Study on Slag Phase Control of Thermal Reduction of Spodumene
Abstract
1. Introduction
2. Materials and Methods
2.1. Spodumene
2.2. Coke
2.3. Other Raw Materials
2.4. Experimental Methods
3. Results
3.1. Theoretical Analysis
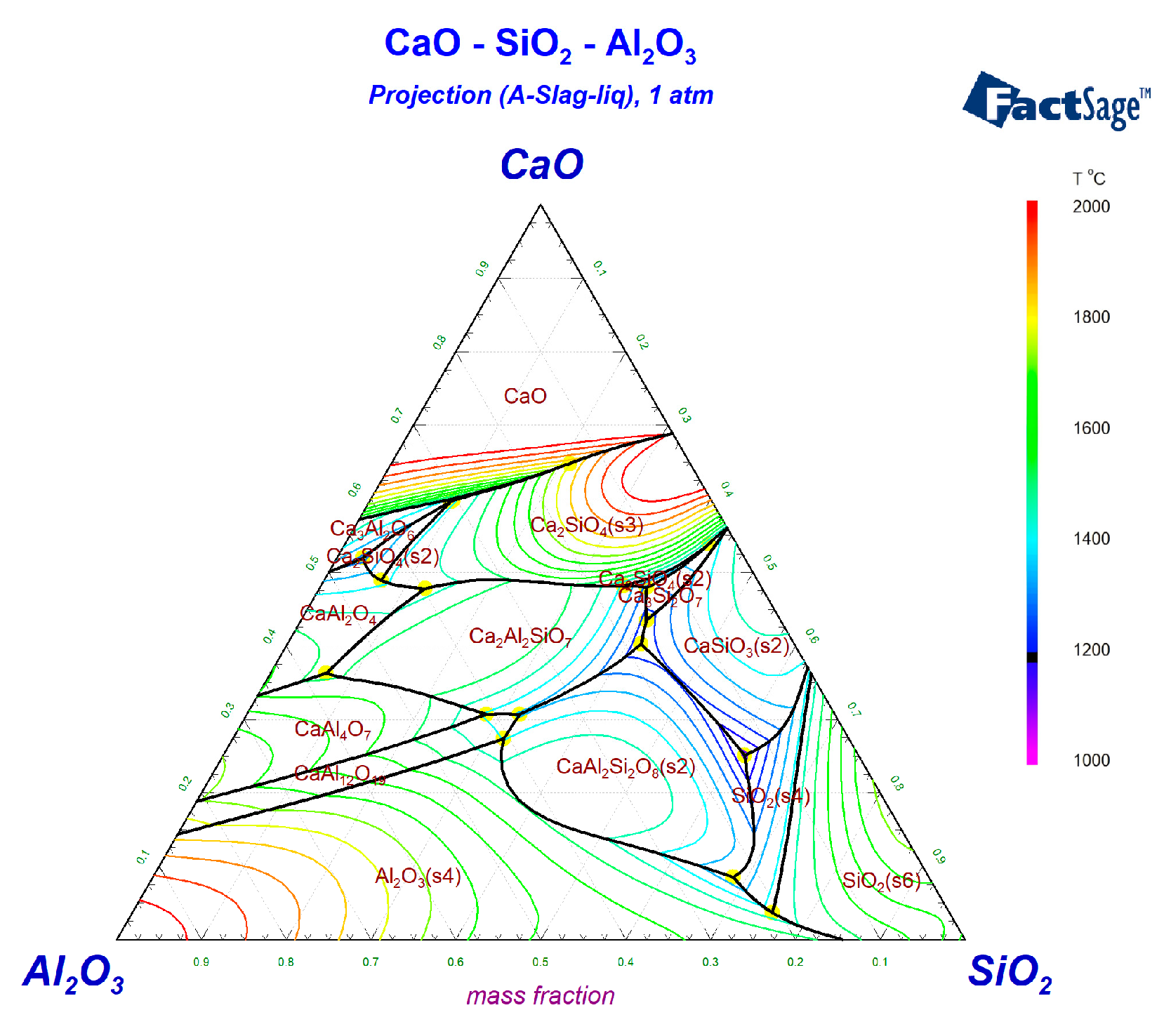
3.1.1. C-A-S Ternary Phase Diagram
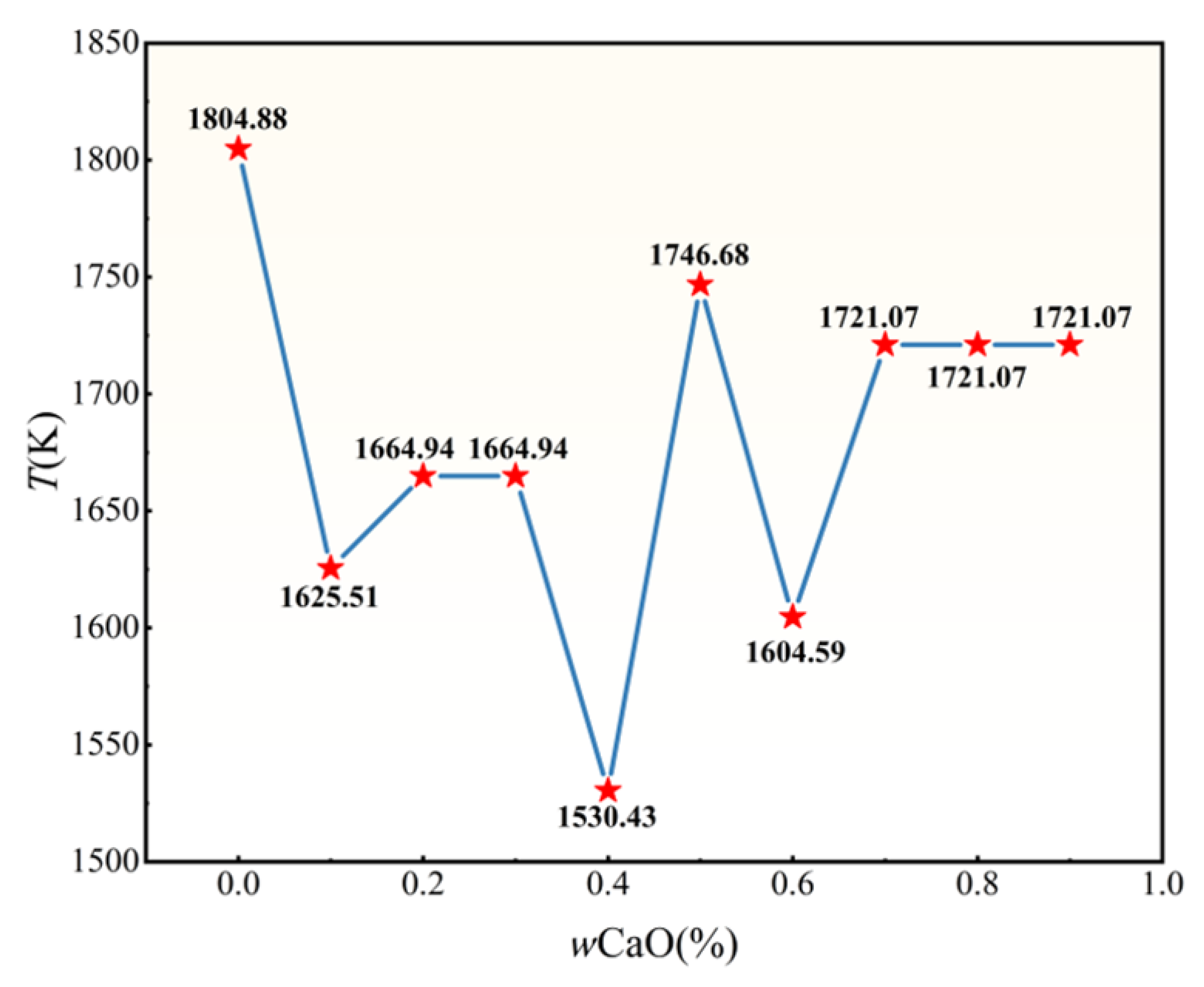
3.1.2. Analysis of the Melting Point of the Slag Phase at Different Calcium Oxide Contents
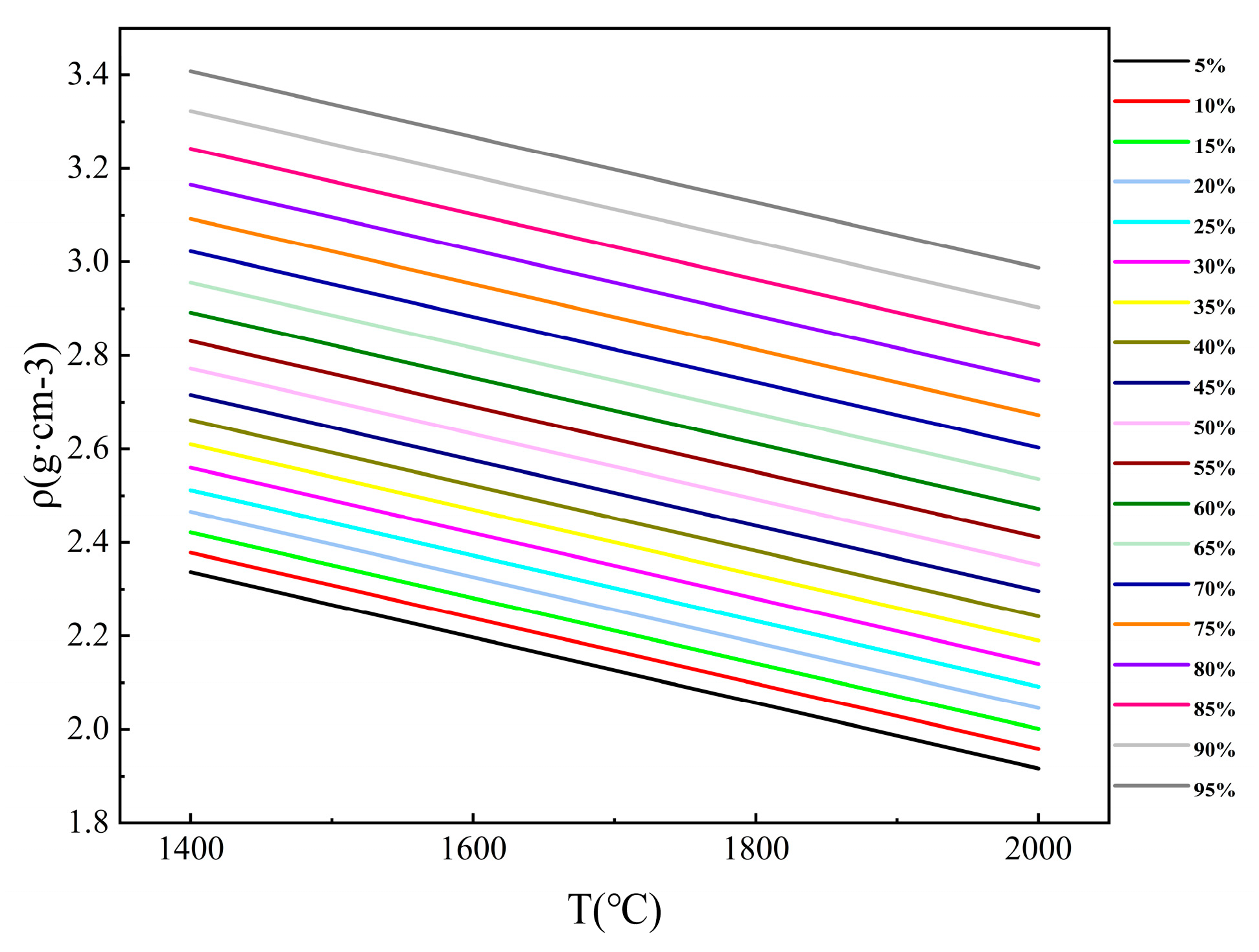
3.1.3. Analysis of Slag Phase Density Under Different Calcium Oxide Content
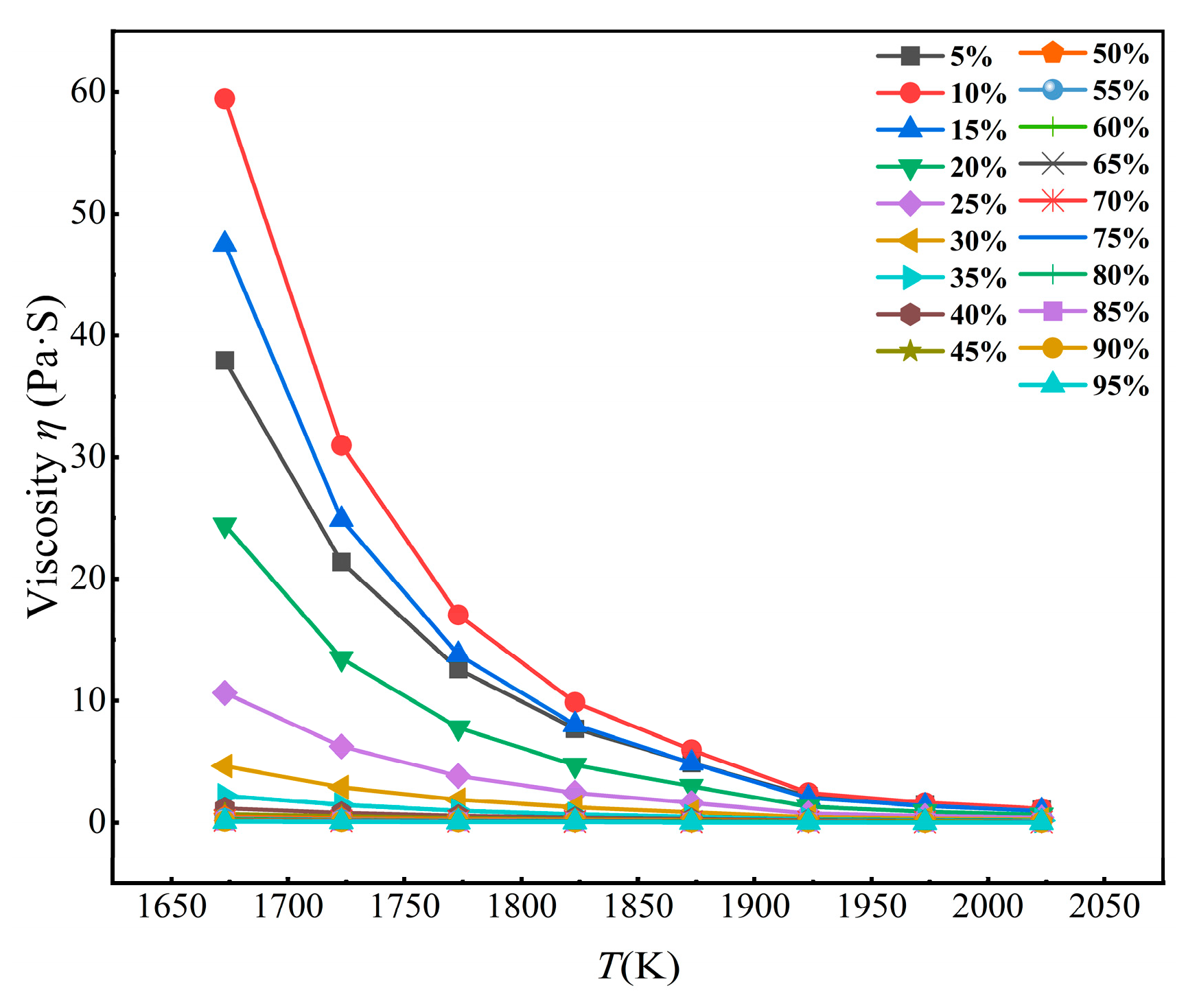
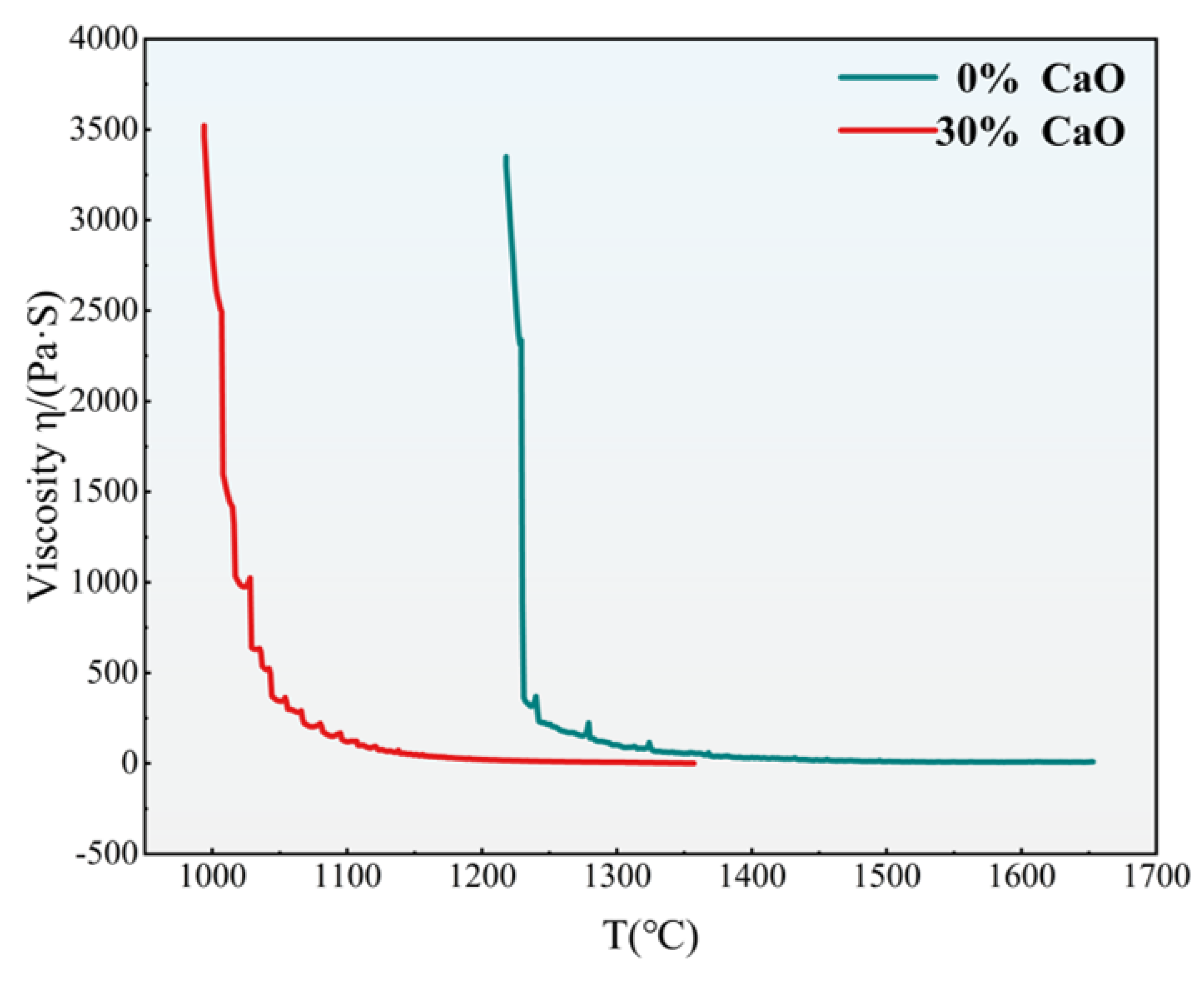
3.1.4. Viscosity Analysis of Slag Phase at Different Calcium Oxide Content
3.2. Effect of Calcium Oxide Slagging Agent on Phase Control of Spodumene Carbothermic Reduction Slag and Condensate Analysis After Reduction
3.3. The Reduction Rate of Lithium and the Direct Yield of Manganese-Silicon Alloy
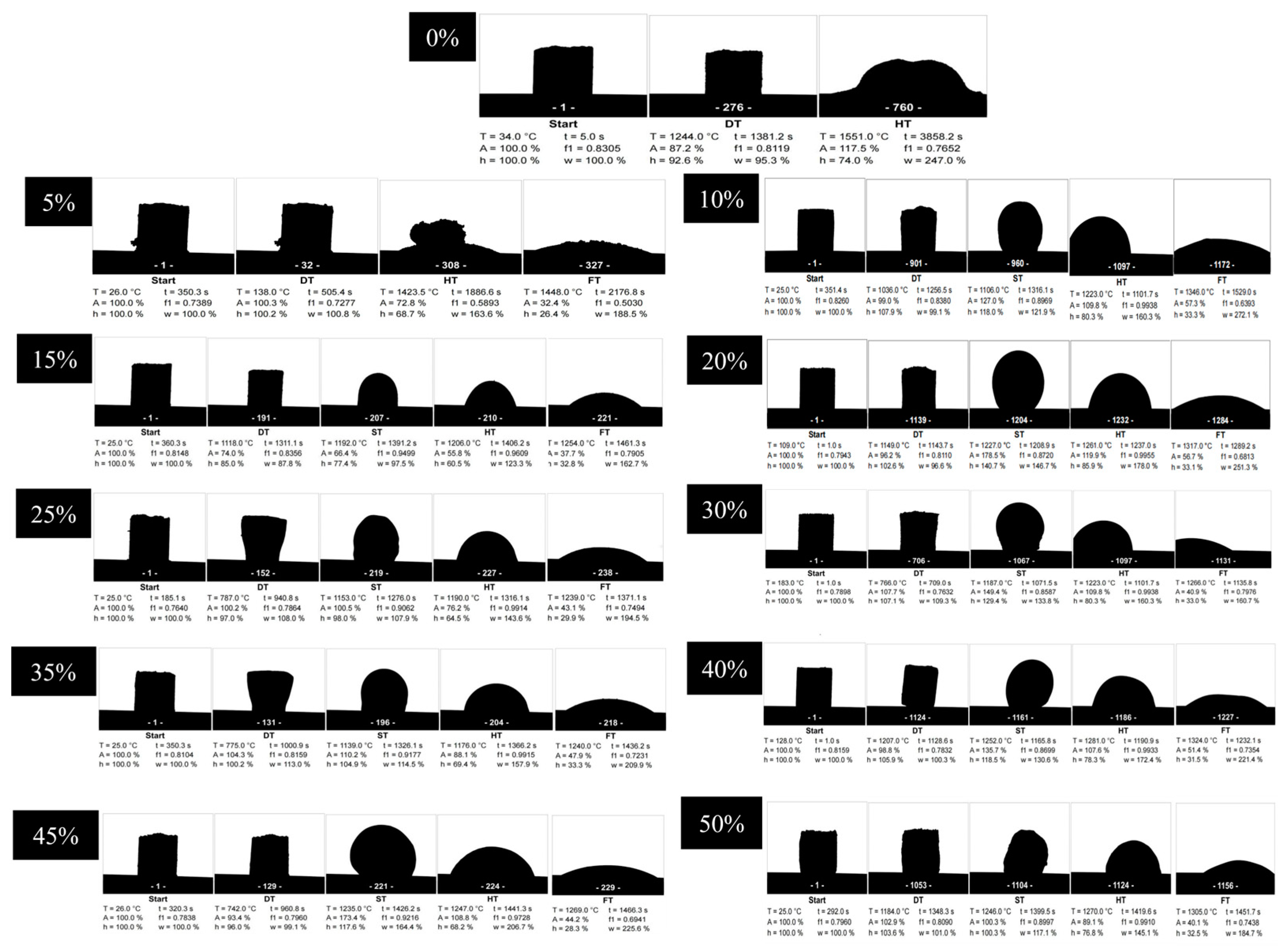
3.4. Melting Point Analysis
3.5. Viscosity Analysis
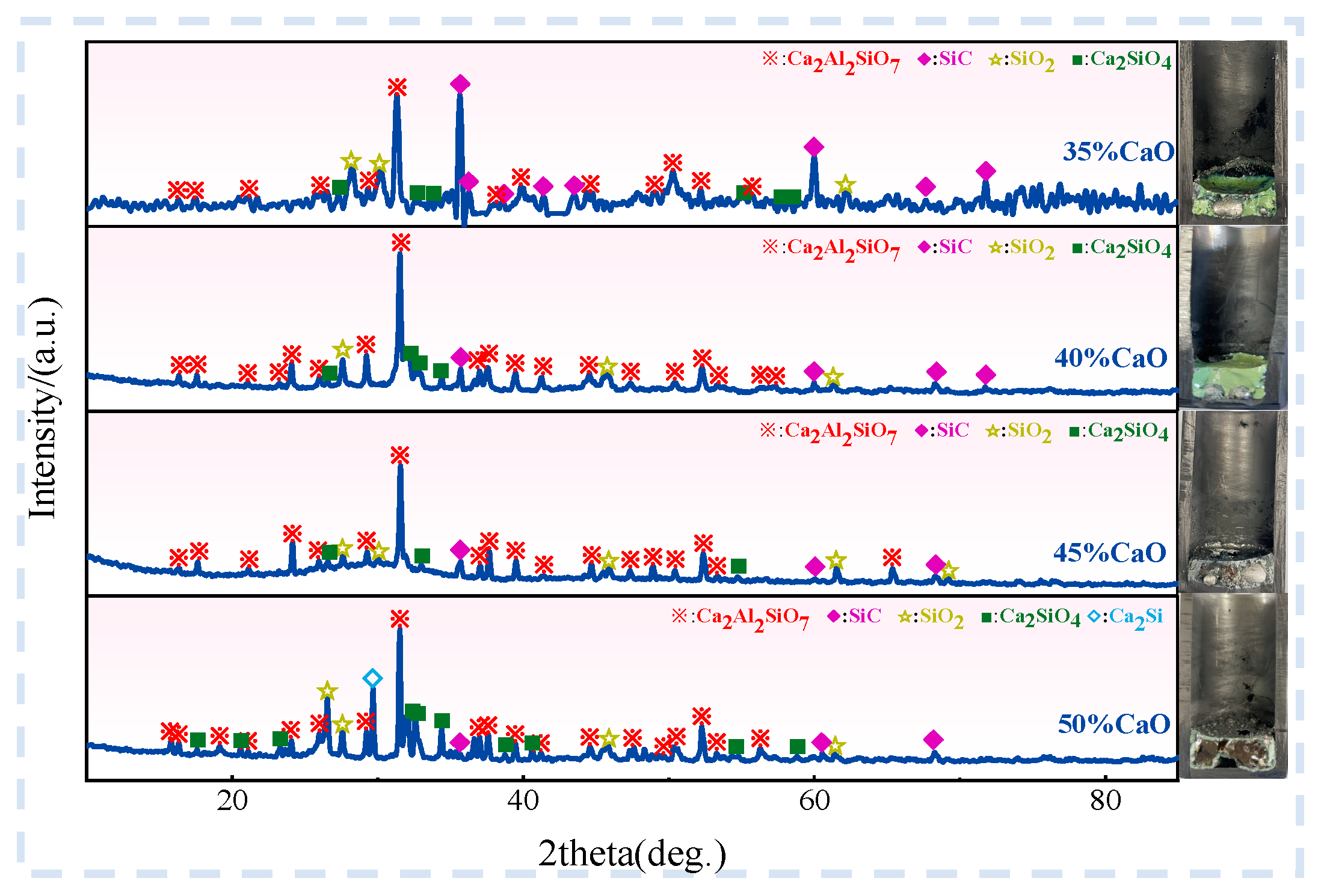
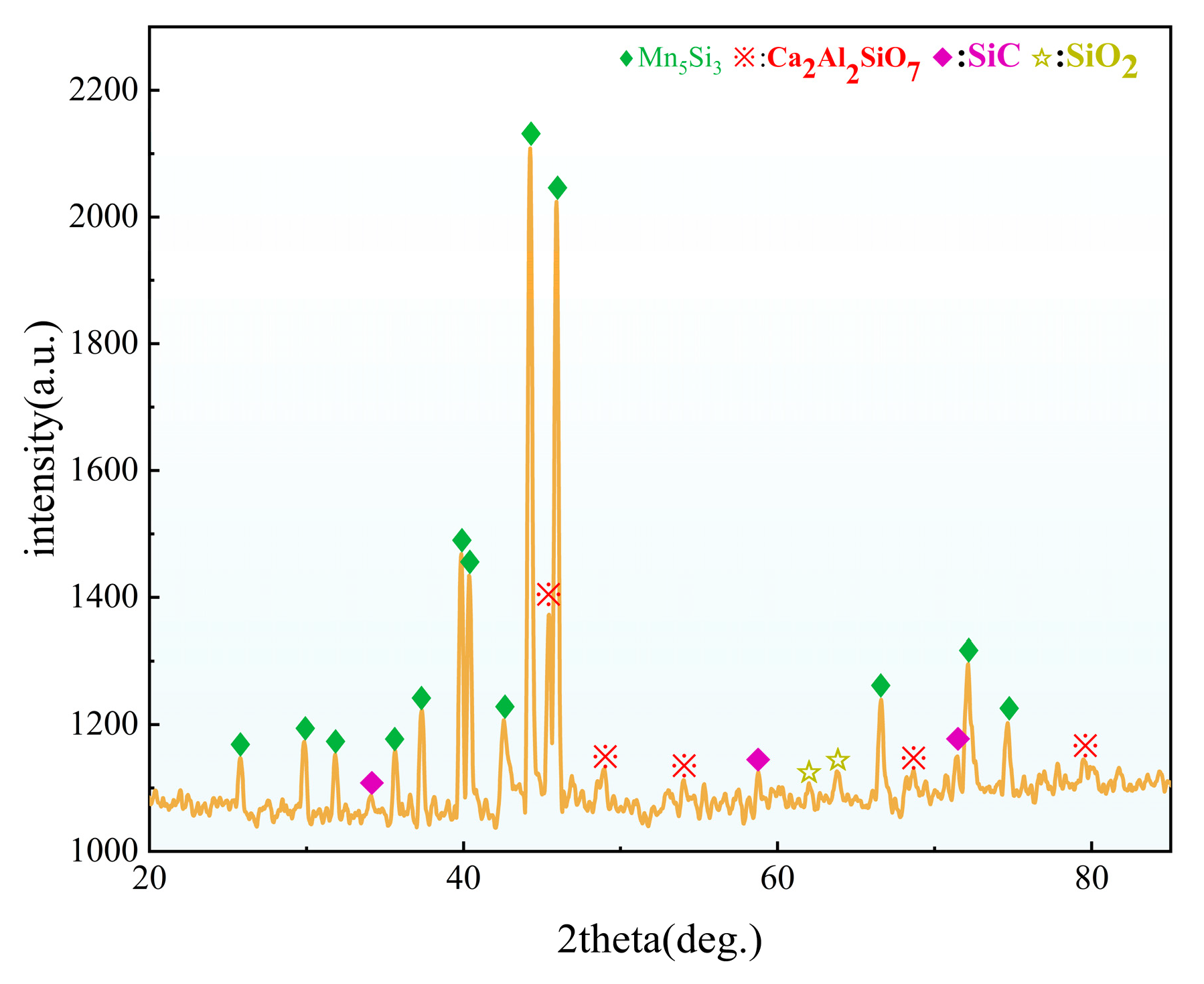
3.6. The Slag Phase and Chemical Composition of the Alloy with 30% CaO Content and the Alloy XRD
3.7. Electron Microscopy Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviation
| C-A-S | CaO-Al2O3-SiO2 |
References
- Vikström, H.; Davidsson, S.; Höök, M. Lithium availability and future production outlooks. Appl. Energy 2013, 110, 252–266. [Google Scholar] [CrossRef]
- Mineral Commodity Summaries 2021; U.S. Geological Survey: Reston, VA, USA, 2021; Volume 200, pp. 82–83. [CrossRef]
- Roy, T.; Plante, B.; Benzaazoua, M.; Demers, I. Geochemistry and mineralogy of a spodumene-pegmatite lithium ore at various mineral beneficiation stages. Miner. Eng. 2023, 202, 108312. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2016, 172, 388–403. [Google Scholar] [CrossRef]
- Yanjia, Z.; Xiaodong, T.; Dayong, Q.; Jingjing, L.; Hong, W. Research progress of technology of lithium extraction. Sep. Purif. Technol. 2024, 359, 130561. [Google Scholar] [CrossRef]
- Tadesse, B.; Makuei, F.; Albijanic, B.; Dyer, L. The beneficiation of lithium minerals from hard rock ores: A review. Miner. Eng. 2019, 131, 170–184. [Google Scholar] [CrossRef]
- Sebastian, A.; Lagache, M.J.A.M. Experimental study of lithium-rich granitic pegmatites; Part I, Petalite + albite + quartz equilibrium. Am. Mineral. 1991, 76, 205–210. [Google Scholar]
- Dessemond, C.; Lajoie-Leroux, F.; Soucy, G.; Laroche, N.; Magnan, J.-F. Spodumene: The Lithium Market, Resources and Processes. Minerals 2019, 9, 334. [Google Scholar] [CrossRef]
- Göd, R. The spodumene deposit at “Weinebene”, Koralpe, Austria. Miner. Depos. 1989, 24, 270–278. [Google Scholar] [CrossRef]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Demartin, F.; Gay, H.D.; Gramaccioli, C.M. The crystal structure of a mineral related to paulkerrite. Z. Fur Krist. Mater. 1993, 208, 57–72. [Google Scholar] [CrossRef]
- Skinner, B.J., Jr. Crystal chemistry of beta-spodumene solid solutions on the join Li2O.Al2O3-SiO2. Am. J. Sci. 1960, 258, 312. [Google Scholar]
- Friedman-Rudovsky, J. Dreams of a Lithium Empire. Science 2011, 334, 896–897. [Google Scholar] [CrossRef]
- Karrech, A.; Azadi, M.R.; Elchalakani, M.; Shahin, M.A.; Seibi, A.C. A review on methods for liberating lithium from pegmatities. Miner. Eng. 2020, 145, 106085. [Google Scholar] [CrossRef]
- Gao, T.; Fan, N.; Chen, W.; Dai, T. Lithium extraction from hard rock lithium ores(spodumene, lepidolite, zinnwaldite, petalite): Technology, resources, environment and cost. China Geol. 2023, 6, 137–153. [Google Scholar]
- Meng, F.; McNeice, J.; Zadeh, S.S.; Ghahreman, A. Review of Lithium Production and Recovery from Minerals, Brines, and Lithium-Ion Batteries. Miner. Process. Extr. Metall. Rev. 2021, 42, 123–141. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Acid roasting of spodumene: Microwave vs. conventional heating. Miner. Eng. 2019, 138, 161–167. [Google Scholar] [CrossRef]
- Kuang, G.; Liu, Y.; Li, H.; Xing, S.; Li, F.; Guo, H. Extraction of lithium from β-spodumene using sodium sulfate solution. Hydrometallurgy 2018, 177, 49–56. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Production of Lithium—A Literature Review Part 1: Pretreatment of Spodumene. Miner. Process. Extr. Metall. Rev. 2019, 41, 335–348. [Google Scholar] [CrossRef]
- Botto, I.L. Structural and spectroscopic properties of leached spodumene in the acid roast processing. Mater. Chem. Phys. 1985, 13, 423–436. [Google Scholar] [CrossRef]
- Rosales, G.D.; Ruiz, M.d.C.; Rodriguez, M.H. Novel process for the extraction of lithium from β-spodumene by leaching with HF. Hydrometallurgy 2014, 147–148, 1–6. [Google Scholar] [CrossRef]
- Talens Peiró, L.; Villalba Méndez, G.; Ayres, R.U. Lithium: Sources, Production, Uses, and Recovery Outlook. JOM 2013, 65, 986–996. [Google Scholar] [CrossRef]
- Tian, Q. Study on Extraction Technology of Lithium Carbonate from Spodumene Ore. Master’s Thesis, Central South University, Changsha, China, 2012. [Google Scholar]
- Tian, Q.; Chen, B.; Chen, Y.; Ma, L.; Shi, X. Study on sulfuric acid roasting and leaching process of spodumene. Rare Met. 2011, 35, 6. [Google Scholar]
- Lajoie-Leroux, F.; Dessemond, C.; Soucy, G.; Laroche, N.; Magnan, J.-F. Impact of the impurities on lithium extraction from β-spodumene in the sulfuric acid process. Miner. Eng. 2018, 129, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Hu, H.; Liu, M.; Li, Y.; Tang, Y.; Zhuang, L.; Tian, B. Comprehensive utilization of waste residue from lithium extraction process of spodumene. Miner. Eng. 2021, 170, 106986. [Google Scholar] [CrossRef]
- Xiao, M.S.; Wang, S.H.; Zhang, Q.F.; Zhang, J.W. Leaching mechanism of the spodumene sulphuric acid process. Rare Met. 1997, 16, 37–45. [Google Scholar]
- Yang, M.; Yan, K.; Ji, R.; Cui, X.; Zhang, W.; Qu, T. Lithium Enrichment by the Carbothermal Reduction of Spodumene Ore and the Preparation of Manganese-Silicon Alloy. Minerals 2022, 12, 1324. [Google Scholar] [CrossRef]
- Du, H.; Shi, L.; Lyu, F.; Zhang, X.-P.; Yang, M.-l.; Yan, K.; Qu, T. Comprehensive utilization of spodumene ore through pyrometallurgical process with Fe2O3 addition. J. Cent. South Univ. 2023, 30, 1831–1840. [Google Scholar] [CrossRef]
- Fei, L.; Hao, D.; Lei, S.; Yuan, T.; Xiao-Pan, Z.; Tao, Q.; Bin, Y.; Yong-Nian, D. Experimental and mechanism research on carbothermal reduction of spodumene ore via vacuum. Mater. Res. Express 2021, 8, 016505. [Google Scholar] [CrossRef]
- Ma, S.F.; Zhang, G. Leaching process of vanadium from argillaceous vanadium ore using calcified roasting study on roasting technology. Chin. J. Rare Met. 2007, 31, 813–817. [Google Scholar]
- He, Q.; Wen, J.; Yang, G.; Xu, F.; Xu, M.; Chen, Z.; Wu, J.; Ma, W. Impurity Aluminum Removal in Industrial Silicon by CaO-SiO2-CaF2 Slagging Refining. Silicon 2023, 15, 5445–5453. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, T.; He, L.; Zhao, Z.; Liu, X. A promising approach for directly extracting lithium from α-spodumene by alkaline digestion and precipitation as phosphate. Hydrometallurgy 2019, 189, 105141. [Google Scholar] [CrossRef]
- Bronsch, A. Viscosity of Slags. 2017, 72, 86–90. Available online: https://nbn-resolving.org/urn:nbn:de:bsz:105-qucosa-229196 (accessed on 12 March 2025).
- Kracek, F.C. The Binary System Li2O–SiO2. J. Phys. Chem. 1929, 34, 2641–2650. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, C. Practical Technical Manual of Ferroalloy Production; Metallurgical Industry Press: Beijing, China, 2003. [Google Scholar]
- Hu, A.M.; Li, M.; Mao, D.L. Growth behavior, morphology and properties of lithium aluminosilicate glass ceramics with different amount of CaO, MgO and TiO2 additive. Ceram. Int. 2008, 34, 1393–1397. [Google Scholar] [CrossRef]
- Maramba, B.; Eric, R.H. Phosphide capacities of ferromanganese smelting slags. Miner. Eng. 2008, 21, 132–137. [Google Scholar] [CrossRef]
- Qiu, S.; Liu, C.; Yu, J. Conversion from α-spodumene to intermediate product Li2SiO3 by hydrothermal alkaline treatment in the lithium extraction process. Miner. Eng. 2022, 183, 107599. [Google Scholar] [CrossRef]





| Component | Li2O | SiO2 | A12O3 | Fe2O3 | Na2O | K2O | CaO | Others |
|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 3.83 | 72.50 | 18.60 | 0.95 | 0.36 | 0.62 | 0.01 | 3.13 |
| Component | Fixed Carbon | Ash | Volatiles | Moisture |
|---|---|---|---|---|
| Content (wt%) | 86.04 | 12.03 | 1.44 | 0.49 |
| Component | SiO2 | Al2O3 | CaO | Fe2O3 |
|---|---|---|---|---|
| Content (wt%) | 1.61 | 1.91 | 0.93 | 0.31 |
| Al2O3 (wt%) | SiO2 (wt%) | SiC (wt%) | CaO (wt%) | MnO (wt%) | Fe2O3 (wt%) | Others (wt%) |
|---|---|---|---|---|---|---|
| 35.262 | 47.984 | 7.8 | 2.695 | 2.800 | 2.119 | 1.34 |
| Ca2Al2SiO7 (wt%) | SiO2 (wt%) | Ca2SiO4 (wt%) | Fe2O3 (wt%) | MgO (wt%) | Li2O (wt%) | Other (wt%) |
|---|---|---|---|---|---|---|
| 78.71 | 12.45 | 1.50 | 0.51 | 0.29 | 0.009759 | 6.53 |
| Mn5Si3 (wt%) | Ca2Al2SiO7 (wt%) | SiO2 (wt%) | Other |
|---|---|---|---|
| 87.93 | 7.88 | 3.13 | 1.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yang, M.; Zhong, Y.; Wang, S.; Qu, T.; Deng, Y. Study on Slag Phase Control of Thermal Reduction of Spodumene. Metals 2025, 15, 322. https://doi.org/10.3390/met15030322
Liu X, Yang M, Zhong Y, Wang S, Qu T, Deng Y. Study on Slag Phase Control of Thermal Reduction of Spodumene. Metals. 2025; 15(3):322. https://doi.org/10.3390/met15030322
Chicago/Turabian StyleLiu, Xuefeng, Mingliang Yang, Yuncheng Zhong, Shichao Wang, Tao Qu, and Yong Deng. 2025. "Study on Slag Phase Control of Thermal Reduction of Spodumene" Metals 15, no. 3: 322. https://doi.org/10.3390/met15030322
APA StyleLiu, X., Yang, M., Zhong, Y., Wang, S., Qu, T., & Deng, Y. (2025). Study on Slag Phase Control of Thermal Reduction of Spodumene. Metals, 15(3), 322. https://doi.org/10.3390/met15030322







