Enrichment Methods for Metal Recovery from Waste from Electrical and Electronic Equipment: A Brief Review
Abstract
1. Introduction
| Region | Waste Generated (Mt) | Waste Recycled (Mt) | Recycling Rate (%) | Source |
|---|---|---|---|---|
| Asia | 26.2 | 3.1 | 11.7 | [6,8] |
| America | 14.3 | 1.3 | 9.4 | [1,9] |
| Europe | 12.2 | 5.2 | 42.5 | [1,10] |
| Africa | 3.1 | 0.03 | 0.9 | [11] |
| Oceania | 0.8 | 0.07 | 8.8 | [9,12] |
2. Research Methodology
3. Composition of Metals in WEEE
4. Current Methods for Metal Recovery
4.1. Size-Reduction Techniques
4.2. Physical Separation Technologies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hubau, A.; Chagnes, A.; Minier, M.; Touzé, S.; Chapron, S.; Guezennec, A.G. Recycling-oriented methodology to sample and characterize the metal composition of waste Printed Circuit Boards. Waste Manag. 2019, 91, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Charitopoulou, M.A.; Kalogiannis, K.G.; Lappas, A.A.; Achilias, D.S. Novel trends in the thermo-chemical recycling of plastics from WEEE containing brominated flame retardants. Environ. Sci. Pollut. Res. 2021, 28, 59190–5921328. [Google Scholar] [CrossRef] [PubMed]
- Achilias, D.S.; Antonakou, E.V. Chemical and Thermochemical Recycling of Polymers from Waste Electrical and Electronic Equipment, in: Recycling Materials Based on Environmentally Friendly Techniques; InTech: Nappanee, IN, USA, 2015. [Google Scholar] [CrossRef]
- Martelo, L.M.; Bastos, M.M.S.M.; Soares, H.M.V.M. Separation of the metallic and non-metallic fractions of waste printed circuit boards—A review focused on the organic swelling. Miner. Eng. 2024, 206, 108529. [Google Scholar] [CrossRef]
- Adrian, S.; Drisse, M.B.; Cheng, Y.; Devia, L.; Deubzer, O.; Goldizen, F.; Gorman, J.; Herat, S.; Honda, S.; Iattoni, G.; et al. Quantities, flows, and the circular economy potential. Glob. E-Waste Monit. 2020, 2020, 13–15. [Google Scholar]
- Tiwary, C.S.; Kishore, S.; Vasireddi, R.; Mahapatra, D.R.; Ajayan, P.M.; Chattopadhyay, K. Electronic waste recycling via cryo-milling and nanoparticle beneficiation. Mater. Today 2017, 20, 67–73. [Google Scholar] [CrossRef]
- Baldé, C.P.; Kuehr, R.; Yamamoto, T.; McDonald, R.; Althaf, S.; Bel, G.; Deubzer, O.; Fernandez-Cubillo, E.; Forti, V.; Gray, V.; et al. The Global E-Waste Monitor 2024. 2024. Available online: https://www.itu.int/itu-d/sites/environment (accessed on 1 December 2024).
- Hadi, P.; Gao, P.; Barford, J.P.; McKay, G. Novel application of the nonmetallic fraction of the recycled printed circuit boards as a toxic heavy metal adsorbent. J. Hazard. Mater. 2013, 252–253, 166–170. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, G.; Hao, J.; He, Y.; Zhang, T.; Yang, X. Morphology, mineralogy and separation characteristics of nonmetallic fractions from waste printed circuit boards. J. Clean. Prod. 2018, 170, 1501–1507. [Google Scholar] [CrossRef]
- Gallegos-Acevedo, P.M.; Espinoza-Cuadra, J.; Olivera-Ponce, J.M. Conventional flotation techniques to separate metallic and nonmetallic fractions from waste printed circuit boards with particles nonconventional size. J. Min. Sci. 2014, 50, 974–981. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Feng, Y.; Zhang, T.; Wang, H.; Zhu, X. Recovery of residual metals from fine nonmetallic fractions of waste printed circuit boards using a vibrated gas-solid fluidized bed. Sep. Purif. Technol. 2018, 207, 321–328. [Google Scholar] [CrossRef]
- Ellamparuthy, G.; Angadi, S.I.; Rao, D.S.; Ghosh, M.K.; Basu, S. Separation and characterization studies of end-of-life mobile printed circuit boards. Part. Sci. Technol. 2020, 39, 467–474. [Google Scholar] [CrossRef]
- Xiong, S.; Peng, Y.; Chen, K.; Lu, S.; Jiang, W.; Li, X.; Wang, F.; Cen, K. Phase distribution, migration and relationship of polychlorinated dibenzo-p-dioxins and dibenzofurans and heavy metals in a large-scale hazardous waste incinerator. J. Clean. Prod. 2022, 341. [Google Scholar] [CrossRef]
- Holgersson, S.; Steenari, B.M.; Björkman, M.; Cullbrand, K. Analysis of the metal content of small-size Waste Electric and Electronic Equipment (WEEE) printed circuit boards—Part 1: Internet routers, mobile phones and smartphones. Resour. Conserv. Recycl. 2018, 133, 300–308. [Google Scholar] [CrossRef]
- Otsuki, A.; Gonçalves, P.P.; Leroy, E. Selective Milling and Elemental Assay of Printed Circuit Board Particles for Their Recycling Purpose. Metals 2019, 9, 899. [Google Scholar] [CrossRef]
- Ernst, T.; Popp, R.; Wolf, M.; van Eldik, R. Analysis of eco-relevant elements and noble metals in printed wiring boards using AAS, ICP-AES and EDXRF. Anal. Bioanal. Chem. 2003, 375, 805–814. [Google Scholar] [CrossRef]
- Ou, Z.; Li, J.; Wang, Z. Application of mechanochemistry to metal recovery from second-hand resources: A technical overview. Environ. Sci. Process. Impacts 2015, 17, 1522–1530. [Google Scholar] [CrossRef]
- Li, J.; Shrivastava, P.; Gao, Z.; Zhang, H.C. Printed circuit board recycling: A state-of-the-art survey. IEEE Trans. Electron. Packag. Manuf. 2004, 27, 33–42. [Google Scholar] [CrossRef]
- Mir, S.; Dhawan, N. A comprehensive review on the recycling of discarded printed circuit boards for resource recovery. Resour. Conserv. Recycl. 2021, 178, 106027. [Google Scholar] [CrossRef]
- Lee, J.C.; Song, H.T.; Yoo, J.M. Present status of the recycling of waste electrical and electronic equipment in Korea. Resour. Conserv. Recycl. 2007, 50, 380–397. [Google Scholar] [CrossRef]
- Otsuki, A.; La Mensbruge, L.D.; King, A.; Serranti, S.; Fiore, L.; Bonifazi, G. Non-destructive characterization of mechanically processed waste printed circuit boards—Particle liberation analysis. Waste Manag. 2020, 102, 510–519. [Google Scholar] [CrossRef]
- Yamane, L.H.; de Moraes, V.T.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of WEEE: Characterization of spent printed circuit boards from mobile phones and computers. Waste Manag. 2011, 31, 2553–2558. [Google Scholar] [CrossRef]
- Kaya, M. Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Manag. 2016, 57, 64–90. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, M.; Yaghmaei, S.; Mousavi, S.M. Content evaluation of different waste PCBs to enhance basic metals recycling. Resour. Conserv. Recycl. 2018, 139, 298–306. [Google Scholar] [CrossRef]
- Ogunniyi, I.O.; Vermaak, M.K.G.; Groot, D.R. Chemical composition and liberation characterization of printed circuit board comminution fines for beneficiation investigations. Waste Manag. 2009, 29, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, V.; Wen, X.; Li, S.; Tao, D. Recovery of copper from waste printed circuit boards. Min. Metall. Explor. 2004, 21, 99–102. [Google Scholar] [CrossRef]
- Zhang, S.; Forssberg, E. Mechanical separation-oriented characterization of electronic scrap. Resour. Conserv. Recycl. 1997, 21, 247–269. [Google Scholar] [CrossRef]
- Wen, X.; Zhao, Y.; Duan, C.; Zhou, X.; Jiao, H.; Song, S. Study on Metals Recovery from Discarded Printed Circuit Boards by Physical Methods. In Proceedings of the 2005 IEEE International Symposium on Electronics and the Environment, New Orleans, LA, USA, 16–19 May 2005. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.; Cho, H.; Ok, J.; Kim, S. Shredding and liberation characteristics of refrigerators and small appliances. Waste Manag. 2017, 59, 409–421. [Google Scholar] [CrossRef]
- Chancerel, P.; Meskers, C.E.M.; Hagelüken, C.; Rotter, V.S. Assessment of precious metal flows during preprocessing of waste electrical and electronic equipment. J. Ind. Ecol. 2009, 13, 791–810. [Google Scholar] [CrossRef]
- Touze, S.; Guignot, S.; Hubau, A.; Devau, N.; Chapron, S. Sampling waste printed circuit boards: Achieving the right combination between particle size and sample mass to measure metal content. Waste Manag. 2020, 118, 380–390. [Google Scholar] [CrossRef]
- Oliveira, P.C.; Cabral, M.; Nogueira, C.A.; Margarido, F. Printed circuit boards recycling: Characterization of granulometric fractions from shredding process. Mater. Sci. Forum 2010, 636–637, 1434–1439. [Google Scholar] [CrossRef]
- Li, J.; Duan, H.; Yu, K.; Liu, L.; Wang, S. Characteristic of low-temperature pyrolysis of printed circuit boards subjected to various atmosphere. Resour. Conserv. Recycl. 2010, 54, 810–815. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.; Park, J. Grinding characteristics of waste printed circuit boards in hammer mill using population balance model. Geosystem Eng. 2021, 24, 173–179. [Google Scholar] [CrossRef]
- Gao, R.; Xu, Z. Pyrolysis and utilization of nonmetal materials in waste printed circuit boards: Debromination pyrolysis, temperature-controlled condensation, and synthesis of oil-based resin. J. Hazard. Mater. 2019, 364, 1–10. [Google Scholar] [CrossRef]
- Guo, C.; Wang, H.; Liang, W.; Fu, J.; Yi, X. Liberation characteristic and physical separation of printed circuit board (PCB). Waste Manag. 2011, 31, 2161–2166. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Ruiz, R.H.; Flores-Campos, R.; Gámez-Altamirano, H.A.; Velarde-Sánchez, E.J. Separation of the metallic and non-metallic fraction from printed circuit boards employing green technology. J. Hazard. Mater. 2016, 311, 91–99. [Google Scholar] [CrossRef]
- Hanafi, J.; Jobiliong, E.; Christiani, A.; Soenarta, D.C.; Kurniawan, J.; Irawan, J. Material Recovery and Characterization of PCB from Electronic Waste. Procedia Soc. Behav. Sci. 2012, 57, 331–338. [Google Scholar] [CrossRef]
- Gharde, S.; Kandasubramanian, B. Mechanothermal and chemical recycling methodologies for the Fibre Reinforced Plastic (FRP). Environ. Technol. Innov. 2019, 14, 100311. [Google Scholar] [CrossRef]
- Al Razi, K.M.H. Resourceful recycling process of waste desktop computers: A review study. Resour. Conserv. Recycl. 2016, 110, 30–47. [Google Scholar] [CrossRef]
- Qiu, R.; Lin, M.; Ruan, J.; Fu, Y.; Hu, J.; Deng, M.; Tang, Y.; Qiu, R. Recovering full metallic resources from waste printed circuit boards: A refined review. J. Clean. Prod. 2020, 244, 118690. [Google Scholar] [CrossRef]
- Duan, H.; Hou, K.; Li, J.; Zhu, X. Examining the technology acceptance for dismantling of waste printed circuit boards in light of recycling and environmental concerns. J. Environ. Manag. 2011, 92, 392–399. [Google Scholar] [CrossRef]
- Franke, D.M.; Suponik, T.; Nuckowski, P.M.; Dubaj, J. Evaluation of the Efficiency of Metal Recovery from Printed Circuit Boards using Gravity Processes. Physicochem. Probl. Miner. Process. 2021, 57, 63–77. [Google Scholar] [CrossRef]
- Nekouei, R.K.; Pahlevani, F.; Rajarao, R.; Golmohammadzadeh, R.; Sahajwalla, V. Direct transformation of waste printed circuit boards to nano-structured powders through mechanical alloying. Mater. Des. 2018, 141, 26–36. [Google Scholar] [CrossRef]
- Suponik, T.; Franke, D.M.; Nuckowski, P.M.; Matusiak, P.; Kowol, D.; Tora, B. Impact of grinding of printed circuit boards on the efficiency of metal recovery by means of electrostatic separation. Minerals 2021, 11, 281. [Google Scholar] [CrossRef]
- Zhou, C.; Pan, Y.; Lu, M.; Yang, C. Liberation characteristics after cryogenic modification and air table separation of discarded printed circuit boards. J. Hazard. Mater. 2016, 311, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, K. Shaking Table. In The ECPH Encyclopedia of Mining and Metallurgy; Xu, K., Ed.; Springer Nature: Singapore, 2022; pp. 1–2. [Google Scholar] [CrossRef]
- Arshadi, M.; Yaghmaei, S.; Mousavi, S.M. Optimal electronic waste combination for maximal recovery of Cu-Ni-Fe by Acidithiobacillus ferrooxidans. J. Clean. Prod. 2019, 240, 118077. [Google Scholar] [CrossRef]
- Burat, F.; Özer, M. Physical separation route for printed circuit boards. Physicochem. Probl. Miner. Process. 2018, 54, 554–566. [Google Scholar] [CrossRef]
- Liu, W.; Liang, C.; Qin, W.Q.; Jiao, F. A new technology for recovery of metals from waste printed circuit boards. Appl. Mech. Mater. 2014, 675-677, 698–703. [Google Scholar] [CrossRef]
- Suponik, T.; Franke, D.; Nuckowski, P. Electrostatic and magnetic separations for the recovery of metals from electronic waste. IOP Conf. Series Mater. Sci. Eng. 2019, 641, 012017. [Google Scholar] [CrossRef]
- Veit, H.M.; Diehl, T.R.; Salami, A.P.; Rodrigues, J.S.; Bernardes, A.M.; Tenório, J.A.S. Utilization of magnetic and electrostatic separation in the recycling of printed circuit boards scrap. Waste Manag. 2005, 25, 67–74. [Google Scholar] [CrossRef]
- Hamerski, F.; Krummenauer, A.; Bernardes, A.M.; Veit, H.M. Improved settings of a corona-electrostatic separator for copper concentration from waste printed circuit boards. J. Environ. Chem. Eng. 2019, 7, 102896. [Google Scholar] [CrossRef]
- Kelly, E.G.; Spottiswood, D.J. The theory of electrostatic separations: A review part II. Particle charging. Miner. Eng. 1989, 2, 193–205. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Z. Precious metals recovery from waste printed circuit boards: A review for current status and perspective. Resour. Conserv. Recycl. 2016, 113, 28–39. [Google Scholar] [CrossRef]
- Ghosh, B.; Ghosh, M.K.; Parhi, P.; Mukherjee, P.S.; Mishra, B.K. Waste Printed Circuit Boards recycling: An extensive assessment of current status. J. Clean. Prod. 2015, 94, 5–19. [Google Scholar] [CrossRef]
- Huang, K.; Guo, J.; Xu, Z. Recycling of waste printed circuit boards: A review of current technologies and treatment status in China. J. Hazard. Mater. 2009, 164, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Richard, G.; Medles, K.; Zeghloul, T.; Dascalescu, L. Distinct recovery of copper and aluminum from waste electric wires using a roll-type electrostatic separator. Waste Manag. 2018, 76, 207–216. [Google Scholar] [CrossRef]
- Lungu, M. Separation of small nonferrous particles using an angular rotary drum eddy-current separator with permanent magnets. Int. J. Miner. Process. 2005, 78, 22–30. [Google Scholar] [CrossRef]
- Rolicz, P. Eddy currents generated in a system of two cylindrical conductors by a transverse alternating magnetic field. Electr. Power Syst. Res. 2009, 79, 295–300. [Google Scholar] [CrossRef]
- Cui, J.; Forssberg, E. Mechanical recycling of waste electric and electronic equipment: A review. J. Hazard. Mater. 2003, 99, 243–263. [Google Scholar] [CrossRef]
- Ruan, J.; Li, J.; Xu, Z. An environmental friendly recovery production line of waste toner cartridges. J. Hazard. Mater. 2011, 185, 696–702. [Google Scholar] [CrossRef]
- Ruan, J.; Xu, Z. Environmental friendly automated line for recovering the cabinet of waste refrigerator. Waste Manag. 2011, 31, 2319–2326. [Google Scholar] [CrossRef]
- Smith, Y.R.; Nagel, J.R.; Rajamani, R.K. Eddy current separation for recovery of non-ferrous metallic particles: A comprehensive review. Miner. Eng. 2019, 133, 149–159. [Google Scholar] [CrossRef]
- Schlett, Z.; Lungu, M. Eddy-current separator with inclined magnetic disc. Miner. Eng. 2002, 15, 365–367. [Google Scholar] [CrossRef]
- Rem, P.; Leest, P.; van den Akker, A. A model for eddy current separation. Int. J. Miner. Process. 1997, 49, 193–200. [Google Scholar] [CrossRef]
- Maraspin, F.; Bevilacqua, P.; Rem, P.C. Modelling the throw of metals and nonmetals in eddy current separations. Int. J. Miner. Process. 2004, 73, 1–11. [Google Scholar] [CrossRef]
- Ogunniyi, I.O.; Vermaak, M.K.G. Froth flotation for beneficiation of printed circuit boards comminution fines: An overview. Miner. Process. Extr. Metall. Rev. 2009, 30, 101–121. [Google Scholar] [CrossRef]
- Ari, V.; Vidyadhar, A.; Das, A. Kinetics and efficacy of froth flotation for the recovery of metal values from pulverized printed circuit boards. In Proceedings of the XXVI International Mineral Processing Congress (IMPC-2012), New Delhi, India, 24–28 September 2012; pp. 236–242. [Google Scholar]
- Kumar, V.; Lee, J.C.; Jeong, J.; Jha, M.K.; Kim, B.S.; Singh, R. Recycling of printed circuit boards (PCBs) to generate enriched rare metal concentrate. J. Ind. Eng. Chem. 2015, 21, 805–813. [Google Scholar] [CrossRef]
- He, J.; Duan, C. Recovery of metallic concentrations from waste printed circuit boards via reverse floatation. Waste Manag. 2017, 60, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Pawliszak, P.; Bradshaw-Hajek, B.H.; Skinner, W.; Beattie, D.A.; Krasowska, M. Frothers in flotation: A review of performance and function in the context of chemical classification. Miner. Eng. 2024, 207, 108567. [Google Scholar] [CrossRef]
- Flores-Campos, R.; Estrada-Ruiz, R.H.; Velarde-Sánchez, E.J. Study of the physicochemical effects on the separation of the non-metallic fraction from printed circuit boards by inverse flotation. Waste Manag. 2017, 69, 400–406. [Google Scholar] [CrossRef]
- Mallampati, S.R.; Heo, J.H.; Park, M.H. Hybrid selective surface hydrophilization and froth flotation separation of hazardous chlorinated plastics from E-waste with novel nanoscale metallic calcium composite. J. Hazard. Mater. 2016, 306, 13–23. [Google Scholar] [CrossRef]
- Güney, A.; Özdilek, C.; Kangal, M.O.; Burat, F. Flotation characterization of PET and PVC in the presence of different plasticizers. Sep. Purif. Technol. 2015, 151, 47–56. [Google Scholar] [CrossRef]
- Bueno-Tokunaga, A.; Pérez-Garibay, R.; Martínez-Carrillo, D. Zeta potential of air bubbles conditioned with typical froth flotation reagents. Int. J. Miner. Process. 2015, 140, 50–57. [Google Scholar] [CrossRef]
- Kaya, M. Sorting and Separation of WPCBs. In Electronic Waste and Printed Circuit Board Recycling Technologies; Springer International Publishing: Cham, Switzerland, 2019; pp. 143–176. [Google Scholar] [CrossRef]
- Bilesan, M.R.; Makarova, I.; Wickman, B.; Repo, E. Efficient separation of precious metals from computer waste printed circuit boards by hydrocyclone and dilution-gravity methods. J. Clean. Prod. 2021, 286, 125505. [Google Scholar] [CrossRef]
- Bae, M.; Kim, S.; Lee, J.C. Gold Recovery from Waste Solutions of PCBs Gold Plating Process Using Hydro Cyclone Reactor for Demonstration Study. In Proceedings of the 3rd Pan American Materials Congress, San Diego, CA, USA, 26 February–2 March 2017. [Google Scholar] [CrossRef]
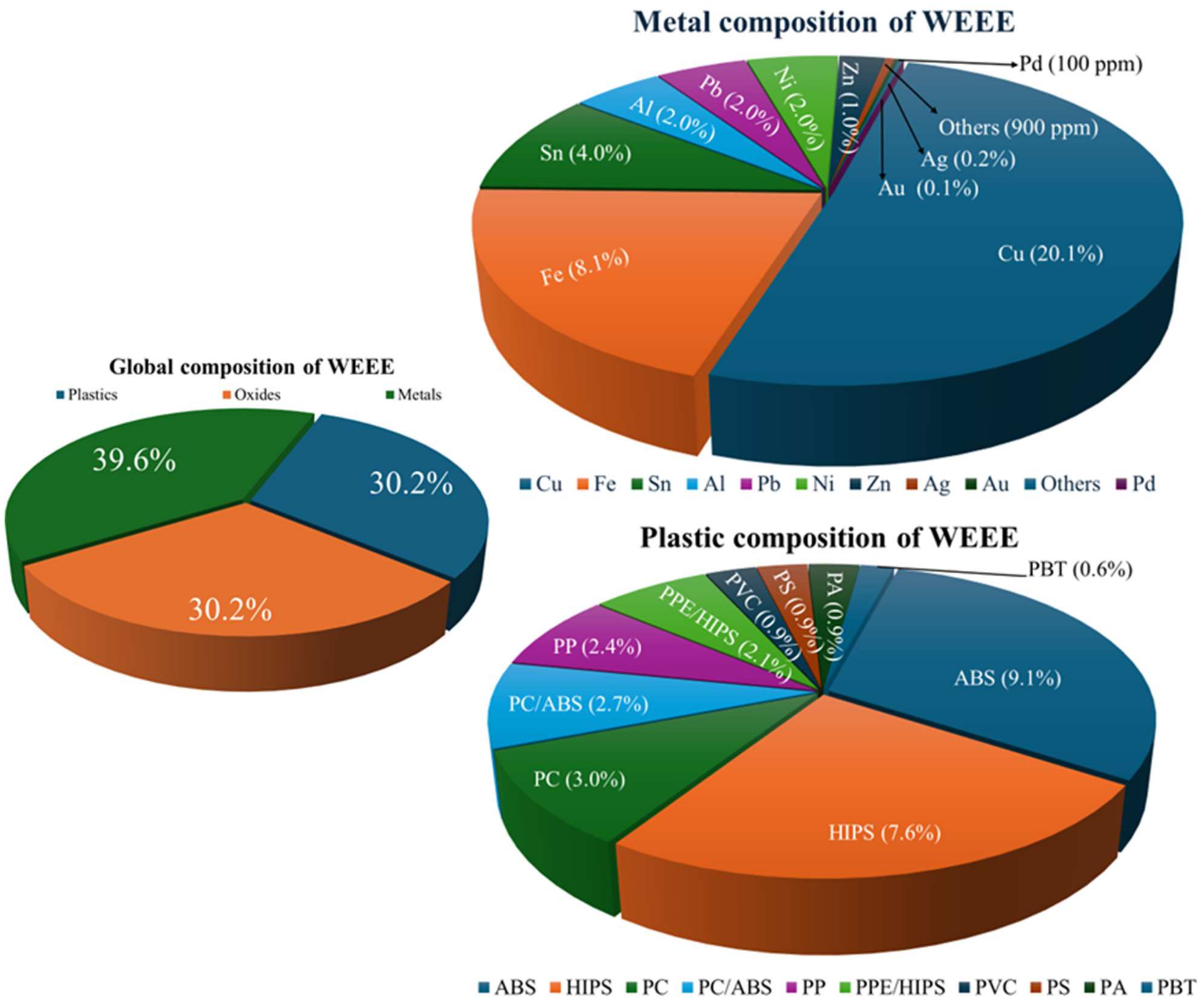


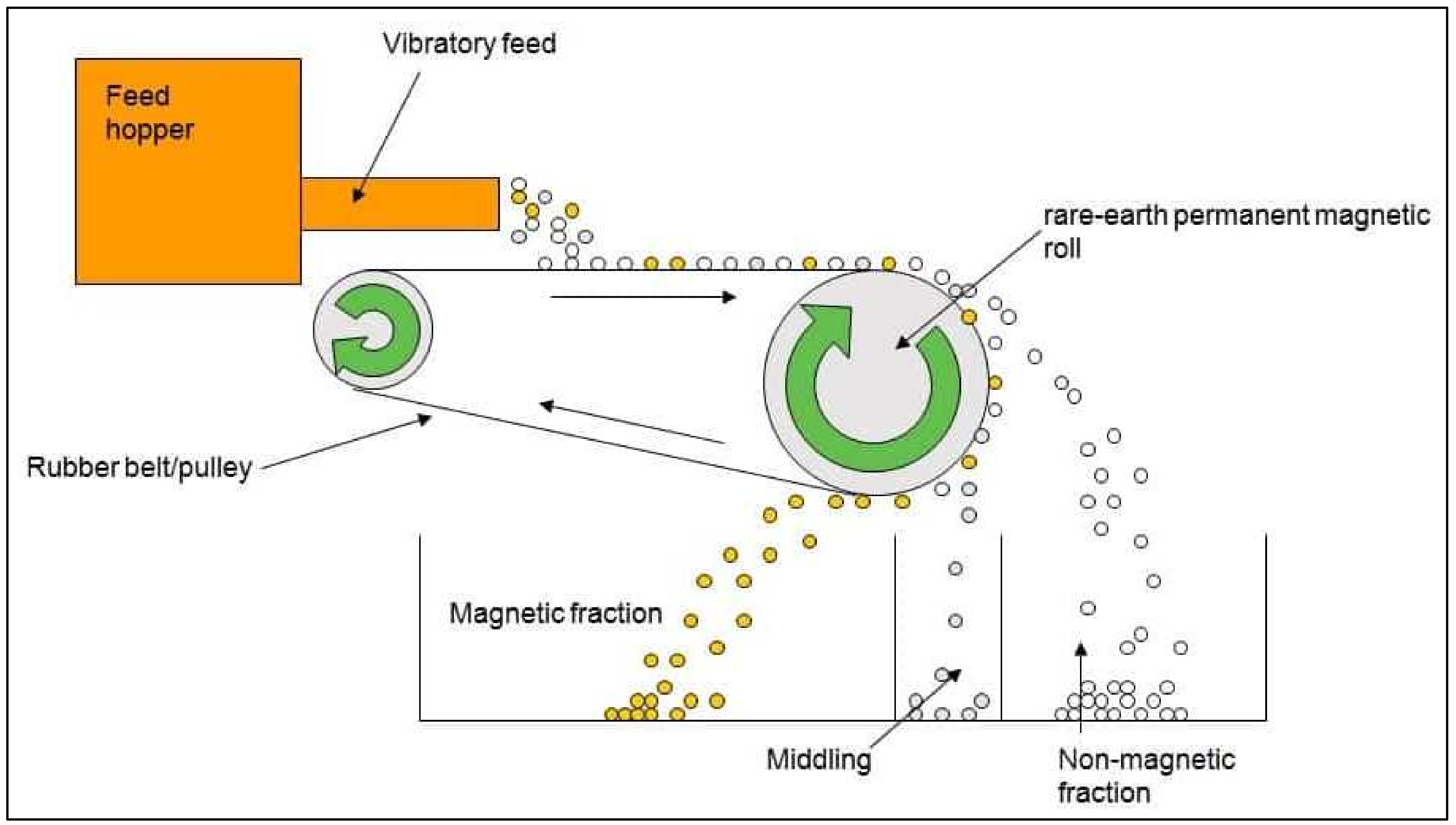
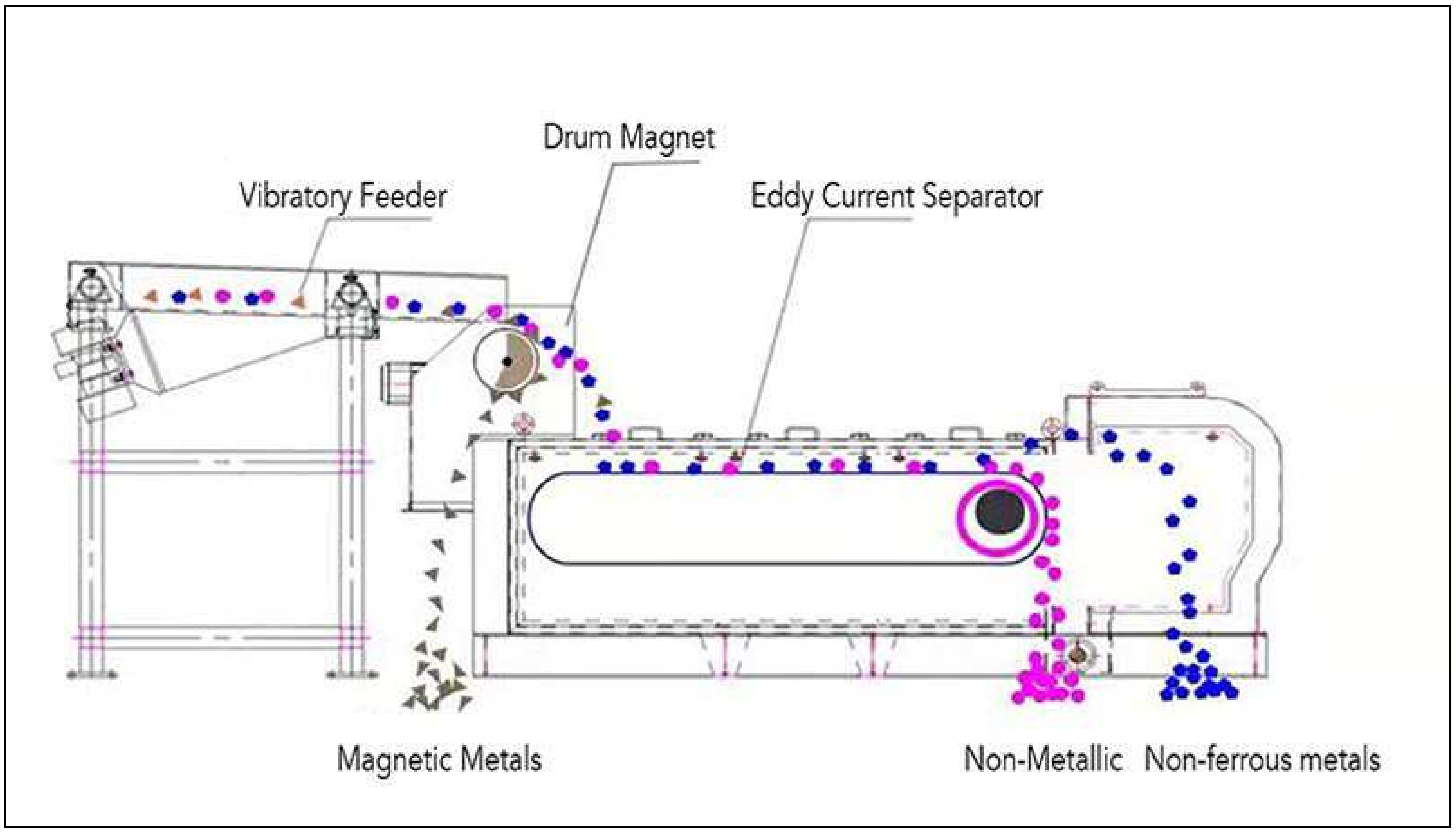
| Metal | Large Household Appliances | Small Household Appliances | IT and Telecommunications Equipment | Consumer Equipment |
|---|---|---|---|---|
| Cu (%) | 15–30 | 12–25 | 20–45 | 18–30 |
| Au (ppm) | 20–100 | 3–100 | 80–300 | 5–200 |
| Ag (ppm) | 200–1000 | 100–800 | 30–1500 | 30–1000 |
| Pd (ppm) | 1–5 | 1–3 | 3–7 | 2–5 |
| Pb (%) | 2–5 | 3–7 | 5–8 | 3–6 |
| Ni (%) | 1–5 | 0.5–3 | 1–4 | 1–3 |
| Sn (%) | 1–4 | 2–6 | 2–5 | 1.5–4 |
| Fe (%) | 25–45 | 20–30 | 10–20 | 15–25 |
| Zn (%) | 2–5 | 1–4 | 1–3 | 2–4 |
| Al (%) | 5–15 | 3–12 | 7–15 | 4–10 |
| Pt (%) | 1–10 | 1–5 | 2–20 | 1–10 |
| Author | Reduction Technology | Equipment | Particle Size, Min–Max (mm) | |
|---|---|---|---|---|
| Gao [35] | Crusher cut and impact action | SCP180-2 plastic crusher and FZ102 micro-plant | 1.25 | |
| Guo Chao [36] | Crushing | 0.5 | 1.25 | |
| Estrada-Ruiz [37] | Crushing | 0.25 | ||
| Hanafi [38] | Hammer mill | 0.25 | 0.5 | |
| Ellamparuthy [12] | Hammer mill | 0.015 | 0.02 | |
| Gharde [39] | Hammer Mill | Rivakka | 0.25 | |
| Al Razi [40] | Shredding | 3 | 5 | |
| Chancerel [30] | Shredding | 2.5 | 9 | |
| Lee [20] | Shredding | 3 steps | 19 | 45 |
| Qiu [41] | Shredding/Impact grinding | 0.6 | 1.2 | |
| Duan [42] | Wet impact crusher | Hammer mill with a water medium | 0.25 | 2.2 |
| Franke [43] | Knife mill | LMN-100 knife mill | <0.09 | 1.4 |
| Lee [34] | Hammer mill | Lab scale mill (not commercial mill) | 0.1 | 1.3 |
| Electrostatic Separator | Conditions | Reference |
|---|---|---|
| Boxmag-rapid LTC | Drum speed: 50 rpm Drum-to-electrode distance: 5 cm Electrode voltage: 20 kV | Suponik et al. [51] |
| Corona ES Inbraz-Eriez ESP-14/01S | Drum speed: 50–80 rpm Drum-to-corona-electrode distance: 4–6 cm Drum-to-electrostatic-electrode distance: 5–7 cm Corona electrode angle: 20–40° Electrostatic electrode angle: 55–75° Electrode voltage: 20–30 kV Feedstock rate: 30 g/min Relative humidity: 40–50% | Hamerski et al. [53] |
| Corona ES Equimag ES 1010 | Drum speed: 85 rpm Drum-to-corona-electrode distance: 2.5 cm Drum-to-electrostatic-electrode distance: 2.5 cm Corona electrode angle: 52.5° Electrostatic electrode angle: 80° Electrode voltage: 45–46 kV | Veit et al. [52] |
| Corona ES | Drum-to-corona-electrode distance: 9.6 cm Drum-to-electrostatic-electrode distance: 7.7 cm Corona electrode angle: 20° Electrostatic electrode angle: 60° Electrode voltage: 20–30 kV | Huan et al. [57] |
| Corona ES | Corona electrode angle: 20° Electrostatic electrode angle: 60° Electrode voltage: 20–30 kV | Kaya et al. [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chicardi, E.; Lopez-Paneque, A.; García-Orta, V.H.G.; Sepúlveda-Ferrer, R.E.; Gallardo, J.M. Enrichment Methods for Metal Recovery from Waste from Electrical and Electronic Equipment: A Brief Review. Metals 2025, 15, 140. https://doi.org/10.3390/met15020140
Chicardi E, Lopez-Paneque A, García-Orta VHG, Sepúlveda-Ferrer RE, Gallardo JM. Enrichment Methods for Metal Recovery from Waste from Electrical and Electronic Equipment: A Brief Review. Metals. 2025; 15(2):140. https://doi.org/10.3390/met15020140
Chicago/Turabian StyleChicardi, Ernesto, Antonio Lopez-Paneque, Victoria Humildad Gallardo García-Orta, Ranier Enrique Sepúlveda-Ferrer, and Jose Maria Gallardo. 2025. "Enrichment Methods for Metal Recovery from Waste from Electrical and Electronic Equipment: A Brief Review" Metals 15, no. 2: 140. https://doi.org/10.3390/met15020140
APA StyleChicardi, E., Lopez-Paneque, A., García-Orta, V. H. G., Sepúlveda-Ferrer, R. E., & Gallardo, J. M. (2025). Enrichment Methods for Metal Recovery from Waste from Electrical and Electronic Equipment: A Brief Review. Metals, 15(2), 140. https://doi.org/10.3390/met15020140







