Effect of Extrusion Temperature on the Microstructure and Properties of Biomedical Mg-1Zn-0.4Ca-1MgO Composite
Abstract
1. Introduction
2. Material and Methods
2.1. Material Preparation
2.2. Microstructure Characterization
2.3. Mechanical Properties Test
2.4. Corrosion Properties Test
3. Results
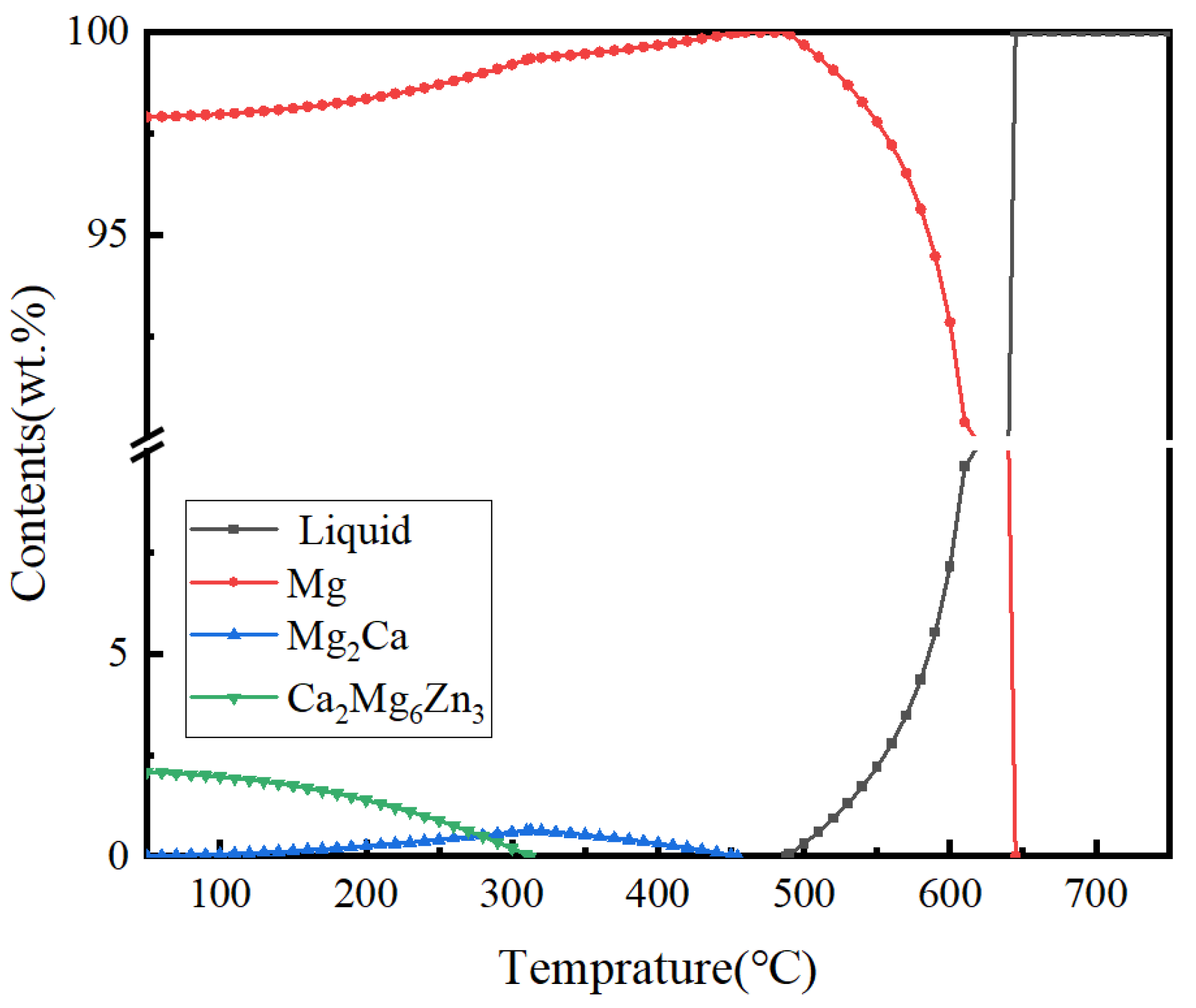
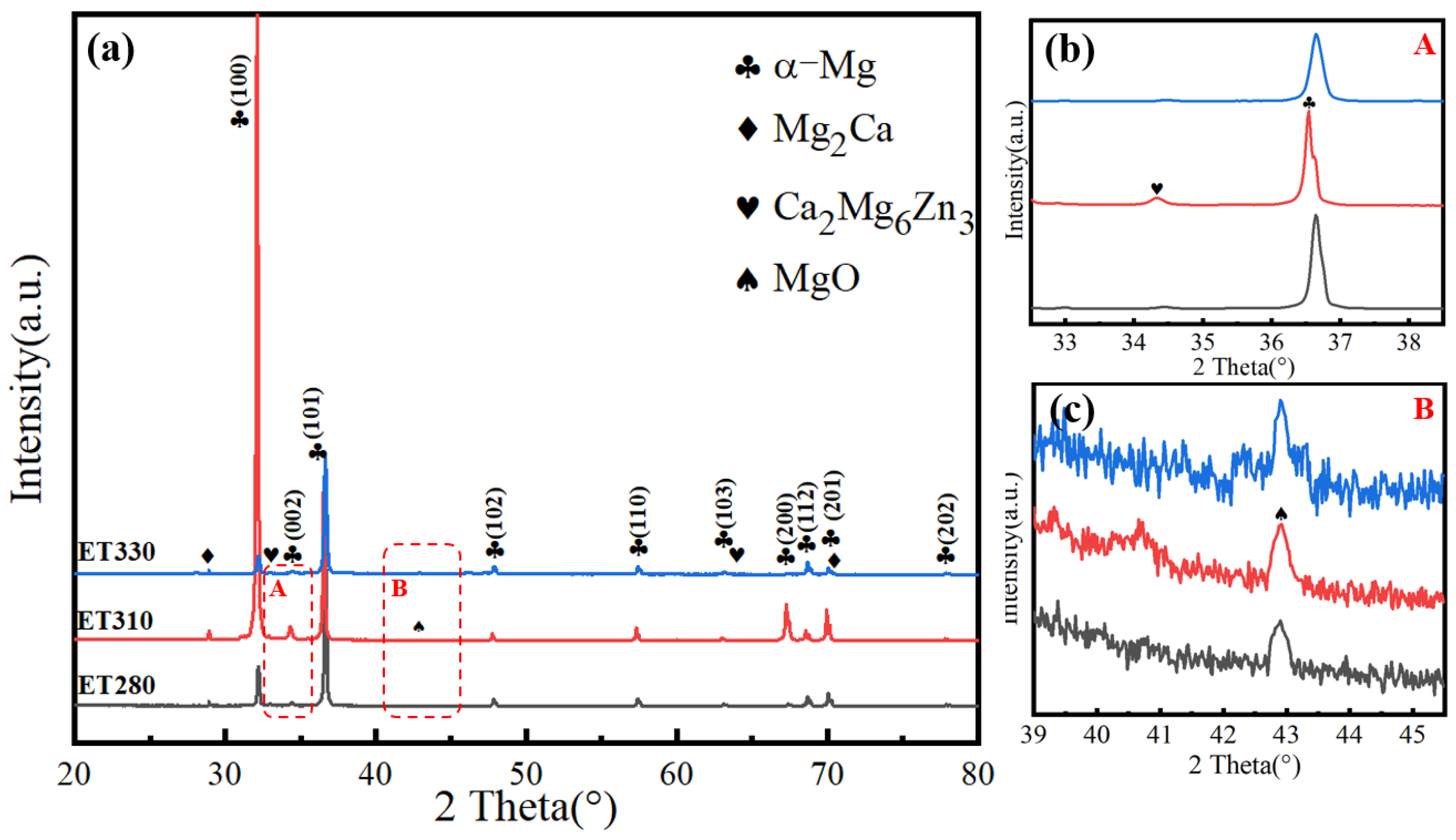
3.1. Microstructure of Extruded Composites
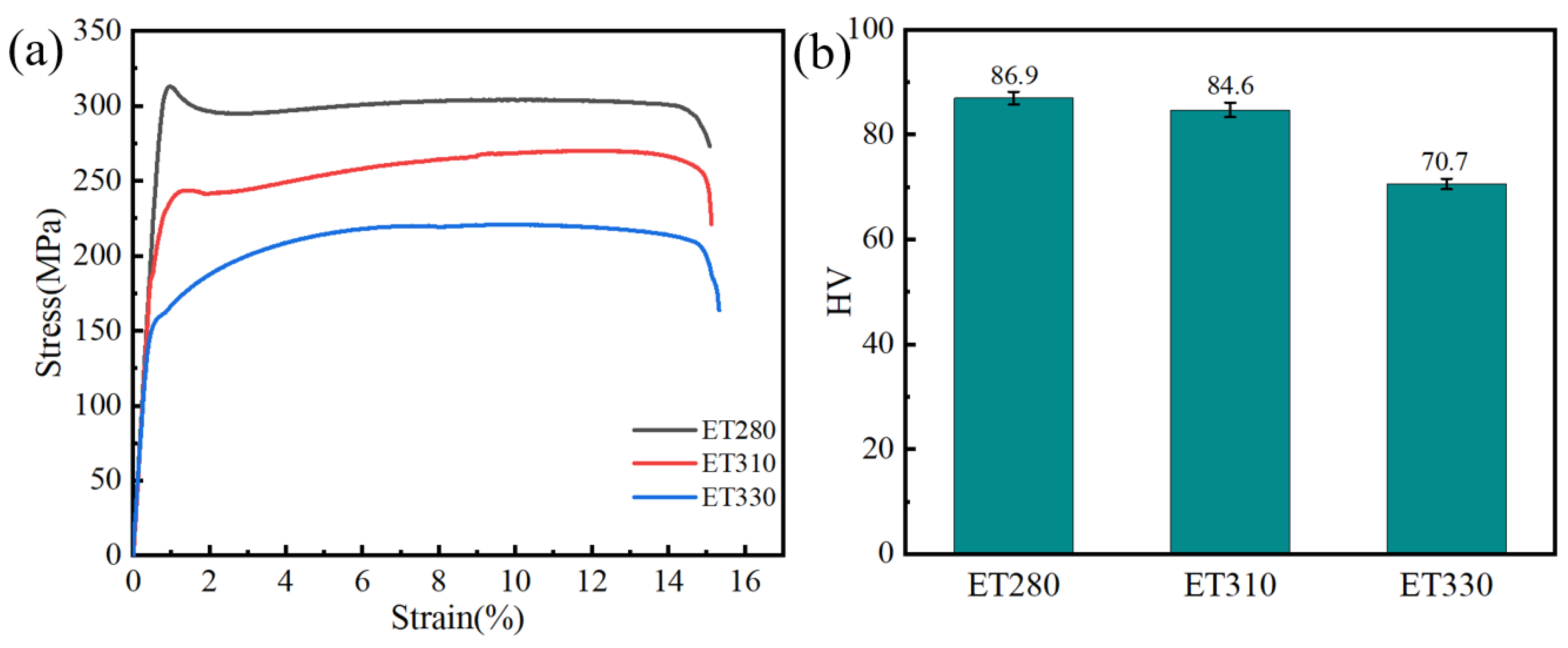
3.2. Mechanical Properties
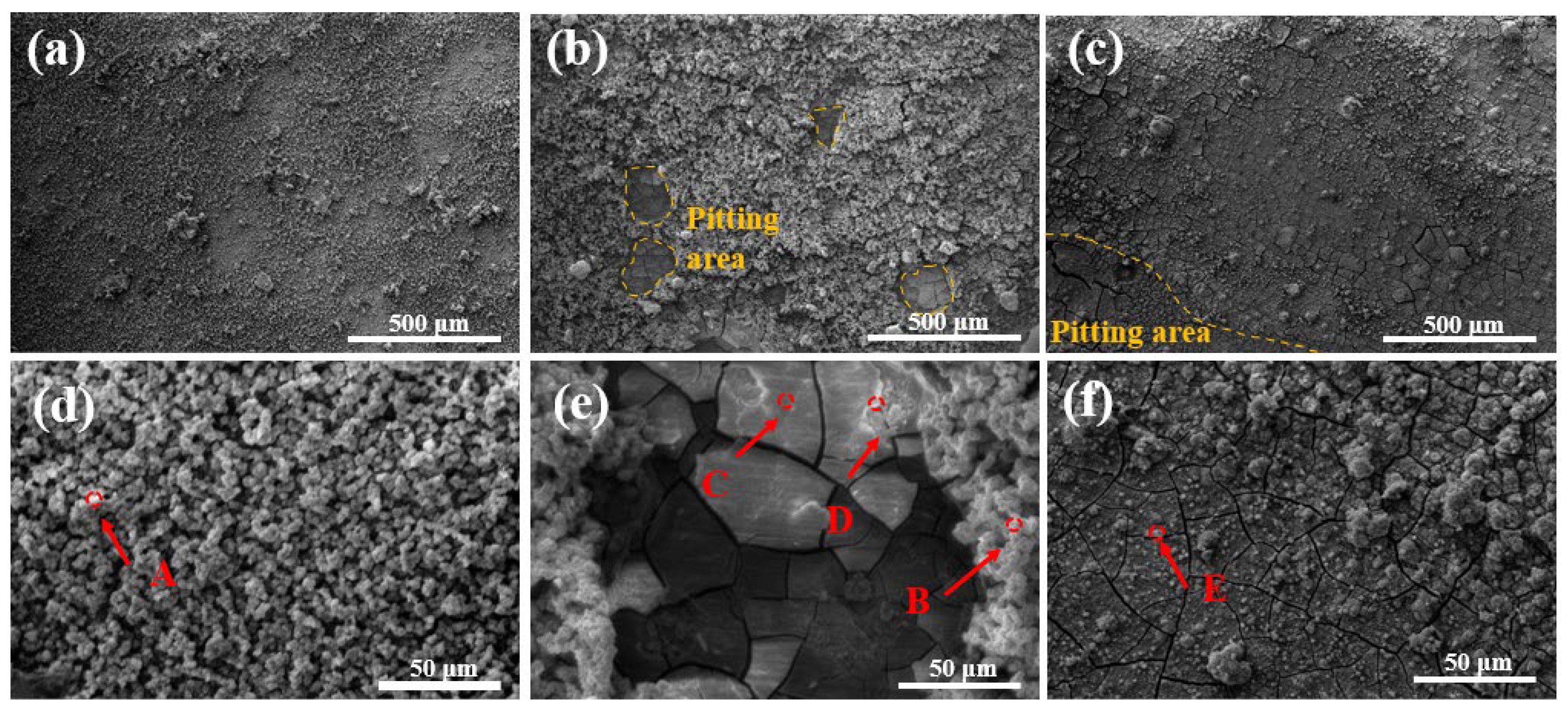
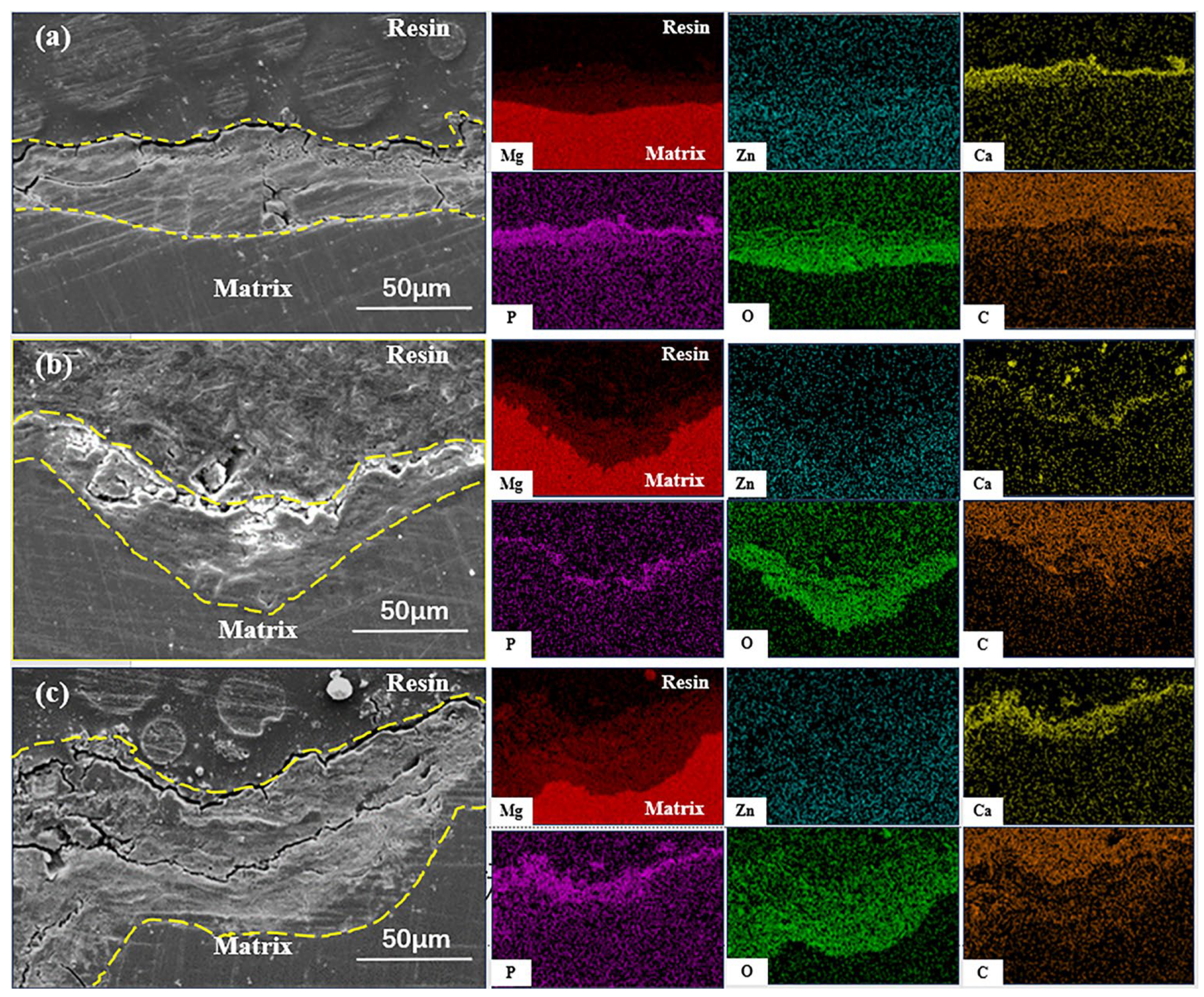
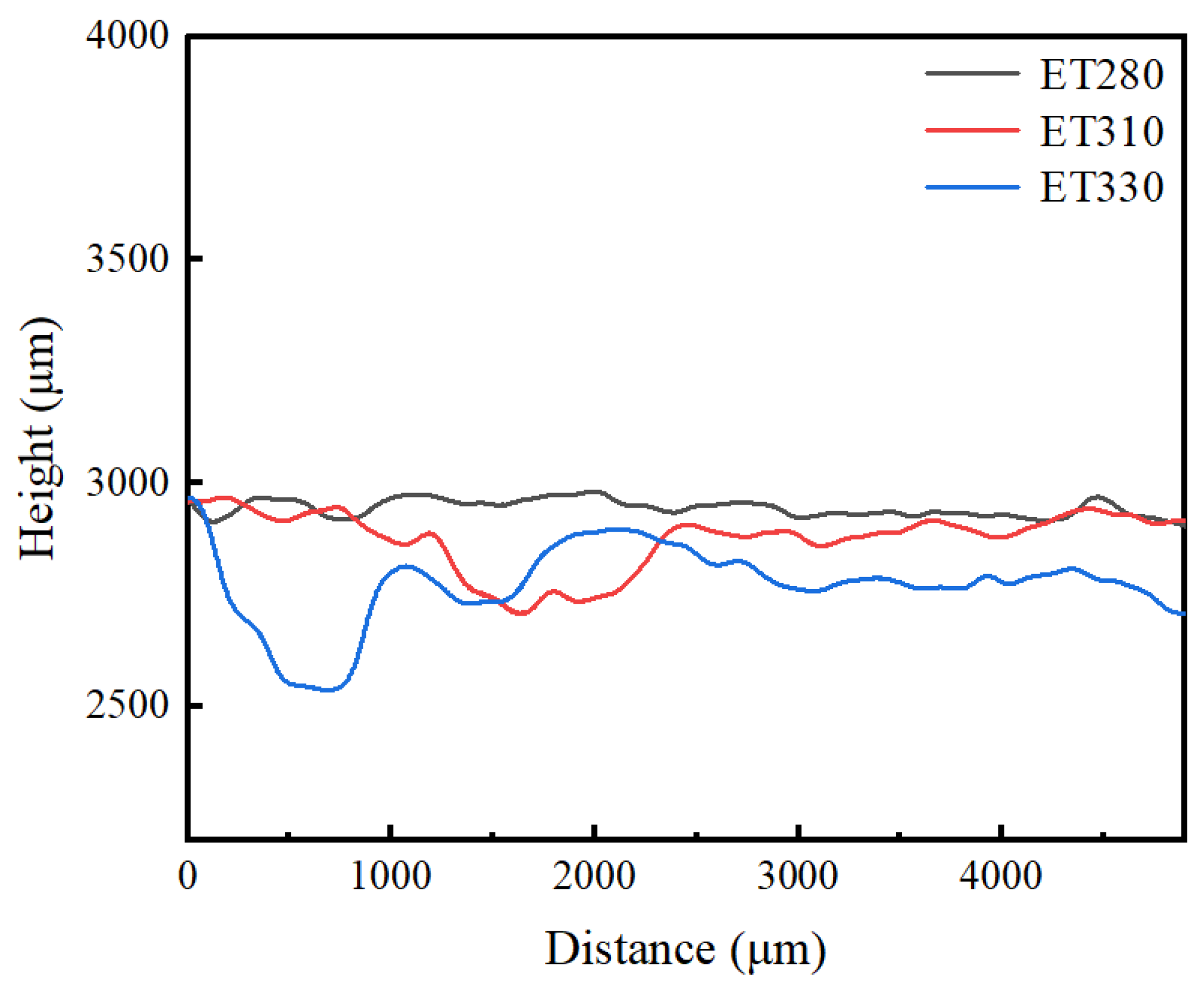
3.3. Corrosion Performance
4. Discussion
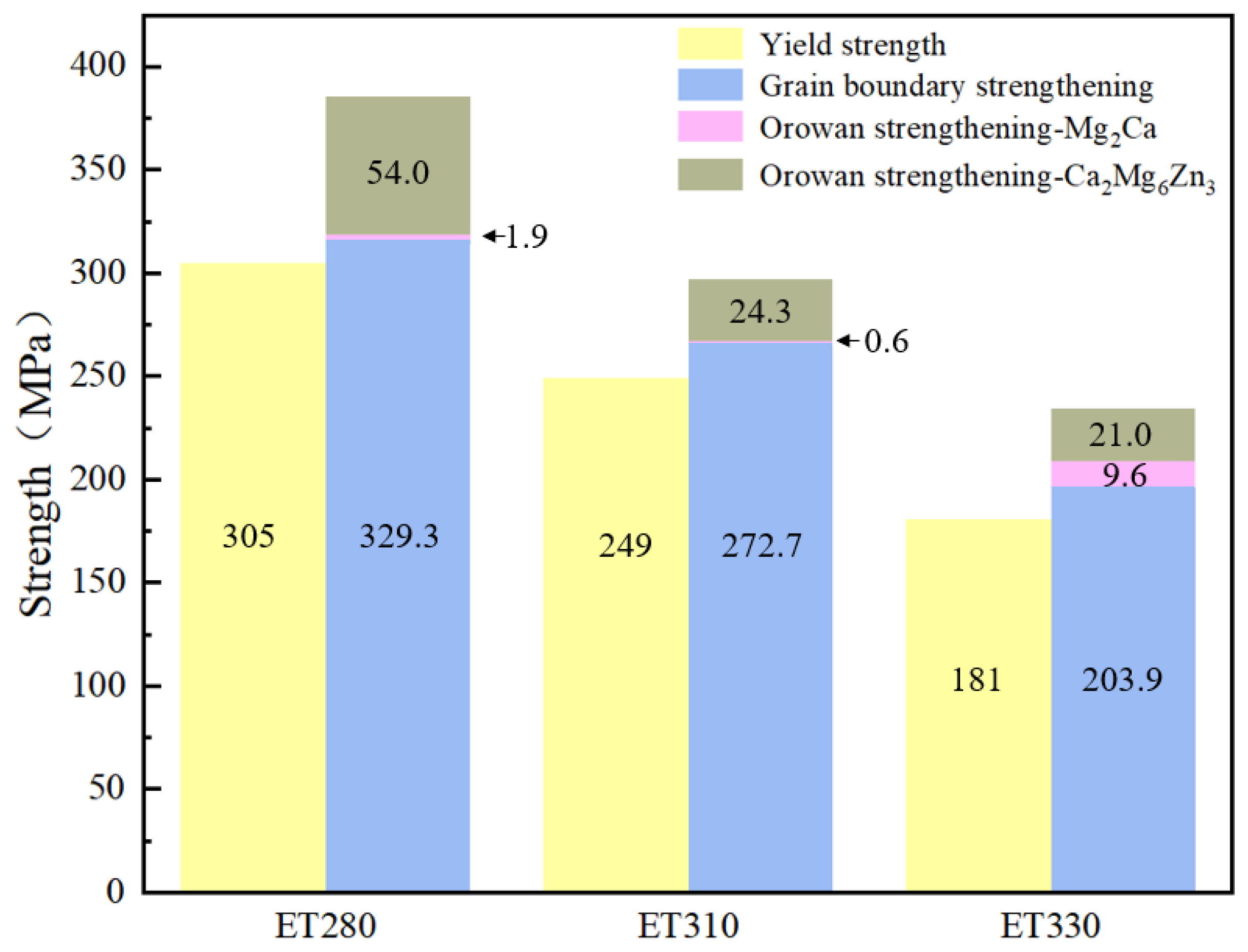
4.1. Mg-1Zn-0.4Ca-1MgO Mechanical Properties of Composites
4.2. Corrosion Mechanism of Mg-1Zn-0.4Ca-1MgO Composites
5. Conclusions
- Extrusion temperature significantly affects the grain size and second phase of Mg-1Zn-0.4Ca-1MgO composite materials. At lower temperatures, the grain size decreases, and a large number of nanoscale second-phase particles precipitate in the matrix. Specifically, as the extrusion temperature increases from 280 °C to 330 °C, the grain size increases from 0.77 μm to 3.44 μm, and a fully recrystallized structure was obtained at 330 °C.
- ET280 extruded at 280 °C achieves tensile strength, yield strength, and elongation of 312 MPa, 305 MPa, and 15.2%, respectively. The highest microhardness of 86.9 HV was also obtained among the three materials extruded at different temperatures. Grain refinement and increased fine second-phase content are the main reasons for the improved performance.
- In electrochemical tests, ET280 exhibits a corrosion potential and current density of −1.2 V and 2.01 μA/cm2, respectively, indicating good corrosion resistance. Meanwhile, the annual corrosion rate after 14 days of immersion is only 0.14 mm/y for ET280, which is lower than that of ET310 and ET330. It is consistent with the electrochemical results. The combination of the rapidly forming layers of corrosion products in the early stages of corrosion and the dense Ca-P and Mg(OH)2 product layers in the later stage contributed to the good corrosion resistance of ET280.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, J.; Lin, T.; Shao, H.; Wang, H.; Wang, X.; Song, K.; Li, Q. Advances in degradation behavior of biomedical magnesium alloys: A Review. J. Alloys Compd. 2022, 908, 164600. [Google Scholar]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [PubMed]
- Yamasaki, Y.; Yoshida, Y.; Okazaki, M.; Shimazu, A.; Kubo, T.; Akagawa, Y.; Uchida, T. Action of fgmgco3Ap-collagen composite in promoting bone formation. Biomaterials 2003, 24, 4913–4920. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, Y.; Yoshida, Y.; Okazaki, M.; Shimazu, A.; Uchida, T.; Kubo, T.; Akagawa, Y.; Hamada, Y.; Takahashi, J.; Matsuura, N. Synthesis of functionally graded MgCO3 apatite accelerating osteoblast adhesion. J. Biomed. Mater. Res. 2002, 62, 99–105. [Google Scholar]
- Chen, J.; Tan, L.; Yu, X.; Etim, I.P.; Ibrahim, M.; Yang, K. Mechanical properties of magnesium alloys for medical application: A Review. J. Mech. Behav. Biomed. Mater. 2018, 87, 68–79. [Google Scholar] [CrossRef]
- Shi, Y.; Qi, M.; Chen, Y.; Shi, P. Mao-Dcpd composite coating on Mg alloy for degradable implant applications. Mater. Lett. 2011, 65, 2201–2204. [Google Scholar]
- Cao, X.; Xu, C.; Zhang, Z.; Yang, W.; Zhang, J. Effect of I-Phase morphology and microstructure transformation in biomedical Mg-3Zn-1Mn-1Y alloys on vitro degradation behavior in dynamic simulated body fluid. J. Mater. Sci. 2021, 56, 12394–12411. [Google Scholar]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A Review From Clinical Translational Perspective. Biomaterials 2017, 112, 287–302. [Google Scholar]
- Saris, N.L.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium: An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar]
- Williams, K.; Sobol, R.W. Mutation research/fundamental and molecular mechanisms of mutagenesis: Special issue: DNA repair and genetic instability. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2013, 743–744, 1–3. [Google Scholar]
- Niranjan, C.A.; Raghavendra, T.; Rao, M.P.; Siddaraju, C.; Gupta, M.; Jain, V.K.S.; Aishwarya, R. Magnesium alloys as extremely promising alternatives for temporary orthopedic implants—A Review. J. Magnes. Alloys 2023, 11, 2688–2718. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, J.K.; Hopkins, C.; Chow, D.H.K.; Qin, L. Biodegradable magnesium-based implants in orthopedics—A general review and perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yuan, K.; Li, X.; Qiao, Y. Microstructure and properties of biomedical Mg-Zn-Ca alloy at different extrusion temperatures. Mater. Today Commun. 2023, 35, 105578. [Google Scholar] [CrossRef]
- Kumar, N.; Duhan, R.K.; Kandpal, B.C. Stir casting technology for fabrication of biodegradable magnesium metal matrix composites containing different percentages of hydroxyapatite reinforcement material for biomedical purposes. Mater. Today Proc. 2023, 10, 058. [Google Scholar] [CrossRef]
- Li, N.; Zheng, Y. Novel magnesium alloys developed for biomedical application: A Review. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Kubásek, J.; Vojtěch, D. Structural characteristics and corrosion behavior of biodegradable Mg–Zn, Mg–Zn–Gd alloys. J. Mater. Sci. Mater. Med. 2013, 24, 1615–1626. [Google Scholar] [CrossRef]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg–Zn Alloys. Mater. Sci. Eng. C 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Yin, P.; Li, N.F.; Lei, T.; Liu, L.; Ouyang, C. Effects of Ca on microstructure, mechanical and corrosion properties and biocompatibility of Mg–Zn–Ca alloys. J. Mater. Sci. Mater. Med. 2013, 24, 1365–1373. [Google Scholar] [CrossRef]
- Pulido-González, N.; Torres, B.; García-Rodríguez, S.; Rodrigo, P.; Bonache, V.; Hidalgo-Manrique, P.; Mohedano, M.; Rams, J. Mg–1Zn–1Ca alloy for biomedical applications. Influence of the secondary phases on the mechanical and corrosion behaviour. J. Alloys Compd. 2020, 831, 154735. [Google Scholar] [CrossRef]
- Du, Y.; Du, W.; Zhang, D.; Ge, Y.; Jiang, B. Enhancing mechanical properties of an Mg–Zn–Ca alloy via extrusion. Mater. Sci. Technol. 2021, 37, 624–631. [Google Scholar] [CrossRef]
- Kang, Q.; Jiang, H.; Zhang, Y.; Xu, Z.; Li, H.; Xia, Z. Effect of various Ca content on microstructure and fracture toughness of extruded Mg-2Zn alloys. J. Alloys Compd. 2018, 742, 1019–1030. [Google Scholar]
- Yurchenko, N.Y.; Stepanov, N.D.; Salishchev, G.A.; Serebryany, V.N.; Martynenko, N.S.; Lukyanova, E.A.; Rokhlin, L.L.; Birbilis, N.; Dobatkin, S.V.; Estrin, Y.Z. Effect of multiaxial deformation on structure, mechanical properties, and corrosion resistance of a Mg-Ca alloy. J. Magnes. Alloys 2022, 10, 266–280. [Google Scholar]
- Zeng, R.; Qi, W.; Cui, H.; Zhang, F.; Li, S.; Han, E. In vitro corrosion of as-extruded Mg–Ca alloys—The influence of Ca concentration. Corros. Sci. 2015, 96, 23–31. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Abdul-Kadir, M.R.; Idris, M.H.; Farahany, S. Relationship between the corrosion behavior and the thermal characteristics and microstructure of Mg–0.5Ca–xZn alloys. Corros. Sci. 2012, 64, 184–197. [Google Scholar]
- Wang, J.; Ferdowsi, M.R.G.; Kada, S.R.; Hutchinson, C.R.; Barnett, M.R. Influence of precipitation on yield elongation in Mg-Zn alloys. Scr. Mater. 2019, 160, 5–8. [Google Scholar]
- Radha, R.; Sreekanth, D. Insight of magnesium alloys and composites for orthopedic implant applications—A Review. J. Magnes. Alloys 2017, 5, 286–312. [Google Scholar] [CrossRef]
- Yim, C.D.; Kim, Y.M.; You, B.S. Effect of Ca addition on the corrosion resistance of gravity cast AZ31 magnesium alloy. Mater. Trans. 2007, 48, 1023–1028. [Google Scholar]
- Ma, Y.; Yang, C.; Liu, Y.; Yuan, F.; Liang, S.; Li, H.; Zhang, J. Microstructure, mechanical, and corrosion properties of extruded low-alloyed Mg-xZn-0.2Ca alloys. Int. J. Miner. Metall. Mater. 2019, 26, 1274–1284. [Google Scholar]
- Guan, H.; Xiao, H.; Ouyang, S.; Tang, A.; Chen, X.; Tan, J.; Feng, B.; She, J.; Zheng, K.; Pan, F. A review of the design, processes, and properties of Mg-based composites. Nanotechnol. Rev. 2022, 11, 712–730. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, B.; Zhao, R.; Yang, X.; Xiao, Z.; Aurora, A.; Iulia, B.A.; Zhu, X.; Iulian, A.V.; Zhang, X. Evaluation on the corrosion resistance, antibacterial property and osteogenic activity of biodegradable Mg-Ca and Mg-Ca-Zn-Ag alloys. J. Magnes. Alloys 2022, 10, 3380–3396. [Google Scholar]
- Zhao, J.; You, C.; Chen, M.; Lyu, S.; Tie, D.; Liu, H. Effect of calcium oxide particle size on microstructure and properties of AZ91 Mg alloy. J. Alloys Compd. 2021, 886, 160970. [Google Scholar]
- Gu, X.; Zhou, W.; Zheng, Y.; Dong, L.; Xi, Y.; Chai, D. Microstructure, mechanical property, bio-corrosion and cytotoxicity evaluations of Mg/HA composites. Mater. Sci. Eng. C 2010, 30, 827–832. [Google Scholar]
- Lin, G.; Liu, D.; Chen, M.; You, C.; Li, Z.; Wang, Y.; Li, W. Preparation and characterization of biodegradable Mg-Zn-Ca/MgO nanocomposites for biomedical applications. Mater. Charact. 2018, 144, 120–130. [Google Scholar]
- Tang, C.; Lyu, S.; Zhao, Z.; Chen, M. Effects of MgO nano particles on the mechanical properties and corrosion behavior of Mg–Zn–Ca alloy. Mater. Chem. Phys. 2023, 297, 127380. [Google Scholar]
- Fan, Z.; Gao, F.; Wang, Y.; Wang, S.H.; Patel, J.B. Grain refinement of Mg-alloys by native MgO particles: An Overview. J. Magnes. Alloys 2022, 10, 2919–2945. [Google Scholar] [CrossRef]
- Zhao, Q.; Lyu, S.; Zhang, G.; Chen, M. Effect of MgO contents on the microstructure, mechanical properties and corrosion behavior of low-alloyed Mg-Zn-Ca alloy. Metals 2024, 14, 274. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, L.; Li, X.; Zhang, Z.; Sun, Y.; Wang, J. Notoginsenoside R1 suppresses wear particle-induced osteolysis and rankl mediated osteoclastogenesis in vivo and in vitro. Int. Immunopharmacol. 2017, 47, 118–125. [Google Scholar]
- Wang, H.; Zhang, D.T.; Qiu, C.; Zhang, W.W.; Chen, D.L. Microstructure and tensile properties of a low-alloyed magnesium alloy: Effect of extrusion temperature. J. Mater. Sci. 2023, 58, 13502–13517. [Google Scholar]
- Hu, H.; Ying, Y.; Zhang, D. Relationship between extrusion temperature and corrosion resistance of magnesium alloy AZ61. Mater. Test. 2018, 60, 325–332. [Google Scholar] [CrossRef]
- GB/T 16865-2013; National Standard of the People’s Republic of China. Standardization Administration of PRC (SAC): Beijing, China, 2017.
- ASTM G31-72; Standard Practice for Laboratory Immersion Corrosion Testing of Metals. American Society for Testing and Materials: West Conshohocken, PA, USA, 2004.
- Fu, J.; Du, W.; Liu, K.; Du, X.; Zhao, C.; Liang, H.; Mansoor, A.; Li, S.; Wang, Z. Effect of the Ca2Mg6Zn3 phase on the corrosion behavior of biodegradable Mg-4.0Zn-0.2Mn-xCa alloys in Hank’s solution. Materials 2022, 15, 2079. [Google Scholar] [CrossRef]
- Li, S.; Sun, B. Preparation of corrosion-resistant surface of magnesium alloy and its performance study. Mater. Res. Express 2023, 10, 96402. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, Z.; Li, G.; Yao, Y.; Yu, H.; Liu, Y.; Zheng, T.; Xiong, H. Microstructure, mechanical and corrosion properties of Mg-1.61Al-1.76Ca alloy under different extrusion temperatures. J. Mater. Eng. Perform. 2020, 29, 672–680. [Google Scholar] [CrossRef]
- Nie, K.; Guo, Y.; Munroe, P.; Deng, K.; Kang, X. Microstructure and tensile properties of magnesium matrix nanocomposite reinforced by high mass fraction of nano-sized particles including TiC and MgZn2. J. Alloys Compd. 2020, 819, 153348. [Google Scholar] [CrossRef]
- Habibi, M.K.; Joshi, S.P.; Gupta, M. Hierarchical magnesium nano-composites for enhanced mechanical response. Acta Mater. 2010, 58, 6104–6114. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. Magnesium in the murine artery: Probing the products of corrosion. Acta Biomater. 2014, 10, 1475–1483. [Google Scholar] [CrossRef]







| Samples | Extrusion Temperature (°C) | Extrusion Speed (mm/s) | Extrusion Ratio |
|---|---|---|---|
| ET280 | 280 | 2 | 29:1 |
| ET310 | 310 | 2 | 29:1 |
| ET330 | 330 | 2 | 29:1 |
| Reagent Name | C6H₃N₃O₇ | C2H5OH | CH₃COOH | H2O |
|---|---|---|---|---|
| Content | 2.75 g | 45 mL | 15 mL | 5 mL |
| Reagent | Hank’s |
|---|---|
| NaCl | 8.00 |
| MgSO4·7H2O | 0.10 |
| KCl | 0.40 |
| MgCl2·6H2O | 0.10 |
| CaCl2 | 0.14 |
| Na2HPO4·12H2O | 0.12 |
| KH2PO4 | 0.06 |
| C6H12O6 | 1.00 |
| NaHCO3 | 0.35 |
| Samples | Total Content of the Second Phase (%) | Mg2Ca (%) | Ca2Mg6Zn3 (%) |
|---|---|---|---|
| ET280 | 2.16 ± 0.11 | 0.46 ± 0.06 | 1.70 ± 0.08 |
| ET310 | 1.51 ± 0.08 | 0.41 ± 0.04 | 1.10 ± 0.03 |
| ET330 | 0.28 ± 0.01 | 0.08 ± 0.01 | 0.20 ± 0.01 |
| Samples | Average Grain Size/μm | Average Mg2Ca Size/μm | Average Ca2Mg6Zn3 Size/nm |
|---|---|---|---|
| ET280 | 0.77 ± 0.01 | 1.58 ± 0.15 | 71.07 ± 1.75 |
| ET310 | 1.32 ± 0.01 | 3.07 ± 0.21 | 139.45 ± 3.22 |
| ET330 | 3.44 ± 0.02 | 0.08 ± 0.01 | 55.39 ± 1.82 |
| Samples | Yield Strength (YS) | Ultimate Tensile Strength (UTS) | Elongation (EL) |
|---|---|---|---|
| ET280 | 305 | 312 | 15.1 |
| ET310 | 249 | 271 | 15.2 |
| ET330 | 181 | 240 | 15.4 |
| Samples | Ecorr (V) | Icorr (μA/cm2) | Rs (Ω) | CPEct-C | Rct (Ω) | CR (mm/y) |
|---|---|---|---|---|---|---|
| ET280 | −1.2 | 2.01 | 40.48 | 4.126 × 10−5 | 6832 | 0.46 |
| ET310 | −1.198 | 2.98 | 38.62 | 4.17 × 10−5 | 4484 | 0.68 |
| ET330 | −1.223 | 3.35 | 53.35 | 2.320 × 10−5 | 3263 | 0.77 |
| Samples | /MPa | /MPa | /MPa | |
|---|---|---|---|---|
| ET280 | 329.3 | 1.9 | 54.0 | 385.2 |
| ET310 | 272.7 | 0.6 | 24.3 | 297.6 |
| ET330 | 203.9 | 9.6 | 21.0 | 234.5 |
| Points | Samples | Elements (at%) | ||||
|---|---|---|---|---|---|---|
| Mg | Zn | Ca | P | O | ||
| A | ET280 | 8.24 | 0.27 | 25.31 | 14.54 | 51.64 |
| B | ET310 | 2.04 | 0.20 | 42.61 | 15.32 | 39.83 |
| C | ET310 | 42.15 | 0.73 | 0.96 | 0.84 | 55.32 |
| D | ET310 | 12.08 | 0.43 | 26.62 | 9.23 | 51.64 |
| E | ET330 | 10.80 | 0.36 | 21.07 | 12.00 | 55.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Lyu, S.; Zhao, Q.; Chen, M. Effect of Extrusion Temperature on the Microstructure and Properties of Biomedical Mg-1Zn-0.4Ca-1MgO Composite. Metals 2025, 15, 337. https://doi.org/10.3390/met15030337
Gao S, Lyu S, Zhao Q, Chen M. Effect of Extrusion Temperature on the Microstructure and Properties of Biomedical Mg-1Zn-0.4Ca-1MgO Composite. Metals. 2025; 15(3):337. https://doi.org/10.3390/met15030337
Chicago/Turabian StyleGao, Shuaipeng, Shaoyuan Lyu, Qian Zhao, and Minfang Chen. 2025. "Effect of Extrusion Temperature on the Microstructure and Properties of Biomedical Mg-1Zn-0.4Ca-1MgO Composite" Metals 15, no. 3: 337. https://doi.org/10.3390/met15030337
APA StyleGao, S., Lyu, S., Zhao, Q., & Chen, M. (2025). Effect of Extrusion Temperature on the Microstructure and Properties of Biomedical Mg-1Zn-0.4Ca-1MgO Composite. Metals, 15(3), 337. https://doi.org/10.3390/met15030337






