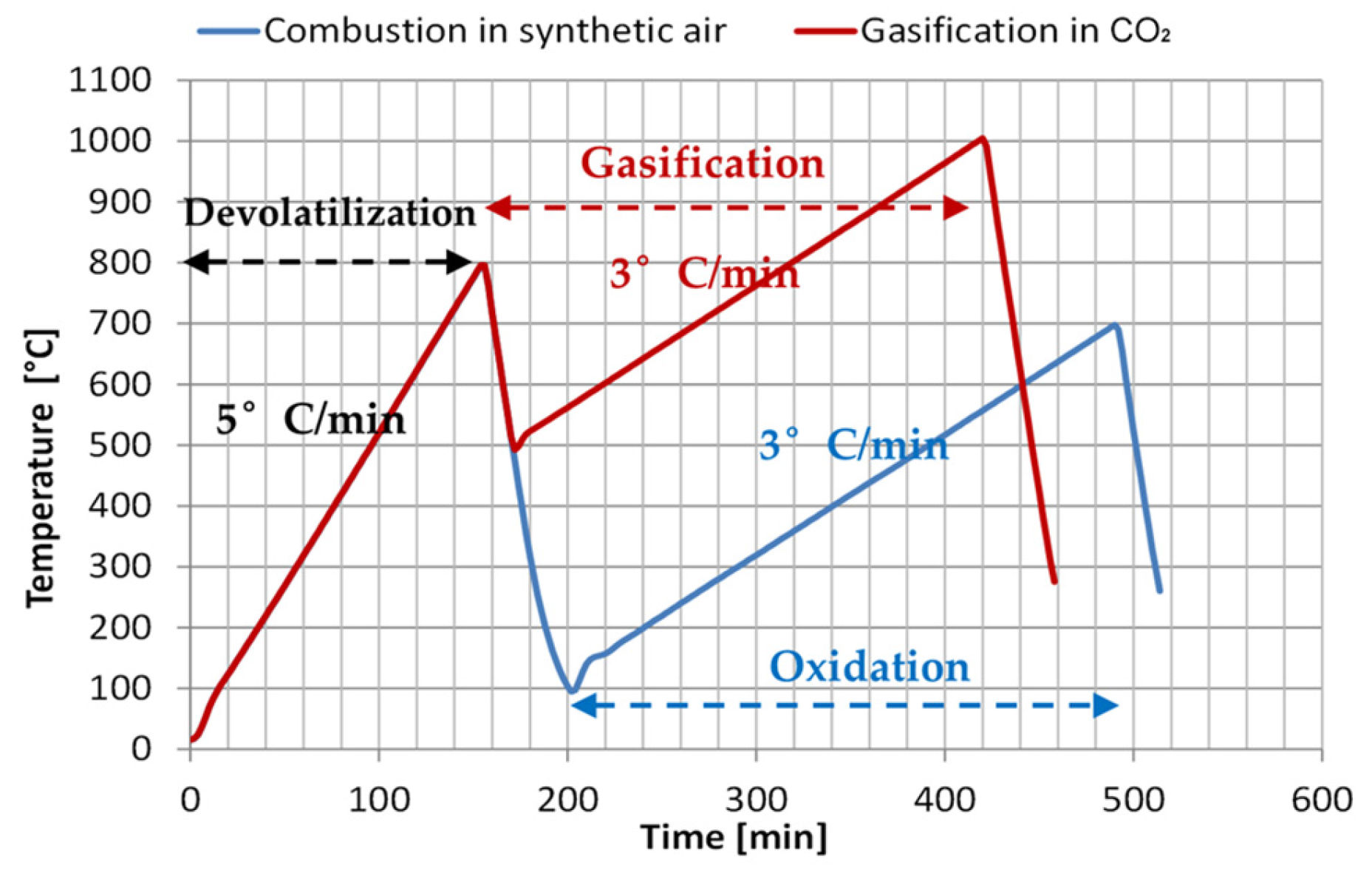
2.2.5. Reaction Modelling of Carbonaceous Materials
2.2.6. CFD Modelling on Pulverized Injection of Bio-Coal with PC
3. Results
3.1. BF Operational Trials Results
3.2. Effect on the PCI Plant
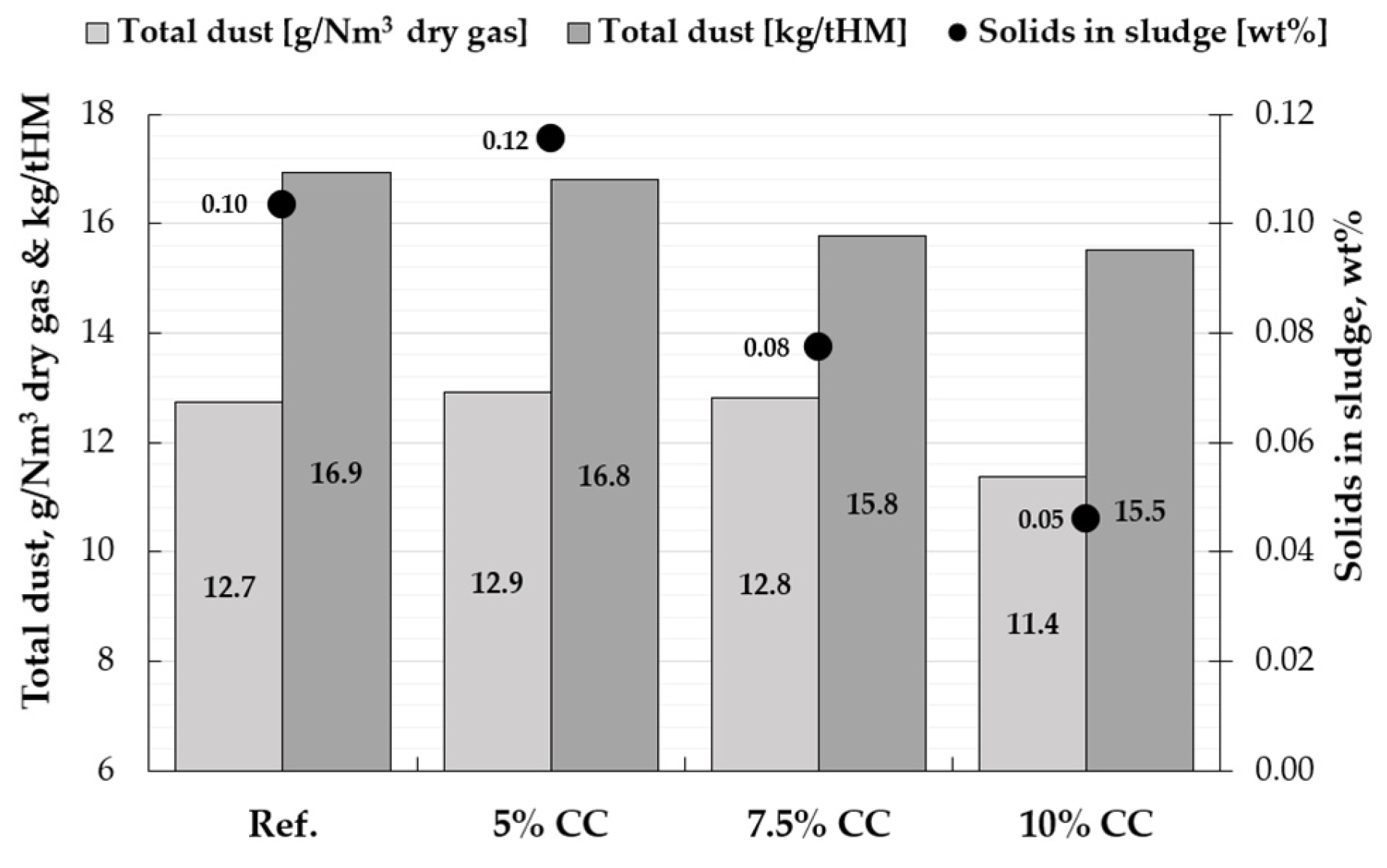
3.3. Dust Formation and Characteristics
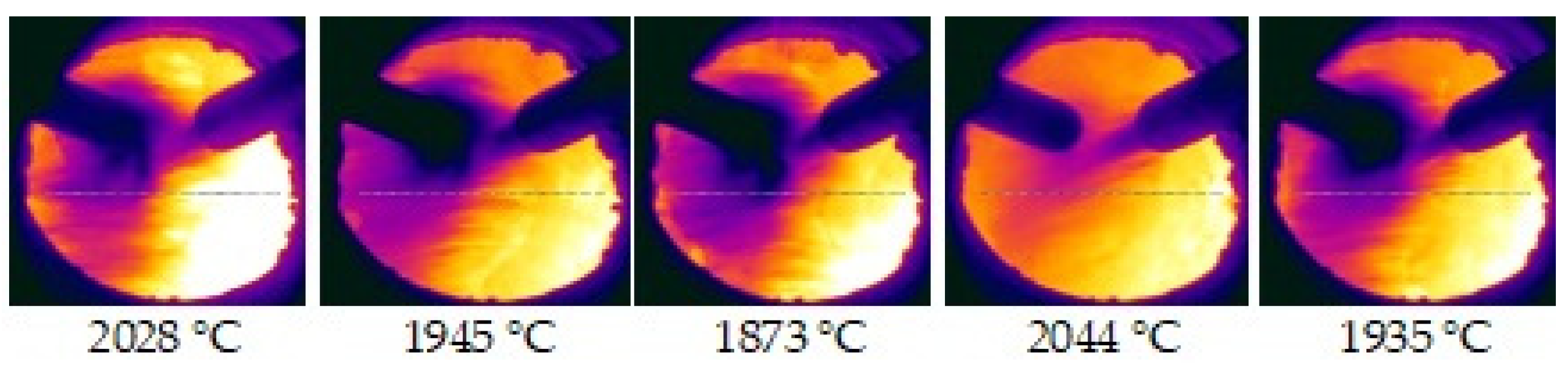
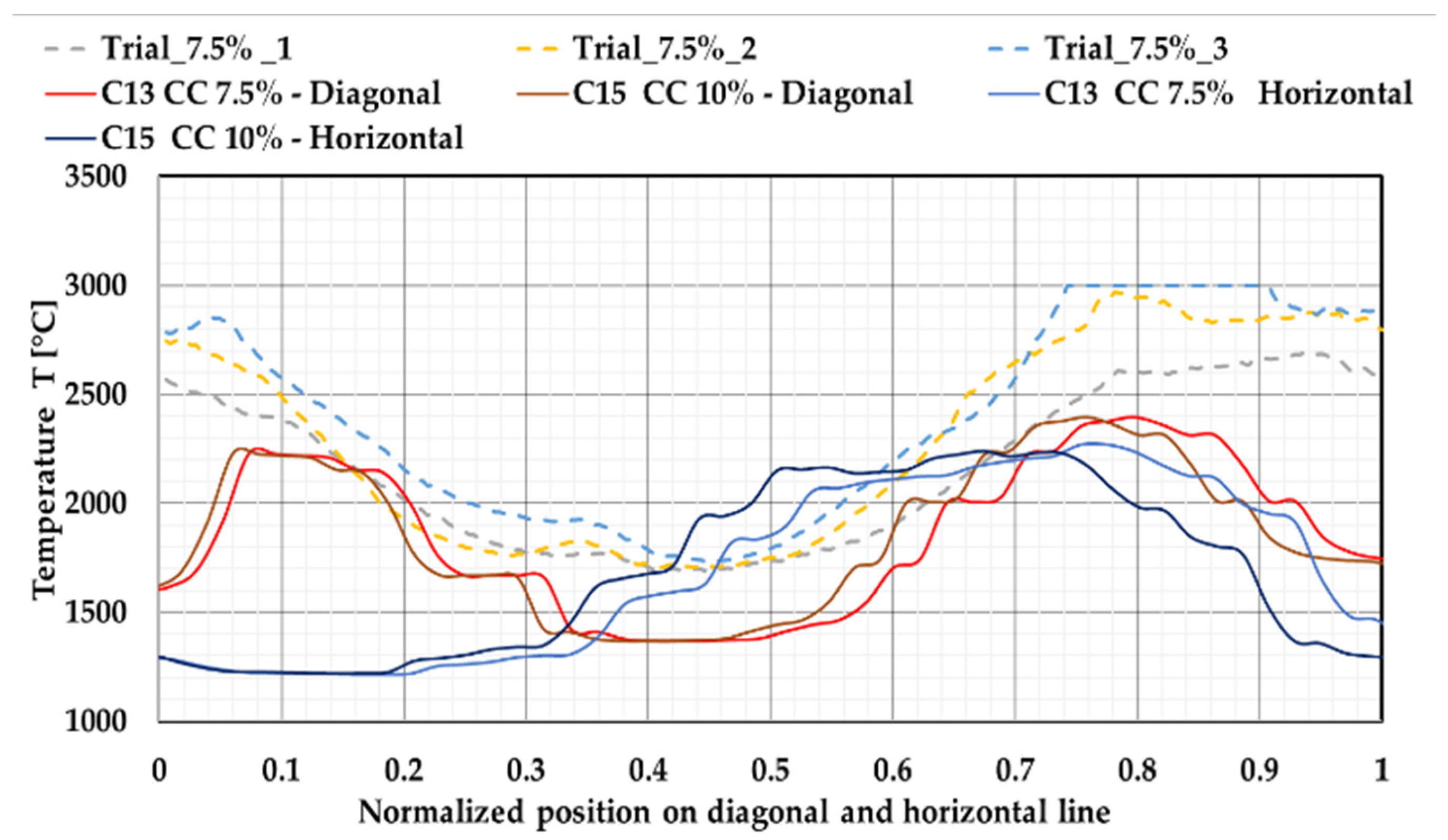
3.4. Raceway Monitoring
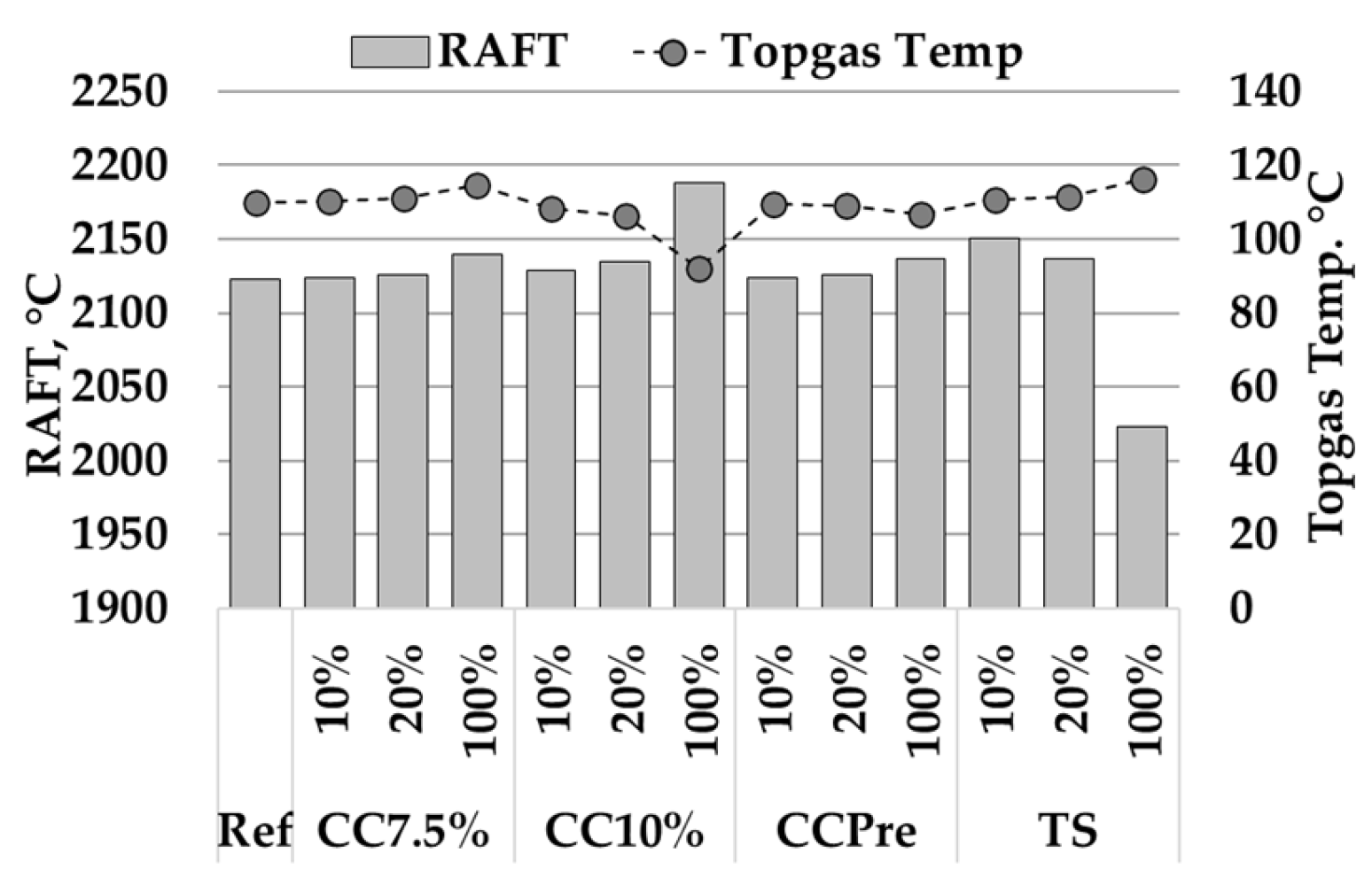
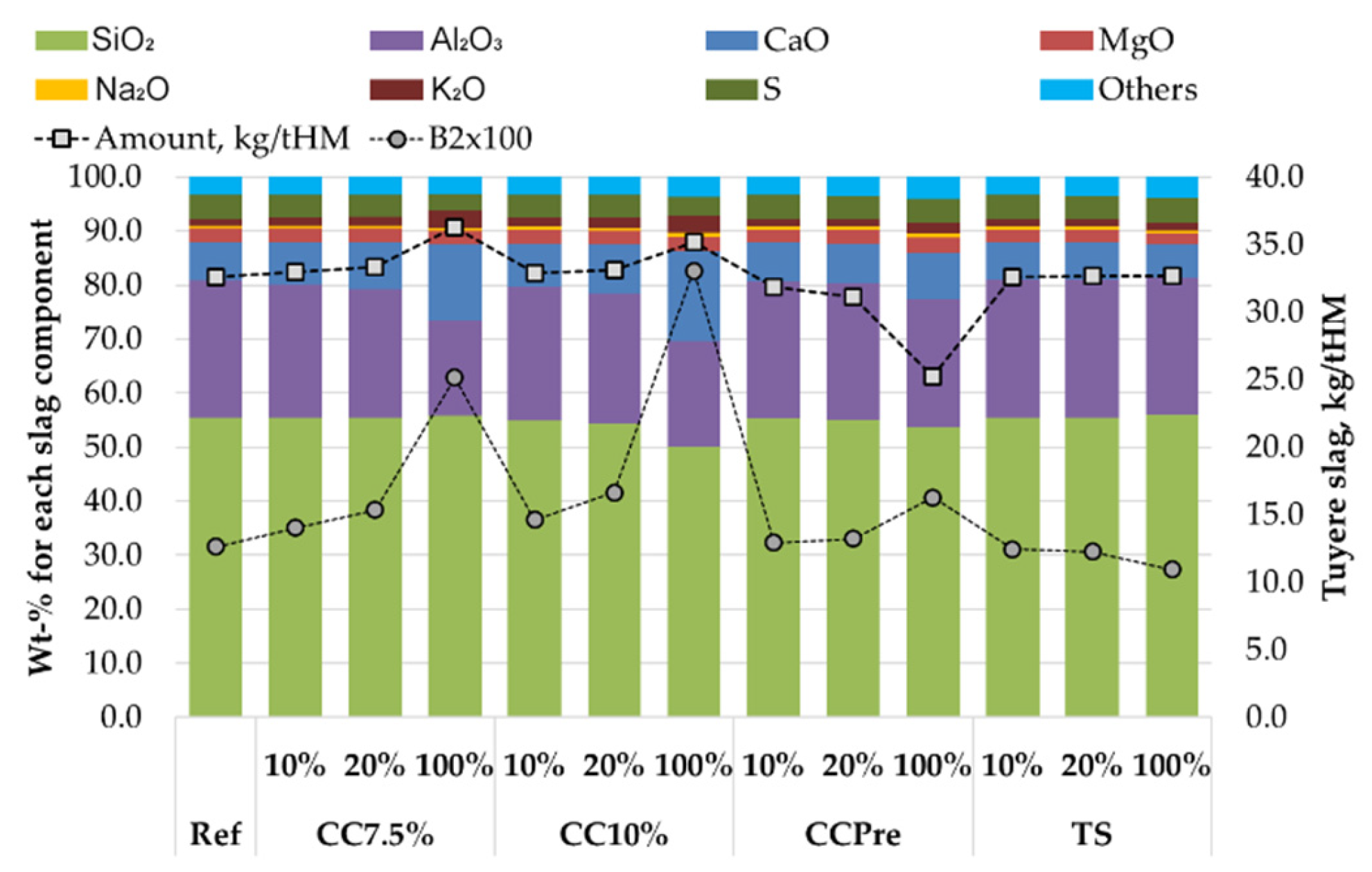
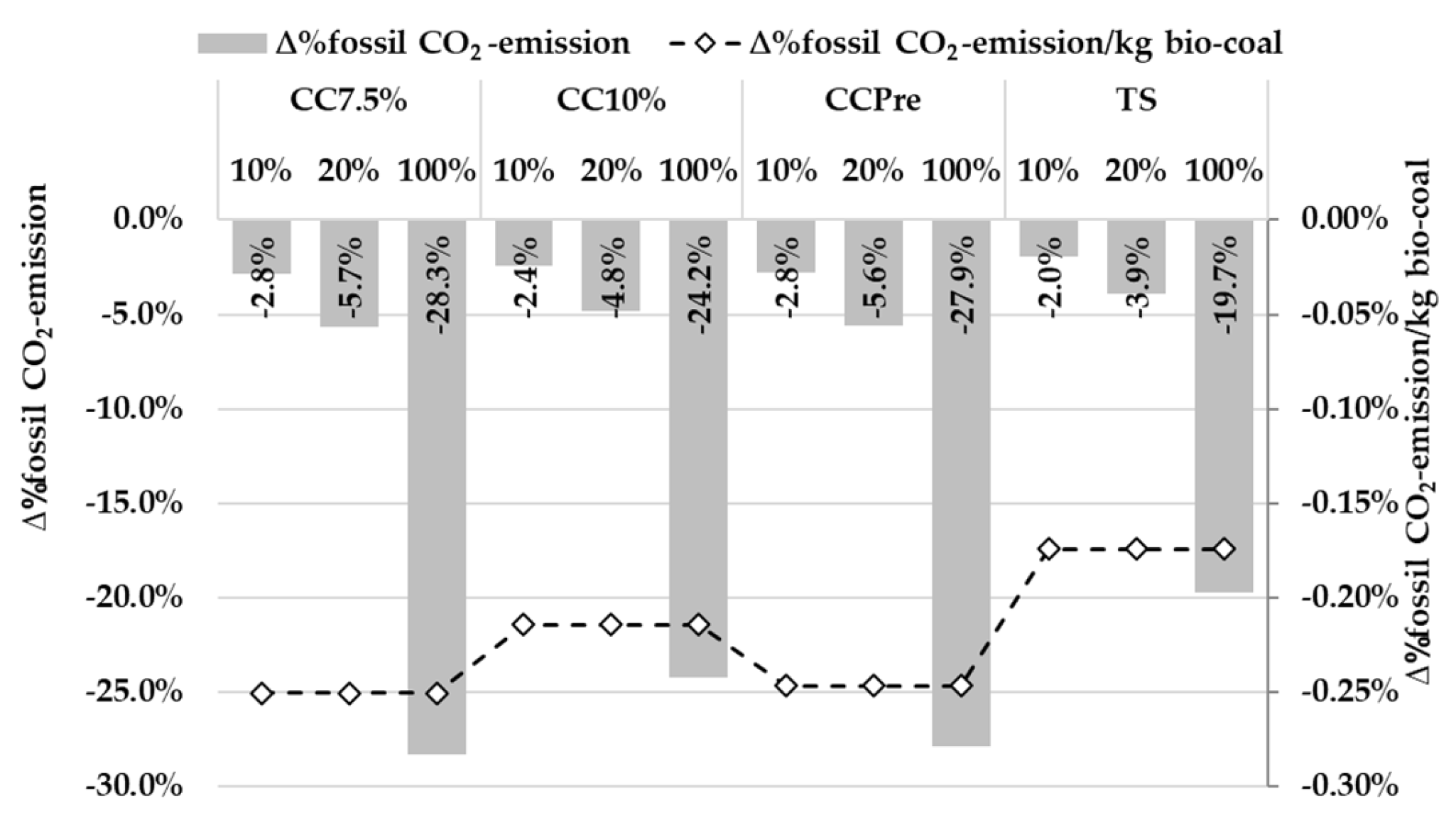
3.5. Evaluation of Bio-Coal Impact on the BF in Heat and Mass Balance Model
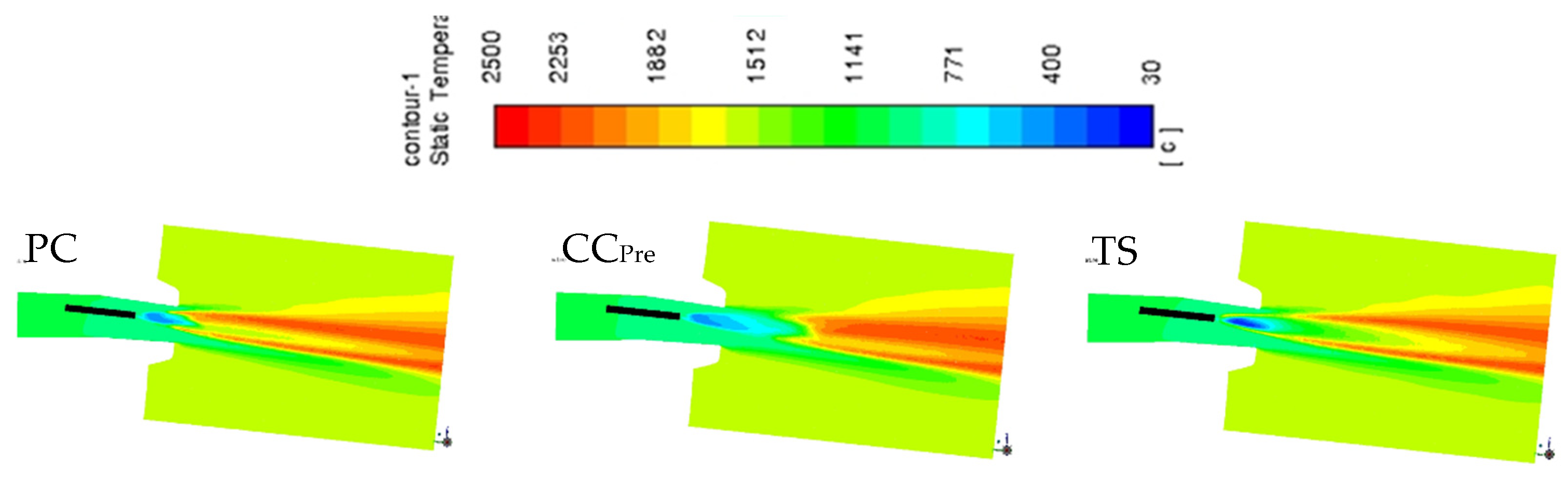
3.6. CFD Modelling
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- 2021 World Steel in Figures; World Steel Association: Brussels, Belgium, 2021.
- Steel’s Contribution to A Low Carbon Future and Climate Resilient Societies—World Steel Position Paper. World Steel Association. 2020. Available online: https://www.worldsteel.org/en/dam/jcr:7ec64bc1-c51c-439b-84b8-94496686b8c6/Position_paper_ climate_2020_final.pdf (accessed on 28 November 2021).
- European Commission Energy 2030 Strategy and the 2050 Energy Roadmap. Available online: https://ec.europa.eu/energy/taxonomy/energy-strategy_en (accessed on 28 November 2021).
- Fact Sheet. Hydrogen (H2)-Based Ironmaking; World Steel Association: Brussels, Belgium, 2021. [Google Scholar]
- HYBRIT. Available online: https://www.hybritdevelopment.com/ (accessed on 20 December 2020).
- H2 Green Steel. Available online: https://www.h2greensteel.com/ (accessed on 28 November 2021).
- Available online: https://corporate.arcelormittal.com/climate-action/decarbonisation-technologies/hamburg-h2-working-towards-the-production-of-zero-carbon-emissions-steel-with-hydrogen (accessed on 1 August 2024).
- Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 28 November 2021).
- Available online: https://www.voestalpine.com/greentecsteel/en/breakthrough-technologies/ (accessed on 1 August 2024).
- Lavelaine de Maubeuge, H.; van der Laan, S.; Hita, A.; Olsen, K.; Serna, M.; Haarberg, G.M.; Frade, J. Iron Production by Electrochemical Reduction of Its Oxide for High CO2 Mitigation (IERO); EUR 28065 EN; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Available online: https://www.siderwin-spire.eu/ (accessed on 1 August 2024).
- Available online: https://www.thyssenkrupp-steel.com/en/newsroom/press-releases/thyssenkrupp-steel-concludes-first-test-phase-successfully.html (accessed on 1 August 2024).
- Watakabe, S.; Miyagawa, K.; Matsuzaki, S.; Inada, T.; Tomita, Y.; Saito, K.; Osame, M.; Sikström, P.; Sundqvist Ökvist, L.; Wikström, J.-O. Operation Trial of Hydrogenous Gas Injection of COURSE50 Project at an Experimental Blast Furnace. ISIJ Int. 2013, 53, 2065–2071. [Google Scholar] [CrossRef]
- Bittencourt Marques, M.; Rodrigues Assis, A.; Benício Dias, S.M.; Harley Araújo, F.; Junqueira dos Santos, R. Co-injeção de gás natural moinha de carvão vegetal e carvão mineral no alto-forno “A” da Arcelormittal Monlevade. In Proceedings of the 41 Seminário de Redução de Minério de Ferro e Matérias-Primas Conference, Vila Vehla, Brazil, 12–16 September 2011. [Google Scholar]
- Mathieson, J.G.; Somerville, M.; Deev, A.; Jahanshahi, S. Chapter 19 Utilization of biomass as an alternative fuel in ironmaking. In Iron Ore: Mineralogy. Processing and Environmental Sustainability, 1st ed.; Lu, L., Ed.; Woodhead Publ. Elsevier Ltd.: Cambridge, UK; Waltham, MA, USA, 2015; pp. 581–609. [Google Scholar] [CrossRef]
- Mousa, E.; Lundgren, M.; Sundqvist Ökvist, L.; From, L.-E.; Robles, A.; Hällsten, S.; Sundelin, B.; Friberg, H.; El-Tawil, A. Reduced Carbon Consumption and CO2 Emission at the Blast Furnace by Use of Briquettes Containing Torrefied Sawdust. J. Sustain. Met. 2019, 5, 391–401. [Google Scholar] [CrossRef]
- Sundqvist Ökvist, L.; Hu, X.; From, L.-E.; Sandström, D.; Ölund, M.; Hirsch., A.; Mittelstädt, M.; Bolle, M.; Hensmann, M.; Möhring, S.; et al. Improved Coal Combustion under Variable Blast Furnace Conditions (IMPCO); Final Report EUR 29519 EN; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-98296-5. [Google Scholar]
- Sundqvist Ökvist, L.; From, L.-E.; Ölund, M.; Orre, J.; Sundelin, B.; Ahmed, H. Lowering of CO2 Emissions at the BF by Using Bio-coal—Theoretical and Practical Possibilities and Limitations. In Proceedings of the Association for Iron and Steel AISTech 2018 Conference, Philadelphia, PA, USA, 7–10 May 2018. [Google Scholar]
- Swedish Energy Agency. Reduced CO2 Emission through Designed Bio-Coal in the Residue Briquette for the Blast Furnace (MICO); 48843-1; Swedish Energy Agency: Eskilstuna, Sweden, 2023. [Google Scholar]
- El-Tawil, A. Influence of the Properties of Bio-Coal as a Substitute for Fossil Coal in Carbon Composite Agglomerates and in Coke. Ph.D. Thesis, Luleå University of Technology, Luleå, Sweden, 2022. Available online: https://urn.kb.se/resolve?urn=urn:nbn:se:ltu:diva-88817 (accessed on 1 August 2024).
- Available online: https://cinea.ec.europa.eu/featured-projects/fresme_en (accessed on 28 November 2021).
- Available online: https://www.thyssenkrupp.com/en/newsroom/content-page-162.html (accessed on 1 August 2024).
- Available online: http://www.steelanol.eu/en (accessed on 28 November 2021).
- Available online: https://www.initiate-project.eu/initiate/ (accessed on 28 November 2021).
- Noldin, J.H. Energy efficiency and CO2 reduction in the Brazil steel industry. In Proceedings of the METEC InsteelCon 2011, 1st International Conference on Energy Efficiency and CO2 Reduction in the Steel Industry, Dusseldorf, Germany, 28–30 June 2011. [Google Scholar]
- Nascimento, R.; Almeida, A.; Olivera, E.; De Jesus, A.; De Moraes, A. 18 months of charcoal fines injection into Gusa Nordeste’s (1) blast furnaces. In Proceedings of the 3rd International Meeting on Ironmaking, ABM, Sao Paolo, Brazil, 1–4 October 2018. [Google Scholar]
- Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quiro, M. Crystallography open database: An open-access collection of crystal structures cif applications. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar]
- Hooey, P.L.; Bodén, A.; Wang, C.; Grip, C.-E.; Jansson, B. Design and Application of a Spreadsheet-based Model of the Blast Furnace Factory. ISIJ Int. 2010, 50, 924–930. [Google Scholar] [CrossRef]
- Badzioch, S.; Hawksley, P.G.W. Kinetics of thermal Decomposition of Pulverized Coal Particles. Ind. Eng. Chem. Process. Des. Develop. 1970, 9, 521–530. [Google Scholar] [CrossRef]
- Magnussen, B.F.; Hjertager, B.H. On mathematical models of turbulent combustion with special emphasis on soot formation and combustion. In Proceedings of the 16th Symposium International on Combustion, Cambridge, MA, USA, 15–20 August 1976. [Google Scholar]








| Pre-Trial Evaluated Material | BF Trial Materials | |||||
|---|---|---|---|---|---|---|
| PC 1 | TS | CCPre | PC 2 | CC7.5% | CC10% | |
| CaO | 0.65 | 0.16 | 0.53 | 0.58 | 3.09 | 3.70 |
| MgO | 0.24 | 0.023 | 0.14 | 0.24 | 0.35 | 0.40 |
| SiO2 | 3.84 | 0.03 | 0.50 | 4.01 | 6.55 | 3.23 |
| Al2O3 | 1.92 | 0.01 | 0.12 | 1.99 | 0.52 | 0.47 |
| K2O | 0.14 | 0.07 | 0.22 | 0.15 | 0.75 | 0.73 |
| S | 0.28 | 0.01 | 0.02 | 0.26 | 0.04 | 0.05 |
| P | 0.022 | 0.006 | 0.034 | 0.018 | 0.08 | 0.11 |
| Fe | 0.61 | 0.01 | 0.08 | 0.54 | 0.36 | 0.36 |
| C | 81.7 | 57.7 | 87.2 | 80.6 | 88.7 | 75.4 |
| H | 4.1 | 5.8 | 3.2 | 4.1 | 2.7 | 2.5 |
| O | 4.8 | 36.7 | 6.6 | 4.6 | 3.1 | 11.9 |
| N | 2.0 | 0.1 | 0.6 | 2.2 | 0.15 | 0.23 |
| VM | 18.2 | 70.8 | 13.9 | 18.5 | 17.8 | 18.3 |
| CFix | 70.6 | 24.5 | 82.4 | 70.6 | 69.2 | 71.5 |
| Ash | 10.6 | 0.4 | 2.4 | 8.2 | 12.6 | 10.1 |
| H2O | 1.2 1 | 1.2 1 | 1.2 1 | 0.51 2 | 0.51 2 | 0.51 2 |
| HHV db 3 | 31.3 | 21.8 | 32.3 | 32.3 | 31.6 | 29.9 |
| Parameter | Value | Unit | Parameter | Value | Unit |
|---|---|---|---|---|---|
| Production rate | 3100 | tHM/24 h | BF dust inj. | 0–25 | kg/tHM |
| Blast flow | 117.65 | kNm3/h | Blast moisture | 10 | g/Nm3 |
| O2 to blast | 2.64 | kNm3/h | Blast temp. | 1040 | °C |
| O2 to lance | 2.51 | kNm3/h | Coke rate | ~355 | kg/tHM |
| PC and CC inj. | 115 | kg/tHM | Top-gas temp. | 125 | °C |
| O2 enrichment (blast + oxy-coal) | 4.3 | % |
| Devolatilization | (R1) | |
| Homogenous reactions | (R2) | |
| (R3) | ||
| (R4) | ||
| (R5) | ||
| (R6) | ||
| Heterogeneous reactions | (R7) | |
| (R8) | ||
| (R9) |
| PC1 | TS | CCPre | PC2 | CC7.5% | CC10% | ||
|---|---|---|---|---|---|---|---|
| Devolatilization | Start of reaction [°C] | 379 | 220 | 499 | 340 | 341 | 341 |
| Exp. factor [1/s] | 1.11 × 104 | 6.22 × 106 | 2.96 × 104 | 1.80 × 103 | 3.09 × 10−2 | 2.22 × 10−2 | |
| Norm. Exp. factor [1/s] | 3.49 × 109 | 8.22 × 1014 | 6.86 × 109 | 1.96 × 108 | 1.55 | 8.24 × 10−1 | |
| Activation energy [kJ/mol] | 102 | 111 | 126 | 89 | 30 | 28 | |
| Combustion | Exp. factor [s/m] | 2.03 × 10−3 | 8.55 × 10−2 | 1.44 × 10−2 | 5.55 × 10−3 | 3.61 × 10−3 | 3.98 × 10−3 |
| Norm. Exp. factor [s/m] | 2.42 × 102 | 5.16 × 105 | 2.89 × 103 | 2.18 × 103 | 4.39 × 103 | 4.94 × 103 | |
| Activation energy [kJ/mol] | 103 | 114 | 114 | 109 | 94 | 94 | |
| Gasification | Exp. factor [s/m] | 1.54 × 10−3 | 4.77 × 10−3 | 5.66 × 10−2 | 1.01 × 10−3 | 3.66 × 10−3 | 3.82 × 10−3 |
| Norm. Exp. factor [s/m] | 1.16 10 | 4.35 × 102 | 2.80 × 103 | 7.62 | 3.78 × 10 | 1.92 × 102 | |
| Activation energy [kJ/mol] | 184 | 176 | 201 | 170 | 159 | 157 |
| Homogenous Reactions | Heterogeneous Reactions | |||
|---|---|---|---|---|
| A(R2) | 2.12 × 1011 | PC, TS, CCPre, CC7.5%, CC10% | Coke | |
| E(R2) | 2.03 × 108 | Ac(R7) | See Table 4 | 234 |
| A(R3) | 2.24 × 1012 | Ec(R7) | 9.00 × 107 | |
| E(R3) | 1.70 × 1018 | Ac(R8) | 11.0 | |
| A(R4)–(R6) | 1.00 × 1015 | Ec(R8) | 2.40 × 108 | |
| E(R4)–(R6) | 1.00 × 1018 | Ac(R9) | 1.50 | - |
| AEDM | 4.00 | Ec(R9) | 1.50 × 108 | - |
| BEDM | 0.50 | C1 | 5.00 × 10−11 | 7.50 × 10−8 |
| Pre-Trial Evaluation by CFD Modelling | Trial Evaluation by CFD Modelling | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Injection Mix | PC wt.% | Bio wt.% | Energy (kJ/s) | Energy Bio, % | Case | Injection Mix | PC wt.% | Bio wt.% | Energy (kJ/s) | Energy Bio, % |
| 1 | PC1 | 100 | 0 | 5734 | --- | 7 | PC2 | 100 | 0 | 5404 | --- |
| 2 | PC1 and CCPre | 95 | 5 | 5740 | 5.16 | 8 | PC2 and CC7.5% | 92.5 | 7.5 | 5395 | 7.35 |
| 3 | PC1 and CCPre | 80 | 20 | 5757 | 20.2 | 9 | PC2 and CC7.5% | 80 | 20 | 5381 | 19.7 |
| 4 | PC1 and TS | 80 | 20 | 5423 | 15.4 | 10 | PC2 and CC10% | 90 | 10 | 5364 | 9.33 |
| 5 | CCPre | 0 | 100 | 5851 | 100 | 11 | PC2 and CC10% | 80 | 20 | 5364 | 18.65 |
| 6 | TS | 0 | 100 | 4178 | 100 | ||||||
| Ref. | 5% CC | 7.5% CC | 10% CC | Unit | |
|---|---|---|---|---|---|
| Production rate | 3076 | 3082 | 3118 | 3025 | Ton/24 h |
| Blast flow | 117.7 | 117.7 | 117.7 | 115.4 | kNm3/h |
| Blast moisture | 20.9 | 18.3 | 13.2 | 12.8 | g/Nm3 |
| Blast temperature | 1047 | 1039 | 1039 | 1040 | °C |
| O2 to blast | 2.56 | 2.45 | 2.44 | 2.36 | kNm3/h |
| O2 to lance | 2.51 | 2.51 | 2.52 | 2.50 | kNm3/h |
| Total O2 enrichm. | 4.4 | 4.3 | 4.3 | 4.3 | % |
| Total injection rate * | 113.0 | 113.4 | 119.6 | 114.7 | kg/tHM |
| BF dust injection | 15.9 | 16.4 | 15.3 | 15.8 | kg/tHM |
| Coke rate | 353.8 | 352.9 | 348.6 | 351.4 | kg/tHM |
| TGT | 117.1 | 110.6 | 109.9 | 111.3 | °C |
| EtaCO | 55.4 | 55.6 | 55.3 | 55.0 | % |
| RAFT | 1938 | 1937 | 1966 | 1961 | °C |
| HM temperature | 1477 | 1461 | 1471 | 1474 | °C |
| HM Si | 0.68 | 0.62 | 0.60 | 0.63 | % |
| Slag amount # | 150 | 151 | 152 | 152 | kg/tHM |
| Basicity B2 | 0.90 | 0.88 | 0.90 | 0.91 | - |
| Ref. | 5% CC to PC | 7.5% CC to PC | 10% CC to PC | |||
|---|---|---|---|---|---|---|
| CaO | 5.09 | 4.64 | 5.71 | 5.30 | Wt.% | |
| MgO | 2.48 | 2.04 | 2.25 | 2.50 | ||
| SiO2 | 7.71 | 6.96 | 7.20 | 6.86 | ||
| Al2O3 | 4.44 | 3.25 | 3.62 | 3.62 | ||
| Na2O | 0.34 | 0.20 | 0.22 | 0.25 | ||
| K2O | 0.52 | 0.40 | 0.39 | 0.43 | ||
| S | 0.36 | 0.38 | 0.41 | 0.44 | ||
| Fe | 27.8 | 27.9 | 27.0 | 24.1 | ||
| Ctot | 35.8 | 39.0 | 38.5 | 43.1 | ||
| Zn | 0.28 | 0.21 | 0.19 | 0.26 | ||
| Fe | Fe2O3 | 10.9 | 11.5 | 13.6 | 10.1 | |
| Fe3O4 | 89.1 | 88.5 | 86.4 | 89.9 | ||
| C | CPC | 8.1 | 8.2 | 7.2 | 9.7 | |
| CCC | - | - | - | - | ||
| CCoke | 31.0 | 27.5 | 28.5 | 27.4 | ||
| Cases | Injected PC and Bio | Including Coke | Raceway Characteristics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Injected Carb. Mtrl. | Ratio [%] | Devolatilization Degree [%] | Reaction Eff. VM [%] | Reaction Eff. C(s) [%] | Conv. C+H2 (VM, C(s)) [g/s] | Reaction Eff. C+H2 [%] | Conv. C<s> in Coke [g/s] | Tot Conv. C+H2 [g/s] | Total Reaction Eff. C+H2 [%] | Comb. Start from Lance Inlet [mm] | Reaction Heat Complete Comb. of Injected [MW] | Max. Raceway Temp. [°C] | Mean Raceway Temp. [°C] | ||
| 1 | PC1 | 100 | 100 | 83.1 | 45.8 | 77.5 | 51.3 | 50.3 | 128 | 23.4 | 226 | 3.9 | 2405 | 1811 | |
| 2 | PC1 | 95 | 100 | 83.6 | 46.5 | 79.0 | 52.1 | 50.1 | 129 | 23.6 | 222 | 4.0 | 2338 | 1810 | |
| CCPre | 5 | 100 | 97.1 | 51.9 | |||||||||||
| 3 | PC1 | 80 | 99.9 | 84.5 | 45.9 | 80.1 | 52.1 | 50.3 | 130 | 23.7 | 194 | 4.0 | 2334 | 1806 | |
| CCPre | 20 | 99.9 | 92.0 | 51.1 | |||||||||||
| 4 | PC1 | 80 | 100 | 80.4 | 42.7 | 78.7 | 54.8 | 49.5 | 128 | 23.8 | 226 | 3.7 | 2306 | 1785 | |
| TS | 20 | 100 | 94.6 | 81.7 | |||||||||||
| 5 | CCPre | 100 | 97.1 | 82.9 | 46.4 | 80.9 | 49.1 | 53.0 | 134 | 23.9 | 218 | 4.2 | 2344 | 1812 | |
| 6 | TS | 100 | 100 | 80.4 | 84.7 | 94.0 | 82.2 | 48.2 | 142 | 27.9 | 230 | 3.6 | 2339 | 1811 | |
| 7 | PC2 | 100 | 97.4 | 83.9 | 48.0 | 81.6 | 52.8 | 51.7 | 133 | 24.3 | 226 | 4.2 | 2347 | 1835 | |
| 8 | PC2 | 92.5 | 99.5 | 77.9 | 49.6 | 85.5 | 55.1 | 50.8 | 136 | 24.8 | 274 | 4.2 | 2324 | 1819 | |
| CC7.5% | 7.5 | 99.5 | 97.1 | 62.7 | |||||||||||
| 9 | PC2 | 80 | 99.5 | 81.7 | 50.2 | 88.1 | 57.1 | 50.9 | 139 | 25.4 | 240 | 4.3 | 2343 | 1822 | |
| CC7.5% | 20 | 99.5 | 88.5 | 63.2 | |||||||||||
| 10 | PC2 | 90 | 99.6 | 81.8 | 51.3 | 85.3 | 56.1 | 50.6 | 136 | 24.9 | 203 | 4.2 | 2340 | 1832 | |
| CC10% | 10 | 99.6 | 97.9 | 59.9 | |||||||||||
| 11 | PC2 | 80 | 99.5 | 82.9 | 52.6 | 88.1 | 58.0 | 51.3 | 139 | 25.5 | 233 | 4.3 | 2371 | 1825 | |
| CC10% | 20 | 99.5 | 93.2 | 61.4 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundqvist Ökvist, L.; Lundgren, M.; From, L.-E.; Eck, J.; Kjellberg, M.; Ahmed, H. Realization of Bio-Coal Injection into the Blast Furnace. Metals 2024, 14, 969. https://doi.org/10.3390/met14090969
Sundqvist Ökvist L, Lundgren M, From L-E, Eck J, Kjellberg M, Ahmed H. Realization of Bio-Coal Injection into the Blast Furnace. Metals. 2024; 14(9):969. https://doi.org/10.3390/met14090969
Chicago/Turabian StyleSundqvist Ökvist, Lena, Maria Lundgren, Lars-Erik From, Joakim Eck, Martin Kjellberg, and Hesham Ahmed. 2024. "Realization of Bio-Coal Injection into the Blast Furnace" Metals 14, no. 9: 969. https://doi.org/10.3390/met14090969
APA StyleSundqvist Ökvist, L., Lundgren, M., From, L.-E., Eck, J., Kjellberg, M., & Ahmed, H. (2024). Realization of Bio-Coal Injection into the Blast Furnace. Metals, 14(9), 969. https://doi.org/10.3390/met14090969






