Use of Sn91Zn9 Lead-Free Solder in Resistance Element Soldering Technology
Abstract
:1. Introduction
2. Materials and Methods
| Material | C (wt.%) | Si (w.%) | Mn (wt.%) | P (wt.%) | S (w.%) | Nb (wt.%) | Ti (wt.%) | Al (wt.%) |
|---|---|---|---|---|---|---|---|---|
| HX220BD-100MBO | 0.1 | 0.5 | 0.7 | 0.08 | 0.025 | 0.09 | 0.12 | 0.1 |
| Material | RP0.2 (MPa) | Rm (MPa) | A80 (%) |
|---|---|---|---|
| HX220BD-100MBO | 220–280 | 320–400 | 32 |
| Tensile Strength ISO 527-2 [20] (MPa) | Flexural Strength ISO 178 (MPa) | Elongation at Break ISO 527-2 (%) | Impact Strength Charpy Unnotched ISO 179-1 (KJ/m2) | Vicat Temperature (B50) * ISO 306 (°C) | Maximal Temperature for Short Term Use (°C) | Degradation Temperature (°C) |
|---|---|---|---|---|---|---|
| 70 | 115 | 4 | 17 | 105 | 90 | >280 |
| Material | Sn | Pb | Cu | Zn | Ag | Sb | Bi | Cd |
|---|---|---|---|---|---|---|---|---|
| (wt.%) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | |
| Sn91Zn9 | 91 | 0.1 | 0.05 | 8.5–9.5 | 0.1 | 0.1 | 0.1 | 0.002 |
| Material | Density (g/cm3) | Yield Strength Re (MPa) | Ultimate Tensile Strength Rm (MPa) | Elongation at Break A (%) |
|---|---|---|---|---|
| Cu | 8.96 | 55.5 | 238 | 43.7 |
| Sn91Zn9 | 7.2 | 41.5 | 58.8 | 52.2 |
- -
- amount of heat on the volume of remelted solder in the bimetallic elements;
- -
- amount of heat on the appearance of the joined materials;
- -
- process parameters on the strength of the joints;
- -
- process parameters on the structure of the joints and the fracture surfaces.
3. Results and Discussion
4. Conclusions
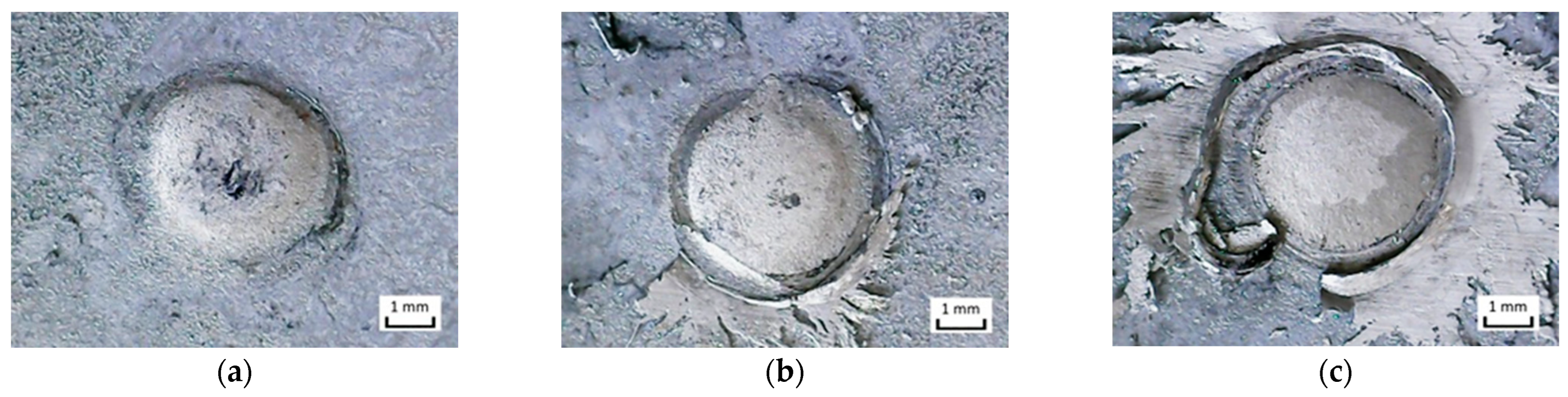
- The soldering time (heat input) has a significant effect on the spattering of Sn91Zn9 solder around the joint. At soldering times of 0.1 to 0.3 s (Q = 291 to 901 J), spatter appeared only occasionally. At the heating time t = 0.4 s, the solder spatter was already a permanent manifestation of the heating of the bimetallic element.
- The flux used as well as the soldering conditions (process parameters) ensured good wetting of the base material with molten solder without the appearance of cracks or other imperfections at the solder/steel sheet interface.
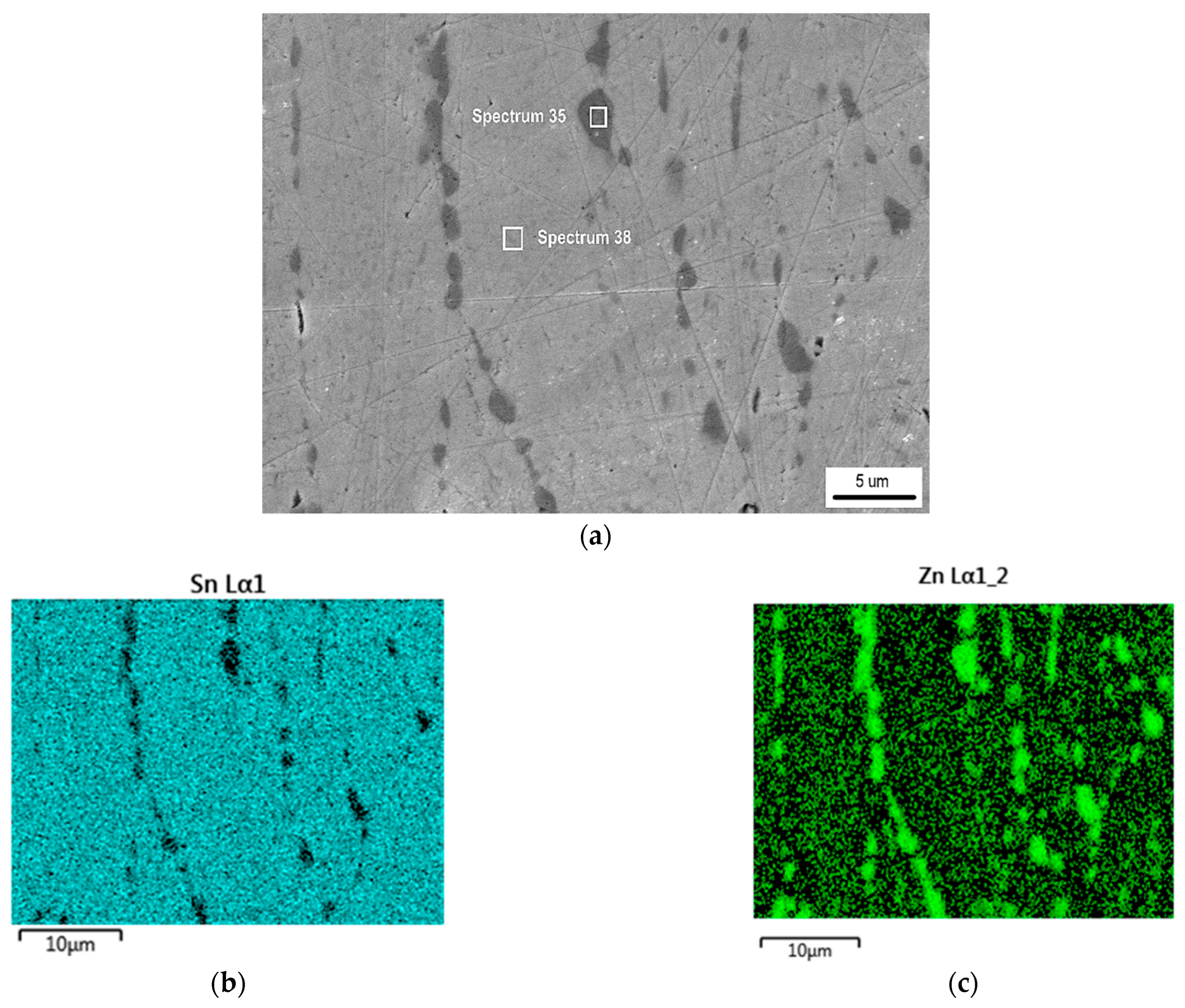
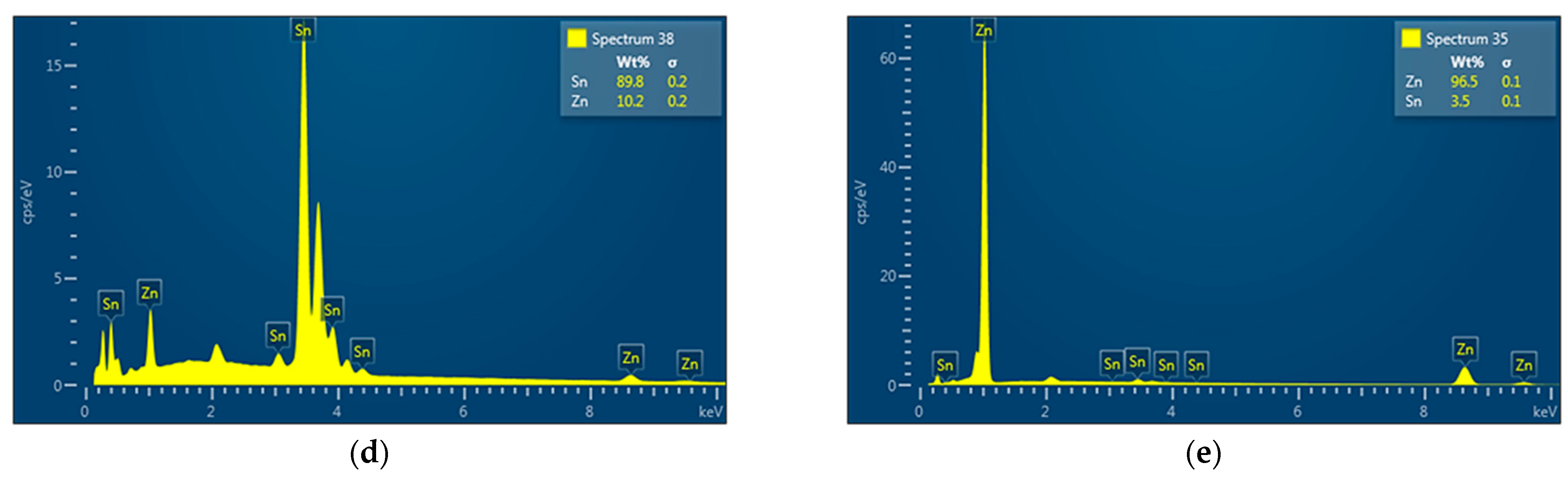
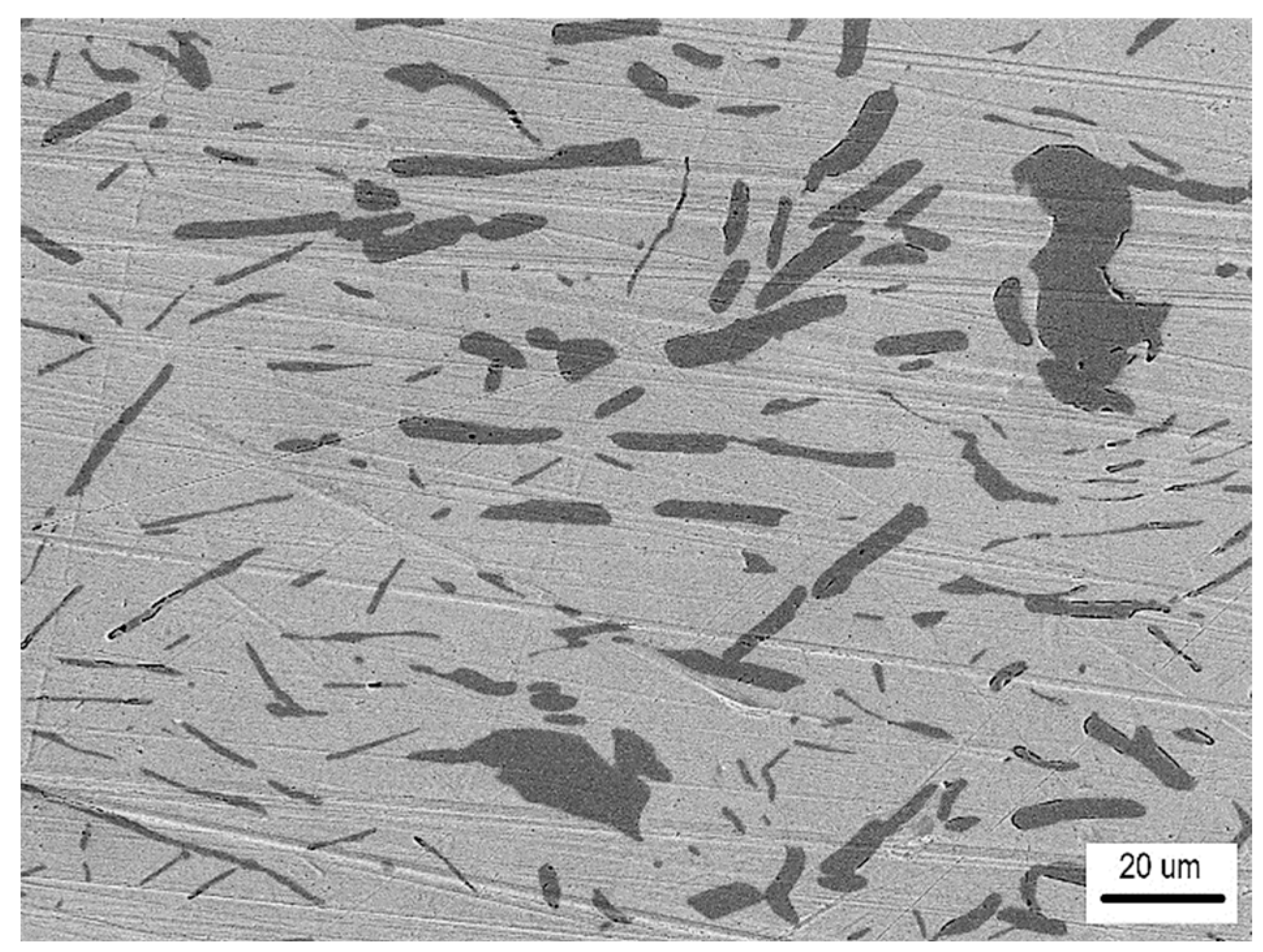
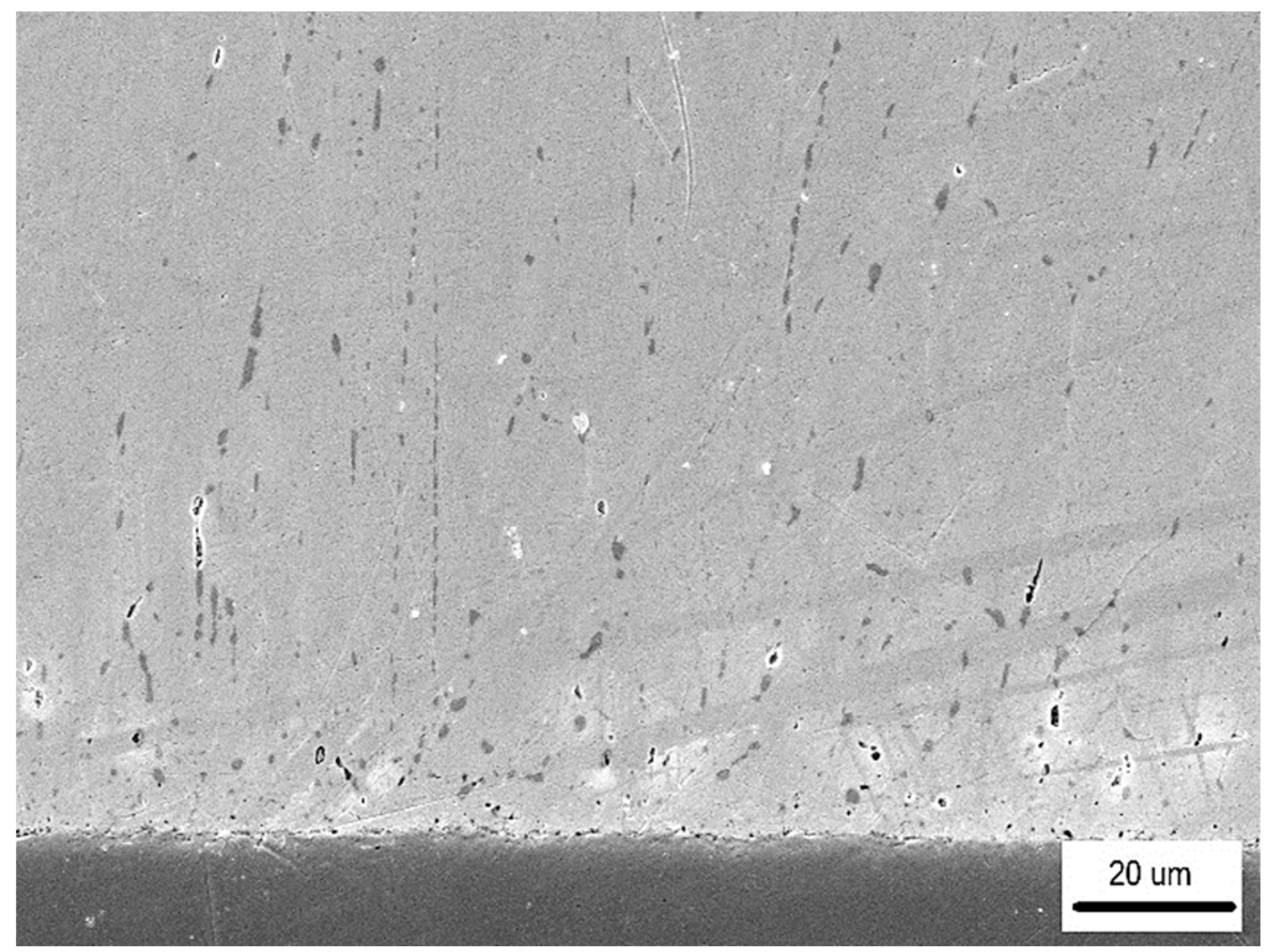
- The detected Sn91Zn9 solder structure in the core of the bimetallic element corresponded to the Sn–Zn equilibrium binary diagram. Due to the almost eutectic composition, the structure of the solder was formed by a mechanical mixture of solid solutions α-Zn and β-Sn. The difference between the structure of the solder before and after remelting under resistance heating was in the grain size. In the non-remelted (as-cast and unfused) core volume of the bimetallic element, the zinc phases were 3.1 to 46.6 µm in size, in the remelted core volume they were 1.4 to 7.2 µm in size. Differences in grain size were caused by different temperature cycles during the production of semi-finished products for bimetallic elements by centrifugal casting and during soldering. The different grain sizes enabled a relatively accurate evaluation of the influence of the amount of heat generated during soldering on the volume of remelted solder in the core of the bimetallic element. It ranged from 11.2% (of the total core volume) at a soldering time of 0.1 s (Q = 291 J) up to 52.1% at a soldering time of 0.3 s (Q = 901 J).
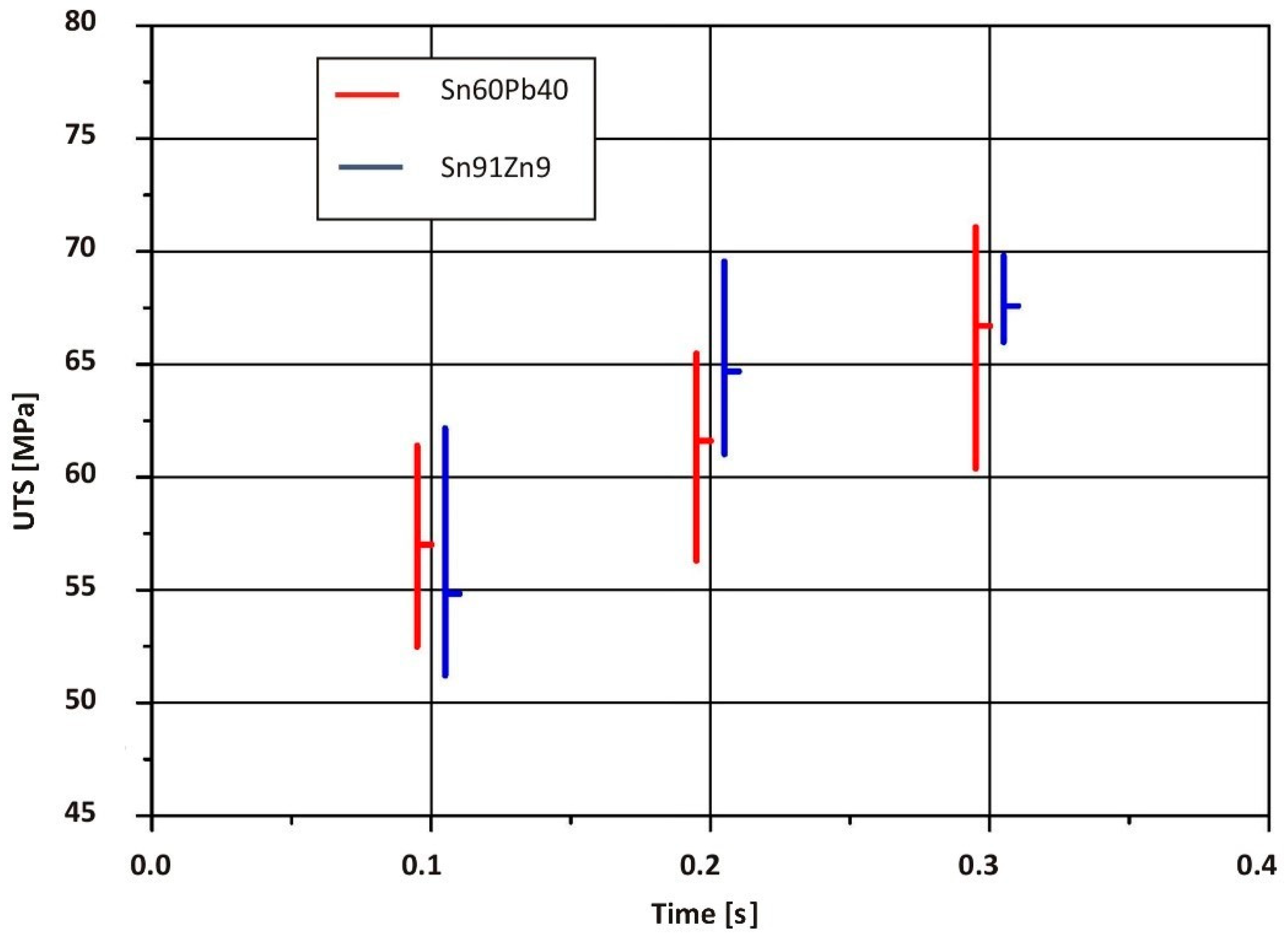
- Due to the solder joint being greater in strength than the of the PMMA thermoplastic sheet, the plastic on the tensile test samples was replaced with AW-1050A aluminum alloy sheet. The results showed that the strength of RES joints is primarily determined by heat input during soldering. The highest average ultimate tensile strength of joints made with a Cu/Sn91Zn9 bimetallic element (55.14 MPa) was obtained at a soldering time of 0.3 s (Q = 901 J). The obtained value in this case represented 94% of the Sn91Zn9 solder strength. After comparing the average ultimate tensile strength of RES joints, which were made using Cu/Sn91Zn9 and Cu/Sn60Pb40 bimetallic elements, we can conclude that the strength of the joints when using Sn91Zn9 lead-free solder was slightly higher. When Sn60Pb40 solder was used, the maximum joint strength was 53.4 MPa, which represented 87% of the solder strength [1].
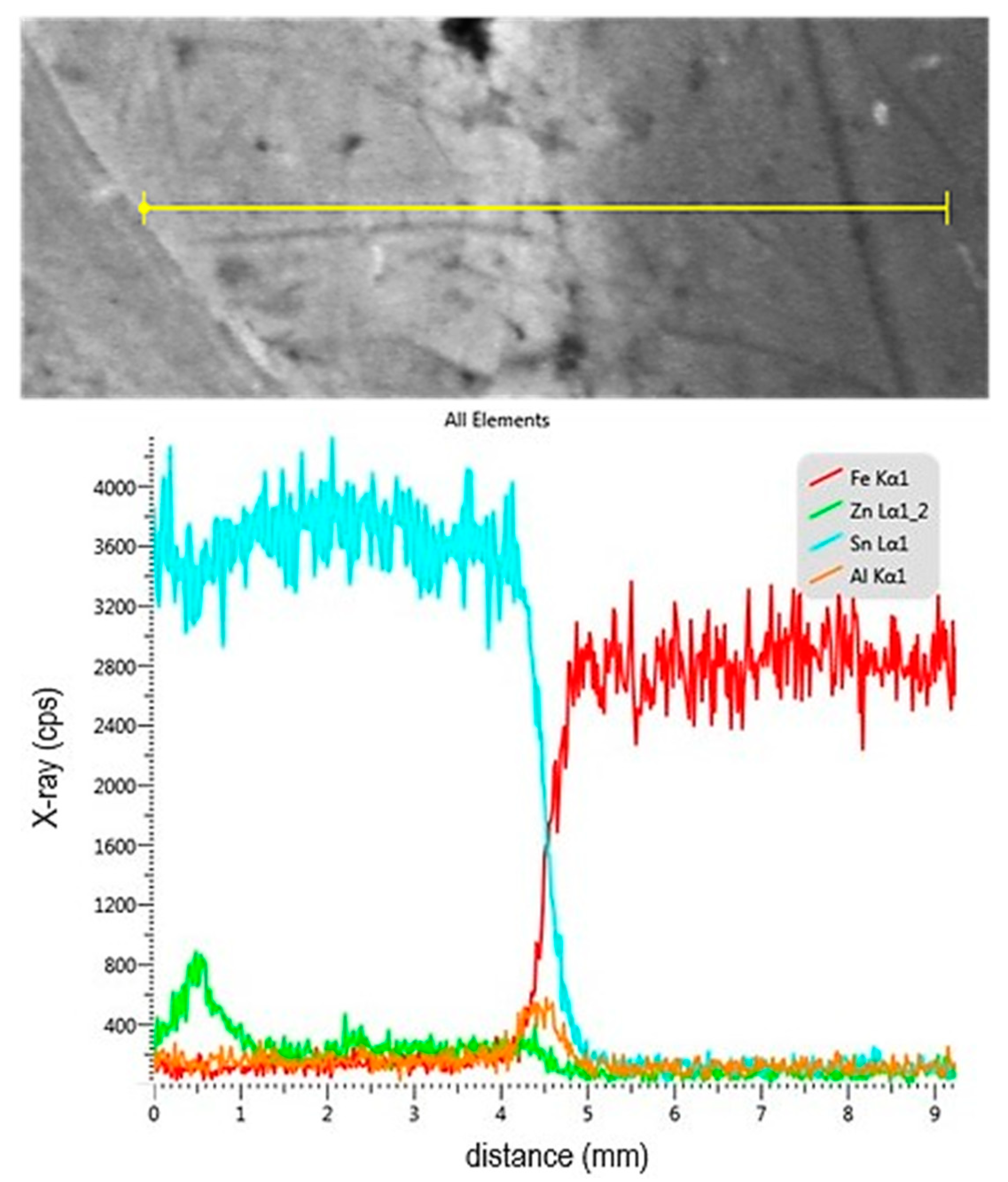
- The topography of the fracture surface of the Sn91Zn9 solder showed the character of a ductile fracture. By dissolving the zinc coating of the steel sheet with liquid solder, the diffusion zone of the solder was saturated with zinc and aluminum because aluminum is an important element in galvanizing baths. However, the diffusion area was very narrow, reaching a thickness of about 1 µm. Zinc saturation was significant, and the portion of the element increased from 9 to 28%. By increasing the content of Zn in the diffusion area of the solder, a decrease in the deformation capabilities of the Sn91Zn9 solder can be expected.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sejč, P.; Vanko, B.; Schrek, A.; Gábrišová, Z. Use of Sn60Pb40 Solder in Resistance Element Soldering Technology. Metals 2023, 13, 1313. [Google Scholar] [CrossRef]
- Meschut, G.; Hahn, O.; Janzen, V.; Olfermann, T. Innovative joining technologies for multi-material structures. Weld. World 2014, 58, 65–75. [Google Scholar] [CrossRef]
- Soldering Handbook, 3rd ed.; American Welding Society: Doral, FL, USA, 1999; ISBN 0-87171-618-6.
- Directive 2002/95/EC of the European Parliament and of the Council of 27 January 2003 on the Restriction Of the use of certain Hazardous Substances in Electrical and Electronic Equipment (OJ L 37, 13.2.2003, p. 19). Available online: https://eur-lex.europa.eu/eli/dir/2002/95/oj/eng (accessed on 15 March 2024).
- Directive 2002/96/EC of the European Parliament and of the Council of 27 January 2003 on Waste Electrical and Electronic Equipment (WEEE) (OJ L 37, 13.2.2003, p. 24). Available online: https://eur-lex.europa.eu/eli/dir/2002/96/oj/eng (accessed on 15 March 2024).
- Directive 2005/32/EC of the European Parliament and of the Council of 6 July 2005 Establishing a Framework for the Setting of ECO Design Requirements for Energy-Using Products and Amending Council Directive 92/57/EC and 2000/55/EC of the European Parliament and of the Council (OJ L 191, 22.7.2005, p. 29). Available online: https://eur-lex.europa.eu/eli/dir/2005/32/oj/eng (accessed on 15 March 2024).
- Directive 2011/65/EU of the European Parliament and of the Council of 8 June 2011 on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment (Recast). Official Journal of the European Union, 1.7.2011, L 174/88. Available online: https://eur-lex.europa.eu/eli/dir/2011/65/oj/eng (accessed on 15 March 2024).
- Available online: https://www.designchainassociates.com/ (accessed on 30 April 2024).
- China ROHS Solutions by Design Chain Associates, Retrieved 3 July 2015. Available online: http://www.chinarohs.com (accessed on 30 April 2024).
- Available online: https://calrecycle.ca.gov/electronics/statutes/ (accessed on 30 March 2024).
- Bath, J. Lead-Free Soldering; Springer Science+Business Media: Berlin, Germany, 2007; ISBN 978-0-387-32466-1. [Google Scholar]
- Plevachuk, Y.; Sklyarchuk, V.; Svec, P.S.; Svec, P.; Janickovic, D.; Illekova, E.; Yakymovych, A. Thermophysical structure-sensitive properties of Tin–Zinc alloys. J. Mater. Sci. Mater. Electron. 2017, 28, 750–759. [Google Scholar] [CrossRef]
- Miyazaki, M.; Ogata, S.; Katayama, N. Influences of Impurities on Wave Soldering Properties for Sn-9Zn Solders. In Proceedings of the 4th International Symposium on Environmentally Conscious Design and Inverse Manufacturing (EcoDesign 05), Tokyo, Japan, 12–14 December 2005. [Google Scholar] [CrossRef]
- Subramanian, K.N. Lead-Free Electronic Solders. A Special Issue of the Journal of Materials Science: Materials in Electronics; Springer Science+Business Media, LLC: Berlin, Germany, 2007; ISBN 978-0387-48431-0. [Google Scholar]
- Suganuma, K. Advances in lead-free electronics soldering. Curr. Opin. Solid State Mater. Sci. 2001, 5, 55–64. [Google Scholar] [CrossRef]
- Abtew, M.; Selvaduray, G. Lead-free Solders in Microelectronics. Mater. Sci. Eng. R Rep. 2000, 27, 95–141. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, K.S.; Inoue, M.; Jiang, J.; Suganuma, K. Effects of Ag and Cu addition on microstructural properties and oxidation resistance of Sn–Zn eutectic alloy. J. Alloys Compd. 2008, 454, 310–320. [Google Scholar] [CrossRef]
- Available online: http://www.steelnumber.com/en/steel_composition_eu.php?name_id=614 (accessed on 30 April 2023).
- Available online: http://vascony.com/wp-content/uploads/2014/01/en-quinn-xt-10-07.pdf (accessed on 7 May 2024).
- ISO 527-2:2012; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. International Organization for Standardization: Geneva, Switzerland, 2012.
- Available online: https://www.kovot.sk (accessed on 15 March 2023).
- Okamoto, H.; Schlesinger, M.E.; Mueller, E.M. Alloy Phase Diagrams. In ASM Handbook; ASM International: Detroit, MI, USA, 2016; Volume 3, ISBN 978-1-62708-070-5. [Google Scholar]
- Schrek, A.; Vanko, B.; Sejč, P. Forming of a bimetallic element for the resistance element soldering method. J. Met. Mater. 2022, 74, 8–20. [Google Scholar] [CrossRef]
- Available online: https://weld.sk/produkt/spajkovacie-tavidlo-sf-601-letol-05-kg (accessed on 15 May 2024).
- ISO 6892-1:2019(en); Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. ISO: Geneva, Switzerland, 2019. Available online: https://www.iso.org/obp/ui/#iso:std:iso:6892:-1:ed-3:v1:en (accessed on 30 June 2023).
- Available online: https://imagej.en.softonic.com/download (accessed on 5 June 2024).
- Available online: https://www.alumeco.com/aluminium/plates/raw/en-aw-1050a/05-x-1000-x-2000-mm/p/390/3714 (accessed on 1 May 2024).
- Zhang, H.; Senkara, J. Resistance Welding. Fundamentals and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Marder, A.R. The metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Fourmentin, R.; McDermid, J.; Gertsman, V.; Dionne, S.; Goodwin, F. Properties of galvanized and galvannealed high strength hot rolled steel. In Materials Science and Technology, Proceedings of the Zinc Coated Sheet Steels Session 1, Detroit, Michigan, 16–20 September 2007; pp. 535–546. Available online: https://www.researchgate.net/publication/288612164_Properties_of_galvanized_and_galvannealed_high_strength_hot_rolled_steel (accessed on 30 June 2024).
- Lee, J.E.; Kim, K.S.; Suganuma, K.; Inoue, M.; Izuta, G. Thermal Properties and Phase Stability of Zn-Sn and Zn-In Alloys as High Temperature Lead-Free Solder. Mater. Trans. 2007, 48, 584–593. [Google Scholar] [CrossRef]
- Chen, K.I.; Cheng, S.C.; Wu, S.; Lin, K.L. Effects of Small Additions of Ag, Al, and Ga on the Structure and Properties of the Sn-9Zn Eutectic Alloy. J. Alloys Compd. 2006, 416, 98–105. [Google Scholar] [CrossRef]
- Das, S.K.; Sharif, A.; Chan, Y.C.; Wong, N.B.; Yung, W.K.C. Influence of Small Amount of Al and Cu on the Microstructure, Microhardness and Tensile Properties of Sn-9Zn Binary Eutectic Solder Alloy. J. Alloys Compd. 2009, 481, 167–172. [Google Scholar] [CrossRef]

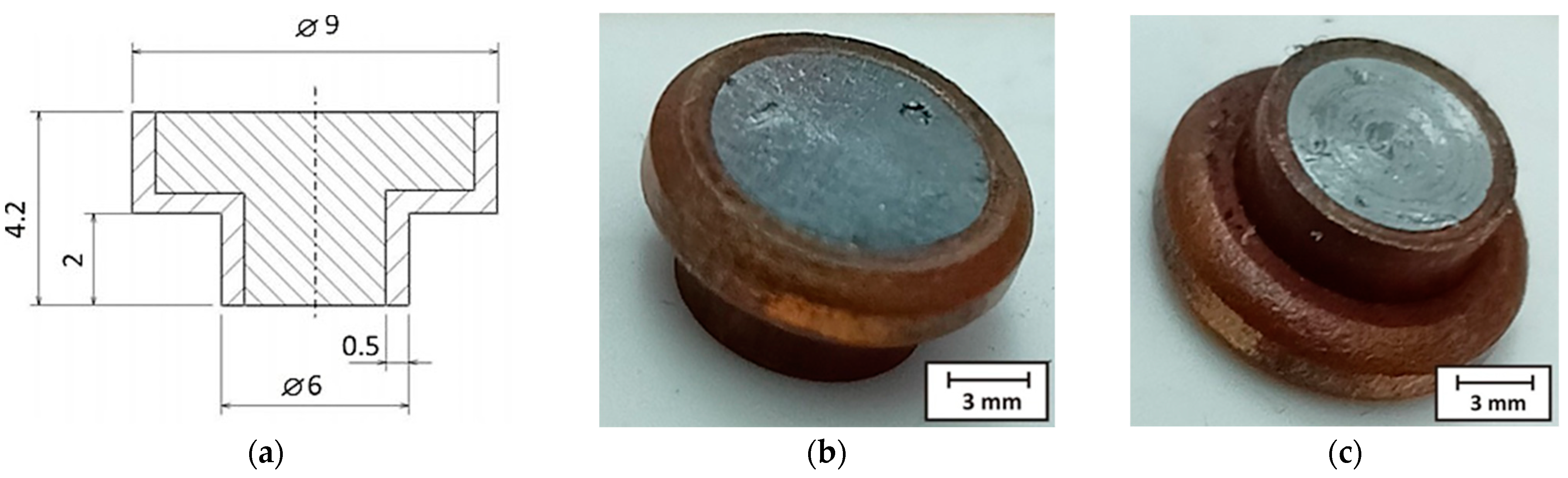

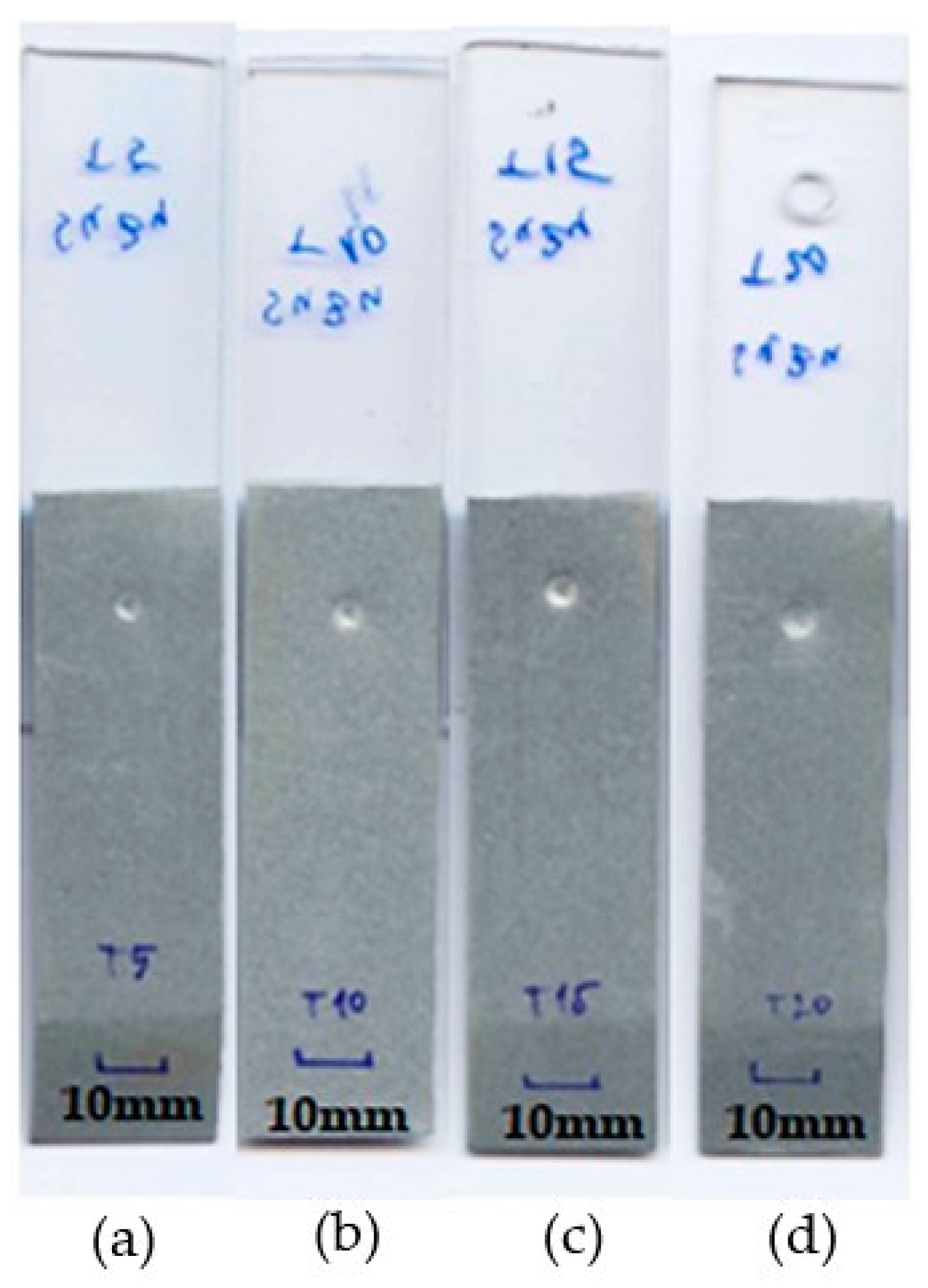
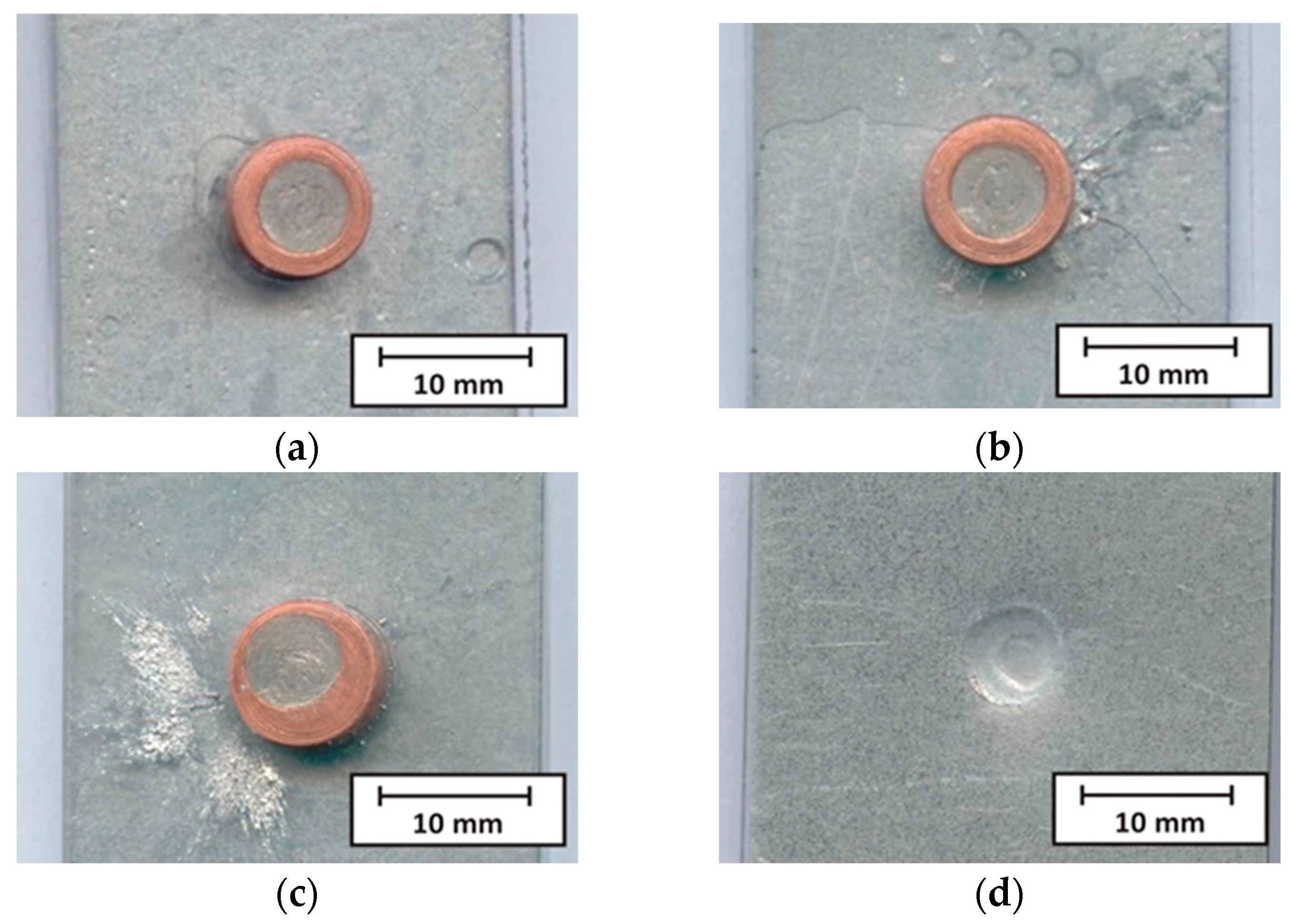
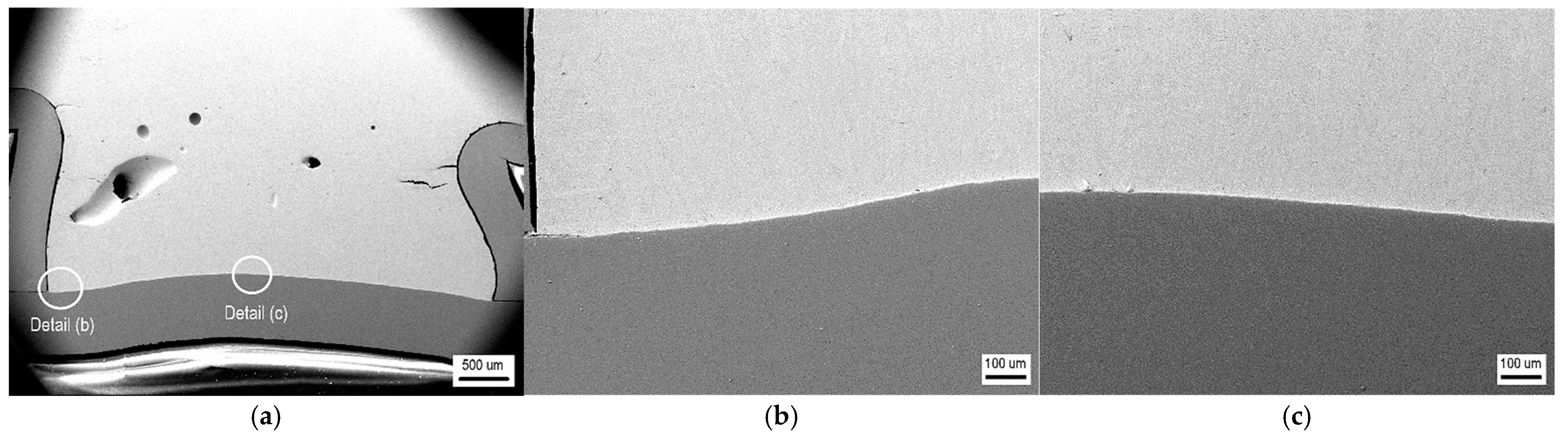

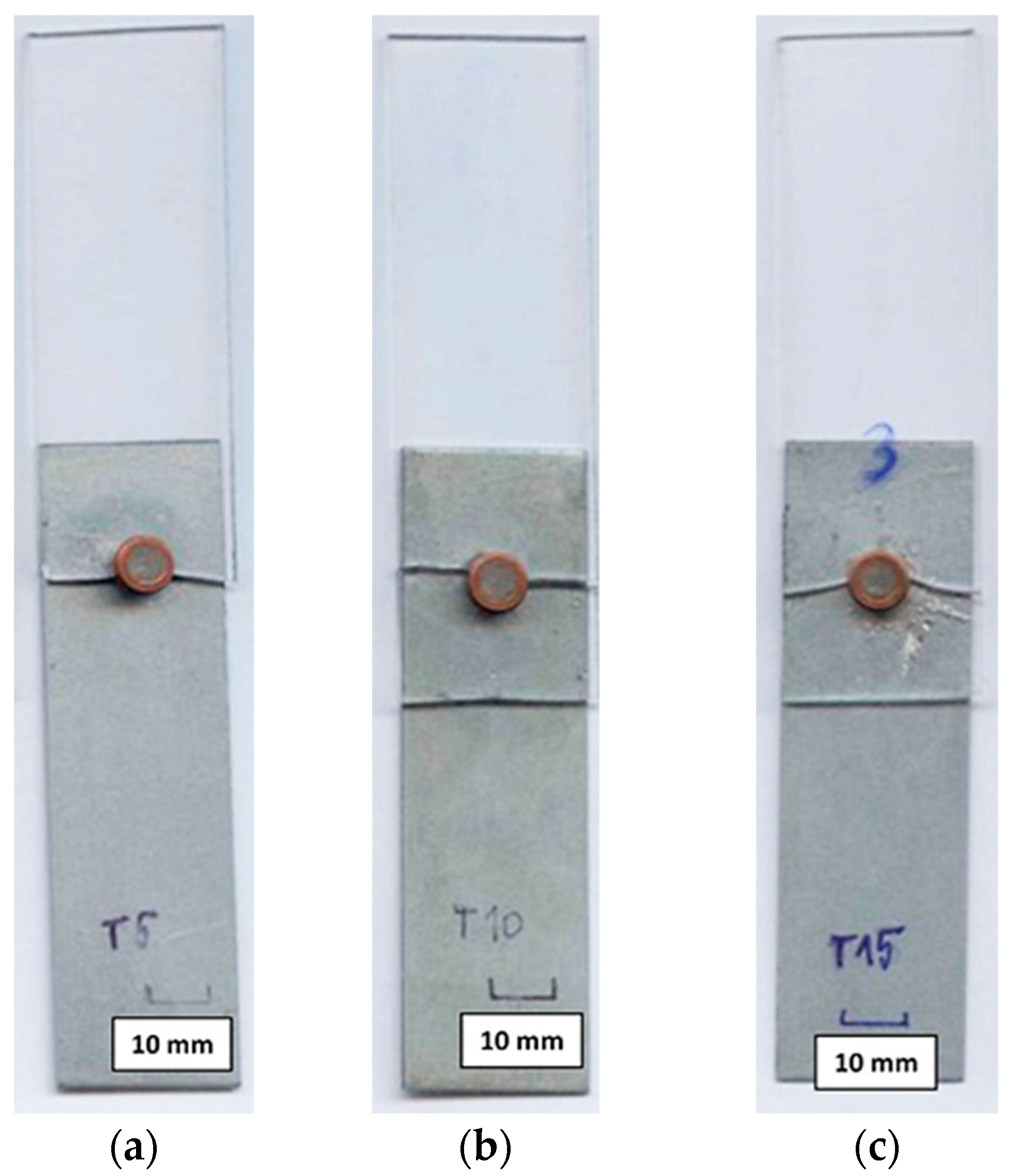
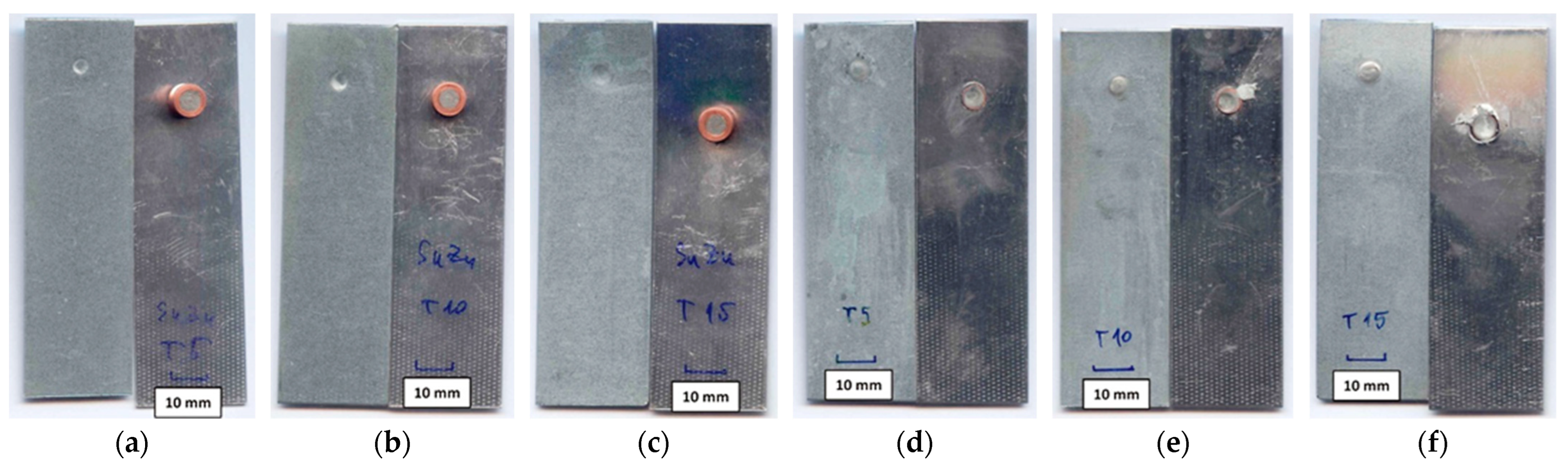

| Sample Number | Joining Parameters | Note | |||
|---|---|---|---|---|---|
| Electric Current I (kA) | Voltage U (V) | Soldering Time t (s) | Heat Input Q (J) | ||
| T1 | 6.07 | 0.48 | 0.1 | 291 | None |
| T2 | 6.18 | 0.48 | 0.2 | 593 | None |
| T3 | 6.13 | 0.49 | 0.3 | 901 | None |
| T4 | 6.25 | 0.45 | 0.4 | 1125 | Intensive spatter of the solder |
| Soldering Time (s) | Volume of Remelted Solder (%) |
|---|---|
| 0.1 | 11.2 |
| 0.2 | 35.5 |
| 0.3 | 52.1 |
| Material | Al (wt.%) | Si (wt.%) | Fe (wt.%) | Cu (wt.%) | Mn (wt.%) | Cr (wt.%) | Zn (wt.%) | Ti (wt.%) |
|---|---|---|---|---|---|---|---|---|
| AW-1050A | rest | 0.25 | 0.40 | 0.05 | 0.01 | 0.01 | 0.07 | 0.05 |
| Material | Yield Strength Rp0.2 (MPa) | Ultimate Tensile Strength Rm (MPa) | Elongation at Break A (%) |
|---|---|---|---|
| AW-1050A | min. 85 | 105–145 | 2 |
| Sample Number | Soldering Time t (s) | Loading Force Fmax (N) | Average Value of Loading Force Fmax (N) | Ultimate Tensile Strength Rm (MPa) | Average Value of Tensile Strength Rm (MPa) | Standard Deviation of Tensile Strength Rm (MPa) | (Rm (joint)/Rm (solder)) × 100 (%) |
|---|---|---|---|---|---|---|---|
| 1 | 0.1 | 870 | 582 | 44.33 | 29.66 | 14.78 | 50.4 |
| 2 | 440 | 22.42 | |||||
| 3 | 440 | 22.42 | |||||
| 4 | 620 | 31.59 | |||||
| 5 | 540 | 27.52 | |||||
| 1 | 0.2 | 1160 | 969 | 59.11 | 49.38 | 6.95 | 84.0 |
| 2 | 990 | 50.45 | |||||
| 3 | 850 | 43.31 | |||||
| 4 | 825 | 42.04 | |||||
| 5 | 1020 | 50.45 | |||||
| 1 | 0.3 | 1080 | 1082 | 55.05 | 55.14 | 8.37 | 93.9 |
| 2 | 1120 | 57.07 | |||||
| 3 | 1020 | 51.97 | |||||
| 4 | 1170 | 59.62 | |||||
| 5 | 1020 | 51.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sejč, P.; Vanko, B.; Gábrišová, Z.; Schrek, A. Use of Sn91Zn9 Lead-Free Solder in Resistance Element Soldering Technology. Metals 2025, 15, 306. https://doi.org/10.3390/met15030306
Sejč P, Vanko B, Gábrišová Z, Schrek A. Use of Sn91Zn9 Lead-Free Solder in Resistance Element Soldering Technology. Metals. 2025; 15(3):306. https://doi.org/10.3390/met15030306
Chicago/Turabian StyleSejč, Pavol, Branislav Vanko, Zuzana Gábrišová, and Alexander Schrek. 2025. "Use of Sn91Zn9 Lead-Free Solder in Resistance Element Soldering Technology" Metals 15, no. 3: 306. https://doi.org/10.3390/met15030306
APA StyleSejč, P., Vanko, B., Gábrišová, Z., & Schrek, A. (2025). Use of Sn91Zn9 Lead-Free Solder in Resistance Element Soldering Technology. Metals, 15(3), 306. https://doi.org/10.3390/met15030306






