Impurity Behavior in Cast Copper Anodes: Implications for Electrorefining in a Circular Economy
Abstract
1. Introduction
2. Materials and Methods
Doped Anodes with Ni, Sn and Sb
3. Results and Discussion
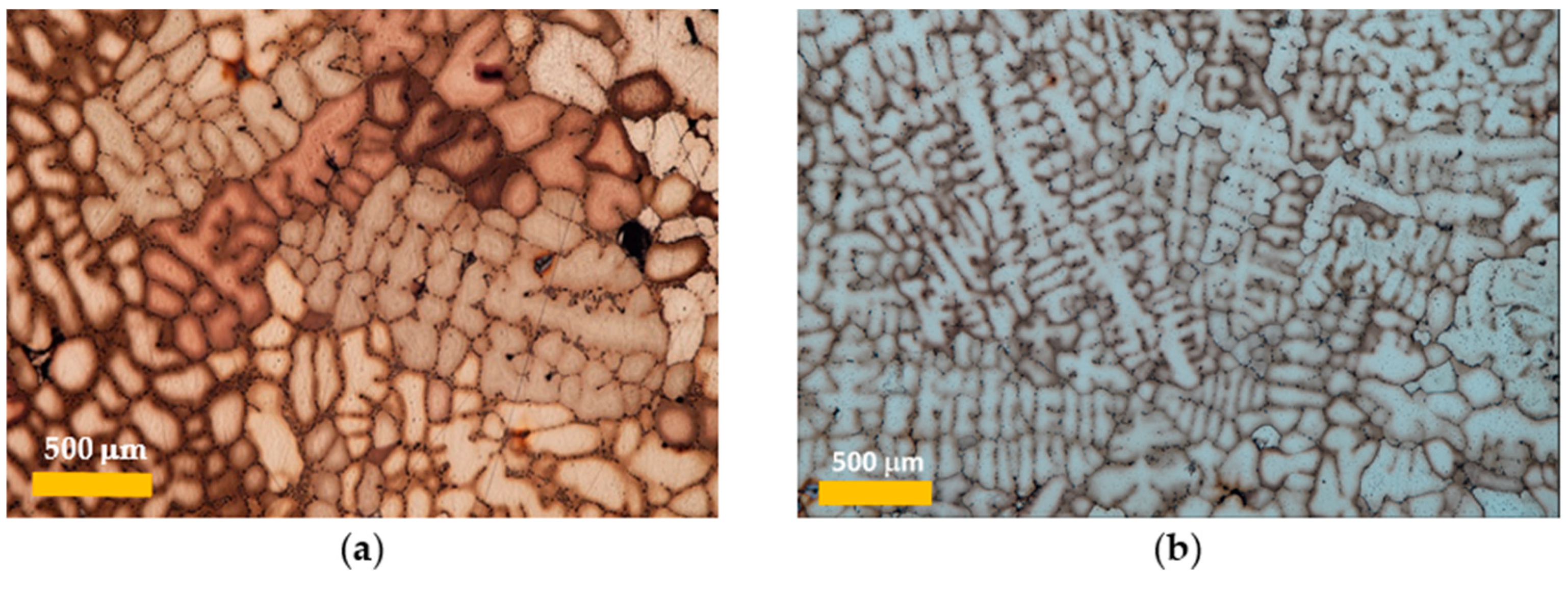
3.1. Grain Size Distribution
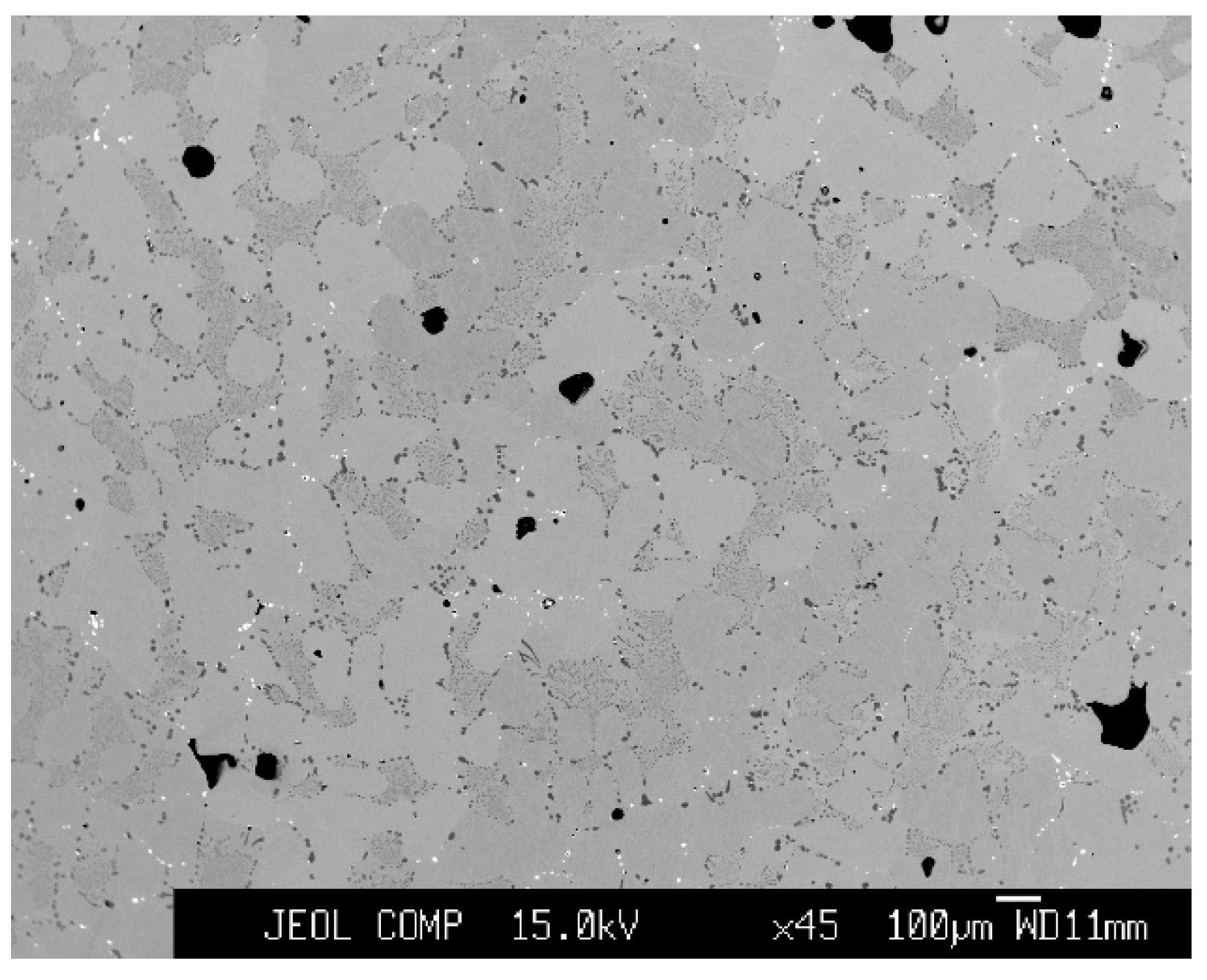
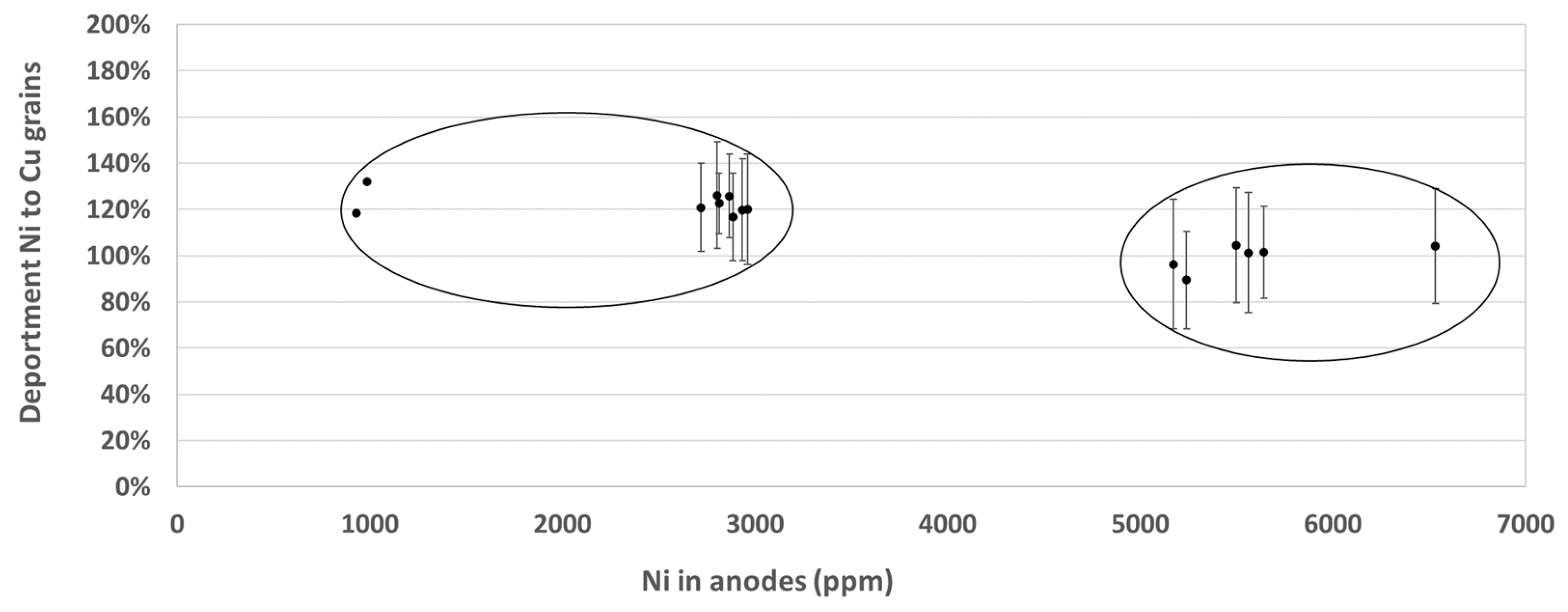
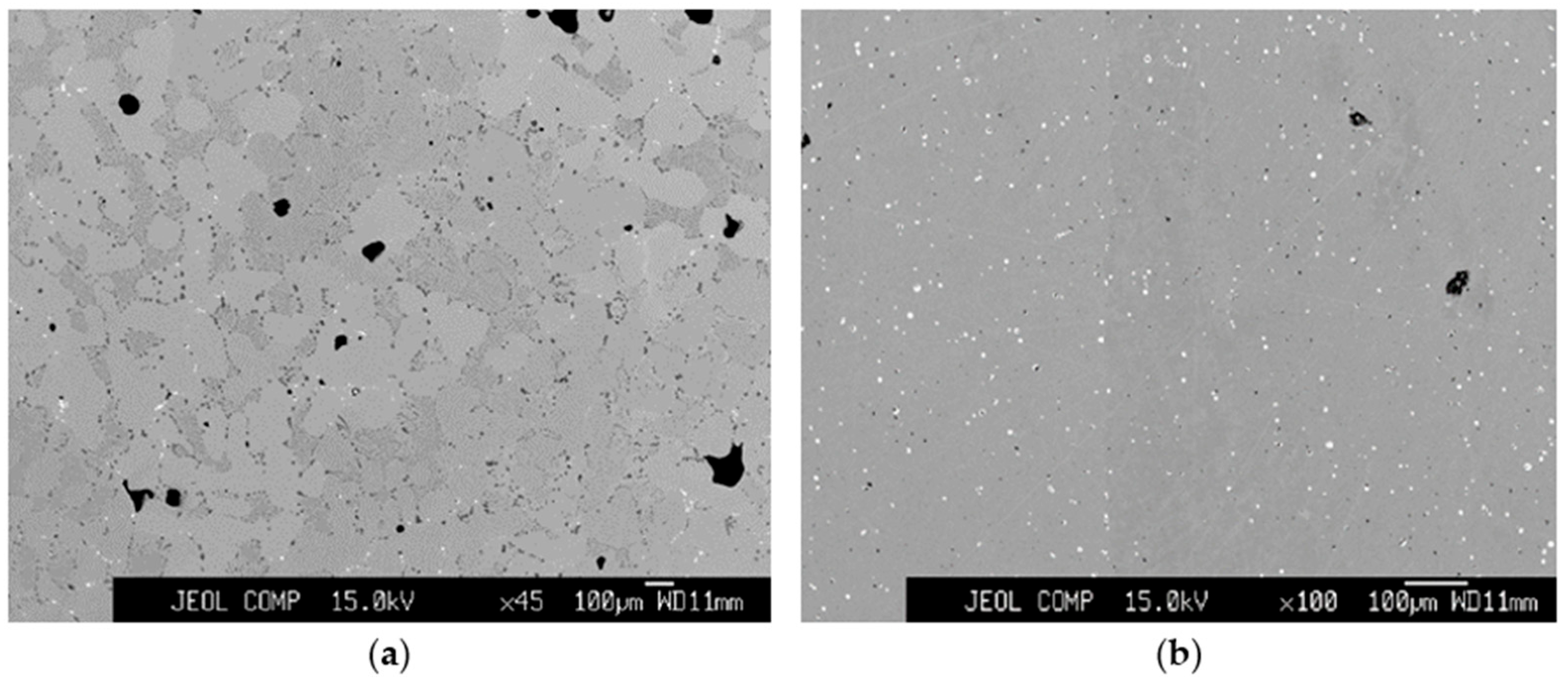
3.2. Chemical Distribution of Impurities in Cu Grains
3.3. Chemical Distribution of Impurities in Inclusions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reuter, M.A.; Van Schaik, A.; Gutzmer, J.; Bartie, N.; Abadías-Llamas, A. Challenges of the Circular Economy: A Material, Metallurgical, and Product Design Perspective. Annu. Rev. Mater. Res. 2019, 49, 253–274. [Google Scholar] [CrossRef]
- Salviulo, G.; Lavagnolo, M.C.; Dabalà, M.; Bernardo, E.; Polimeno, A.; Sambi, M.; Bonollo, F.; Gross, S. Enabling Circular Economy: The Overlooked Role of Inorganic Materials Chemistry. Chem.-A Eur. J. 2021, 27, 6676–6695. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, E. Towards the Circular Economy: Opportunities for the Consumer Goods Sector; Ellen MacArthur Foundation: Cowes, UK, 2013; ISBN 0102603022. [Google Scholar]
- IEA Global Critical Minerals Outlook 2024. Available online: https://www.iea.org/reports/global-critical-minerals-outlook-2024?utm_content=buffer3599f&utm_medium=social&utm_source=linkedin.com&utm_campaign=buffer (accessed on 1 December 2024).
- Elshkaki, A.; Graedel, T.E.; Ciacci, L.; Reck, B.K. Resource Demand Scenarios for the Major Metals. Environ. Sci. Technol. 2018, 52, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Pietrzyk, S.; Tora, B. Trends in global copper mining—A review. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 427. [Google Scholar]
- Ciacci, L.; Fishman, T.; Elshkaki, A.; Graedel, T.E.; Vassura, I.; Passarini, F. Exploring future copper demand, recycling and associated greenhouse gas emissions in the EU-28. Glob. Environ. Chang. 2020, 63, 102093. [Google Scholar] [CrossRef]
- Zhang, J.; Hua, P.; Krebs, P. The build-up dynamic and chemical fractionation of Cu, Zn and Cd in road-deposited sediment. Sci. Total Environ. 2015, 532, 723–732. [Google Scholar] [CrossRef]
- ICSG. The World Copper Factbook 2020; ICSG: Lisbon, Portugal, 2020; ISBN 9780874216561. [Google Scholar]
- Lennartsson, A.; Engström, F.; Samuelsson, C.; Björkman, B.; Pettersson, J. Large-Scale WEEE Recycling Integrated in an Ore-Based Cu-Extraction System. J. Sustain. Metall. 2018, 4, 222–232. [Google Scholar] [CrossRef]
- Loibl, A.; Tercero Espinoza, L.A. Current challenges in copper recycling: Aligning insights from material flow analysis with technological research developments and industry issues in Europe and North America. Resour. Conserv. Recycl. 2021, 169, 105462. [Google Scholar] [CrossRef]
- Siegmund, S.M.S.D.A. Challenges and opporunities with WEEE treatment in Cu smelting processes. In Proceedings of the Copper 2022, Santiago de Chile, Chile, 13–17 November 2022; pp. 17–33. [Google Scholar]
- Van Yken, J.; Cheng, K.Y.; Boxall, N.J.; Sheedy, C.; Nikoloski, A.N.; Moheimani, N.R.; Kaksonen, A.H. A comparison of methods for the characterisation of Waste-Printed Circuit Boards. Metals 2021, 11, 1935. [Google Scholar] [CrossRef]
- Ohtsuka, T.T.H.S.K.A.N. Development of physical separation process of Electronic Scrap for copper smelting and refining. In Proceedings of the Copper International Conference 2022, Santiago de Chile, Chile, 13–17 November 2022; Volume 9, pp. 45–57. [Google Scholar]
- Fountain, C. The Whys and Wherefores of Penalty Elements in Copper Concentrates. In Proceedings of the MetPlant 2013: Metallurgical Plant Design and Operating Strategies, Perth, Australia, 15–17 July 2013. [Google Scholar]
- Nakawara, K.W.S. The behavior of impurities at Kosaka Smelter. In Proceedings of the Metallurgical and Materials Processing: Principles and Technologies; The Minerals, Metals and Materials Society: Pittsburgh, PA, USA, 2003; Volume II, pp. 521–531. [Google Scholar]
- Schlesinger, M.E.; Sole, K.C.; Davenport, W.G.; Flores, G.R. Extractive Metallurgy of Copper; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 2013206534. [Google Scholar]
- Adam, F.; Wood, J.; Andrews, R. Processing of Electronic Scrap with Ausmelt TSL Technology. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2024; Volume 543, pp. 4–9. [Google Scholar] [CrossRef]
- Chen, T.T.; Dutrizac, J.E. A mineralogical study of the deportment of impurities during the electrorefining of secondary copper anodes. In Proceedings of the Copper 99-Cobre 99 International Conference; Dutrizac, J.E., Ji, J., Ramachandran, V., Eds.; The Minerals, Metals and Materials Society: Pittsburgh, PA, USA, 1999; Volume 111, pp. 437–460. [Google Scholar]
- Chen, T.T.; Dutrizac, J.E. Mineralogical characterization of anode slimes: Part v-nickel-rich copper anodes from the ccr division of noranda minerals inc. Can. Metall. Q. 1990, 29, 27–37. [Google Scholar] [CrossRef]
- Miyamoto, M.; Mitsuno, S.; Kitada, A.; Fukami, K.; Murase, K. Mechanism of nodular growth in copper electrorefining with the inclusion of impurity particles under natural convection. Hydrometallurgy 2023, 216, 106013. [Google Scholar] [CrossRef]
- Mori, K.; Yamakawa, Y.; Oue, S.; Taninouchi, Y.K.; Nakano, H. Effect of Impurity Ions and Additives in Solution of Copper Electrorefining on the Passivation Behavior of Low-Grade Copper Anode. Mater. Trans. 2023, 64, 242–251. [Google Scholar] [CrossRef]
- Miyamoto, M.; Ishikawa, Y.; Fukami, K.; Murase, K. Surface Roughening and Growth-Promoting Effects of Nickel and Antimony on Nodules in Copper Electrorefining. Metall. Mater. Trans. B 2023, 54, 3579–3590. [Google Scholar] [CrossRef]
- Markovic, R.; Krstic, V.; Friedrich, B.; Stopic, S.; Stevanovic, J.; Stevanovic, Z.; Marjanovic, V. Electrorefining process of the non-commercial copper anodes. Metals 2021, 11, 1187. [Google Scholar] [CrossRef]
- Sahlman, M.; Aromaa, J.; Lundström, M. Detachment and flow behaviour of anode slimes in high nickel copper electrorefining. Physicochem. Probl. Miner. Process. 2024, 60, 186194. [Google Scholar] [CrossRef]
- Djokić, J.; Radovanović, D.; Nikolovski, Z.; Andjić, Z.; Kamberović, Ž. Influence of electrolyte impurities from e-waste electrorefining on copper extraction recovery. Metals 2021, 11, 1383. [Google Scholar] [CrossRef]
- Wenzl, C. Structure and Casting Technology of Anodes in Copper Metallurgy. Ph.D. Thesis, University of Leoben, Leoben, Austria, 2008. [Google Scholar]
- Chen, T.T.; Dutrizac, J.E. Mineralogical characterization of anode slimes-i. anode copper from inca’s copper cliff copper refinery. Can. Metall. Q. 1988, 27, 91–96. [Google Scholar] [CrossRef]
- Marković, R.; Friedrich, B.; Stajić-Trošić, J.; Jordović, B.; Jugović, B.; Gvozdenović, M.; Stevanović, J. Behaviour of non-standard composition copper bearing anodes from the copper refining process. J. Hazard. Mater. 2010, 182, 55–63. [Google Scholar] [CrossRef]
- Möller, C.A.; Bayanmunkh, M.; Friedrich, B. Influence of As, Sb, Bi and O on copper anode behaviour—Part 1: Passivation characteristics. World Metall.-Erzmetall 2008, 61, 357–367. [Google Scholar]
- Möller, C.A.; Bayanmunkh, M.; Friedrich, B. Influence of As, Sb, Bi and O on copper anode behaviour—Part 2: Anode dissolution behaviour and anode sludge generation. World Metall.-Erzmetall 2009, 62, 6–16. [Google Scholar]
- Möller, C.A.; Bayanmunkh, M.; Friedrich, B. Influence of As, Sb, Bi and O on copper anode behaviour—Part 3: Elemental distribution. World Metall.-Erzmetall 2009, 62, 70–80. [Google Scholar]
- Dupont, D.; Arnout, S.; Jones, P.T.; Binnemans, K. Antimony Recovery from End-of-Life Products and Industrial Process Residues: A Critical Review. J. Sustain. Metall. 2016, 2, 79–103. [Google Scholar] [CrossRef]
- Mitan, M. Microstructure and Morphology of Impure Copper Anodes. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1997. [Google Scholar]
- Chen, T.T.; Dutrizac, J.E. Mineralogical characterization of anode slimes-iv. copper-nickel-antimony oxide (“kupferglimmer”) in ccr anodes and anode slimes. Can. Metall. Q. 1989, 28, 127–134. [Google Scholar] [CrossRef]
- Forsén, O. The Behaviour of Arsenic, Antimony and Bismuth in the Solidification and Electrolysis of Nickel-Oxygen-Bearing Copper Anodes; The Minerals, Metals and Materials Society: Pittsburgh, PA, USA, 1985; pp. 353–377. [Google Scholar]
- Chen, T.T.; Dutrizac, J.E. Mineralogical characterization of a copper anode and the anode slimes from the La Caridad copper refinery of Mexicana de Cobre. Metall. Mater. Trans. B 2005, 36, 229–240. [Google Scholar] [CrossRef]
- Chen, T.T.; Dutrizac, J.E. Mineralogical characterization of anode slimes: Part 7-copper anodes and anode slimes from the chuquicamat a division of codelco-Chile. Can. Metall. Q. 1991, 30, 95–106. [Google Scholar] [CrossRef]
- Kurimoto, K.; Aoki, H.; Yamaguchi, Y.; Hattori, Y. High current density operation at the toyo tank house #2. In Proceedings of the Copper 2016, Kobe, Japan, 13–16 November 2016; Volume EL2-4, pp. 2001–2012. [Google Scholar]
- Tayler, P.L.; Van Wagoner, D.L.; Pitt, C.H. Solubility limits of selenium in copper. Metall. Trans. B 1976, 7, 103–106. [Google Scholar] [CrossRef]
- Forsén, O. Solidification and Electrolysis of Copper Anodes Containing Nickel, Arsenic, Antimony and Bismuth; The Minerals, Metals and Materials Society: Pittsburgh, PA, USA, 1987; pp. 47–69. [Google Scholar]
- Forsen, O.; Tikkanen, M.H. On the dissolution of Copper Anodes in Electrolytic Refining Part I: The behaviour of nickel in oxygen-bearing copper anodes. Scand. J. Metall. 1981, 10, 109–114. [Google Scholar]

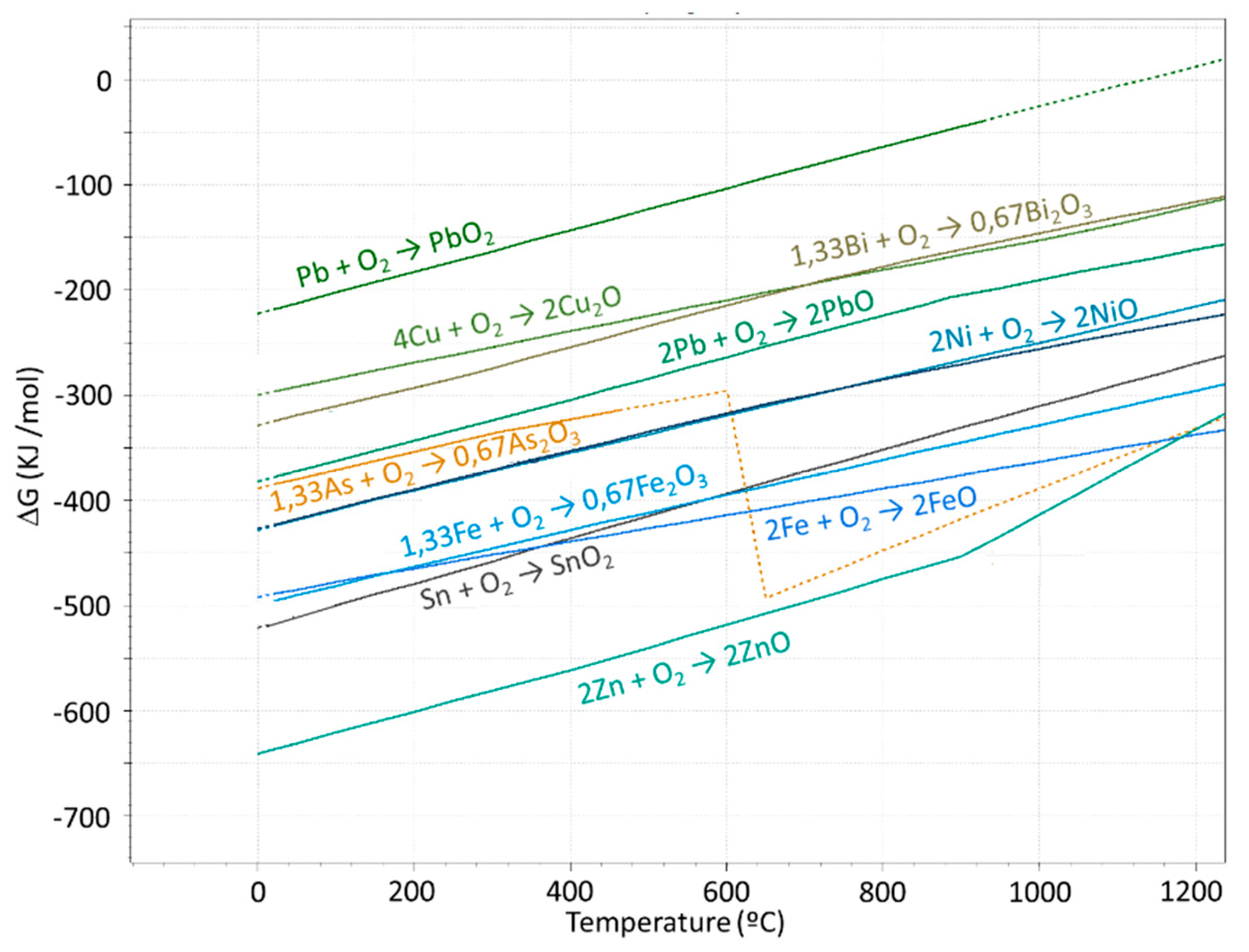

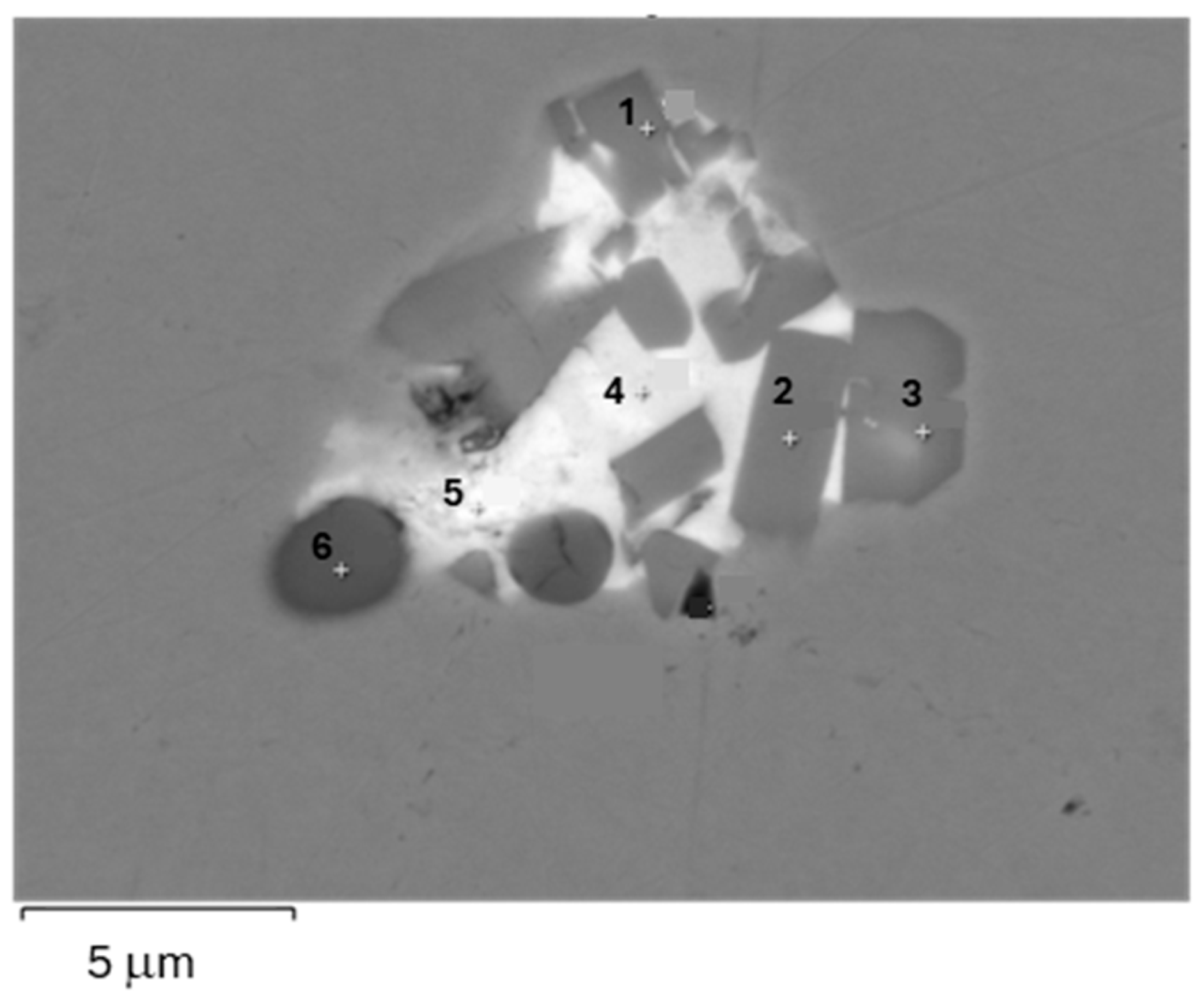

| Anode | Ni | Sn | Sb | As | O | Ag | Bi | Pb | Se | Te |
|---|---|---|---|---|---|---|---|---|---|---|
| AC1 | 929 | 113 | 194 | 861 | 1545 | 300 | 250 | 1106 | 396 | 60 |
| AC2 | 985 | 30 | 159 | 1744 | 1135 | 302 | 340 | 397 | 355 | 52 |
| DA1 | 2962 | 324 | 481 | 843 | 1944 | 254 | 364 | 1072 | 372 | 58 |
| DA2 | 2813 | 342 | 593 | 698 | 2100 | 219 | 385 | 1135 | 505 | 109 |
| DA3 | 2804 | 355 | 501 | 1508 a | 1576 | 205 | 258 | 960 | 312 | 45 |
| DA4 | 5562 a | 306 | 457 | 783 | 1630 | 251 | 314 | 919 | 366 | 53 |
| DA5 | 5241 a | 305 | 429 | 586 | 1900 | 209 | 392 | 996 | 480 | 108 |
| DA6 | 2885 | 360 | 741 a | 842 | 2070 | 268 | 366 | 1015 | 370 | 56 |
| DA7 | 2935 | 690 a | 477 | 831 | 2134 | 269 | 361 | 970 | 374 | 59 |
| DA8 | 2868 | 748 a | 520 | 758 | 1466 | 244 | 504 | 1078 | 358 | 49 |
| DA9 | 2721 | 744 a | 709 a | 617 | 2203 | 207 | 437 | 926 | 480 | 117 |
| DA10 | 5643 a | 398 | 526 | 1555 a | 1522 | 215 | 296 | 904 | 340 | 50 |
| DA11 | 5500 a | 395 | 753 a | 815 | 2036 | 255 | 336 | 1031 | 358 | 55 |
| DA12 | 5172 a | 769 a | 930 a | 684 | 2026 | 236 | 514 | 1005 | 531 | 127 |
| DA13 | 6533 a | 829 a | 881 a | 1466 a | 1543 | 200 | 696 | 1162 | 308 | 43 |
| Low Concentration Level | High Concentration Level | |||
|---|---|---|---|---|
| Element | Concentration (g/t) | Percentage to Solid Solution (%) | Concentration (g/t) | Percentage to Solid Solution (%) |
| Ni | 2900 | >100 | 5600 | 100 |
| As | 755 | 40 | 1570 | 60 |
| Sn | 350 | 32 | 750 | 9 |
| Sb | 490 | 0 | 800 | 0 |
| Anode | Impurity Phases |
|---|---|
| AC1, AC2 | AC inclusions |
| DA1 | AC inclusions + SnO2 + Kupferglimmer |
| DA2 | AC inclusions + SnO2 + Kupferglimmer |
| DA3 | AC inclusions + SnO2 + Kupferglimmer |
| DA4 | AC inclusions + SnO2 + Kupferglimmer + NiO |
| DA5 | AC inclusions + Kupferglimmer + NiO |
| DA6 | AC inclusions + SnO2 + Kupferglimmer |
| DA7 | AC inclusions + SnO2 + Kupferglimmer |
| DA8 | AC inclusions + SnO2 + Kupferglimmer |
| DA9 | AC inclusions + SnO2 + Kupferglimmer |
| DA10 | AC inclusions + SnO2 + Kupferglimmer + NiO |
| DA11 | AC inclusions + SnO2 + Kupferglimmer + NiO |
| DA12 | AC inclusions + SnO2 + Kupferglimmer + NiO |
| DA13 | AC inclusions + SnO2 + Kupferglimmer + NiO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Aragon, A.; Sánchez-Rodas, D.; Ríos, G.; Moats, M.S. Impurity Behavior in Cast Copper Anodes: Implications for Electrorefining in a Circular Economy. Metals 2025, 15, 113. https://doi.org/10.3390/met15020113
Morales-Aragon A, Sánchez-Rodas D, Ríos G, Moats MS. Impurity Behavior in Cast Copper Anodes: Implications for Electrorefining in a Circular Economy. Metals. 2025; 15(2):113. https://doi.org/10.3390/met15020113
Chicago/Turabian StyleMorales-Aragon, Agustin, Daniel Sánchez-Rodas, Guillermo Ríos, and Michael S. Moats. 2025. "Impurity Behavior in Cast Copper Anodes: Implications for Electrorefining in a Circular Economy" Metals 15, no. 2: 113. https://doi.org/10.3390/met15020113
APA StyleMorales-Aragon, A., Sánchez-Rodas, D., Ríos, G., & Moats, M. S. (2025). Impurity Behavior in Cast Copper Anodes: Implications for Electrorefining in a Circular Economy. Metals, 15(2), 113. https://doi.org/10.3390/met15020113






