Influence of Chemical Composition and Microstructural Transformation of Two Low-Carbon Steels on Fine Blanking and Further Carbonitriding Heat Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Details
2.2. Conception of a Characterization Tool for Fine Blanking
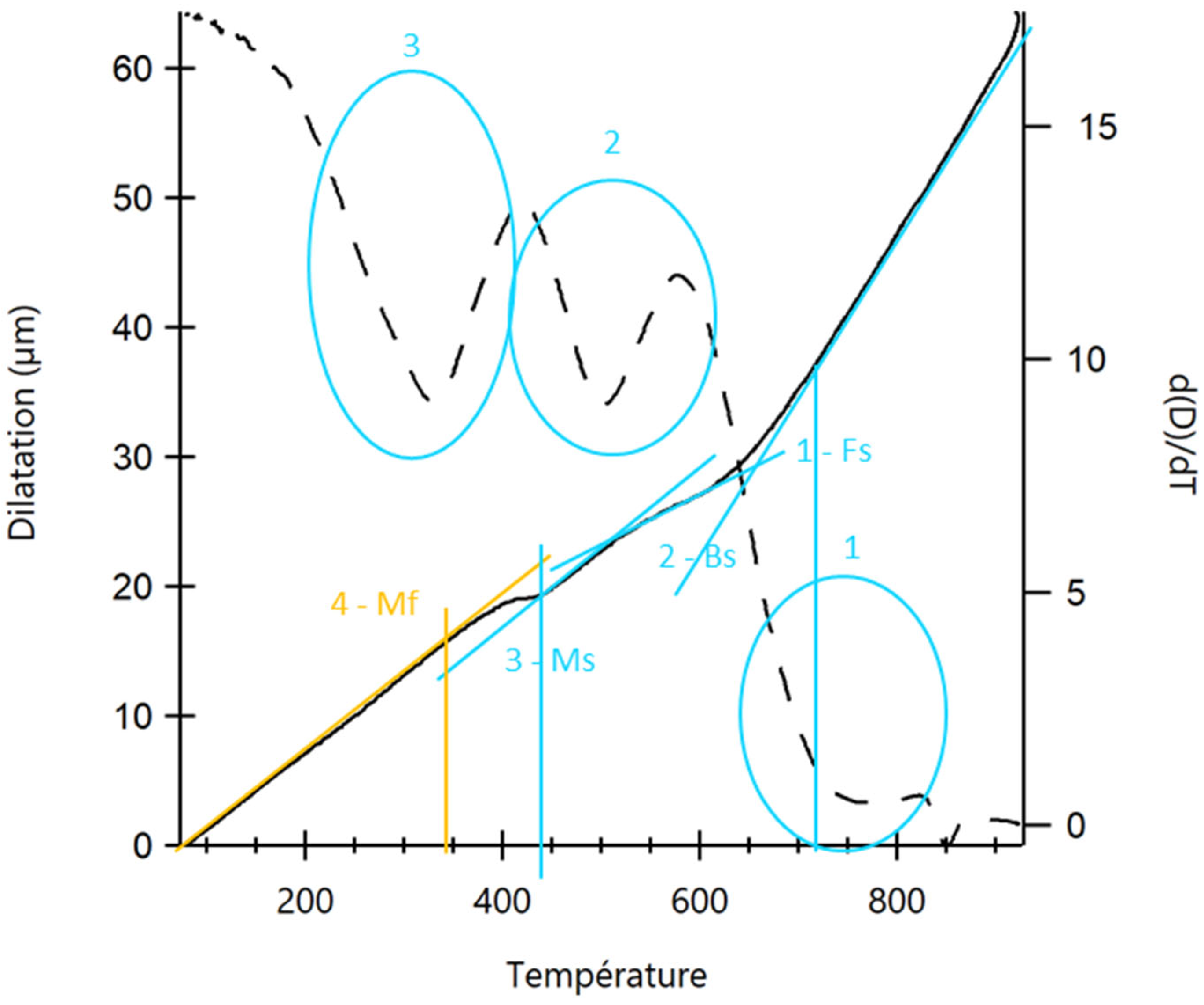
2.3. Carbonitriding Treatment
3. Results and Discussion
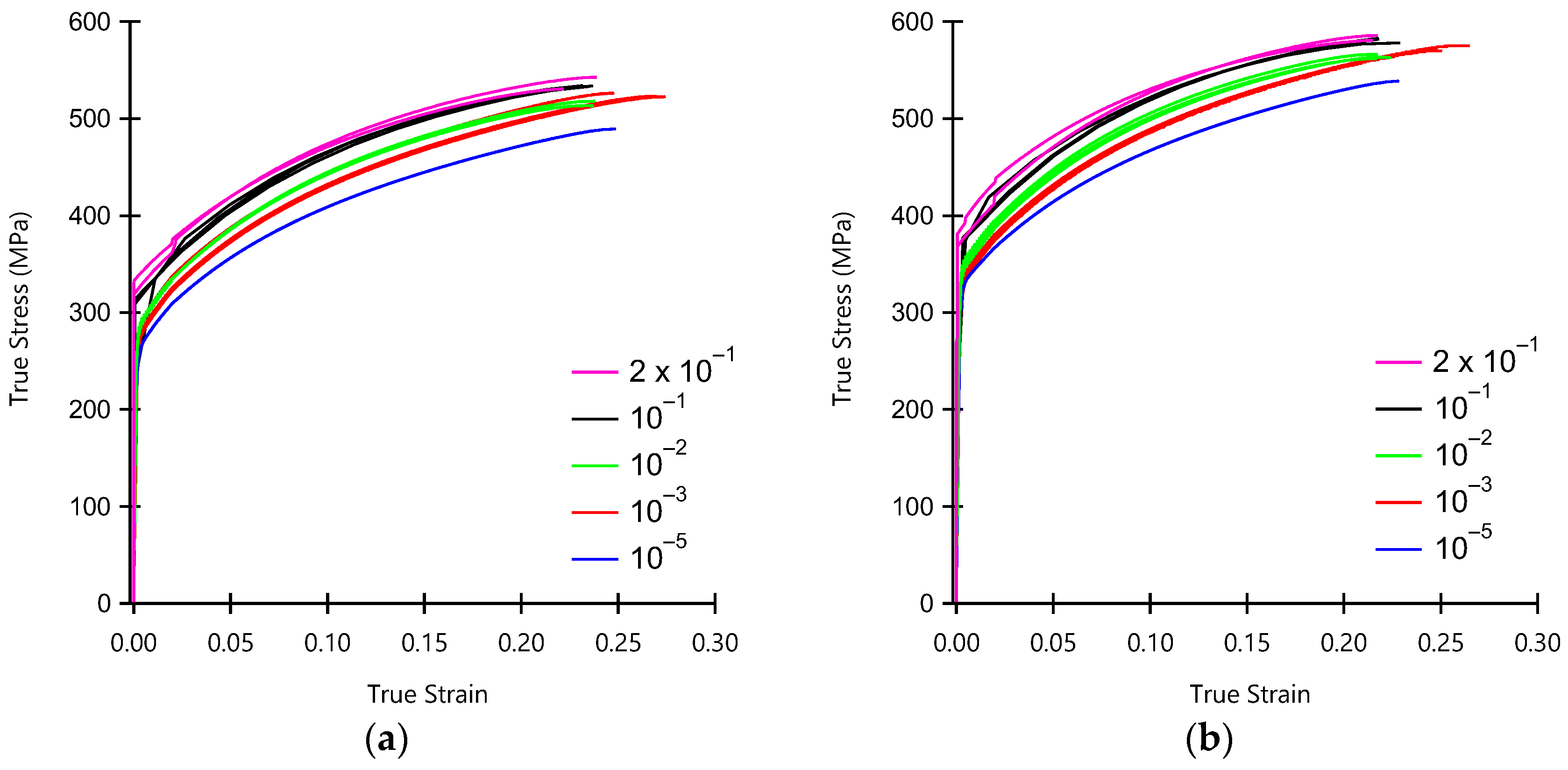
3.1. Mechanical Properties and Fine Blanking Behavior
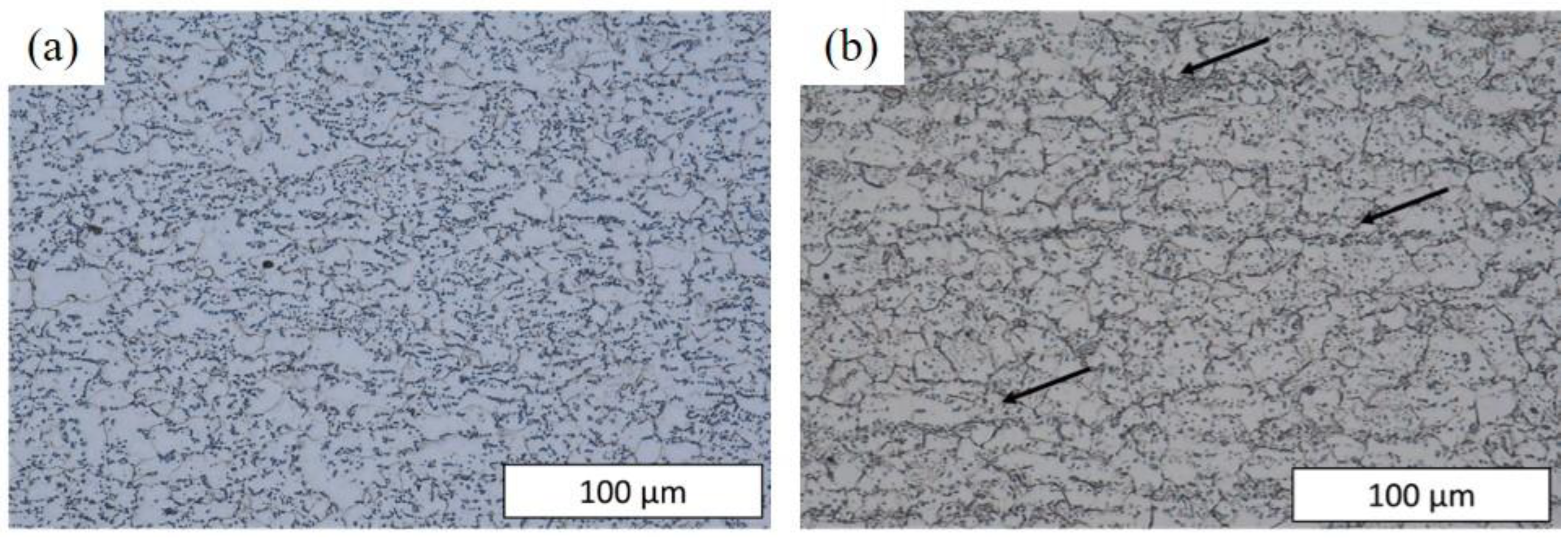
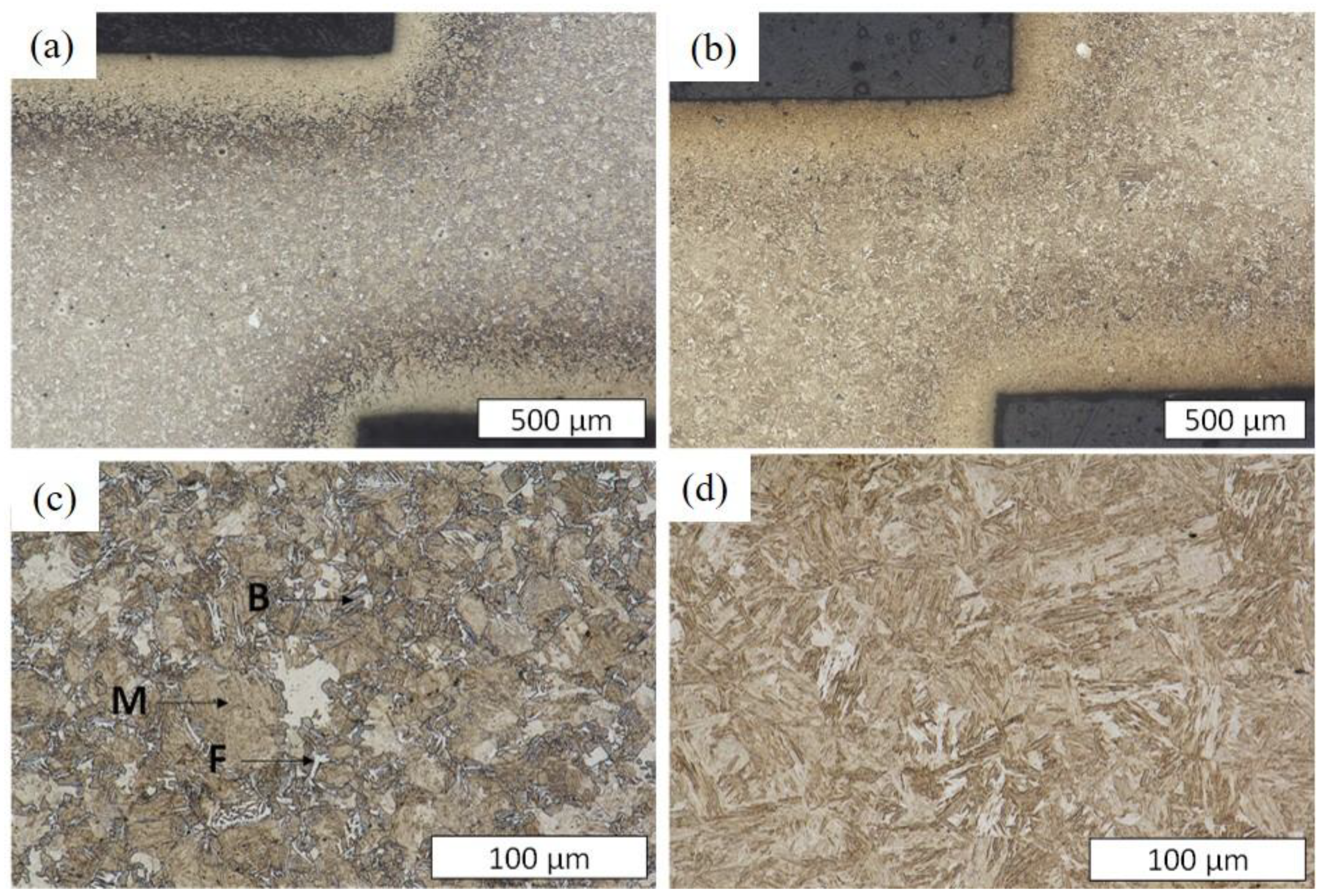
3.2. Microstructural Analysis
3.3. Impact of a Carbonitriding Heat Treatment on the Fracture Resistance During Fine Blanking
4. Conclusions
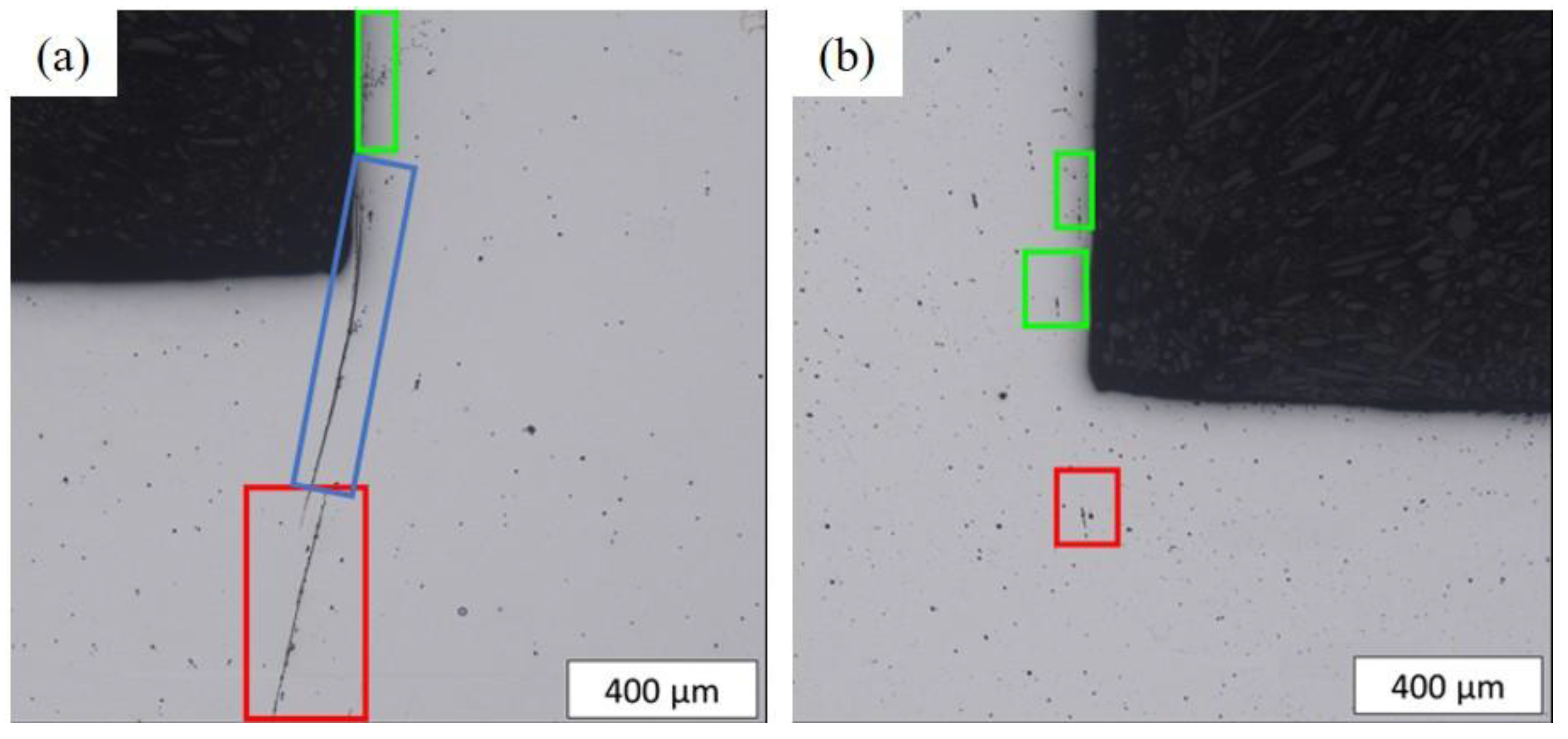
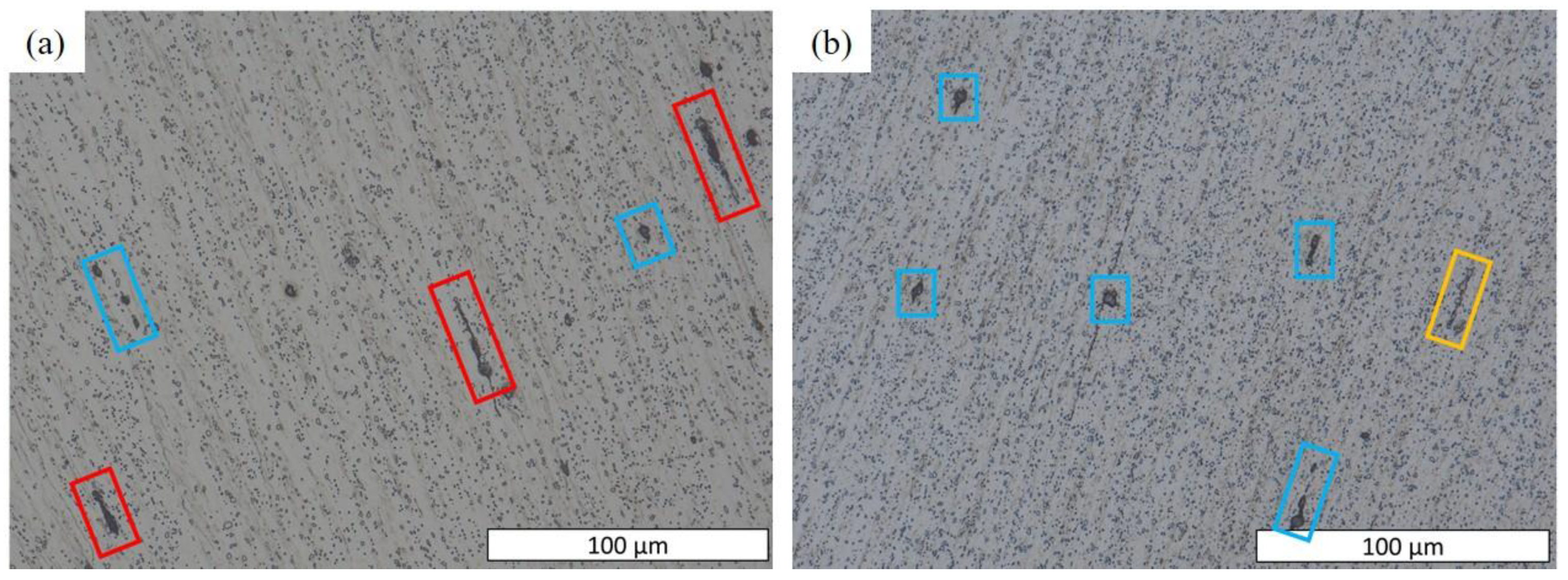
- Numerous cracks were observed in C18E at a medium half-cutting height, while only a few crack initiations were detected in 22MnB5 at a higher half-cutting height.
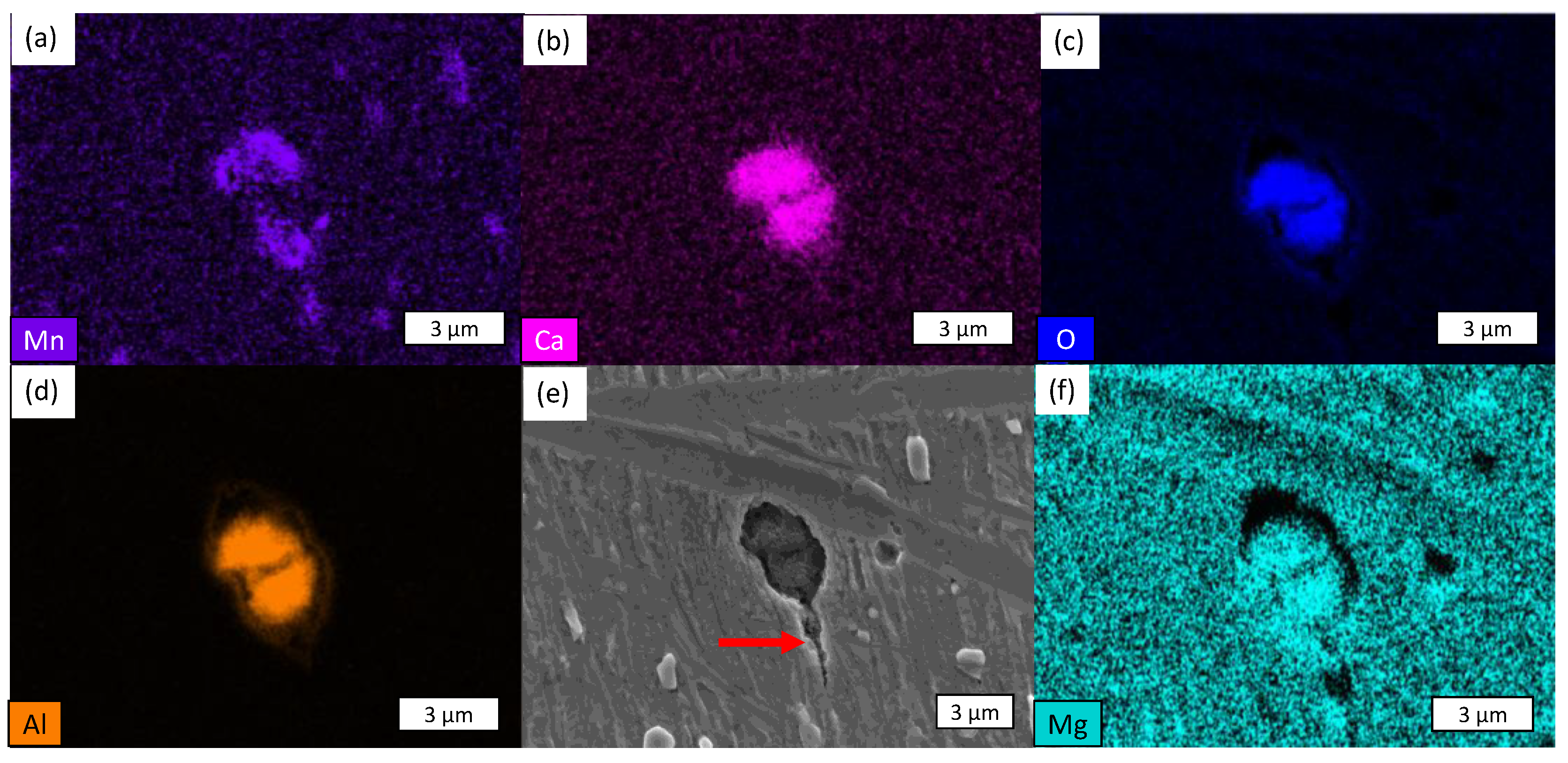
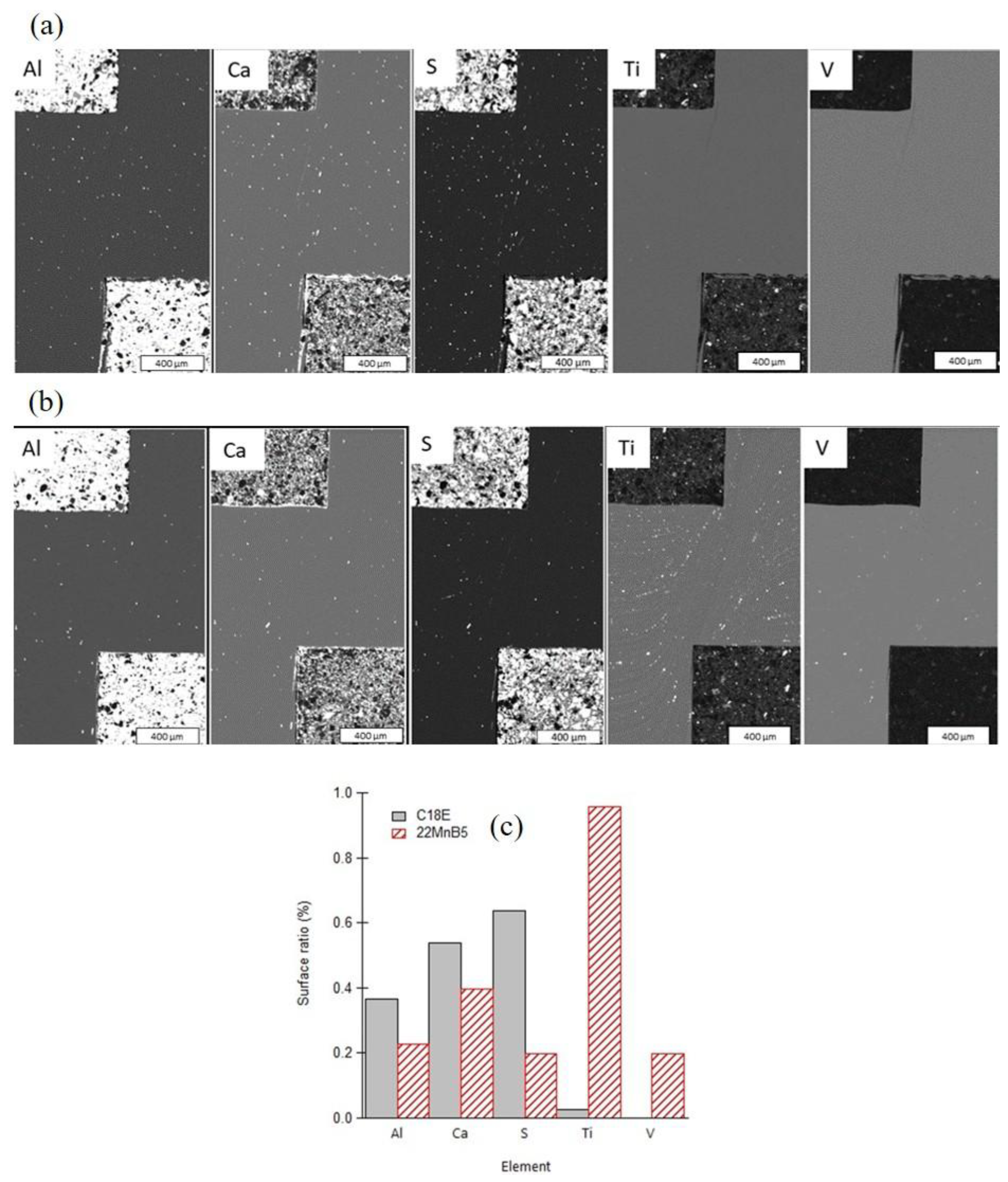
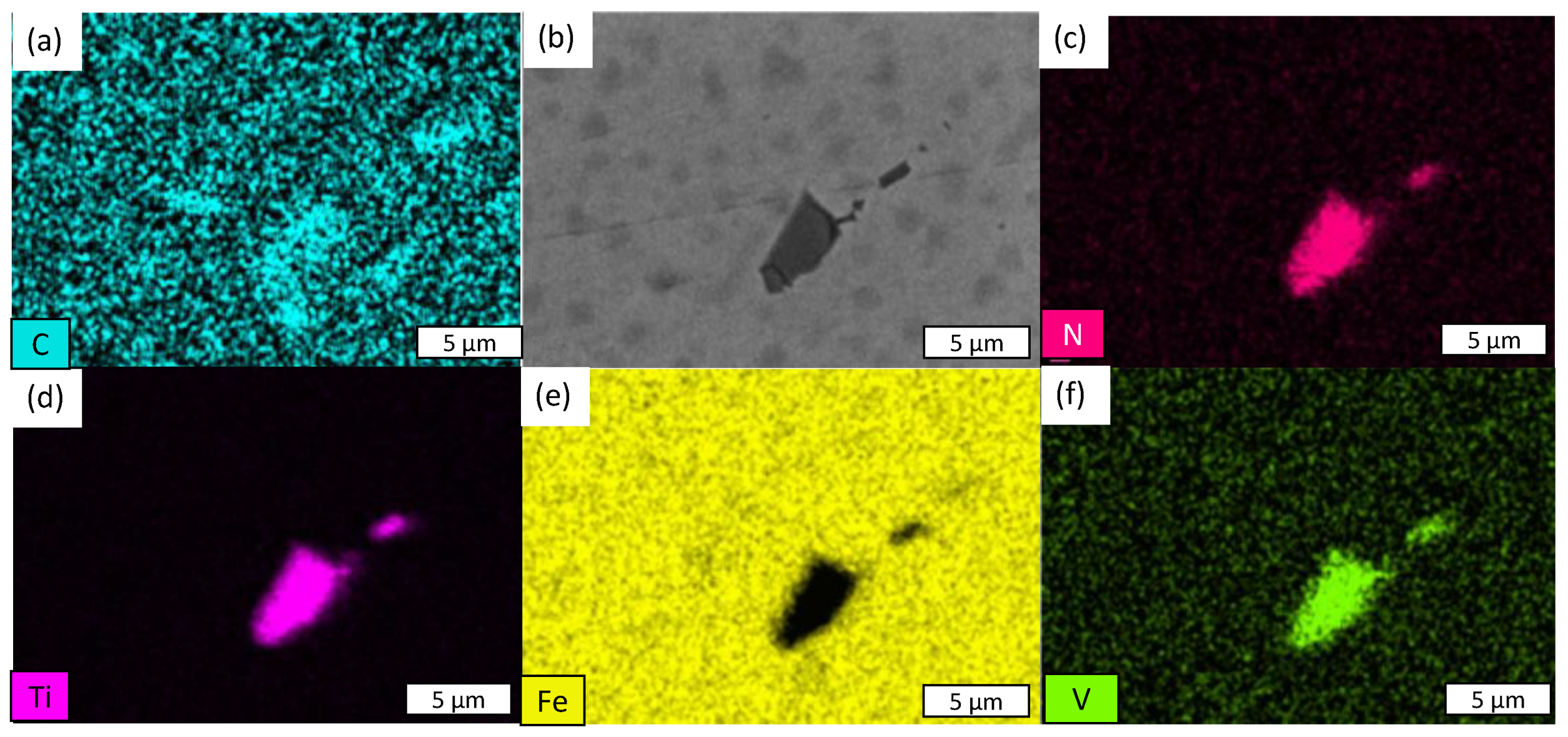
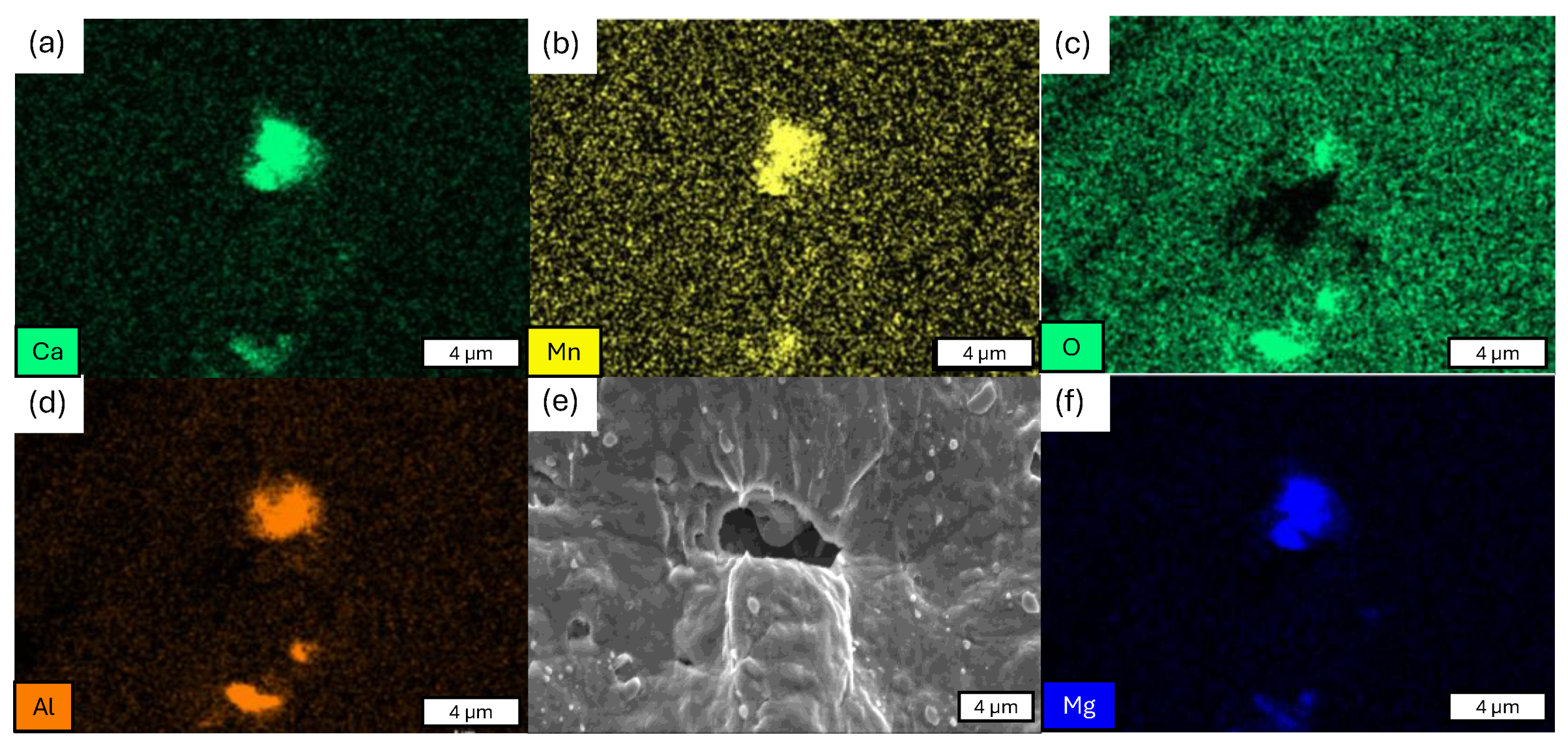
- The presence of cracks in the shear areas is correlated with the different types of inclusions present in the two steels. Cracks originate from cavities caused by brittle alumino-calcite inclusions. Manganese sulfide inclusions promote the propagation of previously formed cracks by delaminating from the metallic matrix. These inclusions are more prevalent in the considered C18E than in 22MnB5, resulting in greater cracking after blanking.
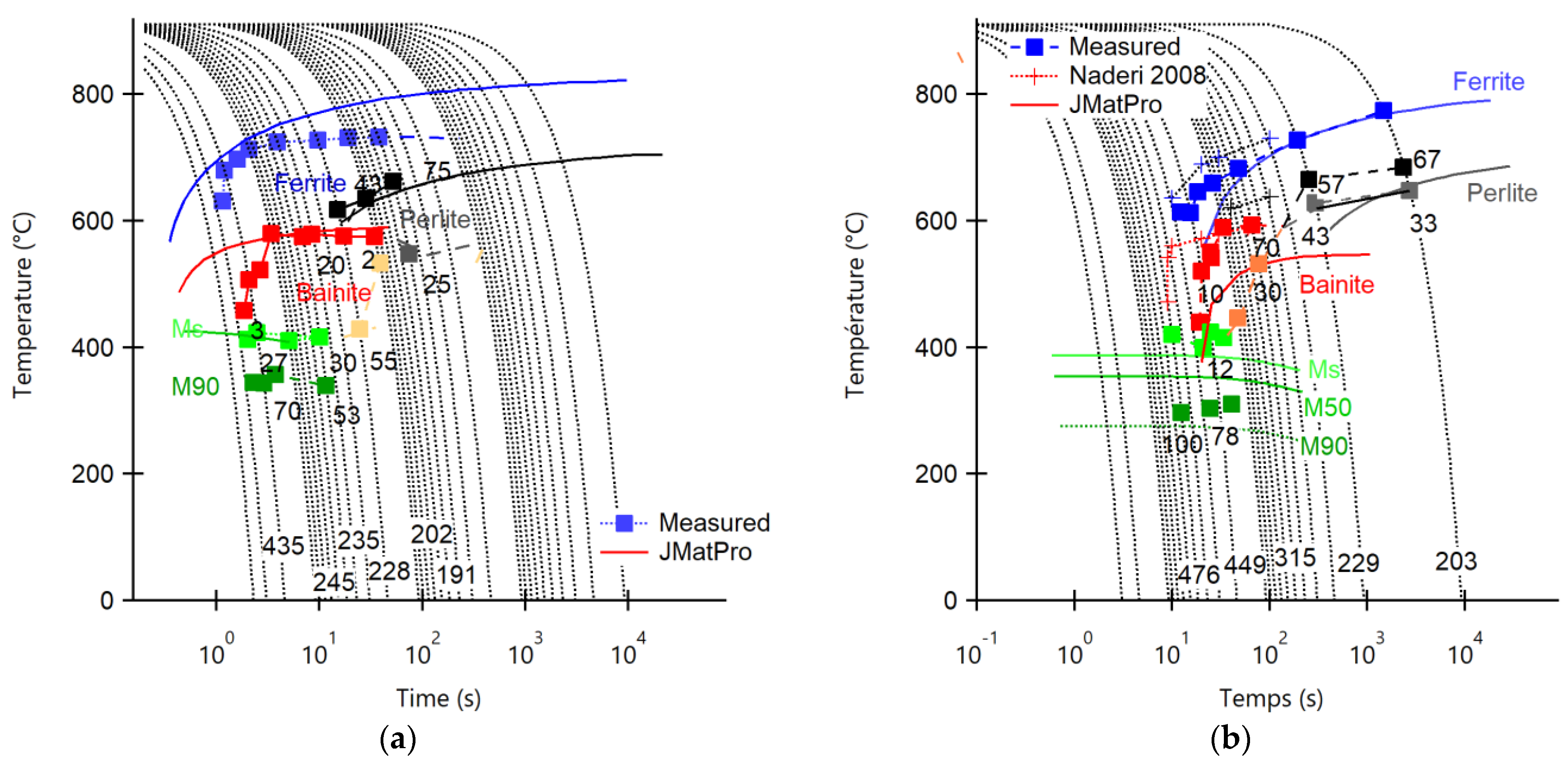
- Compared to the C18E steel, 22MnB5 exhibits better hardenability with carbonitriding thermal treatment. This treatment creates the martensite structure necessary to meet the surface hardness requirements of parts used in the automotive industry. A quench rate of 25 °C/s is sufficient for the formation of martensite in 22MnB5. However, C18E never obtains a full martensitic structure, even with quench rates as high as 250 °C/s, leading to dimensional instability of parts.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCT | Continuous Cooling Transformation |
| GDOES | Glow Discharge Optical Emission Spectroscopy |
| SEM | Scanning Electron Microscopy |
| EDS | Energy Dispersive Spectroscopy |
References
- Zheng, Q.; Zhuang, X.; Zhao, Z. State-of-the-art and future challenge in fine-blanking technology. Prod. Eng. 2019, 13, 61–70. [Google Scholar] [CrossRef]
- Quazi, P.; Shaikh, R. An overview of clearance optimization in sheet metal blanking process. Int. J. Mod. Eng. Res. 2012, 2, 4547–4558. [Google Scholar]
- Fan, W.; Li, J. An investigation on the damage of AISI-1045 and AISI-1025 steels in fine-blanking with negative clearance. Mater. Sci. Eng. A 2009, 499, 8–251. [Google Scholar] [CrossRef]
- Hartmann, C.; Weiss, H.; Lechner, P.; Volk, W.; Neumayer, S.; Fitschen, J.; Steidl, G. Measurement of strain, strain rate and crack evolution in shear cutting. J. Mater. Process Technol. 2021, 288, 116872. [Google Scholar] [CrossRef]
- Aoki, I.; Takahashi, T. Material flow analysis on shearing process by applying Fourier phase correlation method-analysis of piercing and fine-blanking. J. Mater. Process Technol. 1996, 134, 45–52. [Google Scholar] [CrossRef]
- Da Costa, E.; Silva, A. The effects of nonmetallic inclusions on properties relevant to the performance of steel in structural and mechanical applications. J. Mater. Res. Technol. 2019, 8, 2408–2422. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kurokawa, T.; Shimomura, T.; Matsudo, K.; Miyahara, S. Effect of non-metallic inclusion of flange cracking of drawn and ironed can from tinplate. Trans. Iron Steel Inst. Jpn. 1983, 23, 410–416. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.-G.; Gui, J.-T.; Du, Z.-L.; Xu, Z.-F.; Guo, B.-F. Effect of MnS inclusions on plastic deformation and fracture behavior of the steel matrix at high temperature. Vacuum 2020, 174, 109209. [Google Scholar] [CrossRef]
- Wang, Y. Effects of MnS inclusions on mechanical behavior and damage mechanism of free-cutting steel: A molecular dynamics study. J. Mol. Graph. Model. 2023, 118, 108354. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, A.; Pokorny, J. Inclusions non métalliques dans l’acier. Technol. L’ingénieur 1998, 2, 1–36. [Google Scholar] [CrossRef]
- Muan, A.; Osborn, E. Phase Equilibria Among Oxides in Steelmaking; Addison-Wesley Publishing Company: Boston, MA, USA; Reading, MA, USA, 1965. [Google Scholar]
- Kiessling, R.; Lange, N. Non-metallic inclusions in steels. Lond. Met. Soc. 1978, 2, 190–194. [Google Scholar]
- Shiraiwa, T.; Fujino, N.; Matsuno, F. Properties of non metallic inclusions in steels. Sumitomo Search 1974, 11, 85–100. [Google Scholar]
- Yu, H.-L.; Bi, H.-Y.; Liu, X.-H.; Tu, Y.-F. Strain distribution of strips with spherical inclusion during cold rolling. Trans. Nonferrous Met. Soc. China 2008, 18, 919–924. [Google Scholar] [CrossRef]
- Yu, H.; Liu, X.; Bi, H.; Chen, L. Deformation behavior of inclusions in stainless Steel strips during multi-pass cold rolling. J. Mater. Process Technol. 2009, 209, 455–461. [Google Scholar] [CrossRef]
- Kaushik, P.; Lehmann, J.; Nadif, M. State of the art in control of inclusions, their characterization, and future requirements. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2012, 43, 710–725. [Google Scholar] [CrossRef]
- Ervasti, E.; Ståhlberg, U. Void initiation close to a macro-inclusion during single pass reductions in the hot rolling of steel slabs: A numerical study. J. Mater. Process Technol. 2005, 170, 142–150. [Google Scholar] [CrossRef]
- Wu, M.; Fang, W.; Chen, R.M.; Jiang, B.; Wang, H.B.; Liu, Y.Z.; Liang, H.L. Mechanical anisotropy and local ductility in transverse tensile deformation in hot rolled steels: The role of MnS inclusions. Mater. Sci. Eng. A 2019, 744, 324–334. [Google Scholar] [CrossRef]
- Ghosh, A.; Modak, P.; Dutta, R.; Chakrabarti, D. Effect of MnS inclusion and crystallographic texture on anisotropy in Charpy impact toughness of low carbon ferritic steel. Mater. Sci. Eng. A 2016, 654, 298–308. [Google Scholar] [CrossRef]
- Zhuang, X.; Ma, S.; Zhao, Z. A microstructure-based macro-micro multi-scale fine-blanking simulation of ferrite-cementite steels. Int. J. Mech. Sci. 2017, 128–129, 414–427. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, L.; Lu, S.; Lin, Z.; Wen, T.; Chen, Z. Progress and perspective of ultra-high-strength martensitic steels for automobile. Metals 2022, 12, 2184. [Google Scholar] [CrossRef]
- Karolak, C.; Montmitonnet, P.; Moore Parks, D.; Delattre, G.; Bouchard, P.O. Analysis and modeling of the failure behavior of carbonitrided parts. Procedia Eng. 2017, 207, 2030–2035. [Google Scholar] [CrossRef]
- Krauss, G. Heat Treated Martensitic Advanced Manufacture Systems for Advanced Manufacture. ISIJ Int. 1995, 35, 349–359. [Google Scholar] [CrossRef]
- Naderi, M.; Saeed-Akbari, A.; Bleck, W. The effects of non-isothermal deformation on martensitic transformation in 22MnB5 steel. Mater. Sci. Eng. A 2008, 487, 445–455. [Google Scholar] [CrossRef]
- Bialobrzeska, B. Effect of alloying additives and microadditives on hardenability Increase Caused by Action of Boron. Metals 2021, 11, 589. [Google Scholar] [CrossRef]
- ISO 6892-1:2019; Metallic Materials—Tensile Testing Part 1: Method of Test at Room Temperature. ISO: Geneva, Switzerland, 2019.
- Fields, D.; Backofen, W. Determination of the strain hardening characteristics by torsion testing. Am. Soc. Test. Mater. Proceeding Sixtieth Annu. Meet. Soc. 1957, 57, 1259–1272. [Google Scholar]
- Lange, K.; Bizer, F.; Höfel, P.; Mukhoty, A.; Singer, H. Cold Forming and Fineblanking; Edelsthalwerke Buderus AG: Hessen, Germany; Feintool AG: Lyss, Switzerland; Lyss Hoesch Hohenlimburg GmbH: Hagen, Germany; Kallwalzwerk Brockhaus GmbH: Lüdenscheid, Germany, 1997. [Google Scholar]
- Tong, C.; Zhu, G.; Rong, Q.; Yardley, V.A.; Shi, Z.; Li, X.; Luo, J.; Lin, J. Investigation of austenitising behaviour of medium-Mn steel in the hot-stamping heating process. J. Mater. Process Technol. 2021, 297, 117269. [Google Scholar] [CrossRef]
- Qian, L.; Ji, W.; Sun, C.; Fang, G.; Lian, J. Prediction of edge fracture during hole-flanging of advanced high-strength steel considering blanking pre-damage. Eng. Fract. Mech. 2021, 248, 107721. [Google Scholar] [CrossRef]
- Llewellyn, D.; Marriott, J.; Naylor, D.; Thewlis, G. The effects of residual elements on the properties of engineering steels. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1980, 295, 69–85. [Google Scholar]



| C | N | Cr | Mn | Si | P | S | Mo | Ca | Al | Ti | B | V | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C18E | 0.194 | 0.012 | 0.019 | 0.667 | 0.183 | 0.010 | 0.003 | 0.006 | 0.002 | 0.009 | 0.000 | 0.000 | Traces |
| 22MnB5 | 0.224 | Traces | 0.183 | 1.235 | 0.213 | 0.011 | Traces | 0.006 | 0.001 | 0.036 | 0.009 | 0.002 | Traces |
| σy (MPa) | σm (MPa) | εf | |
|---|---|---|---|
| C18E | 236 ± 2 | 500 ± 6 | 0.31 ± 0.01 |
| 22MnB5 | 286 ± 12 | 549 ± 12 | 0.27 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiavazza, T.; Marnier, M.; Achille, A.; Eve, S.; Hug, E. Influence of Chemical Composition and Microstructural Transformation of Two Low-Carbon Steels on Fine Blanking and Further Carbonitriding Heat Treatment. Metals 2025, 15, 1173. https://doi.org/10.3390/met15111173
Chiavazza T, Marnier M, Achille A, Eve S, Hug E. Influence of Chemical Composition and Microstructural Transformation of Two Low-Carbon Steels on Fine Blanking and Further Carbonitriding Heat Treatment. Metals. 2025; 15(11):1173. https://doi.org/10.3390/met15111173
Chicago/Turabian StyleChiavazza, Thomas, Margaux Marnier, Aurélie Achille, Sophie Eve, and Eric Hug. 2025. "Influence of Chemical Composition and Microstructural Transformation of Two Low-Carbon Steels on Fine Blanking and Further Carbonitriding Heat Treatment" Metals 15, no. 11: 1173. https://doi.org/10.3390/met15111173
APA StyleChiavazza, T., Marnier, M., Achille, A., Eve, S., & Hug, E. (2025). Influence of Chemical Composition and Microstructural Transformation of Two Low-Carbon Steels on Fine Blanking and Further Carbonitriding Heat Treatment. Metals, 15(11), 1173. https://doi.org/10.3390/met15111173






